Abstract
Pyrolysis of biomass feedstocks in the absence or limited supply of air/oxygen yields solid (char), liquid (oils), and gaseous products (flue gas). Biomass pyrolysis is gaining attention due to the vast array of products as output and offers a wide range of applications in both energy and environmental arena. Biochar is being used as a soil amendment in the agricultural sector, carbon sequestration, and adsorbing pollutants from the land, whereas pyrolysis oil can be upgraded to the application in the internal combustion (IC) engines. Flue gas has various constituents of hydrocarbons in the form of hydrogen, methane, and ethylene and demands the sophisticated purification for their specific application. This paper attempts to model the pyrolysis process using different biochemical and empirical approaches to evaluate the product yield along with its composition. Since the biomass feedstocks are mainly composed of carbon, hydrogen, and oxygen, the kinetic model has been developed to utilize the elemental characteristics as inputs to predict the product composition. The pyrolysis operational parameter of temperature, at which the reaction occurs, has been considered in the model used in the present investigation. This modeling approach would help in comprehending the pyrolysis process and for the efficient utilization of biomass feedstocks to derive the desired product yield.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
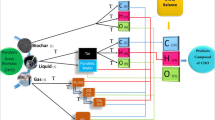
Pyrolysis is a process of thermal decomposition of biomass in the oxygen-free or oxygenless environment. During the pyrolysis, biomass is converted into different products of (a) a solid product—biochar; (b) a liquid product—bio-oil; and (c) a gaseous product syngas which consists of CO, CO2, hydrogen gas, and other hydrocarbons [1]. Various process parameters such as feedstock type, pyrolysis temperature, heating rate, and the pressure parameter for the gaseous and liquid product yield [2].
Different compositions of cellulose, hemicellulose, and lignin in the biomass affect the yield of charred product. Lignin-rich biomass yields a higher amount of charred product than the biomass which is rich in cellulose and hemicellulose [3]. The temperature and the heating rate affect the char yield inversely. Higher heating rate condition during fast pyrolysis yields lesser biochar than the biomass pyrolyzed at as lower heating rate in slow pyrolysis [4]. Thus, the yield of different pyrolysis products can be controlled by process parameters.
Biochar holds potential application in agriculture as a soil amendment for enhanced crop production [5] and simultaneously acts as an anthropogenic climate mitigation strategy [6]. The upgraded bio-oil (which is the combination of tar and pyrolysis water from the reaction) can be used as liquid fuel for the internal combustion engines. Shihadeh and Hochgreb [7] had also shown that heat release profiles of bio-oil are consistent with the slow combustion and rapid mixing as relative to the diesel engine. The volatiles are mainly composed of carbon monoxide, carbon dioxide, hydrogen gas, and hydrocarbons such as methane, ethylene which are liberated as flue gas [8]. Methane, one of the volatiles, is used as natural gas fuel.
Modeling of pyrolysis system is used to predict the product distribution based on reactions. Pyrolysis modeling helps to get the required outcome through steering the process toward the specific product. The manipulation can be done on different parameters, heating rate, pyrolysis temperature, etc.
A number of models have been developed to simulate the pyrolysis process for the prediction of products yield. But each one has its own limitation. Sharma et al. [1] have modeled the slow pyrolysis of wood and predicted the biochar yield and the flue gas composition. In this model, the authors have used the plots of experimental data of CO/CO2, H2/CO2, and total hydrocarbons/CO2 from [8, 9] to solve the set of equations with zero degrees of freedom. But in the model developed, temperature parameter has not been taken into account for the prediction of char yield.
Song [2] has taken into account of the kinetic parameters used in a gasifier reactor by considering the empirical relationships previously developed by Neves et al. [10]. One of the advantages of this model is being the temperature factor is taken into account in calculating the biochar amount and accordingly the proportion of carbon, hydrogen, and oxygen ratio in the char is obtained. Thus, the char composition varies with the temperature. Char yield is directly obtained from temperature parameter and not through the biomass composition as it has been taken an average from all the biomass composition. The biochar yield is further used to calculate the volatiles and tar yield.
