Abstract
Tobacco plants (Nicotiana tabacum cv Samsun NN) have been transformed with the gene encoding the type-2 ribosome-inactivating protein (RIP) SNA-I′ from elderberry (Sambucus nigra) under the control of the Cauliflower Mosaic Virus 35S promoter. Previous research confirmed that these plants synthesize, correctly process and assemble a fully active RIP. Variability in protein expression was observed within the transgenic lines. The effects of the type-2 RIP SNA-I′ delivered through a leaf feeding assay were evaluated in the laboratory on two economically important pest insects belonging to the orders of Hemiptera, the tobacco aphid (Myzus nicotianae) and Lepidoptera, the beet armyworm (Spodoptera exigua). In the experiment with aphids, significant effects were observed on the life parameters, such as survival, intrinsic rate of increase, net reproductive rate, mean generation time and mean daily offspring, whereas with caterpillars significant reduction in fresh weight as well as retardation in development were observed. In addition, significant increases in mortality were noted for insects fed on the transgenic lines as compared to wild type plants. This information provides further support for RIPs having a role in plant resistance to insect pest species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant genetic engineering offers opportunities for the creation of insect-resistant plants by insertion and expression in plants of entomopathogenic proteins (Jouanin et al. 1998). Genetic engineering of crop plants has been developed as an alternative method to the use of chemical insecticides for protection against insect pests (Ranjekar et al. 2003). Transgenic plants currently commercialized have been engineered with a gene encoding a toxin from the bacterium Bacillus thuringiensis (Bt), which targets Lepidoptera, Coleoptera and Diptera.
Ribosome-inactivating proteins (RIPs) are a group of plant proteins, which possess highly specific rRNA N-glycosidase activity and are capable of catalytically inactivating eukaryotic ribosomes (Barbieri et al. 1993; Stirpe 2004). RIPs are classically divided into two categories. Type-1 RIPs are single chain proteins (approximately 30 kDa) with enzymatic activity whereas type-2 RIPs consist of an enzymatic A-chain which is linked through a disulfide bridge to a B-chain (approximately 35 kDa) with lectin properties. Lately a type-3 RIP has been identified in maize. This RIP is activated after proteolytic removal of a peptide segment with unknown function. It is now generally accepted that all RIPs are enzymes and that some of them have multiple enzymatic activities. RIPs display adenine polynucleotide glycosylase activity on different nucleic acid substrates, which at the ribosomal level is responsible for the arrest of protein synthesis (Peumans et al. 2001; Van Damme et al. 2001).
Because of their inability to bind to the cell surface type-1 RIPs cannot enter the cytoplasm, and consequently have a relatively low toxicity for cells and animals. Type-2 RIPs owe their carbohydrate binding activity to the B-chain, which contains two or possibly three binding sites (Frankel et al. 1996; Steeves et al. 1999). Most RIPs specifically recognize galactosyl terminated glycoproteins on the cell surface and as such facilitate the entry of the A-chain into the cell where it can exert its enzymatic activity on ribosomes or other cellular structures. Recently Baykal and Tumer (2007) have demonstrated that a conserved sequence at the C-terminus of the pokeweed antiviral protein (type-1 RIP) mediates its transport to the cytosol suggesting that type-1 and type-2 RIPs may use a common signal to enter the cytosol.
Many plants express several type-2 RIPs in seeds and/or vegetative tissues. Elderberry bark (Sambucus nigra) contains two NeuAc(α-2,6)Gal/GalNAc-specific agglutinins referred to as Sambucus nigra agglutinin I and I′. Both RIPs have been recognized as a type-2 RIPs and share 77% sequence similarity. SNA-I′ is a dimer of two [A-s-s-B] pairs. Compared to SNA-I, SNA-I′ lacks one cysteine residue in the B-chain, which is involved in the disulfide bridge formation between the B-chains of SNA-I (Van Damme et al. 1996, 1997).
RIPs have various degrees of toxicity both in vitro and in vivo (Nielsen and Boston 2001; Barbieri et al. 2004; Stirpe and Battelli 2006). Ricin, a RIP from castor beans (Ricinus communis), is highly cytotoxic, but RIPs from edible grains such as wheat (Triticum aestivum) and barley (Hordeum vulgare) have no reported toxicity in vivo (Stirpe et al. 1992). RIPs are widely distributed in the plant kingdom (Girbés et al. 2004), but the number of very toxic ones is restricted (Barbieri et al. 2004; Stirpe and Battelli 2006).
Insecticidal activity of type-2 RIPs has been demonstrated towards some insects (Gatehouse et al. 1990; Dowd et al. 1998, 2003; Wei et al. 2004). Although the enzymatic mechanism of RIP activity is well defined, the physiological steps by which ribosome inactivation leads to cell death are not well understood. In the case of ricin and mistletoe lectin I it was shown that RIP-treated cells exhibit the morphological features characteristic of apoptosis including condensation and fragmentation of cell nuclei, cytoplasmatic densification, breakdown of nuclear DNA into discrete fragments, and mitochondrial membrane alterations (Williams et al. 1997; Vervecken et al. 2000).
