Abstract
Discovering of new industrial applications from microorganisms is diverse as they came from variety of environmental niches. The majority of existing biotechnological applications are of microbial origin and enzymes are the most important among them. Microbial enzymes surpass those from animals and plant sources since their ease of production and genetic manipulation, diverse catalytic activities, etc. The role of enzymes in many processes has been known for a long time, in which the enzymes from microorganisms, used particularly for baking, brewing, alcohol production, cheese making etc. Starch represents one of the most pervasive and an important renewable biological resource that forms a major source of food to a large population. Starch hydrolysis forms the basis of many industrial processes and acid hydrolysis was significant during the earlier days. However, this was almost completely replaced by enzymatic hydrolysis, nowadays, since the availability and abundance of starch hydrolasing microorganisms, corrosion-free reaction, and specificity of the reaction. One of the major applications of these enzymes is in the food industry and starch hydrolysis yields a diverse range of products such as glucose, maltose and fructose syrups, cyclodextrins, fat mimetics substances, etc. They also find application as brewing and baking agents. Enzymatic liquefaction and saccharification of starch require higher temperatures; that demands novel thermostable amylases. In this chapter, we are discussing about various aspects of amylase enzymes, their sources, application in the food industry and future prospects of thermostable amylase from mesophilic organisms, etc.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Amylolytic enzymes also known as amylases act on starch and related oligo/polysaccharides. The history of amylases was began with the discovery of the first starch degrading enzyme by Kirchhoff in 1811. Followed by this, many other digestive amylases and malt amylases were discovered in succession. Amylases are significant enzymes for their specific use not only in the industrial starch conversion but also in other commercial process. Last century witnessed the emergence of large-scale starch processing industries. For the last few decades, a change from starch acid hydrolysis toward its enzymatic hydrolysis for the production of modified starch and other products such as glucose and fructose syrups, dextrins, etc., has been noticed. Roughly about 30% of the global enzyme production is constituted by these starch-hydrolyzing enzymes. Apart from starch hydrolysis, these enzymes find their applications in industries such as food industry, detergent industry, paper and textile industry, etc. Amylases are one of the most widely distributed enzymes and it is also one of the most well-studied enzymes. Microbial amylases have potential benefit over chemical starch hydrolysis such as specificity and corrosion less mechanism. This is especially useful in the production of products with distinct qualities.
Prior to going in detail about amylases and their applications, let us go through the features of its natural substrate starch. As mentioned before, starch is one of the predominant naturally occurring carbohydrates on earth apart from cellulose. Potatoes, cassava, maize (corn), rice, and wheat are important starch-containing crops and are important constituents for the human diet and a large proportion of the world’s population consume it as the major food (Van Der Maarel et al. 2002; Myat and Ryn 2013). Starch is frequently isolated and is used in food industries to impart the desirable functional properties, and to modify food texture and consistency (Kuttigounder et al. 2011). Apart from the usage of starchy plant material directly as food, starch is harvested and used as such or chemically or enzymatically processed into a variety of different products such as starch hydrolysates, glucose syrups, fructose, starch or maltodextrin derivatives, and cyclodextrins (El-Fallal et al. 2012). Starch is widely used in the laundry sizing of fine fabrics in textile industry, skin cosmetics in cosmetic industry and for enhancing the paper strength and printing properties in paper industry. Amylolytic enzymes de-polymerize the starch, which forms the basis of these industries (Vaidya et al. 2015). Starch is also considered as a substrate in fuel production by enzymatic processes (Vengadaramana 2013), and the sugars produced can be fermented to produce ethanol.
Commercially, starch can be extracted in pure form from a variety of natural sources. Maize is the predominant source, but wheat, rice, potato, and sago also have significant contributions for the commercial production (Vengadaramana 2013). Native starch is a semicrystalline material synthesized as roughly spherical granules in plant tissues. It is a white, tasteless, and odorless powder that is insoluble in cold water or alcohol. Starch has been used as stiffening or thickening agent while dissolved in warm water. The origin of the word “starch” came from a Middle English word “sterchen” which means to stiffen. The Greek word “amulon” means “not ground at a mill” from which the Latin word “Amylum” was derived meaning starch. Pure starch is a heterogeneous polysaccharide consisting predominantly of α-glucan in the form of amylose and amylopectin. These two polymers have different structure and physical properties (Mojsov 2012). Starch is a polymer formed of d-glucose units linked together by glycosidic bonds. At alkaline pH, the glycosidic linkage is stable, however, at acidic pH, the bond become instable resulting in hydrolysis (Aiyer 2005). Acid hydrolysis was routinely used for conversion of starch earlier.
The amylose and amylopectin molecules are polymers of α-d-glucose units but are fundamentally different. Amylose is usually a linear unbranched chain of glucose units ranging from 500 to 20,000 in number and it chiefly contains α-(1–4) linkage (approximately 99%). However, a small number of α-(1–6) branches (approximately 1%) and linked phosphate groups are also found. Due to the peculiar molecular shape and structure, amylose is not stable in aqueous solution. However, it has a considerable viscosity in alkaline solutions due to its molecular shape. The average amylose content in starch is 20–25% depending on the source (Amoozegar et al. 2003).
Amylopectin is a branched polymer consisting of linear chains of 10–60 glucose units linked by α(1–4) glycosidic bond and side chains of 15–45 glucose units linked by α(1–6) glycosidic bond. In this molecule, the branching points are roughly about 5%. However, it may vary depending on the source (Thompson 2000). Amylopectin is one among the largest molecule occurring in nature comprising of about 2,000,000 glucose units (Vengadaramana 2013). Side chains of amylopectin are ordered on the longer backbone to form a clustered appearance (Thompson 2000; Bertoft 2007). In aqueous solution, amylopectins are relatively stable due to branched molecules and are not able to form compact aggregates. Starch contains nearly 75–80% amylopectin depending on the source (Amoozegar et al. 2003).
11.2 Amylolytic Enzymes
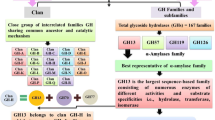
As discussed above, starch is an important energy source for animals, plants, and microorganisms and its hydrolysis are achieved by α-glycoside hydrolases. Since these cleaves starch (amylose and amylopectin), they are called amylolytic enzymes often indicated as amylases. Amylolytic enzymes are the most widely distributed enzymes, making its presence in all three domains of life, i.e., Bacteria, Archaea, and Eucarya. Best known and characterized among the amylolytic enzymes are α-amylase, β-amylase and glucoamyase. Though they are similar in their functionalities, they show significant dissimilarity in structure and reaction mechanism owing to the larger evolutionary distance between them (Janecek 1994). Thus, they form their own independent glycoside hydrolase (GH) families such as α-amylases forming GH family 13, whereas β-amylases constituting GH family 14 and glucoamylases forming GH family 14 (Henrissat and Bairoch 1993).
11.2.1 α-Amylase Glycoside Hydrolase Family 13
α-amylases along with related enzymes constitute the GH family 13. Two related families GH family 70 and 77 are considered under α-amylases (Stam et al. 2006). These enzymes retain the α-configuration after hydrolysis. Apart from hydrolases belonging to class 3 (EC 3), the family also containing transferases and isomerases from classes 2 and 5, thus indicating that all the members of the family not necessarily cleave the glycoside bonds in starch. However, they exhibit certain common features thus clustering them in the same family (Horvathova et al. 2001). These features includes (i) they should contain same conserved regions (sequence similarity), (ii) catalytic centre should contain Asp, Glu and Asp at β strands (i) they have ability to act on α-glucosidic linkages; (iii) should hydrolyse or form by transglycosylation of α-glucosidic linkages; (v) should contain four conserved regions and (v) catalytic centre should contain Asp, Glu and Asp residues like Asp206, Glu230 and Asp297 of Taka-amylase A (Janecek 2002). Binding of substrate to the catalytic site makes the breakage of the glycoside links possible (Vaidya et al. 2015). Taka-amylase A, from Aspergillus oryzae was the first α-amylase enzyme to get structurally resolved (Matsuura et al. 1984). One of the most important characteristics of α-amylase family is its catalytic domain formed of (α/β) 8-barrel. C-terminal end of the β-barrel in (α/β) 8-structure constitute the active center. C-terminal ends of the three β-strands (β4, β5, and β7) carries catalytic triad, i.e., Asp206, Glu230, and Asp297. The distance between carboxylate group and the catalytic triad ranges from 5 to 7 Å (Matsuura 2002).
