Abstract
The photosynthetic performance of a microalgal biofilm colonizing a building facade was investigated between February and July 2004, with an emphasis on changing water availability and air humidity. The fluorimetric measurements of the quantum efficiency (F v/F m) indicated diurnal activity patterns. At most sampling dates the algal biofilm photosynthesized particularly in the morning and substantially less in the afternoon. As long as liquid water was present, the microalgae exhibited at least some degree of photosynthesis. However, F v/F m values never exceeded 0.4, pointing to slight photoinhibition or damage of the cells. Dried cells without photosynthesis could recover within minutes after artificial moistening.
Three microalgal strains were isolated from aeroterrestrial biofilms and established as unialgal cultures. Their photosynthesis and growth were characterized under different air humidities and temperatures. Photosynthesis and growth of strain ROS 55/3 (Stichococcus sp.) showed similar patterns with decreasing relative air humidity. Positive growth and optimum photosynthesis were recorded at 100% relative air humidity. At air humidities below 93%, both processes were strongly inhibited. All studied strains grew between 1 and 30°C with optimum rates at 20–23°C, indicating eurythermal features.
The data indicate that liquid water or 100% air humidity are the prerequisite for optimum photosynthesis and growth of aeroterrestrial microalgae. However, when dried and consequently inactive, these microorganisms can recover quickly if water is suddenly available, e.g., after rain events. These physiological capabilities explain well the ecological success of aeroterrestrial microalgae in occupying many man-made substrata such as building facades and roof tiles in urban areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aeroterrestrial microalgae typically colonize in biofilms the interface between all kinds of hard substrata and the atmosphere. The surface can be of natural origin, e.g., tree barks, soil, and rocks, or of artificial origin, e.g., roof tiles, concrete, or building facades in urban areas. Additionally, some taxa are known as photobionts of lichens [7]. On man-made surfaces, aeroterrestrial microalgae often cause aesthetically unacceptable discoloration known as incrustations and patinas [8, 34]. Whether these particular microorganisms also actively corrode the materials being colonized remains controversial [26]. However, the principal potential to corrode hard substrata is biologically realized in the green algal genus Ostreobium, which lives endolithically by boring into the calcified structures of coral reefs or molluskan shells using organic acids [10, 35]. Acid production is documented for various algal taxa as the possible underlying mechanism for biodegradation. The aeroterrestrial green microalga Stichococcus bacillaris is a growing cryptoendolithic in granite, particularly found in historic cathedrals and other monuments [26], indicating at least some deteriorative activity [36].
Although aeroterrestrial microalgae are interesting and important from an ecological as well as an applied point of view, their ecophysiology is poorly investigated so far. The colonization of terrestrial habitats involves exposure to much harsher conditions, such as desiccation and high insolation [photosynthetic active radiation (PAR) and UV], compared to typical freshwater and marine environments. Although at least 150 unicellular species of aeroterrestrial Chlorophyta are morphologically described [7], neither their adaptation to environmental conditions nor their phylogenetic relationships are well understood.
In a recent study, various aeroterrestrial microalgal species colonizing building facades were investigated for the presence of UV-absorbing mycosporine-like amino acids (MAAs) [15], which are considered as highly effective sunscreen compounds [4, 5]. Green microalgae that occur in such urban biofilms are often exposed to high UV radiation and, thus, certain protection mechanisms that may involve MAAs could be expected. Most interestingly, the aeroterrestrial strains studied produce a unique MAA that was exclusively found in the green algal class Trebouxiophyceae [15]. This particular UV sunscreen was first described in the subaerial green macroalga Prasiola crispa ssp. antarctica [12]. These authors reported high concentrations of a UV-absorbing compound with an absorption maximum at 324 nm, which was characterized as a putative MAA due to its chromatographic properties and called it 324 nm-MAA. Experiments indicated that all species containing this MAA showed a strong accumulation of the compound after UV exposure, thus supporting their function as putative UV sunscreens [15].
Although aeroterrestrial microalgae are poorly studied, the ecophysiology of related organisms such as terrestrial cyanobacteria and lichens is much better understood [22, 30]. In particular, the responses of organisms from arid areas indicate that dry green algal lichens are capable of reactivating an inhibited photosynthesis solely by water vapor from the atmosphere, whereas cyanobacterial lichens rely on fluid water [18].
