Abstract
Nowadays pollution control and abatement are critical issues faced by environmental scientists due to rapid industrialization. Petroleum industry is one of the major industries which release hydrocarbon pollutants in environment. Polycyclic aromatic hydrocarbons (PAHs) are the priority pollutants which are released into the environment by exploration activities of petroleum industries. The indiscriminate accumulation of petroleum hydrocarbon pollutants can be hazardous to the human life and aquatic biota. Due to toxicity of these pollutants, establishing efficient and environment-friendly method to degrade and detoxify these pollutants is an important research challenge. Various physiochemical methods are applied all over the world to remediate of petroleum hydrocarbon pollutants. Bioremediation technique has been developed for treatment of crude oil pollutants using biological agents like bacteria, fungi, algae, and plants. Applications of certain microorganisms have gained importance in the field of applied environmental microbiology. The application of microbes to degrade pollutants is getting attention due to its environmental and economic benefits. They can be used to change bioavailability and toxicity of petroleum hydrocarbons present in polluted soil and aqueous environment. This paper explores hydrocarbons present in petroleum crude. The effect of petroleum hydrocarbon pollutants on human health and environment is also discussed. This chapter also explains microbial degradation of these pollutants.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Polyaromatic hydrocarbons (PAHs) are chemical compounds that contain more than one fused benzene ring (Bisht et al. 2010; Nikitha et al. 2017) which consists of numerous carbon atoms joined together to form multiple rings. More than 10,000 different PAH compounds exist in environment (Rubailo and Oberenko 2008; Abdel-Shafy and Mansour 2016). They are commonly found in petroleum fuels, coal products, and tar (Rubailo and Oberenko 2008; Varjani and Upasani 2016c). Different types of anthropogenic activities are the sources of PAHs present in environment (Samanta et al. 2002; Varjani and Upasani 2017c) such as fuel combustion, pyrolytic processes, spillage of petroleum products, waste incinerators, and domestic heaters (Bamforth and Singleton 2005). Mostly, PAHs are formed from the incomplete combustion of plant or animal matter, or carbon fuels, such as coal or petroleum (Varjani and Upasani 2017b). PAHs such as naphthalene, anthracene, phenanthrene, fluoranthene, and pyrene having four or more fused ring structure are typically recalcitrant to biodegradation (Haritash and Kaushik 2009; Varjani 2017a).
They are also called polynuclear aromatic hydrocarbons (Stogiannidis and Laane 2015). Other activities that release PAHs include driving, agricultural burning, roofing or working with coal tar products, sound- and water-proofing, coating pipes, steel making, and paving with asphalt (Choi et al. 2010). PAHs are persistent organic pollutants (POPs) which are toxic, carcinogenic, and mutagenic so their presence in environment is the harmful effect on human health (Haritash and Kaushik 2009; Gupte et al. 2016; Varjani and Upasani 2017a). Chemical, physical, and biological processes are used in remediation of PAHs (Ukiwe et al. 2013; Varjani 2017b). Among all these techniques, biological processes by application of microorganisms for bioremediation of pollutants are preferred as they are economically viable and environment-friendly (Varjani and Upasani 2016c). In this chapter sources, applications, types, characteristics and properties, and toxicity of PAHs are discussed. It also includes the recent literature available for degradation of PAHs by bacteria and fungi.
2 Source and Uses of PAHs
Different industrial, agricultural, and medical activities are involved in releasing large quantity of hazardous hydrocarbon substances in the environment and create pollution problems (Kim et al. 2013; Sarria-Villa et al. 2015; Varjani and Upasani 2016b). PAHs are the product of incomplete combustion of organic compounds. PAHs are formed as a result of incomplete combustion of organic compound at high temperature (500–800 °C) to low temperature (100–300 °C) for long duration (Stogiannidis and Laane 2015; Sarria-Villa et al. 2015). They are also known as polyarenes or polynuclear aromatic hydrocarbons (Haritash and Kaushik 2009; Ganesh et al. 2014). They are organic compound which is toxic and persistent for the environment (Varjani et al. 2015; Ghosal et al. 2016) which are released from different natural and anthropogenic activities.
