Abstract
Regular depletion of fossil fuels urges human society to depend on renewable resources seriously and invest more on biofuels sector. Recently generation of bioethanol from algal feedstock or algal waste has been an interesting research. Unlike fossil fuels, production of bioethanol from algal feedstock or waste will take less time and expensive. In the present study, an important green alga Chlorella vulgaris (C. vulgaris) was selected for ethanol production. Chlorella vulgaris cultures were initiated under in vitro conditions using universal tris-acetate-phosphate (TAP) medium along with various concentrations and combinations of vitamins such as thiamin, biotin and cobalamin (B1, B7 and B12) to enhance the biomass in turn ethanol production. Optimal level of vitamins i.e. CV2 medium (TAP with 0.4 g/L of B1, 0.002 g/L of B7 and 0.002 g/L of B12) augmented the biomass production including lipid contents. Later all the algal feedstocks were used for production of ethanol in the company of Saccharomyces cerevisiae (S. cerevisiae) in both light and dark fermentations. Higher levels of ethanol production was achieved with the feedstock generated from CV2 medium at 48 h in dark fermentation and compared with other feedstocks as well with light fermentation yield at different time intervals. The results of the present investigation may grab the attention of investors in bioenergy sector for the production of bioethanol at commercial level from algal feedstock or algal waste.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The potential of ethanol generation from seaweed or algal feedstock or waste from algal industry is increasing regularly due to enriched sugars and lipids in algae, thereby cutting down the initial expenses (John et al. 2011). Moreover, several countries are not properly using algae and are remained as waste in coastal areas. In addition, research on renewable energy is one of the most concern issues recently due to exhausting fossil fuels. Hence, entire world is trying to invest more on alternate energy sources including production of ethanol from biological sources and their waste (Gray et al. 2006). Moreover, addition of bioethanol to conventional fuel reduces the cost and pollution led to sustainable development with respect to energy sector. Specifically, production and transportation use of the bioethanol will also reduce the greenhouse gas emissions (Hirayama et al. 1998; Balat 2009). In addition, production of biofuels from feedstock of advanced plants such as corn, sorghum and other agricultural wastes is common practice (Kim and Dale 2004; Prasad et al. 2007). Algae or fresh waste of algae is one of the best alternate sources for biofuel production in recent years apart from edible and medicinal uses (Daroch et al. 2013). Growth of algae varies depends on the species, their metabolic activities and environmental conditions. In addition, various algae possess significant carbohydrate and lipid content along with useful biomolecules (Sanchez and Cardona 2008).
Rate of biofuel production from algae or advanced plants through fermentation completely depends on quantity and quality of biomass (Wyman 1994; Hirano et al. 1997). Specifically, starch or sugar or cellulose level decides the bioethanol production. Various forms of carbohydrates such as starch, cellulose, other sugars and lipids are synthesized in algae, but these profiles are not same as in the case of land plants (Chen et al. 2013). Basic works have been done on bioethanol production using both fresh and marine algae for the improvement of output (Gfeller and Gibbs 1984; Kosaric and Velikonja 1995; Gupta et al. 2012; Hossain et al. 2015). It is a well-known fact that the addition of growth factors, vitamins and hormones improves the algal biomass in turn ethanol. In general, most of the vitamins have been found to act as coenzymes, and some also act as growth regulators (Croft et al. 2006). Among vitamins A, B, C, D, E and K, B-complex vitamins were focused more in the present study. Thiamin (vitamin B1), biotin (vitamin B7) and cobalamin (vitamin B12) were available as prototroph or auxotroph for plants including primitive algae and are involved in biosynthesis of certain amino acids, acts as co-factors in metabolic and enzymatic activities (Grossman 2016; Hansoz et al. 2018; Ruangsomboon et al. 2018). The green alga Chlorella vulgaris from chlorellaceae family lives in both fresh and marine water conditions depends on the strain with unicellular in nature (Cha et al. 2010). It is a well-known fact that phototrophic organism such as algae and their fresh waste are useful for ethanol production through fermentation process in the presence of yeast accompanier. Few works on bioethanol production using feedstock of C. vulgaris through enzymatic hydrolysis and fermentation were carried out (Ho et al. 2013; Moncada et al. 2013; Kim et al. 2014). Similarly, Salman and Mohammed Ali (2014) also noticed the improved ethanol content in this alga. But all the previous works were done without vitamin assistance. But till date, there was no information on biomass enhancement through exogenous supply of vitamin assistance in this species. For the first time, ethanol production has been carried out using various feedstocks of C. vulgaris obtained from different vitamin assisted tris-acetate-phosphate (TAP) media. Further, we also standardized the media to enhance the biomass leading to high-level production of ethanol from the feedstocks by yeast fermentation.
