Abstract
Amongst the many approaches being tried out to contain plant viruses, induced systemic resistance (ISR) is one that finds its basis in the induction of antiviral resistance in susceptible plants against viruses by application of sap from certain non-host plants. Phytoprotein based antiviral researches in India started with the first study published in 1952. Progress on such research in India has been focused on screening plants for potential antiviral activity and/or induction of resistance, purification of the active principles and their characterization, and insights into possible mechanisms of action. However, not much success has been achieved at the field level. Resistance induced by plant proteins has been found to be either local or systemic, and in a majority of cases, is reversed by actinomycin D, suggesting host transcriptional involvement. The major plants harbouring such proteins are Clerodendrum inerme, C. aculeatum, Boerhaavia diffusa, Bougainvillea spectabilis, B. xbuttiana, and Celosia cristata. Most proteins inducing resistance fall in the molecular mass range of 25–35 kDa, are heat tolerant, and basic glycoproteins. A few possess ribosome-inactivating properties and share amino acid sequence homologies with other known ribosome-inactivating proteins. Systemic resistance inducing proteins have been shown to induce the production of a virus inhibitory agent (VIA) in the susceptible plant. The VIAs are also proteins, in the range of 30–65 kDa, and are tolerant to conditions that would degrade normal cellular proteins. One such VIA has exhibited homologies to a lectin. A few studies have suggested that at least some of the resistance inducing proteins suppress virus replication.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Systemic induced resistance
- Virus inhibitory agent
- Antiviral activity
- Ribosome-inactivating protein
- Clerodendrum inerme
- Clerodendrum aculeatum
- Boerhaavia diffusa
1 Introduction
Plant virus control through the use of endogenous virus inhibitory plant proteins holds a lot of promise, as it comes without the involvement of microbial infection or chemical toxicity (Verma et al. 1998; Prasad et al. 2012; Srivastava and Prasad 2014). Plant extracts exhibiting antiviral activity have been known since long, and this topic opened up with the observation that the virus causing a mosaic disease on pokeweed (Phytolacca sp.) could be transmitted through sap inoculation to pokeweed but not to tobacco (Allard 1918). Experimental evidence for the same came in 1925 when the transmission of tobacco mosaic virus (TMV) onto healthy tobacco was inhibited following inoculation of TMV mixed with Phytolacca sap (Duggar and Armstrong 1925). The studies logically moved towards characterization of these inhibitors, and the virus inhibitor in Phytolacca esculenta was identified (Kassanis and Kleczkowski 1948). Studies on the mode of action of these inhibitors were a more challenging area and the importance of the host species in determining the action of the virus inhibitors became apparent (Gendron and Kassanis 1954; Grasso 1977). The virus inhibitors were then classified either as inhibitors of virus infection in vitro/in vivo, or as inhibitors of virus replication (Gianinazzi 1982). Another milestone was achieved with the demonstration of inhibition of polypeptide synthesis in vitro by the purified antiviral protein from P. americana (PAP) (Obrig et al. 1973; Owens et al. 1973). Thus PAP became the centre of all initial path breaking researches on plant virus inhibitors.
As of now, all well characterized inhibitors of virus infection from higher plants are able to cleave the N-glycosidic bond of the adenine residue in the highly conserved sequence (5′AGUACGAGAGGA3′) located on the α-sarcin/ricin loop of both eukaryotic (28S) and prokaryotic (23S) rRNAs, and thereby inhibit protein synthesis on ribosomes. They depurinate eukaryotic ribosomes and are hence classified as ribosome-inactivating proteins (RIPs) with rRNA N-glycosidase activity (Endo et al. 1987; Endo and Tsurugi 1988), or more appropriately with polynucleotide:adenosine glycosidase activity since a few RIPs can also deadenylate substrates such as tRNA, mRNA, viral RNA, poly A as well as DNA (Olivieri et al. 1996; Barbieri et al. 1997). RIPs are widespread in the plant kingdom (Barbieri et al. 1993, 2006; Stirpe 2004, 2013; Stirpe and Lappi 2014; Schrot et al. 2015) and were initially discovered as proteins present in different plant tissues that could inhibit viruses (Nielson and Boston 2001; Girbés et al. 2004; Di and Tumer 2015). Their antiviral activity was routinely ascertained by co-inoculating the inhibitor with a plant virus and determining the reduction in lesion number on the test host (Barakat and Stevens 1981; Stevens et al. 1981). This review will focus on inhibitors of plant viruses and inducers of systemic antiviral resistance in susceptible plants, with special emphasis on researches carried out in India in the last few decades.
2 Early Reports and Screening for Virus Inhibitors
Given the potential that these inhibitors carried in the realm of plant virus control methods, reports on the presence of antiviral substances in higher plants soon surfaced in India as well. In early screenings, the plant tissue was homogenized in water or buffer and the extract was either co-inoculated with the virus or applied shortly before or after virus challenge. One of the earliest reports was on inactivation of Cucumis virus 2C by plant extracts (Vasudeva and Nariani 1952). A very narrow host range of the virus prompted the investigators to study the effect of leaf extracts of a few solanaceous plants, such as Nicotiana glutinosa, N. tabacum and Datura stramonium, to name a few, on the infectivity of the virus on bottlegourd (Lagenaria leucantha). Leaf extracts of D. stramonium, Capsicum annuum and Lycopersicon esculentum markedly inhibited this virus (Vasudeva and Nariani 1952). Initial studies made no attempt towards characterization of these inhibitors but instead, focussed on screening of plants carrying inhibitory principles in their various parts, viz., roots, flowers, fruits and bark, but mostly leaves. In a few cases the physico-chemical attributes of the inhibitors were included, studied through effects on the antiviral activity, of factors like temperature, pH, dilution, chemicals, organic solvents, enzymes, aging in vitro, precipitation by ammonium sulfate and dialysis. The results were a pointer towards the proteinaceous or non-proteinaceous nature of the inhibitors.
Thus, inhibitors reported from several higher plants soon stood partially (Paliwal and Nariani 1965a, b; Verma et al. 1969; Roychoudhury and Basu 1983; Verma and Baranwal 1983; Verma et al. 1984; Baranwal and Verma 1997), or completely characterized. The latter are dealt with separately in this review. Though reported mostly from angiosperms, culture filtrates of fungi, Trichothecium roseum and Aspergillus niger (Rao and Raychaudhuri 1965; Sharma and Raychaudhuri 1965), and some pteridophytes and gymnosperms, were also found to contain antiviral substances (Pandey and Bhargava 1980, 1983; Rao and Shukla 1985). Plant extracts inhibiting a number of animal viruses are known as well (Vijayan et al. 2004; Bhanuprakash et al. 2007, 2008; Bag et al. 2012; Nutan et al. 2013) along with several instances of antibacterial and antifungal activity (Guleria and Kumar 2006; Sundaram et al. 2011; Yadav et al. 2011).
In the early studies, inhibition of plant viruses by extracts from higher plants, including several medicinal plants such as Cinchona ledgeriana, Emblica officinalis, Chrysobalanus icaco, Terminalia chebula and Ocimum sanctum, was demonstrated against a number of mechanically transmitted viruses. While an exhaustive list of plants and the viruses inhibited has been variously incorporated in earlier reviews (Verma 1982; Narayanasamy 1990; Verma and Prasad 1992; Verma et al. 1995; Verma and Baranwal 2011), a chronological list of antiviral research in India has been included here as Table 28.1. Plant extracts were reported to inhibit viruses like TMV, potato virus X (PVX), Radish mosaic virus, Chilli mosaic virus, Sunnhemp mosaic virus, Watermelon mosaic virus, Pea top necrosis virus, etc. (Rao and Raychaudhuri 1965; Raychaudhuri and Prasad 1965; Raychaudhuri and Chadha 1965; Paliwal and Nariani 1965a, b; Bhargava and Singh 1965; Singh 1969, 1972; Verma et al. 1969; Dhaliwal and Dhaliwal 1971; Gupta and Raychaudhuri 1971; Lal et al. 1973; Roy et al. 1979).
Virus inhibitory activity was soon reported in seed, root and floral extracts of flowering plants (Khurana and Bhargava 1970; Sharma and Chohan 1973; Verma et al. 1975; Murty and Nagarajan 1980; Rao et al. 1985). Though virus inhibitors were rarely reported in monocotyledons, an inhibitor of TMV infectivity was detected in wheat seed extract that caused a reduction in local lesions on N. glutinosa (Verma and Verma 1965). Seed extracts of Argemone mexicana, Datura fastuosa, Raphanus sativus and Rhynchosia asnaris were inhibitory to PRSV and PapMV on Carica papaya (Khurana and Bhargava 1970), while in a related study, the seed extracts of Syzygium cumini, Callistemon citrinus and Mangifera indica were found to inhibit Cucumis virus I on its local lesion host Chenopodium amaranticolor (Sharma and Chohan 1973). Unripe Lawsonia alba and germinating seeds of Phaseolus radiatus (black gram) showed a strong anti-TMV activity on N. glutinosa (Verma et al. 1975; Murty and Nagarajan 1980). Flower extracts of Azadirachta indica, Euphorbia milii and Vinca rosea were effective at inhibiting PVX lesions on C. amaranticolor, whereas only A. indica extract prevented systemic PVX infection on L. esculentum (Rao et al. 1985). Inhibitors of PVX and watermelon mosaic virus infectivity were also identified in bark extracts of Ficus elastica, Prunus persica and some other plant species (Singh and Singh 1973, 1975; Tewari 1976). Later, inhibition of turnip mosaic virus (TuMV), peanut green mosaic virus, tobacco ringspot virus, urdbean leaf crinkle virus, bean common mosaic virus, bottle gourd mosaic virus and rice tungro virus by various other plant extracts was also reported (Bose et al. 1983; Chowdhury and Saha 1985; Pandey and Mohan 1986; Saigopal et al. 1986; Srinivasulu and Jeyarajan 1986; Darekar and Sawant 1989). Pumpkin mosaic and Pumpkin yellow vein mosaic could be inhibited by extracts from several medicinal plants as well as their derivatives, such as neem oil and neem seed kernel extract (Louis and Balakrishnan 1996; Jayashree et al. 1999). Upon screening of leaf extracts from several plants, only the extract from Psidium guajava was found to inhibit transmission of Brassica isolate of Turnip mosaic potyvirus (TuMV-B) on Brassica juncea var. rugosa. Complete inhibition of transmission of TuMV-B and Chilli mosaic virus was recorded when these viruses were mixed with an equal volume of the guava leaf extract (Mandal and Singh 2001). Such screenings for inhibitors continued, and occasionally the focus shifted towards use of inhibitors for plant virus control in glass houses and open fields, where the pathogen stress was undeniably higher. Extracts from Psidium guajava, Leucas aspera, O. sanctum, Tridax procumbens, Phyllanthus niruri, Thuja occidentalis, Azadirachta indica, Pongamia glabra and Bougainvillea spectabilis were effective in controlling TMV and Tomato mosaic virus (ToMV) on bell pepper and tomato plants (Deepthi et al. 2007; Madhusudhan et al. 2011). Spraying of leaf extracts from A. indica, P. glabra and B. spectabilis on N. glutinosa 1 h prior to inoculation, inhibited TMV and ToMV by 53–62%, while reduction in virus concentration, as determined by ELISA, was noted in bell pepper/TMV and tomato/ToMV combinations. Treatment of seeds of bell pepper and tomato with these extracts enhanced seedling vigour and seed germination (Madhusudan et al. 2011). Inhibition of safflower mosaic virus and sunflower mosaic virus by plant extracts from M. jalapa, B. spectabilis and Prosopis chilensis was also noted (Devi et al. 2004; Kulkarni and Byadagi 2004; Lavanya et al. 2009).