Natural sources include forest and grass fires, oil seeps, volcanoes, chlorophyllous plants, fungi, and bacteria. Anthropogenic sources of PAHs include: (a) Petroleum, combustion of fossil fuel—including motor vehicle emission and power generation, (b) Effluent from industries and wastewater treatment plant, (c) Electric power generation, (d) Refuse incineration, (e) Home heating, (f) Production of coke, carbon black, coal tar, asphalt roads, roofing tar, (g) Internal combustion engines, (h) Smoked foods (meat, fish, etc), (i) Cigarette and tobacco smoke, (j) Wood burning (Freeman and Cattell 1990), and (k) Hazardous waste sites, coal gasification sites, chimneys of industries as well as aluminum production industrial units (Rubailo and Oberenko 2008; Abdel-Shafy and Mansour 2016; Nikitha et al. 2017).
2.1 Characteristics and Properties of PAHs
Many PAHs have toxic, mutagenic, and carcinogenic properties. Polycyclic aromatic hydrocarbons are lipophilic, nonpolar molecules (Lundstedt et al. 2003; Bojes and Pope 2007). PAHs are not very soluble in water hence they persist in the environment. PAHs are highly lipid soluble and thus readily absorbed from gastrointestinal tract of mammals (Choi et al. 2010; Bisht et al. 2010). Polycyclic aromatic hydrocarbons (PAHs) are organic compounds that are mostly colorless, white, or pale-yellow solids (Lundstedt et al. 2003; Bojes and Pope 2007). In PAHs structure mainly carbon and hydrogen are present. But it also contains N, S, and O atoms which are heteroatoms. It is also considered to be known as PAH (Clemente et al. 2014; Varjani et al. 2015). Generally, their molecular weight ranges from 166 to 328 (Chiou et al. 1998). It generally depends on number of carbon it possesses as well as the position of fused rings and elements present in its structure. They are group of several hundred chemically related compounds (Haritash and Kaushik 2009; Choi et al. 2010). They have toxic effects on organisms through various ways (Arulazhagan and Vasudevan 2011; Varjani and Upasani 2016c). PAHs enter in the environment through various routes and are usually found as a mixture containing two or more of PAHs. According to genotoxicity and abundance, US Environmental Protection Agency (USEPA) have listed 16 PAH compounds as priority pollutants (Lundstedt et al. 2003; Bojes and Pope 2007).
2.2 Types of PAHs
Chemically PAHs are comprised of two or more benzene rings. Polycyclic aromatic hydrocarbons have two or more single or fused aromatic rings with a pair of carbon atoms shared between rings in their molecules. According to their arrangements, they are defined as linear, cluster, or angular (Rubailo and Oberenko 2008; Sarria-Villa et al. 2015). Types of PAH according to ring arrangement are summarized in Table 1. Commonly there are two types of categories small PAHs having six or less than six fused aromatic rings and large PAHs having more than six aromatic rings in their structure (Mohsen et al. 2009; Ghosal et al. 2016).
2.3 Uses of PAHs
Apart from industrial emissions or effluents, PAHs can be released in the environment through incomplete combustion of organic material, viz., coal, oil, and wood. PAHs are used as intermediates in thermosetting plastics, lubricating materials, and other chemical industries (Rubailo and Oberenko 2008; Gupte et al. 2016). PAHs are present in asphalt, which is used for roads construction (Nikitha et al. 2017). Precise PAHs and specific refined products are also used in the field of electronics, functional plastics, and liquid crystals. Table 2 represents industrial application(s) of some polyaromatic hydrocarbons (PAHs).
3 Toxicity of PAHs
Different types of petroleum components are directly and indirectly more or less harmful for living organisms and surrounding environment (USEPA 2012; Varjani 2017a) as they are reported as potential carcinogens. Some are mutagenic and teratogenetic. The most dangerous PAHs are benzo[a]pyrene, benz[a]anthracene, and dibenz[a,h]anthracene (ATSDR 1995; USEPA 2012; Lammel et al. 2015; Varjani and Upasani 2017a). These compounds also appear to be less bioavailable (Varjani and Upasani 2017b). There solubility rate in water is very low (Samanta et al. 2002; Nikitha et al. 2017). In a study, benzo[a]pyrene was estimated to be responsible for 48–52% of the added risk of sediment and for 44–54% sediment in another. Benzo[a]pyrene is responsible for DNA binding, sister chromatid exchange, chromosomal aberrations, point mutation, and transformations in mammalian cell cultures (Bamforth and Singleton 2005). When concentrated benz[a]anthracene and dibenz[a,h]anthracene were included 90% of the added risk could be estimated. According to this estimation, these compounds are very easily combined with environmental factors. Several attempts have been made to classify PAHs, and one of this is called as toxicological equivalent factor (TEF). Benzo[a]pyrene is often assumed to be the most harmful PAH (Kim et al. 2013; Sarria-Villa et al. 2015).
