Abstract
Micro determination of Zirconium (IV) has been carried out using 3-Hydroxy-2-[2′-(5′-methylthienyl)]-4H-chromen-4-one (HMTC) as an analytical reagent. Zr (IV) forms a 1:4 (M:L) yellow coloured complex with HMTC extracted into dichloromethane from ammoniacal medium (pH 7.05–7.09). The complex system shows a maximum at 424–440 nm and follows Beer’s law in the range 0.0–0.9 µg Zr (IV) ml−1 with an optimum range of determination as 0.27–0.79 µg Zr (IV) ml−1as detected from Ringbom plot. Zr (IV)-HMTC complex has molar absorptivity of 8.22 × 104 Lmol−1 cm−1, specific absorptivity of 0.900 ml g−1 cm−1 and Sandell’s sensitivity value 0.0011 µg Zr (IV)cm−2; the linear regression equation being Y = 0.981X − 0.036 (Y = absorbance, X = µg Zr (IV) ml−1) with the correlation coefficient 0.9987. Detection limit of the procedure is 0.0174 µg ml−1. The repercussions obtained are highly consistent with the standard deviation of ±0.0039 absorbance unit and has been confirmed by student’s t-test with 0.5% limit. The proposed technique has been successfully applied in diverse synthetic and industrial samples.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Zirconium (Zr) with atomic number 40 is a lustrous grey white transition element. It has no biotic role known so far. But depending on the nutritional custom human body contains 250 mg of Zr with a daily consumption of 4.1 mg, i.e., 3.5 mg from food and 0.65 mg from water, respectively. Zirconium is extensively scattered in nature and is found in all biological systems, for example: 2.86 μg g−1 in whole wheat, 3.09 μg g−1 in brown rice, 0.55 μg g−1in spinach, 1.23 μg g−1 in eggs, and 0.86 μg g−1 in ground beef [1]. Additionally, zirconium has been frequently used in commercial products like deodorant sticks, aerosol antiperspirants such as the aluminium zirconium tetrachlorohydrex gly or AZG, is used as an antiperspirant in many deodorant products. It has the ability to obstruct pores in the skin and prevent sweat from leaving the body. Other use of zirconium is in water purification, for example, control of phosphorus pollution, bacteria and pyrogen-contaminated water [2]. Zirconium has not been assessed as health or environmental hazard and is not carcinogenic or genotoxic. However, at levels of 25 mg m−3, zirconium has been detected to be immediately dangerous to life and health.
Zirconium bearing compounds have various biomedical applications, including dental implants and crowns, knee and hip replacements, middle-ear ossicular chain reconstruction and other restorative and prosthetic devices [2]. Sodium zirconium cyclosilicate has been used in the treatment of hyperkalemia [3].
Due to such vast applications of the element in commercial industry, it is needed that we develop a procedure which has good reliability, less time consuming and good sensitivity and selectivity for micro-determination of the same. Many approaches have been evaluated so far in this field where their pertinence has been diminished in terms of sensitivity, selectivity and rapidity using UV-VIS spectrophotometery [4,5,6,7,8]. The presented procedure manifests the complexation of new benzopyran named 3-hydroxy-2-[2′-(5′-methylthienyl)]-4H-chromen-4-one (HMTC) with Zr (IV) for its trace analysis, deriving preferable outcomes.
2 Experimental
2.1 Equipments, Reagents and Solutions
A UV-VIS spectrophotometer (2375; Electronics India) with 10 mm matched quartz cells was used for absorbance measurements and spectral analysis.
A stock solution of Zr (IV) containing 1 mg ml−1 of the metal ion was attained by solvation of promptly weighed amount of ZrOCl2.8H2O (CDH® ‘AR’) in 2 M solution of HCl and making up the volume by same in 100 ml volumetric flask. Lower concentrations like that of μg ml−1 level were obtained by appropriate dilutions therefrom.
