Abstract
Objectives
To date, there is no approved second-line treatment for patients dismissing sorafenib or ineligible for this treatment, so it would be useful to find an effective alternative treatment option. The aim of our study was to evaluate safety, feasibility and effectiveness of transarterial chemoembolisation with degradable starch microspheres (DSM-TACE) in the treatment of patients with advanced hepatocellular carcinoma (HCC) dismissing or ineligible for multikinase-inhibitor chemotherapy administration (sorafenib) due to unbearable side effects or clinical contraindications.
Methods
Forty consecutive BCLC stage B or C patients (31 male; age, 70.6 ± 13.6 years), with intermediate or locally advanced HCC dismissing or ineligible for sorafenib administration, who underwent DSM-TACE treatment cycle via lobar approach were prospectively enrolled. Tumour response was evaluated on multidetector computed tomography based on mRECIST criteria. Primary endpoints were safety, tolerance and overall disease control (ODC); secondary endpoints were progression-free survival (PFS) and overall survival (OS).
Results
Technical success was achieved in all patients. No intra/peri-procedural death/major complications occurred. No signs of liver failure or systemic toxicity were detected. At 1-year follow-up, ODC of 52.5% was registered. PFS was 6.4 months with a median OS of 11.3 months.
Conclusions
DSM-TACE is safe and effective as a second-line treatment in HCC patients dismissing or ineligible for sorafenib.
Key Points
• DSM-TACE is safe and effective as second-line treatment in HCC patients dismissing or ineligible for sorafenib
• DSM-TACE allows the temporary occlusion of the smaller arterial vessels, improving overall therapeutic effectiveness by reducing the immediate wash-out of the cytostatic agent
• DSM-TACE also decreases the risk of systemic toxicity and post-embolic syndrome
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sorafenib treatment is the only available option for well-moderate compensated cirrhotic patients with Barcelona Clinic Liver Cancer stage B (BCLC-C) advanced hepatocellular carcinoma (HCC) stage or with BCLC-B intermediate HCC with tumour progressing after ineffective loco-regional therapies [1,2,3,4,5,6,7,8,9]. However, sorafenib treatment has no effects on the symptomatic time to progression of tumour and shows a low rate of objective responses [7]. Furthermore, the SHARP Investigators Study Group reported an overall occurrence of treatment-related adverse events, such as diarrhoea and hand-foot skin reaction, as high as 80% in the sorafenib group [7, 8]. This results in permanent treatment discontinuation in about 15% of the cases in the phase III trials leading to drug authorisation in USA and Europe [7, 8]. The rate of treatment discontinuation due to adverse events seems to be higher in field practice studies, as also confirmed by SOFIA study group [10]. In detail, in this study, 269 Child-Pugh A-B patients treated with sorafenib for advanced HCC had to interrupt treatment because of the onset of adverse events or liver dysfunction. In another recent study, the rate of permanent sorafenib discontinuation due to adverse events was 23% in a cohort of 140 patients in whom the beneficial impact of sorafenib dose reduction was evaluated in a subgroup of patients [11]. Furthermore, in clinical practice, some patients cannot be safely referred to this therapy because of advanced age, poor compliance or coexistence of severe comorbidities such as heart or respiratory failure.
However, to date, there is no approved second-line treatment in patients dismissing sorafenib or ineligible for this treatment so that it would be useful to find an effective alternative treatment option.
A new technique of intra-arterial hepatic chemoembolisation with degradable starch microspheres (DSM-TACE) was introduced; only few published data are available on the therapeutic efficacy of DSM chemoembolisation for HCC treatment, all obtained with the only degradable microspheres (EmboCept; PharmaCept, Berlin-Schöneberg, Germany) currently available in the market. Degradable starch microspheres (DSMs) consist of a three-dimensional, cross-linked hydrophilic starch matrix, which swells heavily in a water suspension environment and are completely degradable by amylase, allowing to obtain a transient occlusion of small arteries reaching the arteriolar or capillary level, due to their 50 μm diameter (45 ±7 μm), at which they lodge. With a half-life of approximately 40 min, the arterial occlusion provided by DSMs is limited, decreasing the risk of systemic toxicity and post-embolic syndrome. In detail, epirubicin co-administered with DSM is selectively trapped with DSM in small arteries, and is concentrated in areas of tumour. Based on pharmacokinetic, a specific accumulation of the drug in the tumour-affected area is obtained, with a lower systemic level of the active substance, and a consequent potential significant reduction of the side effects’ rate. On the other hand, the reduced or halted blood flow increases in situ time and the tumour exposure, and thereby the efficacy of any co-administered drug. Furthermore, it could also be obtained a tumour damage due to the transient ischaemic effect.