The aim of the present study was to follow, for the first time, the physiological status of an aeroterrestrial microalgal community growing on top of a building facade over a period of several months in situ, especially concerning desiccation and water availability. The effect of water availability was evaluated using chlorophyll fluorescence of photosystem II by PAM fluorometry as an indicator for photosynthetic efficiency. In addition, growth and photosynthesis of selected aeroterrestrial microalgal strains were investigated at different controlled air humidities and temperatures. The results indicate the ability of the tested isolates to use high air humidities for physiological processes, as well as their eurythermal nature.
Methods
Study Area
The investigated building is situated in the town of Rostock (northeast Germany) in a spaciously arranged estate. Most buildings were constructed using precast concrete modules in the 1970s and were renovated in 1993 by adding insulation and artificial resin plaster on the surface. Only a few years later, these coatings became conspicuously infested by green microalgae, mainly on the north and west sides. The facade studied was oriented toward the northeast, and was protected from radiation by adjacent trees, i.e., the aeroterrestrial algal community was never exposed to full solar radiation.
Climatic conditions of the study area are moderate as a result of coastal influence. Annual mean relative humidity is 83% in Rostock. The annual temperature averages at 8.9°C [Deutscher Wetterdienst (DWD), Warnemünde]. The study period, covering February through July 2004, was more humid compared to other years, mainly because of heavier rains. Photosynthetic efficiency was measured in situ every 2 weeks. (Because the in situ measurements were part of a diploma thesis of the first author, it was not possible to cover a longer period).
Abiotic Parameters on the Facade
Aside from measurement of the physiological state of the microalgal community, moisture and temperature of the facade, relative air humidity, and PAR were also determined. Available water on the facade was estimated by absorption into a 1 cm2 piece of a commercial nappy (Pampers, Germany). This measurement was always performed before recording photosynthesis. The nappy pieces were dried for 8 h at 45°C, weighed afterwards (MC 210 P, Sartorius AG Göttingen, Germany), and kept in 15-mL tightly closed tubes. After pressing the dried nappy pieces onto the plaster surface for 5 s, it was again placed in the 15-mL tubes. Water content of the nappy pieces was gravimetrically measured within an hour. The difference between wet and dry values equaled the area-based available water on the facade. PAR was determined before and after each fluorescence measurement by a light detector (Li 250, Li-Cor, USA). The relative air humidity and air temperature was directly recorded in front of the facade and in 1.50 m horizontal distance from the building, by using a combined temperature/air humidity sensor (P670, Dostmann, Wertheim-Reichholzheim, Germany).
Photosynthetic Performance of the Algal Community in Situ
To check for possible diurnal effects on photosynthesis, measurements were always undertaken at four different periods of the day at three measuring areas. These were 1 h before and after sunrise, at noon, and 1 h before dawn. The physiological status of the algal community was determined as the photosynthetic efficiency using a PAM-2000 (Walz, Effeltrich, Germany). The light conductor was placed by a tripod onto the substrate (141RC, Fa. Manfrotto, Italy) (Fig. 1). Instead of a leaf clip holder, a distance clip was used. The light conductor was always fixed onto the substrate with the same angle of 60° at a distance of 7 mm. It was necessary to keep this angle unchanged to prevent shading because natural sunlight was used as actinic light source. By using the saturation pulse method, the minimum (F 0) and maximum (F m) fluorescence before sunrise, and the minimum (F 0′) and maximum (F m′) fluorescence after light acclimation, respectively, were determined. At each sampling date, 15 light saturation pulses were applied at each of the same three measuring areas. The first 10 pulses were randomly placed in two semicircles onto the untreated facade within an area of 400 cm2. The last five pulses were applied immediately, within seconds, after moistening the microalgal biofilm by tap water to evaluate the influence of water stress. In all experiments for the diurnal and the moistening measurements, different locations in the algal community were used. Fluorescence data were used to calculate the maximum and effective quantum efficiency of photosystem II according to Kromkamp and Forster [17]. We also determined the growth rates of samples taken from the measuring area according to Karsten et al. [13].
