Abstract
Synthetic chlorinated organic compounds—polychlorinated biphenyls (PCBs)—have been used in several industrial applications for over 50 years and are among the most persistent classes of xenobiotic pollutants. PCBs remain in the environment for a long period due to their low reactivity and stability in harsh environmental conditions. Samples of PCBs can be analysed using chromatographic methods (gas or liquid) coupled with mass spectrometry after various pre-treatment and extraction methods. Hydrophobicity and a chemically stable nature cause them to break down very slowly under natural conditions. Catabolism by microbial enzymes is an efficient route for environmental biodegradation of PCBs, but as chlorination substitution in the biphenyl ring increases, the microbial degradation rate decreases. Different types of microbes are reported to degrade PCBs under anaerobic and/or aerobic conditions by reducing and oxidizing dechlorination mechanisms, respectively. Four main enzymes are reported for the biodegradation pathway of PCBs: biphenyl dioxygenase (bphA), dihydrodiol dehydrogenase (bphB), 2,3-dihydroxybiphenyl dioxygenase (bphC) and 2-hydroxyl-6-oxo-6-phenylhexa-2,4-dienoic acid hydrolase (bphD). Different types of bacteria are reported to successfully degrade PCBs, but only a few fungi are possible degraders in the absence of alternative carbon sources.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Polychlorinated biphenyls
- Stir-bar sorptive extraction
- Solid-phase microextraction
- Extraction syringe
- Matrix solid-phase dispersion
- Ultrasonic extraction
8.1 Introduction
The industrial boom after World War II saw a range of recalcitrant chemical compounds enter into environment, and each year over thousands of different xenobiotic chemicals are added to the market. Such compounds have poor degradability and thus accumulate in air, water and soil. Polychlorinated biphenyls (PCBs), also called polychlorobiphenyls, are among the most recalcitrant of these pollutants. They have been used for more than 50 years in different industrial applications (Abramowicz 1990). Schmidt and Schultz (1881) first reported the synthesis of PCBs; they are a group of synthetic chlorinated organic compounds in which chlorine is attached to the basic structural unit, the biphenyl, which is composed of two benzene rings. Benzene is a by-product of gasoline, which is extracted from crude oil and heated under a controlled condition to form biphenyls, which by electrophilic chlorination use chlorine gas to produce PCBs. The degree of chlorination determines the chemical and physical properties of PCBs. PCB compounds have quite low reactivity and are non-flammable; they have high electrical resistance and good insulating properties and are quite stable at high temperatures and pressures. They enter into the environment through spills, leaks and improper disposal of electrical and other equipment, where they persist for an extended period, since they are not readily degradable. Because of their chemical stability and low degradability, PCBs possess long distance mobility and are highly accumulative in organisms. For this reason, PCBs have been designated as persistent organic pollutants (POPs), together with dioxins and nine chlorine-based agricultural chemicals (such as, DDT and chlordane) with properties similar to PCBs. An international convention concerning usage regulations and disposal of POPs (The Stockholm Convention on POPs 2010) was adopted in May 2001 in order to prevent this pollution spreading globally. In year 2007, a methodology was developed by the United Nations Environment Programme (UNEP) Chemicals Branch, by which countries following its protocol released their POPs inventories as a starting point for developing interventions that would reduce or eliminate these pollutants altogether (Fiedler 2007).
Toxicity of PCBs has been reported in incidents such as the Kanemi oil poisoning syndrome, caused by PCB-contaminated edible oil (Miyata et al. 1977). These toxic contaminants have the ability to bioaccumulate in the food web. Larsson (1987) reported the bioaccumulation of PCBs from the sediments of an artificial freshwater pool containing PCB concentration of 2.7 μg/g by macroalgae Cladophora glomerata to a level of 3.6 μg/g dry in a period of 2 months. PCBs have a range of toxicity, and people exposed to high levels of them respond with skin and other conditions, such as chloracne (Ju et al. 2009) and liver damage. PCBs are recycled between air, water and soil. Their airborne deposit onto plants and aquatic sources cause PCBs to be assimilated by surrounding fauna, and since they are lipophilic in nature they accumulate in fatty biological tissue and the food web (Cohen 2010). The main source of exposure to PCBs for the general public is via consumption of food, particularly animal and dairy products. Toxicity varies depending on the specific PCB. For these reasons, the study of different methods of degradation to remove PCBs is of great significance, and microbial biodegradation plays a major role.
8.2 Chemical Properties of PCBs
PCBs are synthetic organic chemical compounds in which up to 2–10 chlorine atoms have attached to two linked benzene rings (the biphenyl) and some or all of the hydrogen atoms have been substituted by chlorine atoms. The basic chemical structure is shown in Fig. 8.1.
The chemical formula of PCBs is C12H10-nCln, where n ranges from 1 to 10. More than 209 distinct PCB congeners are reported. Lipophilicity and melting point increase with increasing degree of chlorination, whereas water solubility and vapour pressure decrease. PCBs with a lower degree of chlorination are more volatile than those with a higher degree. PCB congeners in pure form are colourless, often crystalline and have high flashpoints (170–380 °C); they are either oily liquids or solids with no discernable taste or odour. As the number of chlorines in a PCB mixture increases, flashpoint also rises, leading to less combustible substances. PCBs have low electrical conductivity, high resistance to thermal degradation and high thermal conductivity. The International Union of Pure and Applied Chemists (IUPAC) adopted the PCP numbering system proposed by Ballschmiter and Zell (1980).
8.3 Production and Application
The production of PCBs began in1929, manufactured as mixtures of 60–90 different congeners. In that year, Monsanto was the only American company to manufacture PCBs, selling them in the USA under the trade name Aroclor. An Aroclor PCB mixture contains over 100 different individual PCBs. As the number of chlorines in PCBs increases, the compound becomes more stable and thus resistant to biodegradation. PCBs produced commercially are used in transformers, printing inks, plasticizers in paints and cements, capacitors, pesticides, hydraulic fluids, lubricating and cutting oils, reactive flame retardants and sealants for caulking, wooded floor finishes, as de-dusting agents, waterproofing compounds and casting agents.
8.4 Analytical Methods to Determine PCBs
Many analytical methods for PCB analysis from different samples are approved by federal agencies and organizations such as Environmental Protection Agency (EPA), the American Public Health Association (APHA) and the National Institute for Occupational Safety and Health (NIOSH). For food and feed sample analysis, the Association of Official Analytical Chemists (AOAC) has standardized methods. Additionally, modified analytical methods are included to obtain higher sensitivity and/or to improve accuracy and precision. In general, PCB analysis methods can be categorised into: specific methods (gas and liquid chromatography coupled with mass spectrometry-MS); and non-specific methods, which includes PCB screening kits, X-ray fluorescence (XRF) spectrometry, and micro coulometric titration, which provides total PCBs. PCB analysis includes several steps as shown in Figs. 8.2 and 8.3 and as briefly reported in the following section of this chapter (Barska et al. 2005; Cranor et al. 2005; Rejczak and Tuzimski 2015; Ahmed 2003; Muir and Sverko 2006; Sanchez-Rojas et al. 2009; Silva et al. 2012; Namiesnik and Szefer 2009; National Research Council 2001; Riaz and Zamorani 1988; Tan and Chai 2011; Llompart et al. 1998; Bjoerklund et al. 2002; Halfadji et al. 2003; Criado et al. 2003).
8.4.1 Extraction
PCBs are extracted from the sample matrix by liquid/liquid extraction (LLE), solid-phase extraction (SPE), Soxhlet extraction, stir-bar sorptive extraction (SBSE), solid-phase microextraction (SPME), extraction syringe (ESy), matrix solid-phase dispersion (MSPD), ultrasonic extraction (USE), supercritical fluid extraction (SFE), microwave-assisted extraction (MAE) and automated accelerated solvent extractor. MAE has already been successfully applied in the extraction of PCBs and other semi-volatile pollutants from solid matrices and samples. Quantitative extraction of solutes from solid matrices is possible using MAE by employing microwave energy as a source of heat in closed vessels, with comparable extraction efficiency in shorter extraction time and with smaller solvent volume. Fully automated methods such as accelerated solvent extraction considerably simplify sample preparation, using less time for fast and easy extraction with lower solvent consumption. In this method, the extraction solvent is pumped into an extraction cell containing the sample, and temperature and pressure are increased. The extract is further transferred to a typical collection vial for the next step of clean-up or analysis.