The treatment has been shown potentially useful and an alternative option for the treatment of HCC patients with intermediate or advanced disease by controlling tumour progression and enhancing local hepatic chemotherapy drug activity, without triggering liver function failure [12,13,14,15].
Based on this background, the aim of the study was to evaluate the safety, feasibility, and effectiveness of DSM-TACE in patients with intermediate-advanced HCC, ineligible for sorafenib administration due to clinical contraindication or dismissing it for unbearable side effects or progression of disease.
Material and methods
Study design
This is a prospective single-centre study to test the safety, feasibility, and efficacy of DSM-TACE. The study was followed the protocol and the principles of the Declaration of Helsinki, in accordance with the International Conference on Harmonization Tripartite Guideline for Good Clinical Practice and was approved by our Local Ethics Committee. Informed consent was obtained from all subjects prior to any treatment.
All patients were evaluated by a multidisciplinary tumour board, composed by all medical specialists involved in the HCC patients’ management (hepatologist, oncologist, hepatic and transplant surgeon, nuclear physician, radiotherapist, radiologist and interventional radiologist), based on clinic-laboratoristic parameters and computed tomography (CT) and/or magnetic resonance imaging (MRI) examinations.
Inclusion criteria: (1) intermediate-stage HCC (BCLC-B) refractory to transcatheter arterial chemoembolisation (TACE) or locally advanced HCC (BCLC-C) in patients dismissing sorafenib due to adverse events or tumour progression or ineligible for sorafenib administration; (2) liver cirrhosis classified as Child-Pugh score A or B; (3) tumour volume ≤70% of liver volume; (4) no confirmed extrahepatic metastases, except for limited extra-hepatic spread defined as portal or mesenteric lymph nodes at imaging up to 2.5 cm each based on measurement of the short axis, focal lung lesion single <1.5 cm or multiple lesions for a total diameter <2 cm; (5) performance status (ECOG) classified as 0-1 category.
Definition of ‘refractoriness or failure to TACE’ was based on the JSH Consensus Guidelines [16, 17], described as follows: ≥2 consecutive ineffective responses of treated tumours (residual viable lesions >50%), or ≥2 consecutive progressive increases in total tumour count (tumour number increases compared to tumour number before the previous TACE procedure), despite a change of chemotherapeutic agent or selection of the feeding artery, and/or continuous elevation of tumour marker levels (AFP) immediately after the TACE, and/or new emergence of vascular invasion and extrahepatic spread after the procedure.
Exclusion criteria: (1) Child-Pugh score C; (2) performance status (ECOG) = 2; (3) platelet count <40,000/μL and/or international normalised ratio >1.5; (4) serum creatinine levels ≥ 2 mg/dL, (5) doxorubicin administration contraindications. Tumourous macrovascular invasion (MVI) of hepatic and/or portal vein branches, as well as non-neoplastic portal vein thrombosis were not considered exclusion criteria. For portal vein tumour thrombus (PVTT), the classification was performed using the Liver Cancer Study Group of Japan, which divides PVTT into four classes according to the extent of the thrombus (peripheral, in the second-order, in the first-order branches, or in the main portal trunk) [18]. On the other hand, hepatic vein tumour thrombus (HVTT) was also categorised by the Japanese staging system in three categories based on the extent (peripheral or major hepatic vein, or inferior vena cava) [16]. Diagnosis of tumourous/non-tumourous thrombosis was based on CT/MR imaging findings. In detail, tumourous thrombosis can generally be expected to follow enhancement characteristics similar to primary parenchymal HCC, when there is a definite soft tissue arterial enhancement not attributable to mixing artefacts and/or enlargement of the portal vein and/or hepatic vein. On the other hand, non-tumourous thrombus does not enhance and usually does not expand the lumen [19].