Apart from α-amylase, few other enzymes such as isoamylase, pullanase, amylomaltase, and cyclodextrin glycosyltransferase also belong to this family (Horvathova et al. 2001). However, the glucosyltransferases and alternansucrase were placed under the GH family 70 as it is assumed that (β/α)8 barrel of the enzyme shows a slight variation from the α-amylases (MacGregor et al. 1996; Argüello-Morales et al. 2000). GH family 77 carries amylomaltases which exhibits low sequence similarities with α-amylases. However, they retained (α/β)8 barrel and catalytic triad (Asp-Glu-Asp) exclusive to α-amylases (Przylas et al. 2000). Three of the related α-amylases families together constitute clan GH-H (Stam et al. 2006). All these enzymes are α-retaining enzymes thus the resulting hydroxyl group retains the α-configuration. The reaction mechanism is double displacement two-step mechanism where covalent glycosil enzyme intermediate will be formed. One of the carboxylic acid residues acted as nucleophile and act upon the anomeric center of sugar to form glycosil-enzyme intermediate. Second carboxyl residue meanwhile acts as acid–base catalyst. In the first step, they protonate glycosidic oxygen and in the second step they protonate water. Figure 11.1 represents the double displacement retaining mechanism of α-amylases. During the reaction, an oxocarbenium ion-like transition state will also be achieved (square brackets) (Horvathova et al. 2001).
Double displacement retaining mechanism of α-amylases (Horvathova et al. 2001)
11.2.1.1 α-Amylase
The α-amylases (1,4-α-d-glucan glucanohydrolases) acts upon 1,4-α-d-glucosidic bonds to cleave them, however, they cannot act upon 1,6-α-d-glucosidic branch points. These enzymes are endo-acting enzymes and are also called endo-amylases (Naidu and Saranraj 2013). Starch gets hydrolyzed to low molecular weight units such as maltotriose, maltose, and glucose by α-amylases (Mobini-Dehkordi and Javan 2012). Based on the degree of hydrolysis, the α-amylases can be categorized into two. (i) Saccharifying α-amylases that make 50–60% hydrolysis of starch. (ii) Liquefying α-amylases that could achieve about 30–40% starch hydrolysis. α-amylases are present in seeds with starch as reserve food and also microorganisms (Tiwari et al. 2015). Microbial amylases will be discussed below. Even though α-amylases are endo-acting, they are nonspecific and act randomly during hydrolysis. This yield many low molecular weight products such as maltose, maltotriose, and dextrins. Since these enzymes can act at random sites, α-amylases tends to be faster than β-amylase in enzymatic reaction (Liu et al. 2010) (Fig. 11.2).
Homology Model of soyabean α-amylase (pdb id: 2TAA) (Matsuura et al. 1984)
Direct displacement inverting mechanism of β and γ-amylases (adopted from Horvathova et al. 2001)
11.2.2 GH Families 14 and 15: (β-Amylase and Glucoamylase)
Members of these two families show a similar reaction mechanism, although they are structurally distinct from each other. On the other hand, these enzymes have a different catalytic mechanism when compared to that of α-amylases. They exhibit an inverting reaction mechanism by which the configuration of the resulting hydroxyl group will be changed to β-configuration. The catalytic mechanism involves a simple direct displacement mechanism (Fig. 11.4). One of the carboxylic acid residues at the catalytic site acts as the base during the attack of water. Meanwhile, the second active site residue acts as the general acid during the cleavage of glycosidic bond. Here also, during the reaction an oxocarbenium ion-like transition state will be achieved (square brackets) (Horvathova et al. 2001). Two of the well-known members of these families are discussed below (Fig. 11.3).
Crystal structure of soyabean β-amylase mutant (pdb id: 1Q6G) (Hirata et al. 2004)
11.2.2.1 GH Family 14/β-Amylase (EC 3.2.1.2)
β-amylase (1,4-d-glucan maltohydrolase) have wider distribution, produced by bacteria, fungi, and plants. However, animal tissue does not contain β-Amylase, but microflora of the gastrointestinal tract may possess these enzymes. They are also known as glycogenase or saccharogen amylase, and cleaves second α-(1,4) linkages at nonreducing end leaving maltose as the end product. Maltose formed has β-configuration in glucose units. Hence, they are β-amylase. They are also called exo-acting enzymes (Horvathova et al. 2001). Soybean β-amylase was first crystallized structure of β-amylase to be resolved (Mikami et al. 1994). They also carry a TIM-barrel [(β/α)8-barrel] structure like α-amylase, however, they both are differing from each other (Horvathova et al. 2001). Catalytic domain of β-amylase carries two Glu residues (Mikami et al. 1994; Totsuka and Fukazawa 1996). The sweetness of ripened fruit is imparted by maltose formed by β-amylase. Seeds usually contain β-amylase apart from α-amylase. However, β-amylase occurs in inactive form before seed germination. Malt is produced by amylase in cereal grain (Kaplan and Guy 2004).
11.2.2.2 GH Family 15/Glucoamylase/γ-Amylase
γ-amylase is also known as glucan 1,4-α-glucosidase, amyloglucosidase, exo-1,4-α-glucosidase, glucoamylase, lysosomal α-glucosidase, or 1,4-α-d-glucanglucohydrolase. They are generally multi-domain enzymes structurally very distinct from both α-amylases and β-amylases and consisting of a catalytic domain folded as a twisted (α/α)6 barrel with a central funnel-shaped active site connected to a starch binding domain (Aleshin et al. 1992; Sevèík et al. 1998) Glucan 1,4-α-glucosidase acts on both α-(1–6) glycosidic linkages and terminal α(1–4) glycosidic linkages of starch molecules to yield glucose as the end product. Here also, catalysis is characterized by two Glu residues (Harris et al. 1993). Most of the γ-amylases are active at acidic pH, however, the search of newer and improved γ-amylases with ability to act on higher pH is in demand (Kumar and Satyanarayana 2009) (Fig. 11.5).
Homology Model of glucoamylase from aspergillus niger (pdb id: 3EQA) (Hirata et al. 2004)
11.2.3 Types of Amylases
Another classification of amylases is based on the manner in which the glycosidic bond is attacked. Accordingly, starch-hydrolyzing enzymes are grouped into four such as (i) endo-amylases; (ii) exo-amylases; (iii) de-branching enzymes; and (iv) transferases which are used to hydrolyze starches particularly for the production of dextrins and glucose (Marc et al. 2002).
11.2.3.1 Endo-amylases
Endo-amylases are able to cleave α-(1–4) glycosidic bonds present in the inner part (endo) of the amylose or amylopectin chain. α-amylase is a well-known endo-amylase and characteristics were discussed above.
11.2.3.2 Exo-Amylases
The exo-amylases, either exclusively cleave α(1–4) glycosidic bonds such as β-amylase (EC 3.2.1.2) or cleave both α-(1–4) and α-(1–6) glycosidic bonds like amyloglucosidase or glucoamylase and α-glucosidase. These enzymes act upon the terminal glucose residues of starch molecules and thereby yielding maltose and limit dextrin (e.g., β-amylase) or glucose (glucoamylase and α-glucosidase) (Pandey et al. 2000). Two of the members of this group β-amylase and γ-amylase have already been discussed above.
11.2.3.3 The De-branching Enzymes
De-branching enzymes act on branching points and hydrolyze α-(1–6) glycosidic bonds. Few of the examples are pullulanase enzymes and isoamylase. Both act on amylopectin and hydrolyze to form linear polysachharides. Apart from that pullulanase could cleave α-(1–6) glycosidic bond in pullulan. These de-branching enzymes along with other amylolytic enzymes have been proved significant in the food industry, especially during saccharification process (Hii et al. 2012).
11.2.3.4 Transferases
Transferases not only hydrolyze the glycosidic linkage but they also transfer the glycoside bonds. They act by the cleavage of α-(1–4) glycosidic bond of donor molecule and transfer fragments of them to glycosidic acceptor molecule. Few of the members are amylomaltase and cyclodextrin glycosyltransferase that forms new α-(1–4) glycosidic bond. However, new α-(1–6) glycosidic bond will be established by branching enzymes. They form cyclic molecule such as cyclodextrin (Horvathova et al. 2001).
