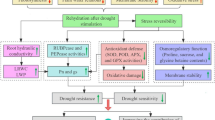
Abstract
In a greenhouse study, we aimed to determine whether a temporary water deficit induces ‘drought memory’ in sugar beet (Beta vulgaris L.), and whether this effect can be quantified by alterations in the fluorescence signature of the leaves. Plants were subjected to three consecutive water deficit phases, each followed by a recovery period, and in each cycle new, fully developed leaves were analyzed. Changes in the photosynthetic performance and pigment fluorescence were recorded with a hand-held fluorescence sensor, a laser-induced fluorescence spectrometer, and a leaf gas exchange analyzer. Parameters such as osmotic potential, proline, and chlorophyll content were used as indicators for biochemical modifications and quantification of stress intensity. In general, the evaluated cultivars showed a similar response pattern to water deficit, although the intensity of the stress-induced modification was not always on the same level in the distinct parameters. The long-term and repeated drought caused a decrease of net photosynthesis, increase of far-red fluorescence, and a decrease of both the ‘Simple Fluorescence Ratio’ and the fluorescence lifetime (LT mean) in the blue spectral region. In the second drought cycle, changes in osmotic potential and proline content were lower, but alterations in photosynthesis and fluorescence were as strong as in the first and third drought cycles. This indicates that even if a drought stress memory might occur, it was not possible to precisely identify it using gas exchange and pigment fluorescence determinations. Irrespective of that, the photosynthesis and chlorophyll fluorescence-based parameters (RF, SFR) clearly indicated with high temporal resolution the response of sugar beet plants to the stress, and their partial recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In their life cycle, plants commonly face a number of stress situations that negatively influence their development and agronomic performance. In general, water deficit is one of the main environmental factors limiting growth and productivity of crops (Montesinos-Pereira and others 2014). Under drought, photosynthetic efficiency is decreased as stomatal closure restricts CO2 uptake (Pantin and others 2013). Under extended drought, several non-stomatal mechanisms inhibit essential cellular processes required to maintain photosynthesis (Flexas and Medrano 2002). Alterations in the photosynthetic activity are also associated with a decrease of chlorophyll concentration as a consequence of membrane disturbances in the mesophyll cells (Cornic and Masacci 1996). Finally, several physiological, biochemical and molecular responses occur to maintain plant vitality and survival during drought (Reddy and others 2004).
In general terms, a better understanding of the response mechanisms of plants to water deficit is essential to enhance their drought tolerance (Thapa and others 2011). In their evolution, plants have developed morphological, physiological, and biochemical mechanisms to adapt and overcome stress phases (Biswal and others 2011; Conde and others 2011), as reported for different plant species under different conditions. Moreover, plants might have some kind of stress ‘memory’, which could support their fitness in response to recurrent environmental stresses (Thellier and others 1982, 2013). Although the precise mechanisms involved in regulation of physiology and molecules is poorly understood (Hu and others 2015), epigenetic changes and sustained alterations of important metabolites or transcription factors support the transgeneration of stress memory in plants (Bruce and others 2007; Kinoshita and Seki 2014; Hu and others 2015; Molinier and others 2006). In Arabidopsis, the involvement of abscisic acid (ABA)-dependent pathways for stress memory was proven (Goh and others 2003). Specifically on water deficit, plants with transcriptional stress memory displayed an increase in the rate of transcription, and elevated transcript levels, of a subset of the stress–response genes (Ding and others 2012). Similarly, it had been shown that even perennial plants and long-lived trees (Thellier and Lüttge 2013) as well as grasses (Hu and others 2015; Walter and others 2011) might store and recall stress imprints.
Although the absolute majority of ‘stress memory’ studies focus on responses at the transcript level, there are only a few publications on the effects on plant physiology or in a more applied sense, the agronomic performance of crops. In one of the rare examples, it has been proposed that the grass Arrhenatherum elatius might have a drought memory over an entire vegetation period, as demonstrated using chlorophyll a fluorescence as an indicative technique (Walter and others 2011). In sugar beet (Leufen and others 2013, 2014), physiological measurements as well as visual observations clearly demonstrate a fast recovery of the stressed plants when the optimum growing conditions were re-established. In this context, it is also not clear if stress imprints might also be stored in roots, such as the pronounced storage root of sugar beet.
