Abstract
Dengue fever is a leading cause of illness and mortality in the tropics and subtropics. There are no therapeutics currently available and a recently approved vaccine is not very efficacious demanding an urgent need to develop an effective antiviral. The path to successful dengue drug development depends on availability of relevant preclinical testing models and better understanding of dengue pathogenesis. In recent years, efforts to develop dengue therapeutics have focused on both repurposing approved drugs as well as discovery of new chemical entities that act via virus or host targeted mechanisms. Here, we discuss the various innovative approaches, their outcome, and the lessons gleaned from the development efforts.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
22.1 Introduction
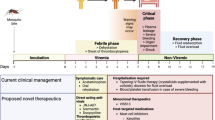
Dengue fever is the most prevalent mosquito-borne viral disease globally. It is endemic in more than 100 countries, and causes an estimated 400 million infections and 25,000 deaths every year [5]. As these numbers have been rising steadily over the past decades, developing efficacious antiviral agents and vaccine is imperative to control the disease burden. Current treatment guidelines rely entirely on supportive care and aggressive monitoring [23]. Recently, a tetravalent vaccine was licensed in several dengue-endemic countries, which is a considerable breakthrough [63]. Its efficacy, however, varies widely hence there remains a strong need to develop effective therapeutic modalities for dengue virus (DENV) infections [27].
Since the early 2000s, several compounds have been tested in early proof-of-concept trials but, none was able to demonstrate clinical efficacy. In this chapter, we discuss the progress that has been made and the main challenges faced in dengue therapeutics research and attempt to draw a parallel to the development of other antivirals like oseltamivir.
22.2 Candidate Drugs
Consideration of the nature of the disease and the impacted demographics is essential for drug development – and defining a Target Product Profile (TPP) is a key step [30]. Dengue fever (DF) results in an acute self-limiting disease, with a small proportion of patients, mostly children, developing constellations of life threatening complications referred to as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). DF mostly affects low-income, tropical countries. Consequently, the ideal drug should have an excellent safety profile not to surpass the low risk posed by uncomplicated DF. In addition, it should be an oral drug, require minimum frequency dosing with total course of therapy not to exceed 5 days duration given the acute nature of the disease, and produce measurable clinical benefits. It should be easily manufactured and distributed at affordable cost, and be able to withstand temperatures and high humidity prevalent in tropical regions. These general TPP goals should be kept in mind but they should not stand in the way of new innovations such as prophylactic drugs or biologics in the form of potent therapeutic antibodies that target the viral envelope protein to block its infectivity.
22.2.1 Repurposed Agents
Starting from scratch, drug development is a long and expensive process, and for dengue the experiences of Novartis pharmaceutical company which formed the first major industrial effort to develop a drug that could be used as defined in the TPP has been reviewed [34]. The lack of any compounds from these ventures and the urgent need for affordable anti-dengue drugs has resulted in several attempts at repurposing drugs that were developed for other indications. The main advantage of this approach is that it has a relatively short development path as the initial safety data in animals and humans are already established. In fact, all seven compounds evaluated in dengue clinical trials to date were repurposed. These agents were either already approved for different indications or were discontinued for further development due to reasons other than acute safety and tolerability. There have been 5 completed and published and 2 ongoing randomized clinical trials for anti-dengue agents since the early 2000s.
22.2.1.1 Chloroquine
Tested by the Oxford University Clinical Research Unit in Vietnam (OUCRU), chloroquine is a 4-amino-quinoline derivative with lysosomotropic and weak base properties (Trial Identifier: ISRCTN38002730). It has evidence of in vitro and in vivo (aotus monkey) anti-viral activity that is thought to be mediated by disrupting endosomal fusion and viral maturation [21]. This oral drug is cheap and widely available, with a strong safety profile. The clinical trial for this drug monitored time to resolution of viremia and NS1 antigenemia as primary measures of drug efficacy. There was no observed difference in efficacy between the drug and placebo, but chloroquine was associated with a higher incidence of adverse events [61].
22.2.1.2 Prednisolone
Prednisolone is a corticosteroid with potent anti-inflammatory activity. Some non-randomized trials have reported possible benefits of corticosteroids as a rescue treatment for severe DF, but this remains controversial [47]. It is, however, universally accepted that inflammatory factors play a crucial role in the pathogenesis of DSS. On this ground, the OUCRU in Vietnam conducted a Randomized Controlled Trial (RCT) to assess the safety of corticosteroids and outcome in viremia but was not powered to measure its therapeutic efficacy, although clinical outcomes such as DSS, ICU admission, and bleeding were measured (Trial Identifier: ISRCTN39575233). The safety profiles were similar in both groups but no clinical benefit was detected [57].
22.2.1.3 Balapiravir
A prodrug of nucleoside analogue 4′-azidocytidine, balapiravir was originally developed as a therapeutic agent against hepatitis C virus (HCV), another flavivirus. Balapiravir development against HCV was halted after evidence of excessive toxicity upon prolonged exposure. Due to the known similarities between DENV and HCV RNA-dependent RNA polymerases, this drug was evaluated as an anti-dengue agent by OUCRU in Vietnam (Trial identifier: NCT01096576). The phase II study monitored time to fever clearance, viremia and NS1 antigenemia to measure the drug efficacy. The results showed no significant difference between control and treated arms [15, 41].
22.2.1.4 Ribavirin
Ribavirin is a drug commonly used to treat HCV and also sometimes used as a broad-spectrum antiviral for RNA viruses. It has RNA-dependent RNA polymerase inhibitory activity, but its anti-flaviviral activity is believed to be mediated by intracellular GTP depletion. Ribavirin is used in conjunction with interferon-α against HCV as they show synergistic effect. Ribavirin was evaluated against DENV infection in combination with traditional Chinese medicine by the Guangzhou 8th People’s Hospital, but the results have not been made publicly available yet (Trial identifier: NCT01973855).
22.2.1.5 Celgosivir
Celgosivir is another drug that was originally developed for HCV. It is an inhibitor of α-glucosidase, a host enzyme necessary for glycosylation of viral coat proteins that aids in proper protein folding. It was tested in a RCT as an anti-dengue agent by Duke-National University of Singapore (Duke-NUS)/Singapore General Hospital (SGH) in Singapore using fever and viremia reduction as measures of drug efficacy. No statistically significant difference was seen between control and treatment groups, but celgosivir was found to have a good safety profile in dengue patients [36]. Extended pharmacokinetics studies and alternative regimen testing in mouse models of dengue infection predicted that increased exposure may impact efficacy [55, 70]. To test this possibility a small pharmaceutical company 60° Pharma has partnered with Singapore General Hospital to test an altered regimen that will result in an almost 300% increase in exposure with only a modest increase in dose. The clinical trial is scheduled to start recruiting in 2017 (Trial Identifier: NCT02569827).
