Abstract
Virus-induced gene silencing (VIGS) is widely used to analyse the gene functions in model plants and in the plant species where generation of stable genetic transformants to downregulate gene expression is laborious and time-consuming. Plant viruses serve as a suitable candidate for understanding functional genomics by their modification as Virus Induced Gene Silencing vectors. Recent advancements in genetic engineering tools have made a significant contribution to their use as vectors. Here in this chapter, we have tried to discuss about the use of various plant viruses as gene silencing vectors and the next-generation vectors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Viruses cause numerable diseases in plants as well as in animals. They have DNA or RNA as their genetic material with a protein coat protecting it. The majority of the viruses infecting plants have RNA as their genetic material. They have simple mode of replication and virus completes its life cycle after the viral RNA translates itself and complete formation of viral particle occurs (Hull 2008). Virus utilizes host machinery for its survival and can trigger number of reactions for their movement and replication inside, leading to an array of disease symptoms by hampering plant physiology.
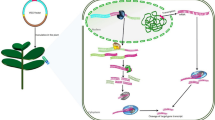
With the recent significant advancements, genome sequencing through NGS and cloning full-length viruses with diverse genetic engineering tools has become much easier. Generating infectious clones of these viruses and using them for gene silencing has now become a choice for functional genomics. VIGS is a reverse genetic strategy that can allow us to functionally annotate the genes. VIGS utilizes an RNA-mediated antiviral defence mechanism of host which silences virus encoded transcripts. The technology has been exploited with viruses amenable of carrying gene sequences that are identical to the endogenous host target gene/s to silence them and deciphering their function. The viral double-stranded RNA (dsRNA) formed during the replication of viral RNAis identified by plant’s defence machinery and the DICER proteins come into play to degrade them into small-interfering RNAs (siRNAs), which further leads to down-regulate the gene of interest via this mechanism. VIGS has been successfully used in cases where genetic transformation was not successful. Plant viruses serve an important VIGS vectors because they can lead to systemic infections which makes the functional annotation of genes easier. Large number of viruses have been developed and now extensively used for functional genomics. VIGS vectors are used for studying gene functions in tomato, pepper, soybean, barley, potato, pear etc. (Liu et al. 2002; Wang et al. 2013; Zhang et al. 2013; Scofield et al. 2005; Faivre-Rampant et al. 2008; Sasaki et al. 2011).
VIGS provide a most suitable way of transformation of the plant transiently. It has many advantages over any other known methods of transformation. It’s low cost and more robustness adds to its quality.
2 VIGS Technology
VIGS technology is entirely based upon the backbone of the infectious viruses clones modified using one or more mutations/modifications. There are certain properties of the virus to serve as candidate to be developed into VIGS vector. The candidate virus must have a broader host range, should amount infection with good virus titre and without obvious symptoms are few among the list. The infectious clones of the Virus genome are primary requirement to develop VIGS vectors. There are two popular methods to construct infectious clones: (1) constructing full length cDNA clone of virus and obtaining RNA transcripts from it under the influence of a bacteriophage RNA polymerase promoters like T7, T3 or SP6, and (2) The expression of infectious viral RNA’s by in vivo transcription of cDNA-containing vectors through a constitutive CaMV35S promoter. There are many viral infectious clones developed and used using these two techniques (Table 22.1). In later case, many steps of transcription can be avoided. This is particularly important for RNA viruses for which the production of good yield transcripts can be problematic. It is quite inexpensive as no RNA cap analogs or RNA polymerases are required. VIGS utilizes the plant virus genomes along with the target genes are transformed via Agrobacterium. Viral DNA under the influence of CaMV35S promoter transcribes into RNA which then gets replicated via host RNA dependent RNA polymerase enzyme leading to an event in which double stranded RNA (dsRNAs) formation occurs and which ultimately triggers RNA silencing machinery. These dsRNAs are cleaved to small interfering RNAs by the activity of DICER-like proteins (DCLs). These siRNAs are recruited by the RNA induced silencing complex (RISC), turning them into single-stranded RNAs. These single-stranded RNAs search for complementary RNA sequences of the host which upon complementation are degraded. These developed infectious clones are used to carry foreign gene inserts resembling the endogenous host gene targets which can then be silenced through RNA silencing. The first RNA virus to be used as VIGS vector was Tobacco mosaic virus (TMV; Kumagai et al. 1995). The simplest way of inoculating the virus on the test plants makes virus a subtle choice to be used as gene silencing vectors. Phytoene desaturase gene was silenced using TMV based VIGS vector in Nicotiana benthamiana by cloning of partial cDNA encoding PDS gene in viral vector(Kumagai et al. 1995), 1 week after inoculation systemically infected N. benthamiana plants. This work was first of its kind demonstrated that a viral vector can be used to silence any target gene. Tobacco rattle virus based vectors have been efficiently used to silence genes in N. benthamiana and Tomato as well and the modified version is now the choice for its use to modify model plant Arabidopsis thaliana (Liu et al. 2002; Turnage et al. 2002).