Generally, that PAHs compounds have more toxicity which contain more number of benzene rings. PAHs toxicity is measured by using LD50 values (Bamforth and Singleton 2005). In toxicological studies, it is also important to consider the possible toxic metabolites of PAHs. The first step in degradation of aromatic hydrocarbons in eukaryotes is formation of trans-dihydrodiol (Das and Chandran 2011; Gupte et al. 2016). These trans-dihydrodiols are in balance with trans-diol epoxides. The dihydrodiols are converted to catechols. Catechol is then converted into to intermediates of tricarboxylic acid cycle (ATSDR 1995). Toxic metabolites affect DNA which leads to DNA damage. These effects are shown in animals very slowly. The reactive species formed, quinones and trans-diol epoxides, can also cause DNA scission (Bamforth and Singleton 2005; Varjani and Upasani 2017a).
4 Effect of PAHs on Human Health and Environment
Petroleum hydrocarbon pollutants are mostly known as carcinogens (Nikitha et al. 2017). They can also damage other body parts of human beings. They are reported to be harmful for either short term or long term (Varjani et al. 2015; Varjani 2017a). They can lead to genetic and immunotoxic effects.
Short-term effects of petroleum hydrocarbon pollutants are symptoms such as eye irritation, nausea, skin irritation, vomiting, diarrhea, and inflammation (Kim et al. 2013; Yan et al. 2015; NHHS 2017; Varjani and Upasani 2017a). Anthracene, benzo(a)pyrene, and naphthalene are known as direct skin irritants. Anthracene and benzo(a)pyrene are reported as skin sensitizers means they can cause an allergic skin response in animals and humans (Oluwaseun et al. 2017).
Long-term effects persist for long time period and slowly damage body parts. The symptoms are categorized as chronic effect such as decreased immune function, cataracts, kidney and liver damage (e.g., jaundice), breathing problems, asthma-like symptoms, and lung function abnormalities. Repeated contact of PAHs with skin may induce redness and skin inflammation (ATSDR 1995; Ghosal et al. 2016). Naphthalene is responsible for red blood cells’ breakdown if inhaled or ingested in large amounts (Bisht et al. 2010; Olajire and Essien 2014).
Genotoxic effect causes genetic damage in living beings. Mainly PAHs are not genotoxic by themselves. But they need to be metabolized to diol epoxides. Diol epoxides react with DNA, thus inducing genotoxic damage (Bamforth and Singleton 2005).
Mostly PAHs are organic compound which are playing role as carcinogenic compounds. Epoxides and dihydrodiols of some PAHs bind with cellular proteins and DNA. Because of this activity cell is damaged. It causes mutations, developmental malformations, tumors, and cancer (NAHH 2017). It also has increased risk of mostly skin, lung, bladder, gastrointestinal cancers, and stomach cancer, etc. PAHs are directly or indirectly connected in food, skin contacts, and respiration, etc. Hence it causes lung cancer by inhalation, stomach cancer by ingesting PAHs in food, and skin cancer by skin contact (Haritash and Kaushik 2009).
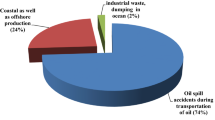
Different sources of PAHs and their effect(s) on human health are shown in Fig. 1. PAHs cause harmful effect on male or female fertility organs. Embryo-toxic effects occur due to benzo(a)anthracene, benzo(a)pyrene, and naphthalene. High amount of benzo(a)pyrene during pregnancy is responsible for birth defects and decreased body weight in the offspring in mice (ATSDR 1995; Digg et al. 2012). It is not known that this type of effect particularly occurs in humans or not. The effects of PAHs in living organisms are low birth weight, premature delivery and heart malformations, lower intelligence quotient at age three, increased behavioral problems at age six and eight, and childhood asthma (ATSDR 1995; Kim et al. 2013; Yan et al. 2015). Presence of PAHs is suppressing immune reaction in rodents. The defined mechanisms of PAH-induced immunotoxicity are still not clear; however, it appears that immunosuppressant which may be involved in mechanism by which PAH induces cancer (Kim et al. 2013).
