Abstract
In recent years, an increasing number of studies have shown that fibroblast growth factor 12 (FGF12) plays important roles in regulating neural development and function. Importantly, changes of FGF12 expression are thought to be related to the pathophysiology of many neurological diseases. However, little research has been performed to explore the protective effect of FGF12 on nerve damage. This study aims to explore its neuroprotective effects using our recombinant humanized FGF12 (rhFGF12). The hFGF12 gene was cloned and ligated into an expression vector to construct a recombinant plasmid pET-3a-hFGF12. Single colonies were screened to obtain high expression engineering strains, and fermentation and purification protocols for rhFGF12 were designed and optimized. The biological activities and related mechanisms of rhFGF12 were investigated by MTT assay using NIH3T3 and PC12 cell lines. The in vitro neurotoxicity model of H2O2-induced oxidative injury in PC12 cells was established to explore the protective effects of rhFGF12. The results indicate that the beneficial effects of rhFGF12 were most likely achieved by promoting cell proliferation and reducing apoptosis. Moreover, a transgenic zebrafish (islet) with strong GFP fluorescence in the motor neurons of the hindbrain was used to establish a central injury model caused by mycophenolate mofetil (MMF). The results suggested that rhFGF12 could ameliorate central injury induced by MMF in zebrafish. In conclusion, we have established an efficient method to express and purify active rhFGF12 using an Escherichia coli expression system. Besides, rhFGF12 plays a protective effect of on nerve damage, and it provides a promising therapeutic approach for nerve injury.
Key points
• Effective expression and purification of bioactive rhFGF12 protein in E. coli.
• ERK/MAPK pathway is involved in rhFGF12-stimulated proliferation on PC12 cells.
• The rhFGF12 has the neuroprotective effects by inhibiting apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibroblast growth factors (FGFs) belong to a large family of growth factors containing 22 members with similar structural polypeptide (Beenken and Mohammadi 2009). FGFs play important roles in normal physiological processes and pathological conditions, including embryonic development, angiogenesis, metabolism regulation, and wound healing (Hui et al. 2018). At present, FGFs are mainly purified by overexpression in E. coli, such as FGF2, FGF9, FGF13, FGF17, and FGF20 (Alibolandi and Mirzahoseini 2011; Tian et al. 2016; Wang et al. 2017; Wu et al. 2017). FGF homologous factors (FHFs, including FGF11-FGF14) are a subfamily of FGF proteins that share similar sequences and structures with the FGF family (Goldfarb 2005; Hennessey et al. 2013). In the previous studies, FHF proteins were considered to be intracellular factors, which could not bind and activate FGF receptors (FGFRs) (Olsen et al. 2003). However, in a previous study, we have demonstrated that FGF13 is able to bind directly to FGFR under a certain concentration of heparin, which in turn transmit the signal inside the cell (Lin et al. 2019). It is possible that other members of the FHF family may have similar biological functions as FGF13.
FGF12 is expressed mainly in the nervous system, heart, connective tissue, and pancreas (Goldfarb 2005; Hartung et al. 1997). It was found to be an intracellular modulator of voltage-gated sodium channel essential to modulate its properties (Nav channels). Moreover, it was reported that FGF12 could bind to the C terminus of the cardiac voltage-gated sodium channel to modulate its properties (Liu et al. 2003; Nakayama et al. 2011; Wang et al. 2011). Nav channels are considered to be the backbone of generation and transmission of neuron action potentials (Al-Mehmadi et al. 2016; Goldfarb et al. 2007). Further study found that FGF12 could interact with Nav and regulate the channel activity in neurons (Zhang et al. 2012). In addition, it was revealed that a mutation in the FGF12 gene in patients with epileptic encephalopathy elevated the voltage dependence of neuronal sodium channel fast inactivation (Guella et al. 2016; Takeguchi et al. 2018). Although the above studies have reported mechanisms for neural regulation via FGF12, it is still unclear whether it has a protective effect on nervous systems (Smallwood et al. 1996).
There are increasing evidences showing that changes of FGF12 expression have been associated with various neuropsychiatric illnesses and brain diseases including depression, anxiety, epilepsy, psychosis, and Alzheimer’s disease (AD) (Burgess and Granato 2007; Cunliffe 2016; Kim et al. 2010; Marcon et al. 2016; Stewart et al. 2012). FGF12 is highly expressed in the nervous system and involved in the regulation of channel activity in neurons (Hanada et al. 2018). The previous studies mainly focus on endogenous FGF12 expression in nerve-related diseases, while there are few studies to explore the function of exogenous FGF12 in neuroprotection. In this study, rhFGF12 was highly expressed in E. coli using genetic engineering methods and purified to obtain a biologically active recombinant protein. The rhFGF12 protein promoted the proliferation of PC12 cells and had a significant protective effect on PC12 cells exposed to H2O2. At the same time, mechanistic studies showed that the neuroprotective effects of rhFGF12 are related to anti-apoptosis and proliferation-promoting pathways. In addition, rhFGF12 could induce protection against MMF injury in central nervous system of zebrafish, which is a transgenic zebrafish strain (Islet) with neuronal fluorescence (Brennan 2011; Stewart et al. 2015). In conclusion, this study investigated a simple method for rhFGF12 expression and revealed that rhFGF12 has a good protective effect against nerve damage via anti-apoptosis and promoting proliferation. These findings laid a foundation for further study of the role of rhFGF12 in neuroprotection.
Materials and methods
The E. coli strain harboring pET-3a was stored in Zhejiang Provincial Key Laboratory, Wenzhou Medical University (Wenzhou, China). The E. coli strains DH5α and BL21 (DE3) pLysS and isopropyl β-d-1-thiogalactopyranoside (IPTG) were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). The plasmid extraction kit was purchased from Axygen (USA). The restriction endonucleases BamH I and Nde I were purchased from NEB (USA). Dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and Coomassie brilliant blue G-250 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), and Roswell Park Memorial Institute 1640 (RPMI 1640) were purchased from Gibco (Thermo Fisher Scientific, Inc., Carlsbad, CA, USA). NIH 3T3 cells and PC12 cells were obtained from Zhejiang Provincial Key Laboratory, Wenzhou Medical University (Wenzhou, China). Primary antibodies against FGF12, β-actin, Bcl-2, Bax, Caspase-3, and PCNA were purchased from Abcam (Cambridge, MA, USA). Primary antibodies against phospho-ERK1/2, ERK1/2, phosphor-AKT, and AKT were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Protein molecular weight standard was purchased from Thermo Fisher Scientific, Inc. (Carlsbad, CA, USA). The zebrafish (Islet) was introduced by the University of Oregon’s Tanguay (USA) Zebrafish Toxicology Laboratory and was raised by the Zebrafish Model Biological Laboratory of the Zhejiang Provincial Key Laboratory of Biotechnology and Applications at Wenzhou Medical University. Mycophenolate mofetil (MMF) was purchased from Yuanye Co., Ltd. (Shanghai, China).