3 Inducers of Systemic Antiviral Resistance in Plants
It soon became apparent that a few of these inhibitors could also function as inducers of systemic antiviral resistance. Application of pepper extract at some distance from the point of virus inoculation inhibited lesion formation on cowpea and Chenopodium was a first in the demonstration of systemic resistance inducing ability associated with virus inhibitory sap (McKeen 1956), a finding that was later substantiated (Apablaza and Bernier 1972). In India, extract from Solanum melongena was seen to prevent infection of TMV and mosaic disease of sunnhemp and Gomphrena on treated as well as non-treated leaves of the test hosts (Verma and Mukerjee 1975). Thus, a new era of research opened up at the Lucknow University, following the demonstration of induction of resistance in plants against viruses by pre-inoculation treatment with plant extracts from several non-host plants. In all such cases, the extracts or purified proteins were applied onto the lower leaves of a test host, 6–24 h prior to virus challenge on the lower (treated) as well as upper (un-treated) leaves of the host plants. Induced resistance was routinely assayed on a test host which gave a hypersensitive response to virus infection. A decrease in the number of local lesions in the untreated leaves was taken as a measure of systemic induced resistance, a host-mediated response that could be reversed by simultaneous application of actinomycin D. In hosts that allowed the virus to spread systemically, a decrease in symptom severity or delay in symptom production post-inoculation was generally noted. The resistance inducing proteins were purified by using a series of column chromatography matrices, which included ion-exchange, hydrophobic-interaction, reverse-phase and molecular sieving, with an occasional use of adsorption chromatography on Hydroxyapatite matrix (Fig. 28.1). Almost all the antiviral resistance inducing proteins were highly stable to thermal denaturation and proteolytic degradation. Some also inhibited protein synthesis, and, hence, at a functional level, were RIPs. Barring very few exceptions, none of the vast majority of RIPs were reported to induce systemic resistance. A few resistance inducers (both systemic and localized inducers) that have been well characterized at the physico-chemical as well as the molecular level are described below and compared in Table 28.2.
Purification scheme for antiviral proteins from Clerodendrum aculeatum (CAP-34), Boerhaavia diffusa (BDP-30), C. inerme (CIP-29, CIP-34, Crip-31), Bougainvillea xbuttiana (BBP-24, BBP-28), Chenopodium amaranticolor (CAP-I, CAP-II), Amaranthus tricolor (AAP-27), Celosia cristata (CCP-25, CCP-27) and Bougainvillea spectabilis (BAP1)
4 Plant Species as Antiviral Sources
4.1 Clerodendrum inerme
Leaf extracts from Clerodendrum inerme (renamed as Volkameria inermis, family Lamiaceae) could inhibit inhibit TMV, SRV and TmYMV on C. amaranticolor by nearly 70–75% and TMV infection on C. amaranticolor, D. stramonium, D. metel, N. glutinosa and N. tabacum var. Ky-58 by almost 100%. A complete inhibition of SRV was noted on C. tetragonoloba when the time interval between application of the leaf extract and virus challenge was 24 h. The inhibitor could induce systemic resistance and was reported to be heat stable and non-dialyzable in nature (Verma et al. 1984). Following these preliminary findings, two antiviral resistance inducing proteins, CIP-29 and CIP-34, were recovered from C. inerme leaves (Prasad et al. 1995). These proteins were basic glycoproteins with molecular masses of 29 and 34 kDa, respectively. Stability of both proteins was evident due to their resistance to digestion with proteinase K and exposure to temperatures up to 80 °C. No serological relatedness to RIPs like dianthin, momordin and saporin could be demonstrated (Prasad et al. 1995). Of the two, CIP-29, a monomeric protein, possessed better resistance inducing ability and a concentration as low as 16 μgmL−1 effectively induced systemic antiviral resistance (Prasad et al. 1995). Subsequently, CIP-34 was shown to have inhibitory effect at extremely high concentrations and comprised a mixture of proteins, with low levels of RIP activity (Olivieri et al. 1996). CIP-29 inhibited protein synthesis by various cell lines, with BeWo and NB100 being the most sensitive, though at concentrations higher than those required for inhibition of in vitro protein synthesis in a rabbit reticulocyte lysate system. CIP-29 was classified as a polynucleotide:adenosine glycosidase since it released adenine not only from rRNA but also from tRNA, poly(A) as well as DNA, with the effects being catalytic (Olivieri et al. 1996). Leaves of C. inerme were reported to contain another systemic resistance inducing protein of 31 kDa, named Crip-31. This too was a basic antiviral protein with no effect on its resistance inducing ability following its incubation with proteinase K (Praveen et al. 2001).
4.2 Clerodendrum aculeatum
Leaf extract from Clerodendrum aculeatum was found to offer complete protection against TMV on D. stramonium, D. metel and N. glutinosa, and against SRV on C. tetragonoloba. However, its inhibitory effect against viruses like TMV, SRV, TmYMV and GMV on C. amaranticolor was less obvious, varying between 43 and 50%. A heat-stable and resistance inducing nature was also indicated through these preliminary studies on the inhibitory principal contained in the extract (Verma et al. 1984). The systemic antiviral resistance inducer present in the C. aculeatum leaf extract was later purified as a 34 kDa basic glycoprotein, CA-SRI (CAP-34), possessing a pI of pH 8.65. In early works it was referred to as CA-SRI, however later the purified protein was renamed as CAP-34 on the basis of host from which derived and molecular weight. A concentration of 64 μgml−1 of the purified inducer afforded complete protection against TMV infection on N. tabacum Samsun NN plants. An overnight incubation of CAP-34 with proteinase K, pronase and trypsin failed to abolish its antiviral resistance inducing activity (Verma et al. 1996). Digestion of CAP-34 with endoproteinase arg-C yielded biologically active fragments of 14, 16, 20, and 28 kDa each. The full length cDNA (1218 bp) with an ORF of 906 bp encoding a 33.9 kDa protein was cloned and sequenced (Kumar et al. 1997). Its N terminus, with highly hydrophobic residues, comprised the secretory signal. The deduced amino acid sequence showed 11–54% homology with other antiviral/ribosome-inactivating proteins, such as PAP, MAP, dianthin, trichosanthin, luffin A chain, abrin A chain, ricin A chain and α-momorcharin, exhibiting a maximum with PAP. However, there was no hybridization seen between CAP-34 gene and Mirabilis genomic DNA despite there being 21% homology between MAP and the deduced amino acid sequence of CAP-34. The CAP-34 gene also did not hybridize with the genomic DNA extracted from Bougainvillea, indicating absence of significant homology. In vitro protein synthesis was completely inhibited by CAP-34 as well as the recombinant protein in a rabbit reticulocyte lysate system, while it was less efficiently inhibited in a wheat germ lysate system (Kumar et al. 1997). In a separate study, CAP-34 was also produced in consistent amounts in the micropropagated plants of C. aculeatum (Srivastava et al. 2004).
4.3 Boerhaavia diffusa
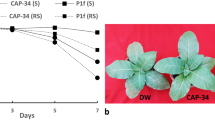
Boerhaavia diffusa, family Nyctaginaceae, is a perennial herbaceous plant with immense medicinal value (Sreeja and Sreeja 2009). Aqueous root extract of B. diffusa could induce systemic resistance and inhibit several viruses on different hosts when applied 24 h prior to virus inoculation (Verma and Awasthi 1979). The extract could check the infectivity of TMV, SRV, GMV and TRSV on C. amaranticolor by almost 70–95%. Virus infectivity dipped by almost 85–100% for tubular viruses like TMV and SRV on their several hosts as compared to 55–73% for spherical viruses like GMV and TRSV on their respective hosts (Verma and Awasthi 1979). The inducer was also present in the plantlets established in vitro from the callus cultures of B. diffusa (Gupta et al. 2004), and transfer of resistance in the explants regenerated from tobacco plants treated with B. diffusa root extract was also demonstrated (Lohani et al. 2007). An attempt at characterization of the inducer was made in an early study which pointed to its heat-stable glycoprotein nature (Verma and Awasthi 1979; Verma et al. 1979a, b, c). Following its purification to homogeneity, the resistance inducing protein, BDP-30, was shown to be a 30 kDa basic glycoprotein, with pI equal to or greater than pH 9.0. BDP-30 showed thermal stability upto 80 °C and its antiviral activity remained unaltered even after an overnight incubation with proteinase K, a non-specific protease. Failure to locate TMV coat protein as well as TMV RNA in induced resistant N. tabacum cv. Xanthi, strongly suggested inhibition of TMV replication in the treated plants due to BDP-30 application (Srivastava et al. 2015b). The BDP-30-treated plants also remained symptompless (Fig. 28.2). In-gel proteolytic digestion of BDP-30 yielded two peptides, KLYDIPPLR and KVTLPYSGNYER, that shared complete sequence identity with α-Trichosanthin (TCS), an RIP present in the roots of Trichosanthes kirilowii, while sharing homologies of 78% and 100%, respectively, with Bryodin, another RIP contained in the roots of Bryonia dioica (Srivastava et al. 2015b).
(a) Induction of systemic antiviral resistance in Nicotiana tabacum cv. Xanthi. Basal leaves of tobacco Xanthi were treated with DW (control) and BDP-30, the purified antiviral protein from Boerhaavia diffusa roots (treated). The upper (untreated) leaves of both plants were inoculated with TMV, 18 h post-treatment, and plants observed for development of mosaic after 21 days. (b) Detection of TMV RNA by RT-PCR. RT-PCR was carried out with TMV coat protein specific primers using the total RNA extracted from the DW treated set after 7 days of TMV inoculation (lane 1) and BDP-30-treated sets, after 7, 14 and 21 days of TMV inoculation (lanes 2, 3 and 4, respectively). The expected 480 bp amplified product can be seen in the control set (lane 1). DNA ladder (lane 5) (c) Detection of TMV coat protein by immunoblot. Leaf sap from control (C) and BDP-30 treated (T) plants was analyzed by SDS-PAGE after Coomassie staining (Gel) and Immunoblotting (Blot). Lanes were loaded with leaf saps from 1, 3, 7, 14, and 21 days after TMV inoculation
4.4 Bougainvillea spectabilis and B. xbuttiana
Antiviral activity in the leaf extract of B. spectabilis, family Nyctaginaceae was first reported in 1983 (Verma and Dwivedi 1983). Multiple sprays of the extract afforded complete protection to Crotalaria juncea and Cucumis melo against infection by SRV and CGMMV, while TMV, TmYMV and PhySMV were completely inhibited on L. esculentum as evident from the local lesion bioassay on C. amaranticolor (Verma and Dwivedi 1983). An RIP was purified from the leaves of B. spectabilis (Bolognesi et al. 1997), but its ability to induce systemic antiviral resistance was not studied. A 28 kDa basic (pI >8.6) antiviral protein, called BAP I, was purified from the roots of B. spectabilis that could inhibit mechanical transmission of TSWV and also interfered with in vitro protein synthesis (Balasaraswathi et al. 1998). A partial BAP-cDNA was synthesized from the leaf mRNA, cloned and sequenced. The probable ORF was translated and showed a poor relatedness to MAP, PAP and CAP-34 (Rajesh et al. 2005).
Antiviral principals and systemic resistance inducers were also detectable in the leaf extract from B. xbuttiana. Two highly basic glycoproteins of 24 kDa (pI 10.5) and 28 kDa (pI 10.0), named BBP-24 and BBP-28, respectively, were reported (Narwal et al. 2001a). An antiviral protein (AVP) that could deadenylate rRNA, and hence possibly functioned as a ribosome-inactivating protein, was also detected but its molecular weight was not specified (Narwal et al. 2001b). Subsequently, AVPs from B. xbuttiana were reported to exhibit RNase as well as DNase activity against viral RNA and supercoiled plasmid DNA, respectively (Bhatia and Lodha 2005). In this case, though not mentioned, the tests were perhaps conducted using a mixture of the two proteins that had been purified earlier (Narwal et al. 2001a). A full-length cDNA sequence (1364 bp) encoding a 35.49 kDa protein of 319 amino acids was also isolated from the leaves of B. xbuttiana. The deduced protein, termed BBAP1, possessed the catalytic RIP domain and was phylogenetically more closely related to RIPs from Nyctaginaceae family, but distantly related to PAP (Choudhary et al. 2008). The purified recombinant protein exhibited rRNA N-glycosidase activity (Choudhary et al. 2008) and demonstrated antiviral activity against Groundnut bud necrosis virus, antifungal activity against Trichoderma harzianum and Rhizoctonia solani as well as insecticidal activity against a voracious insect pest, Helicoverpa armigera (Lodha and Choudhary 2011; Lodha et al. 2010).