Study population
Based on inclusion and exclusion criteria, a total of 40 consecutive BCLC-B or -C patients, with intermediate or locally advanced HCC dismissing or ineligible for sorafenib administration, were recruited. The main features of patients and tumours are reported in Table 1.
A total of 18 patients (15 men, 3 women) had intermediate-stage BCLC-B HCC and were non-responders to at least two selective DEB-TACE, whereas the remaining 22 patients (16 men, 6 women) had advanced multifocal HCC, classified as BCLC-C due to macrovascular invasion. Eighteen patients dismissed sorafenib administration. This was due to side effects such as gastrointestinal symptoms of grade 2 diarrhoea in eight patients and progression of HCC in ten patients. The remaining 22 patients were considered ineligible for sorafenib administration because of deteriorated liver function indicated by total bilirubin of higher than 2 mg/dL and/or liver cirrhosis classified as Child-Pugh score B.
Treatment
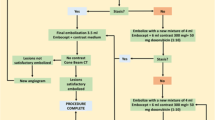
DSM-TACE was performed in an angiography suite monitoring vital signs during anaesthesia, by the same experienced interventional radiologist (15 years of experience). The treatment was performed under local anaesthesia through a femoral approach, with a Seldinger needle, by using a 5-Fr 12-cm arterial introducer sheath (Terumo, Tokyo, Japan). The selective celiac trunk catheterisation and the cannulation of common hepatic artery were performed with a 5-Fr diagnostic catheter (Cobra, Simmons; Terumo). A hepatic angiography was used to identify the appropriate anatomy of the hepatic artery and of any possible branches related to non-target structures, and exclude any arteriovenous fistulae. After diagnostic angiography, a selective lobar catheterisation was performed with a coaxial technique, placing a 2.7-Fr microcatheter (Progreat; Terumo) in the right or left hepatic artery that was feeding the involved lobe. A selective lobar angiography was then performed to confirm the correct position of microcatheter, to identify non-hepatic arteries and limit any possible extrahepatic diffusion of the degradable microspheres. In particular, identification of cystic artery was recommended to ensure that the catheter tip would bypass this anatomical point to avoid non-target embolisation.
Under fluoroscopic guidance, a solution of 3.5 mL Embocept (PharmaCept) loaded with 50 mg Epirubicin (Farmorubicin, 50 mg powder; Pfizer, Rome, Italy), combined with 5 mL saline solution, and 15 mL contrast medium (Iomeron, 300 mgI/mL; Bracco, Milan, Italy), followed by 4 mL of unloaded Embocept mixed with 6 mL contrast medium was slowly and continuously infused until a “stop flow” was observed. In detail, a “two step” infusion technique was used: “drug uptake” phase in which a 20–30 mL of “ready-to-use” solution (3.5 mL of microparticles + 50 mg Epirubicin in 5 mL saline solution + 15 mL contrast medium) was slowly intra-arterially injected, followed by a “stop flow” phase in which starch microspheres (4 mL) mixed with contrast medium (6 mL) was slowly injected.
In order to prevent infections, subjects were given antibiotics before and after the treatment for 7 days. According to the extent and distribution of disease, it was decided to carry out a single lobe (two treatments at 4 weeks’ interval) or a bilobar treatment (four treatments, at 2 weeks’ interval; the first and third treatment were targeted to the lobe more involved by disease). It is mandatory to underline that we usually performed a treatment cycle composed of two or four treatments if we have a unilobar or bilobar disease, respectively, and not a treatment “on demand”, in which the number of sessions is based on tumour response after each treatment. In detail, we performed a regular predefined scheduled strategy with a predefined number of sessions regardless of the “interim” response, checking results only after 1 month from the last procedure. This strategy is more concordant with the general principle of oncologic therapy, being possible for the low-risk of adverse events obtained with DSM-TACE, when compared with cTACE or DEB-TACE, for which there is evidence suggesting that the repetition of procedures increases the incidence of adverse events.