In our previous studies, we demonstrate the potential of non-invasive fluorescence techniques for the characterization of physiological responses of different plant species such as tomatoes (Kautz and others 2014), cereals (Bürling and others 2013), and sugar beet (Leufen and others 2013, 2014) when exposed to water deficit. In this scope, instead of using the classical pulse-amplitude chlorophyll fluorescence we adopted either the spectrally resolved fluorescence, the fluorescence lifetime, or the multiparametric fluorescence technique, all of them providing precise indications of stress-induced alterations of plant physiology as well as the type and content of pigments in the cells. Spectrally resolved fluorescence lifetime provides precise information on the stress-induced alteration of the amount and composition of fluorescing pigments in the plant tissue (Bürling and others 2011, 2012; Cerovic and others 1994; Morales and others 1994; Cerovic and others 1996). The intensity of red fluorescence, that is, its change under stress, is a fast indicator of alterations in the photochemistry, while the Simple Fluorescence Ratio (SFR), an index derived from the far-red and red fluorescence, strongly correlates with the chlorophyll content but also reflects changes of the photochemistry in plants under stress (Leufen and others 2013; Ben Ghozlen and others 2010).
In commercial cultivations, sugar beet is grown over a comparatively long period (until 8 months), and has a prominent root system which could, in addition to the leaves, store stress imprints for later recall. However, to our knowledge, it is still unknown whether sugar beet plants possess such a ‘memory’ which could enable them to better overcome recurrent stresses. The objective of the trial was to study the existence of drought stress memory in sugar beet in a long-term greenhouse experiment under semi-controlled conditions, with focus on physiological parameters. Based on our previous work and indications from the literature we hypothesized that, if existent, the memory effect in sugar beet plants would be identified by less accentuated changes in the fluorescence signature in recurrent stress phases.
Materials and Methods
Plant Material and Growth Conditions
The experiment was conducted from October 2011 to March 2012 in a heated greenhouse (temperature range at plant level: daytime 20–22 °C, nighttime 18–20 °C; photoperiod: 16 h enabled by supplemental light). Seeds of the sugar beet (Beta vulgaris L.) cultivars Pauletta, OVK and 8GK were provided by the company KWS Saat AG (Einbeck, Germany). These cultivars were selected because of their differences in leaf morphology and general plant performance as indicated by the plant breeder (personal communication Dr. Britta Schulz, KWS Saat AG, Einbeck, Germany), and confirmed in our preliminary trials. Seeds without any agrochemical treatment were germinated in a sowing tray, and after one week uniform plants were transplanted into 4 l plastic pots (0.233 m high, 0.157 m diameter) evenly filled with peat substrate (Einheitserde Typ VM, Einheitserde- und Humuswerke Gebr. Patzer GmbH & Co.KG, Sinntal-Altengronau, Germany). Plants were assigned to the experimental treatments (n = 4 per cultivar, treatment and evaluation date) and placed at random on two benches (10.5 × 1.65 m) with automatic nutrient supply (pH 6.5 and an EC 180 mS cm−1). The photoperiod (16 h) and photosynthetic active radiation (250–350 µmol−2s−1) were enabled by supplemental light from high-pressure sodium lamps (Philips SON-T Agro 400 W, Philips Electronics N.V., Hamburg, Germany). Water deficit was induced by withholding irrigation on three consecutive phases, considering the number of days after sowing (DAS) as the time-reference: 35–54 DAS, 86–102 DAS, and 135–151 DAS. Cessation of irrigation caused increasing water deficit stress in the time course of the experiment until rewatering of plants. In the periods between the two water deficit phases, plants were allowed to recover under full irrigation.
Non-Destructive Determinations
Multiparametric Fluorescence
Fluorescence recordings were done in the laboratory at leaf level using a hand-held optical fluorescence sensor (Multiplex® 3, Force-A, Orsay, France), as previously described (Leufen and others 2013, 2014). Briefly, light-emitted-diodes (LED) excite sequentially the fluorescence with UV (peak at 375 nm), green (peak at 518 nm) and red (peak at 630 nm) light and the fluorescence signals are recorded in the blue (425–475 nm), red (680–690 nm), and far-red (720–755 nm) spectral regions. A grid in front of the sensor enabled a constant distance of 0.10 m between sensor and leaves; thereby, an area of approximately 50 cm2 was illuminated. Recordings were always taken on the two upper, fully expanded opposite leaves of each plant. As target parameters, we selected the red fluorescence (RF_G), and the Simple Fluorescence Ratio (SFR) after excitation with green light.
