Abstract
This chapter will explore whether the differences in the process development for phosphorus recovery and recycling and its implementation in Europe and Japan are linked to the waste flows and the regulative framework. The main waste flows and their qualities are summarized for the two geographical areas. Then a comparative overview of the full-scale applications and their importance in relation to the potential is presented. The drivers for phosphorus recycling and the expected further development in Europe and Japan are described.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Phosphorus (P) has important biological functions as the building block of DNA, cell membranes, energy-storing ATP, and bones. It is necessary for growth and development of all living organisms and is present in most organic materials. More than 80% of mined phosphate rock is used for fertilizer, providing about half of the fertilizer P consumed globally. The remaining P comes from recycled organic waste, mainly manure (Scholz et al. 2014). The fossil phosphorus reserves are concentrated in Morocco and Western Sahara (>70%) (USGS 2018), and their share of reserves is increasing as other countries mine their deposits at a higher rate. Therefore, numerous initiatives are emerging which aim at increasing the fraction of recycled phosphorus. On a global scale, Japan and Europe are front-runners in phosphorus recovery technologies.
From a material flow perspective, phosphorus recycling requires to bridge the gap from several distinctive P-containing waste streams with characteristic impurity profiles to well-defined outputs responding to market demand. Numerous technical processes have been proposed which achieve one or several of the following objectives:
-
Purification
-
Improvement of plant availability
-
Concentration
This book presents a selection of processes which were developed in Europe or Japan and which reached a certain level of technical maturity, mostly at least pilot scale. By processing, the P can in many cases be recycled more effectively and diminish the consumption of limited and geographically critical fossil P reserves. In addition, often costs and other resources can be saved. Through the extraction of P from the waste and the reduced input of fossil P, the risk of diffuse emissions into the environment diminishes, and especially the risk for eutrophication can be reduced.
This chapter will explore whether the differences in recovery process development and implementation in Europe and Japan is linked to the waste flows and the regulative framework. The main waste flows and their qualities are summarized for the two geographical areas. Then a comparative overview of the full-scale applications and their importance in relation to the potential is presented. The drivers for P recycling and the expected further development in Europe and Japan are also described.
2 P Flow and Hot Spots
2.1 Europe
The European P flows were studied by various authors (Ott and Rechberger 2012; van Dijk et al. 2016). In 2015, EU28 used 1480 kt of mineral P (76% fertilizer, 17% feed, 7% others (including phaseout of detergents)) (Hukari et al. 2015). Ninety-two percent of the mineral P inputs such as phosphoric acid, phosphate (Pi) rock, and fertilizers are imported (European Commission 2013a). White phosphorus, which is used as a starting point for specialty chemicals synthesis and high-purity phosphoric acid (see also Chap. 5), is 100% imported.
The main P-rich waste streams are manure, sewage sludge, slaughterhouse waste (P almost exclusively in meat and bone meal), and food waste. Food waste from household and retail represents a large amount of P (187 kt/a). However, the sources are diffuse and heterogeneous, and the concentration is also usually low (average 0.4% on dry matter (DM)). Therefore, there has been little development of processes for recovery of mineral P materials from food waste.
The composition and volumes of the other three main waste streams are described in Table 1.1. Manure is the largest stream (1810 kt P/a) and is today mostly recycled directly to fields. Sewage sludge (374 kt P/a) is also spread on fields to a large extent (39%). Meat and bone meal (312 kt P/a) was used in animal feed, but because of the mad cow (BSE) disease outbreak, this use was prohibited by the EU Animal By-Products (ABP) Regulation (1774/2002). Sewage sludge and manure pose less danger of pathogens if used correctly but contain other organic contaminants that might cause concern.
In EU28 most of the meat and bone meal (MBM), about a quarter of the sewage sludge and a small fraction of the manure are incinerated. The MBM ash has the highest P content (18%), the municipal sewage sludge ash about half as much and the manure ash about a quarter (3.9%). The MBM has also the advantage of low heavy metal content. The sewage sludge ash has much higher heavy metal content. Yet, sewage sludge ash on German average just fulfills the limits of Cd, Cu, Ni, and Pb in the German fertilizer legislation, which is one of the strictest in Europe.
Expressed in absolute figures, manure ash contains less heavy metals, but Cd, Cu, and Ni levels are relatively high in comparison to its limited P content. Manure is mostly recycled to fields, but there are large surpluses in some regions. Pig factories cause most of these problems. Poultry factories also produce large amounts of manure, but it has a much higher dry matter content and can thus be transported further. In contrast, cattle is usually raised more in balance with cropping agriculture and pastures and does not cause nutrient hot spots (Schoumans et al. 2010).
In Europe, waste streams present a large unused potential for P recycling. Unused manure, sewage sludge, and MBM together contain over 500 kt P/a. Several properties of these streams can be improved by processing:
-
Removing contaminants is important for part of the sewage sludge (heavy metals), for meat and bone meal (prions), and to some extent for manure (heavy metals).
-
Improving plant availability is important since sewage sludge (unless EBPR) and ashes excepting chicken litter ash have low plant availability which makes processing worthwhile.
-
Finally, the dry matter and nutrient concentrations are low compared to mineral fertilizers, and the materials are biologically active and with varying concentrations with regard to both the source and the time period. Thus, processing is useful to facilitate storage and transport and reach a more uniform quality.
2.2 Japan
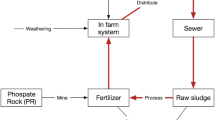
Figure 1.1 shows the estimated P flows in Japan in 2016. As the primary resource of P, Pi rock is a significant starting material for P-related products. Since Japan has no significant Pi rock reserve, all the ore is imported from abroad. The imported ore is mostly used in the chemical industry as a raw material, amounting to an estimated 41 ktP/a. The fertilizer industry produces commercial and special fertilizers by using raw materials and commodity chemicals produced by the chemical industry. This P throughput in the fertilizer industry is reported as 126.9 ktP/a.