Construction of the pET3a-hFGF12 recombinant plasmid
According to the hFGF12 gene sequence obtained by GenBank (GenBank accession number NM_021032.5) and the codon preference of E. coli, the optimized gene sequence of FGF12 (including BamH I and Nde I restriction enzyme sites, MW459160) was synthesized and then cloned into the pUC57 vector by Suzhou GENEWIZ Biotechnology Co., Ltd. The amplified fragments that were cut with the restriction enzymes BamH I and Nde I were purified with a gel extraction kit followed by ligation into the predigested pET3a vector. The pET3a-hFGF12 recombinant plasmid was transformed into E. coli DH5α competent cells, and the plasmid was extracted and introduced into E. coli BL21 (DE3) pLysS expression strain.
Screening for rhFGF12 high expression engineering strains
Different positive single colonies were screened then grown in LB liquid medium containing ampicillin (100 mg/l) and chloramphenicol (34 mg/l) and incubated at 37 °C with shaking at 180 rpm until the A600 value reached 0.8~1.0. Then, isopropylthio-β-D-galactoside (IPTG) was added to a final concentration of 0.5 mmol/l. After another 4-h induction, the cells were collected by centrifugation. The concentrations of the bacterial solutions were normalized to each other, and the protein expression was analyzed by 12% SDS-PAGE after sample preparation. The strain with the highest expression was selected to identify the recombinant protein of interest by Western blot, and then the glycerol strain (20% glycerol as a preservation solution) was prepared and stored at −80 °C.
Expanded culture of rhFGF12-expressing bacteria
The highly expressing pET3a-hFGF12-BL21 (DE3) pLysS glycerol stock was inoculated at 1:100 (v/v) into LB liquid medium containing chloramphenicol and ampicillin and cultured at 37 °C for 4 h. When A600 reached 0.8~1.0, it was used as a seed liquid. The bacterial solution was inoculated to the fermentation medium at 1:10 (v/v) to expand the culture to A600 of 1.2~1.5. After IPTG was added to a final concentration of 0.5 mmol/l, the bacterial liquid was divided into two parts. Part of the cells was collected after induction at 37 °C for 4 h, and the bacterial solution was taken after induction. The other part was collected after induction at 16 °C for 24 h, and the induced bacterial solution was collected. After centrifugation at 9000 rpm at 4 °C for 15 min, the collected cells were cryopreserved at −80 °C. The induced expression was examined by 12% SDS-PAGE. For the pre-treatment of the cells, the OD value is classified to 1.0, take 1ml for centrifugation and discard the supernatant, and then add 80 μl of purified water and 20 μl of 5× sample treatment solution. After mixing, boil in boiling water for 10 min, centrifuge at 12000 rpm for 10 min, and take 10 μl of the supernatant for SDS-PAGE electrophoresis.
Purification of rhFGF12
The cells were thoroughly stirred and mixed with lysis buffer and then ultrasonically disrupted. After confirming that the disruption was sufficient by microscopic observation, the supernatant and the pellet were collected using an ultralow temperature high speed centrifuge, and it was determined whether the expressed rhFGF12 was soluble by 12% SDS-PAGE. Based on the theoretical isoelectric point of rhFGF12 (IP = 9.98) and its heparin affinity, a Tris-HCl buffer system (pH = 7.5) was selected, and a two-step purification protocol using a cation exchange column (SP) and heparin affinity column chromatography was designed for protein purification. Firstly, the supernatant was loaded to the column which had been pretreated with equilibration buffer (20 mM Tris, 2 mM EDTA, 0.1 M NaCl, pH = 7.5). The eluting buffers for SP was buffer A (20 mM Tris, 2 mM EDTA, 0.6 M NaCl, pH = 7.5). The SP eluted protein fractions were diluted to 0.15 M NaCl con-centration by equilibration buffer (20 mM Tris, 2 mM EDTA, pH = 7.5) before bounding to the heparin affinity column. Then the supernatant was loaded to the column. The following elution buffers for rhFGF12 were buffer A (20 mM Tris, 2 mM EDTA, 0.6 M NaCl, pH = 7.5) and buffer B (20 mM Tris, 2 mM EDTA, 0.8 M NaCl, pH = 7.5). After purification, the purified sample containing the protein of interest was again subjected to Western blot verification. The rhFGF12 sample was ultrafiltered through an ultrafiltration tube at low temperature. The total protein concentration was determined using the BCA method. After sterilization and filtration, cryopreservation was performed at −80 °C.
The effect of rhFGF12 on the proliferation of PC12 cells and the related indexes of cell proliferation
PC12 cells growing in wells were digested with 0.05% trypsin. Digestion was stopped with 1640 complete medium, and cells were harvested and counted using a hemocytometer plate and seeded onto 96-well plates in a cell culture incubator (37 °C, 5% CO2). The cells were cultured for 24 h and then starved for 24 h in DMEM starvation medium containing 0.5% FBS. The rhFGF12 protein was added to a 96-well plate to final concentrations of 0, 6.25, 12.5, 25, 50, 100, 200, 400, and 800 ng/ml. After incubation for 24 h in a cell culture incubator, 25 μl of MTT was added to each well and incubated for 4 h in a cell culture incubator. The liquid in the wells was aspirated with a syringe, and then 150 μl of DMSO was added to each well and shaken on a plate shaker for 5 min. Absorbance values were measured at a wavelength of 490 nm. In addition, the purified rhFGF12 protein was assayed for biological activity using NIH3T3 cells in the same manner as above.
