Abstract
Background
We designed a novel transcatheter arterial infusion chemotherapy (TAI) using iodized oil (lipiodol) and degradable starch microspheres (DSM) for hepatocellular carcinoma (HCC) patients. In this study, we investigated the efficacy of TAI using lipiodol and DSM in a prospective randomized trial.
Methods
We randomly divided 45 patients with HCC into 3 groups: TAI using lipiodol (lipiodol group, n = 15), TAI using DSM (DSM group, n = 15), and TAI using lipiodol and DSM (lipiodol + DSM group, n = 15). In the lipiodol group, a mixture of cisplatin and lipiodol was administered. In the DSM group, a mixture of cisplatin and DSM was administered. In the lipiodol + DSM group, a mixture of cisplatin and lipiodol was administered, followed by DSM.
Results
The response rates were 40% in the lipiodol group, 53.4% in the DSM group, and 80% in the lipiodol + DSM group, respectively. The response rate tended to improve in the lipiodol + DSM group (lipiodol group vs. lipiodol + DSM group, P = 0.07). The median progression-free survival time was 177 days in the lipiodol group, 287 days in the DSM group, and 377 days in the lipiodol + DSM group. The progression-free survival in the lipiodol + DSM group was significantly better than those in the DSM group (P = 0.020) and the lipiodol group (P = 0.035). There were no serious adverse effects among the 3 groups.
Conclusions
TAI using lipiodol and DSM was superior to TAI using lipiodol only and TAI using DSM only because of improvements in therapeutic effects and progression-free survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer in the world [1]. Deaths due to HCC are increasing in almost all countries worldwide, including Japan [2–4]. Recent advancements in several therapeutic techniques such as hepatic resection, percutaneous ethanol injection, radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), sorafenib, and transplantation have improved the prognosis of HCC patients [5–10].
Of these treatments, TACE has become one of the most popular for HCC patients. TACE in Japan has generally used several anticancer agents, iodized oil (lipiodol) and gelatin sponge particles [11]. On the other hand, polyvinyl alcohol (PVA), drug-eluting beads (DEB), and embospheres have been used as embolizing agents in Europe and the United States [12]. Studies prior to 2000 failed to prove a survival benefit of TACE in the treatment of HCC [13, 14]. However, the survival benefit of TACE was proven by meta-analysis in recent reports [15, 16]. In addition, with the development of the microcatheter, the catheter can be inserted in the segmental or subsegmental hepatic artery, and segmental or subsegmental TACE has been reported to be a useful treatment [7, 17]. On the other hand, transcatheter arterial infusion chemotherapy (TAI) using an emulsion of lipiodol and an anticancer agent (without gelatin sponge particles) has usually been performed for HCC patients in whom the catheter could not be inserted in the targeted segment or a feeding artery was not detected in the tumor. In addition, TAI without gelatin sponge particles has been also used for HCC in high-risk patients (for example, main portal vein occlusion, Child–Pugh B or C) [18]. We have also experienced that repeated TACE therapy is not possible due to obstruction of the hepatic artery in HCC patients. Therefore, we have been performing segmental or subsegmental TACE for selected HCC patients. However, it has been reported that the effect of TAI using lipiodol was lower than that of TACE in local tumor control [19]. Many interventional radiologists desire a novel therapy that is both more effective than TAI using lipiodol in local tumor control and is less damaging to the hepatic artery than TACE.
Degradable starch microspheres (DSM) were developed to provide transient occlusion of small arteries [20, 21]. The duration of occlusion in the hepatic arteries by DSM is limited to 80 min [22]. Several studies of metastatic liver tumors indicate that intra-arterial therapy with DSM and an anticancer agent improves the therapeutic effects compared with therapy using an anticancer agent alone [22–24]. However, few studies have evaluated TAI using DSM in HCC patients [25–27].