The main input flows of P in the agricultural sector originate from fertilizers used in farms and ranches. About 127 ktP/a was applied to farms and ranches in the form of fertilizer. This value is one of the largest input flows in the entire domestic P flow. Input flows to the food and feed sector also have large values. They flow in mainly from world imports and marine resources (121 ktP/a) and domestic crop production from farmlands (52 ktP/a). This P is mainly consumed by humankind and livestock (84 ktP/a and 48 ktP/a, respectively). The P flows associated with cattle, pigs, chickens, horses, sheep, goats, and other livestock are considered in this sector. Livestock grow by eating grass and feed on ranches, and the P in livestock manure ends up accumulating in the soil. The amount of P accumulated in the soil (123 ktP/a) is nearly equal to the input from fertilizer to farms and ranches.
The main path for P return from the economy to farms and ranches is in the form of compost and livestock manure, amounting to about 95 ktP/a. Almost all the P in livestock manure is likely utilized as fertilizing materials in the farm and ranch sector intentionally or not, only 1% is clearly utilized as a resource of the fertilizer production sector, and the rest is assumed to be treated in well-managed landfill site. Other relevant P flows come from the iron and steel industry in Japan. Iron ore, coal, and limestone are major mineral resources for the iron and steel industry, amounting to 120 ktP/a. Japan produces more than 100 million tons of steel materials annually, while generating a huge amount of steelmaking slag at the same time. Of the several types of steelmaking slag, the slags which are generated after dephosphorization in torpedo cars and basic oxygen furnaces (BOFs) have approximated 5.0 and 3.0 mass% of P2O5, respectively (Matsubae-Yokoyama et al. 2009).
The total P input to Japanese society is estimated to be 520 ktP/a. Of this, 227 ktP/a is supplied to the economic activities as a valuable resource, while the remainder is not recognized as a resource flow. Most of the P resources are used in fertilizers. Though approximately 80–100 ktP/a of the P cycle is involved in the farm/ranch sector, the remaining P diffuses into the environment. The P flow into the steel industry is estimated to be approximately 114 ktP/a, most of which is accumulated in steelmaking slag through the dephosphorization of molten iron. Although the main flows of P finally reach the soil, river, and sea, approximately 42 and 114 ktP/a end up in sewage sludge and steelmaking slag, respectively. Concerning the restricted supplies of P resource, it is important to consider the quantity and availability of P resources that currently remain untapped.
3 Full-Scale Plants
Examples of full-scale implementation of recovery processes are presented in this section. In the text below and in this book, they are grouped according to raw materials and process type (Table 1.2).
3.1 Europe
Previous summaries of European processes were made by Egle et al. (2016) and Kabbe et al. (2015). A few key characteristics of each group in Table 1.2 are given below together with a short description of an implementation example.
3.1.1 Ash for Direct Fertilizer Use
Ashes from for example poultry litter (PLA) and MBM can be directly used to produce a fertilizer.Footnote 1
Advantages
are that incineration is a simple and relatively low-cost process that eliminates pathogens and organic contaminants and provides a more concentrated and storable output.
Limitations
are that most ashes have limited plant availability and some have high heavy metal loads. MBM combustion ash produced by the Saria group in the UK is recycled in agriculture, having gained end of waste status, since 2014. About 12,000 t/year with the with average NPK content 0-21-4Footnote 2 (and 37% CaO) is currently sold under name “Kalfos” in the form of a coarse powder that is best spread using a lime spreading machine. The product is not soluble in water, but about 20% of the phosphate is soluble in neutral ammonium citrate. It has been demonstrated over several years to be effective at promoting grass growth in a range of farm and controlled laboratory conditions.
3.1.2 Ash as a Feedstock in Fertilizer Production
Ashes and other mineral P-rich materials can be combined with fossil feedstocks in fertilizer production.
Advantage
is a simple process that uses existing infrastructure and distribution channels in the fertilizer industry.
Limitation
is that such a process can increase plant availability through acidulation, but does not concentrate phosphorus or reduce contaminant load. The iron and aluminum coagulants used in wastewater treatment (unless EBPR is used) create processing problems with these ashes.
Cost
is close to break-even, making the process economically viable in certain cases depending on ash phosphorus concentration, contaminants as well as waste disposal and transport costs. ICL, Amsterdam processed a few hundred tons of MBM ash in 2016.Footnote 3
3.1.3 Ash Leaching
Sewage sludge ash, MBM ash, and other ashes can be leached to produce a purified, concentrated, bioavailable material.
Advantages
are that the processes have several possible outputs (CaP, H3PO4, etc.) which can be tailored to different markets (fertilizer, feed, technical).
Limitation
is the process complexity, the availability of ash, and the dilution of some ashes through co-incineration of phosphorus-rich and phosphorus-poor materials.
Cost
of the process is close to breakeven, as indicated by the construction of full-scale production lines and cost estimations (Nättorp et al. 2017).
Ecophos is constructing a plant for production of 200 kt of dicalcium phosphate (DCP) for the feed market in Dunkerque, France. Production should start in autumn 2017, and in 2019 a production line from sewage ash should follow. Ecophos has a supply contract with the Dutch incineration operators SNB and HVC for 60 kt/a of sewage sludge ash, approximately 4 kt P/a. The Ecophos process uses sulfuric acid, which is reacted with calcium chloride to gypsum and hydrochloric acid, which in turn is used in the ash leaching. The leachate is purified, and phosphate is precipitated with calcium carbonate yielding also calcium chloride (see Chap. 14).
Hamburg water and REMONDIS are planning a plant with capacity to process 20 kt of sewage sludge ash/a using the REMONDIS TetraPhos® leaching process with intended start in 2019.Footnote 4
3.1.4 Wastewater and Sludge Precipitation
Struvite or calcium phosphate is precipitated from an orthophosphate-rich process stream (concentration usually >100 g P/m3).
Advantages
of struvite precipitation are operational benefits in WWTP with EBPR and anaerobic digestion. Precipitation is also used to valorize P from industrial wastewater rich in P (e.g., potato processing, dairy).
Limitations
are that the process is limited to the few WWTP which use EBPR (e.g., <10% of the wastewater in Germany) and to certain industrial streams. In municipal wastewater applications, only a small fraction of the phosphorus is recovered (5%–15%), but processes with higher yield are in the process of being implemented.