4.5 Celosia cristata
Aqueous leaf extract of Celosia cristata, family Amaranthaceae, was reported to inhibit plant virus infection, offering localized resistance to the host plant (Baranwal and Verma 1992). The inhibitor was partially characterized as a protease resistant and thermostable glycoprotein of M r 21–22 kDa (Baranwal and Verma 1997). Subsequently, two glycoproteins, CCP-25 (M r 25 kDa) and CCP-27 (M r 27 kDa), were purified from the leaves and shown to inhibit several mechanically transmitted viruses on hosts responding hypersensitively or systemically to virus infection. A growth-stage dependent variation was observed in the concentration of the two proteins and they were able to withstand protease digestion in their native state (Balasubrahmanyam et al. 2000). Depurination studies carried out with CCP-25 yielded a diagnostic fragment from yeast rRNA, indicating its RIP nature. It was also shown to inhibit the in vitro translation of Brome mosaic virus and Pokeweed mosaic virus RNA (Baranwal et al. 2002). Through a modification in the purification protocol, the N-terminally free proteins were obtained which allowed partial N-terminal sequencing of CCP-25 (Gholizadeh and Kapoor 2004). Degenerate primers designed against the conserved RIP domain yielded a small cDNA fragment (150 bp) from C. cristata leaves, which when expressed in E. coli yielded a fusion protein of 57 kDa. The purified recombinant protein was reported to inhibit plant viruses as well (Gholizadeh et al. 2005). Post-flowering stage of C. cristata gave a full-length cDNA clone (1015 bp), encoding an ORF deduced to yield 283 amino acids. The purified recombinant protein (reCCP-27) inhibited in vitro protein synthesis in a rabbit reticulocyte lysate as well as on tobacco ribosomes, in addition to exhibiting antiviral activity towards TMV and SRV (Begam et al. 2006).
4.6 Chenopodium album
Leaf extract from Chenopodium species, family Chenopodiaceae, inhibits plant viruses (Smookler 1971; Alberghina 1976). C. ambrosoides aqueous leaf extract inhibited TMV and SRV on different hosts, but not on C. amaranticolor. Furthermore, like C. cristata, the extract could not induce systemic antiviral resistance (Verma and Baranwal 1983). Antiviral proteins that could impart resistance in hypersensitive hosts against several viruses were purified from C. album and also partially characterized (Dutt et al. 2000). This study was followed by a more comprehensive report of two antiviral proteins from the same source, CAP-I and CAP-II, which inhibited virus replication in systemic and hypersensitive host/virus combinations and worked as resistance inducing proteins. Both proteins were basic in nature (pI~10.2), contained no carbohydrate, and inhibited virus infection to different extents, CAP-I was at least 2.5-fold more effective than CAP-II. Despite possessing the same molecular weight of 24 kDa, both proteins differed in their amino acid composition and N-terminal sequence (Dutt et al. 2003). They catalyzed the depurination of rRNA extracted from tobacco ribosomes and hence exhibited N-glycosidase activity. Since they could also degrade TMV RNA, they were thought to be associated with RNase activity as well (Dutt et al. 2004).
4.7 Amaranthus tricolor
Amaranthus tricolor, family Amaranthaceae, antiviral protein (AAP-27) was isolated from dried leaves of the plant and characterized as a 27 kDa basic monomeric glycoprotein (pI 9.8) that could inhibit plant viruses. It also exhibited N-glycosidase and RNase activities. Its full-length cDNA comprised 1058 bp and encoded an ORF of 297 amino acids. The deduced amino acid sequence contained the RIP domain and also exhibited varied levels of homology with other antiviral proteins (Roy et al. 2006).
4.8 Cuscuta reflexa
Filaments of Cuscuta reflexa, family Cuscutaceae, parasitizing Zizyphus jujube, contained a proteinaceous inhibitor of plant viruses. The inhibitor was isolated following fractionation of the extract with a series of organic solvents and subsequent precipitation with a saturated solution of ammonium sulphate. Molecular sieving on Sephadex G-200 column yielded a virus inhibitory fraction with characteristics of a protein, and a molecular weight between 14 and 18 kDa. The inhibitor induced both local and systemic antiviral resistance and operated through an AMD-sensitive mechanism (Awasthi 1981, 1982).
5 Antiviral Activity of Other Plant Constituents
Apart from proteins, inhibitory activity was also identified in polysaccharides, plant latex, alkaloids, flavonoids, phenolic acids, tannins or essential oils, with effects on viruses, bacteria and fungi. A polysaccharide, T-poly, obtained from culture filtrates of a fungus Trichothecium roseum, could inhibit viruses on hypersensitive as well as non-hypersensitive hosts and could induce systemic resistance in these plants (Gupta et al. 1973; Chandra and Gupta 1981). In addition to being proficient in exhibiting antioxidant activity, flavonoids are also known to inhibit animal viruses (Kumar and Pandey 2013). Flavonoids (quercetin) and coumarins inhibited the infectivity of Southern Sunnhemp mosaic virus on its local lesion host Cyamopsis tetragonoloba (Chandra et al. 1975). Tannins from Terminalia chebula and Chrysobalanus icacao also functioned as inhibitors of viruses (Verma and Raychaudhuri 1970, 1972). Plant latex from Calotropis procera, Ficus elastica, F. nitida, Euphorbia pulcherrima, inhibited TMV (Khurana and Singh 1972; Lal and Verma 1974; Nagarajan and Murty 1975; Rafiq et al. 1985) and Tobacco necrosis virus (TNV) on bean, Bean yellow mosaic virus (BYMV) on broad bean, and Zucchini yellow mosaic virus (ZYMV) on squash (Mahmoud et al. 2010). The antiviral activity perhaps stemmed from proteases and other defence-related proteins which the latex contain (Kim et al. 2003). Sterols from Artemisia annua were identified as virus inhibitory agents (Khan et al. 1991). Effect of neem and custard apple oil was studied on rice tungro virus transmission by Nephotettix virescens (Mariappan and Saxena 1983), while neem and Phyllanthus oils were also studied for the inhibition of TMV and ToMV on tobacco, bell pepper and tomato (Madhusudhan et al. 2005, 2011). Neem oil could also inhibit PYVMV on pumpkin under glass house conditions, with over 78% reduction in virus transmission (Jayashree et al. 1999). Essential oils present in Foeniculum vulgare and Pimpinella anisum were also associated with virus inhibitory properties (Shukla et al. 1989) and essential oil extract of Chenopodium ambrosoides (EOCA) was found to be effective against Myzus persicae (Rajapakse and Janaki 2006).
6 Control of Plant Viruses by Inhibitors/Systemic Resistance Inducers
Plasticity of the viral genome poses a major challenge in protecting crops from viral infection. One of the finest approaches for durable resistance is offered by the traditional method of breeding, while development of transgenic crops resistant to plant viruses is a relatively novel approach (Cillo and Palukaitis 2014). Induction of systemic resistance in plants by application of phytoproteins/inhibitors for disease control has also been successfully used for some time now, especially where virus resistant cultivars are not available. Viral mosaic on papaya, cucurbits, urdbean, mungbean, and okra is a very common sight and several efforts are on to prevent infection on these important crops. In general, the resistance inducing proteins or inhibitors are administered as a foliar spray, repeated at specified intervals, often over a period of time till the crop produce is ready for harvesting. Sometimes, the resistance inducers are coupled with primers such as bovine serum albumin, milk proteins, oils or detergents, etc. for enhanced and durable resistance. The treatments work well under both glass house and field conditions, and are effective even under intense pathogen pressure. The effect that these inducers have on the sprayed plants varies, depending upon the host-virus system. In general, the plants show more luxuriant growth, along with a several fold increase in crop yield and improved response to varied stresses. Legumes, in addition, show improved nodulation (Verma et al. 1985b). With the defence responses switched on quickly, the sprayed plants are able to protect themselves, thus lowering the incidence of disease. Treated plants either show absence of virus or a low virus titre in ELISA or immunoblots. Often the viral RNA transcripts remain absent in the RT-PCR reactions set up to detect the viral coat protein genes (Srivastava et al. 2009, 2015a, b).
Aqueous extracts from B. diffusa, Cuscuta reflexa, Datura metel and Solanum melongena induced systemic antiviral resistance against TMV and TRSV in N. tabacum var. NP-31, with the active virus being assayed on N. glutinosa. Such plants were resistant for up to three days (Verma et al. 1979b). This demonstration of antiviral resistance induction in a host showing a non-hypersensitive reaction to virus infection was soon followed by experiments designed to control virus infection on economically important crops. Foliar sprays were given at regular intervals either to prevent natural virus infections or infections following challenge inoculation. Effective control of yellow mosaic disease on mung and urdbeans was reported with aqueous leaf extracts of C. fragrans and Aerva sanguinolenta and root extract of B. diffusa. The extracts were administered as foliar sprays at intervals of 3–4 days spanning a period of 6 weeks (Verma et al. 1985b). The protection exhibited an interesting pattern, not being apparent in the initial stages of the experiment, but checking the virus infection and spread in the treated plants between fourth and fifth week, when the control set of plants displayed a sharp rise in the disease incidence. The protection afforded against viral infection and improvement in growth, yield and nodulation was maximal by C. fragrans extract (Verma et al. 1985b). Similarly, a lowering of disease incidence by 90% and the accompanying delay in the onset of yellow mosaic disease together with mitigation of symptom severity was reported on mungbean (Vigna radiata) by B. diffusa root extract, with plants showing improved nodulation and yield (Singh et al. 2004). B. diffusa root extract could also induce resistance against TMV, CMV, SRV, GMV, Cucumber green mottle mosaic virus (CGMMV) on tobacco, tomato, C. juncea, G. globosa and Lagenaria spp., respectively. The most effective spray regimen consisted of six foliar sprays, spread over three days, followed by virus inoculations 24 h after the last spray. The treated plants showed absolute protection from virus infection even after 45 days of virus inoculation (Awasthi et al. 1984). B. diffusa root extract and C. aculeatum leaf extract, either used in combination or alone, were effective in inducing resistance against PRSV (Awasthi and Singh 2009; Singh et al. 2011a, b, c). C. aculeatum leaf extract was used to control Tomato leaf curl virus (Baranwal and Ahmad 1997), and when primed with proteinaceous additives, it could successfully manage SRV and Tobacco leaf curl virus infection on C. juncea and tobacco (Verma and Varsha 1995a, b). CAP-34 (previously called CA-SRI), the purified resistance inducing protein from C. aculeatum, could prevent PRSV infection on papaya as well. Only 10% of the CAP-34 treated plants came down with low level symptoms of mild mosaic, with no observable virus, viral protein or viral RNA in the remaining plants of the treated set (Srivastava et al. 2009). Pre-inoculation sprays with extracts from B. spectabilis and Prosopis chilensis worked against Okra yellow vein mosaic virus (Pun et al. 1999) and Sunflower necrosis virus (Lavanya et al. 2009). The virus titer was slashed in sunflower and cowpea plants treated with these extracts. Reduced disease incidence (33%) was observed in bitter gourd plants treated with B. spectabilis and challenge inoculated with bean golden yellow mosaic virus (BGYMV) (Rajinimala et al. 2009). B. spectabilis and M. jalapa leaf extracts reduced leaf crinkle disease on blackgram caused by the urdbean leaf crinkle virus (Karthikeyan et al. 2009). Leaf extracts of M. jalapa, Datura metel and Azadirachta indica (neem) reduced mungbean yellow mosaic infection on black gram (Venkatesan et al. 2010). Viral diseases on cucumber were prevented by extracts from A. indica, C. auleatum and Terminalia arjuna, and it was also observed that seed treatment with neem extract followed by foliar sprays provided good protection (Kumar and Awasthi 2009). Leaf and seed kernel extract of neem also interfered with aphid transmission of a virus causing mosaic disease on Brassica juncea, reducing disease incidence by 46–54%, while extract from Jatropha curcas was not as effective (Devi et al. 2008).