22.2.1.6 Lovastatin
Lovastatin is a 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor, widely used for its lipid lowering properties. Among their other properties, statin drugs improve endothelial function and stabilize lipid membranes, two mechanisms known to be disrupted in DF pathogenesis. On this ground, a clinical trial was conducted in Vietnam by OUCRU to assess the safety of lovastatin as an anti-DENV agent. The safety outcome was measured clinically by trending liver and muscle injury serum markers, two commonly encountered side effects of lovastatin. Lovastatin was reported to be a safe and tolerable treatment in dengue patients, but there was no evidence of beneficial effect in terms of clinical progress or viremia reduction [71].
22.2.1.7 Ivermectin
This anti-parasitic agent commonly used to treat nematode infections, scabies, and lice has been shown to have anti-viral activities by inhibiting host nuclear import receptors importin-α and importin-β [64]. These receptors are necessary for DENV nonstructural protein 5 (NS5) migration to the nucleus for efficient replication. Nevertheless the precise mechanism by which ivermectin inhibits dengue is still unclear because the nuclear localization of NS5 is not required for viral RNA replication [59]. On the positive side, the drug has been identified as a potent inhibitor of the related flavivirus, zika virus in recent screens with FDA approved drugs [2, 74]. A clinical trial as a treatment for dengue fever with ivermectin is being carried out by the Mahidol University in collaboration with the Ministry of Health of Thailand (clinical identifier: NCT02045069). Preliminary results suggest reduction in serum NS1 antigenemia and body temperature despite the lack of detectable difference in viremia levels [1].
22.2.1.8 Ketotifen
Ketotifen is a non-competitive anti-histamine and a mast cell stabilizer generally used to treat atopic conditions like asthma and allergic rhinitis. It is being evaluated as an anti-dengue agent based on preclinical data suggesting that mast cell degranulation is an important mediator of DF pathogenesis [16, 22]. A clinical trial (NCT026773840) is currently being conducted by Duke-NUS, SGH, and the National University Hospital in Singapore to assess its clinical safety and efficacy.
22.3 DENV Specific Drug Development
Typically, new drug discovery involves identifying a target; developing an assay to assess activity against the target; high-throughput screening of potential candidate compounds; and chemically modifying the successful candidates to optimize activity, pharmacokinetics and toxicology in in vitro and in animal models of infection. Once a lead candidate is identified, manufacturing methods are adapted to produce the drug candidate in sufficient quantities to be tested in clinical trials [54].
Usually, the target is either a viral or host protein required for entry, proteolytic processing of the newly translated polyprotein, RNA replication, viral genome packaging and virion release from infected cells. DENV proteome comprises of 3 structural proteins: Capsid (C), premembrane (prM), and envelope (E) proteins; and 7 nonstructural (NS) proteins involved in protein processing and viral replication: NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. The replication apparatus, especially NS3 and NS5 have been the most intensively investigated targets in dengue drug development [37]. Commonly studied host targets include α-glucosidase, inosine monophosphate dehydrogenase, and dihydroorotate dehydrogenase among others [7].
These endeavors require large amounts of investments and carry high financial risks. Like many other neglected tropical diseases, dengue has been understudied consequently. To date, no candidate drug developed specifically for dengue has had positive results in a phase II human trial. This section highlights the considerable milestones achieved in this field and the promising projects underway.
NITD-008, a nucleoside inhibitor that targets viral RNA-dependent RNA polymerase (RdRp) is a potent antiviral developed by the Novartis Institute of Tropical Diseases in Singapore. It has remarkable nanomolar efficacy against all four DENV serotypes and other Flaviviruses in vitro and in vivo [76]. Toxicology studies conducted in animals revealed significant renal toxicity hence further development of this candidate was halted. Nonetheless, NITD-008 is still considered the standard of preclinical efficacy in dengue drug development and widely used to benchmark new drug development.
Recently, Emergent BioSolutions Inc. developed UV-4B, an antiviral agent that shows in vitro and in vivo activity against DENV. The proposed mechanism of action is through the inhibition of the enzymatic activities of host endoplasmic reticulum α-glucosidases. Viruses require these cellular enzymes for proper processing of their proteins. Since this is a host targeted mechanism of action, it is anticipated that development of viral resistance to UV-4B is less likely to occur than with directly acting antiviral agents [44, 66, 67]. This hypothesis was tested in vitro where DENV-infected cells treated with 38 cycles of UV-4B showed no drug-induced resistance. In vivo, UV-4B efficacy was maintained through 5 DENV passages in a mouse model [45].
An investigational new drug application has been opened for UV-4B based on preclinical safety and efficacy data. A phase 1 clinical trial completed in 2016 documented good tolerability and no serious adverse events after administration of single doses of UV-4B ranging between 3 and 1000 mg (NCT02061358). The pharmacokinetics data showed low inter-individual variability and linearity over a broad dose range. Another phase 1 clinical study has been initiated to determine the safety and pharmacokinetics of UV-4B administered orally as multiple ascending doses to healthy volunteers (NCT02696291). UV-4B is also being evaluated in preclinical studies for the treatment of influenza as an additional indication [66, 67].
Siga Technologies reported the development of the inhibitor ST-148, which acts by inducing structural rigidity. In a nonlethal model of DENV infection in AG129 mice, ST-148 reduced viremia and viral load and lowered cytokine levels in the plasma [6].
In recent years several groups have reported the discovery and development of NS4B inhibitors. NITD reported the activity of spiropyrazolopyridone compound NITD-618. Although, the compound showed reduced virema against DENV-2 in AG129 model of infection they were unable to identify a candidate that could show pan serotypic activity. In another screening effort, van Cleef et al. reported a new inhibitor, SDM25N, from screening the NIH Clinical Collection (NCC); a library of drug-like small molecules a stably replicating DENV serotype 2 (DENV2) subgenomic replicon. SDM25N, which restricts genomic RNA replication by – directly or indirectly – targeting the viral NS4B protein [62, 65, 72]. Janssen is also currently developing a NS4B inhibitor [43].