Diversified viruses have served as candidate because of certain viral properties of which small size of the virus, broader host range, absence of severe infection symptoms and its infectivity matters the most. Now there are ranges of viral vectors which have been developed for different crop systems. We are covering several important viruses with the technologies that have been used to develop them, their uses and future applications.
3 Viruses Modified in to VIGS
Over the past few years, many vectors have been developed for use in VIGS, which includes Tobacco Mosaic Virus (TMV), Potato virus X (PVX), Tomato golden mosaic virus (TGMV), Tobacco rattle virus (TRV), satellite virus-induced silencing system (SVISS), Barley stripe mosaic virus (BSMV), Apple latent spherical virus (ALSV) and Cabbage leaf curl virus (CbLCV). Selection of viral species efficiently working as VIGS vector was based upon the host they can infect. Viruses with broader host range enabled the use of them as VIGS vector for many plant species.
Tobacco Mosaic Virus (TMV)
The use of Tobacco mosaic virus as VIGS vector for silencing ChIHgene (Magnesium- chelatase gene) in N. benthamiana plants is one example of VIGS vectors. The gene which encoded the H-subunit of the magnesium-chelatase enzyme was silenced in the apical tissues, where suppression of both the TMV vector construct and the ChlH mRNA results in a strong reduction of the virus vector in the shoot apex. Lack of white and yellow phenotypes was initially observed on the growing apex, young leaves and stems, forming mosaic tissues thereby exhibiting reduced amount of ChIH mRNA in the affected tissue. Consequently, the plant apex partially recovered from the silenced phenotype leading to the increase in levels of both the ChlH mRNA and the TMV RNA and therefore, the silenced phenotype was re-established. Although the fluctuation of the silenced and recovered phenotypes appeared to be regulated in a feedback loop by a reduction and increase of the target mRNA and viral RNA levels, it also indicated that the TMV VIGS-mediated silencing was not systemically spread in the plants. It was observed that although TMV-ChlH–mediated silencing led to strong suppression of the virus vector, it did not lead to stable recovery of the plant from virus infection or from the VIGS-mediated silencing of the target gene (Hiriart et al. 2003)
Potato Virus X (PVX)
PVX based VIGS vector is another example which triggered a VIGS response in both diploid and cultivated tetraploid Solanum species. The PVX construct with GFP insert was used to investigate the infectivity of the virus in these species. Both infiltrated and systemic upper-uninoculated leaves were harvested after agro-inoculation and the efficiency of gene silencing by binary PVX vector was assessed using RT-PCR by its ability to silence an endogenous pds gene in these species. Down-regulation of pds gene expression resulting in a characteristic photobleaching phenotype exhibits gene silencing. Photobleaching was observed on all N. benthamiana plants by 12–15 days post inoculation when the cDNA region was sub-cloned in antisense orientation into the PVX vector, which is an indicative of pds silencing. The VIGS-based approach was also studied in in vitro grown potato species by assessing the down regulation of pds in S. tuberosum L. cvs Desiree micro propagated plants. Reproducible PVX infections were generated after the leaves of these plants were stab-agro inoculated with plated agrobacteria transformed with either PVX-GFP or PVX-PDSAS (pds anti-sense) constructs. After 4 weeks of inoculation, photobleaching phenotype was observed on systemic areas of leaves, indicating that silencing of endogenous genes (pds)in potato can potentially extend through the whole plant, including tubers. Although this vector is more stable than the TMV-based vector, PVX has a more limited host range than TMV, with only three plant families having members that are susceptible to PVX infection. Also, both TMV and PVX-based vectors can cause viral disease symptoms on inoculated plants thereby interfering with gene silencing effects (Ratcliff et al. 2001).