Given this background, we designed a novel TAI using lipiodol and DSM for use in HCC patients [28]. After a mixture of an anticancer agent and lipiodol is injected, DSM is administered until stasis or reflux of the arterial flow. We postulate that TAI using two occlusion materials may be beneficial because of the tight interruption of blood supply for HCC. In this study, we investigated the efficacy of a novel TAI using lipiodol and DSM in a prospective randomized trial.
Materials and methods
Patients
The eligibility criteria for inclusion in this study were as follows: (1) age 20–80 years; (2) Child–Pugh score of A or B; leukocyte count ≥3000/mm3; (3) hemoglobin level ≥9.5 g/dL; (4) platelet count ≥50000/mm3; (5) serum creatinine level <1.2 mg/dL; (6) total bilirubin <3.0 mg/dL; (7) locally nodular disease without extrahepatic metastasis and/or vascular tumor thrombosis (portal vein, hepatic vein, and bile duct); (8) no indication for surgical resection and local ablation, or patients rejected surgical resection; and (9) Eastern Cooperative Oncology Group (EOGG) performance status of 0–1 [29].
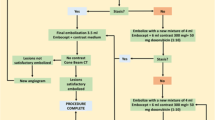
We studied 45 patients with HCC who had been admitted to the Department of Gastroenterology and Hepatology, Yamaguchi University Graduate School of Medicine, between February 2006 and May 2008. We randomly divided the patients into 3 groups before the angiography: TAI using lipiodol (lipiodol group, n = 15), TAI using DSM (DSM group, n = 15), and TAI using lipiodol and DSM (lipiodol + DSM group, n = 15). The primary outcome measure was tumor response. Secondary outcome measures included progression-free survival and toxicity (Fig. 1). HCC was diagnosed on the basis of imaging results (hyperattenuation in the arterial phase and hypoattenuation in the portal-venous phase) and elevated serum levels of α-fetoprotein (AFP) and/or des-γ-carboxyprothrombin (DCP).
Patients provided their written informed consent before participating in the study, which was approved by the Institutional Review Board of Yamaguchi University Hospital.
Table 1 summarizes the clinical profiles of the patients in the 3 groups. There were no significant differences between the 3 groups with regard to age, gender ratio, proportion of patients with hepatitis B virus and hepatitis C virus infections, Child–Pugh score, maximum tumor size, tumor stage, number of tumors, or previous treatment. Tumor stage was determined according to the criteria of the Liver Cancer Study Group of Japan [30, 31]. Tumor staging was based on the following 3 parameters (T factor): solitary tumor, <2 cm in diameter and no vessel invasion. Stage I was defined as one fulfilling all of the above 3 criteria (T1); stage II as one fulfilling 2 of the above 3 criteria (T2); stage III as one fulfilling 1 of the above 3 criteria (T3); stage IV A as one fulfilling none of the above 3 criteria (T4) with no distant metastasis or as one with any T factor with lymph node metastasis; and stage IV B as one with any T factor with distant metastasis.
Embolization technique
Hepatic angiography was performed with a 4-French (4-Fr) or 5-Fr angiographic catheter. After digital subtraction angiography (DSA), angiography combined with a computed tomography (angio-CT) [32] system using a Somatom plus 4 (Siemens, Erlagen, Germany) was performed to carefully evaluate HCC tumors. In this study, a fine-powder formulation of cisplatin (IA-call; Nippon Kayaku Co., Tokyo, Japan) was used as the anticancer agent. The dose of cisplatin was limited to 80 mg. According to the tumor vascularization and distribution, TAI was performed by selectively introducing a catheter into the right or left hepatic artery or a segmental branch of the hepatic artery. Gelatin sponge particles were not used in this study.
In the lipiodol group, a mixture of cisplatin and lipiodol (Lipiodol Ultra Fluid; Andre Guerbet, Paris, France) was administered through the tumor-supplying vessels. In the DSM group, a mixture of cisplatin and emulsion obtained by mixing DSM (Spherex; Yakult Honsha Co., Tokyo, Japan) and contrast agent was administered. If this procedure was insufficient, lipiodol or DSM alone was injected until stasis and reflux were achieved.