Fluorescence Lifetime
Leaves were fixed horizontally on a sample holder by maintaining a constant distance (3.95 mm) between sample and fiber-optical probe. Fluorescence lifetimes were recorded on the leaf tip, about 2 cm from the leaf margin, by avoiding major veins. Fluorescence lifetime was recorded with a compact fiber-optic spectrometer (IOM GmbH, Berlin Germany), as described elsewhere (Bürling and others 2011, 2012). Briefly, a pulsed nitrogen laser (MNL 100, LTB Lasertechnik Berlin GmbH, Berlin, Germany) excites the plant tissue (337 nm, repetition rate of 30 Hz). The pulse energy at the probe exit was adjusted to be 8–8.5 µJ. A photomultiplier (PMT, H5783-01, Hamamatsu, Hamamatsu City, Japan), with a sensitivity of 800 Volt, was used as the detector. The fluorescence lifetime was recorded in a range of 410–560 nm in the interval of 30 nm. The detection gate was opened from 0.0 to 16 ns following excitation and the step width of the integrator gate was set to 0.4 ns. Each single data point was averaged from 16 pulse counts. Fluorescence decays were analyzed using deconvolution software (DC4, V. 2.0.6.3, IOM GmbH, Berlin, Germany).
Gas Exchange
Gas exchange was measured with a portable infrared gas analyzer (CIRAS-1, PP Systems, United Kingdom) equipped with a leaf cuvette (PLC B, PP Systems, United Kingdom) covering an area of 2.5 cm2. Net photosynthetic rate (Pn), stomatal conductance (G), internal CO2 partial pressure (CI), and transpiration rate (E) were measured at the leaf tip by avoiding major veins. Readings were performed in the greenhouse under standardized conditions at a measuring station to minimize the effect of the environment. Recordings were done from 17:00 h to 19:30 h to avoid midday-depression and minimize the impact of external light. Equipment settings were adjusted at an internal CO2 concentration of 350 ± 5 ppm, light irradiation on the leaf surface was about 250–350 μmol m−2 s−1 PAR and the air flow entering the chamber was 200 ± 5 ml min−1. For the measurements, pots of plants were taken randomly to avoid systematic errors concerning the measuring time, and transported to the measuring station. Although the light intensity for photosynthesis determination was not saturating, the conditions corresponded to those find on the cultivation tables. Values of photosynthesis were recorded when steady-state was attained.
Reference Parameters
Sampling Method
Leaves were harvested at irregular intervals in the timeframe from 43 to 159 DAS. Thereby, the leaves previously used for the fluorescence recordings were stored in plastic bags at −21 °C for later quantification of chlorophyll and proline contents. The underlying leaf pair was stored for the consecutive determination of osmotic potential. Samples for determination of chlorophyll and proline were lyophilized, ground, and stored in the dark at room temperature.
Chlorophyll Concentration
Chlorophyll was extracted from 50 mg lyophilized material by 5 ml methanol, and filled up to 50 ml. After extraction the absorbance of extracts was measured at 665 nm (A665) and 650 nm (A650) with a UV–VIS spectrophotometer (Perkin-Elmer, Lambda 5, Massachusetts, USA). The concentration of chlorophyll a (Ca), b (Cb), and total chlorophyll content (Ct) was calculated with the following equations, as published by Hoffmann and others (2015):
Proline Concentration
Determination of proline followed the well-established method (Bates and others 1973) which was adjusted and optimized to experimental conditions (Hoffmann and others 2015). Of each sample, 20 mg lyophilized material was mixed with 3 ml sulfosalicylic acid (3 %), homogenized and afterwards centrifuged at 4000×g for 15 min. under room temperature. Thereafter, 0.4 ml of the supernatant was added to 1.6 ml sulfosalicylic acid (3 %) by gently shaking while adding 2 ml of glacial acetic and ninhydrin acid. The solution was placed for 1 h in a 100 °C water bath; after cooling down, 4 ml toluene was added. The upper part of the solution was pipetted and the absorbance of the extracts was analyzed at 520 nm with a UV–VIS spectrophotometer (Perkin-Elmer, Lambda 5, Massachusetts, USA). The concentration of proline was calculated with the following equation:
Osmotic Potential
Osmotic potential was determined according to Kautz and others (2014). Samples were placed in bags (Bioreba, Switzerland) and extruded with a hand homogenizer. Thereafter, 2 ml of the extract was collected, filled and centrifuged (Eppendorf, Centrifuge 5417 R, Hamburg, Germany) for 10 min (25,000×g min−1 at 4 °C). From the supernatant, 15 µl were pipetted into tubes and the osmolality measured with a freezing-point depression osmometer (Osmomat 030-D, Genotec GmbH, Berlin, Germany). At the beginning of the measurements, the osmometer was calibrated using preformed Genotec vials (850 mmol kg−1 H2O) and distilled water (0 mmol kg−1 H2O).