An economic trade-off model as developed by Yoder et al. [11] also dealt with the prediction of biochar and bio-oil yield percentage. Biomass has been broadly classified into four main categories, namely agricultural residues, other agricultural residues, forest residues, and industrial residues. Also, in this paper, pyrolysis process has been broadly classified into fast and slow pyrolysis based on the heating rate which adds one more set of parameter that needs to be considered apart from pyrolysis temperature. The model developed by Yoder et al. [11] has been given importance on economic aspects of the pyrolysis process rather than the flue gas composition. Biomass composition has not been considered for the prediction of biochar yield is one of the disadvantages of this model.
In this research manuscript, the pyrolytic kinetics model designed by Song [2] has been further modified to predict the yield of the biochar, tar, and the flue gas composition in the pyrolytic reactor. The feedstock selection of different straw biomass has been considered. The comparison of the results was performed to understand the temperature and biomass effect on the pyrolysis products yield.
2 Model Development for Biomass Pyrolysis
The model designed by Song [2] used to predict the yields of biochar, tar, and volatile gas composition essentially derived and advanced from [10]. The mathematical model to predict the components yield based on the following assumptions: Gasification of carbon takes place in an isothermal reactor, and the biomass is well mixed; oxygen supplied is consumed entirely, and nitrogen remains inert throughout the reaction; pyrolysis of carbon takes place in the isothermal reactor condition; thus, kinetic model can be designed, and drying takes place at the start of the reaction itself (i.e., at the inlet).
In the first stage of pyrolysis, given biomass fuel (F) is pyrolyzed and converted into char (ch), volatile gas, and liquid. Liquid consists of tar and pyrolytic water (H2O) as given in Eq. 1. The volatile gas compositions include carbon dioxide (CO2), carbon monoxide (CO), methane (CH4), hydrogen gas (H2), as described in Eq. 2.
Yi,j is the expression to denote yield of ith element in kilograms from jth element in kilograms. Temperature parameter input is given in Celsius, and the yield is obtained in units, kg/kg of biomass. For instance, the char yield is obtained as a kilogram of biochar obtained per kilogram of biomass.
In the second stage, i.e., during the increase in temperature, the tar again gets cracked into volatiles called secondary cracking of tar particles. During this phase, the produced targets cracked into different gaseous substance based on the composition of biomass. Moreover, the increased pressure in response to increased gaseous level in the reactor causes the recompression to tar (liquid).
To apply the pyrolysis model, the elemental composition of different biomasses should be known. These elemental compositions are given as input in the current model under discussion in order to predict the pyrolytic yields. The elemental composition of biomass is obtained from [12]. Elemental compositions of different straw biomasses are used in this model which are: Rice straw, Corn straw, Wheat straw, Barley straw and Oat straw. The elemental composition on dry ash free basis is given in Table 1. Different biochemical compositions of straw biomass are given in Table 2.
The char yield is only related to temperature and not of the biomass in the kinetic model designed by Song [2] as described in Eq. 3:
Equations (4), (5), and (6) give the composition of CHO in the biochar which is also related to the temperature as follows:
The elemental composition ratio of tar to the input biomass/fuel in relation to temperature is obtained from [10] as Eqs. (7), (8), and (9) describe.
To nullify the degrees of freedom, further three more empirical equations were introduced as Eqs. (10), (11), and (12).
The general mass balance equations for the determination of carbon, oxygen, and hydrogen in the pyrolysis reaction are expressed as Eqs. (13), (14), and (15).
Carbon Balance
Oxygen Balance
Hydrogen Balance
3 Results and Discussion
The present developed model is programmed and executed on ‘MATLAB R2015b’ platform in order to predict the yields of different pyrolysis products. The effects of temperature and biomass compositions on the yield of different pyrolysis products are analyzed. The model coded for the pyrolysis temperature range from 200 to 1000 °C, and the results are obtained as the program output, exported in the form of excel sheet.
3.1 Effect of Temperature
The developed model depicts the overall trend of the yield of char, tar, and volatile components obtained from rice straw with respect to temperature in Fig. 1. The volatiles components are , namely carbon dioxide, carbon monoxide, methane, and hydrogen gas. The char yield decreases in an exponential way and becomes steady at higher temperature due to the fixed carbon content in the biomass. The tar quantity increases at the initial pyrolysis process and then declines due to secondary cracking. Among the volatiles, the levels of hydrogen, methane, and carbon monoxide increased with increase in temperature at the later stage of pyrolysis. From Fig. 2, it is observed that at a higher yield of water is obtained at the pyrolysis temperature around 300 °C; the results are due to the release of bound moisture in the biomass. In case of the volatile compounds, the increasing trend of carbon dioxide release was observed up to 800 °C and a further increase in temperature presented sudden drop of carbon dioxide yield (Fig. 3). Similar trends have been observed in the other four types of straws such as barley, oat, corn, and wheat. It might be due to the fact that all the studied agro-residues belonged to the category of straw that comes under the lignocellulosic biomass.