Apple Latent Spherical Virus (ALSV)
The use of ALSV as VIGS vector has overcome the limitations of viral symptoms which often interfere the silencing of target genes as seen mostly in soybean (Glycine max). The viral symptoms because of some VIGS vector include necrosis, chlorosis and leaf distortion along with limited movement within the leaf tissue causing uneven phenotypes. ALSV, isolated from apple (Malus pumila) in Japan, has recently emerged as an efficient candidate for reverse-genetic tool of VIGS (Kasajima et al. 2017). The ALSV cDNA was mobilized into a binary vector compatible with Agrobacterium tumefaciens-mediated delivery into Nicotiana benthamiana leaves by the process of agro-infiltration. This inoculation process upon modification by using infected N. benthamiana homogenate to directly rub-inoculate the first unifoliate of young soybean seedlings have proved to be an improved method as it bypassed the need for particle bombardment along with rapid propagation of inoculum (5–10 days). The result was evaluated in 19 soybean genotypes among which photo-bleaching indicative of Phytoene desaturase gene (GmPDS1), silencing was observed in nine and two of them exhibiting photobleaching in 100% of the inoculated individuals (Gedling et al. 2018). The virus is serving as a very good candidate as by now the infection of the virus can be eliminated from infected plants by thermotherapy.
Sugarcane Mosaic Virus (SCMV)
SCMV based VIGS vector is another potential vector to knock down the expression of endogenous and exogenous genes in Nicotiana benthamiana. In the experiment conducted by Ali et al. (2017), SCMV-VIGS vector construct was developed by replacing the RNA 1 and the two non-structural proteins of the RNA 2 with SCMV CP gene segment. This construct was cloned into an agrobacterium-mediated binary vector and were transformed by electroporation into Agrobacterium tumefaciens strain. Inoculation of SCMV based VIGS construct in N. benthamiana leaves was done using a needleless syringe above the cotyledon. Semi-quantitative RT-PCR analysis was done to evaluate the level of the target gene. Successful post-transcriptional gene-silencing (PTGS) of the target genes, GFP and ChlI was observed. The ChlI gene is responsible for carotenoid biosynthesis when silenced causes photobleaching and characteristic loss of chlorophyll as a result of reduced carotenoid level and initiation of photo-oxidation. The inoculated plants exhibited more than 95% reduction in the transcript levels which confirmed the capability of this vector of endogenous gene silencing. Also, silencing of GFP causes phenotypic changes in the plants resulting in a change of colour from green to red under UV illumination due to chlorophyll autofluorescence. The inoculated plants were observed to successfully maintain the gene silencing for a couple of years as well as in the next progeny. Hence, SCMV-VIGS vector with its ability to exhibit visual phenotypic changes makes it a potential candidate for systemic gene silencing in plants.
Barley Stripe Mosaic Virus (BSMV)
Until the 90s, VIGS was limited to dicot plant species. But with the advancement in the studies of genetic engineering and plant virology, gene silencing could also be achieved even in monocots. One such example was the use of BSMV based VIGS vector to knock down the expression of Phytoene desaturase endogenous gene (PDS) in barley, a monocot host. Holzberg et al. (2002) designed the BSMV based vector construct to express a pds fragment in both the presence and absence of coat protein. The constructs having three RNA transcripts (RNA a, RNA b and GFP-anti sense) were inoculated in barley pants. The spread of virus was monitored at regular intervals post inoculation. The appearance of mosaic symptoms progressed between 8 and 10 days where the barley leaves showed white streaks and patches lacking necrosis. The degree of photo-bleaching exhibited by the plants was assessed to evaluate the gene silencing by this vector construct. Holzberg and his team also inoculated the BSMV-bPDS construct in four different plant species (Hordeum vulgare, Oryzae sativa, Zea mays and Nicotiana benthamiana), which has its coat protein deleted. On regular monitoring of the effects of inoculation, it was observed that the infected plants showed consistent photo-bleaching, except for N. benthamiana suggesting that coat protein deletion caused a significant gene silencing of the PDS endogenous gene. The nPDS fragment expressed by the viruses exhibited neither visual change in phenotype nor any accumulation of phytoene, indicating that N. benthamiana having the least homology to barley species cannot silence barley PDS. BSMV-based VIGS vector could be a powerful tool to study gene silencing in monocot species.