11.3 Sources of Amylases
11.3.1 Bacterial Amylases
Bacteria are one of the major sources of amylase enzyme. Bacterial enzymes are more preferred since its ease of production, and genetic manipulation. Among bacterial amylolytic enzyme producers, Bacillus sp. is a predominant group and most of the commercially applied enzymes are obtained from the genus. Few of the industrially significant producers are Bacillus licheniformis, Bacillus amyloliquefaciens, and Bacillus stearothermophilus (Konsoula and Liakopoulou-Kyriakides 2007; Pandey et al. 2000). Among α-amylases from these strains, B. licheniformis was turned to be the most stable enzyme (Weemaes et al. 1996). B. licheniformis AI20 was reported with thermostable α-amylase of molecular weight 55 kDa (Abdel-Fattah et al. 2012). Bacillus subtilis 168 (1A1) is another bacterial amylase producer yielding a 55 kDa amylolytic enzyme with an optimum activity at 37 °C (Sumrin et al. 2011).
Amylases are preferably metal ion-dependent enzymes and few of them include divalent cations like Ca2+, Mg2+, Mn2+, Zn2+, Fe2+, etc. (Pandey et al. 2000). One or more Ca2+ has been reported near the active site of most of the α-amylases and is required for proper folding and stability of the enzyme making it vital for enzyme activity (Linden et al. 2002; Prakash and Jaiswal, 2010). However, the secondary calcium binding sites of amylases are assumed to be linked with thermostability. α-amylase of B. amyloliquefaciens with 17 Ca2+ showed stability toward thermal and surfactant denaturation (Saboury et al. 2005). An increase in enzyme activity was shown by α-amylase from Bacillus sp. ANT-6, whereas, the Zn2+ showed decremented activity (Burhan et al. 2003). However, few of the other enzymes showed calcium independency. B. thermooleovorans NP54 produced a thermostable α-amylase which showed calcium independency (Malhotra et al. 2000). Another calcium-deficient amylase was reported from Bacillus WN11 (Pandey et al. 2000).
Halophiles are also known to produces enzymes suiting the industrial needs where process occur at extreme conditions such as highly saline solutions (de Lourdes Moreno et al. 2013). Most of the halobacterial enzymes are assumed to be thermostable and can be stored at room temperature for longer periods. Halophilic amylases were reported from halophilic bacteria such as Halo arculahispanica and Thalassobacillus sp. LY18 (Hutcheon et al. 2005; Li and Yu 2012). Alkaline and thermotolerant amylases were produced by B. licheniformis and Bacillus halodurans (Setyorini et al. 2006). Thermostable amylase of Bacillus sp. from salt farm showed maximum hydrolysis at a temperature of 110 °C and pH 8.0 (Pancha et al. 2010).
Archaea such as Pyrococcus furiosus and Thermococcus kodakarensis also produces amylase enzyme that is highly thermostable. P. furiosus yielded a 66 kDa amylase which showed significant thermal stability at 100 °C (Laderman et al. 1993). Amylase derived from T. kodakarensis was having a molecular weight of 80 kDa. The enzyme showed optimum activity at 95–100 °C and pH 3.5. Even though it was more active at acidic pH, the enzyme retained most of its activity in alkaline pH also (Ahmad et al. 2014). The bacterial amylase enzyme with its characteristics is shown in Table 11.1.
11.3.2 Actinobacterial Amylases
Streptomyces sp. is another important microbial producer of amylolytic enzymes. Streptomyces erumpens MTCC 7317 produced a thermostable α-amylase of molecular weight 54.5 kDa which is Ca2+ deficient (Kar and Ray 2008). Another amylolytic enzyme was reported from Streptomyces sp. MSC702 and enzyme derived was stable in presence of metal ions such as K+, Co2+, and Mo2+ and showed maximum stability at a temperature of 60 °C (Singh et al. 2014). Streptomyces gulbargensis DAS 131 produced amylase of molecular weight 55 kDa. The optimum pH of the enzyme was about 9.0 and optimum temperature was about 55 °C (Syed et al. 2009). Streptomyces strain A3, Streptomyces avermitilis, etc., were few among the other producers from the genus (Chakraborty et al. 2012; Hwang et al. 2013). Streptomyces megasporus produced thermostable amylase which showed optimum activity at about 85 °C (Dey and Agarwal 1999). Streptomyces fragilis DA7-7 a desert isolate showed thermostable amylase enzyme production that was stable up to 85 °C (Nithya et al. 2017). Amylases from actinomyces is shown in Table 11.2.
11.3.3 Fungal Amylases
Fungus represents another important microbial producer of amylase enzymes. The advantage of solid-state fermentation and economical production of enzyme makes fungus a better choice. Moreover the agro-industrial residues rich in starch undergo bioconversion upon solid-state fermentation using fungi. Aspergillus sp. is a known fungal producer of amylolytic enzymes. Aspergillus niger produced a 43 kDa amylase which showed enhanced the activity in presence of Ca2+ and Co (Varalakshmi et al. 2009), whereas Aspergillus flavus F2Mbb yielded amylase enzyme of molecular mass 56 kDa (Sidkey et al. 2011). A thermotolerant amylase was isolated from A. penicillioides which showed optimum activity at 80 °C. The enzyme was stabilized in presence of CaCl2, while ZnCl2, FeCl2 and EDTA turned to be its inhibitors (Ali et al. 2015). Penicillium citrinum HBF62 is another known fungal amylase enzyme producer which yielded 65 kDa amylolytic enzymes. The enzyme retained 50% of its activity up to 60 °C upon 34 h incubation (Metin et al. 2010). Details of fungal amylolytic enzymes are shown in Table 11.3:
11.4 Recombinant Amylase
Genetic manipulation of microorganisms brings out improved production of enzymes.α-amylase AmyM is a maltohexaose-forming exo-amylase produced by Corallococcus sp. strain EGB. The gene encoding α-amylase AmyM was identified and cloned in E. coli, overexpressed and purified using Ni-NTA affinity chromatography (Lia et al. 2015). Another recombinant protein α-amylase from B. subtilis was cloned and transformed into Saccharomyces cerevisiae (Afzal-Javan and Mobini-Dehkordi 2013). Recombinant production of α-amylase from B. subtilis PY22 was successfully cloned and overexpressed in Pichia pastoris (Karakaş et al. 2010). Thermophilic alpha-amylase gene of B. licheniformis was transformed to B. licheniformis B0204 for hyperproduction (Niu et al. 2009). amy TO1 gene encoding amylase from Streptomyces strain sp. TO1 was successfully cloned into S. lividans for hyper production (Mellouli et al. 1999). amyR4 from B. subtilis KCC103 is a catabolite repression-resistant promoter of alpha-amylase and is useful in hyperproduction of recombinant enzymes in B. subtilis (Nagarajan and Krishnan 2010). Cloning and overexpression of thermo acidophilic, organic solvent-tolerant α-amylase from Bacillus sp. DR90 was successfully achieved in E. coli by Asoodeh et al. (2014). Double deletion mutants of A. oryzae which was lacking genes responsible for carbon catabolite repression showing enhance amylase enzyme production (Ichinose et al. 2014). Directed evolution has emerged as a pivotal tool gene manipulation experiments. Desired properties can be tailor-made by application of these techniques. pH stability and specific activity of α-amylase from B. amyloliquefaciens were enhanced by directed evolution. The mutant enzyme showed fivefold increased activity when compared with wild type (Bessler et al. 2003). Random mutagenesis was achieved by DNA shuffling to attain thermostability of a maltogenic amylase derived from Thermus sp. strain IM6501. The optimum temperature for amylolytic activity was incremented from 50 to 75 °C in the mutant strain (Kim et al. 2003). Acid stability of amylase from B. licheniformis was enhanced by site-directed mutagenesis (Liu et al. 2017). Recent advancements in improvising the characteristics of α-amylase such as pH stability, temperature stability and acid stability has helped in acquiring definite properties to the enzyme.
11.5 Applications of Microbial Amylase
Amylase is one of the most important hydrolytic enzymes used in all starch-based industries and has been in practice since 1984, as a pharmaceutical aid for the treatment of digestive disorders. Amylases are applied in all the industrial processes such as food, detergents, textiles and paper industries, for the hydrolysis of starch. Nowadays, chemical hydrolysis of starch is interchanged with enzymatic hydrolysis using microbial amylases in starch processing industries. One of the major applications of amylases is in food industry and starch processing industry.