Different sources of PAHs and their effect(s) on human health (Dudhagara et al. 2016)
5 Remediation of PAHs or Degradation of PAHs
Crude oil is a mixture of hydrocarbons composed of mainly heteroatomic and non-heteroatomic hydrocarbons (Arulazhagan and Vasudevan 2011; Kostka et al. 2011; Varjani and Upasani 2017a). PAHs typically enter in the environment through various natural and anthropogenic activities. Petroleum industry is one of them (Das and Chandran 2011; Lammel et al. 2015; Varjani and Upasani 2016a, b). PAHs are found in soil and river sediment near industrial sites (Wilson and Jones 1993; Yan et al. 2015). Oil spills, creosote, coal mining dust, and other fossil fuel sources also include pollution of PAHs in the environment (Okparanma et al. 2011 Varjani 2017b). Two- and three-ring PAHs can spread widely while dissolved in water or as gases in the atmosphere (Leahy et al. 1990; Sakari 2012). PAHs have higher molecular weight as compared to alkanes hence they can disperse locally or regionally, adhere to particulate matter that is suspended in air or water until the particles land or settle out of the water column (Bamforth and Singleton 2005; Haritash and Kaushik 2009). PAHs have a strong attraction for organic carbon, and thus highly organic sediments in rivers, lakes, and ocean can be a substantial sink for PAHs (Yan et al. 2015; Azab et al. 2016; Varjani 2017a).
Chemical, physical, and biological processes are used in degradation of PAHs (Chaillan et al. 2004; Ukiwe et al. 2013). All techniques are involved to decrease the level of hydrocarbon pollutants in the environment (Varjani and Upasani 2017a). Biological processes mainly apply living organisms for remediation of pollutants (Varjani and Upasani 2016c). In this process, plants are used which is known as phytoremediation (Ukiwe et al. 2013; Yan et al. 2015; Varjani and Upasani 2017b).
Biodegradation is one of the best suitable techniques for treating soil, water, or sediments contaminated with PAHs, which is used to degrade or detoxify environmental pollutants. It can be aerobic and/or anaerobic (Chaillan et al. 2004; Varjani 2017a). Biodegradation is considered as a clean up method that presents possibility to eliminate organic contaminants with help of natural biological activity available in substrate (Qi et al. 2017; Varjani et al. 2015). In this process, indigenous microorganisms are used to remove pollutants from the polluted site (Okparanma et al. 2011; Varjani and Upasani 2016c). Biodegradation leads complete mineralization of pollutants which is affected by different factors such as soil type, temperature, soil pH, type of pollutant(s), oxygen level of soil, nutrient content of soil (Zohair et al. 2006; Yan et al. 2015; Varjani 2017a). These factors are capable to degrade pollutants and show effect on microbial growth (Arulazhagan and Vasudevan 2011; Lammel et al. 2015; Varjani and Upasani 2017a).
There are number of bacteria living in environment which play role in degradation of PAHs. Some bacteria can utilize low molecular weight (LMW) PAHs as their carbon source. The main mechanism requires the presence of molecular oxygen which initiates enzymatic attack of PAHs ring (Butler and Mason 1997). In the starting phase, oxidation of arenes and catalyzed dioxygenase takes place in aerobic bacterial system. In this system, cis-dihydrodiols are the primary by-products by a multicomponent enzyme system (Shuttleworth and Cerniglia 1995). These dihydrodiols are replaced by intradiol or extradiol ring cleaving dioxygenases through either a meta-cleavage or an ortho-cleavage pathway. It plays an important role as center intermediates such as protocatechuate and catechols. These are converted into tricarboxylic acid (TCA) intermediates for further process (Shuttleworth and Cerniglia 1995; Gibson and Parales 2000). The bacterial naphthalene dioxygenase system is useful in oxidizing bi- and tri-cyclic PAH substrates, i.e., LMW PAHs such as naphthalene, anthracene, and phenanthrene. (Gibson and Parales 2000). High molecular weight (HMW) PAHs exhibit more persistence in contaminated environment and genotoxicity due to increased molecular weight. HMW PAHs contain more benzene ring, so these PAHs take more time period for degradation as compared with LMW PAHs, e.g., half-life of 3-ring molecule phenanthrene in soil and sediment range is 16–126 days, while half-life of 5-ring molecule benzo[a]pyrene range is 229–1400 days (Shuttleworth and Cerniglia 1995; Vasconcelos et al. 2011).