Tobacco Rattle Virus (TRV)
TRV uses two vector systems both of which are required for infection. Ratcliff F. and his teammates in 2001 assessed the potential of TRV to silence gene by infiltrating 3-weeks-old N. benthamiana leaves with agrobacterium pBINTRA6 and pTV00 using needleless syringe. The accumulation of virus RNA was monitored regularly post inoculation. The affected tissues such as leaves, stems, shoots and roots showed loss of green fluorescence and exhibited red phenotypic change under UV illumination, suggesting the silencing of GFP gene. TRV has an upper advantage from other viruses such as PVX as the effect of gene silencing is persistent affecting more number of tissues with greater reduction in accumulation of mRNA. Although TRV is able to infect growing points and the spread of infection is more uniform by cell division and transport, this vector induces very mild symptoms, making it a potential VIGS vector for gene silencing (Valentine et al. 2004). TRV has been successfully used as VIGS vector in N. benthamiana, tomato, chilli pepper and rose (Senthil-Kumar et al. 2007; Li et al. 2013; Choi and Hwang 2012; Dai et al. 2012).
Tobacco Ringspot Virus (TRSV)
Recently, TRSV-based vectors were developed for efficient VIGS in N. benthamiana, A. thaliana, legumes and cucurbits. In the first advancement, the GFP gene was inserted between the movement protein and coat protein region at MP-CP cleavage site for release of GFP. Efficient VIGS of phytoene desaturase (PDS) in plants demonstrated the use of TRSV as VIGS vector (Zhao et al. 2016).
4 Next-Generation Vectors and Limitations
The current VIGS approach and system has undergone several improvements for functional characterization of the host genes. The current system involving two RNAs of TRV for successful infection was reduced to single RNA (RNA1) component of the virus by removing 16 K cysteine rich protein (Deng et al. 2013). The protein has role in pathogenicity and removal of which created space for cloning new genes to be silenced. Some VIGS vectors have now been developed to target transcriptional level of silencing the gene for which the endogenous targeted gene promoters are cloned and expressed via VIGS which facilitates RNA directed DNA methylation resulting in gene silencing (Kanazawa et al. 2011). A modified virus vector has been developed for the expression of artificial micro RNAs (miRNAs) in plants (Tang et al. 2010).
VIGS utilizes the post-transcriptional gene silencing (PTGS) machinery of host plants to down-regulated the expression of the targeted gene by degrading the targeted plant gene expression along with the viral RNAs. However, the complete elimination of the transcripts of desired gene is not achievable by this method. Therefore, attempts were also made to develop an easy and efficient technique that causes silencing of the targeted gene and they can be good replacement of the VIGS vectors. DNA viruses such as bean yellow dwarf virus (BYDV); wheat dwarf virus (WDV) and cabbage leaf curl virus (CaLCV) and RNA virus as tobacco rattle virus (TRV), cucumber mosaic virus (CMV) or tobacco mosaic virus (TMV) have been modified successfully in transient gRNA delivery vectors and demonstrated efficient gene targeting N.benthamiana, potato, tomato, rice, and wheat (Zaidi and Mansoor 2017). Yin et al. (2015) has developed a Cabbage leaf curl virus (CaLCuV)-based guide RNA delivery system for CRISPR/Cas9 mediated plant genome editing (VIGE) and demonstrated the powerful silencing of NbPDS3 and NbIspH genes which cause photo-bleached phenotype on leaves. These advancements in techniques offered an excellent chance to directly manipulate plant genomes. With recent advancements in the VIGS approach, the deliverance of the viral vectors still remains as a challenge and needs further improvements.
5 Conclusions
With the advancements in NGS technologies, genome sequencing has become much easier. Many genome databases of different plant species are now available. But the sequences remain as text until they have a function annotated to them. With large sized eukaryotic genomes, the functional annotation is a cumbersome and laborious task. With the development of genetic engineering tools, things have become much easier via development of these VIGS vectors. For instance, earlier amplifying and cloning the complete genome of viruses in an instance was difficult. But with the availability of advanced Taq and cloning tools, things have become much easier.