7 Mode of Action of SRIs
The antiviral resistance induced by plant extracts or purified resistance inducing proteins was sensitive to treatment with the transcription inhibitor, actinomycin D (AMD). Effect of AMD on induced resistance was routinely evaluated by using AMD concomitantly with the inducers or at various time intervals after the treatment of leaves with the inducer (Verma and Awasthi 1979; Verma and Dwivedi 1984; Verma et al. 1984; Prasad et al. 1995). In all cases, a breakdown in the induction of antiviral resistance was seen upon concomitant application of AMD, as evident from an increase in lesion number to the control level. The effect of AMD on reversal of induced resistance diminished with increase in the time interval between the application of inducer and AMD. Furthermore, in vitro incubation of the virus along with the leaf extract from the treated plants, from both site and remote site leaves, could inhibit TMV, SRV, GMV, TRSV, TmYMV and Physalis shoestring mosaic virus (PhySMV) on their assay hosts (Verma and Dwivedi 1984). This preliminary observation led to the suggestion that phytoprotein-mediated induction of resistance involved host transcription and that induced resistant tissues possibly carried a virus-inhibitory agent (VIA) that could inactivate viruses in vitro (Verma and Awasthi 1980; Mukerjee et al. 1981; Verma and Dwivedi 1984; Verma et al. 1985a; Prasad et al. 1995). A proteinaceous nature of the VIAs induced by B. spectabilis and B. diffusa extracts was proposed (Verma and Awasthi 1980; Verma and Dwivedi 1984). Cyamopsis plants treated with CAP-34 (CA-SRI) accumulated a 34 kDa basic protein, as compared to the DW-treated fraction, which was also unable to inhibit the virus in vitro (Verma et al. 1996). This 34 kDa protein could have been the elusive VIA that could not be purified to homogeneity. A complete characterization of the VIAs isolated from resistant C. tetragonoloba plants pre-treated with CIP-29, the purified inducer from C. inerme leaves, has since been reported (Prasad et al. 2014) and the leaf extract from resistant tissues inhibited SRV, TMV and PRSV in vitro. Two VIAs, CT-VIA-32 (M r 32 kDa) and CT-VIA-62 (M r 62 kDa) were isolated from such tissues. The CIP-29 inducible CT-VIA-62 displayed better antiviral activity and was characterized as a basic glycoprotein. Its peptides sequenced through LC/MS/MS shared homology with a lectin from Medicago truncatula which carried a mannose-binding lectin domain (Prasad et al. 2014). In a related effort, CAP-34 inducible CP-VIA-34, a virus-inhibitory basic protein, was isolated from resistant leaf tissues of Carica papaya (Srivastava et al. 2015a). No protease, DNase or RNase activity was found associated with CP-VIA-34. Such proteins, though antiviral but not labelled as VIA, were also detected in C. tetragonoloba plants treated with the purified antiviral proteins from C. album. Two polypeptides of 17 and 26 kDa accumulated in the un-treated leaves of C. tetragonoloba plants whose basal leaves were treated with the inducer (Dutt et al. 2004). It was obvious that VIAs were accumulating in the plants treated with such inducers, but were either absent or present in very low amounts in the un-treated plants (Verma et al. 1996; Prasad et al. 2014). Furthermore, these VIAs differed from the inducers, in that they were unable to induce resistance in plants, though the polyclonal antiserum raised against CIP-29 did recognize CT-VIA-32 (Prasad et al. 2014). Detection of polynucleotide:adenosine N-glycosidase activity in CIP-29 and inhibition of in vitro protein synthesis by CAP-34, along with the occurrence of inducible proteins (VIAs), has complicated any thoughts on the probable mode of action of the resistance inducing proteins based on the inactivation of the virus by these VIAs alone.
The role of PAP in plant virus inhibition, and in plant disease resistance, has been dealt with in great detail in a recent review (Di and Tumer 2015). RIPs are associated with depurination linked to ribosome inhibition, cytotoxicity and antiviral activity. Experiments were developed to delink these associations so as to pinpoint the reason for virus inhibition. Antiviral activity of PAP was earlier correlated and attributed to its RNA N-glycosidase enzymic activity on ribosomes. PAP is a secretory RIP and exists in several isoforms. It forms homodimer complexes in the cytosol, which are less active on rRNA as compared to the monomeric form present in the apoplasts (Tourlakis et al. 2010), and this was the presumed way to avoid depurination of pokeweed rRNA, a possible explanation to Allard’s findings (Allard 1918). PAP-specific antibodies revealed its extracellular location, with PAP being sequestered in the cell wall matrix (Ready et al. 1986). Inhibition of ribosome-mediated viral protein synthesis by PAP was possible if there was a release of the inhibitor (PAP) from the cell wall into the cytosol following the virus inoculation (Lodge et al. 1993). A positive correlation was also shown between PAP concentration, inhibition of TMV and depurination of ribosomes (Chen et al. 1993).
All this while it was believed that ribosome-inactivation led to virus inhibition. However, this hypothesis was challenged by the finding that the C-terminus of PAP was required for toxicity and depurination of ribosomes but not for antiviral activity. Thus, depurination of ribosomes was not the only mechanism for virus inhibition (Tumer et al. 1997), and soon overwhelming evidence came in support of this possibility (Zoubenko et al. 2000). It was subsequently reported that non-depurinating mutants of PAP could still depurinate capped BMV and PVX viral RNA and inhibit their translation in vitro (Hudak et al. 2000). These mutants had an intact active site but were altered such that they were unable to bind to the ribosomes and cause their depurination. However, given that in a virus infected cell the capped viral RNAs would be present in huge numbers, they would become the preferred substrate for PAP, leading to their depurination. PAP was later shown to directly depurinate BMV viral RNA3, and this depurination was held responsible for decreased efficiency of packaging of the viral genome and consequently generation of fewer infectious particles (Karran and Hudak 2008, 2011). No effect was observed on the quality of the virus. PAP was also able to inhibit uncapped TBSV and satellite panicum mosaic virus (SPMV) in vivo without causing detectable depurination in the viral RNAs (Vivanco and Tumer 2003). Site-directed mutagenesis in the central domain of PAP led to loss of cytotoxicity but not its ability to depurinate indicating that depurination was not the sole reason for cytotoxicity (Hudak et al. 2004). Yeast cells expressing PAP showed apoptotic features and an anti-apoptotic protein reduced cell cytotoxicity of PAP, without affecting ribosomal depurination and translation inhibition (Cakir and Tumer 2015). Finally, RIPs depurinate substrates other than rRNA as well (Olivieri et al. 1996). Thus, RIPs inhibit plant viruses, but not via inactivation of ribosomes, while resistance inducing proteins like CIP-29 and CAP-34, possess RIP function, inhibit viruses and also induce antiviral resistance in plants and VIA. Incubating the virus along with the VIA in vitro was devoid of any detrimental effect on the virus per se, as fully infectious virus particle could be recovered following their separation from the VIA by ultracentrifugation. Hence the VIA participates in vivo in plant defence at some level, its production being triggered by the application of such inducers.
Besides VIA, there are other examples of inducible antiviral proteins in plants. Nagaich and Singh (1970) reported an inhibitory agent in Capsicum pendulum inoculated with PVX that could prevent PVX infectivity on Gomphrena globosa and Solanum tuberosum. RIPs called Beetins (BE27 and BE29) were induced in Beta vulgaris leaves following infection with Artichoke mottled crinkle virus (AMCV) and application of H2O2 and salicylic acid (Iglesias et al. 2005). Its external application was able to control AMCV infection. JIP-60, a jasmonate inducible 60 kDa RIP, was described from barley (Chaudhry et al. 1994; Reinbothe et al. 1994) and shown to alleviate stress in plants and delay the onset of senescence (Rustgi et al. 2014). Induced production of antiviral proteins in tobacco plants displaying SAR is also known, variously termed as the antiviral factor (AVF) (Sela and Applebaum 1962; Mozes et al. 1978) and the inhibitor of replication (IVR) (Loebenstein and Gera 1981; Gera et al. 1990). Tobacco and tomato plants transformed with the IVR gene were resistant to TMV and a variety of fungal pathogens (Loebenstein et al. 2010; Elad et al. 2012). Two forms of AVF, gp22 and gp35, were determined to be closely related to two pathogenesis-related (PR) proteins, PR-5 (chitinases) and PR-3 (1,3-ß-glucanases), although both of these PRs are devoid of any antiviral activity (Edelbaum et al. 1991).
PR-proteins were initially discovered in tobacco plants reacting hypersensitively to TMV infection, and were associated with the development of resistance termed as systemic acquired resistance (SAR) (Gianinazzi et al. 1970; Van Loon and Van Kammen 1970; Stintzi et al. 1993). Induced expression of the PR-proteins in resistant plants, in particular PR 1a, along with an enhanced accumulation of endogenous levels of salicylic acid (SA), was always noticed and hence these came to be viewed as established markers for SAR. SAR engages a number of signalling molecules in a cross-talk and has a huge potential in crop protection due to the broad spectrum nature of resistance conferred on the plants against subsequent invasion by diverse pathogens and even an insect (McIntyre et al. 1981; Kessler and Baldwin 2002; Durrant and Dong 2004; Fu and Dong 2013; Gozzo and Faoro 2013). PR proteins have been assigned to 14 families, some being directly antimicrobial while others with proposed indirect roles in plant defence (Stintzi et al. 1993; Edreva 2005; Van Loon et al. 2006). Enzymic activities attributed to various PRs include β-1,3-glucanase (PR-2), chitinase (PR-3, PR-4, PR-8, PR-11), proteinase (PR-7), peroxidise (PR-9), ribonuclease-like (PR-10), etc. PR-10 family that shows homology to ribonucleases, is of special interest in the context of plant virus control (Park et al. 2004).
Expression of such antimicrobial proteins in plant tissues was expected to curb pathogen invasion and spread. Thus, PR-1, PR-2 and PR-5 genes have been incorporated in crops like cotton, barley, peanut, potato, rice, etc. for resistance against various fungal pathogens (Collinge et al. 2010). Creation of transgenic plants expressing RIPs proved to be relatively difficult because of their cytotoxicity (Lodge et al. 1993). However, a variant of PAP, PAPv, obtained through mutagenesis was less toxic than the wild type PAPw, and was expressed efficiently in transgenic tobacco, although transgenics over-expressing PAP were stunted and exhibited mottled leaves due to apoptosis, while low PAP-expressing plants were normal in appearance (Lodge et al. 1993). Transgenic tobacco and potato plants expressing PAP were resistant to PVX, PVY and CMV, thus PAP provided broad spectrum resistance to plant viruses (Lodge et al. 1993). Similarly, protection against viruses was evident in transgenic plants expressing other RIPs, including type II (Krishna et al. 2002; Chen et al. 2002; Vandenbussche et al. 2004). A few cases of enhanced resistance to important insects species was also reported (Shahidi-Noghabi et al. 2009). In a majority of cases, the transgenic plants expressing RIPs did not produce PR proteins, but PAP was shown to upregulate the expression of PR1 and PR5 genes in transgenic tobacco and such plants displayed enhanced resistance to Tobacco etch virus (TEV) and fungal pathogens (Hu and Reddy 1997; Schaad et al. 2000; Zoubenko et al. 2000). However, the accompanying increase in levels of SA was not always noticed and hence RIPs were purported to follow a SA-independent pathway. Induced systemic resistance (ISR) by rhizobacteria may also involve PR proteins (Kim et al. 2015). Volatiles of Bacillus sp. strain JS induce the expression of several PR genes, causing an up-regulation of glucanases (PR-2), chitinases (PR-3 and PR-4), peroxidise (PR-9) and PR-14 in tobacco plants exhibiting resistance to fungal pathogens (Kim et al. 2015). Thus the differences in the pathways utilized by the various agents may not always be distinct.
Molecular changes associated with the induction of resistance against viruses by phytoproteins like CIP-29 and CAP-34 have not been studied in detail. In addition to the ubiquity of the inducible VIAs, CAP-34 treated C. tetragonoloba plants also yielded two basic isoforms of β-1,3-glucanase (PR-2), possessing an M r of 34 and 36 kDa (Prasad et al. 2001). Enzymic activities need to be assigned to the induced VIAs as well and probable RIP activity cannot be ruled out. Enhancement in the total protein content and activity profiles of oxidoreductases like catalase, polyphenoloxidase and peroxidises was observed in Samsun NN tobacco treated with C. aculeatum leaf extract and C. tetragonoloba plants treated with C. aculeatum, C. fragrans and B. diffusa extracts (Verma and Prasad 1988, 1992). However, the RNase and DNase activity profiles in the resistant plants remained unaltered (Verma and Prasad 1992). Increase in the activity of superoxide dismutase and peroxidise and a decrease in catalase activity was noted in tobacco plants treated with AVPs from B. xbuttiana, whereas a total reversal in the activity pattern was noted for TMV infected tobacco (Bhatia et al. 2004). Antioxidant activity of C. cristata antiviral proteins, CCP-25 and CCP-27, was also reported. Enhancement in the activities of peroxidise, catalase and poly-phenol oxidase were noted in plants treated with these proteins and challenged with TMV (Gholizadeh et al. 2004). Similarly, AVP treated sunflower and black gram plants showed increase in peroxidise, polyphenol oxidase and phenylalanine ammonia lyase activies, along with enhanced phenolic content (Karthikeyan et al. 2009; Lavanya et al. 2009). Thus induction of defence-related enzymes and proteins appears to be generally associated with phytoprotein mediated induced resistance in plants. The observation that virus infection is inhibited in spatially separated tissues that have not received the inducer treatment prompted a study involving calcium-mediated signalling which is thought to be important for viral movement and replication. In a study taken up to determine the effect of a calcium channel blocker, verapamil, on induced resistance, it was noticed that its application increased the calcium efflux from leaf segments, while verapamil itself induced resistance and inhibited TMV infection (Singh et al. 2011c).