Schematic degradation pathway of PAHs by bacteria and fungi is shown in Fig. 2. The principal mechanism for aerobic bacterial metabolism of PAHs is the first oxidation of benzene ring by the action of dioxygenase enzymes and made cis-dihydrodiols (Rubailo and Oberenko 2008). Dihydrodiols are metabolized by series of enzymes converted to catechol to carbon dioxide and water. In the fungal degradation, it is categorized as two types of remediation (a) Lignolytic and (b) non-lignolytic fungi. Lignolytic fungi (white-rot fungi) produce lignin peroxidase enzymes. PAHs are converted into PAH-QUINONES by ring fission and release the CO2 in the environment (Fig. 2). Non-lignolytic fungi (Chrysosporium pannorum, Cunninghamella elegans) produce endo-oxygenase enzymes which take part in oxidation of PAH compounds and covert into ARENE OXIDE. It is then converted into phenol and trans-dihydrodiol (Fig. 2). Organisms from the genus Pseudomonas, Sphingomonas, Acinetobacter, and Rhodococcus can oxidize naphthalene and other PAHs using dioxygenase enzymes (Okparanma et al. 2011; Varjani 2017a). Addition of straw, wood chips, and other lignin-rich substrates help in enhanced degradation of these pollutants by fungi. Methanotrophs also have ability to degrade PAHs via action of methane monooxygenase gene (Okparanmaet al. 2011; Lammel et al. 2015). Species of genera Arthrobacter, Thiobacter, Bacillus, Stenotrophomonas, Escherichia, and Alcaligenes are reported as anthracene and phenanthrene degraders (Gupte et al. 2016; Varjani 2017a). Some microorganisms from genera Pseudomonas and Mycobacterium are capable to transforming and degrading PAHs under aerobic conditions. It is also noted that anthracene could be completely mineralized by Sphingomonas, Nocardia, Beijerinckia, Paracoccus, and Rhodococcus with dihydrodiol as initial oxygenated intermediate (Chaillan et al. 2004; Ukiwe et al. 2013).
The main purpose of remediation is not only to remove of pollutant(s) but also to maintain a quality of the environment. Bioavailability is an important factor for degradation process (Chaillan et al. 2004; Kim et al. 2013; Varjani and Upasani 2017a). Bioavailability of PAHs depends on biotic and abiotic factors interaction. Other living organisms are also used to remove and reduce the level of this type of pollutant in environment. Algae and some invertebrates such as protozoans, mollusks, and many polychaetes have limited ability to metabolize PAHs and bio-accumulate unequal concentrations of PAHs in their tissues. However, PAH metabolism can vary substantially across invertebrate species (Wilson and Jones 1993; Yan et al. 2015). Most vertebrates metabolize and excrete PAHs relatively quickly.
PAHs transform slowly to a wide range of degradation products. Biological degradation by microbes is a dominant form of PAH transformation in the environment. Soil-consuming invertebrates such as earthworms speedup PAH degradation, either through direct metabolism or by improving conditions for microbial transformations (Lundstedt et al. 2003; Stankovic et al. 2014). Abiotic degradation in atmosphere and top layers of surface waters can produce nitrogenated, halogenated, hydroxylated, and oxygenated PAHs; some of these compounds can be more toxic, water-soluble, and mobile than their parent PAHs (Sarria-Villa et al. 2015; Stankovic et al. 2014).
