Abstract
Siderophores are tiny organic molecules secreted by microbes in iron-restricted environment to make iron available for its functioning and growth. Microorganism synthesizes siderophores in order to convert iron from insoluble form (Fe2+) to soluble one (Fe3+) for their uptake inside the intracellular environment. Siderophores have high affinity towards ferric iron (Fe3+) resulting in the formation of iron-siderophore complexes followed by transportation inside the microbial cell where ferric form of iron (Fe3+) is reduced to ferrous form of iron (Fe2+) in order to make iron accessible to microbes. In recent studies, siderophores have gained much attention due to their potentiality in variety of field such as ecology, agriculture (plant growth), biosensing and medicines. Metals are important for all plants and microbes and also play a crucial role between infecting microbes and their hosts as in microbial pathogenesis, where their metal uptake is restricted by the host organism which affects the microbial growth and virulence. The metals such as iron, zinc, copper, chromium, gallium and manganese interact with the siderophore produced by fungi to make stable siderophore-metal complexes resulting in homeostasis regulation. This chapter focuses on various types of metal-siderophore complexes and their binding sites and functionalities along with their biomedical applications. The efforts have been made to highlight the basic understanding of metal coordination with siderophore produced by fungal pathogens which still has immense scope to investigate further in respect to other metals and specific adaptations towards individual species which leads to the development of novel antifungal strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
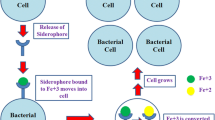
Plants and microbes have vast significance in our day-to-day life. Iron is considered to be an abundant element in the soil (earth’s crust) and essential for all life processes such as respiration, DNA synthesis, tricarboxylic acid cycle and production of various small molecules like amino acids, lipids, and sterols. Being an essential element in earth’s crust, the bioavailability of iron is limited in the habitat (soil and sea) owing to its low solubility. This property of iron results in its poor uptake by plants, which eventually makes iron an essential nutrient for plant growth. In aqueous and oxygenated conditions, the iron is found in its supreme state, which accumulates in the form of minerals such as iron oxides and iron hydroxides and is not ready to be utilized as such by organisms. In order to overcome this restricted process, the microbial flora of soil such as Pseudomonas spp., Enterobacter genus, Bacillus spp. produces special iron-binding tiny carriers, called ‘siderophore’, that help to scavenge iron from these mineral phases (oxides and hydroxides) by forming soluble iron (Fe3+) complexes which are readily taken up by the environment through active transport mechanism (Philpott 2006).
Siderophore came from Greek words sidero that means ‘iron’ and phore that means ‘carriers’, and in combination, it is termed as ‘iron carrier’. Siderophores are small, low-molecular-weight (<10 kDa) iron-chelating compounds, secreted by plants and microorganisms (bacteria and fungi) to maintain their iron requirement. These are also produced by rhizospheric bacteria in iron-limiting conditions in order to increase the plant growth by scavenging iron from the environment and make it available to the cell near the plant roots (Sah and Singh 2015; Li et al. 2016).
7.2 Siderophore-Mediated Iron Transport
Iron transport in siderophore is an energy-dependent mechanism. Type and stereoselectivity of siderophore are specific factors in recognition and transport of iron-siderophore complexes in microbes. The complexation also depends on metal ion coordination geometry as well as N-acyl residues present at the periphery of central metal ion. For instance, the coordination of metal centre and configuration of ligand affect the stability of complex. In case of Rhodotorula pilmanae , configuration of macrocyclic rings of siderophore is favoured, whereas in contrast to this, in Penicillium parvum , Neurospora crassa and Aspergillus quandricinctus , L-cis-ferrichrome is found to be a stable configuration. Further, the geometrical stability of complex also depends on the types and number of N-acyl residues surrounding the iron coordination centre (Huschka et al. 1986).
In spite of having specific transport mechanism of siderophore, many microbes may utilize multiple transport system as well as more than one type of siderophore at a time for efficient transport of metal ion. For example, microorganism like Agaricus bisporus has variable transport systems for fusarinines and ferrichromes, whereas Neurospora crassa represents different recognition sites for coprogen- and ferrichrome-type siderophore system (Howard 1999).