Isolation and Cultivation of Algal Strains
The algal strains used in this study were taken in May and June 2002 from infested facades in Rostock und Wolfsburg (Germany) (Table 1). Although none of these algal isolates were directly taken from the studied facade, strain ROS 47/4 originated from a nearby building. The samples were scratched from the surface and put in 0.85% NaCl solution immediately. After the cell suspension was photographed (Olympus BX 51 equipped with a digital camera SIS Color View 12) at 200–400× magnification, each sample was diluted and spread on agar plates (modified Bolds Basal Medium with 1.5% Agar [32]). These plates were incubated at 22–25 μmol photons m−2 s−1 (continuous light) and 15°C for ca. 2 weeks. Cells were picked from separate green colonies by using micropipettes and transferred to culture tubes filled with modified Bolds Basal Medium in 1.5% agar. The strains were kept in climate cabinets (Vinothek, Liebherr, Germany) at 15°C and ca. 20–25 μmol photons m−2 s−1 in continuous light (Osram Lumilux Deluxe Daylight).
The 18S rDNA region ([15]; Friedl, pers. comm.) of each strain was sequenced. Strains ROS 55/3 and 47/4 were identified as species of the genus Stichococcus and ROS 77/1 as Chlorella luteoviridis.
Photosynthesis and Growth of Selected Algal Strains Under Different Relative Air Humidities
Sealed polyethylene chambers were used to investigate the influence of relative air humidity on photosynthesis and growth of strain ROS 55/3 (Stichococcus sp.). Adjustment of the different relative air humidities was achieved by tap water and saturated salt solutions of NaCl, KNO3, Ca(NO3)2, MgCl2, and AgNO3 [37]. Thus, atmospheres of 100, 93, 85, 76, 55, and 33% relative air humidity were provided, respectively. To produce a dry environment, Silicagel was placed into the containers instead of the salt solutions (Kramer & Martin GmbH, Germany). Perforated metal grilles were placed on top of four glass columns ca. 2 cm above the fluid level to support the test material. To record the conditions inside the chambers a combined sensor (humidity/temperature) was employed (P 670, Dostmann, Wertheim-Reichholzheim, Germany). This experimental design prevented any uncontrolled moistening of the algal cells by condensation water. Glass fiber filters were used as a hard substrate for the algae (∅ 25 mm, Sartorius GMF 5, Germany). The algae were taken from precultures that always grow at logarithmic phase (day 7). Cell suspensions (1–5 mL) were filtered onto the filters followed by the careful addition of 0.1 mL Bolds basal medium to each filter in 10-μL increments. Three replicate filters were always prepared, which were afterwards air-dried in a clean bench for 30 min, then placed in the chambers, and kept in climate cabinets (Vinothek, Liebherr, Germany) at 40–55 μmol photons m−2 s−1 (light/dark rhythm: 12:12 h L:D using an Osram Lumilux Deluxe Daylight) and 17°C. The experiments lasted 10–12 days.
Cell numbers were quantified every 24 h on each filter by counting at least 200 cells on 20 subareas per filter via epifluorescence microscopy (Olympus BX 51, green excitation WG, 200× magnification). Photosynthetic efficiency was measured on the first two of the three replicate filters using the PAM 2000. Samples were incubated for 5 min in the dark. F 0 was determined by a pulsed measuring beam (ca. 0.3–0.4 μmol photons m−2 s−1, 650 nm), followed by short pulses of saturating white light (0.6–0.8 s, 2000–5000 μmol photons m−2 s−1) for recording F m (distance light conductor: 1 mm, angle: 90°). F v/F m values were calculated according to Kromkamp and Forster [17].
For comparison of growth rates, increase in biomass of strain ROS 55/3 was determined in aerated and nonaerated modified Bolds basal medium (35–40 μmol photons m−2 s−1, light/dark rhythm 12:12 h L:D).
Growth of Selected Algal Strains as a Function of Temperature
Five aluminum blocks, each with openings for four cuvettes, were placed on a light bank. The outer block was heated by circulating hot water and the other outermost side was cooled by water from a cryostat (WK 450, MW Lauda, Lauda-Königshofen, Germany). This provided a temperature gradient from 1 to 35°C across all units. The gradient was adjusted by placing plastic sheets between different blocks. Through this arrangement, six different temperatures in two separate experiments were achieved: 1, 7, 15, 24, 28, and 35°C. The algae were incubated in 30-mL polyacryl (transparent, nontoxic) cuvettes (Kleinfeld, Germany) filled with Bold's basal medium at 55–60 μmol photons m−2 s−1 (light/dark rhythm: 12/12 using an Osram Lumilux Deluxe Daylight tubes). Every 24 h, increase in biomass was measured as F 0′ by using an in vivo fluorimeter for phototrophic microorganisms [13]. Growth rate (μ) was calculated by iterative optimization of the exponential (A) and logistic (B) growth curves, respectively. On the basis of these rates an optimum curve was fitted after [2] (C).