PC12 cells in good growth were digested with 0.05% trypsin at room temperature, collected, and pipetted into a single cell suspension. The cells were added to a 6-well plate (3×105 cells/well) and placed in a cell culture incubator for 24 h. The 1640 starvation medium containing 0.5% FBS was used, and the starvation culture was continued for 12 h in the cell culture incubator. Different concentrations of the drug (rhFGF12) were preadministered for 4 h. The experiment was divided into a blank control group, a rhFGF12 (25 ng/ml) administration group, a rhFGF12 (50 ng/ml) administration group, and a rhFGF12 (100 ng/ml) administration group. PC12 cellular proteins were extracted, and the protein concentration was measured by the BCA method to balance the amount of protein loaded. Western blot was used to detect changes in the indexes associated with cell proliferation—PCNA (1 μg/ml) and ERK (1:1000).
Proliferation effect of the rhFGF12 protein on the H2O2-induced PC12 oxidative damage model
After digesting PC12 cells with good growth status, the cells were counted and plated in 96-well plates and incubated in a cell culture incubator for 24 h. After starving the cells, H2O2 was added to the 96-well plates so that the final concentrations were 0, 0.015, 0.03, 0.06, 0.125, 0.25, 0.5, 1, and 2 mmol/ml, and the plates were placed in the cell culture incubators for 2, 4, 8, and 12 h. The absorbance value was measured using the MTT method. The cell injury survival rate was approximately 60% compared with the control group, and the H2O2 oxidative damage cell model was established.
When PC12 cells were in good growth conditions, they were digested and counted in 96-well plates and then incubated for 24 h in a cell culture incubator. After cell starvation, 1640 starvation medium containing different drug (rhFGF12) concentrations was preadministered for 4 h, and then H2O2 was added at a final concentration of 0.06 mmol/l for 2 h. The absorbance value was measured using the MTT method.
Proliferation effect of rhFGF12 on the H2O2-induced PC12 oxidative damage model and the relationship between apoptosis-related indicators
PC12 cells with good growth state were selected for digestion, and the cells were added to a 6-well plate at 3×105 cells/well and cultured in a cell culture incubator for 24 h. The cells were starved for 12 h. Thereafter, the corresponding drug (50 ng/ml rhFGF12) was preadministered, and the cells were damaged with H2O2. The experiment was divided into a blank control group, rhFGF12 administration group, H2O2 injury group, and H2O2+rhFGF12 group. Cellular proteins were extracted, and the protein concentration was measured by the BCA method to balance the amount of protein loaded. Western blotting was used to detect changes in AKT (1:5000), Caspase-3 (1:5000), Bcl-2 (1:10000), and Bax (1:10000) with the associated apoptosis markers.
Establishment of a zebrafish central nervous system injury model and the protective effect of rhFGF12 on the injury model
The healthy and mature Islet transgenic line zebrafish were selected, and males and females were housed separately for 2 days. The male and female fish were placed in a spawning box in a 3:3 ratio one night in advance. The next morning, the zebrafish laid their eggs in the light, and the embryos were collected a half an hour later. After preliminary selection under a microscope, the cells were cultured in a constant temperature incubator for 24 h, and then MMF was added to the final concentrations of 0, 0.5, 5, and 50 nmol/l and 0.5, 5, 50, and 500 μmol/l, which induced zebrafish central nervous system injury for 24, 36, 48, 60, 72, 96, and 120 h. The embryos were observed under a microscope to detect zebrafish mortality and deformity. Compared with the normal control group, the damage concentration and time of treatment with low mortality, slight teratogenicity, and mild central injury were selected to establish a model of MMF central nervous system injury.
The selected transgenic zebrafish Islet embryos were cultured in a constant temperature incubator for 24 h and then replaced with EM medium containing different drug concentrations (FGF12) for 4 h. After 4 h, MMF was added at a final concentration of 50 nmol/l to induce the formation of zebrafish central nervous system injury, and the culture was continued for 48 h. The zebrafish embryos were randomly selected and placed under an inverted fluorescence microscope to observe the fluorescence intensity and central nervous system damage. The experiment was divided into the control group, MMF injury group, and MMF + rhFGF12 (50, 100, 200 ng/ml) group.
Results
Vector construction and expression of rhFGF12
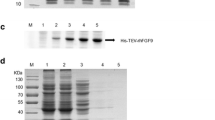
The recombinant plasmid was digested with Nde I and BamH I to obtain two fragments: the pET-3a vector fragment and the FGF12 gene fragment (Fig. 1a). It was initially confirmed that the FGF12 gene had been successfully ligated to the pET-3a vector. The selected positive recombinant strains and plasmids were sequenced, and the results were in line with expectations. The correctly sequenced positive recombinant plasmid was transformed into competent E. coli expressing pLysS, which were cultured after screening, and the expression of rhFGF12 was induced using IPTG. By 12% SDS-PAGE, a significant protein expression band was observed compared to the preinduction control. The molecular weight was approximately 28 kDa (Fig. 1b).
Vector construction and expression of rhFGF12. a The recombinant plasmid was identified by enzyme digestion. 1, pET-3a vector plasmid; 2, pUC57-FGF12 double-digested product; 3, pET3a-hFGF12 recombinant plasmid double-digested product; M1, 15000 bp DNA Marker; M2, 2000 bp DNA Marker. b Screening of rhFGF12 high-expressing single colonies with SDS-PAGE; M, protein molecular weight standard; 1, uninduced; 2~8, expression after induction by different single colonies. c SDS-PAGE analysis of rhFGF12 under 37 °C-induced expression after expanded culture; M, protein molecular weight standard; 1, uninduced; 2, 1 h after induction; 3, 2 h after induction; 4, 3 h after induction; 5, 4 h after induction. d SDS-PAGE analysis of rhFGF12 under 16 °C-induced expression after expanded culture; M, protein molecular weight standard; 1, uninduced; 2, 6 h after induction; 3, 12 h after induction; 4, 18 h after induction; 5, 24 h after induction. e Identification of rhFGF12 under 16 °C-induced expression by Western blot; M, protein molecular weight standard; 1, uninduced; 2, 6 h after induction; 3, 12 h after induction; 4, 18 h after induction; 5, 24 h after induction
Expanded culture of rhFGF12-expressing bacteria
The high-efficiency expression strain was expanded and cultured under different conditions, and then the cells were collected by low-temperature centrifugation. After 12% SDS-PAGE analysis, the results showed that the target protein induced by 16 °C and 37 °C was highly expressed. Interestingly, there was a tendency for the relative expression to increase with time at 16 °C (Fig. 1 c and d). Moreover, the expression of rhFGF12 was further confirmed by Western blotting with hFGF12 monoclonal antibody. The sample treated with the preinduced bacterial solution was used as a negative control. As shown, the rhFGF12 protein was successfully induced and expressed at 16 °C (Fig. 1e).