Cost
of struvite precipitation processes is in general compensated by the aforementioned operational benefits. In industrial streams precipitation is a viable treatment method for high phosphorus concentrations.
Volume in Europe is approximately 1 kt P/a recovered as struvite at >20 WWTP and more than 40 full-scale installations worldwide. The first European high-yield precipitation process installed in Amersfoort, NetherlandsFootnote 5, combines WASSTRIP, Lysotherm thermal sludge disintegration, and an Ostara PEARL precipitation. The plant should reach 30–40% recovery from the sludge according to a first estimation by the operator. It is dimensioned for 900 t/struvite per year (~100 t P/a). The PEARL process produces well-defined struvite prills which Ostara buys from the WWTP for several hundreds of euro per ton and sells as premium fertilizer Crystal Green.
3.1.5 Sludge Leaching
Sewage sludge is leached with acid and phosphorus precipitated as struvite or calcium phosphate, mainly depending on ammonia availability. A demonstration and a full-scale plant are in operation.
Advantages
of sludge dissolution are firstly the higher recovery yield (~50%) compared to precipitation processes (5–15%), although these are catching up. Secondly, these processes, in contrast to precipitation processes, can be applied to sludges containing phosphorus precipitated with iron- or aluminum-based coagulants. Finally, P-depleted sludge allows co-incineration, which is not possible for the higher-yield ash-based routes.
Limitations
are their high demand of mineral acid and large effort for purification. Combined with medium yield, this makes the process comparatively expensive (~10 EUR/kg P).
Seaborne Environmental Laboratory developed and from 2000 onwards piloted a process for nutrient recovery for a great variety of organic materials. The process simultaneously eliminates pollutants of the treated biomass (mainly heavy metals from municipal sewage sludge) and utilizes the energy content of the organic materials. The only full-scale plant of this type was built at the WWTP Gifhorn, Germany, in 2005 (50,000 p.e.). It has a capacity of approx. 1000 tons of dry solids per year (120 m3 digested sludge per day). Full-scale investigations and laboratory scale experiments were conducted to overcome operational problems and to increase the economic efficiency of the plant. To increase robustness, the process was simplified to what became known as the “Gifhorn process.” An efficient separation of iron allowed a safe operation of the ammonia stripping unit and a high phosphorus recovery yield (~50%) with moderate demands of auxiliary chemicals. Currently the ammonia stripping unit and heavy metal separation are not in operation due to economic reasons. As a consequence, the phosphorus yield rate is significantly lower, and the plant availability of the resulting product is reduced (Niewersch et al. 2014).
3.1.6 Manure Processing
When regional manure concentration is high, which is in general due to pig factories, the transport cost for distribution to fields where the manure is allowed to be spread becomes expensive. Approximately 8% of European manure is processed corresponding to 139 kt P/a. Main processes are solid-liquid separation and anaerobic digestion as well as some drying but very little incineration (Foged et al. 2011).
Advantages
of the manure processing are simple low-cost processes to concentrate nutrients and facilitate transport.
Limitations
of these processes are that they do not remove contaminants.
Cost
for separation of pig slurry, drying, and transport of solid fraction is 24–31 EUR/m3 slurry (14–18 EUR/kg P), approximately half for the treatment of the liquid fraction (Schoumans et al. 2010).
Cooperl in Brittany, France, processes 400,000 t/a manure to organic fertilizer products adapted to specific crops. The products are storable and transportable and can thus be sold in all of France.Footnote 6 An example of mineral output is the 50 t P/a recovered as K-struviteFootnote 7 from calf urine, the liquid fraction of manure through addition of magnesium in a CSTR (Schuiling and Andrade 1999).
3.1.7 Recovery with Adsorbents
Solid adsorbents can remove phosphorus from liquid streams. The sorbents can either be regenerated, used as a fertilizer or as a soil improver, or be landfilled.
Advantages
of non-regeneration adsorbents are that they can be made from waste or by-products. Small-scale applications with little operator know-how are possible.
Limitations
of non-regeneration adsorbents are that the loaded material has low nutrient content making it a less efficient fertilizer.
One example of adsorption is filters with Polonite mineral-based reactive filter media marketed by Ecofiltration Nordic AB for households, WWTP, and agricultural runoff. A household filter has a capacity of 3–5 kg of phosphorus for 500 kg of adsorber at a cost of approximately 500 EUR (100–150 EUR/kg P), and 5000–6000 units are currently used, predominantly in Sweden. The adsorber itself is harmless, and approximately 90% of spent filters are recycled as soil improver. The phosphorus mineral phases quantified by X-ray diffraction show about 60% easily mobilizable phosphorus, which is comparable to manure.Footnote 8
3.1.8 Summary
To sum up, over 10 kt P/a is recycled in inorganic form. The largest volumes are by far the direct use of ash (>10,000 t/a of PLA, also MBM ash and others). About 1 kt P/a is recycled as struvite. Two plants for SSA leaching of a total of approximately 5 kt P/a are in planning and construction. This is still low compared to 500 kt P/a from sewage sludge, MBM, and manure that are wasted.
3.2 Japan
Japan has long experience in commercial operation of full-scale plants to recover P from secondary resources such as sewage sludge, sewage sludge ash (SSA), animal manure, and industrial wastewater (Fig. 1.2; Ohtake and Okano 2015). The direct application of sewage sludge to agricultural land is not acceptable in Japan, because of environmental and health securities as well as a shortage of land. Exceptionally, sewage sludge from small WWTP is used as a fertilizing material in some rural areas after being subjected to anaerobic digestion and composting. Hence, in order to recycle P from sewage to farmland, it is essentially necessary to recover it from digested sludge, dewatering liquor, or SSA in WWTP. Meanwhile, manufacturing companies have begun to consider P recycling as an economically beneficial option in waste management, because it leads to saving sludge disposal costs.