Results
Photosynthesis of Aeroterestrial Algae in Situ
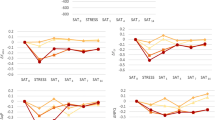
Diurnal changes in the photosynthetic efficiency of an aeroterrestrial microalgal biofilm growing onto a facade depend above all on the surface moisture (Fig. 2). The maximum photosynthetic efficiency (F v/F m) measured in situ never exceeded 0.4. The highest values of photosynthetic activity were often measured in the morning. F v/F m values decreased until noon, followed by some recovery in the late afternoon. This diurnal pattern in photosynthetic activity correlated with the amount of moisture available on the facade. Maximum quantum efficiency was higher with more available water. However, depending on the season, two response patterns, related to changing abiotic parameters during a day, could be distinguished. Although on cold and wet days (e.g., March 2, 2004; Fig. 2) high F v/F m values were measured in the morning, before and after sunrise, as well as in the evening, strong depression was only observed at noon. This activity pattern is typical for days in winter and spring. In contrast, on warm days with elevated irradiances, high F v/F m rates could only be measured in the morning (May 27, 2004; Fig. 2), followed by strong inhibition of photosynthesis for the rest of the day. Besides these two typical activity patterns, changes in photosynthesis could be observed after special events such as strong rains (July 8, 2004; Fig. 2), which lead to immediate recovery of the algal photosynthesis. This observation was further supported by measurements of algal photosynthesis on the facade before and immediately after artificial moistening of the biofilm (Fig. 3). On most dates, the addition of water stimulated photosynthesis, except on February 20 and March 3, 2004. On these days, temperatures were below freezing point, which prevented moistening of the substrate. The highest increases in photosynthetic efficiency were observed on March 15 and July 8, 2004. F v/F m values increased by 300 and 600%, respectively, after adding liquid water to the facade. However, at other days the differences were usually smaller, between 20 and 30%.
Changes in the photosynthetic efficiency (F v/F m) of an aeroterrestrial microalgal community growing as a biofilm on a facade (Germany, Rostock, Südring 21) measured before sunrise in situ and after moistening and surface moisture (mL m−2) during the investigation period February–July 2004. Data are mean values ± SD (n = 10).
Samples of biofilms were taken to the laboratory to determine the growth rate, doubling times, and duration of the lag phase (data not shown). There were no obvious differences in growth rates over the course of the sampling. All rates varied between 0.45 and 0.71, with doubling times of 25–37 h and lag phases always shorter than 1–2 days (data not shown).
Dependence of Microalgal Growth and Photosynthesis on Relative Humidity in Vivo
Growth of the aeroterrestrial microalgal strain ROS 55/3 was determined under submersed conditions in medium, both aerated and not aerated. Although under aerated conditions a maximum growth rate of 0.9 day−1 was measured, the nonaerated culture grew at only 0.6 day−1 (data not shown). On solid substrate (glass fiber filters), which represents a more natural situation, the growth rate was 0.4 day−1 in water-saturated atmosphere (100% relative air humidity) (Fig. 4). A decrease in relative humidity to 93% led to the inactivation of half of the cells after 11 days. Further decrease in humidity to 85% increased the percentage of nongrowing algae. All algal cells were inactive after 6 days. However, adding liquid water to such algae led to growth again (Schumann, unpublished results). Similarly, the photosynthetic efficiency (F v/F m) was almost unchanged in water-saturated air over 11 days (Fig. 5). However, under 93 and 85% relative humidity, photosynthesis strongly decreased after the start of the experiment. Although after 4 days at 93% humidity 10% of the maximum photosynthesis could be recorded, at 85% humidity no activity was detected after the first day.
Abundance (cells mm−2) changes with time (day) at different relative humidities (rh) of Stichococcus sp. ROS 55/3. Symbols represent single values; curves were fitted by median values, except for the values in 100% relative air humidity (logistic curve plotted from average μ determined from three replicates).