22.3.1 Recent Structural Discoveries
Recent advances in immunological, molecular, and structural virology have offered new ways to develop drugs more efficiently. Nuclear magnetic resonance spectrometry, X-ray crystallography, and cryo-electron microscopy have provided detailed structural data of DENV proteins. This information can be combined with molecular tools such as in silico approaches and infectious clone technology to discover new drug targets against DENV and potentially other flaviviridae [6, 33, 35, 73]. The viral processes that can be targeted include entry/fusion (E protein), translation/polyprotein processing of nonstructural (NS) proteins (NS3, NS2B-NS3 complex), replication (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5), and viral packaging (Capsid) [54]. Being essential to replication and infectivity and highly conserved among flaviviruses, NS3 and NS5 have been the most popular targets of DENV antiviral development.
Most notably, this structural information has been used in studies of human antibodies isolated from convalescent patients and yielded greater understanding of the epitopes that need to be targeted for effective virus neutralization [48]. Serotype-specific and cross-reactive neutralizing monoclonal antibodies are both being explored [60]. Ab513, a monoclonal antibody that binds domain III of the E protein of all 4 DENV serotypes was engineered by Visterra (Cambridge, MA). This agent has shown promising preclinical results with the ability to lower viremia and control DHS/DSS features in humanized mouse models without enhancing infection despite the presence of cross-reactive antibodies. It is poised to enter clinical trial by early 2018.
Compound and structure | Mechanism of action | Study site | Preclinical results | Clinical results |
|---|---|---|---|---|
1. Chloroquine | Lysosomal fusion inhibitor | OUCRU, Ho Chi Minh City, Vietnam | Cell-based assay (U937): EC50: 50 μM | No change in viremia or NS1 antigenemia |
Aotus monkey: significant reduction in viremia [21] | ||||
2. Prednisolone | Anti-inflammatory activity | OUCRU, Ho Chi Minh City, Vietnam | NA | No change in hematological virological or clinical endpoints |
3. Balapiravir | Polymerase inhibitor | OUCRU, Ho Chi Minh City, Vietnam | Cell based (Huh-7) | No change in virological and immunological endpoints [41] |
EC50;1.9–11 μM [41] | ||||
4. Ribavirin | Nucleoside analogue | Guangzhou 8th People’s Hospital | Cell-based (LLC-MK2): IC50: 50.9 μM [56] | Pending |
5. Lovastatin | Improving endothelial function and stabilizing lipid membranes | OUCRU, Ho Chi Minh City, Vietnam | AG129 mouse model: Increased survival at dose of 200 mg/kg/day [71] | No evidence of beneficial effect on clinical progress or DENV viremia [71] |
6. Ivermectin | Helicase inhibitor | Mahidol University/Siriraj Hospital, Thailand | Enzyme assay: IC50: 0.5 μM [1] | NS1 antigenemia and fever reduction (preliminary) |
7. Celgosivir | Alpha glucosidase inhibitor | SGH/Duke-NUS, Singapore | Cell-based assay: EC50: 0.2 μM | No statistically significant reduction of viral load or fever [36] |
AG129 mouse model: 100% survival at dose of 50 mg/kg BD for 5 days [67,68,70] | ||||
8. Ketotifen | Mast cell stabilizer | SGH/Duke-NUS, Singapore | Mouse model: significant reduction of severe dengue features [51] | Pending |
9. UV4B | Alpha glucosidase inhibitor | Emergent BioSolutions, Maryland USA | AG129 mouse model: 100-fold viremia reduction at 100 mg/kg PO dosing | Phase 1a Single Ascending Dose Study in healthy volunteers showed UV-4B is well tolerated up to 1000 mg single dose |
10. NITD008 | Nucleoside analogue-polymerase inhibitor | NITD, Singapore | Cell based assay (PBMC) EC50: 0.16–0.85 μM | Excessive nephrotoxicity, development halted |
AG129 mouse model: 100% survival at dose >10 mg/kg PO [76] |
22.4 The Clinical – Preclinical Gap
As discussed above, many compounds have shown good efficacy in preclinical studies. However, none was proven to be superior to placebo in clinical setting. The disparity between preclinical and clinical results is an inherent entity of therapeutics research. Common reasons include the nonspecific and wide spectrum of disease manifestations, the limitations of existing model organisms, and the lack of a biological marker that correlates with clinical results. A factor that makes this even more pertinent for dengue therapeutics, specifically, is the limited financial resources available for research. The recent clinical and preclinical progress achieved gives an insightful perspective on what should be future research directions.
22.4.1 Clinical Manifestation
Dengue fever has extremely nonspecific manifestations making it difficult to study in clinical settings. Contrary to preclinical setting where the time of infection is controlled, patients are recruited into clinical trials when they experience symptoms and present to their healthcare provider. Fever is virtually the only symptom clinicians base they suspicion on when screening for dengue. As with most other febrile illnesses, dengue patients do not present until later in the course of disease. Unless there is an ongoing outbreak, dengue might not be the first impression of the healthcare provider. This presents an additional challenge to clinical trial design and recruitment as administering treatment later in the course of illness when the viremia level has already past the peak can dilute any potential benefit of the therapeutic agent under investigation.
Moreover, the vagueness of early symptoms is a source of inaccuracy based on patient-reported onset of illness/fever. This is a known unreliable measure, but there is usually no better alternative.
22.4.2 Representative Model Organism
DENV is not known to be naturally pathogenic in any species other than humans. This makes it very challenging to obtain representative animal models for research. Efforts have been made to identify the specific characteristics that make us vulnerable to DENV. Multiple animals and DENV strains have been genetically engineered to replicate the identified characteristics, but, to this day, none of the models are able to accurately mimic a human DENV infection.
22.4.3 Nonhuman Primates
Nonhuman primates develop viremia and neutralizing antibody response to DENV infection but do not develop symptoms of DF [75]. They have been used to study antibody-dependent enhancement of infection (ADE) and to test candidate vaccines and antivirals efficacy by assessing viremia levels [12, 13, 24, 25]. As we discuss below, viremia is a suboptimal measure of treatment response. Some nonhuman primate models that express features of human DF have been identified; but their use is hindered by the prohibitive cost and accessibility.
22.4.4 Mouse Models
DENV does not replicate well in rodent cells. To overcome this, extensive work has been done to develop DENV mouse models. The first successful suckling mouse, was developed by serial passage DENV in mice brain, increasing the virus adaptability to mice cells [50]. Intracranial inoculation resulted in DENV encephalitis and paralysis. This model has been used by to evaluate antivirals and vaccines but the altered tropism of the virus makes the relevance to humans questionable [9].
Inoculation with high DENV titers in immunocompetent mice can induce disease comparable to human DF. Different groups have reported hepatic T cell invasion, localized hemorrhage, thrombocytopenia and detectable viral load in serum, spleen, liver, and brain, vascular leakage; all of which are characteristic of human DF/DHS/DSS [9, 10, 12, 13, 52]. This model is useful to gain insight on the pathogenesis of DF but the high viral titer required to induce symptoms is not representative of the infection process in humans; making it inadequate for antiviral development studies.