3.2.1 Ash Leaching for Fertilizer Production
Approximately 75% of sewage sludge is mono-incinerated to reduce volume and for energy recovery. Mono-incinerated SSA has a high P content, typically ranging between 4 and 8 wt% (10–20 wt% as P2O5). Chemical phosphate (Pi) leaching is a technology option for recycling P in SSA. Full-scale plants for recovering Pi from SSA have been implemented at Gifu and Tottori WWTP (see Fig. 1.2; Chaps. 8 and 9). The plants started operation in 2010 and 2013, respectively. Both plants adopted alkaline (NaOH) leaching with subsequent Pi precipitation with Ca2+. Alkaline leaching was chosen because acid leaching inevitably dissolves heavy metals, thereby contaminating recovered products. Recovered P products (i.e., calcium hydroxyapatite (HAP)) are dewatered, dried, and granulated into pellets to generate Pi fertilizer. Approximately 35–40% of Pi (mainly from AlPO4) is recovered from SSA. The Pi fertilizer, whose efficacy has been demonstrated in farmland, is sold to farmers through fertilizer companies. Solid residues after Pi recovery are subjected to weak-acid cleaning to remove heavy metals and then used as soil amendment for street trees.
The Gifu plant supplies approximately 300 t/a (c. 80 t P/a) of Pi fertilizer to local farmers. The P recovery cost accounts for approximately 3% of the total operating cost of Gifu WWTP. The Tottori plant recovers approximately 150 t/a (c. 40 t P/a) of HAP from 500 t/a of SSA with 25–30 wt% P2O5 (Fig. 1.3). Stable channels for the distribution and sale of recovered HAP have been established within the local area. However, mainly due to the expense of chemicals, the cost of P recovery is high compared to the disposal of SSA as industrial waste.
3.2.2 Ash Leaching for Phosphoric Acid Production
Nippon Phosphoric Acid Co. (NPA) produces approximately 90 kt P2O5/a of merchant-grade phosphoric acid (PA) (see Chap. 7). NPA adopts a wet acid process in which sulfuric acid is used to extract PA from Pi rock. NPA started to use SSA, which was transported from a local WWTP, as part of raw materials to generate merchant-grade PA and by-product gypsum (CaSO4) in 2012. NPA accepts SSA from the WWTP equipped with a Bio-P process to remove Pi without using Fe- or Al-based flocculants. The transportation cost is covered by the WWTP. In the PA-manufacturing plant, SSA is blended with roller-milled Pi rock. The mixture is then dissolved in concentrated sulfuric acid to generate phosphogypsum slurry which is continuously filtered to separate PA and gypsum. The by-product gypsum is used as a raw material for the manufacture of plasterboards and cement.
Currently, NPA uses a total of 1000 t/a of SSA for the manufacture of PA. In full operation, the manufacturing plant has the capacity to accept 5000 t/a of SSA. The blend ratio of SSA with roller-milled Pi rock is currently less than 2.5% (<97.5% Pi rock). This is needed to guarantee the quality of both PA and by-product gypsum. Importantly, it was not necessary for NPA to modify its existing facilities to accept SSA as a raw material. NPA could save raw material expenses in proportion to the amount of SSA accepted from the WWTP.
3.2.3 Precipitation from Sewage
Fukuoka and Matsue WWTPs have implemented struvite recovery from rejected water from a membrane-type solid-liquid separator for digested sludge. Struvite particles are formed by the addition of Mg(OH)2 in a fluidized-bed tower reactor (Fig. 1.4). The Fukuoka and Matsue plants started operation in 1997 and 1998, respectively. Both of the plants have been implemented mainly to prevent struvite from forming a damaging build-up in pipe lines downstream of anaerobic sludge digesters (Ueno and Fujii 2001).
The Fukuoka plant recovers a total of 140 t/a (c. 14 t P/a) of struvite which is sold to fertilizer companies. However, the pioneering plant has suffered from the unwanted wall growth of struvite in the fluidized-bed tower reactor from the very beginning. Owing to this obstacle, only one-third of the rejected water is currently processed for struvite recovery. Accordingly, only 2% of influent P is recovered as struvite at the Fukuoka WWTP. On the other hand, the Matsue plant recovers approximately 150 t/a (c. 15 t P/a) of struvite, accounting for about 20% of P flowing into the WWTP. The recovered struvite is sold as a slow-release Pi fertilizer. The sales of struvite cannot cover the expenses of chemicals required for struvite recovery at both Fukuoka and Matsue WWTP.
Another full-scale plant was installed at Kobe WWTP (see Chap. 17) to recover struvite directly from digested sludge in 2012. The Kobe WWTP has three anaerobic sludge digesters. Digested sludge contains Pi at concentrations as high as 600 mgP/L. Mg(OH)2 is supplied to the reactor as a source of magnesium. Digested sludge in the reactor is mechanically mixed to enhance the contact between solid and liquid. The mechanical mixing also causes decarbonization and collision of struvite crystals, thereby controlling their sizes in the reactor. The recovered struvite is washed with water to obtain high-purity products. This plant recovers approximately 360 kg/day of struvite from 239 m3/day of digested sludge with P recovery efficiencies of 30–40%. This amounts to about 130 t/a (c. 13 t P/a) of struvite, which is equivalent to one-tenth of the annual consumption of Pi fertilizer in the Kobe area.
3.2.4 Precipitation from Blackwater
The sludge treatment center at Senboku city (see Fig. 1.2) has a capacity of treating approximately 60 m3/day of blackwater (i.e., night soil and johkasou sludge in Chap. 18). The plant started operation in 2009 to treat blackwater collected from households in a local area where no sewage service was available. It is equipped for nitrogen (N) and P removal in addition to mainstream sludge treatment processes. N is removed from undiluted sludge liquor using a jet aeration system. Pi is precipitated with CaCl2 from the liquid rejected by a membrane-type solid-liquid separator after N removal. Approximately 72% of Pi can be removed from blackwater, thereby reducing the effluent Pi concentration well below the regional Pi discharge standard.
The recovered product (HAP) contains approximately 30 wt% citrate-soluble P2O5 on a dry weight basis. It is shipped out in wet form containing about 30–40% water. The production rate of HAP is approximately 50 kg/day (about 10–15 t/a). Approximately 20% of P is recovered as HAP, whereas the rest ends up in dewatered sludge. Currently, the P recovery cost is less than one-tenth of the total cost of plant operation. However, it is still high compared to simple Pi removal without recycling. Up to now, ten local municipalities have embarked on HAP recovery from blackwater (see Chap. 18).
