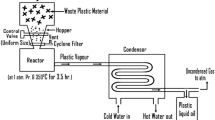
Abstract
In this work, plastic oil derived from the kaoline catalyzed pyrolysis of waste polypropylene plastics are used as alternate fuels along with diesel in CI engine. The performance characteristics of the plastic oil blended with 10, 20, 30, 40, and 50% diesel are analyzed and compared with diesel fuel operation. Experiments have been carried out to analyze the Energy and Exergy characteristics of diesel engine fuelled by plastic oil blended diesel fuel. The energetic and exergetic performance of waste plastic oil blended diesel is found higher than the diesel fuel operation.
Graphical Abstract
The schematic diagram explains the consumption of air-fuel mixture fed to a diesel engine and extraction of useful work from the engine. It also quantifies different losses of energy in different media associated with the engine during power generation.

Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The extensive uses of fossil derived fuels are considered unsustainable as such fuel resources are destined to deplete and cause environmental hazards during their uses [1]. Hence, many investigators have been trying to explore various alternate energy resources and improve efficiency of energy consuming device to sustainably meet the growing global energy demands while minimizing the environmental pollutions [2]. On the other hand, the disposal problems of waste plastics and its sustainable utilization is becoming more and more significant in the present scenario. The catalytic pyrolysis of plastic waste to liquid fuel is one of the remarkable ways to recycle the plastic waste and the plastic pyrolysis oil has the matching characteristics and requisition with that of diesel, and thus acts as a substitute of diesel oil in terms of engine performance and energy output [3].
The engine performance in terms of energy output can be understood through energy and exergy analysis. Study on the energetic and exegetic performance of diesel engine under different operational conditions is reported in the literature [4]. Energy analysis done based on the first law of thermodynamics is not sufficient to understand the sustainability of fuel as the limitations of second law of thermodynamics is not considered. However, another quantity exergy being calculated based on the both first and second laws of thermodynamics is closely related to the concept of renewability and sustainability. Exergy analysis is a robust technique for understanding the unsteadiness of a thermodynamic system for energy conversion. Currently, it has become a popular technique to manage with the problems related to the widespread utilization of different energy resources and their subsequent environmental issues [5]. Exergy analysis is also used by researchers to evaluate and optimize the performance of internal combustion (IC) engines running on different (non-renewable and renewable fuels and their blends) fuels [6,7,8,9] and extensively reported recently and summarized as follows.
Canakci and Hosoz studied the performance of a four-cylinder turbocharged diesel engine using different biodiesels and petro diesel fuel and compared the performance based on their exergy analysis [10]. The energy and exergy analysis of a diesel engine running with diesel, soybean oil methyl ester, and high-oleic soybean oil was done by Caliskan et al. [11]. Da Costa et al. investigated the performance of a dual-fuel diesel engine running on natural gas and diesel [12]. Exergetic performance assessment of a diesel engine running on oliveepomace oil biodiesel, diesel fuel, and their mixtures was reported by Lopez et al. [13]. Aghbashlo et al. reported the improvement of exergetic performance of a diesel engine using expanded polystyrene as an additive [14]. Caliskan et al. reported an increase in the exergetic efficiency with decreased dead state temperature in a diesel engine fuelled with soybean biodiesel by energy exergy analysis [15]. The performance of a four-cylinder SI engine using three different research octane numbers gasoline fuels was studied by Sayin et al. by energy and exergy analysis [16]. Wiley Sekmen and Yilbasi compared the energetic and exergetic performance of diesel and soybean biodiesel in a four-cylinder, direct injection diesel engine [17]. The effect of compression ratio and injection timing on energy and exergy potential of a palm oil methyl ester run diesel engine was studied by Biplab et al. [18]. A comparative performance of a diesel engine with mahua biodiesel blended diesel by energy and exergy analysis was reported by Panigrahi et al. [19]. Energy and exergy analyses diesel engine for 20% blends of neem oil methylester diesel fuel is carried out by Panigrahi et al. and the blended fuel is found to have low exhaust gas energy loss, higher rate of heat transfer and shows similar trends of energetic and exergetic performance with diesel fuel [20]. From the above studies, it can be concluded that, energy and exergy analysis could help understanding the performance of an alternate fuel, identifying optimum operating condition including fuel blends and engine parameters to design more cost-effective and eco-friendly operations. The objective of the present work is to evaluate the performance of the CI engines fuelled with plastic oil blended diesel.