Statistical Analysis
In several cases, results are presented as percent of modification (as compared to the respective control group) to enable more precise comparisons between cultivars and evaluations over time. Data were statistically analyzed with SPSS statistical software (PASW statistics version 19.0, SPSS Inc., Chicago, USA). For each cultivar and evaluation date, control and temporary non-irrigated plants were compared by analysis of variance and paired t test (p ≤ 0.05).
Results
Biochemical Indicators: Osmotic Potential, Proline and Chlorophyll Concentration
Modifications of the leaf osmotic potential, proline concentration and total chlorophyll concentration to temporary water deficit and recovery are shown in Fig. 1. As compared to control plants, water deficit caused a strong increase of leaf osmotic potential (OP) during and immediately after the first drought period (53–65 DAS), reaching a maximum of 150 % as compared to control plants; this alteration was most pronounced in the cultivar OVK (Fig. 1a). At the same time, proline content increased to a much higher extent (Fig. 1b) reaching a maximum of 1000 %, whereas chlorophyll concentration decreased in the worst case to about 60 % (Fig. 1c). In the recovery phase, values of proline approached those of the control plants whereas osmotic potential and chlorophyll content still remained altered.
Influence of temporary water deficit and re-watering on the osmotic potential (a), proline concentration (b), and the total chlorophyll concentration (c) of the cultivars Pauletta, OVK and 8GK. Recordings (here, displayed as relative percent to the irrigated, control plants) were done on selected days (53, 56, 65, 101, 104, 112, 151, 153, and 159 DAS). Asterisks indicate significant differences with a p ≤ 0.05 (t test), between irrigated (control) plants and temporarily non-irrigated (stress, that is, recovery) plants for each cultivar and measuring day (n = 4)
In the second stress cycle, alterations during water deficit were generally less accentuated and the recovery of parameters to ‘normal’ values was also observed not only for proline (Fig. 1b) but also for osmotic potential (Fig. 1a). In the third cycle, the biochemical response of plants was higher than in the second phase, with the exception of chlorophyll content. As observed in the three consecutive cycles, decrease in chlorophyll content was stronger immediately after rewatering the stressed plants, followed by a slight recovery in the following days. In general, we observed significant discrepancies in the intensity and speed of changes of the three parameters (osmotic potential, proline, and chlorophyll content) when plants were exposed to drought stress and recovery. The strongest variations were ascertained for ‘OVK’ during the first and second phases.
Net Photosynthesis and Transpiration
Gas exchange was measured to assess the progress and intensity of drought on the most basic plant process, photosynthesis. In all three stress cycles, photosynthesis decreased very strongly, reaching values close to zero shortly before the recovery phase (Fig. 2a–i). Transpiration was affected in a similar way (Fig. 3a–i), with less pronounced stress-driven alterations in the second phase. As a general pattern, following the rewatering plants reached values similar to those measured on control plants, except in the third phase. In general, no concrete hints of drought memory could be observed here; one exception, however, is found in the third stress cycle for the cultivar 8GK. Here, the minimum net photosynthesis, although very low (about 3 µmol m−2 s−1) was still significantly higher than both the values observed for the other cultivars in the same phase (below 1 µmol m−2 s−1) and the values of the same cultivar in the previous stress cycles (below 0.5 µmol m−2 s−1).