Tobacco plants (Nicotiana tabacum cv Samsun NN) transformed with the gene encoding the type-2 RIP SNA-I′ from S. nigra have previously been shown to synthesize, correctly process and assemble a fully active RIP (Chen et al. 2002b). In this project we evaluated if the constitutive expression of SNA-I′ in tobacco can enhance the plant’s resistance against pest insects. Hereto four transgenic lines (SNA-I′26103, SNA-I′26104, SNA-I′26106, SNA-I′26107) and wild type tobacco plants were exposed to the tobacco aphids (Myzus nicotianae) (Hemiptera: Aphididae) and the beet armyworm (Spodoptera exigua) (Lepidoptera: Noctuidae). M. nicotianae is among the most destructive and widely distributed pests. Serious damage results when a high number of piercing–sucking nymphs and adults remove plant sap from leaves and flower buds. As they feed, aphids also transmit plant viruses to their hosts and these viruses can then kill the plants. S. exigua is an important pest in numerous crops worldwide and originates from south eastern Asia. In northern Europe, it attacks glasshouse ornamentals and vegetables of great economic importance (Smagghe et al. 2003).
Materials and methods
Plants
Tobacco plants have been transformed with the gene encoding the type-2 RIP SNA-I′ from S. nigra. Plants express the SNA-I′ transgene under the control of the 35S promoter from the Cauliflower Mosaic Virus (Chen et al. 2002a). Four independent transgenic tobacco lines (SNA-I′26103, SNA-I′26104, SNA-I′26106, SNA-I′26107) and wild type (N. tabacum cv Samsun NN) plants were grown in a controlled growth chamber.
Insects
Beet armyworms (S. exigua) were kept in a growth chamber at 23 ± 2°C, 65 ± 5% relative humidity and a 16 h light: 8 h dark photoperiod, and the larval stages were reared on an agar-based artificial diet (Hakim et al. 2006). A continuous colony of the tobacco aphids (M. nicotianae) is maintained on tobacco plants (N. tabacum cv Samsun NN) at the Laboratory of Agrozoology, Ghent University, Belgium, under standardized conditions of 25 ± 2°C, 16 h light: 8 h dark photoperiod and 65 ± 5% relative humidity (Sadeghi et al. 2007).
Agglutination assays and estimation of lectin concentrations
Agglutination assays with trypsin-treated rabbit erythrocytes were performed in glass tubes by mixing 10 μl of 1 M ammonium sulfate with 10 μl of crude extract and 30 μl of a 2% solution of rabbit erythrocytes (made up in phosphate-buffered saline containing 137 mM NaCl, 8 mM Na2HPO4 · 2H2O, 3 mM KCl, 1.5 mM KH2PO4). To estimate the lectin content semi-quantitatively, 10 μl of extracts were serially diluted in 10 μl of 1 M ammonium sulfate with 2-fold increments and supplemented with 30 μl of a 2% suspension of trypsin-treated rabbit erythrocytes. The agglutination titre is defined as the highest dilution which still gave a visible agglutination. A dilution series of an SNA-I solution with known concentration purified from elderberry bark as described previously (Van Damme et al. 1996) was used as a control to calculate the absolute lectin content of the extracts. Agglutination was controlled visually after incubation at room temperature for 15 min. All experiments were performed three times taking care to use leaf material of approximately the same age.
Protein extraction and western blot analysis
Crude extracts were prepared by homogenizing N. tabacum leaves in 1.8 ml of 20 mM unbuffered 1,3-propane diamine per g of fresh weight. Homogenates were centrifuged (10 min; 13,000g) and the protein concentration determined using BIO-RAD protein assay kit (Bio-Rad, Hercules, CA, US). The supernatants were used immediately or frozen at −20°C. Approximately 50 μg of total protein and 0.15 μg of purified protein were reduced with 2-mercaptoethanol and separated by SDS-polyacrylamide gel electrophoresis (Laemmli 1970). After electrophoresis, gels were electroblotted on an Immobilon-P membrane (Millipore, Bedford, MA, US) using a semi dry blotting system Multiphor II (Amersham Pharmacia Biotech, Uppsala, Sweden). Before immunodetection the free binding sites on the membrane were blocked with 5% (w/v) bovine serum albumin (BSA) in Trissaline [10 mM Tris–HCl, 150 mM NaCl, 0.1% (v/v) Triton X-100, pH 7.6] for 1 h at room temperature. The membrane was washed with Tris saline for 5 min. Subsequently, the membrane was incubated with primary antibody for 1 h, goat-anti-rabbit antibody for 1 h and finally peroxidase-anti-peroxidase for 1 h at room temperature. Just before the immunodetection the membrane was washed for 5 min with 0.1 M Tris–HCl (pH 7.6). The membrane was placed in detection buffer [0.1 M Tris–HCl pH 7.6 containing 0.7 mM 3,3-diaminobenzidine teterahydrochloride and 0.01% (v/v) H2O2]. The primary antibody directed against native SNA-I was raised in rabbits as reported previously (Chen et al. 2002a).