Similarly, stock solutions of other metal ions at the mg ml−1level were prepared by dissolving their frequently available sodium or potassium salts (‘chemically pure’ grade) in de-ionized water or dilute acids. Dichloromethane (CDH® ‘AR’) and Ammonia (SDFCL ‘AR’) were used as such.
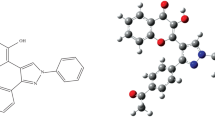
0.1% (m/v) solution of 3-hydroxy-2-[2′-(5′-methylthienyl)]-4H-chromen-4-one (HMTC) [9] [Molar Mass = 258 g mol−1, Melting Point = 203 ℃; Fig. 1) was prepared fresh in acetone.
2.2 Synthetic and Technical Samples
Synthetic samples were brought into solutions by mixing Zr (IV) and different metal ion solutions in desirable quantities with compositions as depicted in Table 1. However, technical samples like reverberatory flue dust and water (tap and well) were dissolved to a definite volume as per the earlier reported work [10]. Suitable aliquots were taken to determine zirconium as portrayed in the methodology.
2.3 Procedure for Extraction and Determination
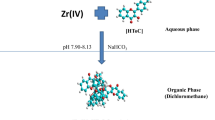
To make final volume of aqueous phase as 10 ml, an aliquot containing ≤9 µg Zr (IV) solution and 0.5 ml of 0.1% HMTC solution were taken in a 125 ml separating funnel and enough amount of ammonia solution was added so as to adjust its pH 7.06. Ample volume of demineralized water was added to make the final volume of aqueous phase 10 ml. It was then equilibrated once with equal volume of dichloromethane for 30 s, releasing the pressure periodically through the stop cork. As the phase gets separated, the yellow coloured organic layer was filtered through Whatman filter paper no. 41 (pretreated with dichloromethane) in a 10 ml volumetric flask and was made up to mark with pure dichloromethane. Absorbance of the yellow coloured extract was measured at 430 nm against the reagent blank prepared in an equivalent manner. The amount of zirconium was determined from the calibration curve obtained by plotting a graph between variable amounts of Zr (IV) and the corresponding absorbance values obtained after applying optimum conditions of procedure.
3 Results and discussion
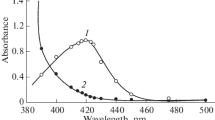
HMTC formed a very stable (stability >4 days) yellow coloured complex with Zr (IV) in an ammoniacal medium (pH 7.05–7.09). From the spectra taken for Zr (IV)—HMTC complex, the absorption maximum was observed in the range 424–440 nm in comparison with the reagent blank that also absorbed to a little extent in the reported range (Fig. 2). Due to this pretext entire measurements were taken at 430 nm against reagent blank. Though similar methodology as applied with ammonia was performed in Na2CO3, CH3COOH, H3PO4, H2SO4, NaHCO3 and HCl, the absorbance observed was comparatively less in the same order, and hence NH3 medium was chosen over others where the complex not only showed high colour intensity but consistency over time.
Extraction conduct of Zr (IV)-HMTC complex was examined in various organic solvents and found to increases in the order: Ethyl acetate < isoamyl alcohol < methyl isobutyl ketone < toluene < benzene < carbon tetrachloride < chloroform < cyclohexane < iso amylacetate < 1,2dichloroethane < dichloromethane. In context to the mentioned order, it can be said that complex should be extracted in dichloromethane as it showed maximum absorbance followed by its stability for more than 4 days. Yellow coloured complex can be wholly uprooted into organic layer of dichloromethane in single step by quantitative (100%) extraction.
The influence of divergent physical variables like pH, HMTC concentration and equilibration time on the extraction of Zr (IV) was studied as summarized in Table 2. After examining Table 2, it can be said that to attain the optimum and constant intensity of the complex containing ≤9 µg Zr (IV) in 10 mL aqueous phase, 0.3–0.8 ml of 0.1% (w/v) HMTC solution in acetone was added maintaining pH 7.05–7.09 of the water solution by adding sufficient ammonia in the same order as per the applied procedure and equilibrating once with same amount (10 ml) of dichloromethane for 15–300 s, are appropriate for the Zr (IV)—HMTC complex formation.