2 Materials and Methods
Chlorella vulgaris cultures were collected from University of Madras, Chennai, and preserved as per the standard protocols. The total glassware (Borosil, India) for media preparation was washed thoroughly using detergents/teepol (10%) solution and then cleaned with running tap water and rinsed with distilled water and kept in an oven for drying purpose (Kemi, K04.3, Ernakulam, India). Tris-acetate-phosphate (TAP) media with different doses of vitamins was prepared as per the procedure and used for in vitro culture. Various media such as CV0 (TAP without vitamins), CV1 (TAP with 0.2 g/L thiamin, 0.001 g/L biotin and 0.001 g/L cobalamin), CV2 (TAP with 0.4 g/L thiamin, 0.002 g/L biotin and 0.002 g/L cobalamin), CV3 (TAP with 0.8 g/L thiamin, 0.004 g/L biotin and 0.004 g/L cobalamin) and CV4 (TAP with 1.6 g/L thiamin, 0.008 g/L biotin and 0.008 g/L cobalamin) were designated based on the vitamin composition (Table 1). The media was adjusted to pH 7.0 using pH meter (Elico limited, India), and all the culture vessels containing media were autoclaved for 15 min at 15 lbs/in2 in an autoclave (Inlab Equipment, Madras, India). Sterilized cultures vessels were removed from the autoclave and cooled down to room temperature and finally were kept in laminar airflow chamber [(LAF) (Hitech products, Chennai, India)] for inoculation. Before going to inoculation, the LAF chamber was sterilized by switching on the ultraviolet lamp for 20 min and later smeared with 70% ethanol.
Inoculation was carried out using sterilized loops and wood sticks. At the time of inoculation, hands were cleaned with 70% frequently. All the inoculated samples were kept in the orbital shaker (Remi Elektrotechnik Limited, Vasai, India) at 120 rpm in continuous light and cultures were grown at 25 ± 1 °C. Haemocytometer was used to know the growth condition of C. vulgaris using cell count experiments. Estimation of chlorophyll was carried out by Arnon’s (1949) spectrophotometric method using UV-Visible spectrophotometer (Shimadzu UV- 1800, India) at 663 and 645 nm. Based on total chlorophyll content, all the samples were set to equal biomass using respective medium (CV0, CV1, CV2, CV3 and CV4). Further, all the algal samples were subcultured by adding 1 ml of culture into 99 ml of respective medium. Lipid contents of C. vulgaris grown in various media were estimated by method of Bligh and Dyer (1959) with minor modification. 10 ml of each algal culture were collected in 15 ml vials and centrifuge at 5000 rpm up to 10 min. Later, add 10 ml mixture of chloroform and methanol (1:1 v/v) to supernatant and kept in ultrasonication for 10 min and finally centrifuge it. Filtrate collected into the pre-weighed beaker and evaporated until the solvent evaporates completely. The weight of the remained lipid content was measured using the standard formula and expressed in g/100 g of algae (percentage).