Humanized mice developed by transplanting human hematopoietic stem cells into severe combined immunodeficient mice have been shown to be susceptible to low passage DENV. They exhibit DF-like feature such as fever, rash and thrombocytopenia, but not DHS/DSS [4]. Kuruvilla and colleagues reported that humanized mice can develop fever and viremia for up to 21 days and produce human anti-DENV Ig-M and Ig-G capable of neutralizing DENV. There was, however, no significant cellular immune response induced [32]. Several groups have tried to replicate a human like T-cell response in humanized mice, but there still has not been any model capable of mimicking severe dengue symptoms, or human-like immune response; limiting their use in therapeutics research [9].
Immunocompromised mice have been the most widely used model thus far. By knocking out key genes involved in immune response, infectible mice models have been engineered. The most established in therapeutics research is the type I and II interferon receptors deficient AG129. This model is susceptible to both mouse-adapted and clinical DENV infection. Concurrently, efforts were also being put into identifying a DENV strain that could successfully infect mice. D2S10 was developed by alternate passaging of PL046, a clinical isolate, between AG129 mice and C3/C6 mosquito cells. This DENV strain was able to cause lethal infection in mice without neurological deficits and; most importantly, D2S10 caused vascular leakage making it very pertinent to study human DF. Of note, celgosivir and lovastatin were proven to be efficacious in AG129 mice, with increased survival and decreased viremia. In clinical trials, however, no significant effect was observed [36, 68, 69, 71].
22.4.5 Need for Human Infection Model
Although there has been reports of certain DENV strains causing some features of DHS and DSS in certain mouse models, the pathogenesis remains substantially different from that in humans [58]. The ideal way around this obstacle is the use of a human infection model (HIM). HIM was an important contributor to the development of oseltamivir. The illness caused by influenza virus infection in healthy young adults is short lived and of mild severity; the use of a HIM was hence accessible to the developers of oseltamivir [28]. That enabled them to control the timing between infection and initiation of therapy, one of the most critical challenges in anti-dengue clinical trials [37]. Perhaps more importantly, it also allowed them to quickly down-select candidate compounds [19]. This was crucial to a remarkably fast drug development; oseltamivir was approved for treatment of influenza only 7 years after the search for an orally available neuraminidase inhibitor was initiated [54].
HIM has been used to study several aspects of dengue before. For instance, Startler and colleagues studied the process of DENV induced plasma leakage in healthy individuals [53]. In 2011, Gunther et al. used HIM to study the immune response to DENV in previously vaccinated people [26]. More recently, Kirkpatrick and colleagues proposed HIM to validate vaccines before moving onto large scale clinical trials [31]. Even though HIM would revolutionize dengue research, there are still strong reservations about its use. DF is typically not associated with mortality in healthy adults, but has a potential for high morbidity requiring hospitalization [19]. Another limitation of HIM is the fact that there is no possibility to access tissue samples for pathological analysis, limiting HIM studies to a descriptive nature [9].
22.5 Objectively Measurable Endpoints
22.5.1 Viremia
Since the earliest dengue studies, viremia as a biomarker for dengue disease has been a universally accepted paradigm [49, 54]. This concept was popularized by the availability of sensitive methods to detect and quantify viral particles in clinical samples. In fact, all trials for candidate antiviral drugs and vaccines have reduction of viremia as one of their primary clinical endpoints, if not the only endpoint. However, it has become evident that measured viremia does not always correlate with clinical outcome. For instance, during secondary infections, measured viremia is often markedly lower than primary despite the higher likelihood of developing severe disease. One of the possible explanations for this phenomenon is intrinsic to the viremia assay methodology itself.
22.5.1.1 qPCR
In the 7 clinical trials mentioned above, viremia was measured using quantitative polymerase chain reaction assay (qPCR). This method detects and quantifies viral RNA in serum, but it does not reflect the amount infective viruses present. RNA copies can exceed infectious viral units by 2–5 logs. This effect has been described for other viral infections as well and was attributed to a probable large quantity of cells infected by defective proviruses, masking the absolute viremia [20].
22.5.1.2 Plaque Assay
A more representative assay would be direct culturing of the clinical samples. However, this is extremely challenging because clinical isolates have widely varying abilities to grow in vitro. Unpassaged viruses, for instance, are known to be less able to infect consistently in vitro despite their potential infectivity in vivo. This makes culture and plaque assays inherently inaccurate. Mosquito inoculation can be used to account for unpassaged DENV, but this technique requires an insectary and highly skilled personnel making it inaccessible to most diagnostic virology laboratories [15].
22.5.2 NS1 Antigenemia
Serum NS1 levels rise early in the infection course making it a good diagnostic tool. However, NS1 antigenemia varies considerably by DENV serotypes and primary versus secondary infection [18, 68, 69]. There is also evidence that it may be involved in DF pathogenesis [3, 39]. Structural and in vivo mice studies suggest that, if combined with a host dependent biomarker, NS1 could be a reliable biomarker and clinical endpoint measure for therapeutic trials [40]. Further work is needed to confirm this.
22.5.3 Host Biomarkers: Cytokine, Endothelial Activation Markers, Cells, Biochemical Markers
It is believed that patients with DHS/DSS experience a “cytokine storm,” causing their clinical symptoms. Trending these cytokines may, consequently, serve as a good prognostic biomarker. Identified markers that correlate with disease progress include interleukin-10, complements C3a and C5a, and macrophage migration inhibitory factor [11, 29, 38].
The correlation between blood cellular components and dengue severity has been extensively studied, platelet and red blood cell counts in particular. Thrombocytopenia is an established hallmark of severe dengue disease [17, 23]. A study conducted in Thailand was able to predict more than 97% subsequent severe disease development using an algorithm based on white blood cell counts, percent monocytes, platelet count, and hematocrit information obtained in the first 72 h of disease [46]. A similar study conducted in Vietnam proposed a prognostic scoring system based on the platelet count, history of vomiting, blood aspartate aminotransferase level, and NS1 rapid test status [42]. This scoring system had a sensitivity of 87% and specificity of 88%; with a negative predictive value of 99% amongst their study participants.
Biochemical markers like liver enzymes, nitric oxide, and lipids are also known to be deranged in severe disease, and have been studied as biomarkers and potential clinical endpoints. Several endothelial activation markers, including angiopoietin, von Willebrand factor, and VEGF, have also been associated with disease severity [29].