Purification of rhFGF12
The cells were subsequently harvested and ultrasonically disrupted on an ice bath. After centrifugation at 12000 rpm, 4 °C for 20 min, the supernatant and the pellet were collected for further analysis on 12% SDS-PAGE. The results showed that in the supernatant, the solubility of recombinant rhFGF12 induced at 16 °C was highly increased compared to that induced at 37 °C (Fig. 2a). It suggests that low-temperature induction promotes the soluble expression of FGF12 protein. Therefore, 16 °C was considered the optimum incubation temperature. Purification was carried out through a cation exchange column using an AKTA Purifier instrument. The loaded protein and elution peaks eluted by SP and heparin column were processed and subjected to 12% SDS-PAGE, and it was found that the rhFGF12 protein was eluted in a buffer with 0.6 mol/l NaCl (Fig. 2b). The samples were diluted with purified working solution A to a final salt concentration of 0.15 mol/l and passed through a heparin affinity column. All collected samples were subjected to 12% SDS-PAGE. As shown in result, the rhFGF12 protein was eluted in a buffer with 0.8 mol/l NaCl (Fig. 2c). RP-HPLC showed that the final protein purity was 98.6% (Fig. 2d). The final concentration of rhFGF12 was determined by the BCA protein concentration assay kit. After sterilization and filtration, the supernatant was then taken and placed in a refrigerator at −80 °C for cryopreservation.
Purification of rhFGF12. a SDS-PAGE analysis of soluble rhFGF12; M, protein molecular weight standard; 1, bacteria expressed at 37 °C; 2, the crushing supernatant of bacteria expressed at 37 °C; 3, the precipitation of bacteria expressed at 37 °C; 4, bacteria expressed at 16 °C; 5, the crushing supernatant of bacteria expressed at 16 °C; 6, the pellet of bacteria expressed at 16 °C. b SDS-PAGE analysis after purification of the cation exchange column; M, protein molecular weight standard; 1, bacteria expressed at 16 °C; 2, pellet; 3, supernatant; 4, elution peak of buffer with 0.6 M NaCl. c SDS-PAGE analysis after purification with the heparin column; M, protein molecular weight standard; 1, the SP eluent protein peak was diluted and loaded; 2, sample penetrating fluid; 3, elution peak of buffer with 0.6 M NaCl; 4, elution peak of buffer with 0.8 M NaCl. d RP-HPLC results after ultrafiltration of the purified recombinant protein.
rhFGF12 promotes NIH3T3 cells and PC12 cells proliferation
The purified rhFGF12 protein was tested for biological activity using NIH3T3 cells. With heparin is 100 μg/ml, the cells were administered rhFGF12 concentrations ranging from 6.25 to 800 ng/ml for 24 h, and the proliferation was determined by MTT assay. The result showed that rhFGF12 promoted cell proliferation in a dose-dependent manner (Fig. 3a). Further, it was further confirmed that in PC12 cells cultured with different concentration of rhFGF12, rhFGF12 also had a pronounced effect on the proliferative activity of PC12 cells (Fig. 3b).
The effect of rhFGF12 on the proliferative effect of NIH3T3 cells, PC12 cells, and related cell proliferation indicators. a Proliferation of NIH3T3 cells by different concentrations of rhFGF12. Note: *** indicates P < 0.001, compared with the control group. b Proliferation of PC12 cells by different concentrations of rhFGF12. Note: *** indicates P < 0.001, compared with the control group. c Western blot analysis of cytoplasmic PCNA in different experimental groups. Note: *** indicates P < 0.001, compared with the control group. d Western blot analysis of cytoplasmic p-ERK and ERK in different experimental groups. Note: *** P < 0.001, compared with the control group.
Cell proliferation is an important feature of living organisms, and the activation and regulation of a series of genes are involved such as PCNA and ERK. PC12 cellular proteins were extracted for Western blot. The results showed that the expression level of PCNA was significantly increased in the rhFGF12-administered group compared with the control group in a dose-dependent (Fig. 3c). On the other hand, the ratio of p-ERK/ERK was significantly increased in the rhFGF12-administered group compared with the control group, which was also significantly dose-dependent (Fig. 3d). These results indicate that rhFGF12 affects the proliferation activity of PC12 cells.
rhFGF12 promotes the proliferation of PC12 oxidative damage induced by H2O2
Previous reports have implicated that H2O2-induced oxidative stress could impair the proliferation. This prompted us to ask whether rhFGF12 could alleviate H2O2-induced proliferation inhibition. First, the oxidative damage model was established in PC12 cells. In brief, we investigated the effect of a series of H2O2 concentration (0, 0.015, 0.03, 0.06, 0.125, 0.25, 0.5, 1, 2 mmol/l) to the cells for 2 h. 0.06 mmol/l H2O2 caused survival rates of up to 53% (Fig. 4a). Therefore, the final modeling method is a concentration of 0.06 mmol/l H2O2 incubated with PC12 cells for 2 h at 37 °C in 5% CO2. Furthermore, cells were preadministered for 4 h with starvation medium containing different concentrations of rhFGF12 (0, 3.125, 6.25, 12.5, 25, 50, 100, 200, 400 ng/ml) (Fig. 4b). Our results showed that oxidative damage induced proliferation inhibition could be ameliorated by treatment of rhFGF12, which was dose related. The optimal concentration of rhFGF12 for protection against oxidative damage was determined to be 50 ng/ml.
rhFGF12 promotes the proliferation of PC12 cells with oxidative damage induced by H2O2, and the effect of rhFGF12 on apoptosis-related indicators. a Effect of H2O2 injury concentration on the PC12 cell survival rate at 2 h. Note: *** P < 0.001, compared with the control group. b Effect of different concentrations of rhFGF12 on the oxidative damage cell model. Note: *** P < 0.001, ** P < 0.01, * P < 0.05, compared with the H2O2 group; ### P < 0.001, compared with the control group. c Western blot analysis of cytoplasmic Bcl-2 and Bax in different experimental groups. Note: * P < 0.05, compared with the control group; ## P < 0.01, compared with the H2O2 group. d Western blot analysis of Caspase-3 in different experimental groups. Note: *** P < 0.001, compared with the control group; ### P < 0.001, compared with the H2O2 group. e) Western blot analysis of p-AKT and AKT in different experimental groups. Note: ** P < 0.01, compared with the control group; ### P < 0.001, compared with the H2O2 group.