8 Concluding Remarks
In a multicomponent defence response to virus infection, the inducers/inhibitors and VIAs are envisaged to play a major role in protecting plants from virus infection and enhancing crop yield. It is entirely possible that ISR, SAR and systemic induced resistance by plant proteins, may run as parallel pathways, destined to converge eventually and give identical end results. Tremendous progress has been made in the recent years in the field of induced resistance in plants as a method of plant virus control. However, at a global level, only compounds like benzothiadiazole and chitosan and seed bacterization by PGPR have been occasionally used for effective control in open fields (Faoro and Gozzo 2015). Phytoprotein-mediated induced systemic protection in plants, having proven its worth in biological control, has the potential to become the much sought-after panacea in the management of plant viruses, with a well deserved place in any IPM programme, if not as a stand-alone practice.
References
Acharya S (2013) Control of the potato virus X through application of root extracts of Chlorophytum nepalense to potato plants and tubers. Potato Res 56:1–10
Alberghina A (1976) The inhibitory activity of extracts of Chenopodium amaranticolor leaves on the infection of tobacco necrosis virus. J Phytopathol 87:17–27
Allard HA (1918) Effect of various salts, acids, germicides, etc., on the infectivity of the virus causing mosaic disease of tobacco. J Agric Res 13:619–637
Apablaza GE, Bernier CC (1972) Inhibition of tobacco mosaic virus infection by plant extracts. Can J Bot 50:1473–1478
Awasthi LP (1981) The purification and nature of an antiviral protein from Cuscuta reflexa plants. Arch Virol 70:215–223
Awasthi LP (1982) Characteristics and mode of action of a virus inhibitor from Cuscuta reflexa plants. Zentt Mikrobiol 137:509–518
Awasthi LP, Chowdhury B, Verma HN (1984) Prevention of plant virus diseases by Boerhaavia diffusa inhibitor. Int J Trop Dis 2:41–44
Awasthi LP, Kumar P (2003) Prevention of infection and multiplication of cucumber green mottle mosaic virus in muskmelon treated with Boerhaavia diffusa. Indian Phytopathol 56:362
Awasthi LP, Singh S (2009) Management of ringspot disease of papaya through plant products. Indian Phytopathol 62:369–375
Awasthi LP, Singh SP, Verma HN, Kluge S (2013) Further studies on the antiviral agent isolated from host plants pretreated with Boerhaavia diffusa glycoprotein. Virol Mycol 3:124
Bag P, Chattopadhyay D, Mukherjee H, Ojha D, Mandal N, Sarkar MC, Chatterjee T, Das G, Chakraborti S (2012) Anti-herpes virus activities of bioactive fraction and isolated pure constituent of Mallotus peltatus: an ethnomedicine from Andaman Islands. Virol J 9:98
Balasaraswathi R, Sadasivam S, Ward M, Walker JM (1998) An antiviral protein from Bougainvillea spectabilis roots: purification and characterization. Phytochemistry 47:1561–1565
Balasubrahmanyam A, Baranwal VK, Lodha ML, Varma A, Kapoor HC (2000) Purification and properties of growth stage-dependent antiviral proteins from the leaves of Celosia cristata. Plant Sci 154:13–21
Barakat A, Stevens WA (1981) Studies on the mode of action of inhibitors of local lesion production by plant viruses. Microbios Lett 16:7–13
Baranwal VK, Ahmad N (1997) Effect of Clerodendrum aculeatum leaf extract on tomato leaf curl virus. Indian Phytopathol 50:297–299
Baranwal VK, Tumer NE, Kapoor HC (2002) Depurination of ribosomal RNA and inhibition of viral RNA translation by an antiviral protein of Celosia cristata. Indian J Exp Biol 40:1195–1197
Baranwal VK, Verma HN (1992) Localized resistance against virus infection induced by leaf extract of Celosia cristata. Plant Pathol 41:633–638
Baranwal VK, Verma HN (1993) Virus inhibitory activity of leaf extracts from different taxonomic group of higher plants. Indian Phytopathol 46:402–403
Baranwal VK, Verma HN (1997) Characteristics of a virus inhibitor from the leaf extract of Celosia cristata. Plant Pathol 46:523–529
Barbieri L, Battelli MG, Stirpe F (1993) Ribosome-inactivating proteins from plants. Biochem Biophys Acta 1154:237–282
Barbieri L, Polito A, Bolognesi A, Ciani M, Pelosi E, Farini V, Jha A, Sharma N, Vivanco JM, Chamberry A, Parente A, Stirpe F (2006) Ribosome-inactivating proteins in edible plants and purification and characterization of a new ribosome-inactivating protein from Cucurbita moschata. Biochem Biophys Acta 1760:783–792
Barbieri L, Valbonesi P, Bonora E, Gorini P, Bolognesi A, Stirpe F (1997) Polynucleotide: adenosine glycosidase activity of ribosome-inactivating proteins: effect on DNA, RNA and poly(A). Nucleic Acids Res 25:518–522
Begam M, Kumar S, Roy S, Campanella JJ, Kapoor HC (2006) Molecular cloning and functional identification of a ribosome-inactivating/antiviral protein from leaves of post-flowering stage of Celosia cristata and its expression in E. coli. Phytochemistry 67:2441–2449
Bhanuprakash V, Hosamani M, Balamurugan V, Gandhale P, Naresh P, Swarup D, Singh RK (2008) In vitro antiviral activity of plant extracts on goatpox virus replication. Indian J Exp Biol 46:120–127
Bhanuprakash V, Hosamani M, Balamurugan V, Singh RK, Swarup D (2007) In vitro antiviral activity of Eugenia jambolana plant extract on buffalo poxvirus: conventional and QPCR methods. Int J Trop Med 2:3–9
Bharathi M (1999) Effect of plant extract and chemical inhibitors on cucumber mosaic virus of brinjal. J Mycology Plant Pathol 29:57–60
Bharathimatha C, Doraiswamy S, Rabindran R, Renukadevi P, Velazhahan R (2003) Existence of antiviral principles (AVPs) in seed extracts of Harpullia cupanioides (Roxb.) against tomato spotted wilt virus (TSWV), rice tungro virus (TRV) and cowpea aphid borne mosaic virus (CABMV). Acta Phytopathol Entomol Hung 38:109–114
Bhargava KS, Singh R (1965) Inactivation of watermelon mosaic virus by juice of Portulacca grandiflora. Curr Sci 11:361
Bhatia S, Kapoor HC, Lodha ML (2004) Modification of antioxidant status of host cell in response to Bougainvillea antiviral proteins. J Plant Biochem Biotechnol 13:113–118
Bhatia S, Lodha ML (2005) RNase and DNase activities of antiviral proteins from leaves of Bougainvillea xbuttiana. Indian J Biochem Biophys 42:152–155
Bolognesi A, Polito L, Olivieri F, Valbonesi P, Barbieri L, Battelli MG, Carusi MV, Benvenuto E, Del Vecchio BF, Di Maro A, Parente A, Di Loreto M, Stirpe F (1997) New ribosome-inactivating proteins with polynucleotide:adenosine glycosidase and antiviral activities from Basella rubra L. and Bougainvillea spectabilis Willd. Planta 203:422–429
Bose K, Kulshreshtha K, Joshi RD (1983) Some aspects of the inhibition of bean common mosaic virus on ornamental plants. Agric Sci Dig 3:195–198
Cakir B, Tumer NE (2015) Arabidopsis Bax inhibitor-1 inhibits cell death induced by pokeweed antiviral protein in Saccharomyces cerevisae. Microbiol Cell 2:43–56
Chandra K, Gupta BM (1981) Acquired local and systemic antiviral (TMV) resistance induced by treatment with T-poly (Trichothecium polysaccharide) in non-hypersensitive host plant Nicotiana tabacum cv. NP31. Curr Sci 50:69–71
Chandra S, Singh BP, Nigam SK, Srivastava KM (1975) Effect of some naturally occurring plant products on Southern sunnhemp mosaic virus (SSMV). Curr Sci 44:511–512
Chaudhry B, Muller-Uri F, Cameron-Mills V, Gough S, Simpson D, Skriver K, Mundy J (1994) The barley 60 kDa jasmonate-induced protein (JIP-60) is a novel ribosome-inactivating protein. Plant J 6:815–824
Chen ZC, Antoniw JF, White RF (1993) A possible mechanism for the antiviral activity of pokeweed antiviral protein. Physiol Mol Plant Pathol 42:249–258
Chen Y, Peumans WJ, Van Damme EJM (2002) The Sambucus nigra type-2 ribosome-inactivating protein SNA-1 exhibits in planta antiviral activity in transgenic tobacco. FEBS Lett 516:27–30
Choudhary NL, Yadav OP, Lodha ML (2008) Ribonuclease, deoxyribonuclease and antiviral activity of Escherichia coli-expressed Bougainvillea xbuttiana antiviral protein 1. Biochem Mosc 73:273–277
Chowdhury AK, Saha NK (1985) Inhibition of urdbean leaf crinkle virus by different plant extracts. Indian Phytopathol 38:566–568
Cillo F, Palukaitis P (2014) Transgenic resistance. Adv Virus Res 90:35–146
Collinge DB, Jorgensen HJL, Lund OS, Lyngkjaer MF (2010) Engineering pathogen resistance in crop plants: current trends and future prospects. Annu Rev Phytopathol 48:269–291
Darekar RN, Sawant DM (1989) Inhibition of bottle gourd mosaic by plant leaf extracts. Indian Phytopathol 42:339
Deepthi N, Madhusudhan KN, Uday Shankar AC, Kumar HB, Prakash HS, Shetty HS (2007) Effect of plant extracts and acetone precipitated proteins from six medicinal plants against tobamovirus infection. Int J Virol 3:80–87
Devi PS, Devi LR, Singh IM (2008) Evaluation of plant products against aphid (Myzus persicae Sultz) transmission of mosaic disease of leaf mustard (Brassica juncea var. rugosa). Indian Phytopathol 61:514–517
Devi PR, Doraiswamy S, Nakkeeran S, Rabindran R, Ganapathy T, Ramiah M, Mathiyazhagan S (2004) Antiviral action of Harpulia cupanioides and Mirabilis jalapa against tomato spotted wilt virus (TSWV) infecting tomato. Arch Phytopathol Plant Protect 37:245–259
Dhaliwal AS, Dhaliwal GK (1971) Inhibition of tobacco mosaic virus multiplication by extract from Allium cepa and Allium sativum. Adv Front Plant Sci 28:305–310
Di R, Tumer NE (2015) Pokeweed antiviral protein: its cytotoxicity mechanisms and applications in plant disease resistance. Toxins 7:755–772
Duggar BM, Armstrong JK (1925) The effect of treating virus of tobacco mosaic with juice of various plants. Ann Mol Bot Gard 12:359–365
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Dutt S, Balasubrahmanyam A, Lodha ML (2000) Purification and partial characterization of antiviral proteins from Chenopodium album L. leaves. J Plant Physiol 156:808–810
Dutt S, Narwal S, Kapoor HC, Lodha ML (2003) Isolation and characterization of two protein isoforms with antiviral activity from Chenopodium album L. leaves. J Plant Biochem Biotechnol 12:117–122
Dutt S, Yadav OP, Kapoor HC, Lodha ML (2004) Possible mechanism of action of antiviral proteins from the leaves of Chenopodium album L. Indian J Biochem Biophys 41:29–33
Edelbaum O, Sher N, Rubinstein M, Novick D, Tal N, Moyer M, Ward E, Ryals J, Sela I (1991) Two antiviral proteins, gp35 and gp22 correspond to 1,3-β-glucanase and an isoform of PR-5. Plant Mol Biol 17:171–173
Edreva A (2005) Pathogenesis-related proteins: Research progress in the last 15 years. Gen Appl Plant Physiol 31:105–124
Elad Y, Rav-David D, Leibman D, Vintal H, Vunsh R, Moorthy H, Gal-On A, Loebenstein G (2012) Tomato plants transformed with inhibitor-of-virus-replication gene are partially resistant to several pathogenic fungi. Ann Appl Biol 161:16–23
Endo Y, Mitsui K, Motizuki M, Tsurugi K (1987) The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes: the site and characteristics of the modification in 28S ribosomal RNA caused by the toxins. J Biol Chem 262:5908–5912