All these host biomarkers are used at varying degrees in dengue research. They all have a common short coming, however; poor specificity. There are many factors that can be responsible for host biomarkers changes and over-relying on them can result in false interpretations [29]. Nonetheless, cautiously combining them with DENV biomarkers greatly improves their prognostic power, making a case for their use in therapeutics trials [37].
22.5.4 18F-Fluorodeoxyglucose Imaging
In addition to developing optimal infection models, identifying new measurable endpoints that are consistent and comparable between animal models and humans would greatly help narrow the aforementioned preclinical-clinical gap. A team of researchers in Singapore is working on identifying new and more representative DF biomarkers by building upon the known pathogenesis. DENV particles preferentially replicate and accumulate in tissues that are not readily accessible. The ability to monitor tissues specifically instead of relying on systemic circulating markers offers a novel way to make early and robust inferences about the course of illness. 18F-Fluorodeoxyglucose uptake monitoring in dengue-infected mice has identified specific inflammation patterns in the intestines that strongly correlate with progression of disease and treatment response [8]. A human proof-of-concept trial is currently recruiting dengue infected subjects in Singapore to validate these findings in human.
22.6 Conclusion
Dengue fever imposes a significant global burden and low-income tropical countries are more heavily impacted. Efforts to develop vaccines and treatment modalities have been very slow until the 2000s. For the most part, this was due to the lack of available resources allocated to the cause. Over the past two decades, more governmental, academic, and private agencies have devoted funds to dengue research; which resulted in considerable advances, most notably a tetravalent vaccine licensed in most endemic countries. Nonetheless, the road to relieving the dengue burden is still long. The available vaccine’s efficacy is not ideal, making it imperative to have effective therapeutic options. Dengue antiviral drug development endeavors with both repurposed and de novo agents have not yet yielded effective products in clinical trials but have bolstered the understanding of dengue pathogenesis and laid up the ground for further projects. Recent technological advances and innovative approaches may be offering faster, targeted, and more cost-efficient models to develop and assess new drugs.
References
Avirutnam P (2016) Ivermectin: a promising anti-dengue replication treatment. Eur Congress Clin Microbiol Infect Dis. (Abstract)
Barrows NJ, Campos RK, Powell ST, Prasanth KR, Schott-Lerner G, Soto-Acosta R, Galarza-Muñoz G, McGrath EL, Urrabaz-Garza R, Gao J, Wu P, Menon R, Saade G, Fernandez-Salas I, Rossi SL, Vasilakis N, Routh A, Bradrick SS, Garcia-Blanco MA (2016) A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe 20(2):259–270. https://doi.org/10.1016/j.chom.2016.07.004
Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E (2015) Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 7(304):304ra141–304ra141. https://doi.org/10.1126/scitranslmed.aaa3787
Bente DA, Melkus MW, Garcia JV, Rico-Hesse R (2005) Dengue Fever in Humanized NOD/SCID mice. J Virol 79(21):13797–13799. https://doi.org/10.1128/JVI.79.21.13797-13799.2005
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI (2013) The global distribution and burden of dengue. Nature 496(7446):504–507. https://doi.org/10.1038/nature12060
Byrd CM, Dai D, Grosenbach DW, Berhanu A, Jones KF, Cardwell KB, Schneider C, Wineinger KA, Page JM, Harver C, Stavale E, Tyavanagimatt S, Stone MA, Bartenschlager R, Scaturro P, Hruby DE, Jordan R (2013) A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob Agents Chemother 57(1):15–25. https://doi.org/10.1128/AAC.01429-12
Caputo AT, Alonzi DS, Marti L, Reca I-B, Kiappes JL, Struwe WB, Cross A, Basu S, Lowe ED, Darlot B, Santino A, Roversi P, Zitzmann N (2016) Structures of mammalian ER α-glucosidase II capture the binding modes of broad-spectrum iminosugar antivirals. Proc Natl Acad Sci U S A 113(32):E4630–E4638. https://doi.org/10.1073/pnas.1604463113
Chacko AM, Watanabe S, Herr KJ, Kalimuddin S, Tham JY, Ong J, Reolo M, Serrano RMF, Cheung YB, Low JGH, Vasudevan SG (2017) 18F-FDG as an inflammation biomarker for imaging dengue virus infection and treatment response. JCI Insight 2(9). https://doi.org/10.1172/jci.insight.93474
Chan KWK, Watanabe S, Kavishna R, Alonso S, Vasudevan SG (2015) Animal models for studying dengue pathogenesis and therapy. Antivir Res 123:5–14. https://doi.org/10.1016/j.antiviral.2015.08.013
Chen HC, Lai SY, Sung JM, Lee SH, Lin YC, Wang WK, Chen YC, Kao CL, King CC, Wu-Hsieh BA (2004) Lymphocyte activation and hepatic cellular infiltration in immunocompetent mice infected by dengue virus. J Med Virol 73(3):419–431. https://doi.org/10.1002/jmv.20108
Chen L-C, Lei H-Y, Liu C-C, Shiesh S-C, Chen S-H, Liu H-S, Lin Y-S, Wang S-T, Shyu H-W, Yeh T-M (2006) Correlation of serum levels of macrophage migration inhibitory factor with disease severity and clinical outcome in dengue patients. Am J Trop Med Hyg 74(1):142–147
Chen HC, Hofman FM, Kung JT, Lin YD, Wu-Hsieh BA (2007a) Both virus and tumor necrosis factor alpha are critical for endothelium damage in a mouse model of dengue virus-induced hemorrhage. J Virol 81(11):5518–5526. https://doi.org/10.1128/JVI.02575-06