Liquid-liquid extraction (LLE) is an extraction method to separate compounds based on their relative solubility in two different immiscible fluids: aqueous and non-polar organic solvent. Generally compounds get transferred from aqueous to organic phase. LLE extraction technique can be performed with a variety of apparatuses, from separatory funnels to counter-current distribution equipment. Samples for PCB analysis are first homogenized with sodium sulfate to remove the water present in the sample, and then dried overnight, followed by extraction with a suitable solvent. Non-polar solvents are used for extractions from oily matrices, and medium-polarity solvents (acetone or dichloromethane) are often combined to achieve the desired viscosity and solvency strength for the particular extraction. Stir bar sorptive extraction (SBSE) is a novel technique for sample enrichment of volatile and semi-volatile organic compounds from aqueous and gaseous media. SBSE consists of a glass-lined magnetic stirring bar covered with a thick layer of polydimethylsiloxane (PDMS). Once exposed to a sample solution, the solution’s components are enriched in the PDMS, which is subsequently removed. The sorbed compounds are then both thermally desorbed by means of a liquid and analysed by GC-MS or LC system. The technique is easy to use, with better sensitivity and accuracy, and it has been applied successfully for trace analysis from different samples.
Soxhlet extraction is one of the most frequently used of liquid-solid extraction methods, being a fairly simple and cheap method that provides good reproducibility. Worldwide, laboratories still routinely use this technique for extraction of PCBs from food matrices. The disadvantages of this extraction method are long extraction time (6–18 h), difficulty of the extraction itself, generation of dirty extracts and extensive clean up. In 1989, Pawliszyn and co-workers invented solid-phase microextraction (SPME) (Kusch 2018). SPME is a simple, efficient and solvent-free sample preparation, which boasts simplicity and low cost. Analytes could be directly extracted and concentrated to the stationary phase (such as polydimethylsiloxane (PDMS)) coated on a fused-silica optical fiber, thus integrating sampling, extraction, concentration and sample introduction in a single step. SPME reduces sample preparation time with improved detection limits. It has been routinely used in combination with gas chromatography (GC-MS) for the extraction of volatile and semi-volatile organic compounds, and with high-performance liquid chromatography (HPLC) and HPLC-MS. SPME is reported to be an ideal choice which yields relatively clean and concentrated extracts. The polarity and volatility of analytes dictates the selection of sampling method and type of chromatography technique to be used.
Ultrasonic extraction is a green, environment-friendly method of extraction. The sample is mixed with anhydrous sodium sulfate in a vessel and submerged in an appropriate organic solvent and placed in an ultrasonic bath. The mixture is extracted using ultrasonic extraction thrice, and the extract is vacuum-filtered or centrifuged. The extract is ready for final concentration, clean-up and/or analysis. The extraction efficiency depends on the solvent polarity, sample homogeneity and extraction time.
Extraction syringe (ESy) is a most recent technique for the handling of liquid samples. It combines online microporous membrane liquid-liquid extraction and GC analysis. ESy integrates sample pre-treatment, enrichment, clean-up and GC injection. In ESy, a mini flat-sheet membrane holds both sides by polypropylene (PP) plastic like a sandwich; this unit is called an ESy extraction card. A syringe pump is attached to one side of the PP plastic—the acceptor side of the ESy card—so as to impregnate and fill the acceptor side with solvent. The sample (1–3 ml) is delivered by a syringe pump to the other side of the extraction card—the donor side. Once the extraction is complete the card holder needle moves down and injects the whole extract directly into the GC system. The amount of solvent used is fairly negligible, and the sample handling does not need extra clean-up steps.
Supercritical fluid extraction (SFE) apparatus contains a stainless steel extraction cell, flash stainless steel extraction collector, cooler, CO2 pump, co-solvent pump and heater. Liquid CO2 (≥ 99.98%, UltraPure) from a cylinder is pumped at 3 × 10−3 kg/min. Extractions are generally carried out at high temperatures and pressure for 1–3 h, as controlled by proprietary software. Solid-phase extraction (SPE) is a sample preparation technique used for the extraction of analytes from liquid samples. This technique facilitates the extraction, clean-up and concentration of analytes. It is extensively used as an alternative extraction or clean-up method to LLE to determine pollutants in liquid samples. The affinity difference between an analyte and interferents for a solid phase allows the separation of the target analyte from the interferents in SPE. Matrix solid-phase dispersion (MSPD) was introduced by Barker (2007). One of the main advantages of this technique is that extraction and clean-up are done at the same time, which helps to reduce sample contamination during the process. This technique has been applied for the extraction of PCBs from different matrices, food samples, biota and environmental samples.
8.4.2 Sample Clean-Up
Different techniques are used to clean extracted sample, either singly or in combination, influenced by the selectivity and sensitivity of the final technique used to detect PCB and by the extraction method employed. Clean-up steps are necessary, since extracted tissue or sediments will contain many compounds other than PCBs; suitable clean-up is necessary to remove interference. Different techniques such as gel permeation, silica gel, florisil, activated carbon and HPLC are often used to remove interference.
Adsorption chromatography is the most commonly used clean-up method. It involves passing extracts through several adsorbent columns such as alumina, silica and florisil. These absorbents are used either separately or in combination, with different mesh sizes, activity and column type. As PCBs are stable under acidic conditions, columns may be used to remove lipid from extract using sulfuric acid or acid-impregnated silica. For sulfur-free sediment, alumina columns give a satisfactorily clean extract for GC analysis. Additional clean-up steps may be required for PCB analysis by ion-trap GC-MS. Activated florisil microcolumns have also been used to separate planar PCBs from non-planar PCBs. The column is first eluted with hexane followed by dichloromethane in order to obtain two fractions: non-planar and planar PCB congeners, respectively. The use of activated carbon and carbon dispersed on silica gel are also reported.
Dialysis is a membrane-based sample preparation technique, which has been used for sample preparation for chromatography applications. It uses a semi-permeable membrane device (SPMD) in an organic solvent phase for the separation of contaminants from lipid matrix prior to organic chemical analysis. This method is quite useful for various types of oily samples and other matrices that are difficult to clean up by traditional clean-up procedure. It is an efficient means of removal of lipids in the determination of bioaccumulative, persistent, halogenated organic compounds. Polychlorinated biphenyls with minimal lipid carryover are determined in lipid matrix. The advantage of this technique is that is has very satisfactory and highly reproducible analyte recoveries in a single operation; however, it requires a large volume of solvent. Acetonitrile-hexane partition is also commonly used to remove lipids from sample extracts, where PCBs get partitioned into the acetonitrile phase, and lipids get partitioned into the hexane phase. Acetonitrile partitioning needs 5 extractions with 10 times the volume of the lipid sample.
Gel-permeation chromatography (GPC) technique is primarily used for the analysis of biological samples. Since PCB is lipophilic in nature, tissue samples are extracted with non-polar solvent for lipid extraction. Cross-linked dextrans (e.g., Sephadex or agarose) are generally used as cross-linked polymer material in GPC columns. GPC method can be fully automated, and it is also more applicable for the isolation of unknown contaminants. GPC can also handle a large quantity of lipid in each sample when a second GPC column is added in parallel.
8.4.3 Determination
Immunoassay methods such as enzyme-linked immunosorbent assay (ELISA) are also reported to screen PCBs from sample, also known as competitive ELISA. This method is based on the ability to bind PCBs to specific binding sites on antibodies in the well plate, which requires less effort for sample clean-up. This is a less expensive, rapid method, useful for screening PCBs from environmental samples in ppm concentration. However, the method has several disadvantages that limit its application for screening PCBs.
‘Competitive ELISA’ is a technique, based on which several immunoassay test kits are commercially available to measure different organic contaminants such as: pesticides, and PCBs from environmental samples.
Gas chromatographic (GC) technique is the most often used technique for identification and quantitation of PCBs, since GC detectors possess high selectivity and sensitivity for the PCBs. Many different detectors are used to determine PCBs by gas chromatography, like electron capture detector (ECD), electrolytic conductivity detector (ELCD), flame ionization detector (FID) and mass spectrometry detector. GC coupled with FID is used much less often than ECD, having exceptional sensitivity to multiple chlorinated compounds compared with FID. Mass spectrometry has sensitivities lower than ECD, but greater selectivity for PCBs, and MS can distinguish and individually measure homologs even if they are co-elutes. Recently, use of the capillary column has made it possible to achieve even lower detection limits and better separation of individual PCBs. LC-MS is becoming an essential technique for the analysis of environmental pollutant, as its user-friendliness, productivity and high selectivity often allows simplified sample preparation. Procedures are relatively highly reproducible and comparatively simpler. However, specific care should be taken on matrix-induced ion suppression, which can be minimized by good sample preparation, good chromatographic separation and optimizing the MS-operating conditions.