Leaf net photosynthesis (µmol m−2 s−1) of the sugar beet cultivars Pauletta (a–c), OVK (d–f), and 8GK (g–i) influenced by water supply. Measurements took place under semi-controlled conditions in the greenhouse in three consecutive phases, 35–58 DAS, 86–120 DAS, and 135–157 DAS, on leaves of irrigated (control) and temporarily non-irrigated (stressed) plants; Asterisks indicate significant differences (t test, p ≤ 0.05) between control and stressed plants. Mean ± SE (n = 4)
Development of leaf transpiration (mol m−2 s−1) on the sugar beet cultivars Pauletta (a–c), OVK (d–f), and 8GK (g–i) influenced by water supply. Measurements took place under semi-controlled conditions in the greenhouse in three consecutive phases, 35–58 DAS, 86–120 DAS, and 135–157 DAS, on leaves of irrigated (control) and temporarily non-irrigated (stressed) plants; Asterisks indicate significant differences (t test, p ≤ 0.05) between control and stressed plants. Mean ± SE (n = 4)
Red Fluorescence (RF) and Simple Fluorescence Ratio (SFR_G)
The development of the green-excited red fluorescence (RF_G, Fig. 4a–c) and the ‘Simple Fluorescence Ratio’ (SFR, Fig. 4d–f) over the three experimental phases (35–65 DAS, 86–121 DAS, and 135–159 DAS) are displayed exemplarily in the cultivar Pauletta. In all experimental cycles, starting at the third recording date, RF_G of stressed plants was higher than RF_G of control plants; in the sequence, values reached or at least approached the level of control plants at the end of the recovery phase (Fig. 4a–c). Nevertheless, the highest increase of RF_G was observed during the second cycle (Fig. 4b), which was, according to the biochemical indicators, the phase with lower stress (Fig. 1). A detailed analysis of the SFR demonstrates a stress-induced decrease of the values, however, following similar trends as observed for RF_G (Fig. 4c–d). Both parameters were also recorded for the cultivars OVK and 8GK (Figs. S1, S2). In general, the chlorophyll fluorescence parameters (FR_G and SFR) of these cultivars follow a similar pattern as the trends reported for Pauletta. Nevertheless, ‘OVK’ responded more sensitively to desiccation than the other cultivars, as FR_G and SFR values declined immediately after the water supply was stopped during the first experimental period (Fig. S1). Irrespective of that, a clear response to the water deficit in the second or third stress cycle could not be related to the plant response in the preceding stress cycle.
Influence of water supply on the red fluorescence (RF_G, a–c) and on the ‘Simple Fluorescence Ratio’ (d–f) of the sugar beet cultivar Pauletta measured after excitation with green light. Fluorescence recordings were taken at leaf level between 41 and 159 DAS in three consecutive phases. Gray regions in the graph illustrate the periods where water supply was stopped for the stress treatment. Values indicate mean ± SE (n = 8). Asterisks indicate significant values with a p ≤ 0.05 (t test) between leaves of irrigated (control) and temporarily non-irrigated (stressed) plants for each measuring day
Fluorescence Lifetime
Fluorescence mean lifetime was lower in plants exposed to water deficit as compared to control plants, mainly in the blue spectral region (410 and 440 nm). Numerical differences were also observed in the green region (500 and 560 nm), but in most cases statistical significance (p < 0.05) was not asserted. This was observed for the cultivar Pauletta (Table 1) as well as for the cultivars OVK and 8GK (data not shown). As observed, in the recovery phase the values measured in the stressed plants could not reach those levels recorded in control plants. For all cultivars, the most suited wavelength to distinguish the experimental treatments was at 410 nm (Table 1). Nevertheless, precise indications of drought memory could not be detected using this spectroscopic method.
Discussion
In our experiments, we used selected non-invasive methods to study plant responses to transient and recurrent water deficit, aiming to exploit and better understand mechanisms of drought memory. Due to the fast setup of the storage organ in beets, starting two weeks after emergence (Rapoport and Loomis 1986), we expected improved stress response by adjusted sink-source regulations in beets, which should become visible through lower stress-related changes in biochemical and physiological parameters during the second and/or third stress period. Moreover, based on plant and leaf morphology, as well as the general plant growth performance under adverse conditions such as drought, we expected pronounced differences in the behavior of the three cultivars to the repeated water deficit cycles. This could not be confirmed because the evaluated cultivars presented a similar response pattern to water deficit, but the degree of changes induced by stress was not always at the same level for the distinct parameters and sampling times.