In fungal species, majority of literature suggested that Saccharomyces cerevisiae has two high-affinity iron transport mechanism. In first reductive mechanism, ferric iron (Fe3+) is identified to be reduced by a number of inducible membrane-bound reductase, namely, Fre1p–4p, usually present at cell surface. Across plasma membrane, the reduced iron (Fe2+) is then transported by involvement of permease-oxidase, namely, Ftr1p and Fet3p. This mechanism is utilized by variety of Fe3+-siderophore complexes such as ferrichrome, triacetyl fusarinine C and rhodotorulic acid for their transportation after being reduced by cell-surface-bound reductase. On the other hand, the second mechanism involves the uptake of iron (Fe3+)-siderophore complex as an intact form into the cell. All the proteins that are involved in the transport of so far identified complexes by this type of mechanism belong to major facilitator superfamily, namely, Sit1p (also known as Arn3p which helps in transporting ferrioxamine B, ferrichrome and ferrichrome A), Arn1p (helps in transporting ferrirubin, ferrirhodin and ferrichrome A), Taf1 (also known as Arn2p, which helps in transporting triacetyl fusarinine C) and Enb1p (helps in transporting enterobactin). The uptake specificity may vary among receptors as well as among strains (Renshaw et al. 2002). For instance, Arn1p specifically transports ferrichrome-type siderophores around the iron centre which have branched-chain ornithine-N5-acyl residues but does not support the short-chain acetyl hydroxamic residues present in siderophore such as ferrirubin and ferrichrome. Likewise, Arn2p is found to specifically transport triacetyl fusarinine C, whereas Sit1p has been found to be less specific. Arn1p and Sit1p transport the complex via cell surface and are then rapidly internalized as both are localized in intracellular vesicle layers (Seneviratne and Vithanage 2015). Table 7.1 represents the list of siderophore transport supported by different receptors.
7.3 Fungal Iron Regulation
In fungi, regulation of iron uptake is necessary to maintain iron homeostatic processes. For this, four different mechanisms of iron uptake have been suggested in fungi at molecular level by different mechanistic-based studies (Haas 2014). These four mechanisms involve (a) ferric iron (Fe3+) uptake through siderophores, (b) reductive iron assimilation, (c) heme uptake and d) direct iron uptake. These four mechanisms are explained as follows.
7.3.1 Iron (Fe3+) Uptake Through Siderophore
Each and every fungal species exhibit siderophore-iron transporter (SIT)-mediated extracellular iron uptake mechanism. SIT constitute majorly facilitator protein family, which acts as a proton-coupled symporters potentiated by plasma membrane. In addition, high solubility and high energy factors render iron-chelated siderophore to combat during microbial growth. On the other hand, triacetyl fusarinine (TAFC) and fusarinine C (FsC) facilitate intercellular release of iron by partial hydrolysis by esterase (Estb) enzyme (Howard 1999).
7.3.2 Reductive Iron Assimilation (RIA)
In fungi, iron acquisition with high affinity is usually achieved either by secreted siderophores (iron chelators) or by reductive iron assimilation (RIA) mechanisms (Fatima et al. 2017). In order to start iron uptake, iron is first reduced from ferric (Fe3+) to more soluble ferrous (Fe2+) form by localized ferrireductases present in the plasma membrane of fungal species. Soon after this, the ferrous iron is re-ionized which is further imported by protein complex consisting of iron permease (FtrA) and ferrioxidase (Fetc) genes. The protein such as permease also transports metals other than iron such as copper and zinc.
7.3.3 Heme Uptake
In contrast to bacteria, binding and uptake of iron, in fungi, are done only with heme component. For instance, Candida albicans heme uptake mechanism involves the glycosylphosphatidylinositol (GPI)-anchored cell surface mannoprotein, namely, Rbt5P, but the details regarding its transport mechanism are still unknown. In order to utilize heme-iron complex, the uptake requires intercellular degradation of heme with heme oxygenase (Hmx1p, localized in endoplasmic reticulum).