8.5 Bacterial Biodegradation
Since PCBs are hydrophobic, inert and stable compounds, their natural breakdown is quite sluggish. Despite its stable nature, much research has been done to study its biodegradation. Bacterial enzymatic degradation is one of the major means of PCB removal (Seeger et al. 1997), but as chlorine substitution increases, the microbial degradation rate of PCBs in soils generally decreases (Furukawa et al. 1979). Several researchers have reported microbial degradation of PCBs in contaminated water, sediments and soils. They have. mainly reported reductive dechlorination of the PCBs leading to less chlorinated compounds, repetitively up to the non-chlorinated biphenyl molecule. Natarajan et al. (1999) reported complete mineralization of the biphenyl congener by a PCB-dechlorinating anaerobic consortium. Dechlorination is reported to occur mainly on meta- and para-chlorine positions and could consequently affect the toxic properties (Lang 1992). Aerobic metabolic pathways as well as the genes involved in biodegradation have also been described (Abramowicz 1990; Chaudhry and Chapalamadugu 1991; Mukerjee-Dhar et al. 1998). Aerobic pathway involves a biphenyl-dioxygenase, which converts PCBs to chlorinated benzoic acids and chlorocatechols (Abramowicz 1990). Bedard et al. (1987) reported the production of a 3,4-dioxygenase by Pseudomonas sp. LB400 and Alcalignes eutrophus H850, and enzymes need a high amount of oxygen to be effective. Those xenobiotic compounds could spread throughout the municipal system, thus, wastewater treatment plants could also play an important role in removal of PCBs from the environment. Due to its hydrophobic properties, it could accumulate by sorption onto sludge particles during settling processes. Microbiological treatment of the contaminated sludge could establish a conjunction point where different bacterial metabolic processes could enable minimizing the release of PCBs to local land or water bodies.
Microorganisms are known to play a key role in PCB biodegradation. Biologically, PCBs can be degraded by two major metabolic steps: anaerobic (reducing) dechlorination and aerobic (oxidising) dechlorination (Aken et al. 2009; Field and Sierra-Alvarez 2008; Vasilyeva and Strijakova 2007; Pieper 2005). Higher chlorinated PCBs are degraded anaerobically and then turned into less chlorinated congeners. These lower-chlorinated PCBs are degraded under aerobic conditions. Thus, either only aerobic or anaerobic or sequential anaerobic and aerobic conditions might also help improve or complete biodegradation (Ahmed and Focht 1973; Bedard et al. 1986; Evans et al. 1996; Furukawa and Matsumura 1976; Furukawa and Chakrabarty 1982; Furukawa 1982; Furukawa and Miyazaki 1986; Fukuda 1993; Kimbara et al. 1988). Aerobic biodegradation of PCBs by various bacteria has been reported (Abramowicz 1990; Abramowicz and Olson 1995; Bedard et al. 1987; Boyle et al. 1992; Chen and Pinatello 1997; Flanagan and May 1993; Yadav et al. 1995). Under anaerobic conditions, PCBs can be reductively dechlorinated by a variety of anaerobes (Berkaw et al. 1996; Beurskens and Stortelder 1995; Kim and Rhee 1997; Nies and Vogel 1990, 1991; Quensen III et al. 1990; Rhee et al. 1993; Tiedje et al. 1993; Williams 1994).
Biodegradation depends on the enzymes produced by microorganisms that can modify organic pollutants into less toxic forms (Dobbins 1995; McEldowney et al. 1993). The process of degradation can be carried out in two ways: as mineralisation when microorganisms use it as a source of carbon and energy; or as co-metabolism where microorganisms depend on the secondary substrate as their source of carbon and energy. For complete degradation the products of co-metabolism are further mineralised. The efficiency of biodegradation depends on several factors (Borja et al. 2005), which include: structure of pollutant; solubility; pollutant concentration; physical and environmental parameters (such as, oxygen content, temperature, intensity of light, pH and conductivity); biological parameters (such as the presence, type and number of microorganisms).
8.5.1 Anaerobic Biodegradation
Anaerobic microorganisms are suitable for the degradation of pollutants with high carbon concentration due to controlled oxygen diffusion. Anaerobic bacteria mainly attack the meta and para positions of PCBs, resulting in the formation of aerobically degradable ortho-chlorinated PCBs. Van Dortand and Bedard (1991) identified that the ortho position was also involved in dehalogenation of PCBs. This is the first experimental report to demonstrate the anaerobic ortho-dehalogenation of PCBs. In anaerobic degradation PCBs act as electron acceptors. During this process hydrogen atoms replace chlorine atoms by removal of chloride ions (R-Cl + 2e– + H+→ R-H + Cl−).
Reductive dechlorination was first observed in the upper Hudson River (Flanagan and May 1993). This process occurs in many environmental matrices like river and pond sediments and flooded soils. Reductive dechlorination of PCB is the only known process to have significant impact on complex PCB mixtures. Several anaerobic bacteria have been identified. These include Dehalococcoides ethenogenes, Desulfomonile tiedjei, Desulfitobacterium, Dehalobacter restrictus, Dehalospirillum multivorans, Desulforomonas chloroethenica ethenica and the facultative anaerobes Enterobacter strain MS-1 and Enterobacter agglomerans.
In sediments, PCB dechlorination was generally carried out by anaerobes like methanogens, sulfate-reducing bacteria (SRBs), etc. The anaerobic reductive paradechlorination of Aroclor 1242 was reported to be inhibited by the addition of eubacterium-inhibiting antibiotics. These antibiotics directly prevented the activity of fermentative bacteria and indirectly the methanogens. Because of inactive bacterial strains, there was no dechlorination and methane production. Dechlorination and methane production could only occur in the presence of substrate for methanogenic bacteria along with antibiotics. The reports suggest that methanogenic bacteria play a vital role in anaerobic reductive dechlorination of PCBs (Dingyi et al. 1995; Adrian et al. 2009; Matturro et al. 2016).
Fava et al. (2003) concluded that the biodegradation of PCBs (such as mono- and dichlorinated PCBs, and compounds similar to Aroclor 1242 and 1254) in contaminated estuarine and marine sediments, was mediated by indigenous SRBs and methanogenic bacteria. For this study three sediments were collected from Porto Marghera contaminated area of Venice Lagoon (Italy). Under anaerobic condition, the sediments were supplemented with sodium bicarbonate and sodium sulfide and monitored for dechlorination. After 6 months of incubation, the highly chlorinated biphenyls were depleted along with the cumulation of low-chlorinated biphenyls. The biologically active microbial consortia utilised sulfate, and the production of methane was also detected.
The addition of a single PCB congener also increases the meta- and ortho-dechlorination of PCBs. In a previous study, Aroclor 1260 was investigated in anaerobic slurries of estuarine sediments from Baltimore Harbor (Wu et al. 1997, 1998). The anaerobic culture was enriched by the addition of 2,3,4,5-tetrachlorobiphenyl (2,3,4,5-CB) or 2,3,5,6-tetrachlorobiphenyl (2,3,5,6-CB). After the incubation period, lag time was depleted in sediments enriched with 2,3,4,5-CB. The 2,3,5,6-CB congener also enhanced dechlorination, though not as 2,3,4,5-CB did. These results suggest that each enrichment contained different organisms with high substrate specificities. As a result, the added congeners enhanced the reductive dechlorination found. The results demonstrated that the addition of single congener also increases the anaerobic reductive dechlorination of PCB.
The genus Clostridium was effectively involved in PCB degradation. Based on the ability of dechlorinating meta and para PCBs, nine species were identified (Clostridium hydroxybenzoicum, Cl. botulinum, Cl. proteolyticum, Cl. beijerinckii, Cl. intestinalis, Cl. thermolacticum, Cl. paraputricum, Cl. cellulosi and Cl. cellobioparum). In this study the phylogenetic relationship with PCB-degrading clostridium using 16S ribosomal RNA (rRNA) genes was identified. The variations in small subunits of rRNA genes were examined using defined operational taxonomic units in samples from contaminated sediments from Lake Medina, New York. Further molecular biology analysis revealed that 75% of all the 16SrRNA clones having anaerobic para- and meta-PCB dechlorinating activity (Hou and Dutta 2000).
Dehalococcoides ethenogenes strain 195 was reported to reductively dechlorinate PCBs (Fennell et al. 2004). In marine sediment, Dehalococcoides mccartyi is also involved in reductive dechlorination of PCB. After incubation for 200 days, the decrements (higher to lower) were observed. In this process the pcbA4 and pcbA5 and, to a lesser extent, pcbA1 genes enriched under saline conditions were observed.
The major microbial biodegradation pathway of PCBs reports four specific enzymes: biphenyl dioxygenase (bphA), dihydrodiol dehydrogenase (bphB), 2,3-dihydroxybiphenyl dioxygenase (bphC) and 2-hydroxyl-6-oxo-6-phenylhexa-2,4-dienoic acid hydrolase (bphD) are sequentially reported for the oxidative degradation of PCBs into chlorobenzoates and 2-hydroxypenta-2,4-dienoate (Furukawa and Miyazaki 1986).
Desulfomonile tiedjei, strain dcB-1, was reported to gain ATP for growth by using aromatic dechlorination as its sole electron acceptor (Dolfing and Tiedje 1987; Dolfing 1990; Mohn and Tiedje 1991). This organism can be grown on hydrogen or acetic acid as its sole electron donor using the conversion of 3-chlorobenzoate to benzoate, and HC1 as its electron acceptor (Dolfing 1990; Mohn and Tiedje 1990). Their report established the basic model and underlying biochemical mechanism, which showed that microorganisms could grow using chlorinated substrates as their sole electron acceptor.