Different plant viruses have been utilized as VIGS vector for different plant species they can infect. The versatility of the VIGS based vectors lie in the fact that now many different plant species which do not serve as host for virus naturally, can be infected by viral VIGS vectors. VIGS approach is a promising tool and will be used widely. The concern regarding certain viruses which do not have infections in different parts of the world can be overcome by developing more virus based VIGS vectors. Virus based vectors have not only been used to silence endogenous genes but also as expression vectors which will be discussed elsewhere.
References
Ali, S., Nasir, I. A., Rafiq, M., Butt, S. J., Ihsan, F., Rao, A. Q., & Husnain, T. (2017). Sugarcane mosaic virus-based gene silencing in Nicotiana benthamiana. Iranian Journal of Biotechnology, 15(4), e1536.
Choi, H. W., & Hwang, B. K. (2012). The pepper extracellular peroxidase CaPO2 is required for salt, drought and oxidative stress tolerance as well as resistance to fungal pathogens. Planta, 235, 1369–1382. https://doi.org/10.1007/s00425-011-1580-z.
Dai, F., Zhang, C., Jiang, X., Kang, M., Yin, X., Lü, P., et al. (2012). RhNAC2 and RhEXPA4 are involved in the regulation of dehydration tolerance during the expansion of rose petals. Plant Physiology, 160, 2064–2082. https://doi.org/10.1104/pp.112.207720.
Deng, X., Kelloniemi, J., Haikonen, T., Vuorinen, A. L., Elomaa, P., Teeri, T. H., et al. (2013). Modification of Tobacco rattle virus RNA1 to serve as a VIGS vector reveals that the 29K movement protein is an RNA silencing suppressor of the virus. Molecular Plant-Microbe Interactions, 26, 503–514. https://doi.org/10.1094/MPMI-12-12-0280-R.
Faivre-Rampant, O., Thomas, J., Allegre, M., Morel, J., Tharreau, D., Notteghem, J., Lebrun, M., Schaffrath, U., & Piffanelli, P. (2008). Characterization of the model system rice-Magnaporthe for the study of non host resistance in cereals. New Phytologist, 180, 592–605. https://doi.org/10.1111/j.1469-8137.2008.02621.x.
Gedling, C. R., Ali, E. M., Gunadi, A., et al. (2018). Improved apple latent spherical virus-induced gene silencing in multiple soybean genotypes through direct inoculation of agro-infiltrated Nicotiana benthamiana extract. Plant Methods, 14, 19. https://doi.org/10.1186/s13007-018-0286-7.
Hiriart, J. B., Aro, E. M., & Lehto, K. (2003). Dynamics of the VIGS-mediated chimeric silencing of the Nicotiana benthamiana ChlH gene and of the Tobacco mosaic virus vector. Molecular Plant-Microbe Interactions, 16, 99–106. https://doi.org/10.1094/MPMI.2003.16.2.99.
Holzberg, S., Brosio, P., Gross, C., & Pogue, G. P. (2002). Barley stripe mosaic virus-induced gene silencing in a monocot plant. The Plant Journal, 30, 315–327.
Kanazawa, A., Inaba, J., Kasai, M., Shimura, H., & Masuta, C. (2011). RNA-mediated epigenetic modifications of an endogenous gene targeted by a viral vector: A potent gene silencing system to produce a plant that does not carry a transgene but has altered traits. Plant Signaling & Behavior, 6, 1090–1093. https://doi.org/10.4161/psb.6.8.16046.
Kasajima, I., Ohtsubo, N., & Sasaki, K. (2017). Combination of Cyclamen persicum Mill. floral gene promoters and chimeric repressors for the modification of ornamental traits in Toreniafournieri Lind. Horticulture Research, 4, 17008. https://doi.org/10.1038/hortres.2017.8.
Kumagai, M. H., Donson, J., della-Cioppa, G., Harvey, D., Hanley, K., & Grill, L. K. (1995). Cytoplasmic inhibition of carotenoid biosynthesis with viral derived RNA. Proceedings of the National Academy of Sciences of the United States of America, 92, 1679–1683.