3.2.5 Precipitation from Industrial Wastewater
Japan Synthetic Alcohol Co., Ltd. (JSA) is a principal supplier of chemically synthesized ethanol for industrial purposes. This company manufactures 95 v/v% ethanol by a direct ethylene hydration process, in which phosphoric acid (PA) is used as a chemical catalyst. Fresh ethylene gas is fed into a tower reactor, where PA is impregnated in silica beads, and converted to ethanol as it passes through the tower reactor. Wastewater from the tower reactor contains high levels of Pi, which leaches from the packed silica beads. JSA needs to remove Pi from the wastewater to meet P discharge regulations in the Tokyo Bay area (less than 18.2 kgP/day). This company implemented P recovery from the wastewater in 1998. Approximately 75% of Pi is recovered from the wastewater as HAP, whose pellets (30 wt% P2O5 and 10% water) are sold to a fertilizer company at a low price. JSA can save sludge disposal costs by turning the waste to a resource, while the fertilizer company benefits from the low-price fertilizing material.
Kyowa Hakko Bio Co., Ltd. (KHB) is a leading fermentation company in Japan. This company manufactures nucleic acids, amino acids and other products for foods, seasonings and pharmaceutical agents. The wastewater from the fermentation processes contains Pi as high as 1000 mg P/L. KHB began to recover Pi from the wastewater to meet regulation standards for total P emissions to the Seto Inland Sea (see Fig. 1.2) in 2006. About 90% of Pi is removed from the wastewater by precipitation with Ca(OH)2. The recovered product is HAP (typically 29 wt% P2O5 and 59 wt% CaO; Fig. 1.5). After drying, HAP is sold to a fertilizer company. At present, approximately 60–90 t/a of P is recovered from fermentation wastewater in KHB.
J-Oil Mills, Inc. (JOM) is a principal supplier of cooking oil in Japan. Edible oil is purified from crude oil extracted from soybeans, grapes, peanuts, and walnuts. Crude vegetable oil contains various phosphatides such as phosphatidylcholine, phosphatidylinositol, phosphatidylethanolamine, and phosphatidic acid. They need to be removed from crude oil, because they are responsible for refining losses due to emulsion formation (van Nieuwenhuyzen and Tomas 2008). The elimination of phosphatides from crude vegetable oil (called degumming) is a key step in vegetable oil refining. To remove nonhydratable phosphatides, high-grade PA is added as an 85% solution to crude oil at a concentration of 0.05–0.15 v/v%. Hence, wastewater from the acid degumming process contains high levels of spent PA. JOM recovers Pi from the wastewater by chemical precipitation with Ca(OH)2. Since the wastewater contains essentially no harmful substances, recovered product (HAP) is suitable for the manufacture of fertilizer. JOM sells the recovered HAP as a by-product Pi fertilizer (approximately 260 t/a).
3.2.6 Manure Processing
Miyazaki Biomass Recycle Co. (MBR) implemented a power plant to generate electricity by incinerating broiler chicken manure in 2005 (Fig. 1.6). Broiler chicken manure with a water content of typically 43 wt% can produce about 1900 kcal/kg when incinerated. The incineration of chicken manure can contribute to solving regional problems such as illegal dumping, offensive odor emission, and groundwater nitrate contamination. The MBR power plant has an incineration capacity of 132,000 t/a of broiler chicken manure. It generates electricity of 74,943 MWh, of which about 80% is sold to a local power company. About 500 t/a of broiler chicken manure (more than 50% water) is transported by road from 400 poultry houses in the Miyazaki area (see Fig. 1.2) to the MBR plant. The collected manure is incinerated in a self-sustaining combustion furnace at 7.43 MPa and 468 °C. After incineration, approximately 13,000 t/a of ash (about 10% of the original weight) is transported by road to a fertilizer company. It is used as a fertilizing material (20 wt% P2O5 and 15 wt% K on average) for compound fertilizers.
3.2.7 Purification of Spent Phosphoric Acid
In the manufacture of liquid crystal glass substrates, phosphoric acid (PA) is commonly used as an aluminum etching agent. Wastewater from wet etching processes contains Pi at concentrations as high as 5000 mgPi/L. Kurita Water Industries Ltd. (KWI) has developed a system for recovering Pi from wastewater in liquid crystal manufacturing processes. After removing suspended solids (SS) by filtration, Al3+ is removed from a wet etching wastewater using an H-type cation exchange column. Then, Pi is concentrated to 4–7% using a two-step reverse osmosis membrane system at 0.7–2.0 and 4 MPa, respectively. Pi can be further condensed to 50% or higher by reduced-pressure distillation. This process has been installed in three liquid crystal manufacturing plants (two in Japan and one in Taiwan).Typically, about 2.7 m3 of 50% Pi solution can be generated from 800 m3/day of wastewater containing 3000 mg Pi/L. More than 1000 t P/a can be recovered, leading to a reduction of 120 t/day of waste product (60% water).
Spent acid etchant (or mixed waste acid) in semiconductor-manufacturing plants typically consists of PA (60–80%), acetic acid (3–5%), nitric acid (3–5%), water (10–30%), and various metallic impurities (Kim 2006). Sanwa Yuka Kogyo Co. (SYK) has developed a commercial process for purifying PA from mixed waste acid using the solvent extraction method (Shin et al. 2009). In this process, mixed waste acid is countercurrently contacted with organic solvent (tri-2-ethylhexyl-phosphate (TOP)) in a multistage mixer-settler, thereby extracting acetic and nitric acids from the aqueous solution. TOP is selected as an organic solvent, because of its high tolerance to hydrolysis. Linear saturated hydrocarbon (C6–C13) is used as the diluent for TOP. The formation of NO3 −-TOP complex enables the extraction of nitric acid by TOP from the aqueous phase. Nitric acid and the protonated form of acetic acid (pKa = 4.7) are extracted by TOP, while PA remains in the aqueous phase at the operating pH. After extraction, organic solvent is washed with water to strip acetic and nitric acids and returned to the extraction process. The commercial process generates approximately 2000 t/a of PA (60%) which is recycled for various industrial applications.