Chen L, Ewing D, Subramanian H, Block K, Rayner J, Alterson KD, Sedegah M, Hayes C, Porter K, Raviprakash K (2007b) A heterologous DNA prime-Venezuelan equine encephalitis virus replicon particle boost dengue vaccine regimen affords complete protection from virus challenge in cynomolgus macaques. J Virol 81(21):11634–11639. https://doi.org/10.1128/JVI.00996-07
Chen YL, Abdul Ghafar N, Karuna R, Fu Y, Lim SP, Schul W, Gu F, Herve M, Yokohama F, Wang G, Cerny D, Fink K, Blasco F, Shi PY (2014) Activation of peripheral blood mononuclear cells by dengue virus infection depotentiates balapiravir. J Virol 88(3):1740–1747. https://doi.org/10.1128/JVI.02841-13, https://www-ncbi-nlm-nih-gov.libproxy1.nus.edu.sg/pubmed/24257621
Choy MM, Ellis BR, Ellis EM, Gubler DJ (2013) Short report: comparison of the mosquito inoculation technique and quantitative real time polymerase chain reaction to measure dengue virus concentration. Am J Trop Med Hyg 89(5):1001–1005. https://doi.org/10.4269/ajtmh.13-0100
Dawicki W, Marshall JS (2007) New and emerging roles for mast cells in host defence. Curr Opin Immunol 19(1):31–38. https://doi.org/10.1016/j.coi.2006.11.006
Dengue: guidelines for diagnosis, treatment, prevention, and control. (2009). World Health Organization, Geneva
Duyen HTL, Ngoc TV, Ha DT, Hang VTT, Kieu NTT, Young PR, Farrar JJ, Simmons CP, Wolbers M, Wills BA (2011) Kinetics of plasma viremia and soluble nonstructural protein 1 concentrations in dengue: differential effects according to serotype and immune status. Journal of Infectious Diseases 203(9):1292–1300. https://doi.org/10.1093/infdis/jir014
Endy TP (2014) Dengue human infection model performance parameters. J Infect Dis 209(SUPPL. 2). https://doi.org/10.1093/infdis/jiu112
Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, Abdel-Mohsen M, Hoh R, Hecht F, Hunt P, Somsouk M, Wong J, Johnston R, Siliciano RF, Richman DD, O'Doherty U, Palmer S, Deeks SG, Siliciano JD (2013) Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 9(2). https://doi.org/10.1371/journal.ppat.1003174
Farias KJS, Machado PRL, Muniz JAPC, Imbeloni AA, da Fonseca BAL (2015) Antiviral activity of chloroquine against dengue virus type 2 replication in Aotus monkeys. Viral Immunol 28(3):161–169. https://doi.org/10.1089/vim.2014.0090
Furuta T, Murao LA, Lan NTP, Huy NT, Huong VTQ, Thuy TT, Tham VD, Nga CTP, Ha TTN, Ohmoto Y, Kikuchi M, Morita K, Yasunami M, Hirayama K, Watanabe N (2012) Association of mast cell-derived VEGF and proteases in dengue shock syndrome. PLoS Negl Trop Dis 6(2). https://doi.org/10.1371/journal.pntd.0001505
Global strategy for dengue prevention and control 2012–2020. (2012). World Health Organization, Geneva.
Goncalvez AP, Engle RE, St. Claire M, Purcell RH, Lai CJ (2007) Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci 104(22):9422–9427. https://doi.org/10.1073/pnas.0703498104
Guirakhoo F, Pugachev K, Arroyo J, Miller C, Zhang ZX, Weltzin R, Georgakopoulos K, Catalan J, Ocran S, Draper K, Monath TP (2002) Viremia and immunogenicity in nonhuman primates of a tetravalent yellow fever–dengue chimeric vaccine: genetic reconstructions, dose adjustment, and antibody responses against wild-type dengue virus isolates. Virology 298(1):146–159. https://doi.org/10.1006/viro.2002.1462
Gunther VJ, Putnak R, Eckels KH, Mammen MP, Scherer JM, Lyons A, Sztein MB, Sun W (2011) A human challenge model for dengue infection reveals a possible protective role for sustained interferon gamma levels during the acute phase of illness. Vaccine 29(22):3895–3904. https://doi.org/10.1016/j.vaccine.2011.03.038
Halstead SB, Russell PK (2016) Protective and immunological behavior of chimeric yellow fever dengue vaccine. Vaccine 34(14):1643–1647. https://doi.org/10.1016/j.vaccine.2016.02.004
Hayden FG, Atmar RL, Schilling M, Johnson C, Poretz D, Paar D, Huson L, Ward P, Mills RG, the Oseltamivir Study, G (1999) Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med 341(18):1336–1343
John DV, Lin Y-S, Perng GC (2015) Biomarkers of severe dengue disease – a review. J Biomed Sci 22(1):83. https://doi.org/10.1186/s12929-015-0191-6
Keller TH, Chen YL, Knox JE, Lim SP, Ma NL, Patel SJ, Sampath A, Wang QY, Yin Z, Vasudevan SG (2006) Finding new medicines for flaviviral targets. In: Novartis Foundation Symposium, vol 277. John Wiley, Chichester, pp 102–114. 1999
Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, Carmolli MP, Luke CJ, Diehl SA, Durbin AP (2016) The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med 8(330):330ra336–330ra336. https://doi.org/10.1126/scitranslmed.aaf1517
Kuruvilla JG, Troyer RM, Devi S, Akkina R (2007) Dengue virus infection and immune response in humanized RAG2(-/-)gamma(c)(-/-) (RAG-hu) mice. Virology 369(1):143–152. https://doi.org/10.1016/j.virol.2007.06.005
Lescar J, Luo D, Xu T, Sampath A, Lim SP, Canard B, Vasudevan SG (2008) Towards the design of antiviral inhibitors against flaviviruses: the case for the multifunctional NS3 protein from Dengue virus as a target. Antivir Res 80(2):94–101. https://doi.org/10.1016/j.antiviral.2008.07.001
Lim SP, Wang Q-Y, Noble CG, Chen Y-L, Dong H, Zou B, Yokokawa F, Nilar S, Smith P, Beer D, Lescar J, Shi P-Y (2013) Ten years of dengue drug discovery: progress and prospects. Antivir Res 100(2):500–519. https://doi.org/10.1016/j.antiviral.2013.09.013
Lim SP, Noble CG, Shi PY (2015) The dengue virus NS5 protein as a target for drug discovery. Antivir Res 119:57–67. https://doi.org/10.1016/j.antiviral.2015.04.010
Low JG, Sung C, Wijaya L, Wei Y, Rathore APS, Watanabe S, Tan BH, Toh L, Chua LT, Hou Y a, Chow A, Howe S, Chan WK, Tan KH, Chung JS, Cherng BP, Lye DC, Tambayah PA, Ng LC, Connolly J, Hibberd ML, Leo YS, Cheung YB, Ooi EE, Vasudevan SG (2014) Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect Dis 14(8):706–715. https://doi.org/10.1016/s1473-3099(14)70730-3