6 Conclusion
Polycyclic aromatic hydrocarbons (PAHs) are a group of compounds known as polycyclic organic matters (POMs) possessing potential health hazards. They play a vital role in environmental pollution and related problems. Besides other approaches, de-aromatization of these pollutants might be a good option to target and curb PAH pollution. It is a difficult task to remove these pollutants totally from the environment, but it is possible to reduce their quantity in the environment. Using living organisms to degrade PAHs and mineralize or transfer them into CO2 and H2O is nowadays considered as the safe option for their remediation. Voluminous research groups have been evolved to work for different bioremediation tools in the form of efficient bacteria and fungi as potential degraders. It is a big challenge for scientists working in the field of bioremediation to achieve complete bioremediation of PAH polluted site(s). More detailed research is necessary to determine exactly what is going on with PAHs in polluted environment. There are still various aspects of bioremediation of these persistent pollutants that remain unknown or otherwise have insufficient information, which requires future attention. The development and newer approaches focus to target specific PAHs. Development of precise, effective, and composite technology to treat complex mixtures is still a thrust area of research.
References
Abdel-Shafy HI, Mansour MSM (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet 25:107–123
Agency for Toxic Substances and Disease Registry (ATSDR) (1995) Toxicological profile for polycyclic aromatic hydrocarbons (PAHs): U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. URL: https://www.atsdr.cdc.gov/toxprofiles/tp69.pdf. Last accessed: 18 Aug 2017
Arulazhagan P, Vasudevan N (2011) Biodegradation of polycyclic aromatic hydrocarbons by a halo tolerant bacterial strain Ochrobactrum sp. VA1. Mar Pollut Bull 62:388–394
Azab AM, Shaban WM, Zaki MS, Authman MMN, Zaher MFA (2016) Monitoring of petroleum hydrocarbons in sediment and gastropods from Suez Gulf. Red Sea Life Sci J 13(7):46–59
Bamforth SM, Singleton I (2005) A Review: Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J Chem Technol Biotechnol 80:723–736
Bisht S, Pandey P, Sood A, Sharma S (2010) Biodegradation of naphthalene and anthracene by chemo-tactically active rhizobacteria of populus deltoids. Braz J Microbiol 41:922–930
Bojes HK, Pope PG (2007) Characterization of EPAs 16 priority pollutant polycyclic aromatic hydrocarbons (PAHs) in tank bottom solids and associated contaminated soils at oil exploration and production sites in Texas. Regul Toxicol Pharmacol 47(3):288–295
Butler CS, Mason JR (1997) Structure-function analysis of the bacterial aromatic ring-hydroxylating dioxygenases. Adv Microb Physiol 38:47–84
Chaillan F, Flèche LA, Bury E, Phantavong YH, Grimont P, Saliot A, Oudot J (2004) Identification and biodegradation potential of tropical aerobic hydrocarbon-degrading microorganisms. Res Microbiol 155(7):587–595
Chiou TC, McGroddy ES, Kile ED (1998) Partition characteristics of polycyclic aromatic hydrocarbons on soils and sediments. Environ Sci Technol 32(2):264–269
Choi H, Harrison R, Komulainen H, Saborit MJ (2010) Polycyclic aromatic hydrocarbons WHO guidelines for indoor air quality: selected pollutants (https://www.ncbi.nlm.nih.gov/books/NBK138709/). Last accessed: 18 Aug 2017
Clemente RA, Torres-Palma RA, Peñuela AG (2014) Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: a review. Sci Total Environ 478:201–225
Das N, Chandran P (2011) Microbial degradation of petroleum hydrocarbon contaminants: an overview. In: Biotechnology research international 2010:13 (doi:https://doi.org/10.4061/2011/941810)
Detoxifying Heterocyclic Aromatic Amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs) with Chlorella vulgaris, A natural approach to human health (NAHH), Biofoundation (https://biofoundations.org/detoxifying-heterocyclic-aromatic-amines-hcas-and-polycyclic-aromatic-hydrocarbons-pahs-with-chlorella-vulgaris/) (Last assessed: 18.08.2017)