4 Perspectives
4.1 Europe
What are the drivers influencing technical P recycling in Europe today and how are they developing? Switzerland and Germany have introduced legislation requiring recycling of P. The Swiss legislation (Schweizerischer Bundesrat 2015) requires recovery from sewage sludge (6 kt P/a) and meat and bone meal (the 1.5 kt P/a that is currently lost) from 2026. The new German sewage sludge ordinance (German federal government 2017) enforces P recovery for all German WWTP not being able to dispose their sludge on arable land. Disposal on arable land is prohibited for all WWTP with more than 50,000 p.e. capacity, producing about two-thirds of the German sludge representing 40 kt P/a. after a transition period. They are thus required to implement P recovery before 2029 (large WWTP >100,000 p.e.) and 2032 (medium-size WWTP >50,000 p.e.). For sludge with more than 2% P on dry matter, a P recovery or mono-incineration of the sludge is required. The recovery from the sludge must have a yield of at least 50% or reduce the concentration below 2%. Recovery from mono-incineration ash must have a yield of at least 80%. The ash may also be separately stored in a landfill for future recovery. Today, 55% of the Swiss sludge and 24% of the German sludge are mono-incinerated. Other countries such as Denmark and Sweden have set more general targets for recycling of P in sludge and other wastes, which include use of sludge in agriculture and thus are not expected to act as drivers for technical recycling of P. Phosphorus rock was added to the EU list of critical raw materials in 2014. In 2017 white phosphorus (P4) was added. These acts by themselves have no legal implications but may serve as a rationale for related policies.
The circular economy package launched by the European Commission in 2015 targets the areas of product design, production processes, consumption, secondary raw materials, and innovation investment. It is an important signal that Europe sees circular economy as a key to prosperity and a potential solution to many resource-related problems such as resource scarcity, resource criticality, and emissions. One prominent part of the circular economy is the revision of the fertilizer regulation, which is currently under way and will bring important drivers for technical phosphorus recycling and recycling of organic phosphorus materials:
-
Product function categories (PFC) and component material categories (CMC) are defined. If these are fulfilled, the outputs have product status and thus are no longer considered waste. This removes an important legal hurdle and facilitates marketing.
-
Several new nutrient-rich CMC are introduced: digestates, compost and food industry by-products, and animal by-products.
-
Additional CMC are in the process of being defined so they can be added: crystallisates like struvite, biochar, and ash.
The Urban Waste Water Treatment Directive (91/271/EEC) (European Commission 2013b) lays out guidelines for treatment of wastewater. Its implementation report reveals a high level of sewerage connection (average 94%) and of treatment more stringent than secondary treatment (77%) in EU27. This category includes not only phosphorus elimination but also nitrogen elimination and reduction of bacteriological pollution. Thus, the level of phosphorus elimination is lower than 77%. The increase in secondary treatment between 2005 and 2009 is <5%, which is hard to distinguish from the methodological uncertainties. Thus, it is not certain if the amount of P ending up in sludge increases. The EU Nitrates Directive (1991), which limits the amount of nitrogen from manure which may be applied, is a constant pressure on the regions with a large amount of livestock, mainly concerning pig factories.
To sum up, the European regulative framework for recovery from the high-potential stream sewage sludge, meat and bone meal, and manure is positive and improving. P is recognized to be of critical importance, and technical recovery has recently been made obligatory in two countries.
4.2 Japan
Although more than ten full-scale P recovery plants are operating in Japan, P recycling is no more than a secondary objective to prevent a damaging buildup of struvite on plant equipment (see Chap. 17) or for saving sludge disposal costs in WWTP (Chap. 9). No regulation requires P recovery and recycling for the wastewater treatment sector. This allows the wastewater treatment sector to consider P recovery as an extra service. On the other hand, fertilizer companies cannot accept recovered P products unless they bring some economic benefits to their business. The low market price of recovered P products makes P recycling business unattractive, thereby demotivating P recovery and recycling in the wastewater treatment sector. P recycling should hold great potential but thus far also significant challenges to establish stable channels for the distribution and sale of recovered P products. While technologies exist, lack of incentives impedes their implementation. To make P recycling business more attractive, it is likely imperative to develop a new value chain that can extract the maximum vale from secondary P resources while taking sustainability and safety into account.
4.2.1 High-Purity P Supply
Although the sustainable management of P is becoming a global concern on food and environmental securities, it is not well recognized that high-purity P becomes increasingly difficult to obtain in the global market. Unlike Pi fertilizer, high-purity P is not a low-cost commodity. In particular, elemental P (P4, also known as white phosphorus) is a strategically important starting material for the synthesis of highly valuable P compounds. They are crucial for the manufacture of high-value added industrial products, including automobiles, electronics, fuel batteries, processed foods, medicines, and fire-retardant plastics (Fertilizer International 2015).
However, since quality matters more than quantity for technical applications, quantitative P flow studies have often failed to find the true importance in the global economy. For example, some researchers have argued that non-fertilizer P applications are in part substitutable and have even advocated seeking to restrict technical use of P (Reijnders 2014). However, it is not easy to find a substitute for P in technical applications in light of its high versatility and safety in handling and use. Needless to say, manufacturing companies are reluctant to substitute P by other elements, because it unavoidably causes serious impacts on cost, quality, and applicable technology in their manufacturing processes. The substitution may also fail to provide the same performance as it is and satisfy consumer demands to products. In reality, the restriction of technical P use is much more difficult than one might imagine.
A more serious but unrecognized challenge is that true risks relevant to P4 supply have not been properly understood by the manufacturing sector. Currently, P4 is available only from China, the USA, Kazakhstan, and Vietnam in the global market. After the world oil shock of the 1970s, Japan ceased the production of P4 because of energy shortages and became entirely dependent on the import from the USA and China (see Fig. 1.7). However, the USA stopped the export of P4 by 2002 to secure domestic supply. The P4 import from China also dramatically declined after the P price shock of 2008 due to the export restriction imposed by China. In addition, Thermophos International B.V., which was the only one P4 manufacturer in Europe, went bankrupt in 2012. To secure the supply P4 for the manufacturing industry, Japan has expanded P4 import from Vietnam since 2007. However, as high-grade Pi rock becomes increasingly scarce in Vietnam, concerns are being raised over the future secured supply of P4. Obviously, this import reliance makes Japan’s industry vulnerable to disruptions in the supply of P4 as it happened in 2008.
