Abstract
The polyphenol compound is extracted from Sargassum tenerrimum with various bioactivities including antibacterial and antioxidant activity and MTT assay for cell cytotoxicity. The total phenolic content was 69.12 ± 0.24%. The S. tenerrimum polyphenol was found to phytochemical constituent’s presence of flavonoids, saponins, tannins, phenolics, alkaloids and steroid. The antibacterial activity of polyphenol presented significant inhibition against ten human pathogen bacterial cultures such as Proteus mirabilis, Klebsiella oxytoca, Escherichia coli, Bacillus cereus, Streptococcus pyogenes, Staphylococcus aureus, Pseudomonas aeruginosa, Vibrio cholerae, Salmonella typhi and Bacillus subtilis. The in vitro antioxidant activity and MTT assay revealed that the polyphenol has anticancer activity against HeLa cells. The polyphenol compound was characterized through HPLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The marine environment is rich in bioactive secondary metabolites, many of which have structural characteristics that are not seen in terrestrial natural products [1]. Bioactive substances such as polyphenols, carotenoids and polysaccharides are abundant in seaweeds. These bioactive chemicals can be used in functional foods, medications and cosmetics because they provide customers with health benefit [2]. Polyphenolic chemicals found in catechins, flavonols and glycosides in methanol extracts of seaweeds have been discovered to exhibit antioxidant and antibacterial activity in vitro [3]. Phenolic chemicals are a major predictor of a food’s antioxidant capacity [4]. Because of their rheological qualities as gelling and thickening agents, seaweeds are abundant sources of polyphenol, making them some of the most desirable additions in the food business (e.g., carrageenan). Polyphenols are known to have a variety of biological activities, including anticoagulant, antiviral and immuno-inflammatory properties, which could be used in nutraceutical/functional food and cosmetic or cosmeceutical and pharmaceutical products [5].
Polyphenolic chemicals can be found in a wide variety of foods. Due to the presence of numerous hydroxyl (OH) groups in polyphenolic compounds, they are likely to have a radical scavenging impact or, in certain cases, an oxidative effect as a generator of reactive oxygen species. Polyphenolic compounds have been shown in animal studies to have favourable health effects due to their antioxidant capabilities and inhibitory involvement in various phases of tumour formation. Polyphenolic content of teas, wines, cacaos, fruits and vegetables has been reported plenty of findings. In comparison to terrestrial plants, seaweeds may have a higher concentration of flavonoid catechins [6]. The effect of antioxidant naturally occurring phenolic components on the protection of cardiovascular diseases and cancer, as well as age-related degenerative brain illnesses, has been examined earlier [7, 8]. Hence, the present investigation was undertaken to value of the Sargassum tenerrimum from Mandapam coast of Gulf of Mannar region South east coast of Tamil Nadu, India and to study the isolation, characterization of bioactive compounds and screening the active principle against antimicrobial and antioxidant activity of different solvent extract of Sargassum tenerrimum.
2 Materials and methods
2.1 Extraction of crude polyphenol compound from S. tenerrimum
The algae was cleaned with sterile distilled water, dried in the sun, chopped into small pieces and pulverized in a mixer grinder. It was kept at room temperature in an airtight polypropylene container. In a Soxhlet extractor, 100 g of S. tenerrimum was extracted for 6 h with 500 ml of methanol (2:1). The entire extract was filtered, and the filtrate produced was concentrated to dryness under reduced pressure. For subsequent study, the concentrated extract was used as the seaweed polyphenol compound [9].
2.2 Estimation of total phenolic content
A phenolic content of methanolic extracts was estimated by the method of Senevirathene et al. [10].
2.3 Phytochemical analysis
The components analysed for were terpenoids, anthraquinones, flavonoids, saponins, tannins, alkaloid, cardiac glycosides, steroid and balsam [11].