2 Experimental Investigation
The waste plastic oil used in this experiment is obtained from the kaolin-catalyzed pyrolysis of waste polypropylene at 500 °C with plastics to catalyst ratio 3:1 using a batch reactor reported in our work [3]. The GC-MS composition and physical properties of the plastic oil is summarized in Tables 1 and 2 respectively. The result indicates the presence of different hydrocarbons, mostly alkenes and alkenes along with some fraction of oxygenated organic compounds, such as alcohols and ketones with C10-C12, in the boiling range of 68–346 °C, which infers the presence of a mixture of different oil components equivalent to gasoline, kerosene, and diesel in the oil [3].
The engine performance was carried out on a compression ignition test engine and is well calibrated to reduce the possible errors during experiment. The diagrammatic representation of the test engine setup is shown in Fig. 1 and the research engine specification and parameters details are given in Table 3. The engine is subjected to different conditions and the performance was measured. The load testing has been conducted using a brake drum dynamometer. A required A/F ratio is maintained for better consumption of the fuel. The air box and U-tube manometer is associated as an integral part of air intake system allows excess air to the engine manifold. The mass flow rate of fuel supplied to the engine is measured with the help of a burette and stop watch. The inlet and outlet temperature of water used in the cooling system, the exhaust gas temperature and ambient air temperature was measured using Chomel-Alumel thermocouple displayed with digital temperature indicator. In addition to this, Automatic emission analyser (AVL analyser) and digital smoke meter was used to measure the exhaust emission and Smoke emitted in the engine.
Blended oils (consists of Diesel and 10, 20, 30, 40, and 50% of WPO) were used for testing in the engine. The properties of the blended oil were summarized in the Table 2. These diesel blend waste plastic oil are denoted as 10% BWPO, 20% BWPO, 30% BWPO, 40% BWPO, and 50% BWPO, with the numbers indicating the percentage of WPO in the blend. Higher blends were not used due to detonation in the engine.
The analysis was carried out both quantitatively and qualitatively with an assumption of control volume in the combustion chamber. The experiment was also conducted under constant ambient temperature and atmospheric pressure conditions. The test fuel was subjected to complete combustion assuming no emission loss in the form of C, H2, CO, H2O, or free O2 as the product.
2.1 Energy Analysis
The energy analysis is carried out to determine the physical significance of thermodynamics laws to access the amount of energy released during combustion. The concept of control volume attributes the significance of the supplied energy and utilized energy in the system. The analysis is well clarified with the following assumptions; engine operates under steady state conditions, the working medium both in the engine and dynamometer assumed under control volume, the gaseous medium in the engine inlet and outlet considered as an ideal gas mixture, potential energy, and kinetic energy of the fluids throughout the flow is neglected. This analysis gives both the amount of energy present and also discusses about the losses of energy in the system.
Figure 2 explains the mechanism of energy transfer in the form of heat transfer and work transfer in different state of the working fluid. The engine is introduced with a mixture of fuel and ambient air in a required proportion and being ready for combustion. This combustible mixture evolves power which can be encountered as shaft work with some form of energy losses to ambient as exhaust gas emission and heat losses to coolant. The following parameters are taken into consideration for energy calculations.
For any thermodynamic open system [21, 22].
Mass balance equation is given by,
According to steady flow energy equation (SFEE) is given by,
For a steady flow system,
Using Eqs. 4, 5 and 6 in Eq. 3 and ignoring the potential energy and kinetic energy being small,
The energy balance per mole of the fuel may be expressed as [23],
where
The heat input energy \(Q_{\text{in}}\) to the diesel engine is the amount of fuel energy content in the supplied fuel and is given by (Eq. 10),
The shaft work can be evaluated by,
Rate of Energy loss in cooling water is given by,
Rate of energy loss in Exhaust gas is given by,
\(C_{pe}\) may be found by equating \(\dot{Q}_{w} \;{\text{and}}\;\dot{Q}_{e}\), and given by
Rate of Unaccounted Energy losses is given by,
2.2 Exergy Analysis
The performance of an engine can be well evaluated by its qualitative and quantitative approach. The exergy associated with energy is a quantitative assessment of its usefulness or quality. Exergy analysis acknowledges that although energy cannot be created or destroyed, it can be degraded in quality, eventually reaching a state in which it is in complete equilibrium with the surroundings and hence of no further use for performing tasks. The assumptions for exergy analysis are:
-
Exergy change of potential, kinetic, electromagnetic and electrostatic are insignificant.