11.5.1 Amylase in Food Industry
Starch is the major carbohydrate source and mainly of plant origin. Starch derivatives also have a momentous role in the food, beverage and feed industries, which include cyclodextrin, glucose syrup, hydrolysates, maltodextrin, and other modified starch. Production of starch derivatives is one of the growing industries, where starch modifying enzymes find substantial roles. Amylases find its applications in many food processing industries, like brewing and baking sectors, preparation of fruit juices and starch syrups, etc. (Mobini-Dehkordi and Javan 2012).
Starch in dough can be broken down to α-limit dextrins, the intermediate product starch hydrolysis; along with fermentable sugars in bread baking process and further fermentation of these yield alcohol and CO2 (Prakash and Jaiswal 2010). The presence of low molecular weight dextrins will reduce bread hardness. In wheat flour, the presence of β-amylases are abundant, have little activity on undamaged native starch granules, while α-amylases are absent. Starch hydrolysis in dough is by the combined action of heterogeneously supplied α-amylases and β-amylase. During milling process, the starch granules in flour are sufficiently damaged and make more susceptible to amylases. During baking process, gelatinization of the starch granules occurs, which together with the action of α-amylase cause liquefaction of the starch. Similarly, the β-amylase present in flour converts the dextrins to maltose, which is subsequently fermented by the baker’s yeast. Only small amounts of fermentable sugars are available in the wheat flour. The enzymatic hydrolysis made available enough fermentable sugars in the dough to sustain vigorous yeast fermentation required to produce lively doughs and large loaf volumes. Fungal α-amylases, mainly from A. oryzae are usually used in bread baking to improve volume, color, and flavor, while bacterial α-amylases are used in the preparation of doughs for cakes, biscuits and crackers, where it adds more sweetness (Dekker 1994a).
There may be staling effects during storage of baked products, causing disagreeable changes affecting crumb firmness, crust crispness, moisture content of the crumb and loss of flavor, etc. Upon storage, the short amylopectin side chains present in soft, fresh bread, is gradually get crystallized to amylopectin network, which accounts for a major role in bread firming. Following starch crystallization, moisture migration within the crumb structure occurs, leads to increased crumb firmness and decreased crumb resilience. Bacterial α-amylases with intermediate thermostability are used in anti-staling agents. These limits recrystallisation of amylopectin, its network formation and consequent water immobilization and help to the retention of softness and improve the shelf life of baked food (Jana et al. 2013). The α-amylase from B. stearothermophilus has been employed in the baking industry as an anti-staling agent (Ogasahara et al. 1970; Mobini-Dehkordi and Javan 2012). However, overuse may result in gumminess of the bread, as it produces more branched dextrins. This can be reduced by the use of thermostable pullulanase along with amylase (Kulp et al. 1981). The pullulanase help to hydrolyze the branched dextrins produced by the α-amylase.
Sweetening agents are the major and expensive elements of large variety of confectionary products. Amylolytic enzymes enable starch from low-cost resources to be transformed into sugar syrup. Sucrose is used as a major sweetening agent and starch syrups (glucose) and dextrose occupy a second position. Glucose, fructose, maltose, and higher oligosaccharides, mainly derived from uncooked starch, mostly from cereal and tuber starches. Starch enzymatic hydrolysis using amylases in the starch liquefaction process converts starch into fructose and glucose syrups (Regulapati et al. 2007). The enzymatic conversion of starch is accomplished in a three-step procedure.
-
Step (i) Gelatinization: Production of viscous suspension by dissolving starch;
-
Step (ii) Liquefaction: Loss of viscosity by partial hydrolysis;
-
Step (iii) Saccharification: Further hydrolysis to form glucose and maltose.
The process liquefaction demands thermostable α-amylase that can act at high temperature ranging from 70 to 100 °C (Mobini-Dehkordi and Javan 2012). Due to thermostability, enzymes from Bacillus sp. are most preferred of the industrial applications. α-amylase from B. amyloliquefaciens was used previously, however, it has been substituted by α-amylase of B. licheniformis or B. stearothermophilus (Prakash and Jaiswal 2010).
High-maltose corn syrup (HMCS) is a food additive used as a sweetening agent as well as a preservative. HMCS contains little to no fructose and is less sweet than high-fructose corn syrup. The β-amylase or fungal α-amylase are used to produce glucose syrups containing over 50–70% maltose. HMCS is used in the production of hard candy. It is useful in frozen desserts, since maltose has a low freezing point. Another use of HMCSs is in food preservation, as it can inhibit bacterial growth and fermentation.
High-fructose corn syrup (HFCS) or high-fructose glucose syrup (HFGS) is a liquid alternative sweetener to sucrose (table), first introduced to the food and beverage industry in the 1970s. HFCS has become a major sweetener used extensively in different variety of processed foods and beverages like soft drinks, candies, jams and jellies, yogurts and breads. It has many advantages compared to sucrose, in its sweetness, solubility, acidity, relative cheapness, and that makes it more attractive to the food industry. It contains either 42% or 55% percent fructose depends upon applications and these are referred to in the industry as HFCS 42 and HFCS 55. The HFCS 42 is mainly used in processed foods, cereals, baked goods, and some beverages, while HFCS 55 is used primarily in soft drinks (https://www.fda.gov/Food/default.htm). Starch liquefaction and saccharification achieved using a combined α-amylase glucoamylase process to yield glucose syrup, containing mostly glucose and a third enzyme (glucose isomerase) is used to isomerize glucose in corn syrup to fructose to yield fructose syrup.
Chocolate syrup is prepared by treating cocoa slurries with amylases, in which chocolate starch is dextrinizing and thus syrup becoming thin. These syrups are having a high cocoa content and excellent stability and flow properties at room temperature. This is useful in the production of cocoa flavored frozen confections (Saini et al. 2017).
Maltooligosaccharides mixture, maltotetraose syrup, and anomalously linked oligosaccharide (Alo) mixture are used by food manufacturers as substitutes for sucrose and other saccharides. The replacement sucrose with these mixtures is useful in controlling microbial contamination as well as retrogradation of starchy foods because they have low water activity and high moisture-retaining capacity and also prevent crystallization of sucrose in foods. These products are attractive due to lower viscosity, less sweet taste, and lower freezing point depression. Moreover, they have low-calorie content and have a great appeal to low-calorie dieters. Maltooligomer mixture is obtained by enzymatic hydrolysis of corn starch with α-amylase, β-amylase, and pullulanase. It usually contains 2.2% of glucose, 37.5% of maltose, 46.4% of maltotriose, and 14% of maltotetrose and larger malto oligosaccharides (Marc et al. 2002).
The “Alo mixture” (anomalously linked oligosaccharide) is a mixture of isomaltose, panose, isomaltotriose and branched oligosaccharide composed of four and five glucose residues. The Alo mixture has mildly sweet, low viscosity, high moisture-retaining capacity, and low water activity and makes it favorable for the food industry. It is manufactured by the dextrinization of starch is using thermostable bacterial α-amylase. In this process, the degree of hydrolysis (DE) of starch is kept between 6 and 10 and a reaction of saccharification and transglucosidation of dextrin is done by using soybean β-amylase and transglucosidase from A. niger (Prasanna 2005).
Maltotetraose, (G4) is an oligosaccharide of four units of α-d-glucopyranose linked by α-(1–4) bond. Maltotetraose-forming amylase is used to convert starch to maltotraose. This product finds potential applications in the food industries. It has high moisture retention power and can be used in baking to prevent retrogradation of starch ingredient. It is being used as a food additive to improve the texture, to reduce the sweetness of foods and in frozen food to depress the freezing point. Besides, the G4 syrup is a partially undigested and unabsorbed substrate in the small intestine, has shown a prebiotic effect by selectively promoting the growth and activity of beneficial bacteria, once it reaches the colon (Malabendu et al. 2013). The α-amylase used for G4 syrup production is mainly from B. licheniformis or B. subtilis (Vaidya et al. 2015).
Replacement of fat molecules in foods by lower caloric value fat mimic is a recent strategy in healthy dietary practices. Carbohydrate-based fat mimetics and starch hydrolysis products (SHP) are recommended as it is generating fatty mouthfeel as a phenomenon of rheology. Different starch hydrolytic enzymes like bacterial α-amylase, β-amylase, glucoamylase, and dextrozyme have been used to prepare fat mimics from corn starch (Ma et al. 2006). These fat mimetic substances find application in food industry. Low-fat mayonnaise (with 60% lower fat) produced by incorporating fat mimetics showed comparable sensory quality (Ma et al. 2006).