A mixture of cisplatin and lipiodol was administered in the lipiodol + DSM group. After that point, emulsion obtained by mixing DSM and contrast agent was injected until stasis and reflux were achieved.
The serotonin antagonist ondansetron hydrochloride was administered intravenously as an antiemetic prior to treatment in all 3 groups. To prevent kidney damage, adequate hydration was ensured before and after the treatment by an intravenous drip infusion of 1000–2000 mL of an infusion solution.
After the treatment, a follow-up examination including CT, tumor marker measurement, and serum biochemistry, was performed, first at 1 month after treatment completion and subsequently every 3–4 months. In principle, the same transcatheter arterial treatments were repeated unless the tumors progressed, when a follow-up CT examination showed new lesions in the liver or regrowth of previously treated tumors.
Response and toxicity evaluation
The antitumor effect was assessed by dynamic CT 1 month or more after treatment. The response was classified according to the Liver Cancer Study Group of Japan criteria [30]. In the response evaluation criteria, lipiodol accumulation in the tumors is regarded as an indication of necrosis because significant positive correlations have been reported between lipiodol accumulation observed on CT images and the necrotic regions in the resected tumors examined pathologically after TACE and TAI [33–35]. Therapeutic effect IV (TE IV) is defined as the disappearance or 100% necrosis of all tumors, and TE III as a greater than 50% reduction in tumor size and/or greater than 50% necrosis. TE I is defined as a greater than 25% increase in tumor size. TE II is defined as disease that does not qualify for classification as TE IV, III, or I.
When repeated TAI was performed, the greatest antitumor effect was assessed as the final response.
The severity of adverse reactions was evaluated during the first treatment cycle according to the Common Terminology Criteria for Adverse Events v.4.0 (CTCAE v.4.0) [36].
Statistical analysis
The data are expressed as the mean ± standard deviation (SD). Statistical analyses were performed using the unpaired t test and the Mann–Whitney U test as appropriate. Progression-free survival and cumulative survival were calculated by the Kaplan–Meier method [37] and significance was determined by the log-rank test. Progression-free survival time was defined as the interval between the first TAI after randomization and death or the progression of the last follow-up period. Survival time was defined as the interval between the first TAI after randomization and death or the last follow-up period. The follow-up period ended on April 30, 2010. Statistical significance was defined as a P < 0.05.
Results
Information on the anticancer agent and embolizing agents
The median doses of cisplatin at first TAI in the lipiodol group, the DSM group, and the lipiodol + DSM group were 64.3 ± 22.0 mg (20–80 mg), 59.4 ± 20.0 mg (20–80 mg), and 60.5 ± 20.1 mg (10–80 mg), respectively. There was no significant difference in cisplatin dose among the 3 groups. In the lipiodol group, the dose of lipiodol at first TAI was 4.8 ± 2.0 mL (1–8 mL). In the DSM group, the dose of DSM at first TAI was 1164.6 ± 1013.1 mg (120–3000 mg). In the lipiodol + DSM group, the doses of lipiodol and DSM at first TAI were 4.1 ± 2.0 mL (0.5–8 mL) and 426.6 ± 404.8 mg (60–1500 mg), respectively.
Response to therapy
The total number of treatment courses was 23 with a mean of 1.5 courses per patient (range 1–5 courses) in the lipiodol group, 29 with a mean of 1.9 courses per patient (range 1–6 courses) in the DSM group, and 29 with a mean of 1.9 courses per patient (range 1–6 courses) in the lipiodol + DSM group.
