Abstract
Microbial pigments are a promising alternative source for natural pigments. They possess a great potential for application due to their natural color, safety, and low production cost. The present investigation is carried out on the antioxidant assays, namely, 2,2-di phenyl-1 picryl-hydrazyl (DPPH), 2,2′-azinobis-3-ehtylbenzthiazoline-6-sulfonate (ABTS), metal chelating activity, reducing power, and ferric reducing antioxidant power (FRAP) of pyocyanin produced from Pseudomonas aeruginosa. Principal antioxidant compounds present in pyocyanin were identified by HPLC. In vitro cytotoxicity study was carried out to evaluate the effect of pyocyanin on MG-63 osteosarcoma cell line.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Microbes have been exploited for pigment production due to factors like relatively large and easily manipulated strands of genes, independent of weather conditions, etc. (Shaikh 2016). The increasing awareness of health and pollution hazards of chemical dye has led to the resurgence of interest in microbial color (Kant 2012). The most characteristic feature of Pseudomonas aeruginosa is the production of a water-soluble blue-green phenazine compound known as pyocyanin (Sudhakar et al. 2013).
The current study aims at the exploitation of different antioxidant activities of pyocyanin, namely, 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity, ferric-reducing antioxidant power (FRAP) activity, 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) activity, reducing power assay (RPA), metal chelating activity, and total phenolic content (TPC) and total flavonoid content (TFC). In vitro cytotoxic effects of pyocyaninon osteosarcoma cell line MG-63 was also studied. An in-depth study on the various antioxidant activities of pyocyanin has been done. We reported for the first time, cytotoxic effect of pyocyanin on MG-63 osteosarcoma cell line.
2 Materials and Methods
2.1 Chemicals
Analytical grade chemicals and solvents were obtained from Sigma Aldrich, Sisco Research Laboratories Pvt. Ltd. (SRL) and Merck, India. Standards like ascorbic acid, ethylene diamine tetra acetic acid (EDTA), beta hydroxy toluene (BHT), gallic acid, and catechin were procured from Sigma Aldrich. MG-63 osteosarcoma cell lines were procured from National Centre for Cell Science (NCCS), Pune, India.
2.2 Screening of Pigment-Producing Microorganism
Screening of the pigment-producing microorganism from soil was previously performed by serial dilution and spread plate method on nutrient agar plates and incubated at 37 °C for 18–24 h. This study had earlier been reported in our previous publication, Chatterjee and Bhowal (2016).
2.3 Identification of the Pigment-Producing Microorganism
Standard biochemical tests and molecular characterization of the microorganism were done for the identification purpose (El-Fouly et al. 2015).
2.4 Production and Extraction of Extracellular Microbial Pigment
Extraction of the pigment was done by solvent (chloroform) extraction method and was stored in a dark-colored glass container at −20 °C for future experimental work. This work had already been reported in our previous publication (Chatterjee and Bhowal 2016).
2.5 Antioxidant Activity of the Pigment
Evaluation of antioxidant potentiality of the pigment was done by various antioxidant assays.
2.5.1 2,2-Di Phenyl-1 Picryl-Hydrazyl (DPPH) Assay
DPPH radical scavenging activity was carried out according to the method reported by Lamien-Meda et al. (2008). DPPH solution in methanol (1:1) served as control. Radical scavenging activity of the pigment was expressed in terms of percentage inhibition of DPPH radical.
where Acontrol is the absorbance of the DPPH+ methanol and Asample is the absorbance of free radical solution with the pigment. Ascorbic acid was used as the positive control.
2.5.2 2,2′-Azinobis-3-Ehtylbenzthiazoline-6-Sulfonate (ABTS) Assay
The ABTS radical scavenging activity was determined by the method described by Miller and Rice-Evans (1997). α-tocopherol was used as the positive control. The scavenging activity on the ABTS radical was measured by the following equation:
2.5.3 Ferric-Reducing Antioxidant Power (FRAP) Assay
The reducing power of the sample was done with the modified method of Benzie and Strain’s (1996). Catechin was used as positive control. The FRAP value was determined from the standard curve of Fe2+ (FeSO4.7H2O).