2.4 Antibacterial activity
In vitro antibacterial test was performed using a panel of ten human pathogenic strains which include Proteus mirabilis, Klebsiella oxytoca, Escherichia coli, Bacillus cereus, Streptococcus pyogenes, Staphylococcus aureus, Pseudomonas aeruginosa Vibrio cholerae, Salmonella typhi and Bacillus subtilis which were obtained from the laboratory in Department of Microbiology, Ayya Nadar Janaki Ammal College, Sivakasi, Tamil Nadu, India.
2.5 In vitro antioxidant activity
The antioxidant activity of S. tenerrimum polyphenol in total antioxidant activity, Reducing power, Hydrogen peroxide assay, DPPH, and ABTS [12,13,14].
2.6 In vitro anticancer activity of polyphenol compound
2.6.1 MTT assay
The cytotoxicity of the polyphenol compound was investigated using Hela cells utilizing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Trypsinization was used to collect the cultivated Hela cells, which were then pooled in a 15-ml tube. The cells were then plated at a density of 1 × 105 cells/ml cells/well (200 l) in a 96-well tissue culture plate in DMEM medium containing 10% FBS and 1% antibiotic solution for 24–48 h at 37 °C in DMEM medium containing 10% FBS and 1% antibiotic solution. In a serum-free DMEM medium, the wells were rinsed with sterile PBS and treated with various concentrations of the polyphenol sample. Each sample was reproduced three times, and the cells were cultured for 24 h at 37 degrees Celsius in a humidified 5% CO2 incubator. MTT (20 µl at 5 mg/ml) was added to each well after the incubation period, and the cells were incubated for another 2–4 h until purple precipitates were visible under an inverted microscope. Finally, the medium was aspirated out of the wells together with MTT (220 µl) and rinsed with 1X PBS (200 µl). DMSO (100 µl) was also added to dissolve formazan crystals, and the plate was agitated for 5 min. Using a micro-plate reader (Thermo Fisher Scientific, USA), the absorbance of each well was measured at 570 nm, and the % cell viability and IC50 value were computed using GraphPad Prism 6.0 software (USA).
2.7 HPLC analysis of polyphenol compound
The polyphenol compound was studied with a HPLC C18 system column (LC- 10VP Shimadzu).
3 Results and discussion
3.1 Yield of the crude extract
The crude polyphenol compound was extracted from 100 g of S. tenerrimum powder using hot water, and the dry weight of the crude polyphenol compound was found to be 12 g, respectively. Erwan et al. [15] report that in the extraction of phlorotannins, a simple process based on the utilization of water and organic solvent combinations is used. Separating methods based on both the polarity and the molecular size of substances are used to purify and fractionate crude extracts.
3.2 Estimation of total phenolic content
The total phenolic content present in the polyphenol compound was found to be 69.12 ± 0.24% of phenolic content present in S. tenerrimum. Similarly, Vijayabaskar and Shiyamala [16] evaluated that the phenolic content of T. ornata was determined to be the highest (43.72 1.63 mg GAE/g extract).
3.3 Phytochemical analysis
The present investigation brings out the S. tenerrimum phytochemical constituents presence of flavonoids, saponins, tannins, Phenolics, alkaloids and steroid is shown in Table 1. Gopalan et al. [17] looked into the alkaloids, terpenoids, flavonoids, tannins, polyphenols, saponins, cardiac glycosides and quinines found in G. corticata. According to Heo et al. [18], marine seaweeds are high in polyphenolic substances such catechins, flavonols and phlorotannins. Bromophenols, phenolic acids and flavonoids make up the majority of the phenolic chemicals found in green and red algae. The primary polyphenolic secondary metabolites found only in maritime brown seaweeds are phlorotannins, a series of complex polymers of phloroglucinol (1,3,5-trihydroxybenzene).