7.3.4 Low-Affinity Iron Uptake
The iron in the form of ferrous (Fe2+) is taken up by permease Fet4p. The system is non-specific for Fe2+ form of iron as it also transports other metals such as zinc and copper. In Saccharomyces cerevisiae , the iron supply is associated with mobilization of iron from vacuole which is facilitated by fluid-phase endocytosis and Smf1p protein belonging to natural resistance-associated macrophage protein (NRAMP) family (Kosman 2003).
All the four iron regulation mechanisms are pictorially represented in Fig. 7.1.
7.4 Types of Siderophore
Chemically based on the interaction sites, siderophore has been categorized into two main groups, namely, first ‘enterobactin’, the strongest iron chelator which shows interaction between iron and catecholate hydroxy groups, and second ‘hydroxamate’ that acquires N-hydroxylated amide bonds (e.g. ferrichromes in fungi). Atoms like nitrogen (N), oxygen (O) and sulphur (S) can also participate in the coordination of iron in carboxylate groups (Drechsel et al. 1995; Butler and Theisen 2010). Mixed type of siderophores involves those donating groups that do not belong to hydroxamates or aromatic hydroxy group category. The description for various types of siderophores (shown in Fig. 7.2) is summarized as follows.
7.4.1 Hydroxamate Siderophores
Chemically, these are specifically tri-hydroxamate type of siderophores reportedly found in fungi and majorly produced by fungal species that belongs to Zygomycotina (Mucorales), Ascomycotina (Aspergillus spp., N. crassa) and Deuteromycotina (Fusarium dimerum) subdivision of fungi. Hydroxamate siderophores have strong correlation among their hydroxamate groups and bound ligands as they known to form hexadentate, tetradentate and bidentate complexes. In hydroxamate class mainly, ferrichromes are of main concern with respect to ecological importance due to their potential to chelate iron from soil and supply to plant species, whereas other naturally occurring siderophores are not as effective because of their affinity towards other metal ions too. From the literature reports, ferrichrome derivative such as ferrioxamines also exhibits antibiotic activity (named as ferrimycines). In addition, rhodotorulic acid, dimerium acid, alkaligens and putrebactin also belong to hydroxamate-type siderophore family (Garnerin et al. 2017).
7.4.2 Carboxylate Siderophores
A new class of siderophore was identified by Winkelmann from fungi Rhizopus microspores belonging to zygomycetes, which contain ‘hydroxyl’ and ‘carboxyl’ moieties as donor groups to iron (Fe3+) which are solely responsible for metal binding. This type of siderophore is termed as ‘carboxylate siderophore’ well known as ‘rhizoferrin’ which is isolated from Rhizopus spp. using ion-exchange column chromatography. Structurally, rhizoferrin and its analogues contain 1,4-diaminobutane symmetrically acylated to the terminal carboxylate of citric acid through amide bonds (Drechsel et al. 1995).
7.4.3 Catecholate Siderophore
This class of siderophores is known to have phenolate or 2,3-dihydroxybenzoate (DHB) as a binding moieties. Catechol (also called as pyrocatechol), naturally occurring organic, colourless compound, is the ortho-isomer of the three isomeric benzenediols and found in trace amounts. Azotobacter vinelandii, in iron-deficient medium, forms various types of catecholate-based siderophores such as monocatecholate aminochelin, dicatecholate azotochelin and tri-catecholate protochelin (Baakza et al. 2004). Basically, all these types of naturally occurring siderophores contain negatively charged oxygen donors as hard Lewis base which binds with Fe3+ which act as hard Lewis acid as per its chemical nature.
7.4.4 Mixed Ligand Siderophores
This class of siderophores, also called heterobactins, contains combined donor groups of hydroxamate and catecholate together. Siderophore of this type includes mixed ligand of lysine, ornithine and histamine derivatives. For instance, mycobactins (Mycobacterium spp.) contain hydroxamate and phenolate donor groups as chelating ligands (Mohammad et al. 2011).
7.5 Mechanism of Binding of Iron in Cell
In fungi, siderophore-mediated iron (Fe3+) uptake can be regulated by four mechanisms, namely, shuttle, hydrolytic, taxicab and reductive. Three of them, i.e. shuttle, taxicab and hydrolytic mechanisms, depend upon the specific recognition of several siderophore. (a) In shuttle mechanism, the iron (Fe3+)-siderophore complex initially enters the cell membrane, and soon after that, it releases the metal from ligand (e.g. ferrichrome in fungal species, viz. Ustilago sphaerogena and Ustilago maydis) which ultimately leads to excretion of free siderophore (Sah and Singh 2015). This mechanism is depicted in Fig. 7.3.