Digg DL, Harris KL, Rekhadevi PV, Ramesh A (2012) Tumor microsomal metabolism of the food toxicant, benzo(a)pyrene, in ApcMin mouse model of colon cancer. Tumor Biol 33(4):1255–1260
Dudhagara DR, Rajpara RK, Bhatt JK, Gosai HB, Sachaniya BK, Dave BP (2016) Distribution, sources and ecological risk assessment of PAHs in historically contaminated surface sediments at Bhavnagar coast, Gujarat, India. Environ Pollut 213:338–346
Freeman Cattell D J (1990) Wood burning as a source of atmospheric polycyclic aromatic hydrocarbons. Environ Sci Technol 24:1581–1585
Ganesh KA, Vijayakumar L, Joshi G, Magesh Peter D, Dharani G, Kirubagaran R (2014) Biodegradation of complex hydrocarbons in spent engine oil by novel bacterial consortium isolated from deep sea sediment. Biores Technol 170:556–564
Ghosal D, Ghosh S, Dutta T K, Ahn Y (2016) Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): a review. Front Microbiol 7:1–27 (Article 1369) (https://doi.org/10.3389/fmicb.2016.01369)
Gibson DT, Parales RE (2000) Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr Opin Biotechnol 11(3):236–243
Gupte A, Tripathi A, Patel H, Rudakiya D Gupte S (2016) Bioremediation of polycyclic aromatic hydrocarbon (PAHs): a perspective. Open Biotechnol J 10:363–378
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15. http://biomonitoring.ca.gov/downloads/polycyclic-aromatic-hydrocarbons-pahs-fact-sheet. Polycyclic Aromatic Hydrocarbons (PAHs) Fact Sheet, biomonitoring, California. Last accessed: 18 Aug 2017
Kim K, Jahan SA, Kabir E, Brown RJC (2013) A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 60:71–80
Kostka JE, Prakash O, Overholt WA, Green SJ, Freyer G, Canion A, Delgardio J, Norton N, Hazen TC, Huettel M (2011) Hydrocarbon-degrading bacteria and the bacterial community response in gulf of Mexico beach sands impacted by the deepwater horizon oil spill. Appl Environ Microbiol 77:7962–7974
Lammel G, Dvorska A, Klanova J, Kohoutek J, Kukuka P, Prokes R, Sehili MA (2015) Long-range atmospheric transport of polycyclic aromatic hydrocarbons is worldwide problem—results from measurements at remote sites and modelling. Acta Chim Slov 62:729–735
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54:305–315
Lundstedt S, Haglund P, Oberg L (2003) Degradation and formation of polycyclic aromatic compounds during bioslurry treatment of an aged gasworks soil. Environ Toxicol Chem 22(7):1413–1420
Mohsen A, Simin N, Chimezie A (2009) Biodegradation of polycyclic aromatic hydrocarbons (PAHs) in petroleum contaminated soils. Iran J Chem Eng 28(3):53–59
Nikitha T, Satyaprakash M, Satya Vani S, Sadhana B, Padal SB (2017) A review on polycyclic aromatic hydrocarbons: their transport, fate and biodegradation in the environment. Int J Curr Microbiol Appl Sci 6(4):1627–1639
Okparanma RN, Ayatamuno MJ, Davis DD, Allagoa M (2011) Mycoremediation of polycyclic aromatic hydrocarbons (PAH) contaminated oil-based drill-cuttings. Afr J Biotech 10(26):5149–5156
Olajire A, Essien JP (2014) Aerobic degradation of petroleum components by microbial consortia. J Pet Environ Biotechnol 5. doi:https://doi.org/10.4172/2157-7463.1000195
Oluwaseun O, Alegbeleye A, Opeolub OB, Jackson V (2017) Bioremediation of polycyclic aromatic hydrocarbon (PAH) compounds: (acenaphthene and fluorene) in water using indigenous bacterial species isolated from the Diep and Plankenburg rivers, Western Cape, South Africa. Braz J Microbiol 48:314–325
Qi Y, Wang C, Lv C, Lun Z, Zheng C (2017) Removal capacities of polycyclic aromatic hydrocarbons (PAHs) by a newly isolated strain from oil field produced water. Int J Environ Res Public Health 14:215
Rubailo AI, Oberenko AV (2008) Polycyclic aromatic hydrocarbons as priority pollutants. J Sib Fed Univ Chem 4:344–354
Sakari M (2012) depositional history of polycyclic aromatic hydrocarbons: reconstruction of petroleum pollution record in peninsular Malaysia. Water Research Unit & School of Science and Technology, Malaysia CHE-6 (http://cdn.intechopen.com/pdfs/29372/InTechDepositional_history_of_polycyclic_aromatic_hydrocarbons_reconstruction_of_petroleum_pollution_record_in_peninsular_malaysia.pdf) (Last assessed: 09/06/2017)
