Abstract
The addition of inorganic spherical nanoparticles fillers to polymers matrix enables in the enhancement in the polymer’s mechanical properties as well as the addition of new features in the polymer matrix. In the present study, ZnO/polystyrene composite was synthesized by bulk polymerization route and the hardness of composites was studied. The prepared nanocomposites were injected into a compression mold in a compression-molding machine. The prepared test specimen was subjected to Rockwell hardness tests. Further, to ensure proper morphology of nanocomposites, scanning electron microscopy (SEM) and X-ray diffraction (XRD) were carried out. Results revealed that by increasing nano-ZnO up to 0.3%, there is a remarkable increment approximately 47% in the hardness. Also, an analysis of variance (ANOVA) has been applied to find the optimal input parameter levels and to analyze the effect of these parameters on hardness. The composite produced is envisioned to have applications as the building blocks for the manufacturing of various automotive parts, sensors, transducers, actuators, UV detectors, and optoelectronic devices.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In today’s industrialization world, polymer-based composites are gaining their popularity as structural materials owing to their versatile properties. In order to enhance various properties like mechanical properties, tribological properties, and thermal stability, polymers are used to filled with nano-sized fibers, metals, and minerals; however, in some previous studies ZnO nanoparticles have been studied as reinforcements for a number of polymers such as polyacrylonitrile [1, 2], polystyrene [3, 4], polystyrene butyl acrylate [5], and polyurethane [6]. However, the degree of property enhancement relies on the polymer matrix and on the particle size, distribution, and dispersion of the nanoparticles and on the adhesion at the interface of the filler matrix. Polystyrene is a widely used polymer, and its main features include low cost, transparency, and ease of processing. Due to its unique characteristics, polystyrene is to be used in a broad spectrum of applications area [7]. Polystyrene finds its applications in various industrial sectors that include electronics appliances, insulation of building and constructions, food utensils, and packaging [8]. The nano-sized zinc oxide (ZnO) has offered great attention as one of the multitask inorganic nanofillers due to its highly significant suitability like infrared adsorption, increased chemical stability, and low dielectric constant [9].
The main issue faced during processing or fabrication of polymer nanocomposites is to disperse the nano-sized fillers in a polymer matrix homogeneously [10]. The nano-sized fillers dispersion originates a greater interface area, and hence, nanocomposites got differentiated from traditional composites and filled plastics. Traditionally, there are usually three methods to fabricate polymer nanocomposites. They are melt intercalation, solution methods, and in situ polymerization. Hardness is purely a relative term, and hence, hardness of materials is impressed in number scale; so, it has no unit. Various evaluation approaches are developed to determine the hardness. Various types of hardness tests are the Rockwell hardness test, Durometer (shore) hardness test, Brinell hardness test, Knoop hardness scale, and Vickers scale.
The objectives of the present work are to synthesize new PS/ZnO nanocomposites using in situ polymerization methods and to analyze the influence of ZnO nanoparticles on the mechanical behavior, i.e., hardness of prepared composites and to characterize the prepared composites using SEM and XRD. The enhanced hardness nanocomposites are expected to be used in various fields such as field emission display, UV shielding, photocatalysis, and gas sensing.
2 Experimental Procedure
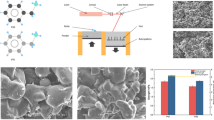
Prior to the fabrication of the nanocomposites, neat polystyrene samples were synthesized at 85 °C with the addition of initiator, namely benzoyl peroxide. The processing time for the polymerization is approximately 120 min, and the level of initiator was taken as 0.40% by weight. The experimental environment was chosen in order to get polystyrene with a melt flow index (MFI) value that is similar to commercially available polystyrene. The synthesis was done using polymerization (in situ) methods which include dispersing the nano-ZnO in monomer styrene and followed up by polymerization (free radical) process initialized by the addition of benzoyl peroxide (BPO). The monomer (styrene) with a suitable amount of ZnO was mixed (mechanically) with stirrer for half an hour. The mixture obtained is further gone in an ultrasonic bath for half an hour in order to obtain better dispersion. The benzoyl peroxide was added to the prepared mixture to initiate the process (polymerization) at 95 °C for 180 min. The composites finally obtained were molded into the molds made up of aluminum and were kept in an oven at 110 °C to accomplish the process.