The hydrolytic mechanism involves the transportation of iron (Fe3+)-siderophore complex in its intact form into the cell (e.g. uptake of ferric triacetyl fusarinine C in mycelia sterilia). Simultaneously, both reductive and degradative steps occur inside the cell that lead to the reduction of iron from ferric (Fe3+) to ferrous (Fe2+) form and also the cleavage of ester bonds in siderophore (triacetyl fusarinine C) in the presence of specific esterase resulting in monomeric fusarinines which are further excreted as shown in Fig. 7.3. This mechanism is helpful for removing toxic metals such as aluminium (Al3+), gallium (Ga3+) and chromium (Cr3+) from the cell as these metal-siderophore chelates have similarity to the prototype iron (Fe3+) complex and their complexes are taken up inside the cell where these metals remain bounded to the monomeric fusarinine, so further reduction of these metals is restricted resulting in their excretion (Sanz-Ferramola et al. 2013), whereas, (c) in taxicab mechanism, iron in ferric form is transferred from extracellular siderophore to intracellular ligands (e.g. ferric rhodotorulate in Rhodotorula pilimanae) across the cell membrane as the extracellular siderophore does not enter the cell membrane (Gerwien et al. 2018). However, (d) in reductive mechanism, the reduction of iron (Fe3+)-siderophore complex occurs at the membranes instead of transporting that complex inside the cell, and the reduced ferrous form is taken up by the cell membrane. This type of mechanism is used for transporting ferrichrome siderophores in some fungal species such as Ustilago maydis (Trivedi et al. 2016).
7.6 Binding Sites in Siderophores
The stability of iron-siderophore complex strongly depends on the nature of binding sites present in varied siderophore structure with different functional motifs. All the different types of siderophores reported till date form extremely stable, highly specific complex containing high-spin ferric iron. The different moieties that explain the anchoring in most siderophore-iron complex belong to hydroxamates, catecholate, carboxylate and mixed-type categories as shown in Table 7.2. The first three types show some similarities in respect to their iron-binding affinity. These three classes hire two sites of the iron centre that form stable five-membered ring structure complexing metal iron to form siderophore-iron complex. Oxygen shows high affinity towards iron (Fe3+), and also classified under hard donor ligand, further, a lone pair of oxygen atom always coordinates with iron cation in order to increase the strength of coordination complex. Using this donor-acceptor interaction, the selectivity of siderophore ligand for iron improves many folds, and this helps in transportation of iron across the cell membrane. Mixed type of siderophore consists of different binding sites. For example, rhizoferrin, a fungal siderophore, contains two molecules of citric acid usually linked with an additional chain. Further, it requires two hydroxycarboxylic acid and two carboxylic acid groups for iron coordination (Neilands 1995). Table 7.2 illustrates the structural representation of iron-siderophore complex among different classes of siderophore.
The physico-chemical parameter, i.e. pKa values of binding groups, has a great influence on the siderophore’s complex stability as the oxygen atom in these complexes is interacting in a non-protonated state. From the available literature, hydroxamic acid has been shown to have pKa values in the range of 8–10; however, acetohydroxamic acid (main compound for monohydroxamate ligand) has shown to have 9.29 pKa value. The pKa values of carboxylates lie in the range of 3.5–5 which contributes to efficient iron imbibitions by carboxylate siderophores under low-pH conditions. The microbes living in acidic medium (such as fungi) use carboxylate siderophores for iron immobilization. In spite of this fact, the carboxylate siderophores couldn’t compete with stronger siderophores such as hydroxamates and catecholates as they are fully protonated at physiological pH. In catecholate, a model monomer has been synthesized in such a way that once nitro group is added on para position, then the pKa values of resulting alcohol groups were found to be 6.69 and 10.83. On the other hand, after substituting it with hydrogen atom, the resulting pKa values were 9.26 and 13.3. The pKa values of binding groups have an impact on the affectivity of siderophores as the oxygen atoms only bind with the groups in non-protonated state. These values are not easily accessible and effected by side chain modifications (Neilands 1995). The pKa values for hydroxamate, catecholate and carboxamate groups exhibit the partly deprotonated states in the presence of iron at neutral pH. Proton-independent pKa values do not demonstrate the actual iron-binding efficiency of siderophores at physiological pH due to incomplete deprotonation. For better analysis, pH analogous to pFe values is considered to be a better approach for comparing the true relative abilities of iron binding with different siderophores by giving negative decadic logarithm of free iron concentration. As per standards, the total ferric concentration and total ligand concentration are considered to be 10−6 M and 10−5, respectively. The chelation efficiency is strongly influenced by pH of medium; thus, pFe value is a pH-dependent value. For instance, at serum pH (i.e. 7.4), in the presence of enterobactin and aerobactin, the concentration of free iron is observed to have different pFe values which are 35.5 and 23.4, respectively (Wilson et al. 2016; Miethke and Marahiel 2007).