Bioassay with aphids (Myzus nicotianae)
The effects of feeding M. nicotianae on four transgenic lines and wild type tobacco plants were tested using bioassays in growth chamber conditions, essentially as reported by Sadeghi et al. (2007). In brief the test was conducted on detached leaves in Petri dishes (90 mm diameter). In the lids one hole was drilled for ventilation and covered by a net cloth. One randomly selected apterous female from the stock culture was transferred to an excised tobacco leaf (from 3 month old plants) placed upside down on wet cotton wool. Thirty young aphids or neonates (aged 0–24 h) were selected and placed individually per Petri dish. This day is considered as day 0. The cotton in the Petri dishes was wetted daily and every 2 days the aphids were transferred to a new tobacco leaf. Nymphal development and survival were scored daily. The presence of exuvia was used to determine moulting. The test was followed for 35 days, by which time no more nymphs were being produced and daily reproduction of all individual adult aphids was recorded. At each daily count, newly deposited nymphs were removed with minimal disturbance to the adult. The experiment was performed two times independently from each other.
Bioassay with caterpillars (Spodoptera exigua)
An experimental cage was constructed using polyethylene containers (9 cm in diameter, 3 cm in height). Six airholes (5 mm in diameter) were drilled in the wall of the container and covered by net cloth for ventilation. In addition, one channel (5 mm in diameter) was drilled to place the leaf petiole that was placed in water. The top of the cup was covered with a piece of the same polyethylene (Van de Veire et al. 1997).
Detached leaves of transgenic tobacco plants expressing RIPs or control tobacco plants were placed in the experimental cages. The bioassay was started with second instar (0–6 h old) larvae of S. exigua with 3 larvae per cage and 10 cages per transgenic line or control plant. Larvae were fed on freshly excised leaves, and cages were cleaned from the excrements daily. Mortality and individual larval fresh weights were followed at 1–2 day-intervals until pupation. In addition to weight gain, development of treated larvae into pupae and adults was followed. The experiment was performed two times independently from each other.
Larval growth and development were measured using a growth index (G.I.), being the ratio of the percentage of larvae that pupated and the larval developmental period in days: G.I. = % pupation/larval period, after Khan and Saxena (1985).
Life table analysis
Four transgenic plants (SNA-I′26103, SNA-I′26104, SNA-I′26106, SNA-I′26107) and the non-transformed wild type tobacco were used for insect feeding. In this paper, the intrinsic rate of increase (r m) is estimated by using iterative bisection method from \( \sum\nolimits_{x = 0}^{\infty } e^{ - r(x + 1)} l_{x} m_{x} = 1 \), with age indexed from 0 (Birch 1948). Finite rate of increase (λ) is a multiplication factor of the original population at each time period, and is calculated based on the equation λ = erm. The age-specific survival rate (L x ; where x = age), the age-specific fecundity (M x : offspring/female/day), and the population parameters (r, the intrinsic rate of increase; R 0, the net reproductive rate; T, the mean generation time) were calculated accordingly; T = ln (R 0)(r m)−1. R 0 was calculated according to the formula, R 0 = ∑ L x M x . Prereproductive time d is the mean number of days from aphid birth to reproduction. The doubling time DT is the time required by the aphid population to double its size, and comes from the standard definition of r m as: DT = ln 2(r m)−1, as used by Deloach (1974). Mean r m values presented in Table 2 are the group values; standard errors were calculated using a jackknife estimator by successive suppression of one individual from group data (Miller 1974). A special program, visual Basic Application version 6.3 in MS-Excel (VBA-macro) was used in order to calculate standard errors for r m (Hulting et al. 1990).
Data analysis
Statistical differences of mean values were analysed by analysis of variance (ANOVA) and significantly different means were separated by a post hoc Tukey test in SPSS v15 (SPSS, Chicago, IL, US). Survival analyses were performed using the standard comparison test of Wilcoxon in SPSS v15. Differences between treatments were considered significant at P = 0.05.
Results
Expression of SNA-I′ in transgenic tobacco plants
Semi-quantitative analyses of SNA-I′ expression in transgenic plants were performed by agglutination assays and revealed a clear agglutination activity in all extracts except those from control plants. The lectin concentration was estimated 21–166 μg/g fresh leaves (Table 1).