Anti-apoptotic effect of rhFGF12 in PC12 cells
To better understand the protective effect of the rhFGF12, Western blot analysis was used to detect Bax, Bcl-2, Caspase-3, and AKT protein levels in PC12 cells. Compared with the control group, the protein expression level of Bcl-2 was significantly decreased in the H2O2 injury group, and the Bax protein level was increased, resulting in a decrease in the Bcl-2/Bax ratio (P < 0.05) (Fig. 4c). rhFGF12 can significantly improve the ratio of Bcl-2/Bax compared with injury group. Moreover, compared with the control group, the expression level of Caspase-3 was increased in the H2O2 injury group (P < 0.001), while these observed alterations were reversed by rhFGF12 treatment (P < 0.001) (Fig. 4d). On the other hand, the ratio of p-AKT/AKT was significantly higher in the H2O2 injury group compared with the control group (P < 0.01) (Fig. 4e). It was also reversed in the rhFGF12 treatment group (P < 0.001). These data suggest that the protective effect of rhFGF12 in PC12 cells may be related to its anti-apoptotic effect.
Protective effect of rhFGF12 on MMF-induced injury in zebrafish central neurons
The zebrafish embryos were cultured to which MMF was added to attain final drug concentrations of 0, 0.5, 5, and 50 nmol/l and 0.5, 5, 50, and 500 μmol/l for 24, 36, 48, 60, 72, 96, and 120 h respectively. The embryo was viewed under the microscope to detect the mortality, deformity rate, and central nervous system damage. The results showed that when the MMF concentration was 50 nmol/l and the injury time was 48 h, the MMF-damaged zebrafish had lower mortality and higher incidence of malformation compared with the normal control group (resulting in mild teratogenicity) (Fig. 5a). Combined with the morphological observation of zebrafish (the left side is a top view, the right side is a side view), 50 nmol/l MMF was determined as the injury concentration (Fig. 5b). Compared with the normal group, the neuronal divergence fluorescence of the MMF central injury group was weak and fragmented. In contrast, the rhFGF12 treatment could reverse MMF-induced neurological manifestations, which was in a dose-dependent manner (Fig. 5c). There was a significant difference between the high-dose rhFGF12 administration group and the injury group (Fig. 5d). These results revealed that rhFGF12 has a significant protective effect on MMF induced central nervous system injury.
Protective effect of rhFGF12 on MMF-induced injury in zebrafish central neurons. a Effects of different MMF administration time and administration concentrations on the mortality and deformity of zebrafish. Note: *** P < 0.001, ** P < 0.01, compared with the control group. b Effects of different MMF concentrations on zebrafish morphology. The left side is a top view, the right side is a side view. c Protective effect of rhFGF12 on MMF-induced injury in zebrafish central neurons (S 50 ng/ml, M 100 ng/ml, L 200 ng/ml). d Fluorescence intensity analysis of zebrafish in each experimental group (S 50 ng/ml, M 100 ng/ml, L 200 ng/ml). Note: *** P < 0.001, ** P < 0.01, compared with MMF group; ### P < 0.001, compared with the control group.
Discussion
Previous study has shown that FGF12 is highly expressed in embryonic development and adult nervous system, and its gene mutations can lead to neurological diseases (Siekierska et al. 2016). This reveals that FGF12 plays an important role in nerve development and mediation, but its mechanism is still unclear. Moreover, current studies have focused on the role of endogenous FGF12, and there is no study on the role of natural exogenous FGF12 in nerves. In this study, the recombinant plasmid pET3a-hFGF12 was transformed into BL21 (DE3) pLysS competent cells, then screen high expression engineering strains (Fig. 1 a and b). They were cultivated and induced expression at 37 °C, and most of the target proteins were expressed as inclusion bodies (Figs. 1c and 2a). When the E. coli system is used to express foreign proteins, the rapid synthesis speed and higher expression are the main reasons leading to the formation of inclusion bodies (Castellanos-Mendoza et al. 2014; Singh et al. 2005; Wei et al. 2020). It is considered to be the main reason for causing insufficient solubility of protein. Lowering the induction temperature and reducing the amount of inducer IPTG can slow down the synthesis level of inclusion bodies and promote the formation of soluble proteins (Zhao et al. 2018). Therefore, the methods of reducing the expression temperature and adding inducers in batches were used to promote the soluble expression of rhFGF12 protein in this study. The results showed that adding the inducer IPTG in two batches to a final concentration of 0.5 mmol/l, and inducing expression at 16 °C for 24 h, effectively increased the bacterial yield and expression of soluble protein (Figs. 1d–e and 2a). The majority of the target protein was present as soluble form and only a small amount of insoluble inclusion bodies was present (< 20%).
In the previous study, it was found that the FGF11 subfamily had a certain affinity with heparin. Compared with the other subfamilies of FGF, the affinity rate with heparin is high. In addition, FGF12 is a positive charge, so we design an SP cation exchange column and heparin affinity column chromatography (with heparin binding properties) two-step purification method (Fig. 2 b and c). Based on this, a high purity of rhFGF12 was obtained. The purified product was identified by RP-HPLC with a purity of 98.6%, indicating that the rhFGF12 protein was successfully purified (Fig. 2d). It lays a foundation for further study on the biological activity of FGF12.