End-points and post treatment follow up studies
A technical success is defined as the ability to deliver the full 7.5-mL planned dose (i.e. 50 mg of Epirubicin-loaded microspheres) and to obtain stop flow [20]. Assessment of safety as well as 1-year overall disease control (ODC) were the primary endpoints of the research, followed by secondary endpoints of progression-free survival (PFS) and overall survival (OS). Primary endpoints were evaluated by analysing occurrence of major/minor complications, whereas ODC was calculated as the sum of objective responses and stable diseases.
Perioperative morbidity and mortality, including major/minor complications and death occurring within 7 days from treatment, were registered. Major complication was defined as an event that engenders substantial morbidity and disability, an increased level of care, or substantially lengthens hospital stay. Other complications were considered minor [20].
Child-Pugh score was used to determine post-treatment evaluation of liver function, serum levels of alanine-aminotransferase (ALT), prothrombin time (PT) and bilirubin [21].
Treatment efficacy includes α-fetoprotein dosage and mRECIST criteria such as complete response (CR), partial response (PR), stable disease (SD) and progression disease (PD). The treatment efficacy was evaluated by multiphasic CT or MRI examinations performed at 1 month after treatment cycle (after a total of two DSM-TACEs for unilobar disease, or four DSM-TACEs for bilobar disease) and every 3 months thereafter [22, 23]. All results obtained in the follow-up were evaluated and discussed by our multidisciplinary tumour board in order to also define the treatment strategy. Re-treatment of the same patient/lobe with further cycles of DSM-TACE was permitted with a maximum of two re-treatment cycles in any patient.
Statistical analysis
All data were reported as the mean ± standard deviation. Differences between groups were evaluated using the Student's t-test and the Fisher exact test correction for small numbers. The p values were judged significant if they were less than 0.05. All analysis was conducted using SAS software (SAS Institute, Cary, USA).
Results
Treatment feasibility and tolerance
A total of 137 treatments were performed with a mean number of treatments per patient of 3.42 ± 0.9; in detail, one patient refused to perform the second treatment and to complete the cycle. Technical success was achieved in all treatments, particularly it was possible to deliver the full 7.5-mL planned dose and to obtain a stop flow in all treatments. No unexpected adverse reactions were noted and no major complications or treatment-related deaths were observed. Minor complications were detected in six patients (15%) and were represented by increased serum level of transaminases in five cases and transient cholecystitis in one case. These complications were completely recovered without any therapy. At discharge time, no significant changes were found in terms of Child-Pugh Score (baseline pre-procedural value of 7.3 ± 1.22 vs discharge value after completion of the treatment of 7.67 ± 1.2) (p = 0.34). No ascites occurred after DSM-TACE. No patients progressed to Child-Pugh class C.
Tumour response
Based on post-treatment 1-month follow-up, 8/40 complete response (20%), 12/40 partial response (30%), 12/40 stable disease (30%) and 8/40 progressed disease (20%) were observed with an overall response rate (ORR) of 50% and ODC of 80%. According to these results, a repeated DSM-TACE schedule treatment was performed in 11 patients with residual viable tumour volume higher than 50% (11/40, 27.5%). The follow-up period was stopped at the time of the last visit, at the time of liver transplantation or at death with an obtained median follow-up of 16.5 months. At 1-year follow-up, ORR of 27.5% (successfully transplanted 3 patients with CR, and 8 patients with PR), and ODC of 52.5% (21 patients with CR/PR in 11 cases or SD in 10 cases) were registered (Fig. 1)
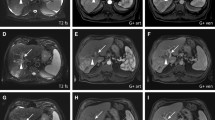
The case of a 76-year-old man. Multinodular BCLC-B HCC mainly located in sVII-VIII and recurrent 2 years after right-lobe radioembolisation in a patient who had to dismiss sorafenib because of tumour progression (a, b arterial phase T1-weighted spoiled gradient-echo MR images). Two right lobar DSM-TACEs were performed with a 4-week interval (c DSA images). In DSA images (c), the lobar position of the microcatheter with coils previously placed for pre-radioembolisation prophylactic embolisation of right gastric (proximal tract) and gastroduodenal arteries is clearly demonstrated. At 6-month follow-up, MR images demonstrated a partial response, with a subtotal necrosis of the main nodules in sVII-VIII (d, e arterial phase T1-weighted spoiled gradient-echo MR images)
Progression-free survival and overall survival rate
PFS, calculated using the Kaplan-Meier method, was 6.4 months. During the follow-up, 15 patients died of tumour progression, 2 of respiratory failure due to chronic pulmonary emphysema and pneumonia and 5 of acute heart failure among the 40 patients. Four patients underwent liver transplantation (three of them with a CR) and one patient underwent surgical resection after a successful downstaging. Seven patients were lost during the follow-up. The remaining six patients were still alive at the censored time.