When drought stress begins, stomatal closure reduces CO2 assimilation (Cornic and Massacci 1996), in our experiments causing a decline in leaf net photosynthesis in all evaluated cultivars (Fig. 2). A decrease in photosynthesis is usually accompanied by increased heat dissipation and chlorophyll fluorescence as immediate mechanisms to avoid damage to the photosystems. Extended and severe water deficit led to further structural and functional disturbances in the photosynthetic apparatus, illustrated by strong alterations in chlorophyll fluorescence indices (Fig. 4, Supplemental Material Figs. S1, S2). Thereby, the increase in the red fluorescence and the simultaneous decrease in the SFR_G throughout the individual stress periods can be linked to an impairment of the photosynthetic quantum conversion and a lower capacity for light-harvesting (Iturbe-Ormaetxe and others 1998; Lichtenthaler and Rinderle 1988; Mafakheri and others 2010). Similar results were also obtained in previous studies (Leufen and others 2013, 2014). Nevertheless, the new information is that these changes follow a similar trend in all three experimental phases, despite the different development stages of the beets.
Even if the physiological readings of drought-exposed plants indicate similar trends in the three cycles (Figs. 2, 3, 4), biochemical parameters respond significantly less intensively to desiccation during the second period (Fig. 1). This effect might be explained by improved osmotic adjustment in beets. It is known that proline acts as a stress indicator but also significantly contributes to osmotic adjustment (Molinari and others 2007); this adjustment happens together with other compounds, increasing the osmotic potential during drought (Ingram and Bartels 1996). The decline in the total chlorophyll concentration is caused by lower chlorophyll synthesis, as well as the formation of reactive oxygen species which induce oxidative stress in proteins, membrane lipids, and other cellular components (Farooq and others 2009; Molinari and others 2007).
In contrast to the chlorophyll content, net photosynthesis and all chlorophyll fluorescence indices recover very soon after re-watering. It is known that severe drought stress might increase synthesis of ABA preventing excessive water loss through excessive transpiration as well as accelerating senescence due to recycling of vital nutrients (Munné-Bosch and Alegre 2004; Wingler and Roitsch 2008). On the other hand, during recovery of plants cytokinins might delay senescence and/or induce stomatal opening (Vomáčka and Pospíšilová 2003).
Mean fluorescence lifetime recordings in the blue-green spectral range did not show any hints of improved stress tolerance in recurrent stress cycles. Thereby, lifetime was numerically and in some cases statistically lower in drought stressed plants than in control plants leaves (Table 1). Differences might be associated with a decrease of blue-green fluorescing compounds, for example, ferulic acid, p-cumaric acid, through dehydration, which finally caused lower mean fluorescence lifetimes (Cerovic and others 1994; Morales and others 1994; Sgherri and others 2004).
Even if we could assess non-invasively drought-related stress patterns in all three sugar beet cultivars, a ‘drought memory’ as indicated on annual grasses using classical chlorophyll fluorescence (Walter and others 2011), could not be proven in our study. Amongst others, morphological and physiological differences between mono and dicotyledonous plants might play a significant role in explaining the weaker stress responses in the second period besides comparable experimental conditions in the three cycles (Stober and Lichtenthaler 1993; Cerovic and others 1999). Further, the sink-source relations in the different stress phases might have influenced both the response of the plants detected with physiological and biochemical parameters. In this context, there is a higher relevance to maintain existing structures in beets under stress than the storage process (Shaw and others 2002). With our experimental setup, plants exposed to drought could effectively accumulate substances either before the trial or during the recovery phases after stress. Particularly in the second stress cycle, the comparatively lower stress-induced alteration of the biochemical parameters might be explained by a decrease in the concentration of sucrose and other compounds in the storage root (Bloch and others 2006). If the storage substances are not available anymore, and starvation happens in the leaves, biochemical parameters might be more strongly affected than physiological parameters, as observed again in the third stress cycle. In this context, the hypothesis of accelerated senescence at the end of the third phase can be excluded because in each cycle new, fully developed leaves were selected for the recordings, whereas the plant of sugar beet, a bi-annual species under natural environments, can continuously produce new leaves.
Taking this fact into consideration, the detection and elucidation of a’memory effect’ in species that build up a pronounced storage organ during their life cycle, as sugar beets do, is particularly difficult. Unfortunately, in our experimental setup, we had no plants exposed to only a single drought event at any one of the stress cycles. Thus, we cannot differentiate if the lower sensitivity to drought during the second period was caused by growth-dependent alterations and/or changes in levels of key signaling metabolites or transcription factors initiated by previous stress (Bruce and others 2007). In this context, a more precise elucidation requires extended destructive analysis of shoot and root components such as ABA, carbohydrates, phenolic compounds and soluble constituents on top of our determinations.