Table 2 shows the final response to therapy. In the lipiodol group (n = 15), 4 (26.7%), 2 (13.3%), 4 (26.7%), and 5 (33.3%) patients exhibited TE VI, III, II, and I, respectively [response rate (patients with TE VI and III/all patients) = 40%; complete response (CR) rate (patients with TE VI/all patients) = 26.7%]. In the DSM group (n = 15), 4 (26.7%), 4 (26.7%), 7 (46.6%), and 0 (0%) patients exhibited TE IV, III, II, and I, respectively (response rate = 53.4%; CR rate = 26.7%). In the lipiodol + DSM group (n = 15), 6 (40%), 6 (40%), 2 (13.3%), and 1 (6.7%) patient exhibited TE IV, III, II, and I, respectively (response rate = 80%; CR rate = 40%). The response rate tended to improve in the lipiodol + DSM group (lipiodol group vs. lipiodol + DSM group, P = 0.07; Mann–Whitney U test). However, no significant differences were seen between the 3 groups (lipiodol group vs. DSM group, P = 0.21; DSM group vs. lipiodol + DSM group, P = 0.25; Mann–Whitney U test).
Progression-free survival
Figure 2 shows the progression-free survival rates for the 3 groups. The 1- and 2-year progression-free survival rates in the lipiodol group were 13 and 13%, respectively. The 1-year progression-free survival rate was 27% in the DSM group. The 1-, 2-, and 3-year progression-free survival rates in the lipiodol + DSM group were 53, 13, and 7%, respectively. The median progression-free survival times were 177 days in the lipiodol group, 287 days in the DSM group, and 377 days in the lipiodol + DSM group. No significant difference in progression-free survival was seen between the lipiodol group and the DSM group (P = 0.515). On the other hand, progression-free survival in the lipiodol + DSM group was significantly better than that in the DSM group (P = 0.020) and the lipiodol group (P = 0.035).
Progression-free survival rates for the 3 groups. The 1- and 2-year progression-free survival rates in the lipiodol group were 13 and 13%, respectively. The 1-year progression-free survival rate was 27% in the DSM group. The 1-, 2-, and 3-year progression-free survival rates in the lipiodol + DSM group were 53, 13, and 7%, respectively. No significant difference in progression-free survival was seen between the lipiodol group and the DSM group (P = 0.515). On the other hand, progression-free survival in the lipiodol + DSM group was significantly better than that in the DSM group (P = 0.020) and the lipiodol group (P = 0.035)
Survival
In the lipiodol group, the 1- and 2-year cumulative survival rates were 80 and 60%, respectively. In the DSM group, they were 87 and 40%, respectively. In the lipiodol +DSM group, they were 87 and 67%, respectively. No significant differences between the 3 groups were seen in survival (lipiodol group vs. DSM group, P = 0.377; lipiodol group vs. lipiodol + DSM group, P = 0.560; DSM group vs. lipiodol + DSM group, P = 0.212).
By the final follow-up, 21 patients remained alive (lipiodol group, n = 8; DSM group, n = 6; lipiodol + DSM group, n = 7), while the other 24 patients had died (lipiodol group, n = 7; DSM group, n = 9; lipiodol + DSM group, n = 8). In the lipiodol group, the cause of death was cancer progression in 6 patients and hepatic failure in 1 patient. In the DSM group, the cause of death was cancer progression in 7 patients, hepatic failure in 1 patient, and another disease in 1 patient. In the lipiodol + DSM group, the cause of death was cancer progression in 3 patients, hepatic failure in 2 patients, another disease in 2 patients, and rupture of esophageal varices in 1 patient.
Adverse effects of therapy
Table 3 shows the adverse effects of therapy. There was no significant difference in thrombocytopenia between the 3 groups, although grade 3 thrombocytopenia occurred in 4 patients of the lipiodol group (26.7%) and grade 3 or 4 thrombocytopenia occurred in 5 patients of the lipiodol + DSM group (33.3%). However, only 1 patient in the lipiodol + DSM group required a blood transfusion. The grade of elevated alanine aminotransferase (ALT) levels was significantly higher in the lipiodol + DSM group than in the lipiodol group (P = 0.043), although there were no significant differences in any other adverse effects between the 3 groups. No treatment-related deaths were observed in the 3 groups.