The production of P4 is an energy-intensive process which is strongly dependent on locally sourced electricity, Pi rock, and cheap labor forces. To generate P4, Pi rock needs to be carbothermally reduced in an electric furnace at temperatures higher than 1300 °C. This requires approximately 14 MWh of electricity for each ton of P4 produced. Furthermore, environmental pollution such as the dispersion of radioactive dust from Pi rock is another problem related to the production of P4 from Pi rock. While P4 becomes increasingly difficult to obtain in the global market, Japan’s industry continues to consume approximately 20,000 tons of P4 annually. Nevertheless, since high-purity P is used only in small quantity per each industrial product, the true value of P4 has not been well recognized by the manufacturing sector. Except the phosphate industry, manufacturing companies are barely motivated by concerns about the looming risk of P4 availability.
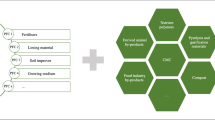
4.2.2 Innovative P Value Chain
Since the legal, social, and economic structures are not fully developed for the sustainable use of P in Japan, it is critical to consider how to call on industry to participate in P recycling business. The future risk for secured P supply alone is not enough to get industry involved in P recycling business. To make P recycling business more attractive, it is necessary to redefine existing P value chains in order to boost technology and business innovation based on P recycling (Fig. 1.8). As mentioned above, P has a wide variety of technical applications. From industrial perspectives, P can be viewed as a technical nutrient which plays a critical role in the manufacturing industry. Accordingly, a new value chain needs to be designed for realizing P recycling not only in the biosphere but also in the technosphere (Fig. 1.8). The innovative P value chain should never restrict the technical use of P but rather maximize synergies of P recycling across biosphere and technosphere. The innovative P value chain needs to boost technology innovation on:
-
Highly efficient P recovery from secondary P resources
-
Wet acid process to generate crude PA from recovered P
-
Carbothermal reduction of crude PA to P4 with minimum electricity consumption and low environmental burden
-
New processes for the manufacture of high-value added specialty P compounds
The highest priority should be given to the development of innovative carbothermal reduction of low-grade phosphoric acid (PA), which enables P4 production with minimum electricity consumption and low environmental burden. The carbothermal reduction of Pi rock in conventional arc processes is prohibitively difficult in Japan because of energy shortage and environmental issues. Without this disruptive technology development, it would be difficult to make the innovative P value chain a reality. Of course, low-grade PA must be generated using P recovered from domestic secondary resources. Regarding secondary P resources, special attention should be given to steelmaking slag. In the steelmaking process, P is present in raw materials such as iron ore, coal, and limestone at concentrations as low as 0.1–0.2 wt% P2O5 (Matsubae-Yokoyama et al. 2009). Since P has detrimental effects on the mechanical properties of steel, it must be removed into hot metal pretreatment slag (dephosphorization slag). Dephosphorization slag contains approximately 2–10 wt% P2O5. In Japan alone, P emitted into dephosphorization slag amounts to 100 kt P/a, which is about two times more than that of sewage sludge. Hence, dephosphorization slag should be a quantitatively important secondary P resource, but it is currently landfilled or used as construction materials.
5 Conclusions
The main three waste streams with large P potential, namely, manure, sewage sludge, and meat and bone meal, have different characteristics regarding contaminants plant availability and nutrient concentration. Together they represent a large unused potential, in the order of 500 kt P/a in Europe, about a third of the mineral P consumption. In Japan similarly these three streams are estimated to approximately 115 kt P/a, steelmaking slag another 115 kt/a, each about a fifth of the total input (520 kt P/a). The different types of P recovery processes implemented in full scale in Europe and Japan have been summarized. These are diverse and can treat the three organic waste streams, whereas processes for steelmaking slag are still in development.
In Europe, the installed capacity is less than 10% of the available potential because the development has so far been limited to profitable niches. Switzerland and Germany have passed legislation in 2016 and 2017, respectively, that makes recovery obligatory, and the fertilizer regulation is expected to open new possibilities for recycling of especially ashes. These regulatory developments together with the fact that ash processes are close to break-even is expected to lead to increased recycling fractions in the future. In Japan, on the other hand, the secured supply of high-purity P is essential for the high-tech industry. The government funded a new research project to develop an innovative carbothermal reduction of low-grade phosphoric acid to P4 with minimum electricity consumption and low environmental burden in 2017.
Technology and business innovation based on P recycling has the potential to make a great contribution not only to the sustainable agricultural production but also to the secured supply of high-purity P for high-tech industries. There should be no significant trade-off between fertilizer use and technical application, because the later needs only a small portion of recovered P. Rather, redefining the supply chain of high-purity P could provide economic incentives to P recovery from secondary resources and help promote P recycling.
The European Sustainable Phosphorus Platform (ESPP) was founded in 2013 to:
-
Define and promote a long-term vision for phosphorus sustainability in Europe
-
Identify and facilitate collaborative actions, policies, business cases, and value chains
-
Address short-term obstacles, propose solutions, identify, and communicate opportunities
ESPP (www.phosphorusplatform.eu) has in the last years gained a solid reputation as an information source brokering ideas between different parties. This is facilitated by the fact that ESPP has numerous important members in all three areas shaping sustainable P use in Europe, namely, industry, science, and policy.
Meanwhile, to promote P recycling in Japan, the Phosphorus Recycling Promotion Council of Japan (PRPCJ) was founded in 2008 with the support from relevant ministries of Japan. Currently, PRPCJ has more than 150 members, including 13 large stakeholder associations and more than 70 private companies. PRPCJ members share their knowledge and information to develop a comprehensive understanding of P recycling, taking into account the social dimension, resource efficiency, and environmental protection. PRPCJ is opening a window of opportunity for getting policy support for P recovery and recycling.
Notes
- 1.
Current production and marketing of poultry litter ash includes among others: Fibrophos UK, BHSL Ireland, BMC Moerdijk Netherlands, Sanders France. MBM ash: SARIA UK, Fibrophos UK, Wykes Engineering UK.
- 2.