Samanta SK, Singh OV, Jain RK (2002) Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol 20(6):243–248
Sarria-Villa R, Ocampo-Duque W, Páez M, Schuhmacher M (2015) Presence of PAHs in water and sediments of the Colombian Cauca river during heavy rain episodes, and implications for risk assessment. Sci Total Environ 540:455–465
Shuttleworth KL, Cerniglia CE (1995) Environmental aspects of PAH biodegradation. Appl Biochem Biotechnol 54(1–3):291–302
Stankovic D, Krstic B, Nikolic N (2014) Effect of traffic on the soil contamination with polycyclic aromatic hydrocarbons PAHs. Biotechnol Equip 22(2):736–741
Stogiannidis E, Laane R (2015) Source characterization of polycyclic aromatic hydrocarbons by using their molecular indices: an overview of possibilities. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology. Springer International Publishing Switzerland (https://doi.org/10.1007/978-3-319-10638-0_2)
Ukiwe LN, Egereonu UU, Njoku CP, Nwoko IAC, Allinor IJ (2013) Polycyclic aromatic hydrocarbons degradation techniques: a review. Int J Chem 5(4):43–55
U.S. Environmental Protection Agency (2012) 2012 edition of the drinking water standards and health advisories: Office of Water, U.S. Environmental Protection Agency, Washington, D.C. EPA 822-S-12-001. URL: https://www.epa.gov/sites/production/files/2015-09/documents/dwstandards2012.pdf. Last accessed: 18 Aug 2017
Varjani SJ (2017a) Review on microbial degradation of petroleum hydrocarbon. Biores Technol 223:277–286
Varjani SJ (2017b) Biodegradation of petroleum hydrocarbon pollutants in marine environments. In: Prasad R, Kumar N (eds) Microbes and sustainable agriculture. IK International, New Delhi, pp 89–99
Varjani SJ, Upasani VN (2016a) Core Flood study for enhanced oil recovery through ex-situ bioaugmentation with thermo- and halo-tolerant rhamnolipid produced by Pseudomonas aeruginosa NCIM 5514. Biores Technol 220:175–182
Varjani SJ, Upasani VN (2016b) Carbon spectrum utilization by an indigenous strain of Pseudomonas aeruginosa NCIM 5514: production, characterization and surface active properties of biosurfactant. Biores Technol 221:510–516
Varjani SJ, Upasani VN (2016c) Biodegradation of petroleum hydrocarbons by oleophilic strain of Pseudomonas aeruginosa NCIM 5514. Biores Technol 222:1950201
Varjani SJ, Upasani VN (2017a) Review on A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int Biodeterior Biodegrad 120:71–83
Varjani SJ, Upasani VN (2017b) Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Biores Technol 232:389–397
Varjani SJ, Upasani VN (2017c) Crude oil degradation by P. aeruginosa NCIM5514: influence of process parameters. Indian J Exp Biol 55:493–497
Varjani SJ, Rana DP, Jain AK, Bateja S, Upasani VN (2015) Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int Biodeterior Biodegrad 103:116–124
Vasconcelos U, Franca FP, Oliveira FJS (2011) Removal of high-molecular weight polycyclic aromatic hydrocarbons. Quim Nova 34(2):218–221
Wilson SC, Jones KC (1993) Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): a review. Environ Pollut 81(3):229–249
Yan Z, Jiang J, Cai H, Zhou Y, Lee Y, Krumholz LR (2015) Complex interactions between macrophyte Acorus calamus and microbial fuel cells during pyrene and benzo(a)pyrene degradation in sediments. Sci Rep (https://doi.org/10.1038/srep10709)
Zohair A, Salim A, Adeola A, Beck JA (2006) Residues of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and organochlorine pesticides in organically farmed vegetables. Chemosphere 63:541–553
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Varjani, S.J. et al. (2018). Polycyclic Aromatic Hydrocarbons from Petroleum Oil Industry Activities: Effect on Human Health and Their Biodegradation. In: Varjani, S., Gnansounou, E., Gurunathan, B., Pant, D., Zakaria, Z. (eds) Waste Bioremediation. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-10-7413-4_9
Download citation
DOI: https://doi.org/10.1007/978-981-10-7413-4_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7412-7
Online ISBN: 978-981-10-7413-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)