Dependence of Algal Growth on Temperature in Vivo
To evaluate the growth performance of three aeroterrestrial microalgal isolates, submersed cultures were exposed to different temperatures. All isolates were able to grow at relatively high rates of ca. 0.2 day−1 (doubling time: ca. 3.5 days) already at the lowest temperatures tested (1–2°C), as well as at about 30°C. The optimum temperature for both Stichoccocus species ROS 47/4 and ROS 55/3 was 21°C, and that for C. luteoviridis ROS 77/1 was 24°C (Fig. 6). Maximum growth rates were different between strain ROS 55/3 and ROS 47/4, with values of 0.82 day−1 (doubling time 20 h) and 0.50 day−1 (doubling time 33 h), respectively (Fig. 6), although both isolates are almost genetically identical [15].
Growth rates (day−1) of three aeroterrestrial algal isolates as a function of the temperature (°C): (A) Chlorella luteoviridis. ROS 77/1, (B) Stichococcus sp. ROS 47/4, (C) Stichococcus sp. ROS 55/3. Symbols represent mean values ± SD (n = 3). Curves fitted after Blanchard et al. [2].
Discussion
Photosynthesis in Situ
Exposure of aeroterrestrial algae to the atmosphere results in dehydration of the cells, which strongly affects photosynthesis and growth. The degree of water loss is generally related to external factors such as temperature and air humidity, but also to internal factors such as the algal surface/volume ratio or morphological features. For example, small organisms are more susceptible and thicker cell walls lead to a higher resistance against water loss [23]. To evaluate possible desiccation stress impinging on the algal community in situ, water availability, air humidity, and temperature have to be recorded. However, the climate data from the building facade studied from February to July 2004 indicate the typically increasing temperatures but scattered rainfall without any significant trend over the course of several months (data not shown). Consequently, water availability on the facade was highly variable at each sampling date, which is also well reflected in the fluctuating photosynthetic activity patterns. However, the nappy method applied to measure water availability will only estimate excess water, i.e., water outside cells, cell walls, and any mucoid layers, such as condensation water, rain, or snow. If such excess water can be determined, it is reasonable to assume that under these conditions all algal cells will be fully hydrated and hence physiologically more or less functioning. Because long-term in situ investigations on photosynthesis in aeroterrestrial microalgal communities are missing, the data can only be compared with other related algal systems such as lichens, which also experience diurnal and seasonal changes in relation to water availability [21]. The cyanobacterial lichen Peltigera rufescens and the green algal lichen Usnea antarctica were photosynthetically active only 50% of the time over the course of 1 year [21, 31]. These authors identified water content of the lichen as the ecological key factor for the seasonally changing activity in the organisms. In the present study, due to methodological considerations (i.e., to keep the microalgal biofilm undisturbed during the investigation period) the surface moisture on facades was chosen as an equivalent for the water status. From mid-March until the beginning of April, lower surface moistures compared to the whole investigation period were determined, which correlated with the decrease in F v/F m values. From our data, we suggest that a minimum of 10 mL liquid water per m2 facade surface should be present to enable photosynthesis. However, even during days without measurable surface moisture, the F v/F m values in the algal community were between 0.12 and 0.18, indicating at least some minor activity and a photosynthesis apparatus not damaged through the dry conditions. It seems that mucoid substances excreted by some aeroterrestrial strains [6, 11] may hinder evaporation and hence support at least some photosynthetic activity, perhaps to guarantee a maintenance metabolism under desiccation.
At each sampling date, diurnal measurements on photosynthetic efficiency were carried out, of which three representative examples are shown in Fig. 2. At 10 out of 11 sampling dates, highest photosynthetic efficiencies were recorded in the morning, and in four out of 11 dates at noon and in the evening. The high morning activity of the algal community correlated with the presence of condensation water on the facade. With increasing solar radiation this water film evaporated, the microalgae became more desiccated, and hence photosynthesis was inhibited. These diurnal patterns are in accordance with those on the aeroterrestrial filamentous green alga Trentepholia odorata [25] and various lichens [19, 21, 28]. The ecological consequence of diurnally and seasonally changing, i.e., regularly inhibited, photosynthesis is a reduced primary production. Moistening the microalgal biofilm on the facade led, in most cases, to an immediate increase in photosynthetic efficiency (Fig. 3). These data indicate that (1) the algae were under desiccation stress before moistening, and (2) photosynthesis can quickly recover after applying water. Similar results have been reported for lichens [18] and the green alga Pleurococcus sp. growing on tree bark [1]. A conspicuous observation was the fact that in the microalgal community the F v/F m values over the whole measuring period never exceeded 0.4 in situ. Typical F v/F m values of physiologically intact green algae under optimum conditions are in the range of 0.8 [3], which supports the view that on facades the algal cells, for most of the year, were—at least to some degree—photoinhibited. Similar data were reported for P. rufescens [18], apart from very wet days. The low F v/F m values point to a situation of continuous stress for the aeroterrestrial microalgal biofilm.