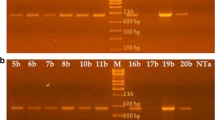
Western blot analysis of crude leaf extracts from transgenic tobacco plants confirmed that SNA-I′ was expressed efficiently from the introduced gene under the control of the CaMV 35S promoter. According to Van Damme et al. (1997) SNA-I′ migrates as two polypeptides of 32 and 35 kDa or a single 60 kDa band in SDS/PAGE under reducing and non-reducing conditions, respectively. Our western blot analyses of crude leaf extract confirmed that the transgenic lines SNA-I′26103, SNA-I′26104 and SNA-I′26106 express polypeptides of approximately 30 kDa that react with antibodies against SNA-I (Fig. 1). Surprisingly, transgenic line SNA-I′26107 yielded a slightly different pattern in that two polypeptides of 30–34 kDa were observed on the western blot. No reacting bands were observed when leaf extracts from untransformed plants were probed with anti-SNA-I antibody.
Western blot analysis of the leaf extracts from the transgenic and untransformed control plants. Lane 1, 0.1 μg of purified SNA-I; Lane 2, Wild type plants (Control); Lane 3–6, crude protein extracts from four transgenic tobacco lines: SNA-I′26103, SNA-I′26104, SNA-I′26106 and SNA-I′26107, respectively; Lane 7, protein marker. Fifty micrograms of crude protein of transformed and untransformed plants were denatured and reduced with 2-mercaptoethanol, and loaded in each lane
Effects on nymphal and adult survival of M. nicotianae
Neonate nymphs (aged 0–24 h) were tested to determine the effect of the type-2 RIP SNA-I′ on nymphal and adult survival for 8 (normal developmental time to adulthood) and 35 days (corresponding to tobacco aphid lifespan), respectively. No effect on survival was observed in nymphal stage for any transgenic line expressing SNA-I′. Compared to non-transformed plants, transgenic lines showed a significant level of reduction in adult aphid survival using Wilcoxon statistics (P < 0.05). In transgenic lines survival reduction started after 6–9 days of adult formation, whereas in the controls this decline started after 13–15 days of adult life (Fig. 2). By day 27, survival of the adult aphids fed on transgenic lines SNA-I′26103, SNA-I′26104, SNA-I′26106 and SNA-I′26107 was reduced significantly (P < 0.05). It is worthy noting that no aphid survival was observed on transgenic lines SNA-I′26103, SNA-I′126104 and SNA-I′26106 after 33 days or 34 days for line SNA-I′26107, whereas in the control more than 35% of aphids were still alive after 34 days.
Life history trails in M. nicotianae
The effects of the four transgenic lines expressing the type-2 RIP SNA-I′ were tested on the development and fecundity of M. nicotianae. The mean duration of the prereproductive period, that is the period from time of birth until onset of reproduction, was significantly affected for aphids fed on transgenic plants SNA-I′26106, SNA-I′26103 and SNA-I′26104 compared to control plants (P < 0.05) (Table 2). A significant decrease was observed for the mean daily offspring of the aphids fed on SNA-I′ plants due to a decrease in mean number of new born nymphs over the assay period. Transgenic lines SNA-I′26106 and SNA-I′26103 showed the strongest effects, followed by SNA-I′26104. In contrast SNA-I′26107 did not negatively affect the offspring. Directly linked with a lower daily fecundity, the intrinsic rate of increase (r m) was also significantly lower in aphids fed on transgenic lines SNA-I′26106, SNA-I′26103 and SNA-I′26104 compared to control plants (P < 0.05). Significant differences were observed between transgenic lines: aphids fed on line SNA-I′26106 showed a lower intrinsic rate of increase than aphids fed on SNA-I′26104 and SNA-I′26107 (P < 0.05). Also aphids fed on transgenic line SNA-I′26103 showed a lower intrinsic rate of increase than those fed on transgenic line SNA-I′26107 (P < 0.05). As a consequence of a lower intrinsic rate on transgenic lines SNA-I′26106, SNA-I′26103 and SNA-I′26104, the finite ratio of increase (λ), that is the number of aphids added to the population per adult that will produce adults was also lower and the doubling times of the population were conspicuously increased (Table 2).
Figure 3 shows the reproductive performance of the aphids as the mean total numbers of nymphs born per adult every single day for the entire reproductive period (R 0 = ∑ L x M x ). Over this period there was a significant effect of transgenic plants on cumulative fecundity and poorer fecundity was observed on aphids fed on transgenic lines SNA-I′26106 and SNA-I′26103 followed by line SNA-I′26104, compared to the ones in the control groups (P < 0.05). By day 32, cumulative nymph production in the transgenic lines SNA-I′26106, SNA-I′26103 and SNA-I′26104 was observed to be 24, 19 and 18% lower than in the control, respectively (Fig. 3). The mean total fecundity of aphids fed on transgenic lines SNA-I′26106, SNA-I′26103 and SNA-I′26104 was significantly lower than that of aphids in the control (P < 0.05) (Table 2). In contrast, line SNA-I′26107 had no remarked negative effects in aphids.