The median overall survival of all patients was 11.3 months and the survival rate for 6 months, 1-, and 2-year rate, calculated with Kaplan-Meier survival analysis, were 78%, 60.7% and 37.7% respectively. When considering the subgroup median survival evaluation (Table 2), no significant differences were found between BCLC-B and BCLC-C patients (p > 0.05) and between Child-Pugh A and Child-Pugh B patients (p > 0.05). Otherwise, observation shows significantly higher median OS for BCLC-B Child-Pugh A patients compared to BCLC-C Child-Pugh B patients (12.9 months vs 6.5 months, p = 0.02).
Discussion
An increasing number of emerging agents, including novel molecular targeted drugs, have been attempted in sorafenib-refractory HCC. Nevertheless, their efficacy was found to be limited with a response rate between 0 to 4.3% and time to progression between 1.6 to 2.7 months [24,25,26,27]. It is notable that in the second-line treatment of HCC, only regorafenib and nivolumab have been recently shown to be the only FDA approved systemic medications. Regorafenib provided survival benefit in HCC patients progressing on sorafenib treatment in a phase III trial [28]; on the other hand, nivolumab, an immunotherapy agent that inhibits PD-1, has been reported to be more beneficial in HCC patients with Child Pugh Class A/B7, and achieved a higher response rate in patients with PD-L1 ≥ 1%, as predictor for tumour response [29].
At present, there is no approved alternative treatment for patients progressing or intolerant to sorafenib, who can only be addressed to the best supportive care or evaluated for recruitment in clinical trials and bear a dismal prognosis with a cumulative 1-year survival of 25% [6]. Therefore, tolerable, life-prolonging strategies or treatments in the second-line setting are needed.
Based on this background, a single-centre study was conducted using DSM-TACE in well-moderately compensated cirrhotic patients with advanced HCC—staged as BCLC stage C—or with tumour progressing after loco-regional therapies—staged as BCLC stage B—ineligible for or dismissing sorafenib. As conventional or drug-eluting beads, application of TACE in a palliative setting is a common procedure and limited toxicity is one of the key conditions to be met in order to maintain a good performance status and compensated liver disease. Chemoembolisation with degradable starch microspheres (Embocept) allows the temporary occlusion of the smaller arterial vessels, so improving the overall therapeutic effectiveness by reducing the immediate wash-out of the cytostatic agent, and decreasing the risk of systemic toxicity and post-embolic syndrome. Indeed, no major complications were experienced despite the fact that our population was at high risk of treatment failure or liver function decompensation for standard TACE; in particular, 22 patients (55%) had vascular invasion with portal vein thrombosis, 19 (47.5%) had mild ascites, 29 (72.5%) were Child-Pugh B class, of whom 21 (52.5%) had B8-9 score and 19 (47.5%) had a total bilirubin level higher than 2 mg/dL. Despite these advanced conditions, DSM-TACE was not offset by any important side effects or worsening of liver function particularly; none of them experienced an increased Child-Pugh score 1 month after treatment. Furthermore, the transitory vascular occlusion generated by DSM allowed the repeat of treatment in 27.5% of patients, reducing the risk of liver toxicity that may occur when repeating conventional TACE [20, 21, 30, 31].