As a best-case scenario, we roughly estimate that the algal community on the studied facade will be productive for only 50% of the time, when sufficient solar radiation for photosynthesis is available.
Temperature is another important abiotic factor for regulating the activity of phototrophic algae. However, there was no correlation between low or high temperature and the photosynthetic efficiencies. During the investigation period, a temperature range between −3.9 and 21.7°C was measured. At all sampling days, photosynthesis of the microalgae could be documented, at least after moistening the facade. Therefore, it can be assumed that temperature is not the limiting factor for photosynthesis at the facade, but rather the availability of liquid water.
At all sampling dates, algal material was taken from the facade to the laboratory to check for growth as an important indicator of vitality. All growth rates measured varied between 0.50 and 0.65 day−1, except in the middle of March (0.9 day−1) (data not shown). These data indicate that the microalgal biofilm was always in a physiological status good enough to allow an immediate growth response following a sudden supply of water, e.g., after rain. Consequently, it seems that aeroterrestrial microalgae are not getting completely desiccated over a longer period. Although irregularly available, water is more or less constantly present on the facade, i.e., in the form of condensation water, snow or rain, fog, or high air humidity—which explains the strong infestation of many buildings with green microalgae. Only longer dry periods in summer should have an influence on the growth and photosynthesis of the aeroterrestrial algal community.
Growth and Photosynthesis of Selected Aeroterrestrial Strains Under Different Air Humidities and Temperatures
Strain ROS 55/3 (Stichococcus sp.) grew well at 100% relative air humidity with a rate of 0.4 day−1 (Fig. 4), indicating for the first time its principal capacity to support this important physiological process by solely taking up vapor from the surrounding air. Growth of this strain on top of a hard substratum must be rated as high, because similar rates (0.5 day−1) have been described for phytoplankton green algae such as Scenedesmus sp. under optimum conditions [29]. Under decreasing air humidities, growth in ROS 55/3 was inhibited. Although considerable literature exists on the ecology, distribution, and taxonomy of aeroterrstrial microalgae (e.g., [7]), ecophysiological data on these interesting organisms are still scarce.
Compared to growth pattern, photosynthetic efficiency in strain ROS 55/3 showed similar responses under different air humidities, i.e., highest activity at 100% relative air humidity and inhibited photosynthesis with decreasing air humidities (Fig. 5). Similar results have been described for the aeroterrestrial green microalga Apatococcus lobatus [1], as well as for green algal lichens from Utah (USA) [20] and cryptoendolithical lichens from hot and cold desserts [27]. However, although ROS 55/3 showed already at 93% relative air humidity a strong decrease in photosynthetic efficiency, A. lobatus was able to take up CO2 down to 68% relative air humidity [1]. Green algal lichens differ physiologically from cyanobacterial lichens by the fact that although the former are able to photosynthesize in the presence of water vapor, the latter are dependent on liquid water [18]. In addition, both lichen types exhibited different speeds in restitution of the photosynthetic apparatus after sudden hydration of dry thalli by liquid water, i.e., the green algal lichen recovered significantly faster, and hence resemble, in this respect, the green microalgal biofilm studied in situ. However, the underlying mechanisms are not well understood.
Growth rates as a function of temperature clearly showed a wide ecological range for the strains ROS 55/3, 47/4 (Stichococcus sp.), and ROS 77/1 (C. luteoviridis). All isolates grew with relatively high rates between 1 and 30°C, with optimum rates at 21–24°C, indicating eurythermal features. The observed growth optimum temperatures are similar to the optimal CO2 uptake rates at 18–20°C determined for A. lobatus [1]. Because the aeroterrestrial strains isolated from facades grew at 0°C, they should be able to grow and photosynthesize well in winter, as long as water is available. This assumption is supported by regular inspection of various buildings in Rostock during the cold season and by irregular determination of F v/F m during the growth experiments, always yielding values of between 0.63 and 0.80 (unpublished results).