3.4 Antibacterial activity of polyphenol compound
The S. tenerrimum polyphenol compound high activity in 18 mm of inhibition zone against Streptococcus pyogenes and minimum of 8 mm of inhibiting zone against Salmonella typhi is shown in Table 2. Rajasulochana et al. [19] determined the antibacterial activity of the experimental brown algae. The brown algae showed maximum antibacterial activity of E. coli (2.2 ± 0.063) and Staphylococcus aureus (6.2 ± 0.128), respectively. Smullen et al. [20] evaluated that the antimicrobial assay of the polyphenol compound was related to their chemical structure and ester sulphate groups. Vijayabaskar and Shiyamala [16] reported that the S. wightii and T. ornate methanolic extracts were tested against a variety of human pathogenic microorganisms. The discovery suggests that T. ornata methanol extracts could be used as a valuable source of antibacterial activity in the pharmaceutical business.
3.5 In vitro antioxidant activity of polyphenol compound
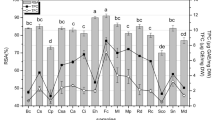
The antioxidant activity of polyphenol compound was present in total antioxidant capacity, reducing power, hydrogen peroxide scavenging activity, DPPH and ABTS shown in Table 3. Riaz et al. [21] found that the total antioxidant of Gardenia jasminoides polyphenolic components may be represented in terms of ascorbic acid equivalents. In a dose-dependent way, total antioxidant activity was raised. According to Valdes [22], the polyphenol molecule found in seaweed can be a possible source of antioxidants with both protective and beneficial properties. Their bioactivity has been mostly attributed to their direct antioxidant, radical scavenging and anti-inflammatory activities for a long time. In the reducing power antioxidant experiment, increased absorbance indicates that materials are capable of donating hydrogen atoms in a dose-dependent way [23, 24]. Audibert et al. [25] investigated the antioxidant activity of pure extracts of phlorotannin fractions and discovered that low molecular weight phlorotannins had increased antioxidant activity, which declined as the polymerization process progressed. Czochra and Widensk [26] have been investigated that the measurement of H2O2 scavenging activity is one of the useful methods of responsible the ability of antioxidants to decrease the level of pro-oxidants in H2O2. Similarly, Vijayabaskar and Shiyamala [16], the DPPH, T. ornata had a high activity (84.27 2.17% scavenging activity on DPPH) when compared to ordinary Gallic acid. In DPPH radical scavenging antioxidant experiments, Riaz et al. [21] reported considerable free radical scavenging capabilities when compared to conventional ascorbic acid. Kajal et al. [27] worked that the ABTS of the MeOH extracts/fractions of the red algae at 0.6 μg/ml are recorded. Among H. musciformis MeOH extracts recorded significantly higher ABTS (19.6%), H. valentiae (14.9%) and J. rubens (8.7%), respectively. The ethyl acetate fraction of S. marginatum was shown to have the highest antioxidant activity (39.62 mg ascorbic acid equivalent/g extract) by Sachindra et al. [28].
3.6 In vitro anticancer activity of polyphenol
3.6.1 MTT assay for cell cytotoxicity
To investigate the effect of polyphenol on colon cancer cell proliferation, Hela cells were grown for 24 h at various concentrations of polyphenol (10, 20, 40, 60, 80 and 100 g/ml), and the vitality of the cells was determined using the MTT assay (Table 4 and Fig. 1). By using an inverted microscope, morphological alterations in Hela cells were observed, indicating that polyphenol reduced the viability of Hela cells at various concentrations (10, 20, 40, 60, 80 and 100 g/ml), as shown in Table 5 and Fig. 2. The presence of both viable cells was demonstrated in Hela cells treated with 10 and 20 g/ml polyphenol. Hela cells treated with 40 and 60 g/ml polyphenol revealed the presence of both viable and non-viable cells. A lower number of viable cells were seen in cells treated with 80 g/ml polyphenol. Figure 3 shows that a 100 g/ml polyphenol treatment resulted in a higher number of nonviable cells. Polyphenol treatment of Hela cells decreased cell proliferation in a dose-dependent manner. The IC50 value (inhibitory concentration) was 23.02 g/ml. The effect showed that as the concentration of polyphenols increased, the cell survival rate reduced. Table 6 shows that polyphenol was more effective against colon cancer cell line Hela cells as a result of the study’s findings. Namvar et al. [29] determined the effect of S. muticum methanolic extract against MCF-7 and MDA-MB-231 breast cancer cell line proliferation. The MTT assay effects indicated that the extract was cytotoxic against breast cancer cell lines in a dose-dependent manner, with IC50 of 22 μg/ml for MCF-7 and 55 μg/ml for MDA-MB-231 cell lines. Apostolidis and Lee [30] reported that the polyphenols extracted from A. nodosum showed significant a-amylase and a-glucosidase inhibitory activity equal to acarbose, a commercially potent inhibitor drug.