Figure 3 shows the changes in serum ALT or platelets before and after treatment in the lipiodol + DSM group. Transient increases in serum ALT concentration were observed in almost all patients; however, 2 weeks after treatment, concentrations decreased almost to pretreatment levels. Transient decreases in platelets were observed in almost all patients, and platelet counts at 3 days after treatment were the lowest before and after treatment; 2 weeks after treatment, the count increased almost to pretreatment levels.
Changes in serum alanine aminotransferase (ALT) (a) or platelet (b) levels before and after treatment in the lipiodol + DSM group. Transient increases in serum ALT concentration were observed in almost all patients; however, 2 weeks after treatment, the concentration decreased almost to pretreatment levels. Transient decreases in platelet levels were observed in almost all patients, and platelet counts at 3 days after treatment were lower than before and after treatment; 2 weeks after treatment, the count increased almost to pretreatment levels
Discussion
We designed a novel TAI using lipiodol and DSM for use in HCC patients, and reported the usefulness of this procedure [28]. In this study, we investigated the efficacy of this novel TAI using lipiodol and DSM in a prospective randomized trial (lipiodol vs. DSM vs. lipiodol + DSM).
We used a fine-powder formulation of cisplatin (IA-call; Nippon Kayaku Co., Tokyo, Japan) as the anticancer agent. The most common single-agent anticancer drug was doxorubicin, followed by cisplatin [12]. Although there is no evidence of the superiority of any chemotherapeutic agents [12], only a nonrandomized trial by Ono et al. [38] showed that cisplatin was better than doxorubicin. A Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin reported that the response rate was 33.8% [39]. Therefore, we selected IA-call as the anticancer agent.
In our study, the response rates (patients with TE VI and III/all patients) in the lipiodol group, DSM group, and lipiodol + DSM group were 40, 53.4, and 80%, respectively. The CR rate (patients with TE IV/all patients) in particular was 40% in the lipiodol + DSM group. Although no significant differences between the 3 groups were seen due to the small population, the response rate tended to improve in the lipiodol + DSM group (lipiodol group vs. lipiodol + DSM group, P = 0.07; Mann–Whitney U test). Because of the response rate results, progression-free survival in the lipiodol + DSM group was significantly better than that in the DSM group (P = 0.020) and the lipiodol group (P = 0.035). On the other hand, no significant difference in progression-free survival was seen between the lipiodol group and the DSM group (P = 0.515).
Previous reports associated with our study are shown in Table 4. The response rate was 51% (CR rate 29%) in TAI using cisplatin and lipiodol [19]. On the other hand, the response rates were 73% (CR rate 32%) [19] and 45% (CR rate 0%) [38] in TACE using cisplatin and lipiodol. Although there is a difference in anticancer drugs, the response rates were 52.9% (CR rate 11.8%) [26] and 26% (CR rate 0%) [27] in TAI using DSM. The present findings showed that the response rates in the lipiodol group and in the DSM group were 40% (CR rate 26.7%) and 53.4% (CR rate 26.7%), respectively. Although it is difficult to compare the response rates of our data with those of previous reports, the response rates of the lipiodol group and the DSM group were similar to those of previous reports. On the other hand, only two clinical studies have evaluated TAI using lipiodol and DSM in HCC patients [40, 41]. However, there were some differences in embolization technique. Although the procedure of Vogl et al. [40] was similar to ours, the DSM dose was low (2–10 mg) compared with our procedure (60–1500 mg; mean, 426.6 ± 404.8 mg). Kirchhoff et al. [41] reported administering a mixture of anticancer drugs, DSM, and lipiodol and seeing a response rate of 36% (CR rate 0%). The particles of the emulsion using anticancer agents and lipiodol (lipiodol emulsion) are <30 μm [42] and those of DSM are 45 ± 7 μm in diameter [43]. Because the DSM particles are larger in diameter than those of the lipiodol emulsion, DSM may cause the occlusion of feeding tumor vessels before the accumulation of lipiodol emulsion by means of a mixture of DSM and lipiodol emulsion. In our study, the response rate was 80% (CR rate 40%). Our response rate is better than that reported by Kirchhoff et al. [41], and is similar to that of TACE reported by Ikeda et al. [19]. Both animal and clinical studies have reported that lipiodol injected into the hepatic artery occasionally appears in the portal veins through multiple arterioportal communications [44, 45], and that lipiodol can be used to temporarily embolize both the hepatic arteries and the portal veins. We speculate that lipiodol emulsion may be pushed out in the portal vein, the drainage vein of HCC, by DSM. Consequently, we may achieve as tight an interruption of blood supply as TACE for HCC.