The treatment was safe and effective in clinical setting as confirmed by 1-month ODC and ORR of 80% and 50%, respectively, with an ODC of 52.5% at the end of the 16.5 months median follow-up period. A median PFS of 6.4 months and a median overall survival of 11.3 months were observed. The 1- and 2-year survival rates were 60.7% and 37.7%, respectively. These results seem to be at least comparable with the obtained sorafenib registration trials [7, 8] and field practice studies [9, 10], despite the deteriorated clinical condition of most patients in this study. As also reported in the paper of Giannini et al [32], it is well known that in a real-world setting, BCLC-C patients showed a markedly different prognosis according to the characteristics that determined the assignment to this stage; in detail, the occurrence of macrovascular invasion and extrahepatic disease on treatment entails the poorest prognostic meaning.
When considering the subgroup evaluation, significantly longer overall survival was obtained for BCLC-B intermediate Child-Pugh A patients compared with BCLC-C advanced Child-Pugh B patients. The outcome justifies the prognostic pivotal role of liver functional reserve in intermediate/advanced HCC patients with liver cirrhosis, which has been previously described by other groups [33, 34]. In particular, another multicentre study reported a poor prognosis in BCLC-C advanced patients with a compromised liver function (Child-Pugh B class) [32].
Furthermore, yttrium-90 transarterial radioembolisation (SIRT) should be considered as potential alternative for patient’s clinical setting so that it would be interesting to compare our data with results of SIRT in terms of response in a similar scenario. However, the role of SIRT in patients with advanced HCC is currently still under investigation and to the best of our knowledge there are no published data on its use in BCLC-C patients excluded from sorafenib therapy due to side effects or serum bilirubin level > 2 mg/dL suggesting impending liver function failure. Furthermore, SIRT is generally contraindicated for patients with decompensated cirrhosis (Child-Pugh ≥ B8).
Both DSM-TACE and SIRT may be used in HCC patients with macroscopic vascular invasion and they act by selectively delivering high-dose anticancer treatments directly to tumour, with a limited embolic effect, so improving the toxicity profiles and decreasing the risk of treatment related worsening of liver function. On the basis of the results of present study, randomised prospective comparative studies performed on a larger population are needed in future to define and determine patient inclusion criteria and the efficiency of these therapeutic techniques. An important point favouring DSM-TACE could be its lower cost when compared with SIRT.
The number of recruited patients is a major limitation in the present study. However, the present research is targeting to define the safety and efficacy of DSM-TACE in BCLC B or C HCC patients. These preliminary findings can be a stepping stone for future researchers to performs a prospective comparative multicentre study covering this scope.
In conclusion, preliminary results of the present study show that TACE with degradable starch microspheres (DSM-TACE) is safe and effective as second-line treatment in HCC patients dismissing or ineligible for sorafenib.
Abbreviations
- BCLC-B:
-
Barcelona Clinic Liver Cancer stage B
- BCLC-C:
-
Barcelona Clinic Liver Cancer stage C
- CR:
-
Complete response
- DSM-TACE:
-
Degradable starch microspheres – transarterial chemoembolisation
- ECOG:
-
Eastern Cooperative Oncology Group
- HCC:
-
Hepatocellular carcinoma
- JSH:
-
Japan Society of Hepatology
- ODC:
-
Overall disease control
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- PR:
-
Partial response
- SD:
-
Stable disease
- SIRT:
-
Selective internal radiotherapy
References
Rinninella E, Zocco MA, De Gaetano A et al (2012) From small nodule to overt HCC: a multistep process of carcinogenesis as seen during surveillance. Eur Rev Med Pharmacol Sci 16:1292–1294
Murata S, Mine T, Sugihara F et al (2014) Interventional treatment for unresectable hepatocellular carcinoma. World J Gastroenterol 20:13453–13465
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Llovet JM, Brú C, Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19:329–338
Falkson G, MacIntyre JM, Moertel CG, Johnson LA, Scherman RC (1984) Primary liver cancer. An Eastern Cooperative Oncology Group Trial. Cancer 54:970–977
Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C (2010) A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 51:1274–1283