-
Steady flow engine system.
-
Available energy is from finite energy source.
-
The kinetic and potential exergies being small were ignored as compared to total exergy value.
For a direct injection diesel engine, the exergy balance equation can be written as
The exergy flow rate of the air to diesel engine can be expressed as follows:
The specific heat capacity of the intake air can be calculated using the following equation:
Considering the chemical exergy only for determining the energy flow rate of fuel,
The value of chemical exergy factor (ρ) of the fuel and can be calculated by knowing mass fraction of H, C, O, and Sas used in the following equation [21, 24];
The lower heating value of diesel and plastic fuel were considered to be 42,400 and 42,600 kJ/kg respectively Moreover, the chemical formula of diesel and plastic fuel were taken into account as C12H24 and C12.66H24.66O0.25.
The summation of physical exergy and chemical exergy flow rates can produce exergy flow rate of the exhaust hot gas from engine.
The physical exergy flow rate and chemical exergy flow rate of exhaust hot gas can be calculated using the following equation
The specific heat capacity of the exhaust hot gas can be specified as [25],
where \(y_{i}\) and \(C_{P,i}\) are the mass fraction and specific heat capacity of each component in the exhaust hot gas from diesel engine.
In order to calculate standard molar percentage of different components in the exhaust gas, it is required to calculate the chemical exergy.
The exergy rate of the shaft work generated is equal to the energy rate of net work
The exergy loss rate because of heat transfer rate to the cooling water can be computed as:
The exergy loss rate to the environment as a result of heat transfer can be assessed as follows:
The heat loss to the environment can be estimated using the energy balance equation (by equating total energy inputs to total energy outputs) as:
The exergy efficiency (Ψ) of the DI diesel engine can be evaluated as follows [26],
3 Result and Discussion
3.1 Brake Thermal Efficiency
The brake thermal efficiency of an engine depends upon engine loads and blend ratio and their variation in the present experiment as shown in Fig. 3. The thermal efficiency found decreasing with increase in blend ratio at full load conditions. It can be observed from Fig. 3 that the thermal efficiency is 20.83% at full load for diesel. In addition to this, the brake thermal efficiency of the waste plastic oil blend found closer or slightly higher to diesel up to 80% load. This may be due to higher calorific value of WPO–diesel blend than diesel.
3.2 Brake-Specific Fuel Consumption
Brake-specific fuel consumption (BSFC) is a more consistent criterion to signify the performance of an engine for blended fuels due to difference in their density and calorific values. Brake-specific fuel consumption of different WPO–diesel blends with diesel is summarized in Fig. 4.
It can be observed from Fig. 4 that the increase in load results the decrease in BSFC for both diesel and various blended fuels. In addition to this, the BSFC is found decreasing within the variation of an increase of concentration of WPO in WPO–Diesel blend. The high calorific value of WPO causes lesser consumption of blended fuel by the engine.
3.3 Energy Analysis of Tested Fuels
The useful energy and energy losses of WPO–diesel blend in comparison with diesel is shown in Fig. 5. The fuel energy generated by WPO–diesel blend is observed very close to diesel at 20% BWPO. The shaft work produced in diesel is found more than WPO. This may be due to higher thermal efficiency of diesel than WPO blend. The heat lost in the cooling water for both fuels are found very close to each other. Energy lost in the form of exhaust emission is found higher in diesel than WPO–diesel blend. This may be due to higher calorific value of diesel than WPO–diesel blend at lower proportion.
3.4 Exergy Analysis of Tested Fuels
The different values of exergy of different fuels are calculated (Eq. 30) and the analysis reveals that the fuel exergy are becoming higher than the corresponding fuel energy inputs.
Due to lower specific heating value of diesel, the fuel exergy of diesel is found lower than that of WPO–diesel blend as explained in Fig. 6. The trend is continued for shaft exergy as the brake thermal efficiency of diesel is higher than the blend. In addition, due to higher calorific value of WPO, the exhaust gas exergy is less than diesel.