8.5.2 Aerobic Biodegradation
Aerobic transformation of PCBs is a widely known and well-studied technique. It occurs in an oxygen-rich environment. Aerobic transformation is a co-metabolic pathway, which means the microorganisms involved require an additional source of carbon apart from PCBs. The common enrichment factor for PCB-degrading bacteria is biphenyl or monochlorobiphenyls. PCB degradation involves two gene clusters: the first involves the sparsely chlorinated PCB congeners formed from anaerobic degradation of the higher congeners being degraded by co-metabolic aerobic oxidation via the 2,3-dioxygenase pathway, converting the low-chlorinated PCBs to the corresponding chlorobenzoic acids. The second involves chlorobenzoic acid, which is completely mineralised to Cl, water, CO2 and cell biomass by indigenous bacteria (Abramowicz 1995; Urbaniak 2013).
Several bacterial strains that mediate PCB degradation have been isolated and characterised; they are Pseudomonas, Alcaligenes, Burkholderia, Comamonas, Sphingomonas, Ralstonia, Cupriavidus, Achromobacter, Acidovorax, Norcardia and Acinetobacter as Gram-negative strains, and Rhodococcus, Corynebacterium and Bacillus as Gram-positive strains. Ahmed and Focht (1973) reported that two species of Achromobacter are suitable for degrading ‘dichlorobiphenyl’ and ‘monochlorobiphenyl’ to ‘chlorobenzoic acid’. Clark et al. (1979) described how the presence of acetate (co-substrate) can increase the co-metabolism of PCB. In their study, three different enriched mixed cultures—Alcaligenes odorans, Alcaligenes denitrificans and an unidentified bacterium—were reported. Several researchers (Abramowicz 1990; Arnett et al. 2000; Bedard et al. 1987; Billingsley et al. 1997; Furukawa 1982) summarised the correlation between PCB structures and microbial breakdown as follows:
-
1.
Low-chlorinated PCB congeners are more easily degraded. As chlorine substitution increases, the degradation rate of PCBs decreases.
-
2.
PCBs containing two chlorines in the ortho-position of a single ring (i.e., 2,6-) and in each ring (i.e., 2,2′) are poorly degraded.
-
3.
PCBs containing all chlorines on a single ring are degraded much more easily than those containing the same number on both rings.
-
4.
The bacterial strains involved are responsible for both the relative rate of primary degradation and the appropriate ring attacked.
-
5.
PCBs having two chlorines at the 2,3-position of one ring are more susceptible to microbial attack than tetra- and penta-chlorobiphenyls. This series of PCBs could be metabolised through an alternative pathway.
-
6.
Initial dioxygenation followed by ring cleavage of the biphenyl molecule occurs with a non-chlorinated or less chlorinated ring.
Degradation of PCB by Alcaligenes xylosoxidans, P. stutzeri, Ochrobactrum anthropi and P. veronii were found to be more efficient with the addition of glucose and biphenyls (Murinova et al. 2014). The addition of glucose alone increases the degradability of PCBs more than the addition of biphenyl. The addition of biphenyl only increases the degradation of highly chlorinated PCBs for some strains. Among other strains Alcaligenes xylosoxidans, was reported to have highest degradability with the addition of glucose.
Generally, bacterial strains with PCB-degrading ability degrade congeners having five or fewer chlorine substitutions and very low or no degrading ability on higher chlorinated PCBs, but Enterobacter (LY 402), a Gram-negative bacterium, could effectively degrade both the higher and lower chlorinated PCBs under aerobic conditions (Cao et al. 2011). Enterobacter (LY 402) strain has the ability to transform 92% of the penta-CBs, 76% of the hexa-CBs and 37% of the hepta-CBs, and it also degrades some of the octa-CBs. On the other hand, the strain LY402 also showed an ability to degrade both ortho- and para-chlorinated congeners. LY402 was able to degrade 97% of 2,3,5,6,2′,5′-CB (ortho-) and 46% of 2,3,4,5,2′,3′,5′-CB (ortho-); 89% of 2,4,5,2′,4′,5′-CB (para-) and 21% of 2,3,4,6,2′,3′,4′-CB (para-); and 73% of 3,4,3′,4′-CB. LY 402 strain also has the ability to degrade the intermediate metabolites (2-CBA and 2,3-CBA) involved in of PCB degradation. Strain LY402 also found to be effective in degrading complicated PCB mixtures, Aroclors 1242, 1254 and 1260. LY402 strain was found to have a stronger PCB transformation activity from the environment.
Metabolism of several types of chlorobiphenyls by the enzymes of the upper biphenyl catabolic pathway encoded by the bph locus of Pseudomonas sp. strain LB400 (Seeger et al. 1995) was reported by using recombinant strains harboring gene cassettes containing ‘bphABC’ or ‘bphABCD’. The enzymes of the upper pathway were generally able to metabolise mono- and dichlorinated biphenyls, but they could only partially transform most trichlorinated congeners.
The strong and unique ability of the transformation of PCB by Rhodococcus species (Strain RHA1) have been identified. The RHA1 strain extensively degrades highly chlorinated congeners, including hepta-chlorobiphenyl. Intermediate metabolites di- and trichlorobenzoic acids were also identified. During incubation, the RHA1 strain gradually degraded these chlorobenzoic acids. Compared with Pseudomonas sp. LB400 and P. pseudoalcaligenes KF707, RHA1 strain exhibited a good transformation activity on both ortho and para positions. The degrading activity was a superior characteristic of RHA1 compared with previously reported strong PCB degraders (Masai et al. 1995; Seto et al. 1995).
Pseudomonas CH07, a marine mercury-resistant bacterium, was able to degrade a variety of highly chlorinated biphenyls from the technical mixture Clophen A-50 (De et al. 2006). Devrukhkar et al. (2017) reported that the benzoate-enriched P. mendocina strain CL-10.4 also has the ability to completely degrade the commercial mixture of PCB-Aroclor 1242.
Based on the examination of 36 pure isomers of PCB, 23 compounds were metabolised by Alcaligenes species Y 42 (Furukawa et al. 1979), and 33 species were metabolised by Acinobacter sp. strain P6. The Alcaligenes sp. converted 2,4,6-trichlorobiphenyl, and Acinobacter converted 2,4,2′,4′- and 3,4,3′,4′-tetrachlorobiphenyls to chlorobenzoic acid, subsequently. The meta cleavage products of PCB were accumulated through the oxidative route of 2,4′-, 2,4,4′- and 2,5,4′-chlorobiphenyls. Those groups of PCBs were chlorinated at the 2,4′-position. The dihydroxy compounds are accumulated by Acinobacter species. Alcaligenes sp. was not capable of metabolising this group of PCBs. PCBs that possess chlorine atoms at the 2,3-position in the molecule were alternatively metabolised. In this reaction, two metabolites were produced and accumulated. One of the products was chlorinated benzoic acid and another is not yet identified. Trichlorobiphenyl was also metabolised by different methods by two organisms. Alcaligenes metabolise very slowly compared to Acinobacter species. In this study it was proved that chlorine substitution also affected the metabolic behaviour of PCB. Tu et al. (2011) investigated rhizobia, nitrogen-fixing bacteria also as an emerging candidate to degrade PCB. Sinorhizobium meliloti, a rhizobial strain, played a key role in the bioremediation of contaminated soil.
8.6 Fungal Biodegradation
Apart from bacteria, fungi also participated in the degradation of PCB. Fungi were rarely reported as PCB degraders in the absence of an alternative carbon source; however, the growth capability on biphenyl has been considered an indicator of the ability to degrade PCBs. Only a little was known about fungal degradation of PCBs, regardless of fungus’ ability to biodegrade a variety of aromatic pollutants, and a few species have been identified which were already involved in the degradation of aromatic pollutants. These include Phanerochaete chrysosporium, Trametes versicolor, Lentinus edodes, Pleurotus ostreatus, Grifola frondosa and Coriolopsis polyzona. In contrast, mitosporic fungi have been rarely reported for PCB biodegradation. In liquid mineral medium, the removal of 2-chlorobiphenyl, 4,4′-dichlorobiphenyl and 2,2′,5,5′-tetrachlorobiphenyl by six selected fungal species (Aspergillus fumigatus, Penicillium chrysogenum, P. digitatum, Fusarium solani and two strains of Scedosporium apiospermum) was reported. PCB biosorption abilities of six fungi were different: where A. fumigatus was the least efficient PCB-removing strain, and the P. chrysogenum strain potentially degraded three PCB congeners with high efficiency. High-chlorinated PCB biodegradation (up to 48%) was also displayed by the two strains of S. apiospermum. The strains P. digitatum and F. solani showed moderate degradation (up to 24%). These strains degrade the highly chlorinated and most recalcitrant biphenyl 2,2′,5,5′-tetrachlorobiphenyl; however, possible routes of biodegradation were not available (Krcmar et al. 1999; Ruiz-Aguliar et al. 2002; Kamei et al. 2006; Takagi et al. 2007; Rabinovich et al. 2004; Singh 2006; Novotny et al. 1997; Seto et al. 1999; Kubatova et al. 2001; Verdin et al. 2004; Sietmann et al. 2006; Mancera-Lopez et al. 2008).