Endo Y, Tsurugi K (1988) The RNA N-glycosidase activity of ricin A-chain. The characteristics of the enzymatic activity of ricin A-chain with ribosomes and with rRNA. J Biol Chem 263:8735–8739
Faoro F, Gozzo F (2015) Is modulating virus virulence by induced systemic resistance realistic? Plant Sci 234:1–13
Fu ZQ, Dong X (2013) Systemic acquired resistance: Turning local infection into global defense. Annu Rev Plant Biol 64:839–863
Gendron Y, Kassanis B (1954) The importance of the host species in determining the action of virus inhibitors. Ann Appl Biol 41:183–188
Gera A, Loebenstein G, Salomon R, Franck A (1990) Inhibitor of virus replication (IVR) from protoplast of a hypersensitive tobacco cultivar infected with tobacco mosaic virus is associated with a 23k protein species. Phytopathology 80:78–81
Gholizadeh A, Kapoor HC (2004) Modification in the purification protocol of Celosia cristata antiviral proteins lead to protein that can be N-terminally sequenced. Protein Pept Lett 11:551–561
Gholizadeh A, Kohnehrouz BB, Santha IM, Lodha ML, Kapoor HC (2005) Cloning and expression of small cDNA fragment encoding strong antiviral peptide from Celosia cristata in Escherichia coli. Biochem Mosc 70:1005–1010
Gholizadeh A, Kumar M, Balasubrahmanyam A, Sharma S, Narwal S, Lodha S, Kapoor HC (2004) Antioxidant activity of antiviral proteins from Celosia cristata. J Plant Biochem Biotechnol 13:13–18
Gianinazzi S (1982) Antiviral agents and inducers of virus resistance: analogies with interferon. In: RKS W (ed) Active defence mechanisms in plants. Plenum Publishing Corp, New York, pp 275–296
Gianinazzi S, Martin C, Vallee JC (1970) Hypersensibilite aux virus, temperature et proteins soluble chez le Nicotiana Xanthi n.c. Apparition de nouvelles macromolecules lors de la repression de la synthese virale. CR Acad Sci D 270:2382–2386
Girbes T, Ferreras JM, Arias FJ, Stirpe F (2004) Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini-Rev Med Chem 4:461–476
Gozzo F, Faoro F (2013) Systemic acquired resistance (50 years after discovery): moving from the lab to the field. J Agric Food Chem 61:12473–12491
Grasso S (1977) Investigations on the action mechanism of a virus inhibitor from Phytolacca Americana. Riv di Patol Veg 13:77–84
Guleria S, Kumar A (2006) Azadirachta indica leaf extract induces resistance in sesame against Alternaria leaf spot. J Cell Mol Biol 5:81–86
Gupta BM, Chandra K, Verma HN, Verma GS (1973) Induction of antiviral resistance in Nicotiana glutinosa plants by treatment with Trichothecium polysaccharide and its reversal by actinomycin D. J Gen Virol 24:211–213
Gupta VK, Raychaudhuri SP (1971) Nature of virus inhibitor in Acacia arabica. Ann Phytopath Soc Jpn 37:124–127
Gupta VK, Raychaudhuri SP (1972) Mechanism of inhibition of Potato virus Y by extracts from leaves of some woody plants. J Phytopathol 73:256–262
Gupta RK, Srivastava A, Verma HN (2004) Callus culture and organogenesis in Boerhaavia diffusa: a potent antiviral protein containing plant. Physiol Mol Biol Plants 10:263–268
Hu X, Reddy AS (1997) Cloning and expression of a PR5-Like protein from Arabidopsis: inhibition of fungal growth by bacterially expressed protein. Plant Mol Biol 34:949–959
Hudak KA, Parikh BA, Di R, Baricevic M, Santana M, Seskar M, Tumer NE (2004) Generation of pokeweed antiviral protein mutations in Saccharomyces cerevisiae: evidence that ribosome depurination is not sufficient for cytotoxicity. Nucleic Acids Res 32:4244–4256
Hudak KA, Wang P, Tumer NE (2000) A novel mechanism for inhibition of translation by pokeweed antiviral protein: depurination of the capped RNA template. RNA 6:369–380
Iglesias R, Perez Y, de Torre C, Ferreras J, Antolin P, Jimenez P, Rojo MA, Mendez E, Girbes T (2005) Molecular characterization and systemic induction of single-chain ribosome-inactivating protein (RIPs) in sugar beet (Beta vulgaris) leaves. J Exp Bot 56:1675–1684
Jayashree K, Pun KB, Doraiswamy S (1999) Effect of plant extracts and derivatives, butter milk and virus inhibitory chemicals on pumpkin yellow vein mosaic virus transmission. Indian Phytopathol 52:357–361
Karran RA, Hudak KA (2008) Depurination within the intergenic region of Brome mosaic virus RNA3 inhibits viral replication in vitro and in vivo. Nucleic Acids Res 36:7230–7239
Karran RA, Hudak KA (2011) Depurination of brome mosaic virus RNA3 inhibits its packaging into virus particles. Nucleic Acids Res 39:7209–7222
Karthikeyan G, Doraisamy S, Rabindran R, Ganapathy T (2009) Evaluation of antiviral principals for the induction of systemic resistance in black gram (Vigna mungo) against urdbean leaf crinkle virus. Arch Phytopathol Plant Protect 42:1172–1186
Kassanis B, Kleczkowski A (1948) The isolation and some properties of a virus-inhibiting protein from Phytolacca esculenta. J Gen Microbiol 2:143–153
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328
Khan MMAA, Jain DC, Khakuni RS, Thakur RS (1991) Occurrence of some antiviral sterols in Artemisia annua. Plant Sci 75:161–165
Khan MMAA, Verma HN (1990) Partial characterization of an induced virus inhibitory protein associated with systemic resistance in Cyamopsis tetragonoloba (L.) Taub. plants. Ann Appl Biol 117:617–623
Khurana SMP, Bhargava KS (1970) Effect of plant extracts on the activity of three papaya viruses. J Gen Appl Microbiol 16:225–230
Khurana SMP, Singh B (1972) Studies on Calotropis procera latex as inhibitor of tobacco mosaic virus. Phytopathol Z 73:341–346
Kim JS, Kim YO, Ryu HJ, Kwak YS, Lee JY, Kang H (2003) Isolation of stress-related genes of rubber particles and latex in fig tree (Ficus carica) and their expressions by abiotic stress or plant hormone treatments. Plant Cell Physiol 44:412–414
Kim JS, Lee J, Lee C-h, Woo SY, Kang H, Seo S-G, Kim S-H (2015) Activation of pathogenesis-related genes by the rhizobacterium Bacillus sp. JS, which induces systemic resistance in tobacco plants. Plant Pathol J 31:195–201
Krishna R, McDonald KA, Dandekar AM, Jackman AP, Falk B (2002) Expression of recombinant trichosanthin, a ribosome-inactivating protein, in transgenic tobacco. J Biotechnol 97:69–88
Kulkarni VR, Byadagi AS (2004) Evaluation of plant extracts against safflower mosaic virus disease. Karnataka J Agric Sci 17:838–840
Kumar P, Awasthi LP (2009) Prevention of infection and spread of viral diseases in cucumber (Cucumis sativus L.) through botanicals. J Plant Dis Sci 4:25–32
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J. doi:10.1155/2013/162750
Kumar H, Singh R, Gupta V, Zutshi SK (2015) Performance of different germplasm, plant extracts and insecticides against yellow vein mosaic of okra (OYVMV) under field conditions. VEGETOS 28:31–37
Kumar D, Verma HN, Tuteja N, Tewari KK (1997) Cloning and characterization of a gene encoding an antiviral protein from Clerodendrum aculeatum L. Plant Mol Biol 33:745–751
Lal R, Verma GS (1974) Effect of plant latex on virus infectivity. Zent Bakteriol Parasit Infect Hyg 129:271–277
Lal R, Verma GS, Verma HN (1973) Effect of some plant extracts on infectivity of tobacco mosaic virus. Indian Phytopathol 26:122–128
Lavanya N, Saravanakumar D, Rajendran L, Ramiah M, Raguchander T, Samiyappan R (2009) Management of sunflower necrosis virus through antiviral substances. Arch Phytopathol Plant Protect 42:265–276
Lodge JK, Kaniewski WK, Tumer NE (1993) Broad spectrum virus resistance in transgenic plants expressing pokeweed antiviral protein. Proc Natl Acad Sci U S A 90:7089–7093
Lodha ML, Agarwal S, Biswas K, Vasudev S, Dubey SC (2010) Antimicrobial activity of native and recombinant antiviral proteins from Bougainvillea xbuttiana leaves against plant pathogenic fungi and viruses. Indian J Agric Biochem 23:83–90
Lodha ML, Choudhary NL (2011) Purification and evaluation of antiviral proteins from Bougainvillea xbuttiana against Helicoverpa armigera. Indian J Agri Sci 81:74–78
Loebenstein G, Gera A (1981) Inhibitor of virus replication released from TMV infected protoplasts of a local lesion responding tobacco cultivar. Virology 114:132–139
Loebenstein G, Rav-David D, Leibman D, Gal-On A, Vunsh R, Czosnek H, Elad Y (2010) Tomato plants transformed with the inhibitor-of-virus-replication gene are partially resistant to Botrytis cinerea. Phytopathology 100:225–229
Lohani S, Jan A, Verma HN (2007) In vivo and in vitro resistance in tobacco by Boerhaavia diffusa systemic resistance inducing protein and transfer of induced resistance in in vitro tobacco plants. Biotechnology 6:389–392
Louis V, Balakrishnan S (1996) Effect of application of selected medicinal plant extracts on the incidence of pumpkin mosaic virus. Indian Phytopathol 49:373–377
Madhusudhan KN, Nalini MS, Prakash HS, Shetty HS (2005) Effect of inducers against tobamovirus infection in tomato and bell pepper. Int J Bot 1:59–61
Madhusudhan KN, Vinayarani G, Deepak SA, Niranjana SR, Prakash HS, Singh GP, Sinha AK, Prasad BC (2011) Antiviral activity of plant extracts and other inducers against tobamoviruses infection in bell pepper and tomato plants. Int J Plant Pathol 2:35–42
Mahmoud SYM, Gad-Rab SMF, Hussein N, Shoreit AAM (2010) Antiviral activity of latex from Ficus nitida against plant viruses. Glob J Biotechnol Biochem 5:198–205
Mandal B, Singh B (2001) Inhibition of virus transmission by guava leaf extract. Indian Phytopathol 54:381–382
Manickam K, Rajappan K (1999) Field efficacy of plant extracts and chemicals against green gram leaf curl disease. Indian J Virol 15:35–37
Mariappan V, Saxena RC (1983) Effect of custard apple oil and neem oil on survival of Nephotettix virescens (Homoptera Cicadellidae) and on rice tungro virus transmission. J Econ Entomol 76:573–576
McIntyre JL, Dodds JA, Hare JD (1981) Effects of localized infections of Nicotiana tabacum by tobacco mosaic virus on systemic resistance against diverse pathogens and an insect. Phytopathology 71:297–301
McKeen CD (1956) The inhibitory activity of extracts of Capsicum frutescens on plant virus infections. Can J Bot 34:891–903
Mozes R, Antignus Y, Sela I, Harpaz I (1978) The chemical nature of an antiviral factor (AVF) from virus-infected plants. J Gen Virol 38:241–249
Mukerjee K, Awasthi LP, Verma HN (1981) The inhibitory activity of an interfering agent, extracted from the leaves of host plants treated with Datura leaf extract, on plant virus infections. Z Pflanzenkr Pflanzensch 88:228–234
Murty NS, Nagarajan K (1980) Virus inhibitory effect of extracts from germinating seeds of flowering plants. Indian Phytopathol 33:615–617
Murty NS, Nagarajan K (1986) Role of plant extracts in the control of TMV infection in nursery and field grown tobacco. Indian Phytopathol 39:98–100
Nagaich BB, Singh S (1970) An antiviral principle induced by potato virus X in Capsicum pendulum. Virology 40:269–271