Toxicity effects on S. exigua
Larval and pupal mortality were measured and a high mortality was observed with transgenic plants as compared to the wild type plants (Table 3). Compared with larvae fed on control plants, the respective percentages of larvae and pupae dropped 28% and 20% on line SNA-I′26103, 17% and 24% on line SNA-I′26107, and 17% and 16% on line SNA-I′26104. For transgenic line SNA-I′26106 no significant reduction in numbers of larvae and pupae was observed compared to the wild type control.
Effects on growth and development in S. exigua
The sublethal effects of the type-2 RIP SNA-I′ on growth and development of caterpillars was followed over a period of 23 days. In feeding bioassays with second instar S. exigua larvae significant weight reduction (P < 0.05) was observed already after 2 days feeding on transgenic line SNA-I′26107 (50%) and after 6 days on lines SNA-I′26103 and SNA-I′26104 (37–40%) compared to the wild type control, and this reduction continued for the entire experiment (Fig. 4a). After 10 days, the fresh weight of S. exigua larvae fed on transgenic lines SNA-I′26107 (59%), SNA-I′26103 (35%), and SNA-I′26104 (30%) was significantly reduced (P < 0.05) as compared to the wild type plants (Fig. 4b). Larvae fed on line SNA-I′26106 did not show any weight reduction compared to wild type control (P > 0.05).
In planta bioassay of beet armyworm Spodoptera exigua on transgenic lines expressing SNA-I′. The inhibitory effect of transgenic lines SNA-I′26103, SNA-I′26104, SNA-I′26106 and SNA-I′26107 on larval weight gain is expressed as means ± SE after feeding for 2, 4 and 6 days (a) and for 8 and 10 days (b). ANOVA resulted in 2 groups for day 2 (F = 5.312; df = 54; P = 0.001), day 4 (F = 2.640; df = 49; P = 0.046) and day 8 (F = 6.592; df = 59; P < 0.001), and in 3 groups for day 6 (F = 6.920; df = 43; P < 0.001) and day 10 (F = 8.055; df = 48; P < 0.001), and was followed by a post-hoc Tukey test to separate means at P = 0.05
Together with weight reduction, the caterpillars fed on leaves of transgenic lines SNA-I′26103, SNA-I′26104 and SNA-I′26107 showed symptoms of retardation in larval development. At day 13, 100% of the living larvae on wild type tobacco plants were in the fifth instar (Fig. 5). In contrast, when larvae were fed on transgenic lines SNA-I′26103, SNA-I′26107 and SNA-I′26104 only 50, 53 and 72% of them were scored in the fifth instar. With SNA-I′26106, there was no effect.
When considering the whole life cycle including larval, pupal and adult development, higher rates of growth indices were obtained with larvae fed on control plants compared to those fed on transgenic plants expressing SNA-I′, except for SNA-I′26106 (Table 3). The time for development from the second instar larvae to pupa, the weight of pupae and the adult emergence were recorded. Larval development was significantly retarded on transgenic lines SNA-I′26107, SNA-I′26103 and SNA-I′26104 (P < 0.05), and the pupal weight was significantly reduced (P < 0.05) (Table 3). With SNA-I′26106 there was no effect as compared to wild type control plants. Likewise, adult emergence rates were highest (97%) in the control, but in larvae fed on transgenic lines significantly lower rates of adult emergence were observed.
Discussion
In recent years there has been much interest in the potential of lectins in crop protection via genetic manipulation and some potent insecticidal effects of lectins have been introduced against pest insects. Chen et al. (2002b) have shown that the type-2 RIP SNA-I′ can successfully be expressed in tobacco and does not affect the growth and fertility of the transgenic plants.
From the results of the western blot it is clear that SNA-I′ accumulated in the leaf tissues of the transgenic plants. It is striking that transgenic line SNA-I′26107 yields a different pattern. This result may be due to incomplete processing of the linker sequence between the A and B chain. Alternatively, this result could also be due to altered glycosylation of one of the two chains. The levels of SNA-I′ vary between lines, reflecting variability between the primary transformants from which the lines are clonally propagated. The observed variability in the levels of expression can be due to different genetic environments as transgenes are randomly inserted in the plant chromosomes. Another factor is that the number of copies of the transgene inserted into the plant genome will vary between transformants. It should be noted that there is no clear correlation between the amount of RIP in the plant (as shown on the western blot, Fig. 1) and the amount of lectin that was calculated based on agglutination assays (Table 1). Possibly not all of the RIP molecules synthesized in the transgenic lines are active.