2.1 Sample Preparation and Testing
In order to prepare the specimens for hardness testing of the synthesized nanocomposite, synthesized composites are compression-molded between two compression-molding plates at a temperature of 160 °C. Before starting compression mold, the PS composite chunks and gauge are heated up to 160 °C. After this, pressure was applied manually in steps by a hand lever projected in the hydraulic press. The full pressure was applied for a minimum of 2 min. When the last step has taken place, the plates were cooled down to room temperature by giving the water bath. Then the compressed sheet of PS composite was taken out from the gauge. Ten sheets were prepared in a similar fashion. The capacity of this hydraulic press was 20 Ton.
2.1.1 Hardness Test Procedure
In Rockwell hardness test for all samples, M scale indenter is used and 100 kgf load is applied. Rockwell hardness test is most commonly used for relatively hard plastics. ASTM D785, standard test method for Rockwell hardness of plastics and electrical insulating materials was adopted for the Rockwell hardness test. ASTM D785 was adopted, and the test was carried out at a controlled atmosphere of 23 ± 2 °C and 50 ± 5% RH. A test scale preselected from the table was taken for measurement. This test was based on ASTM D785 according to the material. Accordingly, the major load was selected and was set, and the indenter was fitted. The test sample was placed on the anvil and minor load was applied by lowering the steel ball indenter onto the surface of the specimen. Minor load ensures good contact of indenter and test specimen. The dial of equipment was set to zero position, and major load is applied within 10 s by releasing the trip leaver. After 15 s, major load is removed and the specimen is allowed to recover for another 15 s. During this period, the needle of the scale passing from zero position is counted. Rockwell hardness was calculated from the dial scale based on the difference in the number of times the needle of dial passes from zero. The Rockwell hardness was reported in number.
3 Results and Discussion
The hardness of the nanocomposites synthesized at a different speed and concentration shows a notable increment as depicted in Fig. 1. This improvement can be attributed to the uniform dispersion of nano-filler in the polymer matrix. The uniform dispersion of nano-PS is also revealed that as the speed increases, the dispersion improves. In the present work, the maximum increment in Rockwell hardness is 47.41% at 1200 rpm with only a 0.3% level of concentration which is comparable with the previous works [11].
3.1 Characterization
The XRD pattern of the ZnO nanoparticles sample is shown in Fig. 2. It can be observed from Fig. 2 that the significant reflections are between 30° and 40° (2θ values), which reveals a more crystalline regime in the zinc oxide sample. Figure 3 shows the XRD pattern of neat polystyrene. Figure 4 shows the XRD pattern of prepared composites at 1200 rpm and its comparison with base and filler materials.
The nano-ZnO peaks in the composite pattern confirm the formation of zinc oxide dispersed PS composite and development of crystallinity in the polystyrene matrix. In this case, all the three composites peaks match with peaks of nano-ZnO, indicating the good dispersion of ZnO in the matrix.
Peak details of ZnO nanopowder obtained by powderx software are summarized in Table 1.
3.1.1 Scanning Electron Microscopy
The scanning electron microscope (SEM) is one of the most flexible instruments for the assessment and investigation of the structural qualities of materials. The crack system of a polymer is a significant subject of intrigue identified with the structure of the material. The initiation of cracks and voids occurs in the weak region when a point load is applied on the surface of the polymer or composites. These voids can be detected by small angle X-ray scattering when they reach a size of about 20 to several hundred Angstrom units, and the structure can be observed directly by SEM. SEM micrographs for nano-ZnO and nanocomposites are shown in Fig. 5a, b, respectively.