3.1 Effect of Anions/Complexing Agents and Cations
To study the effect of diverse ions, the ions were added under ideal conditions of the suggested strategy to 5 µg concentration of Zr (IV) in a 10 ml aqueous volume. The impact of different anions, complexing agents or cations on the extraction and spectrophotometric determination of Zr (IV) was prospected to evaluate the selectivity and tolerance limit as shown in Tables 3 and 4. The tolerance limit was customized as the amount of diverse ion causing an error ≤1% in the extraction of Zr (IV). Some of the samples containing metal ions required addition of the appropriate masking/complexing agents before the addition of HMTC as depicted in Table 4. Out of 22 anions and 32 cations studied, none (alone or after masking) interfered with the determination of Zr (IV)-HMTC complex.
3.2 Spectral Characteristics
The yellow complex of Zr (IV)-HMTC acquired under the optimum conditions of the mechanism showed linear response up to 0. 9 µg ml−1 of Zr (IV) with the optimum limit of determination as obtained from the Ringbom plot [11] falling in the range 0.27–0.70 ppm. Linearity of the calibration plot was confirmed by analysis of the correlation coefficient having the value 0.9987. Student’s t-test was conducted and it was concluded that at 0.5% level, no biasness had occurred in the research process. Various optical and statistical characteristics as calculated by statistical methods are shown in Table 5.
3.3 Stoichiometry of Zr (IV)-HMTC Complex
The stoichiometric ratio of Zr (IV)-HMTC in the extracted species was established as 1:4 by the job’s continuous variations method [12] as revised for a two-phase system by Vosburgh and Cooper [13] and portrayed in Fig. 3. The ratio 1:4 of the complex constituents was further confirmed by mole ratio method [14]. The stability constant as estimated by the mole ratio method was 3.76 × 1015.
Thus, the probable structure of Zr (IV)-HMTC complex as determined by the two methods was suggested as shown in Fig. 4.
3.4 Structural Elucidation by Optimization Technique
The metal complex was demonstrated with Avogadro 1.01 programme [15] and optimized using molecular mechanics. Various cycles of optimization were effectuated. Energy found for the complex was 4257.28 K J mol−1 before optimization and 2275.56 K J mol−1 after 500 cycles of optimization. The optimized structure has coordination number 8 and square antiprismatic geometry. Some optimized bond lengths and bond angles are also calculated and are shown in Table 6. The optimized structure is shown in Fig. 5.
3.5 Analytical Applications
The proposed spectrophotometric method for the micro-determination of zirconium is rapid to operate (takes 2–3 min for a single determination), less equipment requiring, highly sensitive, reproducible and has a vast forbearance limit for the foreign ions including Fe, Cr, Mo, W, Nb, V, Sn and many other important elements including platinum group metals, thereby increasing the purview of application. The extensive usefulness of the technique is further tested by analysing a large variety of real samples and synthetic mixtures of varying composition as in Table 1. The obtained results are in great concurrence with the amount of metal ion initially added. The proposed method is reproducible and accurate with the relative standard deviation of 0.861% for 10 replicates containing 0.5 µg Zr (IV) ml−1 every time. The procedure preferred has been compared with existing methods in respect of rapidity, selectivity and sensitivity as summarized in Table 7.
4 Conclusion
3-Hydroxy-2-[2′-(5′-methylthienyl)]-4H-chromen-4-one has been used as an analytical reagent for the spectrophotometric determination of zirconium. Zirconium (IV) in presence of several cations and anions/complexing agents forms a yellow 1:4 (M:L) complex with HMTC which is extractable into dichloromethane in ammoniacal medium of pH 7.06 and 0.3–0.8 ml of 0.1% HMTC solution in acetone and is stable for more than 4 days. The complex shows an absorption maximum at 424–440 nm with a molar absorptivity of 8.212 × 104 l mol−1 cm−1 and Sandell’s sensitivity equal to 0.0011 µg Zr cm−2. The linear regression equation was Y = 0.981X − 0.036 and the correlation coefficient, r = 0.9987; detection limit of the method being 0.0174 µg ml−1. The optimum range of determination was 0.27–0.79 µg Zr (IV) ml−1. The method has been adopted in account for its simplicity, selectivity, rapidity and precision and has been applied for the determination of zirconium in synthetic and technical samples.