Nagarajan K, Murty NS (1975) Effect of certain plant extracts and plant latex on the inhibition of tobacco mosaic virus infection. Tob Res 1:122–132
Narayanasamy P (1990) Antiviral principles for virus disease management. In: Vidhyasekaran P (ed) Basic research for crop disease management, Daya Publishing House, New Delhi, pp 139–150
Narwal S, Balasubrahmanyam A, Lodha ML, Kapoor HC (2001a) Purification and properties of antiviral proteins from the leaves of Bougainvillea xbuttiana. Indian J Biochem Biophys 38:342–347
Narwal S, Balasubrahmanyam A, Sadhna P, Kapoor HC, Lodha ML (2001b) A systemic resistance inducing antiviral protein with N-glycosidase activity from Bougainvillea xbuttiana leaves. Indian J Exp Biol 39:600–603
Nielsen K, Boston RS (2001) Ribosome-inactivating proteins: a plant perspective. Annu Rev Plant Physiol Plant Mol Biol 52:785–816
Nutan MM, Dezzutti CS, Kulshreshtha S, Rawat AK, Srivastava SK, Malhotra S, Verma A, Ranga U, Gupta SK (2013) Extracts from Acacia catechu suppress HIV-1 replication by inhibiting the activities of the viral protease and Tat. Virol J 10:309. doi:10.1186/1743-422X-10-309
Obrig TG, Irvin JD, Hardesty B (1973) The effect of an antiviral peptide on the ribosomal reactions of the peptide elongation enzymes, EF-I and EF-II. Arch Biochem Biophys 155:278–289
Olivieri F, Prasad V, Valbonesi P, Srivastava S, Ghosal-Chowdhury P, Barbieri L, Bolognesi A, Stirpe F (1996) A systemic antiviral resistance inducing protein isolated from Clerodendrum inerme Gaertn. is a polynucleotide:adenosine glycosidase (ribosome-inactivating protein). FEBS Lett 396:132–134
Owens RA, Bruening G, Shepherd JR (1973) A possible mechanism for the inhibition of plant viruses by a peptide from Phytolacca Americana. Virology 56:390–393
Paliwal YC, Nariani TK (1965a) Effect of plant extracts on the infectivity of sunnhemp (Crotalaria juncea) mosaic virus. Acta Virol 9:261–267
Paliwal YC, Nariani TK (1965b) Properties of the inhibitors of sunnhemp (Crotalaria juncea) mosaic virus in certain plant extracts. Acta Virol 9:455–458
Pandey AK, Bhargava KS (1980) Antiviral activity of crude extracts of some Pteridophytes. Indian Fern J 3:132–133
Pandey AK, Bhargava KS (1983) Isolation and partial characterization of virus inhibitor from the leaf extract of a fern, Ampelopteris prolifera (Retz.) copel. Indian J Plant Pathol 1:193–198
Pandey AK, Bhargava KS (1984) Effect of Ampelopteris prolifera leaf extract on the activity of tobacco mosaic and cucumber mosaic viruses. Indian Phytopathol 37:271–277
Pandey BP, Mohan J (1986) Inhibition of turnip mosaic virus by plant extracts. Indian Phytopathol 39:489–491
Pardeep K, Awasthi LP (2009) Prevention of infection and spread of viral diseases in cucumber (Cucumis sativus L.) through botanicals. J Plant Dis Sci 4:25–32
Park C-J, Kim K-J, Shin R, Park JM, Shin Y-C, Paek K-H (2004) Pathogenesis-related protein 10 isolated from hot pepper functions as a ribonuclease in an antiviral pathway. Plant J 37:186–198
Patel BN, Patel GJ (1979) Inhibition of tobacco mosaic virus infection by some plant extracts. Tob Res 5:33–36
Prasad V (1986) Alterations in enzyme activity during induced antiviral state by leaf extract. J Indian Bot Soc 65:90–94
Prasad V (1988) Association of polyphenoloxidase with induced antiviral resistance in two host-inducer combinations. J Indian Bot Soc 67:53–55
Prasad V, Ghosal-Chowdhury P, Srivastava S (2001) Purification of two basic 1,3-ß-glucanase isoforms from Cyamopsis tetragonoloba (L.) Taub. induced to resist virus infections. Israel J Plant Sci 49:15–19
Prasad V, Mishra SK, Srivastava S, Srivastava A (2014) A virus inhibitory protein isolated from Cyamopsis tetragonoloba upon induction of systemic antiviral resistance shares partial amino acid sequence homology with a lectin. Plant Cell Rep 33:1467–1478
Prasad V, Misra SK, Krishna SK (2012) Induced systemic resistance against viruses in susceptible plants: phytoproteins and rhizobacteria provide two fascinating avenues. In: Rao GP, Baranwal VK, Mandal B, Rishi N (eds) Recent trends in plant virology. Studium Press LLC, Houston, pp 467–482
Prasad HP, Shankar UAC, Kumar BH, Shetty SH, Prakash HS (2007) Management of Bean common mosaic virus strain blackeye cowpea mosaic (BCMV-BICM) in cowpea using plant extracts. Arch Phytopathol Plant Protect 40:139–147
Prasad V, Srivastava S, Varsha, Verma HN (1995) Two basic proteins isolated from Clerodendrum inerme Gaertn. are inducers of systemic antiviral resistance in susceptible plants. Plant Sci 110:73–82
Praveen S, Tripathi S, Varma A (2001) Isolation and characterization of an inducer protein (Crip-31) from Clerodendrum inerme leaves responsible for induction of systemic resistance against viruses. Plant Sci 161:453–459
Pun KB, Doraiswamy S, Jeyarajan R (1999) Screening of plant species for the presence of antiviral principles against okra yellow vein mosaic virus. Indian Phytopathol 52:221–223
Rafiq M, Jabri A, Siddiqui KA, Khan TA, Husain SI, Mahmood K (1985) Inhibitory effect of latex on lesion caused by some plant viruses. Indian Bot Rep 4:213–214
Rajapakse RHS, Janaki CR (2006) Insecticidal properties of Chenopodium-based botanical against virus vectors, Myzus persicae and Frankliniella schultezi. Abstract OP 9/14. In: XVI Annual convention of IVS and International symposium on management of vector-borne viruses, ICRISAT, Patencheru, Hyderabad (India)
Rajesh S, Balasaraswathi R, Doraisamy S, Sadasivam S (2005) Synthesis and cloning of cDNA encoding an antiviral protein from the leaves of Bougainvillea spectabilis Willd. (Nyctaginaceae). World J Agric Sci 1:101–104
Rajinimala N, Rabindran R, Ramaiah M (2009) Management of bittergourd mosaic virus (BGYMV) by using virus inhibiting chemical, biocontrol agents, antiviral principles (AVP) and insecticide. Arch Phytopathol Plant Protect 42:738–750
Rao DG, Raychaudhuri SP (1965) Further studies on the inhibition of ringspot strain of potato virus x by plant extracts, culture filtrates of Trichothecium roseum and chemicals. Indian J Microbiol 5:9–12
Rao GP, Shukla K (1985) Antiviral activity of copra extract and physical properties of the inhibitor. Indian Phytopathol 38:623
Rao GP, Singh RK, Pandey AK, Singh R (1985) Induction of local and systemic resistance of potato virus by flower extracts. Indian J Virol 1:174–182
Raychaudhuri SP, Chadha KC (1965) Deodar fruit extract-an inhibitor of chilli mosaic virus. Indian Phytopathol 18:97–98
Raychaudhuri SP, Prasad HC (1965) Effect of plant extracts and microbial growth products on the infectivity of radish mosaic virus. Indian J Microbiol 5:13–16
Ready MP, Brown DT, Robertus JD (1986) Extracellular localization of pokeweed antiviral protein. Proc Natl Acad Sci U S A 83:5053–5056
Reinbothe S, Reinbothe C, Lehmann J, Becker W, Apel K, Parthier B (1994) JIP60, a methyl-jasmonate induced ribosome-inactivating protein involved in plant stress reactions. Proc Natl Acad Sci U S A 91:7012–7016
Roy S, Sadhana P, Begum M, Kumar S, Lodha ML, Kapoor HC (2006) Purification, characterization and cloning of antiviral/ribosome-inactivating protein from Amaranthus tricolor leaves. Phytochemistry 67:1865–1873
Roy AN, Sinha BP, Gupta KC (1979) The inhibitory effect of plant juices on the infectivity of top necrosis virus of pea. Indian J Microbiol 19:198–201
Roychoudhury R (1984) Virus inhibitor from Solanum torvum. Indian Phytopathol 37:665–668
Roychoudhury R, Basu PK (1983) Characterization of a plant virus inhibitor from two Solanum sp. Indian J Exp Biol 21:213–215
Rustgi S, Pollmann S, Buhr F, Springer A, Reinbothe C, von Wettstein D, Reinbothe S (2014) JIP-60-mediated, jasmonate-and senescence induced molecular switch in translation toward stress and defense protein synthesis. Proc Natl Acad Sci U S A 111:14181–14186
Saigopal DVR, Prasad VS, Sreenivasulu P (1986) Antiviral activity in extracts of Phyllanthus fraternus Webst (P. niruri). Curr Sci 55:264–265
Saveetha K, Sankaralingam A, Ramanathan A, Pant R (2006) Symptom, epidemiology and management of finger millet mottle streak disease. Arch Phytopathol Plant Protect 39:409–419
Schaad MC, Anderberg RJ, Carrington JC (2000) Strain-specific interaction of the tobacco etch virus NIa protein with the translation initiation factor eIF4E in the yeast two-hybrid system. Virology 273:300–306
Schrot J, Weng A, Melzig MF (2015) Ribosome-inactivating and related proteins. Toxins 7:1556–1615
Sela I, Applebaum SW (1962) Occurrence of an antiviral factor in virus infected plants. Virology 17:543–548
Shahidi-Noghabi S, Van Damme EJM, Smagghe G (2009) Expression of Sambucus nigra agglutinin (SNA-I’) from elderberry bark in transgenic tobacco plants results in enhanced resistance to different insect species. Transgenic Res 18:249–259
Sharma YR, Chohan JS (1973) Inhibitors of cucumis virus 1 in extracts of leaf and seeds of different plants. Indian Phytopathol 26:172–173
Sharma DC, Raychaudhuri SP (1965) Antiviral effect of plant extracts and culture filtrate of Aspergillus niger on ringspot strain of potato virus x. Indian J Microbiol 5:41–48
Shukla HH, Dubey P, Chaturvedi RV (1989) Antiviral properties of essential oils of Foeniculum vulgare and Pimpinella anisum L. Agronomie 9:277–279
Singh R (1969) The inhibitory activity of some plant juices on the infectivity of watermelon mosaic virus. Acta Virol 13:244–246
Singh AB (1972) Inhibitory activity of some plant extracts on the infectivity of papaya leaf reduction virus. Acta Phytopathol Acad Sci Hung 7:175–178
Singh S, Awasthi LP, Singh RK (2011a) Induction of systemic resistance through antiviral agents of plant origin against papaya ring spot disease (Carica papaya L.) Arch Phytopathol Plant Protect 44:1676–1682
Singh SK, Awasthi LP, Singh S, Sharma NK (2011b) Protection of mungbean and urdbean crops against vector borne mungbean yellow mosaic virus through botanicals. Curr Bot 2:8–11
Singh S, Awasthi LP, Verma HN (2004) Prevention and control of yellow mosaic disease of mungbean by application of aqueous root extract of Boerhaavia diffusa. Indian Phytopathol 57:303–307
Singh AK, Najam A, Verma HN, Awasthi LP (2009) Control of natural virus infection on okra (Abelmoschus esculentus) by root. Intl J Plant Protect 2:195–198
Singh P, Sharma S, Prasad V (2011c) Verapamil, a calcium channel blocker, induces systemic antiviral resistance in susceptible plants. J Phytopathol 159:127–129
Singh R, Singh R (1973) Properties of an inhibitor of potato virus x from bark of Ficus elastica. Indian Phytopathol 26:560–563
Singh R, Singh R (1975) Inhibition of potato virus x by Rumex hastatus extract. Sci Cult 43:268–269
Smookler MM (1971) Properties of inhibitors of plant virus infection occurring in the leaves of species of Chenopodiaceae. Ann Appl Biol 69:157–158
Sreeja S, Sreeja S (2009) An in vitro study on antiproliferative and antiestrogenic effects of Boerhaavia diffusa L. extract. J. Ethnopharmacol 126:221–225