This study showed that M. nicotianae fed on detached leaves of transgenic lines expressing SNA-I′ reduced adult survival and it was apparent that the type-2 SNA-I′ influenced aphid development by retardation in the onset of reproduction. The fertility parameters of these aphids were also reduced by feeding on transgenic plants compared to control plants. Feeding on transgenic lines also significantly reduced the intrinsic rate of increase of tobacco aphids. In addition, there is an effect of SNA-I′ on the longevity of aphids, since the decline in survival occurs more rapidly after feeding on SNA-I′ expressing plants. Thus, our results clearly demonstrated that aphids fed with transgenic tobacco plants have a lower population size than insects reared on non-transformed plants. The effects of SNA-I′ on S. exigua were shown by a reduction in survival and in the weight of larvae and pupae. Furthermore retardation in development was observed in larvae fed on transgenic plants.
In the present study we could demonstrate the insecticidal activity of SNA-I′ from elderberry against tobacco aphids and beet armyworm caterpillars. It is known that SNA-I′ exhibits an exclusive specificity towards NeuAc(α-2,6)Gal/GalNAc disaccharide (Van Damme et al. 1997). It has been shown that the B-chain of most type-2 RIPs binds to galactosyl-terminated receptors on the surface of the exposed cells, thus allowing entry of the A-chain inside the cell, where it inactivates ribosomes with consequent arrest of protein synthesis and death of the cell. It is evident that the carbohydrate-binding specificity of the B-chain of the type-2 RIPs is the determining factor in the recognition of the target cells (Stirpe and Battelli 2006). It should be noted that the toxicity of type-2 RIPs varies considerably between different RIPs (Barbieri et al. 2004). Fortunately for SNA-I′ this type-2 RIP is not as toxic as ricin which is extremely toxic to animals. Similar to previously reported insecticidal lectins (Fitches and Gatehouse 1998; Sauvion et al. 2004), we can speculate that SNA-I′ may be detrimental towards the insect midgut epithelium that forms the first barrier after feeding by the insect, and as such can be responsible for the observed insecticidal effects.
To date not much is understood on the mechanisms and the potential targets in the insect’s body for RIPs. The aim of our study was to test the activity of SNA-I′ towards insects. We can confirm that two out of the four lines tested showed insecticidal activity in the two pest insects tested, i.e. SNA-I′26103 and SNA-I′26104, and these transgenic lines showed lectin amounts of 21.4 and 166.5 μg/g FW, respectively. Furthermore, it was of interest that in this project striking differences were observed for two SNA-I′ lines (SNA-I′26106 and SNA-I′26107 with respective lectin amounts of 30.2 and 39.8 μg/g FW) towards the two different insect pest species tested: i.e. no toxicity was observed for SNA-I′26107 in Myzus aphids, and for SNA-I′26106 in Spodoptera caterpillars. To help in explaining the discrepancies in response, it is worthy to mention that the beet armyworm, S. exigua, consumes the whole leaf, whereas M. nicotianae, as all aphids, only ingests plant fluids. Also it should be mentioned that the level of expression in the leaf does not necessarily reflect the actual level of SNA-I′ present in the plant fluids. Hence it is possible that the SNA-I′ expression level in the phloem is not the same for the different transgenic lines. Different gut structures may also underlie the diverse effects of SNA-I′ in these two insect pest species. The peritrophic matrix is an anatomical structure that envelopes the food bolus in the majority of insects, including Lepidoptera. In contrast, aphids like Hemipterans are characterized by the absence of the peritrophic membrane (Silva et al. 2004). There are also considerable variations in stomach pH. Whereas the pH in the stomach of aphids has been estimated to be 5.0–6.0 (Deraison et al. 2004), the pH in the midgut of S. exigua is much more alkaline with values of >8 (own unpublished results). In addition, a potential reason for the different toxicity can also be in the binding to cells, entry into the cytoplasm, intracellular routing and degradation of the protein. Although we can postulate different hypotheses, further research has to indicate the crucial processes that are responsible for a different toxicity of SNA-I′ in aphids and caterpillars.
To our knowledge the present results show for the first time that apterous females of M. nicotianae and S. exigua larvae and pupae are affected by the type-2 RIP SNA-I′ through feeding on transgenic tobacco leaves. Although several studies have been performed to demonstrate the activity of RIPs as an alternative insecticide on some insect species, only limited information is available with regard to toxicity of RIPs to insect larvae. Recently Dowd et al. (2006) reported significant feeding reduction and larval mortality in two Lepidoptera species, Helicoverpa zea and Lasioderma serricorne by feeding on transgenic tobacco plants expressing maize RIP. Also in a previous study they found significant mortality on Carpophilus freemani with proenzyme and protease-activated forms of a maize seed RIP (b-32) and 70% mortality in Trichoplusia ni by a protease-activated form (Dowd et al. 1998). RIPs from seeds of dicot plants such as castor bean (R. communis), soapwort (Saponaria officinalis) and bitter gourd (Momordica cochinchinensis) were shown to be toxic towards beetles but not caterpillars (Gatehouse et al. 1990).