Conspicuous was the difference in maximum growth rates of strain ROS 55/3 and 47/4. The first isolate grew at a rate of 0.82 day−1, whereas the second grew at 0.5 day−1 (Fig. 6). Both strains are almost genetically identical in the coding region of the 18S rDNA, except for one nucleotide (Friedl, pers. comm.), and both were investigated under identical, controlled conditions. Therefore, it is possible that strains ROS 55/3 and ROS 47/4 represent separate ecotypes. Ecotypes evolve under the influence of natural selection in response to very local variations in the environment, when the necessary genes of adaptive value are present in or available to the population. The capability of a single species to exist under a wide range of abiotic parameters has been reported for many aquatic micro- and macroorganisms [14, 24, 33].
Our planet is currently facing global warming due to enhanced CO2 emissions. One result for Europe seems to be milder winters, i.e., less days with freezing temperatures. In addition, due to the introduction of efficient filter systems into power plants burning coal, less sulfur is emitted and hence pollution of the atmosphere has been reduced. Both factors may further stimulate the growth of aeroterrestrial microalgae on building facades [9, 16].
References
Bertsch, A (1966) CO2 Gaswechsel der Grünalge Apatococcus lobatus. Planta (Berlin) 70: 46–72
Blanchard, GF, Guarini, JM, Richard, P, Gros, P, Mornet, F (1996) Quantifying the short-term temperature effect on light-saturated photosynthesis of intertidal microphytobenthos. Mar Ecol Prog Ser 134: 309–331
Büchel, C, Wilhelm, C (1993) In vivo analysis of slow chlorophyll fluorescence induction kinetics in algae: progress, problems and perspectives. Photochem Photobiol 58: 137–148
Cockell, CS, Knowland, J (1999) Ultraviolet radiation screening compounds. Biol Rev 74(3): 311–345
Dunlap, WC, Shick, JM (1998) Ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: a biochemical and environmental perspective. J Phycol 34(3): 418–430
Ehling-Schulz, M, Bilger, W, Scherer, S (1997) UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J Bacteriol 179: 1940–1945
Ettl, H, Gärtner, G (1995) Syllabus der Boden-, Luft- und Flechtenalgen. Gustav Fischer Verlag, Stuttgart
Gaylarde, CC, Morton, LHG (1999) Deteriogenic biofilms on buildings and their control: a review. Biofouling 14: 59–74
Govindasamy, B, Thompson, S, Mirin, A, Wickett, M, Caldeira, K, Delire, C (2005) Increase of carbon cycle feedback with climate sensitivity: results from a coupled climate and carbon cycle model. Tellus Ser B Chem Phys Meteorol 57(2): 153–163
Highsmith, RC (1981) Lime-boring algae in hermatypic coral skeletons. J Exp Mar Biol Ecol 55: 267–281
Hokputsa, S, Hu, CX, Paulsen, BS, Harding, SE (2003) A physicochemical comparative study on extracellular carbohydrate polymers from five desert algae. Carbohydr Polym 54: 27–32
Hoyer, K, Karsten, U, Sawall, T, Wiencke, C (2001) Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Mar Ecol Prog Ser 211: 117–129
Karsten, U, Klimant, I, Holst, G (1996a) A new in vivo fluorimetric technique to measure growth of adhering phototrophic microorganisms. Appl Environ Microb 62(1): 237–243
Karsten, U, Koch, S, West, JA (1996b) Physiological responses of the eulittoral macroalga Stictosiphonia hookeri (Rhodomelaceae, Rhodophyta) from Argentina and Chile: salinity, light and temperature acclimation. Eur J Phycol 31(4): 361–368
Karsten, U, Friedl, T, Schumann, R, Hoyer, K, Lembcke, S (2005) Mycosporine-like amino acids and phylogenies in green algae: Prasiola and its relatives from the Trebouxiophyceae (Chlorophyta). J Phycol 41(3): 557–566
Khandekar, ML, Murty, TS, Chittibabu, P (2005) The global warming debate: a review of the state of science. Pure Appl Geophys 1626(8–9): 1557–1586
Kromkamp, JC, Forster, RM (2003) The use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. Eur J Phycol 38: 103–112
Lange, OL, Bilger, W, Schreiber, U (1989) Chlorophyll fluorescence of lichens containing green and blue-green algae during hydration by water vapor uptake and by addition of liquid water. Bot Acta 102: 306–313