After purification, it was necessary to verify whether rhFGF12 was biologically active and biologically functional. FHFs have high sequence and structural homology with other fibroblast growth factors (FGFs) and bind heparin with high affinity, but unlike most FGFs, they do not stimulate FGF receptors (FGFRs) (Beenken and Mohammadi 2009; Nybakken and Perrimon 2002; Ornitz 2000). In the previous study, we found that FGF13, a member of FGF11 subfamily, could promote NIH3T3 cell proliferation via binding the FGFR receptor in a certain heparin concentration-dependent manner, but heparin has no effect on the proliferation of NIH3T3 cells (Lin et al. 2019). Because FGF11 subfamily is highly conserved, we hypothesized that FGF12 may have similar properties. Therefore, we determined the proliferative activity of the purified rhFGF12 protein on NIH 3T3 cells under heparin is 100 μg/ml. The MTT assay showed that the rhFGF12 had a high proliferative activity (Fig. 3a). In the experiment, we found that higher rhFGF12 protein concentration can inhibit cell proliferation rate. The main reason may be that the protein is easy to form dimers or polymers at higher concentrations, which affects the binding to heparin and FGFR and then affects the signal transduction that promotes proliferation (Harada et al. 2009; Hecht et al. 2001; Kalinina et al. 2009).
In recent years, an increasing number of studies have shown that FGF12 has an important relationship with nerves. FGF12 is highly expressed in the developing nerves (Siekierska et al. 2016; Smallwood et al. 1996), and expression changes of FGF12 gene have been detected in many neurological diseases. However, these studies have not explored the protective and repairing effects of FGF12 on nerve damage. To explore the role of FGF12 in repairing nerve damage, PC12 cells was used, which are widely used as in vitro models of neurons (Das et al. 2004). Furthermore, the H2O2 oxidative damage model of PC12 cells was established to explore the protective effects and mechanisms of FGF12. The experiment found that rhFGF12 had a significant proliferative effect on PC12 cells (Fig. 3b), In addition, rhFGF12 also promoted the expression of PCNA (Fig. 3c), which is closely related to the synthesis of cellular DNA and plays an important role in the initiation of cell proliferation (Moldovan et al. 2007). ERK belongs to the MAPK (mitogen-activated protein kinase) pathway, and MAPK can transmit various cell signals, thereby controlling cell proliferation, differentiation, development, stress response, and apoptosis, while ERK plays a major role in proliferation and differentiation (Goshen-Lago et al. 2017; Shin et al. 2018). In our study, the level of p-ERK/ERK was significantly increased in the proliferative experimental study (Fig. 3d). It indicates that the proliferative effect of FGF12 on PC12 might be related to the activation of the ERK signaling pathway.
H2O2 is one of widely used oxidative stressors for studies, and oxidative damage is closely related to the neuronal apoptosis in clinical research (Chen et al. 2019). Therefore, H2O2 was used to induce the apoptosis to examine the ability of FGF12 to protect PC12 cells (Fig. 4 a and b). Previous studies indicated that the pathological state of nerve damage is closely related to apoptosis (Huang et al. 2019; Wang et al. 2018). We analyzed the protective effect of rhFGF12 by detecting apoptosis-related indicators. Bcl-2/Bax is a powerful indicator of cell anti-apoptosis (Thomadaki and Scorilas 2006). The results showed that the rhFGF12 played a protective role by inhibiting apoptosis (Fig. 4c). Caspase-3 is generally considered to be an important terminal cleavage enzyme in the process of apoptosis (Manu et al. 2019). Our study showed the expression level of Caspase-3 was increased by oxidative injury. However, this alteration was alleviated by rhFGF12 treatment, which further proves the protective effect of rhFGF12 (Fig. 4d). AKT is relative to anti-apoptotic process. The ratio of p-AKT/AKT was used to analyze cell apoptosis. In our study, the increased p-AKT/AKT ratio during oxidative injury could be reversed by rhFGF12 administration, further indicating that the protective effect of rhFGF12 achieves an anti-apoptotic effect, which might be relative to the inhibition of AKT phosphorylation levels (Fig. 4e).
As an animal model, zebrafish have many unparalleled advantages compared with traditional animal models and in vitro cell models: small size, fast reproduction, easy to raise, transparent development period, easy to observe, a high degree of similarity with higher animals in terms of neurophysiology and gene structure, and a systemic regulation of neurohumoral fluids (Fontana et al. 2018). Therefore, zebrafish are widely used in the field of neurological disease research. To further investigate the neuroprotective effects of rhFGF12 in vivo, we used a transgenic zebrafish strain (Islet) with neuronal fluorescence, which was treated by MMF to establish a central injury model. Here we found that more than 0.5 nmol/l MMF caused teratogenesis and more than 50 μmol/l caused death (Fig. 5 a and b). After successfully establishing the MMF central injury model, rhFGF12 was used to detect its protective effect on central nervous system injury. Through fluorescence analysis, we found that rhFGF12 had a significant protective effect on zebrafish central injury induced by MMF in a dose-dependent manner (Fig. 5 c and d). It lays a foundation for the study of the neuroprotective effect of FGF12.
In summary, our study was designed to establish of fermentation and purification process for rhFGF12 protein in E. coli. Besides, we found that rhFGF12 had the neuroprotective effects by promoting cell proliferation and inhibiting apoptosis in vitro. Also, rhFGF12 have the proliferative effect on PC12 cells through the ERK/MAPK pathway. In addition, rhFGF12 ameliorated zebrafish central injury induced by MMF in a dose-dependent. However, it remains unclear which deep mechanisms of FGF12 on promoting proliferation and anti-apoptosis for nerve injury. These warrants further investigation.