Phanerochaete chrysosporium was the first white-rot fungus reported to degrade a wide range of PCB congeners. The degradation of three PCB congeners (4,4′-dichlorobiphenyl [DCB], 3,3′,4,4′-tetrachlorobiphenyl, and 2,2′,4,4′,5,5′-hexachlorobiphenyl) by P. chrysosporium in liquid culture were reported. The dichlorobiphenyl mineralised in considerable volume (11%) as compared to tetra and hexachlorobiphenyl. Intermediates like 4-CBA and 4-CBAlc were identified from the degradation of 4,4′-DCB by P. chrysosporium. This is the first report providing information about the intermediates in PCB degradation by white-rot fungus. Based on the degree of chlorination and the PCB metabolism pattern and also the intermediate characteristics, similarities were described between P. chrysosporium and bacterial systems (Dietrich et al. 1995). In the presence of carbon sources and nitrate as a nitrogen source (non-white-rot conditions), the Phanerochaete chrysosporium also able to degrade 2,2′,4,4′,5,5′-hexa-CB by the enzyme nitrate reductase were also determined.
The degradation of lower chlorinated biphenyls by six white-rot fungi was reported by Kubatova et al. (2001). P. chrysosporium and T. versicolor showed no PCB degradation in soil. In contrast, the four strains of P. ostreatus removed 40% of Delor 103 (the commercial mixture of PCB) in 2 months. The rate of degradation decreased with increasing chlorination.
Some studies showed that the filamentous fungi can also degrade PCBs. Their hyphal structure (fungal highways) can easily penetrate environmental matrices. The oxidative enzymes produced by white-rot fungi can degrade a wide range of aromatic organo-pollutants. In extracellular environment, this non-specific, radical-based system of fungi is active, easily penetrating a contaminated matrix and also poorly bioaccessible pollutants (Stellaa et al. 2017). PCB-degrading abilities of two white-rot fungi (Pleurotus ostreatus and Irpex lacteus) in real contaminated soil were investigated. A total of 18.5–50.5% removal was observed after 12 weeks of treatment. Numerous transformation products were also detected, which also indicates that both fungi were able to degrade PCBs in soil. Compared to I. lacteus species, P. ostreatus was more efficient for degrading PCBs. These results demonstrated the capability of using this fungus for possible bioremediation applications.
In another study, three white-rot fungi, T. versicolor, P. chrysosporium and L. edodes, utilized to degrade a mixture of PCBs in the presence of non-ionic surfactant (Tween-80) were examined. Compared with other surfactants, Tween-80 had no inhibitory effect on the growth of fungi. Apart from T. versicolor and L. edodes, P. chrysosporium was the most effective degrader. In contrast to T. versicolor, which degrades both low- and high-chlorinated PCB congeners, P. chrysosporium and L. edodes accumulated low-chlorinated congeners (Ruiz-Aguliar et al. 2002).
8.7 Biochemical Pathway
8.7.1 Aerobic Pathway
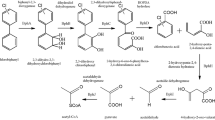
Aerobic degradation of PCB is reported to contain two pathways (Hofer et al. 1994; Seeger et al. 1995; McKay et al. 1997, 2003; Hulsmeyer et al. 1998; Sakai et al. 2002; Taguchi et al. 2001): the biphenyl upper pathway and the biphenyl lower pathway. In general, the lower-chlorinated PCB congeners are more easily transformed than higher-chlorinated PCB congeners; also, chlorines present in one aromatic ring are more easily degraded than chlorines present in both aromatic rings. The degradation is carried out by biphenyl (bph) catabolic enzymes. Many PCB degrading bacteria have been reported and enzymes involved in biphenyl degradation have also been characterised. The bph operon has multiple genes for the degradation of chlorinated biphenyls to chlorobenzoate, followed by pyruvates and acetyl-coA (Fig. 8.4).
8.7.1.1 The Biphenyl Upper Pathway
The upper pathway involves four steps in the transformation of chlorobiphenyls to chlorobenzoic acid. The process is catalysed by four bph enzymes, namely, biphenyl 2,3-dioxygenases (bphA), cis-2,3-dihydro-2,3-dihydroxybiphenyl dehydrogenases(bphB), 2,3-dihydroxybiphenyl-1,2-dioxygenases (bphC) and 2-hydroxy-6-phenyl-6-oxohexa-2,4-dieneoate (HOPDA)hydrolases (bphD). The first step of the PCB metabolic pathway involves the conversion of chlorobiphenyl to 2,3-dihydroxy-1-phenylcyclohexa-4,6-diene (dihydrodiol) by a multicomponent biphenyl dioxygenase (bphA). The enzyme bphA consists of large and small subunits (bphA1–biphenyl dioxygenase large subunit, bphA2–biphenyl dioxygenase small subunit, bphA3 – ferredoxin component and bphA4–ferredoxin reductase. The addition of molecular oxygen at the 2,3-position of the chlorinated ring transforms biphenyl to cis-hydrodiols.
The dehydrogenation of dihydrodiol (chlorinated) to 2,3-dihydroxybiphenyl (chlorinated) is catalyzed by the enzyme bphB. The ring cleavage step is catalysed by bphC resulting in the formation of the meta cleavage product HOPDA (2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid). The final step involves the transformation of HOPDA to chlorobenzoic acid (CBA) and 2-hydroxypenta-2,4-dienoate (HPD).
8.7.1.2 The Biphenyl Lower Pathway
The final products of upper pathway HPD and CBA are further metabolised in the lower pathway. HPD, a five-carbon aliphatic compound, is transformed by 2-hydroxypenta-2,4-dienoate hydratase (bphH(E)) to 4-hydroxy-2-oxo-valerate, which is further converted to acetaldehyde to pyruvic acid by 4-hydroxy-2-oxovalerate aldolase. An acylating acetaldehyde dehydrogenase (bphJ(G)) converts acetaldehyde to acetyl-CoA, which then can enter the Krebs (TCA) cycle. CBA is also transformed to benzoyl-CoA by benzoate-CoA ligase (BCL). The benzoyl-CoA is further mineralised to 2,3-dihydroxydihydrobenzoyl-CoA by benzoyl-CoA dioxygenase. This is followed by non-oxygenolytic cleavage of the aromatic ring and the beta oxidation-like pathway of the ring cleavage product. This pathway produces 3-hydroxyadipyl CoA and 3-ketoadipyl CoA, and then finally is converted to succinyl-CoA and acetyl-CoA. CBA could also be converted to catechol by the enzymes benzoate 1,2-dioxygenase and benzoate dihydrodiol dehydrogenase.
8.7.2 Anaerobic PCB Degradation Pathway
The anaerobic degradation of PCB involves the reductive dehalogenase enzyme resulting in the removal of chlorine, which is based on positions like meta, para, ortho and doubly flanked, singly flanked and unflanked single chlorine. But still, the proper pathway for anaerobic dechlorination of PCB was not elucidated (Bedard et al. 2006). The proposed pathway of the dechlorination of 2,3,4,5,6-pentachlorobiphenyl dechlorinated to 2-chlorobiphenyl by the removal of chlorine atoms from all the three positions of the biphenyl ring was elucidated by M. R. Natarajan et al. (1999). Fennell et al. (2004) described dechlorination of double-flanked chlorines of 2,3,4,5,6-pentachlorobiphenyl by Dehalococcoides ethenogenes strain 195.
A sequential dechlorination 2,3,4,5,6-chlorobiphenyl pathway proceeds as follows (Fig. 8.5): 2,3,4,5,6-chlorobiphenyl to 2,3,4,6-chlorobiphenyl (one meta chlorine removed) → 2,3,4,6-chlorobiphenyl to 2,4,6-chlorobiphenyl (one meta chlorine removed) → 2,4,6-chlorobiphenyl to 2,4-chlorobiphenyl (one ortho chlorine removed) → 2,4-chlorobiphenyl to 2-chlorobiphenyl (one para chlorine removed) → 2-chlorobiphenyl to biphenyl (one ortho chlorine removed).