There were no significant differences between the 3 groups in adverse effects other than the grade of elevated ALT levels. However, we consider that the high level of ALT in the lipiodol + DSM group reflects the effect of embolization. Transient increases in serum ALT concentration decreased almost to pretreatment levels 2 weeks after TAI using lipiodol and DSM. Because no serious adverse effects were seen in the lipiodol + DSM group, we consider TAI using lipiodol and DSM to be a safe treatment.
In conclusion, our developed TAI using lipiodol and DSM was superior to TAI using lipiodol only and TAI using DSM only because of improvements in therapeutic effects and progression-free survival. This procedure is both a safe and an effective therapy for HCC patients. TAI using lipiodol and DSM may be expected to serve as an alternative to TACE. Since our study examined only a small population, further investigations are necessary.
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50.
Kiyosawa K, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Gad A, et al. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127:S17–26.
El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34.
Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe. 1980–2004. Hepatology. 2008;48:137–45.
El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–63.
Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, et al. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458–64.
Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79–83.
Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy with combined angiography and computed tomography assistance for patients with hepatocellular carcinoma. Cancer. 2001;91:1342–8.
Todo S, Furukawa H, Japanese Study Group on Organ Transplantation. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451–61.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9.
Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6–25.
Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578–83.
Pelletier G, Ducreux M, Gay F, Luboinski M, Hagège H, Thong D, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. J Hepatol. 1998;29:129–34.
Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42.
Takayasu K, Muramatsu Y, Maeda T, Iwata R, Furukawa H, Moriyama N, et al. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR Am J Roentgenol. 2001;176:681–8.
Yoon HJ, Kim JH, Kim KA, Lee IS, Ko GY, Song HY, et al. Transcatheter arterial chemo-lipiodol infusion for unresectable hepatocellular carcinoma in 96 high-risk patients. Clin Radiol. 2010;65:271–7.
Ikeda M, Maeda S, Shibata J, Muta R, Ashihara H, Tanaka M, et al. Transcatheter arterial chemotherapy with and without embolization in patients with hepatocellular carcinoma. Oncology. 2004;66:24–31.
Forsberg JO. Transient blood flow reduction induced by intra-arterial injection of degradable starch microspheres. Experiments on rats. Acta Chir Scand. 1978;144:275–81.
Lindell B, Aronsen KF, Rothman U. Repeated arterial embolization of rat livers by degradable microspheres. Eur Surg Res. 1977;9:347–56.
Dakhil S, Ensminger W, Cho K, Niederhuber J, Doan K, Wheeler R. Improved regional selectivity of hepatic arterial BCNU with degradable microspheres. Cancer. 1982;50:631–5.
Ensminger WD, Gyves JW, Stetson P, Walker-Andrews S. Phase I study of hepatic arterial degradable starch microspheres and mitomycin. Cancer Res. 1985;45:4464–7.
Taguchi T. Chemo-occlusion for the treatment of liver cancer. A new technique using degradable starch microspheres. Clin Pharmacokinet. 1994;26:275–91.
Carr BI, Zajiko A, Bron K, Orons P, Sammon J, Baron R. Phase II study of Spherex (degradable starch microspheres) injected into the hepatic artery in conjunction with doxorubicin and cisplatin in the treatment of advanced-stage hepatocellular carcinoma: interim analysis. Semin Oncol. 1997;24(suppl 6):S6-97–9.
Furuse J, Ishii H, Satake M, Onaya H, Nose H, Mikami S, et al. Pilot study of transcatheter arterial chemoembolization with degradable starch microspheres in patients with hepatocellular carcinoma. Am J Clin Oncol. 2003;26:159–64.