References
Alibolandi M, Mirzahoseini H (2011) Purification and refolding of overexpressed human basic fibroblast growth factor in Escherichia coli. Biotechnol Res Int 2011:973741–973746. https://doi.org/10.4061/2011/973741
Al-Mehmadi S, Splitt M, Ramesh V, DeBrosse S, Dessoffy K, Xia F, Minassian BA (2016) FHF1 (FGF12) epileptic encephalopathy. Neurol Genet 2:e115. https://doi.org/10.1212/nxg.0000000000000115
Beenken A, Mohammadi M (2009) The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8:235–253. https://doi.org/10.1038/nrd2792
Brennan CH (2011) Zebrafish behavioural assays of translational relevance for the study of psychiatric disease. Rev Neurosci 22:37–48. https://doi.org/10.1515/rns.2011.006
Burgess HA, Granato M (2007) Sensorimotor gating in larval zebrafish. J Neurosci 27:4984–4994. https://doi.org/10.1523/jneurosci.0615-07.2007
Castellanos-Mendoza A, Castro-Acosta RM, Olvera A, Zavala G, Mendoza-Vera M, García-Hernández E, Valdez-Cruz NA (2014) Influence of pH control in the formation of inclusion bodies during production of recombinant sphingomyelinase-D in Escherichia coli. Microb Cell Factories 13:137. https://doi.org/10.1186/s12934-014-0137-9
Chen L, Wu X, Shen T, Wang X, Wang S, Wang J, Ren D (2019) Protective effects of ethyl gallate on H2O2-induced mitochondrial dysfunction in PC12 cells. Metab Brain Dis 34:545–555. https://doi.org/10.1007/s11011-019-0382-z
Cunliffe VT (2016) Building a zebrafish toolkit for investigating the pathobiology of epilepsy and identifying new treatments for epileptic seizures. J Neurosci Methods 260:91–95. https://doi.org/10.1016/j.jneumeth.2015.07.015
Das KP, Freudenrich TM, Mundy WR (2004) Assessment of PC12 cell differentiation and neurite growth: a comparison of morphological and neurochemical measures. Neurotoxicol Teratol 26:397–406. https://doi.org/10.1016/j.ntt.2004.02.006
Fontana BD, Mezzomo NJ, Kalueff AV, Rosemberg DB (2018) The developing utility of zebrafish models of neurological and neuropsychiatric disorders: a critical review. Exp Neurol 299:157–171. https://doi.org/10.1016/j.expneurol.2017.10.004
Goldfarb M (2005) Fibroblast growth factor homologous factors: evolution, structure, and function. Cytokine Growth Factor Rev 16:215–220. https://doi.org/10.1016/j.cytogfr.2005.02.002
Goldfarb M, Schoorlemmer J, Williams A, Diwakar S, Wang Q, Huang X, D'Angelo E (2007) Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels. Neuron 55:449–463. https://doi.org/10.1016/j.neuron.2007.07.006
Goshen-Lago T, Melamed D, Admon A, Engelberg D (2017) Isolation and characterization of intrinsically active (MEK-independent) mutants of Mpk1/ERK. Methods Mol Biol 1487:65–88. https://doi.org/10.1007/978-1-4939-6424-6_5
Guella I, Huh L, McKenzie MB, Toyota EB, Bebin EM, Thompson ML, Demos M (2016) De novo FGF12 mutation in 2 patients with neonatal-onset epilepsy. Neurol Genet 2:e120. https://doi.org/10.1212/nxg.0000000000000120
Hanada Y, Nakamura Y, Ozono Y, Ishida Y, Takimoto Y, Taniguchi M, Shimada S (2018) Fibroblast growth factor 12 is expressed in spiral and vestibular ganglia and necessary for auditory and equilibrium function. Sci Rep 8:11491. https://doi.org/10.1038/s41598-018-28618-0
Harada M, Murakami H, Okawa A, Okimoto N, Hiraoka S, Nakahara T, Koseki H (2009) FGF9 monomer-dimer equilibrium regulates extracellular matrix affinity and tissue diffusion. Nat Genet 41:289–298. https://doi.org/10.1038/ng.316
Hartung H, Feldman B, Lovec H, Coulier F, Birnbaum D, Goldfarb M (1997) Murine FGF-12 and FGF-13: expression in embryonic nervous system, connective tissue and heart. Mech Dev 64:31–39. https://doi.org/10.1016/s0925-4773(97)00042-7
Hecht HJ, Adar R, Hofmann B, Bogin O, Weich H, Yayon A (2001) Structure of fibroblast growth factor 9 shows a symmetric dimer with unique receptor- and heparin-binding interfaces. Acta Crystallogr D Biol Crystallogr 57:378–384. https://doi.org/10.1107/s0907444900020813
Hennessey JA, Wei EQ, Pitt GS (2013) Fibroblast growth factor homologous factors modulate cardiac calcium channels. Circ Res 113:381–388. https://doi.org/10.1161/circresaha.113.301215
Huang K, Shen L, Niu T, Zhao Y, Fu J, Cao Y (2019) Naomaitai ameliorated brain damage in rats with vascular dementia by PI3K/PDK1/AKT signaling pathway. Evid Based Complement Alternat Med 2019:2702068–2702017. https://doi.org/10.1155/2019/2702068
Hui Q, Jin Z, Li X, Liu C, Wang X (2018) FGF family: from drug development to clinical application. Int J Mol Sci 19. https://doi.org/10.3390/ijms19071875
Kalinina J, Byron SA, Makarenkova HP, Olsen SK, Eliseenkova AV, Larochelle WJ, Mohammadi M (2009) Homodimerization controls the fibroblast growth factor 9 subfamily’s receptor binding and heparan sulfate-dependent diffusion in the extracellular matrix. Mol Cell Biol 29:4663–4678. https://doi.org/10.1128/mcb.01780-08
Kim YH, Lee Y, Kim D, Jung MW, Lee CJ (2010) Scopolamine-induced learning impairment reversed by physostigmine in zebrafish. Neurosci Res 67:156–161. https://doi.org/10.1016/j.neures.2010.03.003
Lin H, Lu P, Zhou M, Wu F, Weng L, Meng K, Tian H (2019) Purification of recombinant human fibroblast growth factor 13 in E. coli and its molecular mechanism of mitogenesis. Appl Microbiol Biotechnol 103:7017–7027. https://doi.org/10.1007/s00253-019-09973-y
Liu CJ, Dib-Hajj SD, Renganathan M, Cummins TR, Waxman SG (2003) Modulation of the cardiac sodium channel Nav1.5 by fibroblast growth factor homologous factor 1B. J Biol Chem 278:1029–1036. https://doi.org/10.1074/jbc.M207074200
Manu TM, Anand T, Pandareesh MD, Kumar PB, Khanum F (2019) Terminalia arjuna extract and arjunic acid mitigate cobalt chloride-induced hypoxia stress-mediated apoptosis in H9c2 cells. Naunyn Schmiedeberg's Arch Pharmacol 392:1107–1119. https://doi.org/10.1007/s00210-019-01654-x