3.7 HPLC analysis of polyphenol
HPLC analysis of polyphenol of S. tenerrimum is given in Fig. 4. The HPLC analysis of the polyphenol presence of various constituents as evidenced by the chromatogram obtained at various retention times (2.050, 2.157, 2.523, 3.023 and 4.377). The polyphenol like p-hydroxybenzoic acid was identified at Rt 3.023. Waghmode and Khilare [31] have been reported that the, measurable analysis of following brown seaweed such as S. cinereum, S. ilicifolium, S. tenerrimum and S. wightii of phenolics were evaluated and studied using RP-HPLC. Jayabarath and Jeyaprakash [32] reported that the high performance liquid chromatography was preferred analytical tool for fingerprints and quantification of marker compounds in seaweed extracts. HPLC analysis of T. conoides methanolic extract showed the presence of different constituents as evidence by the chromatogram obtained at various retention times (3.643, 3.819 and 6.463) at λmax 254 nm.
4 Conclusion
The present study concludes the opportunity to project the importance of brown seaweed Sargassum tenerrimum possessing medicinal. The polyphenol extracted from Sargassum tenerrimum showed higher antibacterial and antioxidant activity and cell line activity.
References
Cantillo-Ciau Z, Moo-Puc R, Quijano L, Freile-Pelegri Y (2010) The tropical brown alga Lobophora variegata: a source of antiprotozoal compounds. Mar Drugs 8:1292–1304
Shibata T, Fujimoto K, Nagayama K (2002) Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int J Food Sci Techn 37:703–709
Sailler B, Glombitza KW (1999) Phlorethols and fucophlorethols from the brown alga Cystophora retroflexa. Phytochemisty 50:869–881
Parr Adrian J, Bolwell GP (2000) Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J Sci Food Agric 80(7):985–1012
Jiao G, Yu G, Zhang J, Ewart HS (2011) Chemical structures and bioactivities of sulphated polysaccharides from marine algae. Mar Drugs 9:196–223
Suzuki M, Daitoh M, Vairappan CS, Abe T, Masuda M (2001) Novel halogenated metabolites from the Malaysian Laurencia pannosa. J Nat Prod 64:597–602
Stoclet JC, Chataigneau T, Ndiaye M, Oak MH, El Bedoui J, Chataigneau M, SchiniKerth VB (2004) Vascular protection by dietary polyphenols. Eur J Pharmacol 500:299–313
Stevenson DE, Hurst RD (2007) Polyphenolic phytochemicals-just antioxidants or much more Cell Mol. Life Sci 64:2900–2916
Vijayabaskar P, Shiyamala V (2011) Antibacterial activities of brown marine algae (Sargassum wightii and Turbinaria ornata) from the Gulf of Mannar biosphere reserve. Adv Biol Res 5(2):99–102
Senevirathene MS, Hyun Kim N, Siriwardhana J, Hwan HK, Wan L, Jin Jeon Y (2006) Antioxidant potential of Eclinia cava on reactive oxygen species scavenging, metal chelating, reducing power and lipid peroxidation inhibition. Food Sci Tech Int 12(1):27–38
Kamba AS, Hassan LG (2010) Phytochemical screening and antimicrobial activities of Euphorbia balsamifera leaves, stems and root against some pathogenic microorganisms. Afr J Pharm Pharmacol 4:645–652
Gulcin T, Irfan KO, Kufrevioglu OM, Buyukokuroglu ME (2004) Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol 90:205–215
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorizing assay. Free Rad Biol Med 26(9):1231–1237
Erwan AG, Florian L, Melanie H, Camille J, Valerie SP (2015) Extraction and purification of phlorotannins from brown algae. Nat Prod Mar Algae 1308:131–143