2.5.4 Reducing Power Assay (RPA)
This assay was carried out according to the method reported by Jayanthi and Lalitha (2011) with slight modifications. Ascorbic acid (AsA) and butylated hydroxy toluene (BHT) were used as a positive and negative controls, respectively.
2.5.5 Metal Chelating Activity
The ability of pyocyanin to chelate ferrous ion was performed according to the method reported by Dinis et al. (1994). EDTA was used as the positive control. The percentage inhibition was calculated as follows:
2.5.6 Total Phenolic Content (TPC)
The concentration of phenolic compounds was measured by the method described by Waterman and Mole (1994). Total phenolic content of the metabolite was calculated from standard curve of gallic acid (1 µg/µl). Total phenolic content of the sample was expressed as mg of gallic acid equivalents (GAEs) per gram of sample.
2.5.7 Total Flavonoid Content (TFC)
This assay was carried out according to the method reported by Quettier et al. (2000). The content of flavonoid was calculated on the basis of the calibration curve of catechin and the results were expressed as mg of catechin equivalents per gram of extract.
2.6 Identification of Principal Antioxidant Compounds by High-Performance Liquid Chromatography (HPLC)
The pigment extract was subjected to HPLC analysis. Ascorbic acid, phenolic acids (gallic acid and ferulic acid), and flavonoids (catechin, myricetin, quercetin and kaempferol) were used as standards. The gradient elution was conducted according to the Evaristo and Leitao (2001) method with minor modifications. Identification of the compounds was done by comparison of their retention’s time and UV absorption spectrum with those of the standards.
2.7 In Vitro Cytotoxic Effect of Pyocyanin on MG-63 Osteosarcoma Cell Lines
The cell viability on MG-63 bone cancer cell lines was determined by standard [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] (MTT) assay (Hassani et al. 2012). Optical density was read at 540 nm with DMSO as blank. The results were expressed in terms of cell viability percentage.
3 Results and Discussion
3.1 Screening of Pigment-Producing Microorganism
Among all the microorganisms that had been screened, a blue-green pigment-producing bacterium was selected for further studies.
3.2 Identification of the Pigment-Producing Microorganism
Standard biochemical tests were done and positive results were recorded with growth on MacConkeys agar medium, methyl red test, citrate test, nitrate reduction test, catalase test, oxidase test, esculine hydrolysis test, and Tween 40 test. The report of the molecular characterization revealed that the sequence obtained was 100% identical to the partial gene sequence of 16S rRNA of P. aeruginosast Hema 10. This strain was accessed in NCBI gene bank for MF419261.1. El-Fouly et al. (2015) found the 16S rRNA gene sequence analysis of two isolates, namely, P. aeruginosa R1 and P. aeruginosa U3 which possessed 97% nucleotide sequence identity to those of P. aeruginosa FPVC 14 and 94% similarity with those of P. aeruginosa 13.A, respectively.
3.3 Production and Extraction of Extracellular Microbial Pigment
Blue-colored microbial pigment was obtained after solvent (chloroform) extraction.
3.4 Antioxidant Assays
3.4.1 DPPH (1,1-Diphenyl-2-Picrylhydrazyl) Free Radical Scavenging Activity
DPPH radical scavenging activity (%) was found to be 47.79 ± 0.05% and the IC50 value was evaluated at 4.75 µg/ml. Ascorbic acid was used as standard. These results are significantly higher than in the study carried out by Dahah et al. (2016) who observed the IC50 value of pyocyaninat 3.15 µg/ml.