Marcon M, Herrmann AP, Mocelin R, Rambo CL, Koakoski G, Piato AL (2016) Prevention of unpredictable chronic stress-related phenomena in zebrafish exposed to bromazepam, fluoxetine and nortriptyline. Psychopharmacology 233:3815–3824. https://doi.org/10.1007/s00213-016-4408-5
Moldovan GL, Pfander B, Jentsch S (2007) PCNA, the maestro of the replication fork. Cell 129:665–679. https://doi.org/10.1016/j.cell.2007.05.003
Nakayama F, Yasuda T, Umeda S, Asada M, Imamura T, Meineke V, Akashi M (2011) Fibroblast growth factor- 12 (FGF12) translocation into intestinal epithelial cells is dependent on a novel cell-penetrating peptide domain: involvement of internalization in the in vivo role of exogenous FGF12. J Biol Chem 286:25823–25834. https://doi.org/10.1074/jbc.M110.198267
Nybakken K, Perrimon N (2002) Heparan sulfate proteoglycan modulation of developmental signaling in drosophila. Biochim Biophys Acta 1573:280–291. https://doi.org/10.1016/s0304-4165(02)00395-1
Olsen SK, Garbi M, Zampieri N, Eliseenkova AV, Ornitz DM, Goldfarb M, Mohammadi M (2003) Fibroblast growth factor (FGF) homologous factors share structural but not functional homology with FGFs. J Biol Chem 278:34226–34236. https://doi.org/10.1074/jbc.M303183200
Ornitz DM (2000) FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays 22:108–112. https://doi.org/10.1002/(sici)1521-1878(200002)22:2<108:Aid-bies2>3.0.Co;2-m
Shin M, Franks CE, Hsu KL (2018) Isoform-selective activity-based profiling of ERK signaling. Chem Sci 9:2419–2431. https://doi.org/10.1039/c8sc00043c
Siekierska A, Isrie M, Liu Y, Scheldeman C, Vanthillo N, Lagae L, Buyse GM (2016) Gain-of-function FHF1 mutation causes early-onset epileptic encephalopathy with cerebellar atrophy. Neurology 86:2162–2170. https://doi.org/10.1212/wnl.0000000000002752
Singh SM, Eshwari AN, Garg LC, Panda AK (2005) Isolation, solubilization, refolding, and chromatographic purification of human growth hormone from inclusion bodies of Escherichia coli cells: a case study. Methods Mol Biol 308:163–176. https://doi.org/10.1385/1-59259-922-2:163
Smallwood PM, Munoz-Sanjuan I, Tong P, Macke JP, Hendry SH, Gilbert DJ, Nathans J (1996) Fibroblast growth factor (FGF) homologous factors: new members of the FGF family implicated in nervous system development. Proc Natl Acad Sci U S A 93:9850–9857. https://doi.org/10.1073/pnas.93.18.9850
Stewart A, Gaikwad S, Kyzar E, Green J, Roth A, Kalueff AV (2012) Modeling anxiety using adult zebrafish: a conceptual review. Neuropharmacology 62:135–143. https://doi.org/10.1016/j.neuropharm.2011.07.037
Stewart AM, Ullmann JF, Norton WH, Parker MO, Brennan CH, Gerlai R, Kalueff AV (2015) Molecular psychiatry of zebrafish. Mol Psychiatry 20:2–17. https://doi.org/10.1038/mp.2014.128
Takeguchi R, Haginoya K, Uchiyama Y, Fujita A, Nagura M, Takeshita E, Sasaki M (2018) Two Japanese cases of epileptic encephalopathy associated with an FGF12 mutation. Brain and Development 40:728–732. https://doi.org/10.1016/j.braindev.2018.04.002
Thomadaki H, Scorilas A (2006) BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci 43:1–67. https://doi.org/10.1080/10408360500295626
Tian H, Zhao Y, Chen N, Wu M, Gong W, Zheng J, Jiang C (2016) High production in E. coli of biologically active recombinant human fibroblast growth factor 20 and its neuroprotective effects. Appl Microbiol Biotechnol 100:3023–3034. https://doi.org/10.1007/s00253-015-7168-y
Wang C, Wang C, Hoch EG, Pitt GS (2011) Identification of novel interaction sites that determine specificity between fibroblast growth factor homologous factors and voltage-gated sodium channels. J Biol Chem 286:24253–24263. https://doi.org/10.1074/jbc.M111.245803
Wang S, Lin H, Zhao T, Huang S, Fernig DG, Xu N, Tian H (2017) Expression and purification of an FGF9 fusion protein in E. coli, and the effects of the FGF9 subfamily on human hepatocellular carcinoma cell proliferation and migration. Appl Microbiol Biotechnol 101:7823–7835. https://doi.org/10.1007/s00253-017-8468-1
Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li H, Chen G (2018) Melatonin alleviates intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl Stroke Res 9:74–91. https://doi.org/10.1007/s12975-017-0559-x
Wei X, Ao Q, Meng L, Xu Y, Lu C, Tang S, Li X (2020) Expression, purification and functional assessment of asprosin inclusion body. Nan Fang Yi Ke Da Xue Xue Bao 40: 67-72. doi: 10.12122/j.issn.1673-4254.2020.01.11
Wu M, Song N, Cheng J, Zhao Y, Chen N, Ma J, Wang H (2017) Increased production of human fibroblast growth factor 17 in Escherichia coli and proliferative activity in NIH3T3 cells. Mol Med Rep 16:447–452. https://doi.org/10.3892/mmr.2017.6575
Zhang X, Bao L, Yang L, Wu Q, Li S (2012) Roles of intracellular fibroblast growth factors in neural development and functions. Sci China Life Sci 55:1038–1044. https://doi.org/10.1007/s11427-012-4412-x
Zhao M, Tao XY, Wang FQ, Ren YH, Wei DZ (2018) Establishment of a low-dosage-IPTG inducible expression system construction method in Escherichia coli. J Basic Microbiol 58:806–810. https://doi.org/10.1002/jobm.201800160
Data availability statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Funding
This work was funded by the Natural Science Foundation of Zhejiang Province (No. LY19H160029) and the Zhejiang Provincial Department of Education (No. Y201839837).
Author information
Authors and Affiliations
Contributions
HT and XL conceived and designed research. MZ, KM, YZ, MZ, PL, YF, and MH conducted experiments. JC and QD contributed new reagents or analytical tools. MZ and KM analyzed data. MZ and KM wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, M., Chen, J., Meng, K. et al. Production of bioactive recombinant human fibroblast growth factor 12 using a new transient expression vector in E. coli and its neuroprotective effects. Appl Microbiol Biotechnol 105, 5419–5431 (2021). https://doi.org/10.1007/s00253-021-11430-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11430-8