0% N, 21% P2O5, 4% K2O.
- 3.
Anthony Zanelli, personal communication, August 2017
- 4.
Patrick Herr, REMONDIS Aqua, personal communication, August 2017
- 5.
- 6.
- 7.
KMg(PO4)•6(H2O)
- 8.
Ecofiltration information sheet “Avsättning änvänd Polonite” Version 2017.
References
Buckwell A, Nadeu E, Six L, Van Keer K, Williams A (2016) Nutrient Recovery and Reuse (NRR) in European agriculture
Campoy M, Gómez-barea A, Ollero P, Nilsson S (2014) Gasification of wastes in a pilot fluidized bed gasifier. 121:63–69
Clarke BO, Smith SR (2011) Review of “ emerging ” organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ Int 37(1):226–247
Coutand M, Cyr M, Deydier E, Guilet R, Clastres P (2008) Characteristics of industrial and laboratory meat and bone meal ashes and their potential applications. J Hazard Mater 150:522–532
Egle L, Rechberger H, Krampe J, Zessner M (2016) Phosphorus recovery from municipal wastewater: an integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci Total Environ 571:522–542
European Commission (2013a) Consultative communication on the sustainable use of phosphorus. http://ec.europa.eu/environment/consultations/phosphorus_en.htm
European Commission (2013b) Seventh report on the implementation of the urban waste water treatment directive (91/271/EEC)
Fertilizer International (2015) Elemental phosphorus market review. 468:52–57
Foged HL et al (2011) Inventory of manure processing
Hua K, Liu L, Bureau DP (2005) Determination of phosphorus fractions in animal protein ingredients. Agric Food Chem 53(5):1571–1574
Hukari S, Nättorp A, Kabbe C (eds) (2015) Phosphorus recycling-now! Building on full-scale practical experiences to tap the potential in European municipal wastewater. P-REX Sustainable sewage sludge management fostering phosphorus recovery and energy efficiency. European Commission no. 308645, P-REX. (2017, February 13). Main P-REX deliverables. Zenodo. https://doi.org/10.5281/zenodo.242550
Kabbe C, Remy C, Kraus F (2015) Review of promising methods for phosphorus recovery and recycling from wastewater. In 763. International Fertiliser Society
Kim K-J (2006) Purification of phosphoric acid from waste acid etchant using layer melt crystallization. Chem Eng Technol 29:271–276
Krüger O, Adam C (2014) Monitoring von Klärschlammmonoverbrennungsaschen hinsichtlich ihrer Zusammensetzung zur Ermittlung ihrer Rohstoffrückgewinnungspotentiale und zur Erstellung von Referenzmaterial für die Überwachungsanalytik (Monitoring of sewage sludge ashes from mono-inc. https://www.umweltbundesamt.de/sites/default/files/medien/378/publikationen/texte_49_2015_monitoring_von_klaerschlammverbrennungsaschen.pdf
Lianfeng D, Wenke L (2012) Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron Sustain Dev 32:309–327
Matsubae-Yokoyama K, Kubo H, Nakajima K, Nagasaka T (2009) A material flow analysis of phosphorus in Japan. J Ind Ecol 13:687–705
Milieu Ltd, WRc, and R (2008) Environmental, economic and social impacts of the use of sewage sludge on land Draft Summary Report 2 Baseline Scenario, Analysis of Risk and Opportunities
Nättorp A, Remmen K, Remy C (2017) Cost assessment of different routes for phosphorus recovery from wastewater using data from pilot and production plants. Water Sci Technol 76:413–424
Niewersch C, Ewert W, Hermanussen O, Kabbe C, Mêlé C, Paillard H, …, Wagenbach A (2014) Description of sludge related processes. P-REX Sustainable sewage sludge management fostering phosphorus recovery and energy efficiency. European Commission no. 308645. P-REX. (2017, February 13). Main P-REX deliverables. Zenodo. https://doi.org/10.5281/zenodo.242550
Ohtake H, Okano K (2015) Development and implementation of technologies for recycling phosphorus in secondary resources in Japan. Glob Environ Res 19:49–65
Ott C, Rechberger H (2012) The European phosphorus balance. Resour Conserv Recycl 60:159–172
Reijnders L (2014) Phosphorus resources, their depletion and conservation, a review. Resour Conserv Recycl 93:32–49
Scholz RW, Roy AH, Brand FS, Hellums DT, Ulrich AE (2014) Sustainable phosphorus management
Schoumans OF, Rulkens WH, Oenema O, Ehlert P. a I (2010) Phosphorus recovery from animal manure. Alterra Report. 2158
Schuiling RD, Andrade A (1999) Recovery of struvite from calf manure. Environ Technol 20(7):765–768
Schweizerischer Bundesrat (2015) Verordnung über die Vermeidung und die Entsorgung von Abfällen (Abfallverordnung, VVEA), 1–46. Retrieved from https://www.admin.ch/opc/de/classified-compilation/20141858/
Shin C-H, Kim J-Y, Kim J-Y, Kim H-S, Lee H-S, Mohapatra D, Ahn J-G, Bae W (2009) Recovery of nitric acid from waste etching solution using solvent extraction. J Hazard Mat 163:729–734
Ueno Y, Fujii M (2001) Three-year experience of operating and selling recovered struvite from full-scale plant. Environ Technol 22:1373–1381
USGS. (2018). Phosphate rock. USGS mineral commodities summaries. Retrieved from http://minerals.usgs.gov/minerals/pubs/mcs/
van Dijk KC, Lesschen JP, Oenema O (2016) Phosphorus flows and balances of the European Union member states. Sci Total Environ 542:1078–1093
van Nieuwenhuyzen W, Tomas MC (2008) Update on vegetable lecithin and phospholipid technologies. Eur J Lipid Sci Technol 110:472–486
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Nättorp, A., Kabbe, C., Matsubae, K., Ohtake, H. (2019). Development of Phosphorus Recycling in Europe and Japan. In: Ohtake, H., Tsuneda, S. (eds) Phosphorus Recovery and Recycling . Springer, Singapore. https://doi.org/10.1007/978-981-10-8031-9_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-8031-9_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8030-2
Online ISBN: 978-981-10-8031-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)