8.8 Conclusion and Future Outlook
PCBs were first synthesized and introduced to the market in the late 1920s. Due to their stability at high temperatures and other harsh environmental conditions, they were widely used in various industries. But this same excellent stability began haunting humankind with its severe negative effects and recalcitrant nature. PCBs were reported to be toxic to the immune system and, as highlighted by EPA, to be a probable human carcinogenic agent, one which could also cause tumors. The main source of exposure to PCBs is either working in an agricultural or at an industrial site already exposed to PCBs, or by consuming food contaminated with it (especially fatty food and meat). Due to those issues, industrial production and usage of PCBs was stopped all over the world, and the EPA phased out PCBs starting in 1979; since 1984, their use has been banned. Several bacteria and fungi are reported to degrade PCBs under aerobic and/or anaerobic conditions. Those microbes produce a specific set of enzymes capable of almost completely degrading PCBs. Although due to its complex structure and very low solubility in water, biodegradation rates and efficiency in large-scale applications are still limited. Further extensive research is still needed to enhance the biodegradation efficiency of PCB microbes and enzymatic degradation using novel technologies to make it work on a large scale with better economics.
References
Abramowicz, D. A. (1990). Aerobic and anaerobic biodegradation of PCBs: A review. Critical Reviews in Biotechnology, 10, 241–251.
Abramowicz, D. A. (1995). Aerobic and anaerobic PCB biodegradation in the environment. Environmental Health Perspectives, 103, 97–99.
Abramowicz, D. A., & Olson, D. R. (1995). Accelerated biodegradation of PCBs. ChemTech, 25, 36–41.
Adrian, L., Dudkova, V., Demnerova, K., & Bedard, D. L. (2009). “Dehalococcoides” sp. strain CBDB1 extensively dechlorinates the commercial polychlorinated biphenyl mixture Aroclor 1260. Applied and Environmental Microbiology, 75, 4516–4524.
Ahmed, F. E. (2003). Analysis of polychlorinated biphenyls in food products. TrAC Trends in Analytical Chemistry, 22, 170–185.
Ahmed, M., & Focht, D. D. (1973). Degradation of polychlorinated biphenyls by two species of Achromobacter. Canadian Journal of Microbiology, 19, 47–52.
Aken, B. V., Correa, P. A., & Schnoor, J. L. (2009). Phytoremediation of polychlorinated biphenyls: New trends and promises. Environmental Science & Technology, 44, 2767–2776.
Arnett, C. M., Parales, J. V., & Haddock, J. D. (2000). Influence of chlorine substituents on the rates of oxidation of chlorinated biphenyls by the biphenyl dioxygenase of Burkholderia sp. strain LB400. Applied and Environmental Microbiology, 66, 2928–2933.
Ballschmiter, K., & Zell, M. (1980). Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography. Fresenius Zeitung der Analytische Chemie, 302, 20–31.
Barker, S. A. (2007). Matrix solid phase dispersion (MSPD). Journal of Biochemical and Biophysical Methods, 70, 151–162.
Barska, I., Guz-Ryczyńska, W., Skrzyński, I., Szlinder-Richert, J., Usydus, Z., Bykowski, P., Hove, H., Heggstad, K., & Bjordal, A. (2005). Non-ortho Polychlorinated biphenyls in Baltic fish in the 1999–2003 period. Bulletin of the Sea Fisheries Institute, 1, 164.
Bedard, D. L., Unterman, R., Bopp, L. H., Brennan, M. J., Haberl, M. L., & Johnson, C. (1986). Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Applied and Environmental Microbiology, 51, 761–768.
Bedard, D. L., Haberl, M. L., May, R. J., & Brennan, J. (1987). Evidence for novel mechanisms of polyclorinated biphenyl metabolism in Alcaligeneseutrophus H850. Applied and Environmental Microbiology, 53, 1103–1112.
Bedard, D. L., Bailey, J. J., Reiss, B. L., & Jerzak, G. V. S. (2006). Development and characterization of stable sediment-free anaerobic bacterial enrichment cultures that dechlorinate Aroclor 1260. Applied and Environmental Microbiology, 72, 2460–2470.
Berkaw, M., Sowers, K. R., & May, H. D. (1996). Anaerobic orthodechlorination of polychlorinated biphenyls by estuarine sediments from Baltimore Harbor. Applied and Environmental Microbiology, 62, 2534–2539.
Beurskens, J. E. M., & Stortelder, P. B. M. (1995). Microbial transformation of PCBs in sediments: What can we learn to solve practical problems? Water Science and Technology, 31, 99–107.
Billingsley, K. A., Backus, S. M., Juneson, C., & Ward, P. (1997). Comparison of the degradation patterns of polychlorinated biphenyl congeners in Aroclors by a Pseudomonas sp. LB400 after growth on various carbon sources. Canadian Journal of Microbiology, 43, 1172–1179.
Bjoërklund, E., Holst, C., & Anklam, E. (2002). Fast extraction, clean-up and detection methods for the rapid analysis and screening of seven indicator PCBs in food matrices. Trends in Analytical Chemistry, 21, 40–53.
Borja, J., Marie-Teleon, D., Auresenia, J., & Gallardo, S. (2005). Polychlorinated biphenyls and their biodegradation. Process Biochemistry, 40, 1999–2013.
Boyle, A. W., Silvin, C. J., Hassett, J. P., Nakas, J. P., & Tanenbaum, S. W. (1992). Bacterial PCB biodegradation. Biodegradation, 3, 285–298.
Cao, Y. M., Xu, L., & Jia, L. Y. (2011). Analysis of PCBs degradation abilities of biphenyl dioxygenase derived from Enterobacter sp. LY402 by molecular simulation. New Biotechnology, 29, 90–98.
Chaudhry, G. R., & Chapalamadugu, S. (1991). Biodegradation of halogenated organic compounds. Microbiological Reviews, 55, 59–79.
Chen, R., & Pignatello, J. (1997). Role of quinone intermediates as electron shuttles in Fenton and photoassisted Fenton oxidations of aromatic compounds. Environmental Science & Technology, 31, 2399–2406.
Clark, R. R., Chian, E. S. K., & Griffin, R. A. (1979). Degradation of polychlorinated biphenyls by mixed microbial cultures. Applied and Environmental Microbiology, 37, 680–685.
Cohen, B. S. (2010). An assessment of historical PCB contamination in Arctic mammals. ENVI Independent Study. Williams College USA. Fall 2009–Winter 2010.
Cranor, W. L., Perkins, S. D., Clark, R. C., & Tegerdine, G. A. (2005). Analysis of SPMD samples from the October/November 2004 deployment in Lake Anna, VA for PCBs as bioavailable organic contaminants. The Columbia Environmental Research Center, 27, 43.
Criado, M. R., Pereiro, I. R., & Torrijos, R. C. (2003). Optimization of a microwave-assisted extraction method for the analysis of polychlorinated biphenyls in ash samples. Journal of Chromatography A, 985, 137–145.
De, J., Ramaiah, N., & Sarkar, A. (2006). Aerobic degradation of highly chlorinated polychlorobiphenyls by a marine bacterium, Pseudomonas CH07. World Journal of Microbiology and Biotechnology, 22, 1321–1327.
Devrukhkar, S., Kothare, A., Kochar, D., & Surti, A. (2017). Aerobic degradation of Aroclor 1242 by Pseudomonas mendocina strain CL-10.4. International Journal of Advanced Research and Development, 2, 128–132.
Dietrich, D., Hickey, W. J., & Lamar, R. (1995). Degradation of 4,49-dichlorobiphenyl, 3,39,4,49-tetrachlorobiphenyl, and 2,29,4,49,5,59-hexachlorobiphenyl by the White Rot fungus Phanerochaetechrysosporium. Applied and Environmental Microbiology, 61, 3904–3909.
Dingyi, Y., Quensen, J. F., III, Tiedje, J. M., & Boyd, S. A. (1995). Evidence for para dechlorination of polychlorobiphenyls by methanogenic bacteria. Applied and Environmental Microbiology, 61, 2166–2171.
Dobbins, D. C. (1995). Biodegradation of pollutants. In Encyclopaedia of environmental biology (Vol. 1). New Delhi: Academic.
Dolfing, J. (1990). Reductive dechlorination of 3-chlorobenzoate is coupled to ATP production and growth in an anaerobic bacterium strain DCB-1. Archives of Microbiology, 153, 264–266.
Dolfing, J., & Tiedje, T. M. (1987). Growth yield increase linked to reductive dechlorination in a defined 3-chlorobenzoate degrading methanogeniccoculture. Archives of Microbiology, 149, 102–105.
Evans, B. S., Dudley, C. A., & Klasson, K. T. (1996). Sequential anaerobic-aerobic biodegradation of PCBs in soil slurry microcosms. Applied Biochemistry and Biotechnology, 57(8), 885–894.
Fava, F., Gentilucci, S., & Zanaroli, G. (2003). Anaerobic biodegradation of weathered polychlorinated biphenyls (PCBs) in contaminated sediments of Porto Marghera (Venice Lagoon, Italy). Chemosphere, 53, 101–109.
Fennell, D. E., Nijenhuis, I., Wilson, S. F., Zinder, S. H., & Häggblom, M. M. (2004). Dehalococcoidesethenogenes strain 195 reductively dechlorinates diverse chlorinated aromatic pollutants. Environmental Science & Technology, 38, 2075–2081.