The present study shows deleterious effects of the type-2 RIP SNA-I′ on two important pest insects, the tobacco aphid M. nicotianae, and the beet armyworm S. exigua, in small-scale trials carried out under controlled conditions. In addition to significant increases in mortality we noted strong developmental effects, reducing the insect populations on the transgenic lines compared to wild type plants. We believe this information provides further support for RIPs having a role in plant resistance to insect pest species. However before claiming firm conclusions, trials under more field related conditions are needed to verify the applicability of SNA-I′ in the control of pest insects.
References
Barbieri L, Battelli MG, Stirpe F (1993) Ribosome-inactivating proteins from plants. Biochem Biophys Acta 1154:237–282
Barbieri L, Ciani M, Girbes T, Liu WY, Van Damme EJM, Peumans WJ, Stirpe F (2004) Enzymatic activity of toxic and non-toxic type 2 ribosome-inactivating proteins. FEBS Lett 563:219–222
Baykal U, Tumer NE (2007) The C-terminus of pokeweed antiviral protein has distinct roles in transport to the cytosol, ribosome depurination and cytotoxicity. Plant J 49:995–1007
Birch LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17:15–26
Chen Y, Peumans WJ, Van Damme EJM (2002a) The Sambucus nigra type-2 ribosome-inactivating protein SNA-I′ exhibits in planta antiviral activity in transgenic tobacco. FEBS Lett 516:27–30
Chen Y, Vandenbussche F, Rougé P, Proost P, Peumans WJ, Van Damme EJM (2002b) A complex fruit-specific type-2 ribosome-inactivating protein from elderberry (Sambucus nigra) is correctly processed and assembled in transgenic tobacco plants. Eur J Biochem 269:2897–2906
Deloach CJ (1974) Rate of increase of population of cabbage, green peach, and turnip aphids at constant temperatures. Ann Entomol Soc Am 67:332–340
Deraison C, Darboux I, Duportets L, Gorojankina T, Rahbé Y, Jouanin L (2004) Cloning and characterization of a gut-specific cathepsin L from the aphid Aphis gossypii. Insect Mol Biol 13:165–177
Dowd PF, Mehta AD, Boston RS (1998) Relative toxicity of the maize endosperm ribosome-inactivating protein to insects. J Agric Food Chem 46:3775–3779
Dowd PF, Zou WN, Gillikin JW, Johnson ET, Boston RS (2003) Enhanced resistance to Helicoverpa zea in tobacco expressing an activated form of maize ribosome-inactivating protein. J Agri Food Chem 51:3568–3574
Dowd PF, Rober AH, Pinkerton TS, Johnson ET, Lagrimini LM, Boston RS (2006) Relative activity of a tobacco hybrid expressing high levels of a tobacco anionic peroxidase and maize ribosome-inactivating protein against Helicoverpa zea and Lasioderma serricorne. J Agr Food Chem 54:2629–2634
Fitches E, Gatehouse JA (1998) A comparison of the short and long term effects of insecticidal lectins on the activities of soluble and brush border enzymes of tomato moth larvae (Lacanobia oleracea). J Insect Physiol 44:1213–1224
Frankel AE, Burbage C, Fu T, Tagge E, Chandler J, Willingham MC (1996) Ricin toxin contains at least three galactose-binding sites located in B chain subdominants 1α, 1β, and 2γ. Biochemistry 35:14749–14756
Gatehouse AMR, Barbieri L, Stirpe F, Croy RRD (1990) Effects of ribosome-inactivating proteins on insect development differences between Lepidoptera and Coleoptera. Entomol Exp Appl 54:43–51
Girbés T, Ferreras JM, Arias FJ, Stirpe F (2004) Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini Rev Med Chem 4:461–476
Hakim RS, Blackburn M, Corti P, Gelman D, Goodman C, Elsen K, Loeb M, Lynn D, Smagghe G (2006) Growth and mitogenic effects of arylphorin in vivo and in vitro. Arch Insect Biochem Physiol 64:63–73
Hulting FL, Orr DB, Obrycki JJ (1990) A computer program for calculation and statistical comparison of intrinsic rates of increase and associated life table parameters. Fla Entomol 73:601–612
Jouanin L, Bonade-Bottino M, Girard C, Morrot G, Giband M (1998) Transgenic plants for insect resistance. Plant Sci 131:1–11
Khan ZR, Saxena RC (1985) Behavioral and physiological responses of Sogatella furcifera (Homoptera: Delphacidae) to selected resistant and susceptible rice cultivars. J Econ Entomol 78:1280–1286