Phytosiderophores are hexadentate ligands that coordinate with Fe3+ from all six coordination sites which explore a variety of combinations at the three binding sites to form a potential iron-siderophore complex. In the contrary, many siderophores use only one type of binding site to form the stable complexes with iron such as tri-hydroxamate siderophore (ferrioxamine) and tri-catecholate siderophores (enterobactin) (Renshaw et al. 2002).
7.7 Metal Ion Complex Formation with Siderophore
Several fungal species have been investigated with complex regulatory arrangements during intake of secondary metabolites, namely, mycotoxins (produced by mycotoxigenic fungi) and its detoxification process of converting these metabolites from more toxic to less toxic form. The metals such as iron, zinc, copper, chromium, gallium and manganese interact with the siderophore produced by different fungi to make stable siderophore-metal complexes resulting in homeostasis regulation. These metal ions may interact with variety of siderophore. Table 7.3 highlights some of the examples of metal-ion-siderophore complexes along with their functions.
7.8 Biological Functions of Siderophore
Siderophore affects the plant and microbes in several significant ways. These are discussed in detail as follows.
7.8.1 An Iron-Scavenging Compound
Siderophores have the ability to support the growth factor in all fungi and auxotrophic organism despite the fact that they are producing or non-producing species. Several auxotrophic organisms found to be in underdeveloped stage in the absence of these compounds require siderophores for their growth. For instance, Pilobolus kleinii utilizes coprogen as an essential growth factor (Dave and Dube 2000). The siderophore-mediated transport system facilitates and enhances the efficient competency among microbes for the consumption of available iron to make survival of one type of microbe over the other. This is advantageous in case of non-pathogenic species producing more siderophores as it competes more efficiently for iron and limiting the growth of pathogenic species (less competent) of the same organism at the same time (e.g. Fusarium species, Aspergillus ochraceus) (Aznar et al. 2014).
7.8.2 Virulence Factor
The siderophore-mediated transport system also plays an important role in microbial pathogenicity. Host pathogenic microorganism acquires iron to survive, and restricting its availability to others is considered to be an effective defence mechanism of host microbes that suggest that siderophores can act as virulence factors. For example, Microbotryum violaceum siderophore (mutant of plant pathogen) accumulates less rhodotorulic acid as compared to its wild type that exhibits minimal pathogenicity. On the other hand, mutant of Ustilago maydis (plant pathogen) showed defectiveness in siderophore production and is found to be as virulent as its wild-type strains. In addition, phototoxic and immunosuppressive effects also contribute to virulence in pathogenic organism in terms of chelating activity in siderophores . For example, Alternaria cassiae produces siderophore such as coprogen and ferricrocin which act as phytotoxins and several other siderophores such as desferrichrome which exhibits immunosuppressive effect in mouse model (Ecker et al. 2018).
7.8.3 Intracellular Iron Storage
Apart from solubilizing and transporting iron, siderophore also facilitates intracellular iron acquisition in mycelia and spores that effect germination. In case of Neurospora crassa , several intercellular siderophores produced by the species such as excretory coprogen and intracellular ferricrocin found in both hyphae and conidia are essential for its germination and iron storage. It is also well established that iron-siderophore complexes maintained in hyphae for the incorporation of spores help in sporulation and also transport iron to mycelial mitochondria. Several fungal species have both types of inter- and intracellular siderophores such examples Penicillium chrysogenum, Aspergillus nidulans, Rhodotorula minuta and Ustilago sphaerogena (Renshaw et al. 2002).