Srinivasulu B, Jeyarajan R (1986) Effect of leaf extract on the infection of rice tungro virus in rice. Indian J Virol 2:176–180
Srivastava KM, Chandra S, Singh BP, SMH A (1976) Induction of local and systemic resistance in Nicotiana glutinosa against TMV by leaf extract of Dahlia. Ind J Exp Biol 4:377–379
Srivastav SK, Ansari NA, Tewari JP (2011) Eco-friendly management of tomato leaf curl disease. Intl J Plant Protect 4:321–323
Srivastava A, Gupta RK, Verma HN (2004) Micropropagation of Clerodendrum aculeatum through adventitious shoot induction and production of consistent amount of virus resistance inducing protein. Indian J Exp Biol 42:1200–1207
Srivastava S, Prasad V (2014) Induction of defence responses for biological control of plant diseases. In: Sharma N (ed) Biological controls for preventng food deterioration. Wiley, UK, pp 321–339
Srivastava A, Srivastava S, Prasad V (2015a) Systemic antiviral resistance induced in papaya by CAP-34, a resistance inducing protein from Clerodendrum aculeatum, is associated with a proteinaceous virus inhibitory activity. J Plant Pathol 97:45–54
Srivastava A, Trivedi S, Krishna SK, Verma HN, Prasad V (2009) Suppression of papaya ringspot virus infection in Carica papaya with CAP-34, a systemic antiviral resistance inducing protein from Clerodendrum aculeatum. Eur J Plant Pathol 123:241–246
Srivastava S, Verma HN, Srivastava A, Prasad V (2015b) BDP-30, a systemic resistance inducer from Boerhaavia diffusa L., suppresses TMV infection, and displays homology with ribosome-inactivating proteins. J Biosci 40:125–135
Stevens WA, Spurdon C, Onyon LJ, Stirpe F (1981) Effect of inhibitors of protein synthesis from plants on tobacco mosaic virus infection. Experientia 37:257–259
Stintzi A, Heitz T, Prasad V, Wiedemann-Merdinoglu S, Kauffmann S, Geoffroy P, Legrand M, Fritig B (1993) Plant ‘pathogenesis-related’ proteins and their role in defence against pathogens. Biochimie 75:687–706
Stirpe F (2004) Ribosome-inactivating proteins. Toxicon 44:371–383
Stirpe F (2013) Ribosome-inactivating proteins: From toxins to useful proteins. Toxicon 67:12–16
Stirpe F, Lappi DA (eds) (2014) Ribosome inactivating proteins: ricin and related proteins. Wiley, USA
Sundaram S, Dwivedi P, Purwar S (2011) In vitro evaluation of antibacterial activities of crude extracts of Withania somnifera (Ashwagandha) to bacterial pathogens. Asian J Biotechnol 3:194–199
Surendran M, Shanmugam V, Rajagopalan B, Ramanian N (1999) Efficacy of botanicals on brinjal mosaic virus. Plant Dis Res 14:63–66
Tewari JP (1976) Inhibition of three strains of watermelon mosaic virus by bark extract. Curr Sci 45:696–697
Thirumalaisamy PP, Rathi YPS, Tripathi HS (2003) Screening of some plant extracts inhibitory to urdbean leaf crinkle virus. Indian Phytopathol 56:233–235
Tiwari P, Khan H, Ansari NA, Tewari JP (2010) Management of pea mosaic virus by leaf extract of some medicinal plants. Intl J Plant Protect 3:117–119
Tourlakis ME, Karran RA, Desouza L, Siu KW, Hudak KA (2010) Homodimerization of pokeweed antiviral protein as a mechanism to limit depurination of pokeweed ribosomes. Mol Plant Pathol 11:757–767
Tumer NE, Hwang D-J, Bonness M (1997) C-terminal deletion mutant of pokeweed antiviral protein inhibits viral infection but does not depurinate host ribosomes. Proc Natl Acad Sci U S A 94:3866–3871
Van Loon LC, Rep M, Pieterse CMJ (2006) Significance of inducible defence-related proteins. Annu Rev Phytopathol 44:135–162
Van Loon LC, Van Kammen A (1970) Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. “Samsun” and “Samsun NN”: changes in protein constitution after infection with tobacco mosaic virus. Virology 40:199–211
Vandenbussche F, Desmyter S, Ciani M, Proost P, Peumans WJ, Van Damme EJ (2004) Analysis of the in planta antiviral activity of elderberry ribosome-inactivating proteins. Eur J Biochem 271:1508–1515
Vasudeva RS, Nariani TK (1952) Host range of bottle gourd mosaic virus and its inactivation by plant extracts. Phytopathology 42:149–152
Venkatesan S, Radjacommare R, Nakkeeran S, Chandrasekaran A (2010) Effect of biocontrol agent, plant extracts and safe chemicals in suppression of mungbean yellow mosaic virus (MYMV) in black gram (Vigna mungo). Arch Phytopathol Plant Protect 43:59–72
Verma HN (1982) Inhibitor of plant viruses from higher plants. In: Singh BP, Raychoudhary SP (eds) Current trends in plant virology. Today and Tomorrow’s printers and publishers, New Delhi, pp 151–159
Verma HN, Awasthi LP (1979) Antiviral activity of Boerhaavia diffusa root extract and the physical properties of the virus inhibitor. Can J Bot 57:926–932
Verma HN, Awasthi LP (1980) Occurrence of a highly antiviral agent in plants treated with Boerhaavia diffusa inhibitor. Can J Bot 58:2141–2144
Verma HN, Awasthi LP, Mukerjee K (1979b) Induction of systemic resistance by antiviral plant extracts in non-hypersensitive hosts. Z Pflanzenkr Pflansensch 86:735–740
Verma HN, Awasthi LP, Mukerjee K (1979c) Prevention of virus infection and multiplication by extracts from medicinal plants. Phytopathol Z 96:71–76
Verma HN, Awasthi LP, Saxena KC (1979a) Isolation of the virus inhibitor from the root extract of Boerhaavia diffusa inducing systemic resistance in plants. Can J Bot 57:1214–1217
Verma HN, Baranwal VK (1983) Antiviral activity and the physical properties of the leaf extract of Chenopodium ambrosiodes L. Proc Indian Acad Sci 92:461–465
Verma HN, Baranwal VK (2011) Potency of plant products in control of virus diseases of plants. In: Dubey NK (ed) Natural products in plant pest management. CAB International, India, pp 149–174
Verma HN, Baranwal VK, Srivastava S (1998) Antiviral substances of plant origin. In: Hadidi A, Khetarpal RK, Koganezawa A (eds) Plant virus disease control. American Phytopathological Society (APS) Press, St Paul, pp 154–162
Verma HN, Chowdhery B, Rastogi P (1984) Antiviral activity of different Clerodendrum L. species. Z Pflanzenkr Pflansensch 91:34–41
Verma HN, Dwivedi SD (1983) Prevention of plant virus diseases in some economically important plants by Bougainvillea leaf extract. Indian J Plant Pathol 1:97–100
Verma HN, Dwivedi SD (1984) Properties of a virus inhibiting agent isolated from plants following treatment with Bougainvillea spectabilis leaf extract. Physiol Plant Pathol 25:93–101
Verma HN, Khan MMAA (1985) Occurrence of a strong virus interfering agent in susceptible plants sprayed with Pseuderanthemum atropurpureum tricolor leaf extract. Indian J Virol 1:26–34
Verma HN, Khan MMAA, Dwivedi SD (1985a) Biophysical properties of highly antiviral agents present in Pseuderanthemum atropurpureum and Bougainvillea spectabilis extracts. Indian J Plant Pathol 3:13–20
Verma HN, Kumar V (1979) Prevention of potato from viruses and insect vectors. J Indian Pot Assoc 6:157–161
Verma HN, Kumar V (1980) Prevention of plant virus diseases by Mirabilis jalapa leaf extract. New Bot 7:87–91
Verma HN, Mukerjee K (1975) Brinjal leaf extract induced resistance to virus infection in plants. Indian J Exp Biol 13:416–417
Verma HN, Prasad V (1987) Systemic induced antiviral resistance by plant extract alters physiology of susceptible test host. Indian J Plant Pathol 5:69–72
Verma HN, Prasad V (1988) Metabolic alterations associated with host mediated systemic antiviral resistance. Indian Phytopathol 41:332–335
Verma HN, Prasad V (1992) Virus inhibitors and inducers of resistance: potential avenues for biological control of viral diseases. In: Mukerji KG, Tewari JP, Arora DK, Saxena G (eds) Recent developments in biocontrol of plant diseases. Aditya Book Pvt Ltd., New Delhi, pp 81–110
Verma HN, Rastogi P, Prasad V, Srivastava A (1985b) Possible control of natural virus infection on Vigna radiata and Vigna mungo by plant extracts. Indian J Plant Pathol 3:21–24
Verma VS, Raychaudhuri SP (1970) Effect of tannins from Terminalia chebula on the infectivity of potato virus. Acta Microbiol Pol 19(B):127–132
Verma VS, Raychaudhuri SP (1972) Effect of catechol tannins isolated from medicinal plant Chrysobalanus icacao on the infectivity of potato virus x Zent Bakenot Abt II 127:178–179
Verma VS, Raychaudhuri SP, Khan AM (1969) Properties and nature of inhibitors of potato virus X in four medicinal plant extracts. Biologia Plantarum (Praha) 11:384–387
Verma HN, Srivastava A (1985) A potent systemic inhibitor of plant virus infection from Aerva sanguinolenta Blume. Curr Sci 54:526–528
Verma HN, Srivastava S, Varsha KD (1996) Induction of systemic resistance in plants against viruses by a basic protein from Clerodendrum aculeatum leaves. Phytopathology 86:485–492
Verma HN, Varsha (1995a) Induction of systemic resistance by leaf extract of Clerodendrum aculeatum in sunnhemp rosette virus. Indian Phytopathol 48:218–221
Verma HN, Varsha (1995b) Prevention of natural occurrence of tobacco leaf curl disease by primed Clerodendrum aculeatum leaf extract. In: Verma JP, Varma A, Kumar D (eds) Detection of plant pathogens and their management. Angkor Publishers (P) Ltd, New Delhi, pp 202–206
Verma HN, Varsha, Baranwal, VK (1995) Agricultural role of endogenous antiviral substances of plant origin. Chessin M, DeBorde D, Zipf A, Antiviral proteins in higher plants, CRC Press, Boca Raton, 23–37
Verma GS, Verma HN (1965) Tobacco mosaic virus inhibitory principle in wheat extract. Indian J Microbiol 5:17–20
Verma GS, Verma HN, Srivastava KM, Mukerjee K (1975) Properties and mode of action of a tobacco mosaic virus inhibitor from seed extract of Lawsonia alba. New Botanist 2:109–113
Vijayan P, Raghu C, Ashok G, Dhanaraj SA, Suresh B (2004) Antiviral activity of medicinal plants of Nilgiris. Indian J Med Res 120:24–29
Vivanco JM, Tumer NE (2003) Translation inhibition of capped and uncapped viral RNAs mediated by ribosome-inactivating proteins. Phytopathology 93:588–595
Yadav S, Kumar S, Jain P, Pundir RK, Jadon S, Sharma A, Khetwal KS, Gupta KC (2011) Antimicrobial activity of different extracts of roots of Rumex nepalensis Spreng. Indian J Nat Prod Resour 2:65–69
Zaidi ZB, Gupta VP, Samad A, Naqvi QA (1988) Inhibition of spinach mosaic virus by extracts of some medicinal plants. Curr Sci 57:151–152
Zoubenko O, Hudak K, Tumer NE (2000) A non-toxic pokeweed antiviral protein mutant inhibits pathogen infection via a novel salicylic acid-independent pathway. Plant Mol Biol 44:219–229
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Prasad, V., Srivastava, S. (2017). Phytoproteins and Induced Antiviral Defence in Susceptible Plants: The Indian Context. In: Mandal, B., Rao, G., Baranwal, V., Jain, R. (eds) A Century of Plant Virology in India. Springer, Singapore. https://doi.org/10.1007/978-981-10-5672-7_28
Download citation
DOI: https://doi.org/10.1007/978-981-10-5672-7_28
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5671-0
Online ISBN: 978-981-10-5672-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)