Conclusion
Our study indicates that the ‘Simple Fluorescence Ratio’ is a reliable parameter to assess the physiological state of sugar beet plants to changing water supply conditions. Nevertheless, similar to the leaf net photosynthesis, fluorescence parameters did not provide strong indications of ‘drought memory’. In general, we observed no clear relation in the different cycles between results of biochemical and physiological parameters. Thus, further studies are needed to clarify the details of plant physiological mechanisms under changing water supply situations, involving also investigations of the root-body, as the main source for providing reserve substances under harmful growth conditions.
References
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Ben Ghozlen N, Cerovic ZG, Germain C, Toutain S, Latouche G (2010) Non-destructive optical monitoring of grape maturation by proximal sensing. Sensors 10:10040–10068
Biswal B, Joshi PN, Raval MK, Biswal UC (2011) Photosynthesis, a global sensor of environmental stress in green plants: stress signalling and adaptation. Curr Sci 101:47–56
Bloch D, Hoffmann CM, Märländer B (2006) Solute accumulation as cause for quality losses in sugar beet submitted to continuous and temporary drought stress. J Agron Crop Sci 192:17–24
Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci 173:603–608
Bürling K, Hunsche M, Noga G, Pfeifer L, Damerow L (2011) UV-induced fluorescence spectra and lifetime determination for detection of leaf rust (Puccinia triticina) in susceptible and resistant wheat (Triticum aestivum) cultivars. Funct Plant Biol 38:337–345
Bürling K, Hunsche M, Noga G (2012) Detection of powdery mildew (Blumeria graminis f. sp. tritici) infection in wheat (Triticum aestivum) cultivars by fluorescence spectroscopy. Appl Spectrosc 66:1411–1419
Bürling K, Cerovic ZG, Cornic G, Ducruet JM, Noga G, Hunsche M (2013) Fluorescence-based sensing of drought-induced stress in the vegetative phase of four contrasting wheat genotypes. Environ Exp Bot 89:51–59
Cerovic ZG, Morales F, Moya I (1994) Time-resolved spectral studies of blue-green fluorescence of leaves, mesophyll and chloroplasts of sugar beet (Beta vulgaris L.). Biochim Biophys Acta 1188:58–68
Cerovic ZG, Goulas Y, Gorbinov M, Briantais J-M, Camenen L, Moya I (1996) Fluorosensing of water stress in plants: diurnal changes of the mean lifetime and yield of chlorophyll fluorescence, measured simultaneously and at distance with a LIDAR and a modified PAM-fluorimeter, in maize, sugar beet, and kalanchoe. Rem Sens Environ 58:311–321
Cerovic ZG, Samson G, Morales F, Tremblay N, Moya I (1999) Ultraviolet-induced fluorescence for plant monitoring: present state and prospects. Agronomie 19:543–578
Conde A, Chaves MM, Gerós H (2011) Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol 52:1583–1602
Cornic G, Masacci A (1996) Leaf photosynthesis under drought stress. In: Baker NR (ed) Photosynthesis and the environment. Kluwer Academic Publishers, Dordrecht, pp 347–366
Ding Y, Fromm M, Avramova Z (2012) Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat Commun 3:740
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212
Flexas J, Medrano H (2002) Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitation revisited. Ann Bot 89:183–189
Goh CH, Nam HG, Park YS (2003) Stress memory in plants: a negative regulation of stomatal response and transient induction of rd22 gene to light in abscisic acid-entrained Arabidopsis plants. Plant J 36:240–255
Hoffmann AM, Noga G, Hunsche M (2015) Acclimations to light quality on plant and leaf level affect the vulnerability of pepper (Capsicum annuum L.) to water deficit. J Plant Res 128:295–306
Hu T, Jin Y, Li H, Amombo E, Fu J (2015) Stress memory induced transcriptional and metabolic changes of perennial ryegrass (Lolium perenne) in response to salt stress. Physiologia Plantarum. doi:10.1111/ppl.12342
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:377–403
Iturbe-Ormaetxe I, Escuredo PR, Arrese-Igor C, Becana M (1998) Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol 116:173–181
Kautz B, Noga G, Hunsche M (2014) Sensing drought- and salinity-imposed stresses on tomato leaves by means of fluorescence techniques. Plant Growth Regul 73:279–288