3.5 Energetic and Exergetic Efficiency of Tested Fuels
Energetic efficiency of the tested fuels agrees at a point and it is resembled with the brake thermal efficiency as shown in Fig. 7. It has been observed that exergetic efficiency of WPO is found more than that of diesel. This may be due to higher BSFC of WPO–diesel blend in comparison to diesel.
4 Conclusion
From the study of conducting performance test using waste plastic oil and diesel on a DI diesel engine, it has been observed that the engine was able to run with maximum 50% of waste plastic oil–diesel blends. The engine is found deteriorating its performance beyond this blend ratio due to knocking and showed better performance up to 30% blend. Brake thermal efficiency of blend is found to be almost the same or marginally higher than diesel up to 80% load and somewhat lower at full load. Exhaust gas temperature is found marginally higher with WPO–diesel blend than diesel operation. Brake specific fuel consumption of WPO is found marginally higher than that of diesel fuel for same rated power. The same trend is followed for destructive exergy of the oil.
Abbreviations
- m a :
-
Mass flow rate of air inducted into the combustion chamber under normal ambient conditions (kg/s)
- \(\dot{m}_{\text{f}}\) :
-
Mass flow rate of fuel mixed with air to maintain a required A/F ratio for ease of combustion (kg/s)
- W :
-
Shaft work produced in BHP
- \(\dot{n}_{\text{F}}\) :
-
Molar rate of the fuel
- hp and hr:
-
The enthalpies of the products and reactants per mole of the fuel respectively
- ΔH :
-
Enthalpy change due to a change of state at a constant composition
- nout and nin:
-
correspond to the relevant coefficients in the reaction equation
- \(H_{f}^{ 0}\) :
-
Enthalpy of formation
- LHVfuel:
-
Lower heating value of the fuel (kJ/kg)
- \(\dot{E}_{xa}\) :
-
Exergy flow rates of inlet air
- \(\dot{E}_{xf}\) :
-
Exergy flow rates of fuel
- \(\dot{E}_{xg}\) :
-
Exergy flow rates of gas
- \(\dot{E}_{xw}\) :
-
Exergy flow rates of shaft work generated
- \(\dot{E}_{xc}\) :
-
Exergy flow rates of heat transfer to coolant and surrounding air
- \(\dot{E}_{xdes}\) :
-
Rate of destructed exergy
- C p, a :
-
Specific heat capacity of air
- \(P_{a}\) :
-
Pressure of the intake air
- \(X_{i}\) :
-
Mass fraction
- \(C_{p,g}\) :
-
Specific heat capacity of exhaust gas generated from engine
- Tg and Pg:
-
Temperature and pressure of exhaust gas generated from engine
- \(\dot{W}\) :
-
The net work rate
- ω :
-
Angular velocity
- T :
-
Torque
References
Hasheminejad M, Tabatabaei M, Mansourpanah Y, Javani A (2016) Effect of an emission reducing soluble hybrid nanocatalyst in diesel/biodiesel blends on exergetic performance of a DI diesel 93(2016):353–368
Voloshin RA, Kreslavski VD, Zharmukhamedov SK, Bedbenov VS, Ramakrishna S, Allakhverdiev SI (2015) Photo electrochemical cells based on photosynthetic systems: a review. Bio fuel Res 227–235
Panda AK, Singh RK (2010) Catalytic performances of kaolin and silica alumina in the thermal degradation of polypropylene. J Fuel Chem Technol 39(3):198–202
Mojarrab M, Aghbashlo M, Mobli H (2016) Exegetic performance assessment of a long-life milk processing plant: a comprehensive survey. J Clean Prod 140:590–607
Rosen MA, Dincer I (2001) Exergy as the confluence of energy, environment and sustainable development. Exergy Int J 1(1):3e13
VanGerpen JH, Shapiro HN (1990) Second-law analysis of diesel engine combustion. J Eng Gas Turb Power 112(1):129–137
Rakopoulos CD, Kyritsis DC (2001) Comparative second-law analysis of internal combustion engine operation for methane, methanol, and dodecane fuels. Energy 26(7):705–722
Rakopoulos CD, Giakoumis EG (2006) Comparative first-and second-law parametric study of transient diesel engine operation. Energy 31(12):1927e1942