Low JG, Ooi EE, Vasudevan SG (2017) Current status of dengue therapeutics research and development. J Infect Dis 215(suppl_2):S96–S102. https://doi.org/10.1093/infdis/jiw423
Malavige GN, Gomes L, Alles L, Chang T, Salimi M, Fernando S, Nanayakkara KDL, Jayaratne SD, Ogg GS (2013) Serum IL-10 as a marker of severe dengue infection. BMC Infect Dis 13(1):341–341. https://doi.org/10.1186/1471-2334-13-341
Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L, Hume DA, Stacey KJ, Young PR (2015) Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med 7(304):304ra142. https://doi.org/10.1126/scitranslmed.aaa3863
Muller DA, Young PR (2013) The flavivirus NS1 protein: molecular and structural biology, immunology, role inpathogenesis and application asadiagnostic biomarker. Antivir Res 98(2):192–208. https://doi.org/10.1016/j.antiviral.2013.03.008
Nguyen NM, Tran CN, Phung LK, Duong KT, Huynh Hle A, Farrar J, Nguyen QT, Tran HT, Nguyen CV, Merson L, Hoang LT, Hibberd ML, Aw PP, Wilm A, Nagarajan N, Nguyen DT, Pham MP, Nguyen TT, Javanbakht H, Klumpp K, Hammond J, Petric R, Wolbers M, Nguyen CT, Simmons CP (2013) A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J Infect Dis 207(9):1442–1450. https://doi.org/10.1093/infdis/jis470
Nguyen MT, Ho TN, Nguyen VVC, Nguyen TH, Ha MT, Ta VT, Nguyen LDH, Phan L, Han KQ, Duong THK, Tran NBC, Bridget W, Wolbers M, Simmons CP (2017) An evidence-based algorithm for early prognosis of severe dengue in the outpatient setting. Clin Infect Dis 64(5):656–663. https://doi.org/10.1093/cid/ciw863
Partnership with the Wellcome Trust to address unmet medical needs. Retrieved from http://www.janssen.com/partnerships/dengue
Perry ST, Buck MD, Plummer EM, Penmasta RA, Batra H, Stavale EJ, Warfield KL, Dwek RA, Butters TD, Alonzi DS, Lada SM, King K, Klose B, Ramstedt U, Shresta S (2013) An iminosugar with potent inhibition of dengue virus infection in vivo. Antivir Res 98(1):35–43. https://doi.org/10.1016/j.antiviral.2013.01.004
Plummer E, Buck MD, Sanchez M, Greenbaum JA, Turner J, Grewal R, Klose B, Sampath A, Warfield KL, Peters B, Ramstedt U, Shresta S (2015) Dengue virus evolution under a host-targeted antiviral. J Virol 89(10):5592–5601. https://doi.org/10.1128/JVI.00028-15
Potts JA, Gibbons RV, Rothman AL, Srikiatkhachorn A, Thomas SJ, Supradish P o, Lemon SC, Libraty DH, Green S, Kalayanarooj S (2010) Prediction of dengue disease severity among pediatric Thai patients using early clinical laboratory indicators. PLoS Negl Trop Dis 4(8):2–8. https://doi.org/10.1371/journal.pntd.0000769
Rajapakse S, Rodrigo C, Maduranga S, Rajapakse AC (2014) Corticosteroids in the treatment of dengue shock syndrome. Infect Drug Resist 7:137–143. https://doi.org/10.2147/IDR.S55380
Robinson LN, Tharakaraman K, Rowley KJ, Costa VV, Chan KR, Wong YH, Ong LC, Tan HC, Koch T, Cain D, Kirloskar R, Viswanathan K, Liew CW, Tissire H, Ramakrishnan B, Myette JR, Babcock GJ, Sasisekharan V, Alonso S, Chen J, Lescar J, Shriver Z, Ooi EE, Sasisekharan R (2015) Structure-guided design of an anti-dengue antibody directed to a non-immunodominant epitope. Cell 162(3):493–504. https://doi.org/10.1016/j.cell.2015.06.057
Sabin AB (1952) Research on dengue during World War II. Am J Trop Med Hyg 1(1):30–50. https://doi.org/10.1086/383418
Sabin AB, Schlesinger RW (1945) Production of immunity to dengue with virus modified by propagation in mice. Science 101(2634):640–642. https://doi.org/10.1126/science.101.2634.640
St John AL (2013) Influence of mast cells on dengue protective immunity and immune pathology. PLoS Pathog 9(12):e1003783. https://doi.org/10.1371/journal.ppat.1003783
St John AL, Rathore APS, Raghavan B, Ng ML, Abraham SN (2013) Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage. eLife 2013(2). https://doi.org/10.7554/eLife.00481
Statler J, Mammen M, Lyons A, Sun W (2008) Sonographic findings of healthy volunteers infected with dengue virus. J Clin Ultrasound 36(7):413–417. https://doi.org/10.1002/jcu.20476
Sung C, Kumar GBS, Vasudevan SG (2014) Dengue Drug Development. In Dengue and Dengue Hemorrhagic Fever (2nd Edn) Gubler DJ, Ooi EE, Vasudevan S, Farrar J (Editors) Chapter 16 p293–321. Oxfordshire, CABI
Sung C, Wei Y, Watanabe S, Lee HS, Khoo YM, Fan L, Rathore AP, Chan KW, Choy MM, Kamaraj US, Sessions OM, Aw P, de Sessions PF, Lee B, Connolly JE, Hibberd ML, Vijaykrishna D, Wijaya L, Ooi EE, Low JG, Vasudevan SG (2016) Extended evaluation of virological, immunological and pharmacokinetic endpoints of CELADEN: a randomized, placebo-controlled trial of celgosivir in dengue fever patients. PLoS Negl Trop Dis 10(8):e0004851. https://doi.org/10.1371/journal.pntd.0004851
Takhampunya R, Ubol S, Houng H-S, Cameron CE, Padmanabhan R (2006) Inhibition of dengue virus replication by mycophenolic acid and ribavirin. J Gen Virol 87(7):1947–1952. https://doi.org/10.1099/vir.0.81655-0
Tam DT, Ngoc TV, Tien NT, Kieu NT, Thuy TT, Thanh LT, Tam CT, Truong NT, Dung NT, Qui PT, Hien TT, Farrar JJ, Simmons CP, Wolbers M, Wills BA (2012) Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo-controlled trial. Clin Infect Dis 55(9):1216–1224. https://doi.org/10.1093/cid/cis655
Tan GK, Ng JKW, Trasti SL, Schul W, Yip G, Alonso S (2010) A non mouse-adapted dengue virus strain as a new model of severe dengue infection in AG129 mice. PLoS Negl Trop Dis 4(4). https://doi.org/10.1371/journal.pntd.0000672
Tay MYF, Fraser JE, Chan WKK, Moreland NJ, Rathore AP, Wang C, Vasudevan SG, Jans DA (2013) Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir Res 99(3):301–306. https://doi.org/10.1016/j.antiviral.2013.06.002