7.9 Applications of Siderophore
Siderophore and its different derivatives have broad scope in medical sciences and offer several applications in the field of biotechnology, microbial ecology and biomedical science. Various studies demonstrate the effective role of some siderophore in the management or treatment of human diseases and infections (Popat et al. 2017; Nagoba and Vedpathak 2011; Ahmed and Holmstrom 2014; Saha et al. 2016; De-Serrano 2017; Dimkpa 2016; Fine 2000; Banner and Woolf 2004). These are as listed in Table 7.4 which are as follows.
7.10 Conclusion
From recent investigations, it can be concluded that siderophores are the key components in iron transport in phototrophs and microorganisms. Structural variation and ligand specificity in siderophore as well as membrane receptors regulate the iron uptake process; hence, this field has immense potential for further exploration in the field of biomolecular science. The siderophore plays significant role in environmental applications and is also investigated as potential strategy in the field of biotechnology (agriculture, bioremediation and biosensor) and medicines (diagnosis and treatment). In a search of advance-level revelations, the metagenomic approach with detailed chemical examinations may be employed to improve the current environmental applications that also give new realm of investigation for siderophores. With the metal-chelating ability, siderophores are known to have potential applications in the field of medicine and biotechnology. Apart from iron binding (Fe3+), the variety of siderophore is also investigated for binding with other metals including Pb2+, Cr3+, Al3+ and actinide ions. The study of metal-microbe conjugation highlights the significance of microbes which provides suitable environment for growth and reproduction of various forms of life. From the established literature, it is clear that siderophores represent the vital organic compounds for iron uptake among microbial and plant species. Siderophore variability in terms of their structural and functional characteristics and membrane receptors involving metal coordination in relation to microbial communities should be thoroughly investigated to establish the role of siderophore at profound level in the field of advance-level therapy in medical science.
References
Ahmed E, Holmstrom SJM (2014) Siderophores in environmental research: roles and applications. Microb Biotechnol 7:196–208
Aznar A, Chen NWG, Rigault M et al (2014) Scavenging iron: a novel mechanism of plant immunity activation by microbial siderophores. Plant Physiol 164:2167–2183
Baakza A, Daave BP, Dube HC (2004) Chemical nature, ligand denticity and quantification of fungal siderophores. Indian J Exp Biol 42:96–105
Baakza A, Dave BP, Dube HC (2005) Chemical properties and NMR spectroscopic identification of certain fungal siderophores. Indian J Exp Biol 43:880–886
Baila S, Garcia M, Baila LC et al (2014) Hydroxamate production as a high affinity iron acquisition mechanism in Paracoccidioides spp. PLoS One 9:1–14
Bairwa G, Jung WH, Kronstad JW (2017) Iron acquisition in fungal pathogens of humans. Metallomics 9:215–227
Banner W Jr, Woolf AD (2004) Antidotes for poisoning by metals and metalloids: deferoxamine. International programme on chemical safety evaluation (WHO/ILO/UNEP), World Health Organization, August 2004
Bernier G, Girijavallabhan V, Murray A et al (2005) Desketoneoenactin-siderophore conjugates for Candida: evidence of iron transport-dependent species selectivity. Antimicrob Agents Chemother 49:241–248
Butler A, Theisen RM (2010) Iron(III)–siderophore coordination chemistry: reactivity of marine siderophores. Coord Chem Rev 254:288–296
Conti M, Eriksson L (2016) Physics of pure and non-pure positron emitters for PET: a review and a discussion. EJNMMI Phys 3:1–17
Crowley DE, Reid CPP, Szaniszlo PJ (1988) Utilization of microbial siderophores in iron acquisition by oat. Plant Physiol 87:680–685