Zheng J, Caton JA (2012) Second law analysis of low temperature combustion diesel engine: effect of injection timing and exhaust gas recirculation. Energy 38(1):78–84
Canakci M, Hosoz M (2006) Energy and exergy analyses of a diesel engine fuelled with various biodiesels. Energy Source Part B 1(4):379–394
Caliskan H, Tat ME, Hepbasli A, Van Gerpen JH (2010) Exergy analysis of engines fuelled with biodiesel from high oleic soybeans based on experimental values. Int J Exergy 7(1):20–36
da Costa YJR, de Lima AGB, Bezerra Filho CR, de Araujo Lima L (2012) Energetic and exergetic analyses of a dual-fuel diesel engine. Renew Sustain Energy Rev 16(7):4651–4660
Lopez I, Quintana CE, Ruiz JJ, Cruz-Perag F, Dorado MP (2014) Effect of the use of oliveepomace oil biodiesel/diesel fuel blends in a compression ignition engine: preliminary exergy analysis. Energy Convers Manage 85:227–233
Aghbashlo M, Tabatabaei M, Mohammadi P, Pourvosoughi N, Nikbakht AM, Goli SAH (2015) Improving exergetic and sustainability parameters of a DI diesel engine using polymer waste dissolved in biodiesel as a novel diesel additive. Energy Converse Manage 10(5):328–337
Caliskan H, Tat ME, Hepbasli A, Gerpen JHV (2010) Exergy analysis of engines fuelled with biodiesel from high oleic soybeans based on experimental values. Int J Exergy 1(7):20–36
Sayin C, Hosoz M, Canakei M, Kilicaslan I (2007) Energy and exergy analysis of a gasoline engine. Int J Energy Res 31(3):259–273
Sekmen P, Yilbasi Z (2011) Application of energy and exergy analyses to a CI engine using biodiesel fuel. Math Comput Appl 6(4):797–808
Biplab KD, Sahoo N, Ujjwal KS (2013) Thermodynamic analysis of a variable compression ratio diesel engine running with palm oil methyl ester. Energy Conserv Manag 65:147–154
Panigrahi N, Mohanty MK, Acharya SK, Mishra SR, Mohanty RC (2014) Experimental investigation of karanja oil as a fuel for diesel engine—using shell and tube heat exchanger, World Academy of Science, Engineering and Technology. Int J Chem Mat Sci Eng 8(1):91–98
Panigrahi N, Mohanty MK, Mohanty RC, Mishra SR (2016) Performance of a C.I. engine with energy and exergy analysis fuelled with neem oil methyl ester. Int J Renew Energy Technol 7(3):264–287
Moran MJ, Shapiro HN, Boettner DD, Bailey M (2014) Fundamentals of engineering thermodynamics, eighth edn. Wiley
Cengel YA, Boles MA (2009) Thermodynamics: an engineering approach, 6th edn. Tata Mc GRAW Hill Companies, New Delhi
Canakci M, Hosoz M (2006) Energy and exergy analysis of a diesel engine fuelled with various biodiesels. Energy Sour Part B 1:379–394
Shamshirband S, Tabatabaei M, Aghbashlo M, Yee L, Petkovi D (2016), Support vector machine-based exergetic modelling of a DI diesel engine running on biodiesel blends containing expanded polystyrene. Appl Therm Eng 727–747
Lopez I, Quintana CE, Ruiz JJ, Cruz-Perag F, Dorado MP (2014) Effect of the use of oliveepomace oil biodiesel/diesel fuel blends in a compression ignition engine. Preliminary exergy analysis. Energy Convers Manage 227–233
Rakopoulos CD, Giakoumis EG (2006) Second-law analyses applied to internal combustion engines operation. Prog Energy Combust Sci 31(12):2–47
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Das, A.K., Panda, A.K., Hansdah, D. (2019). Energetic and Exergetic Performance Analysis of a CI Engine Fuelled with Diesel-Blended Plastic Pyrolytic Oil. In: Chattopadhyay, J., Singh, R., Prakash, O. (eds) Renewable Energy and its Innovative Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-13-2116-0_13
Download citation
DOI: https://doi.org/10.1007/978-981-13-2116-0_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2115-3
Online ISBN: 978-981-13-2116-0
eBook Packages: EngineeringEngineering (R0)