During the early stationary growth phase, all the C. vulgaris cultures were collected and heated at 100 °C for 2 h on the hot plate and later allowed to cool at room temperature. Enzyme hydrolysis was done by adding alpha-amylase enzyme (0.6 g/L) and incubated for 90 min as mentioned by Sulfahri et al. (2011). Later cultures were centrifuged at 5000 rpm and 5 °C for 15 min. The supernatant was collected into fresh tube and filter sterilized and used for further fermentation process. Yeast (S. cerevisiae) was inoculated in yeast peptone medium (10 g/L yeast extract, 20 g/L peptone and 200 g/L glucose) and kept in the orbital shaker at 120 rpm and 27 °C for 24 h. Later, 30 ml serum vials contain 5.0 ml of algal solution along with 10% yeast cultures were used for fermentation. Vials were sealed and maintained in anaerobic conditions with the help of purging process using nitrogen gas. Fermentation was conducted at different time intervals (0, 12, 24, 48, 72 and 96 h) by rotating the vials at 27 °C and 120 rpm in an orbital shaker in both light and dark conditions. Both light and dark cultures were mixed with potassium dichromate and concentrated sulfuric acid, which were later used for test samples. Standard curve was prepared with commercially available ethanol, and final ethanol concentration was calculated by using the standard formula, and units were expressed in g/L.
3 Results and Discussion
By seeing the algal waste in coastal areas, the present work has been initiated and the results obtained were documented as follows. In the first stage, C. vulgaris cultures were initiated under in vitro conditions using various TAP media contain different concentrations and combinations of B1, B7 and B12 vitamins (Table 1). All the media used were tested to know the capacity of improvement of biomass from these C. vulgaris cultures. All the cultures in CV0, CV1, CV2, CV3 and CV4 media were grown well without any microbial contamination, and visually, there was minor differences were observed between the cultures (Fig. 1).
Variations in terms of chlorophyll (biomass) were also noticed in all the cultures grown in different media used. Among all the media tested, C. vulgaris cultures grown in CV2 (TAP with 0.4 g/L of B1, 0.002 g/L of B7 and 0.002 g/L of B12) exhibited more total chlorophyll content when compared to cultures grown in other media (Fig. 2a). In agreement with these results, Croft et al. (2006) emphasized these B vitamins roles in growth and development. Cultures grown in high concentration of vitamins (CV4) displayed less total chlorophyll content when compared to cultures grown without vitamin assistance. Similarly, lipid contents were also varied in C. vulgaris cultures grown in different media. With this experiment, also CV2 medium promotes the lipid content levels (Fig. 2b). The lowest content of lipid level was observed in C. vulgaris grown in CV4 medium. Similarly, Tandon et al. (2017) improved the microalgae productivity with exogenous help of vitamins. It is well-known fact that the inclusion of growth additives/enhancers such as vitamins improves the growth and biomass of both primitive and advanced plants which in turn lead to enhancement in biofuel production in most of the species (Croft et al. 2006; Daroch et al. 2013). To the best of our knowledge, this is the first report on addition of vitamins along with universal TAP medium exhibiting enhanced biomass production in C. vulgaris. Estimation of biomass is the crucial step in the process of ethanol production and comparative studies.
Further, these algal feedstocks obtained from various media were used to produce ethanol in the presence of S. cerevisiae under dark and light fermentation conditions. In light and dark fermentations, variation in production of ethanol was observed at different time intervals (Fig. 3). In dark fermentation, content of ethanol production was more at 48 h in feedstock of C. vulgaris grown in CV2 medium when compared to feedstocks grown in other media (Fig. 3a). Surprisingly, at 72 h, feedstock grown in CV3 medium resulted more ethanol production. Probably, this may be due to variations in cellulose, reducing sugars, lipids, etc., in this feedstock.
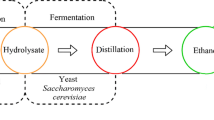
For example, if one feedstock contains xylose, which is difficult to convert into ethanol using S. cerevisiae. So, the type of yeast is also an important factor to convert sugars into ethanol by fermentation process (Bettiga et al. 2009). In light fermentation, feedstock generated from CV2 medium yielded more ethanol at 72 h, and here also CV3 feedstock produced high levels of ethanol (Fig. 3b). Previous works on ethanol production from this alga using various methods were not with vitamin assistance (Moncada et al. 2013; Kim et al. 2014; Salman and Mohammed Ali 2014). Probably, this is the one of the reasons for augmentation in ethanol production in the present investigation. Overall in dark and light fermentations, feedstock of CV2 is best for ethanol production at 48 and 72 h. High vitamin content (CV4 feedstock) and increased fermentation time (96 h) lead to reduced production of ethanol in both light and dark fermentations. Many studies were conducted on ethanol production from bacteria and yeast with the assistance of vitamins but not using algal cultures (Sato et al. 1992; Alfenore et al. 2002; Liu et al. 2018). Recently, Ruangsomboon et al. (2018) used the same combination of vitamins for the production of biodiesel from green alga Botrycoccus braaunii and succeeded. Moreover, in the present work, the standardized media, i.e., CV2 for C. vulgaris can be useful for biomass production at industrial level. Figure 4 illustrates the method developed for ethanol production from algal feedstock, and the same may also benefit for fresh algal waste in coastal areas.