Fiedler, H. (2007). National PCDD/PCDF release inventories under the Stockholm convention on persistent organic pollutants. Chemosphere, 67, S96–S108.
Field, J. A., & Sierra-Alvarez, R. (2008). Microbial transformation and degradation of poly-chlorinated biphenyls. Environmental Pollution, 155, 1–12.
Flanagan, W. P., & May, R. J. (1993). Metabolite detection as evidence for naturally occurring aerobic PCB biodegradation in Hudson River sediments. Environmental Science & Technology, 27, 2207–2212.
Fukuda, M. (1993). Diversity of chloroaromaticoxygenases. Current Opinion in Biotechnology, 4, 339–343.
Furukawa, K. (1982). Microbial degradation of polychlorinated biphenyls. In A. M. Chakrabarty (Ed.), Biodegradation and detoxification of environmental pollutants (pp. 33–57). Boca Raton: CRC Press.
Furukawa, K., & Chakrabarty, A. M. (1982). Involvement of plasmids in total degradation of chlorinated biphenyls. Applied and Environmental Microbiology, 44, 619–626.
Furukawa, K., & Matsumura, F. (1976). Microbial metabolism of polychlorinated biphenyls. Studies on the relative degradability of polychlorinated biphenyl components by Alcaligenes sp. Journal of Agricultural and Food Chemistry, 42, 543–548.
Furukawa, K., & Miyazaki, T. (1986). Cloning of a gene cluster encoding biphenyl and chlorobiphenyl degradation in Pseudomonas pseudoalcaligenes. Journal of Bacteriology, 166, 392–398.
Furukawa, K., Tomizuka, N., & Kamibayashi, A. (1979). Effect of chlorine substitution on the bacterial metabolism of various polychlorinated biphenyls. Applied and Environmental Microbiology, 38, 301–310.
Halfadji, A., Touabet, & Yacine, A. (2003). Comparison of soxhlet extraction, microwave-assisted extraction and ultrasonic extraction for the determination of PCB’s congeners in spiked soils by transformer oil (ASKAREL). International Journal of Advances in Engineering & Technology, 5, 63–75.
Hofer, B., Backhaus, S., & Timmis, K. N. (1994). The biphenyl/polychlorinated Pseudomonas sp. LB400 encodes four additional metabolic enzymes. Gene, 144, 9–16.
Hou, L. H., & Dutta, S. K. (2000). Phylogenetic characterization of several para- and meta-PCB dechlorinating Clostridium species: 16S rDNA sequence analyses. Letters in Applied Microbiology, 30, 238–243.
Hülsmeyer, M., Hecht, H.-J., Niefind, K., Schomburg, D., Hofer, B., Timmis, K. N., & Eltis, L. D. (1998). Crystal structure of cis-biphenyl-2,3-dihydrodiol-2,3-dehydrogenase from a PCB degrader at 2.0 Å resolution. Protein Science, 7, 1286–1293.
Ju, Q., Zouboulis, C. C., & Xia, L. (2009). Environmental pollution and acne: Chloracne. Dermato-endocrinology, 1, 125–128.
Kamei, I., Kogura, R., & Kondo, R. (2006). Metabolism of 4,4′-dichlorobiphenylby white-rot fungi Phanerochaetechrysosporium and Phanerochaete sp. MZ42. Applied Microbiology and Biotechnology, 72, 566–575.
Kim, J., & Rhee, G. Y. (1997). Population dynamics of polychlorinated biphenyl-dechlorinating microorganisms in contaminated sediments. Applied and Environmental Microbiology, 63, 1771–1776.
Kimbara, K., Hashimoto, T., Fukuda, M., Koana, T., Takagi, M., Oishi, M., & Yano, K. (1988). Isolation and characterization of a mixed culture that degrades polychlorinated biphenyls. Agricultural and Biological Chemistry, 52, 2885–2891.
Krcmár, P., Kubatova, A., Votruba, J., Erbanova, P., Novotny, C., & Sasek, V. (1999). Degradation of polychlorinated biphenyls by extracellular enzymes of Phanerochaetechrysosporium produced in a perforated plate bioreactor. World Journal of Microbiology and Biotechnology, 15, 269–276.
Kubatova, A., Erbanova, P., Eichlerova, I., Homolka, L., Nerud, F., & Sasek, V. (2001). PCB congener selective biodegradation by the white rot fungus Pleurotusostreatus in contaminated soil. Chemosphere, 43, 207–215.
Kusch, P. (2018). Headspace solid-phase microextraction coupled with gas chromatography–Mass spectrometry for the characterization of polymeric materials. LCGC North America, 36, 52–61.
Lang, V. (1992). Polychlorinated biphenyls in the environment. Journal of Chromatography, 595, 1–43.
Larsson, P. (1987). Uptake of polychlorinated biphenyls (PCBs) by the macroalga, Cladophoraglomerata. Bulletin of Environmental Contamination and Toxicology, 38, 58–62.
Llompart, M., Li, K., & Fingas, M. (1998). Solid-phase microextraction and headspace solid-phase microextraction for the determination of polychlorinated biphenyls in water samples. Analytical Chemistry, 70, 2510–2515.
Mancera-Lopez, M., Esparza-Garcia, F., Chavez-Gomez, B., Rodriguez-Vazquez, R., Saucedo-Castaneda, G., & Barrera-Cortes, J. (2008). Bioremediation of an aged hydrocarbon-contaminated soil by a combined system of biostimulation-bioaugmentation with filamentous fungi. International Biodeterioration & Biodegradation, 61, 151–160.
Masai, E., Yamada, A., Healy, J. M., Hatta, T., Kimbara, K., Fukuda, M., & Yano, K. (1995). Characterization of biphenyl catabolic genes of gram-positive polychlorinated biphenyl degrader Rhodococcus sp. strain RHA1. Applied and Environmental Microbiology, 61, 2079–2085.
Matturro, B., Di Lenola, M., Ubaldi, C., & Rossetti, S. (2016). First evidence on the occurrence and dynamics of Dehalococcoidesmccartyi PCB-dechlorinase genes in marine sediment during Aroclor 1254 reductive dechlorination. Marine Pollution Bulletin, 112, 189–194.
McEldowney, S., Hardman, D. J., & Wait, S. (1993). Pollution: Ecology and biotreatment. New York: Longman Scientific and Technical.
McKay, D. B., Seeger, M., Zielinski, M., Hofer, B., & Timmis, K. N. (1997). Heterologous expression of biphenyl dioxygenase-encoding genes from a gram-positive broad-spectrum polychlorinated biphenyl degrader and characterization of chlorobiphenyl oxidation by the gene products. Journal of Bacteriology, 179, 1924–1930.
Mckay, D. B., Prucha, M., Reineke, W., Timmis, K. N., & Pieper, D. H. (2003). Substrate specificity and expression of three 2, 3-dihydroxybiphenyl 1, 2-dioxygenases from Rhodococcusgloberulus Strain P6. Journal of Bacteriology, 185, 2944–2951.
Miyata, H., Kashimoto, T., & Kunita, N. (1977). Detection and determination of polychlorinated dibenzofurans in normal human tissues and Kanemi rice oils caused “KanemiYusho” (in Japanese). Journal of the Food Hygienic Society of Japan, 19, 260.
Mohn, W. W., & Tiedje, J. M. (1990). Catabolic thiosulfate disproportionation and carbon dioxide reduction in strain DCB-1, a reductively dechlorinating anaerobe. Journal of Bacteriology, 172, 2065–2070.
Mohn, W. W., & Tiedje, J. M. (1991). Evidence for chemiosmotic coupling of reductive dechlorination and ATP synthesis in Desulfomoniletiedjei. Archives of Microbiology, 1991, 1–8.
Muir, D., & Sverko, E. (2006). Analytical methods for PCBs and organochlorine pesticides in environmental monitoring and surveillance: A critical appraisal. Analytical and Bioanalytical Chemistry, 386, 769–789.
Mukerjee-Dhar, G., Hatta, T., Shimura, M., & Kimbara, K. (1998). Analysis of changes in congener selectivity during PCB degradation by Burkholderia sp. strain TSN101 with increasing concentrations of PCB and characterization of the bph BCD genes and gene products. Archives of Microbiology, 169, 61–70.
Murínová, S., Dercová, K., & Sová, H. D. (2014). Degradation of polychlorinated biphenyls (PCBs) by four bacterial isolates obtained from the PCB-contaminated soil and PCB-contaminated sediment. International Biodeterioration & Biodegradation, 91, 52–59.
Namiesnik, J., & Szefer, P. (2009). Analytical measurements in aquatic environments. Boca Raton: CRC Press.
Natarajan, M. R., Wu, W., Wang, H., Bhatnagar, L., & Jain, M. K. (1999). Dechlorination of spiked PCBs in lake sediment by anaerobic microbial granules. Water Research, 32, 3013–3020.