Kinoshita T, Seki M (2014) Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol 55:1859–1863
Leufen G, Noga G, Hunsche M (2013) Physiological response of sugar beet (Beta vulgaris) genotypes to a temporary water deficit, as evaluated with a multiparameter fluorescence sensor. Acta Physiol Plant 35:1763–1774
Leufen G, Noga G, Hunsche M (2014) Fluorescence indices for the proximal sensing of powdery mildew, nitrogen supply and water deficit in sugar beet leaves. Agriculture 4:58–78
Lichtenthaler HK, Rinderle U (1988) The role of chlorophyll fluorescence in the detection of stress conditions in plants. Crit Rev Anal Chem 19:29–85
Mafakheri A, Siosemardeh A, Bahramnejad B, Struik PC, Sohrabiet Y (2010) Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust J Crop Sci 4:580–585
Molinari HBC, Marur CJ, Daros E, de Campos MKF, de Carvalho JFRP, Filho JCB et al (2007) Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol Plant 130:218–229
Molinier J, Ries G, Zipfel C, Hohn B (2006) Transgeneration memory of stress in plants. Nature 442:1046–1049
Montesinos-Pereira D, Barrameda-Medina Y, Romero L, Ruiz JM, Sánchez-Rodríguez E (2014) Genotype differences in the metabolism of proline and polyamines under moderate drought in tomato plants. Plant Biol 16:1050–1057
Morales F, Cerovic ZG, Moya I (1994) Characterization of blue-green fluorescence in the mesophyll of sugar beet (Beta vulgaris L.) leaves affected by iron deficiency. Plant Physiol 106:127–133
Munné-Bosch S, Alegre L (2004) Die and let live: senescence contributes to plant survival under drought stress. Funct Plant Biol 31:203–216
Pantin F, Monnet F, Jannaud D, Costa JM, Renaud J, Muller B, Simonneau T, Genty B (2013) The dual effect of abscisic acid on stomata. New Phytol 619:67–72
Rapoport HF, Loomis RS (1986) Structural aspects of root thickening in Beta vulgaris L.: comparative thickening in sugar beet and chard. Bot Gaz 147:270–277
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Sgherri C, Stevanovic B, Navari-Izzo F (2004) Role of phenolics in the antioxidative status of the resurrection plant Ramonda serbica during dehydration and rehydration. Physiol Plantarum 122:478–485
Shaw B, Thomas TH, Cooke D (2002) Response of sugar beet (Beta vulgaris L.) to drought and nutrient deficiency stress. Plant Growth Regul 37:77–83
Stober F, Lichtenthaler HK (1993) Characterization of the laser-induced blue, green and red fluorescence signatures of leaves of wheat and soybean leaves grown under different irradiance. Physiol Plantarum 88:696–704
Thapa G, Dey M, Sahoo L, Panda SK (2011) An insight into the drought stress induced alterations in plants. Biol Plantarum 55:603–613
Thellier M, Lüttge U (2013) Plant memory: a tentative model. Plant Biol 15:1–12
Thellier M, Desbiez MO, Champagnat P, Kergosien Y (1982) Do memory processes occur in plants? Physiol Plantarum 56:281–284
Vomáčka L, Pospíšilová J (2003) Rehydration of sugar beet plants after water stress: effects of cytokinins. Biol Plant 46:57–62
Walter J, Nagy L, Hein R, Rascher U, Beierkuhnlein C, Willner E et al (2011) Do plants remember drought? Hints towards a drought-memory in grasses. Environ Exp Bot 71:34–40
Wingler A, Roitsch T (2008) Metabolic regulation of leaf senescence: interactions of sugar signalling with biotic and abiotic stress responses. Plant Biol 10:50–62
Acknowledgments
This study is part of the CROPSENSe.net research project “Networks of excellence in agricultural and nutrition research”, which is financially supported by the German Federal Ministry of Education and Research (BMBF 0315530A) and the European Union for regional development (z1011bc001a). The authors are grateful to Dr. Britta Schulz and the company KWS Saat AG for providing the sugar beet seeds. We would like to express our gratitude to Ms. Libeth Schwager and Ms. Ira Kurth for their help with the chlorophyll and proline determinations. Finally, we acknowledge the anonymous reviewers for their constructive comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leufen, G., Noga, G. & Hunsche, M. Drought Stress Memory in Sugar Beet: Mismatch Between Biochemical and Physiological Parameters. J Plant Growth Regul 35, 680–689 (2016). https://doi.org/10.1007/s00344-016-9571-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9571-8