Vijayabaskar P, Shiyamala V (2012) Antioxidant properties of seaweed polyphenol from Turbinaria ornate (Turner) Agardh, J. (1848). Asian Paci J Trop Biomed 2:90–98
Gopalan R, Periyakali SB, Veeran S, Rajendran U (2017) Phytochemical screenings of the marine red alga, Gracilaria corticata. Noble Inter J Sci Res 1(8):90–97
Heo SJ, Park EJ, Lee KW, Jeon YJ (2005) Antioxidant activities of enzymatic extracts from brown seaweeds. Biores Tech 96(14):1613–1623
Rajasulochana P, Krishnamoorthy P, Dhamotharan R (2012) Isolation, identification of bromophenol compound and antibacterial activity of Kappaphycus sp. Inter J Pharma Biosci 3(2):176–186
Smullen J, Koutsou GA, Foster HA, Zumbé A, Storey DM (2007) The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res 41(5):342–349
Riaz U, Moni RS, Nusrat S, Hemayet H, Ismet AJ, Raushanara A, Ashraful A (2014) HPLC-analysis of polyphenolic compounds in Gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv Pharmal Bull 4(3):273–281
Valdes L, Cuervo A, Salazar N, Ruas-Madiedo P, Gueimonde M, Gonzalez S (2015) The relationship between phenolic compounds from diet and microbiota, impact on human health. Food Funct 6(8):2424–2439
Ganjewala D, Gupta AK (2013) Study on phytochemical composition, antibacterial and antioxidant properties of different parts of Alstonia scholaris Linn. Adv Pharm Bull 3(2):379–384
Asirvatham R, Christina AJ, Murali A (2013) In vitro antioxidant and anticancer activity studies on Drosera Indica L. (Droseraceae). Adv Pharma Bull 3(1):115–120
Audibert L, Fauchon M, Blanc N, Hauchard D, Gall EA (2010) Phenolic compounds in the brown seaweed Ascophyllum nodosum distribution and radical-scavenging activities. Phytochem Anal 21(5):399–405
Czochra MP, Widensk A (2002) Spectrophotometric determination of hydrogen peroxide scavenging activity. J Anlitica Chem Acta 452:177–184
Kajal C, Deepu J, Nammunayathuputhenkotta KP (2015) Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J Food Sci Tech 52(4):1924–1935
Sachindra NM, Airanthi MKWA, Hosokawa M, Miyashita K (2010) Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J Food Sci Tech 47:94–99
Namvar F, Mohamad R, Baharara J, Zafar-Balanejad S, Fargahi F, Rahman HS (2013) Antioxidant, antiproliferative, and antiangiogenesis effects of polyphenol-rich seaweed (Sargassum muticum). Bio Med Res Inter 2013:1–9
Apostolidis E, Lee C (2010) In vitro potential of Ascophyllum nodosum phenolic antioxidant mediated a-amylase inhibition. J Food Sci 75:97–102
Waghmode AV, Khilare CJ (2018) RP-HPLC profile of major phenolics from brown marine macro algae. J Appl Pharma 10(2):1–5
Jayabarath J, Jeyaprakash J (2017) HPLC profiling of brown seaweeds (Turbinaria Conoides). Inter J Lat Engg Manage Res 2(9):75–78
Acknowledgements
I would like to acknowledge the Department of Microbiology, Ayya Nadar Janaki Ammal College (Autonomous) Sivakasi 626 124, Tamil Nadu, for providing the facilities for carrying out this work successfully.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shunmugiah Mahendran, Subbiah Sankaralingam, Senthurpandian Muthuramalinga Sethupathi et al. Evaluation of antioxidant and cytotoxicity activities of polyphenol extracted from brown seaweed Sargassum tenerrimum biomass. Biomass Conv. Bioref. 14, 2063–2069 (2024). https://doi.org/10.1007/s13399-022-02301-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02301-x