3.4.2 Free Radical Scavenging Activity (%) Against ABTS+
The ABTS+ radical scavenging activity was significantly (p ≤ 0.05) high, i.e., 43.63 ± 0.3% at 3.50 µg/ml concentration of pyocyanin which was comparable to that of standard, ascorbic acid. Pawar et al. (2015) reported that the bacterial pigment (PIGB 77) exhibited 38.9 ± 1.98% radical scavenging activity.
3.4.3 Ferric-Reducing Antioxidant Power (FRAP) Assay
FRAP value was recorded as 8.99 ± 0.23 for 1 mg/ml sample concentration. Ferric chloride was used as the standard. This was significantly higher than the results of Pawar et al. (2015) where it was reported that the FRAP value was 7.97 ± 0.12 for 1 mg/ml concentration of pigment produced by Pseudomonas argentinensis (PIGB 46) and 5.90 ± 0.13 for 1 mg/ml in case of the pigment produced by Pseudomonas koreensis (PIGB 77).
3.4.4 Reducing Power Assay (RPA)
RPA (mEq BHT/gm) of the pigment was found to be 34.8 ± 0.00. BHT was used as the standard. Pawar et al. (2015) tested the reducing power activity with the pigment produced by P. koreensis. The highest activity was recorded at 15 mg/ml.
3.4.5 Metal Chelating Activity (MCA)
MCA (%) of the pigment was found to be 20.78 ± 0.02 at 1.36 mg/ml and it represents the greatest inhibition activity when compared to the standard, EDTA. Mani et al. (2015) observed in their study that FC1-3 showed the highest metal chelating activity at 1.53 mg/ml.
3.4.6 Total Phenolic Content (TPC)
TPC was found to be 26.47 ± 0.9 mg GAE/ml. Gallic acid was served as standard. In the study conducted by Mullick et al. (2015), it was reported that the TPC of the pigment extracted from P. aeruginosa MTCC 741 was 23.53 ± 2.5 mg GAE/ml.
3.4.7 Total Flavonoid Content (TFC)
TFC was found to be 32 ± 0.82 mg CAE/ml which is higher than in the study conducted by Mullicket al. (2015) who reported that the TFC of the pigment extracted from P. aeruginosa MTCC 741 was 30 ± 0.99 mg QE/ml.
3.5 Identification of Antioxidant Compounds Present in the Pigment Extract by High-Power Liquid Chromatography (HPLC) Analysis
The phenolic and flavonoid profiling of the pigment extract was identified by HPLC coupled to photodiode array detector (Fig. 1). Aspartic acid, gallic acid, dihydroxy benzoic acid (DHBA), trans cinnamic acid, and ferulic acid were identified as the principal antioxidant compounds present in the pigment extract. This has been reported for the first time.
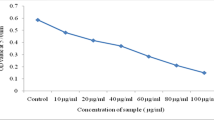
3.6 In Vitro Cytotoxic Effect of Pyocyanin on MG-63 Osteosarcoma Cell Lines
The gradual decline in cell viability percentage established the non-cytotoxic behavior of pyocyanin toward MG-63 osteosarcoma cell lines with respect to control (Fig. 2). On day 1, the cell viability percentage was recorded to be 85.57% and gradually decreased to 11.01% at the end of day 5. Moayedi et al. (2018) reported that pyocyanin was able to reduce human pancreatic cancer (Panc-1) cells, inhibition rate being by 89.88 ± 1.86%. He further added that pyocyanin could also induce dose-dependent apoptosis in Panc-1 cells after 24 h.
4 Conclusion
In vitro investigations showed that pyocyanin exhibits promising antioxidant capacity to scavenge free radicals in biological system. The pigment thus can be used as an alternative natural antioxidant after toxicological examination. This high percentage of free radical scavenging activity of the pigment at very minute concentrations gives us a positive indication for the safe use of the pigment. In addition to this, the pigment showed no cytotoxic effects on cultured MG-63 osteosarcoma cell lines.