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Cheng AL, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Italian Association for the Study of the Liver (AISF); AISF Expert Panel; AISF Coordinating Committee, Bolondi L, Cillo U, Colombo M et al (2013) Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis 45:712–723
Iavarone M, Cabibbo G, Piscaglia F et al (2011) Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology 54:2055–2063
Ponziani FR, Bhoori S, Germini A et al (2016) Inducing tolerability of adverse events increases sorafenib exposure and optimizes patient's outcome in advanced hepatocellular carcinoma. Liver Int 36:1033–1042
Niessen C, Unterpaintner E, Goessmann H et al (2014) Degradable starch microspheres versus ethidol and doxorubicin in transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol 25:240–247
Yamasaki T, Hamabe S, Saeki I et al (2011) A novel transcatheter arterial infusion chemotherapy using iodized oil and degradable starch microspheres for hepatocellular carcinoma: a prospective randomized trial. J Gastroenterol 46:359–366
Iezzi R, Pompili M, Nestola M et al (2016) Transarterial chemoembolization with degradable starch microspheres (DSM-TACE): an alternative option for advanced HCC patients? Preliminary results. Eur Rev Med Pharmacol Sci 20:2872–2877
Schicho A, Pereira PL, Haimerl M et al (2017) Transarterial chemoembolization (TACE) with degradable starch microspheres (DSM) in hepatocellular carcinoma (HCC): multi-center results on safety and efficacy. Oncotarget 8:72613–72620
Kudo M, Izumi N, Kokudo N et al (2011) HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 29:339–364
Kudo M, Matsui O, Izumi N et al (2014) Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 87:22–31
Ikai I, Kudo M, Arii S et al (2010) Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res 40:1043–1059
Costentin CE, Ferrone CR, Arellano RS, Ganguli S, Hong TS, Zhu AX (2017) Hepatocellular carcinoma with macrovascular invasion: defining the optimal treatment strategy. Liver Cancer 6:360–374
Basile A, Carrafiello G, Ierardi AM, Tsetis D, Brountzos E (2012) Quality-improvement guidelines for hepatic transarterial chemoembolization. Cardiovasc Intervent Radiol 35:765–774
Bruix J, Sherman M, Practice Guidelines Committee, American Association for the Study of Liver Diseases (2005) Management of hepatocellular carcinoma. Hepatology 42:1208–1236
Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK (2005) Systematic review: The model for end-stage liver disease–should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther 22:1079–1089
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Terashima T, Yamashita T, Arai K et al (2014) Feasibility and efficacy of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma after sorafenib. Hepatol Res 44:1179–1185
Finn RS, Kang YK, Mulcahy M et al (2012) Phase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res 18:2090–2098
Santoro A, Rimassa L, Borbath I et al (2013) Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomized, placebo-controlled phase 2 study. Lancet Oncol 14:55–63
Yau T, Wong H, Chan P et al (2012) Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Invest New Drugs 30:2384–2390
Bruix J, Qin S, Merle P et al (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:56–66
El-Khoueiry AB, Sangro B, Yau T et al (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389:2492–2502
Wiggermann P, Wohlgemuth WA, Heibl M et al (2013) Dynamic evaluation and quantification of microvascularization during degradable starch microspheres transarterial Chemoembolisation (DSM-TACE) of HCC lesions using contrast enhanced ultrasound (CEUS): a feasibility study. Clin Hemorheol Microcirc 53:337–348
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer (2012) EASL–EORTC Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Giannini EG, Bucci L, Garuti F et al (2018) Patients with advanced hepatocellular carcinoma need a personalized management: A lesson from clinical practice. Hepatology 67(5):1784–1796
Bruix J, Llovet JM (2002) Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 35:519–524
Greten TF, Papendorf F, Bleck JS et al (2005) Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer 92:1862–1868
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Roberto Iezzi, MD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• prospective
• experimental
• performed at one institution
Rights and permissions
About this article
Cite this article
Iezzi, R., Pompili, M., Rinninella, E. et al. TACE with degradable starch microspheres (DSM-TACE) as second-line treatment in HCC patients dismissing or ineligible for sorafenib. Eur Radiol 29, 1285–1292 (2019). https://doi.org/10.1007/s00330-018-5692-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5692-8