The amount of tar produced found increasing up to the temperature around 600 °C (Fig. 1). At a temperature higher than 600 °C, the yield of tar begins to decrease due to the secondary cracking of tar into gaseous species. The main components of tar are benzene, toluene, styrene, phenol compounds, and a meager amount of naphthalene [13]. These compounds of tar are especially phenols and oxygenated tar species get cracked at higher temperatures around 700 °C and produce hydrogen and carbon dioxide gases. These results in increased carbon dioxide and hydrogen gas yields in the temperature range of 700–800 °C [14].
The investigation of the pyrolysis model showed that the considered straw types of biomass produced an almost same quantity of tar. However, oat straw produced a slightly lesser quantity of tar than the other straw types. From the model used, the ratio of oxygen present in tar to the oxygen present in parent biomass was 0.8 [2]. From [12], the concentration of oxygen in the oat straw was found to be higher than the other straw types. Therefore, the presence of a higher proportion of oxygen may affect the overall tar yield of oat straw.
Figure 1 shows the yield of hydrogen and methane for the temperature effect. Both the volatiles are observed increasing in the temperature range of 800–1000 °C. Park et al. [15] showed that due to secondary thermal cracking of tar at 800 °C, there is a sudden increase in methane and carbon monoxide compounds. The carbon monoxide yield increases drastically around the temperature range of 700–800 °C as shown in Fig. 1. Im-orb et al. [16] also reported that carbon monoxide level increases from the range of temperature 500 to 700 °C.
Moisture content present in the biomass contributes to the over all liquid yield of the pyrolysis. Figure 2 indicated that the yield of water increases up to 300 °C, which may be due to the release of bound water molecules from the biomass and then decreases linearly up to 1000 °C. The water vapor produced at higher temperature reformed into hydrogen and methane in the gas phase leading to the decrease in pyrolytic water and increase in the level of hydrogen and methane [16].
Figure 3 depicts the pattern of carbon dioxide yield influenced by the temperature. Around 800 °C, the yield of carbon dioxide found highest and then followed by a decline phase up to 1000 °C. This was due to the partial oxidation of carbon to carbon monoxide at higher temperature rather than complete oxidation to form carbon dioxide.
3.2 Effect of Biomass
In the present model, the char yield is dependent directly on the pyrolytic temperature and independent of the biomass used. Thus, char yield remains almost same for all the types of straw used. However, in the pyrolytic process, the feedstock biomass utilized affects the char yield. By using Eq. (16), the calculated char yield was obtained.
It can be observed from Fig. 1, that with an increase in temperature; the char yield decreases exponentially, and at a higher temperature range, the char yield becomes almost constant. This trend of char yield found similar to the results of [17]. The result observation is because the fixed carbon content increases with increase in temperature [18].
Effect of higher pyrolysis temperature on different straw biomasses has been expressed in Fig. 4. At the higher temperature of pyrolysis, the calculated char yield of rice is higher than the calculated char yield of oats. This can be due to higher fixed carbon content in the rice straw than in comparison to the oat straw. The standard average percentage composition of CHO in the straw biomass assumed to be 49% of carbon, 5.90% of hydrogen and remaining 44% of oxygen. This standard composition is used for determining the char yield in this model. Deviation from this standard biomass composition causes the change in the biochar yield. Higher the deviation from the standard composition causes higher the variation in the biochar yields. Since all the straw biomasses have almost similar elemental (from Table 1) and biochemical composition (from Table 2), the practice of co-pyrolysis process could be promoted. This can be an energy-efficient process of using combined different straw biomasses since the straws do not need to be pyrolyzed separately due to availability restrictions. The characteristics of pyrolysis product-biochar include the particle size, density, porous structure and pH varies based on the feedstock biomass type [19].