Food flavor stability is one of the highly desired factors in food and is highly relevant in with its quality and acceptability. Starch‐based ingredients (modified starches, maltodextrins, β‐cyclodextrins) are widely used in the food industry to retain its natural flavor, as carriers for aroma encapsulation or wall material for spray drying process. Amylase enzymes treated starch granules can create a more highly porous structures and such small starch granules have more ability to combine into potentially useful porous spheres when spray dried with small amounts of bonding agents (Madene et al. 2006). Maltodextrins are manufactured the enzymatic hydrolysis of cornflour and is a good candidate as wall material for encapsulation. Bangs and Reineccius (1981) demonstrated the retention of twelve flavor compounds depends on the dextrose equivalents (DE) of the maltodextrins. Starches modified with octenyl succinic anhydride (OSA) have been used in the food industry and useful stabilizing and encapsulating agent. β-amylolysis is being used under appropriate conditions to modify the structure of gelatinized OSA-modified starches to increase its emulsification properties (Sweedman et al. 2013).
Another major application of amylase enzyme is in beer brewing. Beer is produced by yeast fermentation of sugars. Beer is traditionally based on barley, contain a large quantity of starch and before the yeast fermentation to produce alcohol, starch must be converted to fermentable sugars. Mashing (malting) is the process in which enzymatic degradation of starch into fermentable sugars (maltose) occurs and is a complex process that involves many enzymes like α‐amylase, β‐amylase, α‐glucosidase, and limit dextrinase, etc. (Manners 1985). The α-amylase act on α-1,4 linkages at random, while β-amylases are exo-enzymes which attack the liquefied starch chains forming maltose units from the nonreducing end. Modern brewers usually supplement these enzymes and it is essential when grains other than barley are used. The thermostable α-amylases from B. subtilis, and those from A. oryzae (with glucoamylase activity), and glucoamylases from A. niger, are usually used in mashing for starch hydrolysis (Dekker 1994b).
Enzymes widely used in the maceration of fruit pulps and for clarification of fruit juices and has contributed to improving quality and yield of different types of juices. The application of enzymes fruit juice clarification will depend on the kind of polysaccharides present in different fruit juices. Pectinase plays a major role in clarification process (depectinization), whereas amylase is preferred where the presence of starch. These enzymes are used in combination with cellulose and hemicellulase to effectively reduce haziness in juices. Clarified apple juice is one of the most consumed fruit juices. Raw apple juice obtained as turbid, viscous and tends to settle during storage, due to the presence of polysaccharides (pectin and starch), tannins, proteins, etc. Amylases are used degrade starch present in the apple juice (Carrin et al. 2004). Amylase along with pectinases and other hydrolases are being used in the clarification of banana juice (0.02% amylase and 0.084% pectinase), Kiwifruit juice (0.025% amylases, 0.025% pectinases, 0.05% mash enzyme), and Passion Fruit Juice (0.001% amylase along with cellulase and pectinase) (Singh and Singh 2015).
11.5.2 Non Food Applications
Enzyme-based detergents are currently used in laundry, dishwashing, textile, and other such industries and they are also acknowledged as green chemicals. The amylase in the detergent mainly degrades the residues of starchy food like porridge, potatoes, gravies, custard, chocolate, etc., to dextrins (Kumar et al. 1998). The main advantage of the enzyme application in detergents is that they require only much milder conditions than that with enzyme-free detergents (Kirk et al. 2002). The demand for α-amylase for automatic dishwashing detergents is now growing.
Modern production processes for textiles introduce a considerable strain on the warp during weaving. The yarn must, therefore, be prevented from breaking. Ease of availability, lower cost, and ease of removal makes starch, the most preferred sizing agent. Effective de-sizing without harming the fibers can be attained by α-amylases in starch-sized textiles, where it selectively removes the sizing agent (Feitkenhauer 2003).
The use of α-amylases in the pulp and paper industry is to provide modification on starch coated paper (Van Der Maarel et al. 2002). Another application of α-amylases in the textile industry is in making faded jeans. The process of enzyme fade is also called as bio washing or bio-bleaching retains the softness of the cloth while embossing shades in it (Kumar et al. 1998).
The α-amylases also find its usage in medical applications. Synthetic and natural biodegradable polymers have been a major focus of interest in pharmaceutical research. The biodegradable polymers are used to control the drug release rate from parenteral controlled delivery systems (Dumoulin et al. 1999). Starch-based biodegradable polysaccharide matrix is useful for drugs with lesser solubility. By incorporating α-amylase along with cross-linked amylose (CLA), the drug release can be controlled (Afzal-Javan and Mobini-Dehkordi 2013).
Starch and related polysaccharides in waste material produced by agro-industries causes environmental pollution. The starch pollution can be overcome either by application of purified microbial amylolytic enzymes or by using amylolytic microorganisms directly (Wu et al. 2008; Mobini-Dehkordi and Javan 2012).
Starch is the major substrate for bioethanol production since it is widely distributed and economical. Conventional bioethanol production converts starch to fermentable sugars called saccharification by amylolytic enzyme producers or enzymes, followed by fermentation of the sugar formed to ethanol by microorganisms such as Saccharomyces cerevisiae, Saccharomycopsis fibuligera etc. (Tesfaw and Assefa 2014; Chi et al. 2009).
11.6 Conclusion
Amylases share a major stake in the global enzyme market, and a wide range of applications demands newer enzymes with improved functionalities. Enzymes from Bacillus sp. have been industrially significant for quite a long time. In starch processing industries, thermally stable enzymes are in great demand since starch liquefaction occurs in higher temperatures. Apart from Bacillus sp. few members of Archaea has showed remarkable thermal stability. In fact, thermostable enzymes were obtained not only from extremophiles but mesophiles also. Enzymatic hydrolysis of starch yields high-quality products such as glucose and maltose syrups with definite characteristics. Fat mimetic substances produced by amylases could bring changes in the food industry for the production highly nutritious low-fat diet. With large number of starchy agro-industrial waste, residues expelled to the environment, hyperproducers of amylolytic enzymes are needed. These microorganisms can bring rapid biomass conversion while yielding these enzymes. With advent of recombinant DNA technology, amylases with improved properties can be tailor-made to suit the needs of industry. Increasing demand of these enzymes makes the research on this topic fascinating.