References
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem 239:70–76
Chatterjee S, Bhowal J (2016) Production of microbial color from soil microbes for food use. Materials Today: Proc 3(10):3388–3402
Dahah H, Djibaoui R, Nemmiche S (2016) Antimicrobial, antioxidant and hemolytic effects of Pyocyanin produced by Pseudomonas aeruginosa isolated from saline soil of Mina river. Algeria Int J Biosci 9(5):134–143
Dinis TCP, Madeira VMC, Almeida LM (1994) Action of phenolic derivates (acetoaminophen, salycilate and 5aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. J Architectural Biochem Biophysics 315:161–169
El-Fouly MZ, Sharaf AM, Shahin AAM et al (2015) Biosynthesis of pyocyanin pigment by Pseudomonas aeruginosa. J Rad Res Appl Sci 8(1):1–13
Evaristo IM, Leitao MC (2001) Identification and quantification by DAD-HPLC of the phenolic fraction contained in the leaves of Quercus suber L. Silva Lusitana 9:135–141
Hassani HH, Hasan HM, Al-Saadi A et al (2012) A comparative study on cytotoxicity and apoptotic activity of pyocyanin produced by wild type and mutant strains of Pseudomonas aeruginosa. Euro J Exp Bio 2(5):1389–1394
Jayanthi P, Lalitha P (2011) Reducing power of the solvent extracts of Eichhorniacrassipes (Mart.) solms. Int J Pharm Pharm Sci 3:126–128
Kant R (2012) Textile dyeing industry an environmental hazard. Nat Sci 4:22–26
Lamien-Meda A, Lamien CE, Compaoré MM et al (2008) Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules 13(3):581–94
Mani VM, Priya MS, Dhaylini S et al (2015) Antioxidant and antimicrobial evaluation of bioactive pigment from fusarium sp. isolated from stressed environment. Int J Curr Microbiol App Sci 4(6):1147–1158
Miller N, Rice-Evans C (1997) Factors influencing the antioxidant activity determined by the ABTS radical action assay. Free Radic Res 26:195–199
Moayedi A, Nowroozi J et al (2018) Cytotoxic effect of pyocyanin on human pancreatic cancer cell line (Panc-1). Iran J Basic Med Sci 21:794–799
Mullick A, Shah A, Sett SD (2015) Antimicrobial activity of cell free extract of Pseudomonas aeruginosa MTCC 741 towards opportunistic human pathogens. Int J Pharm Bio Sci 5(3):42–48
Pawar R, Mohandass C, Sivaperuma E et al (2015) Epiphytic marine pigmented bacteria: a prospective source of natural antioxidants. Braz J Microbiol 46(1):29–39
Quettier DC, Gressier B, Vasseur J et al (2000) Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol 72:35–42
Shaikh Z (2016) Biosynthesis of prodigiosin and its applications. IOSR-JPBS 11(6):1–28
Sudhakar T, Karpagam S, Shiyama S (2013) Analysis of pyocyanin compound and its antagonistic activity against phytopathogens. Int J of Chem. Tech Res 5:1101–1106
Waterman PG and Mole S (1994) Analysis of phenolic plant metabolites. Blackwell Scientific Publication, pp 83–85
Acknowledgements
The authors express their sincere thanks to Indian Institute of Chemical Biology (IICB), Kolkata and Centre for Healthcare Science and Technology, IIEST, Shibpur.
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethical Approval
This chapter does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Sengupta, S., Bhowal, J. (2021). Study on the Antioxidant and Cytotoxic Properties of Pyocyanin Extracted from Pseudomonas aeruginosa. In: Ramkrishna, D., Sengupta, S., Dey Bandyopadhyay, S., Ghosh, A. (eds) Advances in Bioprocess Engineering and Technology . Lecture Notes in Bioengineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-7409-2_13
Download citation
DOI: https://doi.org/10.1007/978-981-15-7409-2_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7408-5
Online ISBN: 978-981-15-7409-2
eBook Packages: EngineeringEngineering (R0)