The hydrogen present in the straw contributes to the water yield during pyrolysis since corn straw has higher hydrogen percentage (Fig. 2). Other straws yield less amount of pyrolytic water than corn straw because of the lower hydrogen percentage. Figure 3 indicated that the rice straw produces more amount of carbon dioxide during the pyrolysis reaction due to the higher carbon percentage, whereas corn straw produces lesser amount due to the lower carbon content biomass.
4 Model Validation
The model has been validated by comparing the model predicted value with the actual experimental yield for various straw biomasses as given in Table 3. Different operating temperatures are considered, compared, and validated for each straw biomass. In the case biochar yield from barley and oat straws, model predicts the yield which slightly differs by 20% form the experimental value. In the case of tar, for wheat straw biomass, predicted value slightly differs by 21.5%, for wheat straw biomass, it is 18%, and for maize, it is 13%. Rice and barley straws tar yield prediction is almost similar to the actual experimental value. In the gaseous component yield prediction, result greatly varies for barley around 66%, and for the other straws, the difference is insignificant.
5 Conclusion
The model presents the simplified and generalized equations for predicting the pyrolysis yields of char, tar, and volatiles. The pyrolytic model has been designed and executed in the MATLAB R2015b software platform. The biomass elemental composition is used as input in the present model for the prediction of volatiles gas and tar composition. In this paper, the elemental compositions of straw biomasses such as rice, corn, barley, oat, and wheat were subjected to predict their conversion behaviors at various pyrolytic temperatures. The char and tar yield found almost same for the straw biomasses considered in the present investigation as all belonged to the category of lignocellulosic biomasses. The yield of carbon dioxide obtained highest at 600–700 °C. The yield of other volatiles includes carbon monoxide, hydrogen, and methane increasing around a temperature range of 700–800 °C. Further, validation of the model is also carried out for all the five types of straw biomasses, and difference of mostly around 20% is found in predicted and actual yield. The elemental compositions of different straw biomasses were witnessed equivalent in nature, and these observations encourage the employment of co-pyrolysis for an energy-efficient conversion process.
References
Sharma, A.K., Ravi, M.R., Kohli, S.: Modelling product composition in slow pyrolysis of wood. SESI J. 16(1), 1–11 (2006)
Song, B.: Biomass pyrolysis for biochar production: kinetics, energetics and economics. Biochar 227–238 (2016)
Tillman, D.A.: Wood Combustion: Principle, Processes, and Economics. Elsevier, New York City, New York (2012)
Lehmann, J., Joseph, S.: Biochar for environmental management: science and technology (2009)
Steiner, T., Mosenthin, R., Zimmermann, B., Greiner, R., Roth, S.: Distribution of phytase activity, total phosphorus and phytate phosphorus in legume seeds, cereals and cereal by-products as influenced by harvest year and cultivar. Anim. Feed Sci. Technol. 133(3), 320–334 (2007)
Woolf, D., Amonette, J.E., Street-Perrott, F.A., Lehmann, J., Joseph, S.: Sustainable biochar to mitigate global climate change. Nat. Commun. 1, 56 (2010)
Shihadeh, A., Hochgreb, S.: Diesel engine combustion of biomass pyrolysis oils. Energy Fuels 14(2), 260–274 (2000)
Boroson, M.L., Howard, J.B., Longwell, J.P., Peters, W.A.: Product yields and kinetics from the vapor phase cracking of wood pyrolysis tars. AIChE J. 35(1), 120–128 (1989)
Thunman, H., Niklasson, F., Johnsson, F., Leckner, B.: Composition of volatile gases and thermochemical properties of wood for modeling of fixed or fluidized beds. Energy Fuels 15(6), 1488–1497 (2001)