Li, C., Yan, J. M., Li, Y. Z., Zhang, Z. C., Wang, Q. L., & Liang, Y. (2013). Silencing the SpMPK1, SpMPK2, and SpMPK3 genes in tomato reduces abscisic acid-mediated drought tolerance. International Journal of Molecular Sciences, 14, 21983–21996. https://doi.org/10.3390/ijms141121983.
Liu, Y., Schiff, M., & Kumar, S. P. (2002). Virus-induced gene silencing in tomato. The Plant Journal, 31(6), 777–786.
Ratcliff, F., Martin-Hernandez, A. M., & Baulcombe, D. C. (2001). Technical advance. Tobacco rattle virus as a vector for analysis of gene function by silencing. The Plant Journal, 25, 237–245. https://doi.org/10.1046/j.0960-7412.2000.00942.x.
Roger, H. (2008). Comparative plant virology (2nd ed.). Amsterdam: Elsevier Publications.
Sasaki, S., Yamagishi, N., & Yoshikawa, N. (2011). Efficient virus-induced gene silencing in apple, pear and Japanese pear using Apple latent spherical virus vectors. Plant Methods, 7, 15.
Scofield, S. R., Huang, L., Brandt, A. S., & Gill, B. S. (2005). Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway. Plant Physiology, 138, 2165–2173.
Senthil-Kumar, M., Govind, G., Kang, L., Mysore, K. S., & Udayakumar, M. (2007). Functional characterization of Nicotiana benthamiana homologs of peanut water deficit-induced genes by virus-induced gene silencing. Planta, 225, 523–539. https://doi.org/10.1007/s00425-006-0367-0.
Tang, Y., Wang, F., Zhao, J., Xie, K., Hong, Y., & Liu, Y. (2010). Virus-based microRNA expression for gene functional analysis in plants. Plant Physiology, 153, 632–641.
Turnage, M. A., Muangsan, N., Peele, C. G., & Robertson, D. (2002). Geminivirus-based vectors for gene silencing in Arabidopsis. The Plant Journal, 30, 107–114.
Valentine, T., Shaw, J., Blok, V. C., Phillips, M. S., Oparka, K. J., & Lacomme, C. (2004). Efficient virus-induced gene silencing in roots using a modified tobacco rattle virus vector. Plant Physiology, 136, 3999–4009.
Wang, J. E., Li, D. W., Zhang, Y. L., Zhao, Q., He, Y. M., & Gong, Z. H. (2013). Defence responses of pepper (Capsicum annuum L.) infected with incompatible and compatible strains of Phytophthora capsici. European Journal of Plant Pathology, 136, 625–638. https://doi.org/10.1007/s10658-013-0193-8.
Yin, K., Han, T., Liu, G., Chen, T., Wang, Y., Yu, A. Y., et al. (2015). A geminivirus-based guide RNA delivery system for CRISPR/Cas9 mediated plant genome editing. Scientific Reports, 5, 14926.
Zaidi, S. S.-A., & Mansoor, S. (2017). Viral vectors for plant genome engineering. Frontiers in Plant Science, 8, 539. https://doi.org/10.3389/fpls.2017.00539.
Zhang, W., Zhang, L., Wang, D., Hunter, C., Voogd, N., & Joyce, K. D. (2013). A Narcissus mosaic viral vector system for protein expression and flavonoid production. Plant Methods, 9, 28.
Zhao, F., Lim, S., Igori, D., Yoo, R. H., Kwon, S.-K., & Moon, J. S. (2016). Development of tobacco ring spot virus-based vectors for foreign gene expression and virus induced gene silencing in variety of plants. Virology, 492, 166–178.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dhir, S., Srivastava, A., Yoshikawa, N., Khurana, S.M.P. (2019). Plant Viruses as Virus Induced Gene Silencing (VIGS) Vectors. In: Khurana, S., Gaur, R. (eds) Plant Biotechnology: Progress in Genomic Era. Springer, Singapore. https://doi.org/10.1007/978-981-13-8499-8_22
Download citation
DOI: https://doi.org/10.1007/978-981-13-8499-8_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8498-1
Online ISBN: 978-981-13-8499-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)