Dave BP, Dube HC (2000) Chemical characterization of fungal siderophores. Indian J Exp Biol 38:56–62
De-Serrano LO (2017) Biotechnology of siderophores in high-impact scientific fields. Biomol Concepts 8:169–178
Dimkpa C (2016) Microbial siderophores: production, detection and application in agriculture and environment. Endocyt Cell Res 27:7–16
Drechsel H, Tschierske M, Thieken A et al (1995) The carboxylate type siderophore rhizoferrin and its analogs produced by directed fermentation. J Ind Microbiol 14:105–112
Ecker F, Haas H, Groll M et al (2018) Iron scavenging in Aspergillus species: structural and biochemical insights into fungal siderophore esterases. Angew Chem Int Ed Engl 57:14624–14629
Emery T, Hoffer PB (1980) Siderophore-mediated mechanism of gallium uptake demonstrated in the microorganism Ustilago sphaerogena. J Nucl Med 21:935–939
Farkas E, Szabo O, Gyemant G et al (2018) Complexation of hydroxamate-based siderophores with cobalt(II/III): growth inhibitory effect of cobalt(III)-desferricoprogen complex on fungi. Transit Metal Chem 43:355–365
Fatima N, Javaid K, Lahmo K et al (2017) Siderophore in fungal physiology and virulence. J Pharmacogn Phytochem 6:1073–1080
Fine JS (2000) Iron poisoning. Curr Probl Pediatr 30:71–90
Garnerin T, Dassonville-Klimpt A, Sonnet P (2017) Fungal hydroxamate siderophores: biosynthesis, chemical synthesis and potential medical applications. In: Méndez-Vilas A (ed) Antimicrobial research: novel bioknowledge and educational programs. Formatex Research Center, Badajoz
Gerwien F, Skrahina V, Kasper L et al (2018) Metals in fungal virulence. FEMS Microbiol Rev 42:1–21
Ghosh SK, Banerjee S, Sengupta C (2017) Bioassay, characterization and estimation of siderophores from some important antagonistic fungi. J Biopest 10:105–112
Haas H (2014) Fungal siderophore metabolism with a focus on Aspergillus fumigates. Nat Prod Rep 31:1266–1276
Haas H, Petrik M, Decristoforo C (2015) An iron-mimicking, trojan horse-entering fungi-has the time come for molecular imaging of fungal infections? PLoS Pathog 11:1–7
Howard DH (1999) Acquisition, transport, and storage of iron by pathogenic fungi. Clin Microbiol Rev 12:394–404
Huschka HG, Jalal MAF, Helm DVD et al (1986) Molecular recognition of siderophores in fungi: role of iron-surrounding N-acyl residues and the peptide backbone during membrane transport in Neurospora crassa. J Bacteriol 167:1020–1024
Islam R, Datta B (2015) Influence of copper and zinc on siderophore production of fusarium soil isolates. J Glob Biosci 4:1990–1995
Kosman DJ (2003) Molecular mechanisms of iron uptake in fungi. Mol Microbiol 47:1185–1197
Lesuisse E, Casteras MS, Labbe P (1998) Siderophore-mediated iron uptake in Saccharomyces cerevisiae: the SITl gene encodes a ferrioxamine B permease that belongs to the major facilitator superfamily. Microbiology 144:3455–3462
Li Y, Wang ZK, Liu XE et al (2016) Siderophore biosynthesis but not reductive iron assimilation is essential for the dimorphic fungus Nomuraea rileyi conidiation, dimorphism transition, resistance to oxidative stress, pigmented microsclertium formation, and virulence. Front Microbiol 7:931
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–451
Moerlein SM, Welch MJ, Raymond KN et al (1981) Tricatecholamide analogs of enterobactin as gallium- and indium-binding radiopharmaceuticals. J Nucl Med 22:710–719
Mohammad RS, Matthew FT, Shao-Liang Z et al (2011) Structure and biosynthesis of amychelin, an unusual mixed-ligand siderophore from Amycolatopsis sp. AA4. J Am Chem Soc 133:11434–11437
Nagoba B, Vedpathak D (2011) Medical applications of siderophores. Eur J Gen Med 8:229–235
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270:26723–26726
Petrik M, Haas H, Dobrozemsky G et al (2010) 68Ga-Siderophores for PET imaging of invasive pulmonary aspergillosis: proof of principle. J Nucl Med 51:639–645
Petrik M, Franssen GM, Haas H et al (2012a) Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur J Nucl Med Mol Imaging 39:1175–1183