4 Conclusions
Present work demonstrated that addition of optimal level of vitamins such as thiamin, biotin and cobalamin to TAP medium assisted to produce high amount of biomass in C. vulgaris. The overall content of ethanol produced from feedstock of C. vulgaris grown in CV2 medium through dark fermentation was high at 48 h when compared to feedstock of other media even in light fermentations at different time intervals. The present protocol is also useful for bioethanol production from algal waste.
References
Alfenore, S., Molina-Jouve, C., Guillouet, S., Uribelarrea, J. L., Goma, G., & Benbadis, L. (2002). Improving ethanol production and viability of Saccharomyces cerevisiae by a vitamin feeding strategy during fed-batch process. Applied Microbiology and Biotechnology, 60, 67–72.
Arnon, D. (1949). Estimation of total chlorophyll. Plant Physiology, 24, 1–15.
Balat, H. (2009). Prospects of biofuels for a sustainable energy future: A critical assessment. Energy Education Science and Technology, 24, 85–111.
Bettiga, M., Bengtsson, O., Hahn-Hägerdal, B., & Gorwa-Grauslund, M. F. (2009). Arabinose and xylose fermentation by recombinant Saccharomyces cerevisiae expressing a fungal pentose utilization pathway. Microbial Cell Factories, 24, 40.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Cha, K. H., Lee, H. J., Koo, S. Y., Song, D. G., Lee, D. U., & Pan, C. H. (2010). Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. Journal of Agricultural and Food Chemistry, 58, 793–797.
Chen, C. Y., Zhao, X. Q., Yen, H. W., Ho, S. H., Cheng, C. L., Lee, D. J., et al. (2013). Microalgae-based carbohydrates for biofuel production. Biochemical Engineering Journal, 78, 1–10.
Croft, M. T., Warren, M. J., & Smith, A. G. (2006). Algae need their vitamins. Eukaryotic Cell, 5, 1175–1183.
Daroch, M., Geng, S., & Wang, G. (2013). Recent advances in liquid biofuel production from algal feedstocks. Applied Energy, 102, 1371–1381.
Gfeller, R. P., & Gibbs, M. (1984). Fermentative metabolism of Chlamydomonas reinhardtii: I. Analysis of fermentative products from starch in dark and light. Plant Physiology, 75, 212–218.
Gray, K. A., Zhao, L., & Emptage, M. (2006). Bioethanol. Current Opinion in Chemical Biology, 10, 141–146.
Grossman, A. (2016). Nutrient acquisition: The generation of bioactive vitamin B12 by microalgae. Current Biology, 26, 319–321.
Gupta, R., Biswas, K., Mishra, I., & Suthidiran, K. (2012). Ethanol production from marine algae using yeast fermentation. Research Desk, 1, 17–22.
Hansoz, A. D., Amthor, J. S., JiayiSun, Niehaus T. D., GregoryIII, J. F., Bruner, S. D., & Ding, Y. (2018). Redesigning thiamin synthesis: Prospects and potential payoff. Plant Science, 273, 92–99.
Hirano, A., Ueda, R., Hirayama, S., & Ogushi, Y. (1997). CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy, 22, 137–142.
Hirayama, S., Ueda, R., Ogushi, Y., Hirano, A., Samejima, Y., Hon-Nami, K., et al. (1998). Ethanol production from carbon dioxide by fermentative microalgae. Studies in Surface Science and Catalysis, 114, 657–660.