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Miller RG (1974) The jackknife-a review. Biometrika 61:1–15
Nielsen K, Boston RS (2001) Ribosome-inactivating proteins: a plant perspective. Annu Rev Physiol Plant Mol Biol 52:785–816
Peumans WJ, Hao Q, Van Damme EJM (2001) Ribosome-inactivating proteins from plants: more than RNA N-glycosidases? FASEB J 15:1493–1506
Ranjekar PK, Patankar A, gupta V, Bhatnagar R, Bentur J, Kumar PA (2003) Genetic engineering of crop plants for insect resistance. Curr Sci 84:321–329
Sadeghi A, Broeders S, De Greve H, Hernalsteens JP, Peumans WJ, Van Damme EJM, Smagghe G (2007) Expression of garlic leaf lectin under the control of the phloem-specific promoter Asus1 from Arabidopsis thaliana protects tobacco plants against the tobacco aphid (Myzus nicotianae). Pest Manag Sci 63:1215–1223
Sauvion N, Nardon C, Febvay G, Gatehouse AM, Rahbé Y (2004) Binding of the insecticidal lectin Concanavalin A in pea aphid, Acyrthosiphon pisum (Harris) and induced effects on the structure of midgut epithelial cells. J Insect Physiol 50:1137–1150
Silva CP, Silva JR, Vasconcelos FF, Petretski MDA, DaMatta RA, Ribeiro AF, Terra WR (2004) Occurrence of midgut perimicrovillar membranes in paraneopteran insect orders with comments on their function and evolutionary significance. Arthropod Structure Dev 33:139–148
Smagghe G, Pineda S, Carton B, Del Estal P, Budia F, Viñuela E (2003) Toxicity and kinetics for methoxyfenozide in greenhouse-selected Spodoptera exigua (Lepidoptera: Noctuidae). Pest Manag Sci 59:1203–1209
Steeves RM, Denton ME, Barnard FC, Henry A, Lambert JM (1999) Identification of three oligosaccharide binding sites in ricin. Biochemistry 38:11677–11685
Stirpe F (2004) Ribosome-inactivating proteins. Toxicon 44:371–383
Stirpe F, Battelli MG (2006) Ribosome-inactivating proteins: progress and problems. Cell Mol Life Sci 63:1850–1866
Stirpe F, Barbieri L, Batelli MG, Isoria M, Lappi D (1992) Ribosome-inactivating proteins from plants: recent status and future prospects. BioTechnology 10:405–412
Van Damme EJM, Barre A, Rougé P, Van Leuven F, Peumans WJ (1996) The NeuAc (α–2, 6)-GalNAc-binding lectin from elderberry (Sambucus nigra) bark, a type-2 ribosome-inactivating protein with an unusual specificity and structure. Eur J Biochem 235:128–137
Van Damme EJM, Roy S, Barre A, Citores L, Mostafapous K, Rougé P, Van Leuven F, Girbes T, Goldstein IJ, Peumans WJ (1997) Elderberry (Sambucus nigra) bark contains two structurally different Neu5Ac(α2, 6)Gal/GalNAc-binding type-2 ribosome-inactivating proteins. Eur J Biochem 245:648–655
Van Damme EJM, Hao Q, Chen Y, Barre A, Vandenbussche F, Desmyter S, Rougé P, Peumans WJ (2001) Ribosome-inactivating proteins: a family of plant proteins that do more than inactivate ribosomes. Crit Rev Plant Sci 20:395–465
Van de Veire M, Smagghe G, Degheele D (1997) Laboratory test method to evaluate the effect of 31 pesticides on the predatory bug, Orius laevigatus (Het: Anthocoridae). Entomophaga 41:235–244
Vervecken W, Kleff S, Pfuller U, Bussing A (2000) Induction of apoptosis by mistletoe lectin I and its subunitsNo evidence for cytotoxic effects caused by isolated A- and B-chains. Int J Biochem Cell Biol 32:317–326
Wei GQ, Liu RS, Wang Q, Liu WY (2004) Toxicity of two type II ribosome-inactivating proteins (cinnamomin and ricin) to domestic silkworm larvae. Arch Insect Biochem Physiol 57:160–165
Williams JM, Lea N, Lord JM, Roberts LM, Milford DV, Taylor CM (1997) Comparison of ribosome-inactivating proteins in the induction of apoptosis. Toxicol Lett 91:12–127
Acknowledgements
Sh. Shahidi-Noghabi is recipient of a doctoral grant from the Special Research Council of Ghent University. This research is also supported by project 3G016306 to G. Smagghe and E.J.M. Van Damme from the Fund of Scientific Research (FWO-Vlaanderen, Brussels, Belgium).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahidi-Noghabi, S., Van Damme, E.J.M. & Smagghe, G. Expression of Sambucus nigra agglutinin (SNA-I′) from elderberry bark in transgenic tobacco plants results in enhanced resistance to different insect species. Transgenic Res 18, 249–259 (2009). https://doi.org/10.1007/s11248-008-9215-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-008-9215-2