National Research Council. (2001). A risk-management strategy for PCB-contaminated sediments. Washington, DC: National Academic Press.
Nies, L., & Vogel, T. M. (1990). Effects of organic substrates on dechlorination of Aroclor 1242 in anaerobic sediments. Applied and Environmental Microbiology, 56, 2612–2617.
Nies, L., & Vogel, T. M. (1991). Identification of the proton source for the microbial reductive dechlorination of 2,3,4,5,6-pentachlorobiphenyl. Applied and Environmental Microbiology, 57, 2771–2774.
Novotny, C., Vyas, B. R. M., Erbanova, P., Kubatova, A., & Sasek, V. (1997). Removal of PCBs by various white rot fungi in liquid cultures. Folia Microbiologica, 42, 136–140.
Pieper, D. H. (2005). Aerobic degradation of polychlorinated biphenyls. Applied Microbiology and Biotechnology, 67, 170–191.
Quensen, J. F., III, Boyd, S. A., & Tiedje, J. M. (1990). Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganisms from sediments. Applied and Environmental Microbiology, 56, 2360–2369.
Rabinovich, M. L., Bolobova, A. V., & Vasil’chenko, L. G. (2004). Fungal decomposition of natural aromatic structures and xenobiotics: A review. Applied Biochemistry and Microbiology, 40, 1–17.
Rejczak, T., & Tuzimski, T. (2015). A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chemistry, 13, 980–1010.
Rhee, G.-Y., Sokol, R. C., Bush, B., & Bethoney, C. M. (1993). Long-term study of the anaerobic dechlorination of Aroclor 1254 with and without biphenyl enrichment. Environmental Science & Technology, 27, 714–719.
Riaz, M., & Zamorani, E. (1988). Analytical procedure for SPE of PCBs from water. European Application Research Report EUR 11886 EN, Commission of the European Communities, Luxembourg.
Ruiz-Aguilar, G. M. L., Fernandez-Sanchez, J. M., Rodriguez-Vazquez, R., & Poggi-Varaldo, H. (2002). Degradation by white-rot fungi of high concentrations of PCB extracted from a contaminated soil. Advances in Environmental Research, 6, 559–568.
Sakai, M., Masai, E., Asami, H., Sugiyama, K., Kimbara, K., & Fukuda, M. (2002). Diversity of 2,3-dihydroxybiphenyl dioxygenase genes in a strong PCB degrader, Rhodococcus sp. strain RHA1. Journal of Bioscience and Bioengineering, 93, 421–427.
Sánchez-Rojas, F., Bosch-Ojeda, C., & Cano-Pavón, J. M. (2009). A review of stir bar sorptive extraction. Chromatographia, 69, 79–94.
Schmidt, H., & Schultz, G. (1881). Einwirkung von Fiinffach Chlorphosphor auf das y- diphenol. Annali di Chimica, 207, 338–344.
Seeger, M., Timmis, K. N., & Hofer, B. (1995). Conversion of chlorobiphenyls into phenylhexadienoates and benzoates by the enzymes of the upper pathway for polychlorobiphenyl degradation encoded by the bph locus of Pseudomonas sp. strain LB400. Applied and Environmental Microbiology, 61, 2654–2658.
Seeger, M., Timmins, K. N., & Hofer, B. (1997). Bacterial pathways for the degradation of polychlorinated biphenyls. Marine Chemistry, 58, 327–333.
Seto, M., Kimbara, K., Shimura, M., Hatta, T., Fukuda, M., & Yano, K. (1995). A novel transformation of polychlorinated biphenyls by Rhodococcus sp. strain RHA1. Applied and Environmental Microbiology, 61, 3353–3358.
Seto, M., Nishibori, K., Masai, E., Fukuda, M., & Ohdaira, Y. (1999). Degradation of polychlorinated biphenyls by a ‘Maitake’ mushroom, Grifolafrondosa. Biotechnology Letters, 21, 27–31.
Sietmann, R., Gesell, M., Hammer, E., & Schauer, F. (2006). Oxidative ring cleavage of low chlorinated biphenyl derivatives by fungi leads to the formation of chlorinated lactone derivatives. Chemosphere, 64, 672–685.
Silva, D. J., Pietri, F. V., Ermirio, J., Moraes, F., Bazito, R. C., & Pereira, C. G. (2012). Treatment of materials contaminated with Polychlorinated Biphenyls (PCBs): Comparison of traditional method and supercritical fluid extraction. American Journal of Analytical Chemistry, 3, 891–898.
Singh, H. (2006). Mycoremediation: Fungal bioremediation. Hoboken: Wiley.
Stellaa, T., Covinoa, S., Carová, M. C., Filipová, A., Petruccioli, M., D’Annibale, A., & Cajthamla, T. (2017). Bioremediation of long-term PCB-contaminated soil by white-rot fungi. Journal of Hazardous Materials, 324, 701–710.
Taguchi, K., Motoyama, M., & Kudo, T. (2001). PCB/biphenyl degradation gene cluster in Rhodococcusrhodochrous K37, is different from the well-known bph gene clusters in Rhodococcus sp. P6, RHA1, and TA421. Riken Review, 42, 23–26.
Takagi, S., Shirota, C., Sakaguchi, K., Suzukia, J., Suea, T., Nagasakac, H., Hisamatsua, S., & Sonokia, S. (2007). Exoenzymes of Trametesversicolor can metabolize coplanar PCB congeners and hydroxy PCB. Chemosphere, 67, S54–S57.
Tan, G. H., & Chai, M. K. (2011). Sample preparation in the analysis of pesticides residue in food by chromatographic techniques. In M. Stoytcheva (Ed.), Pesticides – strategies for pesticides analysis. Rijeka: InTech.
The Stockholm Convention on Persistent Organic Pollutants (POPs). (2010). United Nations Environment Programme (UNEP). http://www.pops.int
Tiedje, J. M., Quensen, J. F., III, Chee-Sanford, J., Schimel, J. P., & Boyd, S. A. (1993). Microbial reductive dechlorination of PCBs. Biodegradation, 4, 231–240.
Tu, C., Teng, Y., Luo, Y., Li, X., Sun, X., Li, Z., Liu, W., & Christie, P. (2011). Potential for biodegradation of polychlorinated biphenyls (PCBs) by Sinorhizobiummeliloti. Journal of Hazardous Materials, 186, 1438–1444.
Urbaniak, M. (2013). Chapter 4: Biodegradation of PCDDs/PCDFs and PCBs. In Biodegradation – Engineering and technology (pp. 73–100). Rijeka: InTech.
Van Dort, H. M., & Bedard, D. L. (1991). Reductive ortho and meta-dechlorination of a polychlorinated biphenyl congener by anaerobic microorganisms. Applied and Environmental Microbiology, 57, 1576–1578.
Vasilyeva, G., & Strijakova, E. (2007). Bioremediation of soils and sediments contaminated by polychlorinated biphenyls. Microbiology, 76, 639–653.
Verdin, A., Sahraoui, A. L. H., & Durand, R. (2004). Degradation of benzo[a]pyrene by mitosporic fungi and extracellular oxidative enzymes. International Biodeterioration & Biodegradation, 53, 65–70.
Williams, W. A. (1994). Microbial reductive dechlorination of trichlorobiphenyls in anaerobic sediment slurries. Environmental Science & Technology, 28, 630–635.
Wu, Q., & Wiegel, J. (1997). Two anaerobic polychlorinated biphenyl-dehalogenating enrichments that exhibit different para-dechlorination specificities. Applied and Environmental Microbiology, 63, 4826–4832.
Wu, Q., Sowers, K. R., & May, H. D. (1998). Microbial reductive dechlorination of aroclor 1260 in anaerobic slurries of estuarine sediments. Applied and Environmental Microbiology, 64, 1052–1058.
Yadav, J. S., Quensen, J. F., III, Tiedje, J. M., & Reddy, C. A. (1995). Degradation of biphenyl mixtures (Aroclors 1242, 1254, and 1260) by the white rot fungus Phanerochaetechrysosporium as evidenced by congener-specific analysis. Applied and Environmental Microbiology, 61, 2560–2565.
Acknowledgements
The authors would like to kindly acknowledge Dr. Samuel Premkumar, CAARU, for deducing the chemical structures, and facilities and support provided by Sultan Qaboos University while preparing this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Elangovan, S., Pandian, S.B.S., S. J., G., Joshi, S.J. (2019). Polychlorinated Biphenyls (PCBs): Environmental Fate, Challenges and Bioremediation. In: Arora, P. (eds) Microbial Metabolism of Xenobiotic Compounds. Microorganisms for Sustainability, vol 10. Springer, Singapore. https://doi.org/10.1007/978-981-13-7462-3_8
Download citation
DOI: https://doi.org/10.1007/978-981-13-7462-3_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7461-6
Online ISBN: 978-981-13-7462-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)