Kirchhoff TD, Rudolph KL, Layer G, Chavan A, Greten TF, Rosenthal H, et al. Chemoocclusion vs. chemoperfusion for treatment of advanced hepatocellular carcinoma: a randomised trial. Eur J Surg Oncol. 2006;32:201–7.
Yamasaki T, Saeki I, Harima Y, Okita K, Segawa M, Yamaguchi Y, et al. The pilot study: a novel transarterial chemoembolization using degradable starch microspheres for hepatocellular carcinoma (in Japanese with English abstract). Kanzo. 2008;49:25–7.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 5th edn. Tokyo: Kanehara, 2009 (in Japanese).
Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207–15.
Kanematsu M, Hoshi H, Imaeda T, Murakami T, Inaba Y, Yokoyama R, et al. Detection and characterization of hepatic tumors: value of combined helical CT hepatic arteriography and CT during arterial portography. AJR Am J Roentgenol. 1997;168:1193–8.
Okusaka T, Okada S, Ishii H, Ikeda M, Nakasuka H, Nagahama H, et al. Transarterial chemotherapy with zinostatin stimalamer for hepatocellular carcinoma. Oncology. 1998;55:276–83.
Takayasu K, Arri S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699–704.
Okusaka T, Okada S, Ueno H, Ikeda M, Yoshimori M, Shimada K, et al. Evaluation of the therapeutic effect of transcatheter arterial embolization for hepatocellular carcinoma. Oncology. 2000;58:293–9.
National Cancer Institute (2009) Common Terminology Criteria for Adverse Events v.4.0. National Cancer Institute, Bethesda
Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–81.
Ono Y, Yoshimasu T, Ashikaga R, Inoue M, Shindou H, Fuji K, et al. Long-term results of lipiodol-transcatheter arterial embolization with cisplatin or doxorubicin for unresectable hepatocellular carcinoma. Am J Clin Oncol. 2000;23:564–8.
Yoshikawa M, Ono N, Yodono H, Ichida T, Nakamura H. Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Hepatol Res. 2008;38:474–83.
Vogl TJ, Trapp M, Shroeder H, Mack M, Schuster A, Schmitt J, et al. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success—results from a liver transplantation center. Radiology. 2000;214:349–57.
Kirchhoff TD, Bleck JS, Dettmer A, Chavan A, Rosenthal H, Merkesdal S, et al. Transarterial chemoembolization using degradable starch microspheres and iodized oil in the treatment of advanced hepatocellular carcinoma: evaluation of tumor response, toxicity, and survival. Hepatobiliary Pancreat Dis Int. 2007;6:259–66.
Kanematsu M. Transcatheter arterial chemoembolization therapy with epirubicin hydrochloride, mitomycin C-iohexol-lipiodol emulsion (EMILE) for hepatocellular carcinoma. J Gastroenterol. 1995;30:215–23.
Sigurdson ER, Ridge JA, Daly JM. Intra-arterial infusion of doxorubicin with degradable starch microspheres. Improvement of hepatic tumor drug uptake. Arch Surg. 1986;121:1277–81.
Nakamura H, Hashimoto T, Oi H, Sawada S. Iodized oil in the portal vein after arterial embolization. Radiology. 1988;167:415–7.
Kan Z, Ivancev K, Hägerstrand I, Chuang VP, Lunderquist A. In vivo microscopy of the liver after injection of lipiodol into the hepatic artery and portal vein in the rat. Acta Radiol. 1989;30:419–25.
Acknowledgment
This study was supported by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (Grant No. 20591478).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamasaki, T., Hamabe, S., Saeki, I. et al. A novel transcatheter arterial infusion chemotherapy using iodized oil and degradable starch microspheres for hepatocellular carcinoma: a prospective randomized trial. J Gastroenterol 46, 359–366 (2011). https://doi.org/10.1007/s00535-010-0306-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-010-0306-5