Petrik M, Haas H, Schrett M et al (2012b) In vitro and in vivo evaluation of selected 68Ga-siderophores for infection imaging. Nucl Med Biol 39:361–369
Petrik M, Haas H, Laverman P et al (2014) 68Ga-Triacetylfusarinine C and 68Ga-ferrioxamine E for Aspergillus infection imaging: uptake specificity in various microorganisms. Mol Imaging Biol 16:102–108
Petrik M, Vlckova A, Novy Z et al (2015) 68Ga-siderophores versus 68Ga-colloid and 68Ga-citrate: biodistribution and small animal imaging in mice. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 159:60–66
Petrik M, Zhai C, Novy Z et al (2016) In vitro and in vivo comparison of selected Ga-68 and Zr-89 labelled siderophores. Mol Imaging Biol 18:344–352
Petrik M, Zhai C, Haas H et al (2017) Siderophores for molecular imaging applications. Clin Transl Imaging 5:15–27
Philpott CC (2006) Iron uptake in fungi: a system for every source. Biochim Biophys Acta 1763:636–645
Popat R, Harrison F, Da-Silva AC et al (2017) Environmental modification via a quorum sensing molecule influences the social landscape of siderophore production. Proc R Soc B 284:20170200
Rasha FM (2017) Intracellular siderophore detection in an egyptian, cobalt-treated F. solani isolate using SEM-EDX with reference to its tolerance. Pol J Microbiol 66:235–243
Raymond KN (1994) Recognition and transport of natural and synthetic siderophores by microbes. Pure Appl Chem 66:773–781
Renshaw JC, Robson GD, Trinci APJ et al (2002) Fungal siderophores: structures, functions and applications. Mycol Res 106:1123–1142
Sah S, Singh R (2015) Siderophore: structural and functional characterization – a comprehensive review. Agriculture 61:97–114
Saha M, Sarkar S, Sarkar B et al (2016) Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res Int 23:3984–3999
Sanz-Ferramola MI, Benuzzi D, Calvente V et al (2013) The use of siderophores for improving the control of postharvest diseases in stored fruits and vegetables. In: Microbial pathogens and strategies for combating them: science, technology and education. Formatex Research Center, Badajoz
Seneviratne M, Vithanage M (2015) The role of siderophores on plants under heavy meal stress: a view from the rhizosphere. Res Rev J Bot Sci 4:23–29
Senthilnithy R, De Costa MDP, Gunawardhana HD (2008) The pKa values of ligands and stability constants of the complexes of Fe(III), Cu(II) and Ni(II) with some hydroxamic acids: a comparative study of three different potentiometric methods. J Natl Sci Found 36:191–198
Stintzi A, Barnes C, Xu J et al (2000) Microbial iron transport via a siderophore shuttle: a membrane ion transport paradigm. Proc Natl Acad Sci USA 97:10691–10696
Tamayo E, Gomez-Gallego T, Aguilar CA et al (2014) Genome-wide analysis of copper, iron and zinc transporters in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front Plant Sci 5:1–13
Trivedi HB, Vala AK, Dhrangadhriya JH et al (2016) Marine-derived fungal siderophores: a perception. Indian J Geo-Mar Sci 45:431–439
Velikyan I (2014) Prospective of 68Ga-radiopharmaceutical development. Theranostics 4:47–80
Wilson D, Citiulo F, Hube B (2012) Zinc exploitation by pathogenic fungi. PLoS Pathog 8:e1003034
Wilson BR, Bogdan AR, Miyazawa M et al (2016) Siderophores in iron metabolism: from mechanism to therapy potential. Trends Mol Med 22:1077–1090
Wittenwiler M (2007) Mechanisms of iron mobilization by siderophores. Term paper in Biogeochemistry and pollutant dynamics, December 2007
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bhardwaj, S., Singh, S., Bhatia, S. (2021). Contrasting Role of Fungal Siderophore in Metal Ion Complex Formation. In: Dhusia, K., Raja, K., Ramteke, P. (eds) Fungal Siderophores. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-030-53077-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-53077-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53076-1
Online ISBN: 978-3-030-53077-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)