Neves, D., Thunman, H., Matos, A., Tarelho, L., Gómez-Barea, A.: Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 37(5), 611–630 (2011)
Yoder, J., Galinato, S., Granatstein, D., Garcia-Pérez, M.: Economic tradeoff between biochar and bio-oil production via pyrolysis. Biomass Bioenerg. 35(5), 1851–1862 (2011)
Vassilev, S.V., Baxter, D., Andersen, L.K., Vassileva, C.G.: An overview of the chemical composition of biomass. Fuel 89(5), 913–933 (2010)
Zhang, Y.L., Wu, W.G., Zhao, S.H., Long, Y.F., Luo, Y.H.: Experimental study on pyrolysis tar removal over rice straw char and inner pore structure evolution of char. Fuel Process. Technol. 134, 333–344 (2015)
Nowakowska, M., Herbinet, O., Dufour, A., Glaude, P.A.: Detailed kinetic study of anisole pyrolysis and oxidation to understand tar formation during biomass combustion and gasification. Combust. Flame 161(6), 1474–1488 (2014)
Park, J., Lee, Y., Ryu, C.: Reduction of primary tar vapor from biomass by hot char particles in fixed bed gasification. Biomass Bioenerg. 90, 114–121 (2016)
Im-orb, K., Simasatitkul, L., Arpornwichanop, A.: Analysis of synthesis gas production with a flexible H2/CO ratio from rice straw gasification. Fuel 164, 361–373 (2016)
Peng, X., Ye, L.L., Wang, C.H., Zhou, H., Sun, B.: Temperature-and duration-dependent rice straw-derived biochar: Characteristics and its effects on soil properties of an Ultisol in southern China. Soil Tillage Res. 112(2), 159–166 (2011)
Encinar, J.M., Gonzalez, J.F., Gonzalez, J.: Fixed-bed pyrolysis of Cynaracardunculus L. Product yields and compositions. Fuel Process. Technol. 68(3), 209–222 (2000)
Zhang, Q., Yang, Z., Wu, W.: Role of crop residue management in sustainable agricultural development in the North China Plain. J. Sustain. Agric. 32(1), 137–148 (2008)
Binod, P., Sindhu, R., Singhania, R.R., Vikram, S., Devi, L., Nagalakshmi, S., Kurien, N., Sukumaran, R.K., Pandey, A.: Bioethanol production from rice straw: an overview. Biores. Technol. 101(13), 4767–4774 (2010)
Shawky, B.T., Mahmoud, M.G., Ghazy, E.A., Asker, M.M., Ibrahim, G.S.: Enzymatic hydrolysis of rice straw and corn stalks for monosugars production. J. Genet. Eng. Biotechnol. 9(1), 59–63 (2011)
Sun, Y., Cheng, J.: Hydrolysis of lignocellulosic materials for ethanol production: a review. Biores. Technol. 83(1), 1–11 (2002)
Rowell, R.M.: Opportunities for lignocellulosic materials and composites. 12–27 (1992)
Ji-Lu, Z.: Bio-oil from fast pyrolysis of rice husk: yields and related properties and improvement of the pyrolysis system. J. Anal. Appl. Pyrol. 80(1), 30–35 (2007)
Krishna, B.B., Singh, R., Bhaskar, T.: Effect of catalyst contact on the pyrolysis of wheat straw and wheat husk. Fuel 160, 64–70 (2015)
Mani, T., Murugan, P., Mahinpey, N.: Pyrolysis of oat straw and the comparison of the product yield to wheat and flax straw pyrolysis. Energy Fuels 25(7), 2803–2807 (2011)
Mullen, C.A., Boateng, A.A., Hicks, K.B., Goldberg, N.M., Moreau, R.A.: Analysis and comparison of bio-oil produced by fast pyrolysis from three barley biomass/byproduct streams. Energy Fuels 24(1), 699–706 (2009)
Ravikumar, C., Kumar, P.S., Subhashni, S.K., Tejaswini, P.V., Varshini, V.: Microwave assisted fast pyrolysis of corn cob, corn stover, saw dust and rice straw: Experimental investigation on bio-oil yield and high heating values. Sustainable Mater. Technol. 11, 19–27 (2017)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Swagathnath, G., Rangabhashiyam, S., Parthsarathi, K., Murugan, S., Balasubramanian, P. (2019). Modeling Biochar Yield and Syngas Production During the Pyrolysis of Agro-Residues. In: Drück, H., Pillai, R., Tharian, M., Majeed, A. (eds) Green Buildings and Sustainable Engineering. Springer Transactions in Civil and Environmental Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-13-1202-1_28
Download citation
DOI: https://doi.org/10.1007/978-981-13-1202-1_28
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1201-4
Online ISBN: 978-981-13-1202-1
eBook Packages: EngineeringEngineering (R0)