References
Abdel-Fattah YR, Soliman NA, El-Toukhy NM, El-Gendi H, Ahmed RS (2012). Production, purification, and characterization of thermostable α-amylase produced by Bacillus licheniformis isolate AI20. J Chem
Acer Ö, Bekler FM, Pirinççioğlu H, Güven RG, Güven K (2016) Purification and characterization of thermostable and detergent-stable α-amylase from Anoxybacillus sp. AH1. Food Tech Biotechnol 54(1):70
Afzal-Javan F, Mobini-Dehkordi M, Saffar B (2013) Amplification, sequencing and cloning of iranian native Bacillus subtilis alpha-amylase gene in Saccharomyces cerevisiae. Jundishapur J Microbiol 6(8):1–7
Ahmad N, Rashid N, Haider MS, Akram M, Akhtar M (2014) Novel maltotriose-hydrolyzing thermoacidophilic type III pullulan hydrolase from Thermococcus kodakarensis. Appl Environ Microbiol 80(3):1108–1115
Aiyer PV (2005) Amylases and their applications. Afr J Biotechnol 4(13)
Aleshin A, Golubev A, Firsov LM, Honzatko RB (1992) Crystal structure of glucoamylase from Aspergillus awamori var. X100 to 2.2-A resolution. J Biol Chem 267(27):19291–19298
Ali I, Akbar A, Anwar M, Prasongsuk S, Lotrakul P, Punnapayak H (2015) Purification and characterization of a polyextremophilic α-Amylase from an obligate halophilic Aspergillus penicillioides isolate and its potential for souse with detergents. In: BioMed research international
Ali I, Akbar A, Yanwisetpakdee B, Prasongsuk S, Lotrakul P, Punnapayak H (2014) Purification, characterization, and potential of saline waste water remediation of a polyextremophilic α-amylase from an obligate halophilic Aspergillus gracilis. In: BioMed research international
Amoozegar MA, Malekzadeh F, Malik KA (2003) Production of amylase by newly isolated moderate halophile, Halobacillus sp. strain MA-2. J Microbiol Method 52(3):353–359
Argüello-Morales MA, Remaud-Simeon M, Pizzut S, Sarçabal P, Willemot R, Monsan P (2000) Sequence analysis of the gene encoding alternansucrase, a sucrase glucosyltransferase from Leuconostoc mesenteroides NRRL B-1355. FEMS Microbiol Lett 182(1):81-85
Asoodeh A, Emtenani S, Emtenani S, Jalal R, Housaindokht MR (2014) Molecular cloning and biochemical characterization of a thermoacidophilic, organic-solvent tolerant α-amylase from a Bacillus strain in Escherichia coli. J Mol Catal B Enzym 99:114–120
Banerjee G, Mukherjee S, Bhattacharya S, Ray AK (2016) Purification and characterization of extracellular protease and amylase produced by the bacterial strain, Corynebacterium alkanolyticum ATH3 isolated from fish gut. Arab J Sci Eng 41(1):9–16
Bangs WE, Reineccius GA (1981) Influence of dryer infeed matrices on retention of volatile flavor compounds during spray-drying. J Food Sci 47:254–259
Bano S, Qader SAU, Aman A, Syed MN, Azhar A (2011) Purification and characterization of novel α-amylase from Bacillus subtilis KIBGE HAS. AAPS PharmSciTech 12(1):255–261
Bertoft E (2007) Composition of clusters and their arrangement in potato amylopectin. Carbohyd Polym 68(3):433–446
Bessler C, Schmitt J, Maurer KH, Schmid RD (2003) Directed evolution of a bacterial α-amylase: toward enhanced pH-performance and higher specific activity. Protein Sci 12(10):2141–2149
Bukhari DA, Rehman A (2015) Purification and characterization of α-amylase from Bacillus subtilis isolated from local environment. Pak J Zool 47(4)
Burhan A, Nisa U, Gökhan C, Ömer C, Ashabil A, Osman G (2003) Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. isolate ANT-6. Process Biochem 38(10):1397–1403
Carrin ME, Ceci LN, Lozano JE (2004) Characterization of starch in apple juice and its degradation by amylases. Food Chem 87:173–178
Chakraborty S, Khopade RGA, Mahadik K, Kokare C (2012) Study on calcium ion independent α-amylase from haloalkaliphilic marine Streptomyces strain A3. Indian J Biotechnol 11(4):427–437
Chi Z, Chi Z, Liu G, Wang F, Ju L, Zhang T (2009) Saccharomycopsis fibuligera and its applications in biotechnology. Biotechnol Adv 27(4):423–431
de Lourdes Moreno M, Pérez D, García MT, Mellado E (2013) Halophilic bacteria as a source of novel hydrolytic enzymes. Life 3(1):38–51
Dekker RFH (1994a) Enzymes in food and beverage processing-1. Food Aust 46(3):136–139
Dekker RFH (1994b) Enzymes in food and beverage processing-2 Food Aust 46(4):179–181
Dey S, Agarwal SO (1999) Characterization of a thermostable alpha-amylase from a thermophilic Streptomyces megasporus strain SD12. Indian J Biochem Biophys 36:150–157
Dey TB, Banerjee R (2015) Purification, biochemical characterization and application of α-amylase produced by Aspergillus oryzae IFO-30103. Biocatal Agric Biotechnol 4(1):83–90
Du R, Song Q, Zhang Q, Zhao F, Kim RC, Zhou Z, Han Y (2018) Purification and characterization of novel thermostable and Ca-independent α-amylase produced by Bacillus amyloliquefaciens BH072. Int J Biol Macromol 115:1151–1156
Dumoulin Y, Cartilier LH, Mateescu MA (1999) Cross-linked amylose tablets containing α-amylase: an enzymatically-controlled drug release system. J Controll Release 60(2–3):161–167
El-Fallal A, Dobara MA, El-Sayed A, Omar N (2012) Starch and microbial α-amylases: from concepts to biotechnological applications. In: Carbohydrates-comprehensive studies on glycobiology and glycotechnology. InTech
El-Sayed AK, Dobara MIA, El-Fallal AA, Omar NF (2013) Purification, sequencing, and biochemical characterization of a novel calcium-independent α-amylase AmyTVE from Thermoactinomyces vulgaris. Appl Biochem Biotechnol 170(3):483–497
Feitkenhauer H (2003) Anaerobic digestion of desizing wastewater: influence of pretreatment and anionic surfactant on degradation and intermediate accumulation. Enzyme Microb Technol 33(2–3):250–258
Fincan SA, Enez B, Özdemir S, Bekler FM (2014) Purification and characterization of thermostable α-amylase from thermophilic Anoxybacillus flavithermus. Carbohyd Polym 102:144–150
Harris EMS, Aleshin AE, Firsov LM, Honzatko RB (1993) Refined structure for the complex of 1-deoxynojirimycin with glucoamylase from Aspergillus awamori var. X100 to 2.4 Å. resolution. Biochemistry 32(6):1618–1626
Henrissat B, Bairoch A (1993) New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 293(3):781–788
Hii SL, Tan JS, Ling TC, Ariff AB (2012) Pullulanase: role in starch hydrolysis and potential industrial applications. Enzyme Res
Hirata A, Adachi M, Sekine A, Kang YN, Utsumi S, Mikami B (2004) Structural and enzymatic analysis of soybean β-amylase mutants with increased pH optimum. J Biol Chem 279(8):7287–7295
Horvathova V, Janecek S, Sturdik E (2001) Amylolytic enzymes: molecular aspects of their properties. Gen Physiol Biophys 20(1):7–32
Hutcheon GW, Vasisht N, Bolhuis A (2005) Characterisation of a highly stable α-amylase from the halophilic archaeon Haloarcula hispanica. Extremophiles 9(6):487–495
Hwang SY, Nakashima K, Okai N, Okazaki F, Miyake M, Harazono K, Ogino C, Kondo A (2013) Thermal stability and starch degradation profile of α-amylase from Streptomyces avermitilis. Biosci Biotechnol Biochem 77(12):2449–2453
Ichinose S, Tanaka M, Shintani T, Gomi K (2014) Improved α-amylase production by Aspergillus oryzae after a double deletion of genes involved in carbon catabolite repression. Appl Microbiol Biotechnol 98(1):335–343
Jana M, Maity C, Samanta S, Pati BR, Islam SS, Mohapatra PKD, Mondal KC (2013) Salt-independent thermophilic α-amylase from Bacillus megaterium VUMB109: an efficacy testing for preparation of maltooligosaccharides. Ind Crops Prod 41:386–391
Janeček Š (1994) Parallel β/α-barrels of α-amylase, cyclodextrin glycosyltransferase and oligo-1, 6-glucosidase versus the barrel of β-amylase: evolutionary distance is a reflection of unrelated sequences. FEBS Lett 353(2):119–123
Janeček Š (2002) How many conserved sequence regions are there in the α-amylase family. Biologia 57(Suppl 11):29–41
Kaplan F, Guy CL (2004) β-Amylase induction and the protective role of maltose during temperature shock. Plant Physiol 135(3):1674–1684