Lange, OL, Meyer, A, Büdel, B (1994) Net photosynthesis activation of a desiccate cyanobacterium without liquid water in high air humidity alone. Experiments with Micrcoleus sociatus isolated from a desert soil crust. Funct Ecol 8: 52–57
Lange, OL, Belnap, J, Reichenberger, H, Meyer, A (1997) Photosynthesis of green algal soil crust lichens from arid lands in southern Utah, USA: role of water content on light and temperature responses of CO2 exchange. Flora 192: 1–15
Lange, OL, Leisner, JMR, Bilger, W (1999) Chlorophyll fluorescence characteristics of the cyanobacterial lichen Peltigera rufescens under field conditions—II. Diel and annual distribution of metabolic activity and possible mechanisms to avoid photoinhibtion. Flora 194: 413–430
Lange, OL, Green, TGA (2005) Lichens show that fungi can acclimate their respiration to seasonal changes in temperature. Oecologia 142(1): 11–19
Lobban, C, Harrison, P (1997) Seaweed Ecology and Physiology. Cambridge University Press, Cambridge
Moore, LR, Chisholm, SW (1999) Photophysiology of the marine cyanobacterium Prochlorococcus: ecotypic differences among cultured isolates. Limnol Oceanogr 44(3): 628–638
Ong, BL, Lim, M, Wee, YC (1992) Effects of desiccation and illumination on photosynthesis and pigmentation of an edaphic population of Trentepholia odorata (Chlorophyta). J Phycol 28: 768–772
Ortega-Calvo, JJ, Arino, X, Hernandez-Marine, M, Saiz-Jimenez, C (1995) Factors affecting the weathering and colonisation of monuments by phototrophic microorganisms. Sci Tot Environ 167: 329–341
Palmer Jr, RJ, Friedmann, IE (1990) Water relations and photosynthesis in the cryptoendolithic microbial habitat of hot and cold deserts. Microb Ecol 19: 111–118
Rascher, U, Lakatos, M, Büdel, B, Lu¨ttge, U (2003) Photosynthetic field capacity of cyanobacteria of a tropical inselberg of the Guiana Highlands. Eur J Phycol 38: 247–256
Rhee, GY, Gotham, IJ (1981) The effect of environmental factors on phytoplankton growth: temperature and the interactions of temperature with nutrient limitation. Limnol Oceanogr 26: 635–648
Schlensog, M, Pannewitz, S, Green, TGA, Schroeter, B (2004) Metabolic recovery of continental antarctic cryptogams after winter. Polar Biol 27(7): 399–408
Schroeter, B (1994) Langzeitmessungen von Mikroklima und metabolischer Aktivität von Flechten in der maritimen Antarktis. Mitteilung Kieler Polarforscher 9: 15–18
Starr, RC, Zeikus, J (1993) UTEX—the culture of algae at the University of Texas at Austin. J Phycol 29(supplement): 1–106
Thomas, DN, Kirst, GO (1991) Salt tolerance of Ectocarpus siliculosus (Dillw) Lyngb—comparison of gametophytes, sporophytes of different geographic origin. Bot Acta 104(1): 26–36
Tomaselli, L, Lamenti, G, Bosco, M, Tiano, P (2000) Biodiversity of photosynthetic micro organisms dwelling on stone monuments. Int Biodeterior Biodegrad 46: 251–258
Viles, HA (1987) Blue-green algae and terrestrial limestone weathering on Aldabra Atoll: an SEM and light microscope study. Earth Surf Process Landf 12: 319–330
Welton, RG, Cuthbert, SJ, McLean, R, Hursthouse, A, Hughes, J (2003) A preliminary study of the phycological degradation of natural stone masonry. Environ Geochem Health 25: 139–145
Winston, PW, Bates, DH (1960) Saturated solutions for the control of humidity in biological research. Ecology 41: 232–237
Acknowledgments
We thank Evelyn Lawrenz and Manuela Görs for their assistance during algal cultivation and maintenance of our species collection. Prof. Thomas Friedl, University of Göttingen, helped with the molecular identification of all strains studied. Prof. Gunter Kirst, University of Bremen, provided the temperature aluminum blocks, and Prof. Andreas Wohltmann, University of Bremen, the air humidity chambers. We greatly appreciate the financial support by the Deutsche Forschungsgemeinschaft (Project Ka 899/13-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Häubner, N., Schumann, R. & Karsten, U. Aeroterrestrial Microalgae Growing in Biofilms on Facades—Response to Temperature and Water Stress. Microb Ecol 51, 285–293 (2006). https://doi.org/10.1007/s00248-006-9016-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9016-1