References
Schroeder AH, Balassa JJ (1966) Abnormal trace metals in man: zirconium. J Chronic Diseases 19(5):573–586
Lee DBN, Roberts M, Bluchel CG, Odell RA (2010) Zirconium: biomedical and nephrological. Appl ASAIO J 56(6):550–556
Ingelfinger JR (2016) A new era for the treatment of hyperkalemia. J Med 372(3):275–277
Chawaria M, Sharma HK (2018) A non-extractive spectrophotometric determination of Zirconium with6-Chloro-3-hydroxy-2-(2’-hydroxyphenyl)-4-oxo-4 H-1-benzopyran using propan-1-ol-H2O mixture as solvent. Internat J Green Herbal Chem 7(2):230–236
Chawaria M, Kumar A (2017) Extractive spectrophotometric determination of zirconium with 6-chloro-3-hydroxy-2-phenyl-4H-chromen-4-one as an analytical reagent. J Chem Bio Phy Sci 7(3):600–604
Lasheen TA, Hussein GM, Khawassek YM, Cheira MF (2013) Spectrophotometric determination of zirconium (IV) and hafnium (IV) with pyrazolo (1, 5-a) quinazolin-6-onederivative reagent. Anal Chem Indian J 12(10):368–376
Sumathi G, Sreenivasulu Reddy RT Direct and derivative spectrophotometric determination of zirconium(IV) with 2-hydroxynaphthaldehyde-phydroxybenzoichydrazone. J Appl Chem 2(1):81–85
Jain A, Prakash O, Kakkar LR (2010) Spectrophotometric determination of zirconium with 5,7-dibromo-8-hydroxyquinoline in presence of thiocyanate. J Anal Chem 65(8):820–824
Algar J, Flynn JP (1934) A new method for the synthesis of flavones. Proc Royal Irish Acad 42B:1–8; Oyamada T(1934) A new general method for the synthesis of flavonol derivatives. J Chem Soc Jpn 55:1256–1261
Agnihotri N, Mehta JR (2006) A highly sensitive extractive spectrophotometric determination of zirconium(IV) using 2-(2'-furyl)-3-hydroxy-4-oxo-4H-1-benzopyran. J Indian Chem Soc 83(8):846–848
Ringbom A (1938) On the accuracy of colorimetric analytical methods I. Z Anal Chem 115:332–343
Job P (1928) Formation and stability of inorganic compexes in solution. Ann Chim 9:113
Vosburgh WC, Cooper GC (1941) The identification of complex ion in solution by spectrophotometric measurements. J Am Chem Soc 63:437
Yoe JH, Jones AL (1944) Colorimetric determination of iron with disodium-1,2-dihydroxybenzene-3,5-disulfonate.Ind. Eng Chem (Anal.Ed.) 16:111
Hanwell MD, Curtis DE, lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization and analysis platform. J Cheminform 4(17)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Dhonchak, C., Kaur, N., Agnihotri, R., Berar, U., Agnihotri, N. (2021). Trace Determination of Zirconium (IV) as its 3-Hydroxy-2-[2′-(5′-Methylthienyl)]-4H-Chromen-4-One Complex and Structural Elucidation by Quantization Technique. In: Marriwala, N., Tripathi, C.C., Kumar, D., Jain, S. (eds) Mobile Radio Communications and 5G Networks. Lecture Notes in Networks and Systems, vol 140. Springer, Singapore. https://doi.org/10.1007/978-981-15-7130-5_25
Download citation
DOI: https://doi.org/10.1007/978-981-15-7130-5_25
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7129-9
Online ISBN: 978-981-15-7130-5
eBook Packages: EngineeringEngineering (R0)