Kar S, Ray RC (2008) Statistical optimization alpha-amylase production by Streptomyces erumpens MTCC 7317 cells in calcium alginate beads using response surface methodology. Polish J Microbiol 57(1):49–57
Kar S, Ray RC, Mohapatra UB (2008) Alpha-amylase production by Streptomyces erumpens MTCC 7317 in solid state fermentation using response surface methodology (RSM). Polish J Microbiol 57(4):289-96
Karakaş B, İnan M, Certel M (2010) Expression and characterization of Bacillus subtilis PY22 α-amylase in Pichia pastoris. J Mol Catal B Enzym 64(3–4):129–134
Karmakar M, Ray RR (2011) A maltotriose producing thermostable amylase from Bacillus sp KR11. J Microbiol Biotechnol Res 1(3):91–99
Kim YW, Choi JH, Kim JW, Park C, Kim JW, Cha H, Lee SB, Oh BH, Moon TW, Park KH (2003) Directed evolution of Thermus maltogenic amylase toward enhanced thermal resistance. Appl Environ Microbiol 69(8):4866–4874
Kirk O, Borchert TV, Fuglsang CC (2002) Industrial enzyme applications. Curr Opin Biotechnol 13(4):345–351
Konsoula Z, Liakopoulou-Kyriakides M (2007) Co-production of α-amylase and β-galactosidase by Bacillus subtilis in complex organic substrates. Biores Technol 98(1):150–157
Kulp K, Ponte JG Jr, D’Appolonia BL (1981) Staling of white pan bread: fundamental causes. Crit Rev Food Sci Nutr 15(1):1–48
Kumar CG, Malik RK, Tiwari MP (1998) Novel enzyme-based detergents: an Indian perspective. Curr Sci 75(12):1312–1318
Kumar MDJ, Jayanthisiddhuraj AB, Monica DD, Balakumaran MD, Kalaichelvan PT (2012) Purification and characterization of α-amylase and α–galactosidase from Bacillus Sp. MNJ23 produced in a concomitant medium. Am Eurasian J Agric Environ Sci 12(5):566–573
Kumar P, Satyanarayana T (2009) Microbial glucoamylases: characteristics and applications. Crit Rev Biotechnol 29(3):225–255
Kuttigounder D, Lingamallu JR, Bhattacharya S (2011) Turmeric powder and starch: selected physical, physicochemical, and microstructural properties. J Food Sci 76(9):C1284–C1291
Laderman KA, Davis BR, Krutzsch HC, Lewis MS, Griko YV, Privalov PL, Anfinsen CB (1993) The purification and characterization of an extremely thermostable alpha-amylase from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Biol Chem 268(32):24394–24401
Li X, Yu HY (2012) Characterization of an organic solvent-tolerant α-amylase from a halophilic isolate, Thalassobacillus sp. LY18. Folia Microbiol 57(5):447–453
Li Z, Wu J, Zhang B, Wang F, Ye X, Huang Y, Huang Q, Cui Z (2015) Characterization of a novel maltohexaose-forming α-amylase AmyM from Corallococcus sp. strain EGB. Appl Env Microbiol, pp. AEM-03934
Linden A, Mayans O, Meyer-Klaucke W, Antranikian G, Wilmanns M (2002) Differential regulation of a hyperthermophilic α-amylase with a novel (Ca, Zn)-dimetal centre by zinc. J Biol Chem
Liu Y, Huang L, Jia L, Gui S, Fu Y, Zheng D, Guo W, Lu F (2017) Improvement of the acid stability of Bacillus licheniformis alpha amylase by site-directed mutagenesis. Process Biochem 58:174–180
Liu Y, Lu F, Chen G, Snyder CL, Sun J, Li Y, Wang J, Xiao J (2010) High-level expression, purification and characterization of a recombinant medium-temperature α-amylase from Bacillus subtilis. Biotech Lett 32(1):119
Ma Y, Cai C, Wang J, Sun DW (2006) Enzymatic hydrolysis of corn starch for producing fat mimetics. J Food Eng 73(3):297–303
MacGregor EA, Jespersen HM, Svensson B (1996) A circularly permuted alpha-amylase-type alpha/beta-barrel structure in glucan-synthesizing glucosyltransferases. FEBS Lett 378(3):263-266
Madene A, Jacquot M, Scher J, Desobry S (2006) Flavour encapsulation and controlled release—a review. Int J Food Sci Technol 41:1–21
Maity S, Mallik S, Basuthakur R, Gupta S (2015) Optimization of solid state fermentation conditions and characterization of thermostable alpha amylase from Bacillus subtilis (ATCC 6633). J Bioprocess Biotech 5:218
Malabendu J, Chiranjit M, Saptadip S, Bikas RP (2013) Salt-independent thermophilic α-amylase from Bacillus megaterium VUMB109: an efficacy testing for preparation of maltooligosaccharides. Ind Crops Prod 41:386–390
Malhotra R, Noorwez SM, Satyanarayana T (2000) Production and partial characterization of thermostable and calcium-independent α-amylase of an extreme thermophile Bacillus thermooleovorans NP54. Lett Appl Microbiol 31(5):378–384
Manners DJ (1985) Some aspects of the metabolism of starch. Cereal Foods World 30:722–727
Marc JEC, Maarel V, Veen BV, Uitdehaag JCM, Leemhuis H, Dijkhuizen L (2002) Properties and applications of starch-converting enzymes of the α-amylase family. J Biotechnol 94:137–155
Matsuura Y (2002) A possible mechanism of catalysis involving three essential residues in the enzymes of alpha-amylase family. Biologia 57(Supp 2):21–28
Matsuura Y, Kusunoki M, Harada W, Kakudo M (1984) Structure and possible catalytic residues of Taka-amylase A. J Biochem 95(3):697–702
Mehta D, Satyanarayana T (2014) Domain C of thermostable α-amylase of Geobacillus thermoleovorans mediates raw starch adsorption. Appl Microbiol Biotechnol 98(10):4503–4519
Mellouli L, Guerineau M, Bejar S, Virolle MJ (1999). Regulation of the expression of amy TO1 encoding a thermostable α-amylase from Streptomyces sp. TO1, in its original host and in Streptomyces lividans TK24. FEMS Microbiol Lett 181(1):31–39
Metin K, Ateşlier ZBB, Bıyık HH (2010) Purification and characterization of-amylase produced by Penicillium citrinum HBF62. Afr J Biotech 9(45):7692–7701
Mikami B, Degano M, Hehre EJ, Sacchettini JC (1994) Crystal structures of soybean beta-amylase reacted with beta-maltose and maltal: active site components and their apparent roles in catalysis. Biochemistry 33(25):7779–7787
Mobini-Dehkordi M, Javan FA (2012) Application of alpha-amylase in biotechnology. J Biol Today’s World 1(1):15–20
Mojsov K (2012) Microbial alpha-amylases and their industrial applications: a review. Int J f Manag IT Eng (IJMIE) 2(10):583–609
Myat L, Ryu G (2013) Extrusion with thermostable α-amylase injection as pretreatment method for ethanol production from corn starch. J Microb Biochem Technol 5(2):47–53
Nagarajan DR, Krishnan C (2010) Use of a new catabolite repression resistant promoter isolated from Bacillus subtilis KCC103 for hyper-production of recombinant enzymes. Protein Expr Purif 70(1):122–128
Naidu MA, Saranraj P (2013) Bacterial amylase a review. Int J Pharm Biol Arch 4(2):274–287
Nithya K, Muthukumar C, Kadaikunnan S, Alharbi NS, Khaled JM, Dhanasekaran D (2017) Purification, characterization, and statistical optimization of a thermostable α-amylase from desert actinobacterium Streptomyces fragilis DA7–7. 3 Biotech 7(5):350
Niu DD, Shi GY, Wang ZX (2009) Genetic improvement of alpha-amylase producing Bacillus licheniformis by homolog-mediated alpha-amylase gene amplification. Chin J Biotechnol 25(3):375–380
Obafemi YD, Ajayi AA, Olasehinde GI, Atolagbe OM, Onibokun EA (2018) Screening and partial purification of amylase from Aspergillus Niger isolated from deteriorated tomato (Lycopersicon Esculentum mill.) fruits. Afr J Clin Exp Microbiol 19(1):47–57
Ogasahara K, Imanishi A, Isemura T (1970) Studies on thermophilic α-amylase from Bacillus stearothermophilus. J Biochem 67(1):65–75
Özdemir S, Matpan F, Güven K, Baysal Z (2011) Production and characterization of partially purified extracellular thermostable α-amylase by Bacillus subtilis in submerged fermentation (SmF). Prep Biochem Biotechnol 41(4):365–381
Pancha I, Jain D, Shrivastav A, Mishra SK, Shethia B, Mishra S, Mohandas VP, Jha B (2010) A thermoactive α-amylase from a Bacillus sp. isolated from CSMCRI salt farm. Int J Biol Macromol 47(2):288–291
Pandey A, Nigam PR, Sccol C, Soccol T, Singh V, Mohan DR (2000) Advances in microbial amylases. Biotechnol Appl Biochem 31:135–152
Pasin TM, Benassi VM, Heinen PR, de Lima Damasio AR, Cereia M, Jorge JA, de Moraes MDLT (2017) Purification and functional properties of a novel glucoamylase activated by manganese and lead produced by Aspergillus japonicus. Int J Biol Macromol 102:779–788