3.2 ANOVA Analysis
The two-way ANOVA table for hardness against speed and concentration generated by MINITAB 15 is summarized in Table 2. From the table, it can be inferred that contribution of concentration is 19.93%, and while the contribution of speed is 76.25%, it means that the effect of concentration is more on tensile strength as compared to speed. Their contribution to error is 3.82%. The more the error, lesser will be the simultaneous effect of input parameter on the output parameter. The main effects plot and interaction effects plot are shown in Fig. 6. The concentration and stirring speed have an effect of 19.93% and 76.25%, respectively, as analyzed by ANOVA.
4 Conclusions
This research is focused on an experimental investigation of the effect of nano-ZnO and stirrer speed on the Rockwell hardness of prepared polymer nanocomposites. Polystyrene (PS)–zinc oxide (ZnO) nanocomposites were synthesized at three different concentrations and different stirrer speeds by in situ polymerization. Concentration and speed of stirring during polymerization are important parameters for the enhancement of mechanical properties. Nano-ZnO gives crystalline nature to nanocomposites. The optimum ZnO content to improve Rockwell hardness is 0.3%. At this ZnO content, the increment in Rockwell hardness is 47.41%. The dispersion of nanopowder enhances the crystallinity of PS. ANOVA results exhibit that in the case of Rockwell hardness, the concentration and stirring speed have an effect of 19.93% and 76.25%, respectively.
References
Tang J, Wang Y, Liu H, Xia Y, Schneider B (2003) Effect of processing on morphological structure of polyacrylonitrile matrix nano-ZnO composites. J Appl Polym Sci 90(4):1053–1057
Chae DW, Kim BC (2006) Effects of zinc oxide nanoparticles on the physical properties of polyacrylonitrile. J Appl Polym Sci 99(4):1854–1858
Ma CCM, Chen YJ, Kuan HC (2006) Polystyrene nanocomposite materials—preparation, mechanical, electrical and thermal properties, and morphology. J Appl Polym Sci 100(1):508–515
Hooda A, Goyat M, Gupta R, Prateek M, Agrawal M, Biswas A (2017) Synthesis of nano-textured polystyrene/ZnO coatings with excellent transparency and superhydrophobicity. Mater Chem Phys 193:447–452
Xiong M, Gu G, You B, Wu L (2003) Preparation and characterization of poly (styrene butylacrylate) latex/nano-ZnO nanocomposites. J Appl Polym Sci 90(7):1923–1931
Song H-J, Zhang Z-Z, Men X-H, Luo Z-Z (2010) A study of the tribological behavior of nano-ZnO-filled polyurethane composite coatings. Wear 269(1–2):79–85
Tu Y et al (2010) Transparent and flexible thin films of ZnO-polystyrene nanocomposite for UV-shielding applications. J Mater Chem 20(8):1594–1599
Cao JP, Zhao J, Zhao X, Hu GH, Dang ZM (2013) Preparation and characterization of surface modified silicon carbide/polystyrene nanocomposites. J Appl Polym Sci 130(1):638–644
Ong CB, Ng LY, Mohammad AW (2018) A review of ZnO nanoparticles as solar photocatalysts: synthesis, mechanisms and applications. Renew Sustain Energy Rev 81:536–551
Alam MdA, Arif S, Ansari AH (2017) Mechanical and morphological study of synthesized PMMA/CaCO3 nanocomposites. In: IOP conference series: materials science and engineering, vol 225 no 1. IOP Publishing
Xu J, Pan Q, Tian Z (2000) Grain size control and gas sensing properties of ZnO gas sensor. Sens Actuators B Chem 66(1–3):277–279
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Alam, M.A. et al. (2020). Experimental Investigations on the Surface Hardness of Synthesized Polystyrene/ZnO Nanocomposites. In: Emamian, S.S., Awang, M., Yusof, F. (eds) Advances in Manufacturing Engineering. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-5753-8_32
Download citation
DOI: https://doi.org/10.1007/978-981-15-5753-8_32
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5752-1
Online ISBN: 978-981-15-5753-8
eBook Packages: EngineeringEngineering (R0)