Ho, S. H., Huang, S. W., Chen, C. Y., Hasunuma, T., Kondo, A., & Chang, J. S. (2013). Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresource Technology, 135, 191–198.
Hossain, M. N. B., Basu, J. K., & Mamun, M. (2015). The production of ethanol from micro-algae spirulina. Procedia Engineering, 105, 733–738.
John, R. P., Anisha, G. S., Nampoothiri, K. M., & Pandey, A. (2011). Micro and macroalgal biomass: A renewable source for bioethanol. Bioresource Technology, 102, 186–193.
Kim, K. H., Choi, I. S., Kim, H. M., & Bae, H. J. (2014). Bioethanol production from the nutrient stress-induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresource Technology, 153, 47–54.
Kim, S., & Dale, B. E. (2004). Global potential bioethanol production from wasted crops and crop residues. Biomass and Bioenergy, 26, 361–375.
Kosaric, N., & Velikonja, J. (1995). Liquid and gaseous fuels from biotechnology: Challenge and opportunities. FEMS Microbiology Reviews, 16, 111–142.
Liu, H., Sun, J., Chang, J. S., & Shukla, P. (2018). Engineering microbes for direct fermentation of cellulose to bioethanol. Critical Reviews in Biotechnology, 10, 1–17.
Moncada, J., Jaramillo, J., Higuita, J. C., Younes, C., & Cardona, C. A. (2013). Production of bioethanol using Chlorella vulgaris Cake: A techno economic and environmental assessment in the Columbian context. Industrial and Engineering Chemistry Research, 52, 16786–17794.
Prasad, S., Singh, A., & Joshi, H. C. (2007). Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resources, Conservation and Recycling, 50, 1–39.
Ruangsomboon, S., Sornchai, P., & Prachom, N. (2018). Enhanced hydrocarbon production and improved biodiesel qualities of Botryococcus braunii KMITL 5 by vitamins thiamine, biotin and cobalamin supplementation. Algal Research, 29, 159–169.
Salman, J. M., & Mohammed Ali, M. A. (2014). Bioethanol production from green alga Chlorella Vulgaris under different concentrations of nitrogen. Asian Journal of Natural and Applied Sciences, 3, 27–36.
Sanchez, O. J., & Cardona, C. A. (2008). Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresource Technology, 99, 5270–5295.
Sato, K., Goto, S., Yonemura, S., Sekine, K., Okuma, E., Takagi, Y., et al. (1992). Effect of yeast extract and vitamin B12 on ethanol production from cellulose by Clostridium thermocellum I-1-B. Applied and Environmental Microbiology, 58, 734–736.
Sulfahri, M. S., Sunarto, E., Irvansyah, M. Y., Utami, R. S., & Mangkoedihardjo, S. (2011). Ethanol production from algae Spirogyra with fermentation by Zymomonas mobilis and Saccharomyces cerevisiae. Journal of Basic and Applied Scientific Research, 1, 589–593.
Tandon, P., Jin, Q., & Huang, L. (2017). A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microbial Cell Factories, 16, 219.
Wyman, C. E. (1994). Alternative fuels from biomass and their impact on carbon dioxide accumulation. Applied Biochemistry and Biotechnology, 45, 897–915.
Acknowledgements
The authors are thankful to Prof. Mathivanan, University of Madras, Chennai, India, for algal culture and also Dr. L. V. Reddy and Dr. Veda, Department of Microbiology, Yogi Vemana University, Kadapa, India, for providing yeast and valuable suggestions in this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Varaprasad, D., Ragasudha, N., Paramesh, K., Chandramati Shankar, P., Nazaneen Parveen, S., Chandrasekhar, T. (2020). Production of Bioethanol from Green Alga Chlorella Vulgaris: An Important Approach to Utilize Algal Feedstock or Waste. In: Ghosh, S., Sen, R., Chanakya, H., Pariatamby, A. (eds) Bioresource Utilization and Bioprocess. Springer, Singapore. https://doi.org/10.1007/978-981-15-1607-8_6
Download citation
DOI: https://doi.org/10.1007/978-981-15-1607-8_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1606-1
Online ISBN: 978-981-15-1607-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)