Pavithra S, Ramesh R, Aarthy M, Ayyadurai N, Gowthaman MK, Kamini NR (2014) Starchy substrates for production and characterization of Bacillus subtilis amylase and its efficacy in detergent and bread making formulations. Starch 66(11–12):976–984
Prakash O, Jaiswal N (2010) α-Amylase: an ideal representative of thermostable enzymes. Appl Biochem Biotechnol 160(8):2401–2414
Prasanna VA (2005) Amylases and their applications. Afr J Biotech 4(13):1525–1529
Przylas I, Tomoo K, Terada Y, Takaha T, Fujii K, Saenger W, Sträter N (2000) Crystal structure of amylomaltase from Thermus aquaticus, a glycosyltransferase catalysing the production of large cyclic glucans1. J Mol Biol 296(3):873–886
Ragunathan R, Padhmadas R (2013) Production, purification and characterization of-amylase using Streptomyces spp. PDS1 and Rhodococcus spp. Isolated from Western Ghats. Int J Curr Microbiol Appl Sci 2(8):206–214
Rai S, Solanki MK (2014) Optimization of thermostable alpha-amylase production via mix agricultural-residues and Bacillus amyloliquefaciens. Not Sci Biol 6(1):105–111
Singh R, Singh RK (2015) Role of enzymes in fruit juices clarification during processing: a review. Int J Biol Technol 6(1):1–12
Raul D, Biswas T, Mukhopadhyay S, Kumar Das S, Gupta S (2014) Production and partial purification of alpha amylase from Bacillus subtilis (MTCC 121) using solid state fermentation. Biochem Res Int
Regulapati R, Malav NP, Gummadi NS (2007) Production of thermostable c-amylases by solid state fermentation: a review. Am J Food Technol 2:1–11
Roohi R, Kuddus M, Saima S (2013) Cold-active detergent-stable extracellular α-amylase from Bacillus cereus GA6&58; biochemical characteristics and its perspectives in laundry detergent formulation. J Biochem Technol 4(4):636–644
Saboury AA, Ghasemi S, Dahot MU (2005) Thermodynamic study of magnesium ion binding to alpha-amylase. Indian J Biochem Biophys 42:326–329
Saini R, Saini HS, Dahiya A (2017) Amylases: characteristics and industrial applications. J Pharmacogn Phytochem 6(4):1865–1871
Sajedi RH, Naderi-Manesh H, Khajeh K, Ahmadvand R, Ranjbar B, Asoodeh A, Moradian F (2005) A Ca-independent α-amylase that is active and stable at low pH from the Bacillus sp. KR-8104. Enzyme Microb Technol 36(5–6):666–671
Sarethy IP, Saxena Y, Kapoor A, Sharma M, Seth R, Sharma H, Sharma SK, Gupta S (2012). Amylase produced by Bacillus sp. SI-136 isolated from sodic-alkaline soil for efficient starch desizing. J Biochem Technol 4(2):604–609
Sen SK, Jana A, Bandyopadhyay P, Mohapatra PKD, Raut S (2016) Thermostable amylase production from hot spring isolate Exiguobacterium sp: a promising agent for natural detergents. Sustain Chem Pharm 3:59–68
Setyorini E, Takenaka S, Murakami S, Aoki K (2006) Purification and characterization of two novel halotolerant extracellular proteases from Bacillus subtilis strain FP-133. Biosci Biotechnol Biochem 70(2):433–440
Ševčík J, Solovicová A, Hostinová E, Gašperík J, Wilson KS, Dauter Z (1998) Structure of glucoamylase from Saccharomycopsis fibuligera at 1.7 Å resolution. Acta Crystallogr Sect D 54(5):854–866
Shivlata L, Satyanarayana T (2017) Characteristics of raw starch-digesting α-amylase of Streptomyces badius DB-1 with transglycosylation activity and its applications. Appl Biochem Biotechnol 181(4):1283–1303
Sidkey NM, Abo-Shadi MA, Balahmar R, Sabry R, Badrany G (2011) Purification and characterization of α-amylase from a newly isolated Aspergillus flavus F2Mbb. Int Res J Microbiol 2(3):096–103
Singh R, Kumar V, Kapoor V (2014) Partial purification and characterization of a heat stable α-amylase from a thermophilic actinobacteria, Streptomyces sp. MSC702. Enzyme Res
Smitha RB, Sajith S, Priji P, Unni KNN, Roy TAN, Benjamin S (2015) Purification and characterization of amylase from Bacillus thuringiensis subsp. kurstaki. Bt Res 6
Stam MR, Danchin EG, Rancurel C, Coutinho PM, Henrissat B (2006) Dividing the large glycoside hydrolase family 13 into subfamilies: towards improved functional annotations of α-amylase-related proteins. Protein Eng Des Sel 19(12):555–562
Sumrin A, Ahmad W, Ijaz B, Sarwar MT, Gull S, Kausar H, Shahid I, Jahan S, Asad S, Hussain M, Riazuddin S (2011) Purification and medium optimization of α-amylase from Bacillus subtilis 168. Afr J Biotech 10(11):2119–2129
Sweedman MC, Hasjim J, Tizzotti MJ, Schäfer C, Gilbert RG (2013) Effect of octenylsuccinic anhydride modification on β-amylolysis of starch. Carbohydr Polym 97(1):9–17
Syed DG, Agasar D, Pandey A (2009) Production and partial purification of α-amylase from a novel isolate Streptomyces gulbargensis. J Ind Microbiol Biotechnol 36(2):189–194
Tesfaw A, Assefa F (2014) Current trends in bioethanol production by Saccharomyces cerevisiae: substrate, inhibitor reduction, growth variables, coculture, and immobilization. Int Sch Res Not
Thompson DB (2000) On the non-random nature of amylopectin branching. Carbohyd Polym 43(3):223–239
Tiwari SP, Srivastava R, Singh CS, Shukla K, Singh RK, Singh P, Singh R, Singh NL, Sharma R (2015) Amylases: an overview with special reference to alpha amylase. J Global Biosci 4:1886–1901
Totsuka A, Fukazawa C (1996) Functional analysis of Glu380 and Leu383 of soybean β-amylase: a proposed action mechanism. Eur J Biochem 240(3):655–659
Vaidya S, Srivastava PK, Rathore P, Pandey AK (2015) Amylases: a prospective enzyme in the field of biotechnology. J Appl Biosci 41(1):1–18
Van Der Maarel MJ, Van der Veen B, Uitdehaag JC, Leemhuis H, Dijkhuizen L (2002) Properties and applications of starch-converting enzymes of the α-amylase family. J Biotechnol 94(2):137–155
Varalakshmi KN, Kumudini BS, Nandini BN, Solomon J, Suhas R, Mahesh B, Pavitha AP (2009) Production and characterization of α-amylase from Aspergillus niger JGI24 isolated in Banglore. Polish J Microbiol 58(1):29–36
Vengadaramana A (2013) Industrial important microbial alpha-amylase on starch-converting process. Scholars Acad J Pharm 2(3):209–221
Weemaes C, De Cordt S, Goossens K, Ludikhuyze L, Hendrickx M, Heremans K, Tobback P (1996) High pressure, thermal and combined pressure–temperature stabilities of α-amylases from Bacillus species. Biotechnol Bioeng 50(1):49–56
Wu CH, Mulchandani A, Chen W (2008) Versatile microbial surface-display for environmental remediation and biofuels production. Trends Microbiol 16(4):181–188
Wu X, Wang Y, Tong B, Chen X, Chen J (2018) Purification and biochemical characterization of a thermostable and acid-stable alpha-amylase from Bacillus licheniformis B4-423. Int J Biol Macromol 109:329–337
Xian L, Wang F, Luo X, Feng YL, Feng JX (2015) Purification and characterization of a highly efficient calcium-independent α-amylase from Talaromyces pinophilus 1-95. PLoS ONE 10(3):e0121531
Yassien MAM, Asfour HZ (2012) Improved production, purification and some properties of α-amylase from Streptomyces clavifer. Afr J Biotech 11(80):14603–14611
Yu HY, Li X (2014) Characterization of an organic solvent‐tolerant thermostable glucoamylase from a halophilic isolate, Halolactibacillus sp. SK 71 and its application in raw starch hydrolysis for bioethanol production. Biotechnol progress 30(6):1262–1268
Zhang F, Yang X, Geng L, Zhang Z, Yin Y, Li W (2016) Purification and characterization of a novel and versatile α‐amylase from thermophilic Anoxybacillus sp. YIM 342. Starch 68(5–6):446–453
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Balakrishnan, D., Kumar, S.S., Sugathan, S. (2019). Amylases for Food Applications—Updated Information. In: Parameswaran, B., Varjani, S., Raveendran, S. (eds) Green Bio-processes. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-13-3263-0_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-3263-0_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3262-3
Online ISBN: 978-981-13-3263-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)