Teoh EP, Kukkaro P, Teo EW, Lim AP (2012) The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl 4(139):139ra183
Tricou V, Minh NN, Van TP, Lee SJ, Farrar J, Wills B, Tran HT, Simmons CP (2010) A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis 4(8):e785. https://doi.org/10.1371/journal.pntd.0000785
van Cleef KWR, Overheul GJ, Thomassen MC, Kaptein SJF, Davidson AD, Jacobs M, Neyts J, van Kuppeveld FJM, van Rij RP (2013) Identification of a new dengue virus inhibitor that targets the viral NS4B protein and restricts genomic RNA replication. Antivir Res 99(2):165–171. https://doi.org/10.1016/j.antiviral.2013.05.011
Vannice KS, Durbin A, Hombach J (2016) Status of vaccine research and development of vaccines for dengue. Vaccine 34(26):2934–2938. https://doi.org/10.1016/j.vaccine.2015.12.073
Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans D a (2012) Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J 443(3):851–856. https://doi.org/10.1042/BJ20120150
Wang QY, Dong H, Zou B, Karuna R, Wan KF, Zou J, Susila A, Yip A, Shan C, Yeo KL, Xu H, Ding M, Chan WL, Gu F, Seah PG, Liu W, Lakshminarayana SB, Kang C, Lescar J, Blasco F, Smith PW, Shi PY (2015) Discovery of Dengue virus NS4B inhibitors. J Virol 89(16):8233–8244. https://doi.org/10.1128/JVI.00855-15
Warfield KL, Barnard DL, Enterlein SG, Smee DF, Khaliq M, Sampath A, Callahan MV, Ramstedt U, Day CW (2016a) The iminosugar UV-4 is a broad inhibitor of influenza A and B viruses ex vivo and in mice. Viruses 8(3). https://doi.org/10.3390/v8030071
Warfield KL, Plummer EM, Sayce AC, Alonzi DS, Tang W, Tyrrell BE, Hill ML, Caputo AT, Killingbeck SS, Beatty PR, Harris E, Iwaki R, Kinami K, Ide D, Kiappes JL, Kato A, Buck MD, King K, Eddy W, Khaliq M, Sampath A, Treston AM, Dwek RA, Enterlein SG, Miller JL, Zitzmann N, Ramstedt U, Shresta S (2016b) Inhibition of endoplasmic reticulum glucosidases is required for in vitro and in vivo dengue antiviral activity by the iminosugar UV-4. Antivir Res 129:93–98. https://doi.org/10.1016/j.antiviral.2016.03.001
Watanabe S, Rathore APS, Sung C, Lu F, Khoo YM, Connolly J, Low J, Ooi EE, Lee HS, Vasudevan SG (2012a) Dose- and schedule-dependent protective efficacy of celgosivir in a lethal mouse model for dengue virus infection informs dosing regimen for a proof of concept clinical trial. Antivir Res 96(1):32–35. https://doi.org/10.1016/j.antiviral.2012.07.008
Watanabe S, Tan KH, Rathore APS, Rozen-Gagnon K, Shuai W, Ruedl C, Vasudevan SG (2012b) The magnitude of dengue virus NS1 protein secretion is strain dependent and does not correlate with severe pathologies in the mouse infection model. J Virol 86(10):5508–5514. https://doi.org/10.1128/JVI.07081-11
Watanabe S, Chan KWK, Dow G, Ooi EE, Low JG, Vasudevan SG (2016) Optimizing celgosivir therapy in mouse models of dengue virus infection of serotypes 1 and 2: the search for a window for potential therapeutic efficacy. Antivir Res 127:10–19. https://doi.org/10.1016/j.antiviral.2015.12.008
Whitehorn J, Nguyen CVV, Khanh LP, Kien DTH, Quyen NTH, Tran NTT, Hang NT, Truong NT, Hue Tai LT, Cam Huong NT, Nhon VT, Van Tram T, Farrar J, Wolbers M, Simmons CP, Wills B (2016) Lovastatin for the treatment of adult patients with dengue: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 62(4):468–476. https://doi.org/10.1093/cid/civ949
Xie X, Wang Q-Y, Xu HY, Qing M, Kramer L, Yuan Z, Shi P-Y (2011) Inhibition of dengue virus by targeting viral NS4B protein. J Virol 85(21):11183–11195. https://doi.org/10.1128/JVI.05468-11
Xie X, Zou J, Wang QY, Shi PY (2015) Targeting dengue virus NS4B protein for drug discovery. Antiviral Res 118:39–45. https://doi.org/10.1016/j.antiviral.2015.03.007
Xu M, Lee EM, Wen Z, Cheng Y, Huang W-K, Qian X, Tcw J, Kouznetsova J, Ogden SC, Hammack C, Jacob F, Nguyen HN, Itkin M, Hanna C, Shinn P, Allen C, Michael SG, Simeonov A, Huang W, Christian KM, Goate A, Brennand KJ, Huang R, Xia M, Ming G-l, Zheng W, Song H, Tang H (2016) Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med 22(10):1101–1107. https://doi.org/10.1038/nm.4184
Yauch LE, Shresta S (2008) Mouse models of dengue virus infection and disease. Antiviral Res 80(2):87–93. https://doi.org/10.1016/j.antiviral.2008.06.010
Yin Z, Chen YL, Schul W, Wang QY, Gu F, Duraiswamy J, Kondreddi RR, Niyomrattanakit P, Lakshminarayana SB, Goh A, Xu HY, Liu W, Liu B, Lim JY, Ng CY, Qing M, Lim CC, Yip A, Wang G, Chan WL, Tan HP, Lin K, Zhang B, Zou G, Bernard KA, Garrett C, Beltz K, Dong M, Weaver M, He H, Pichota A, Dartois V, Keller TH, Shi PY (2009) An adenosine nucleoside inhibitor of dengue virus. Proc Natl Acad Sci U S A 106(48):20435–20439. https://doi.org/10.1073/pnas.0907010106
Acknowledgements
UV-4B development was supported by a contract award from the National Institute of Allergy and Infectious Diseases, HHSN272201100030C. The National Medical Research Council of Singapore is gratefully acknowledged for the support of dengue translational research grants (NMRC/CTGCoD/0001/2015 (JGL) and NMRC/CBRG/0103/2016 (SGV)).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Low, J.G., Gatsinga, R., Vasudevan, S.G., Sampath, A. (2018). Dengue Antiviral Development: A Continuing Journey. In: Hilgenfeld, R., Vasudevan, S. (eds) Dengue and Zika: Control and Antiviral Treatment Strategies. Advances in Experimental Medicine and Biology, vol 1062. Springer, Singapore. https://doi.org/10.1007/978-981-10-8727-1_22
Download citation
DOI: https://doi.org/10.1007/978-981-10-8727-1_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8726-4
Online ISBN: 978-981-10-8727-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)