Abstract
Dissolved ions concentration in groundwater beyond the recommended limits is a major problem as they make the water unsuitable for drinking purpose. Fluorine commonly found in certain rocks is released into groundwater due to the processes of rock–water interaction. This leads to increase in the concentration of fluoride in groundwater which is a major problem in several parts of the world including India. Presence of fluoride beyond the prescribed limits causes health problems to humans due to prolonged consumption of water, which is common in many parts of India. Dental and skeletal fluorosis is observed due to prolonged drinking of water with fluoride concentration above 1.5 mg/l. The objective of the study is to know how fluoride get released from the host rock and spot out suitable location for installing a dug well recharge system to decrease the fluoride concentration in groundwater. Several methodologies exist for in situ or exsitu removal of fluoride from groundwater. Exsitu methods can be enforced at community level or even at household level for the reduction of fluoride before its consumption, through ion exchange, reverse osmosis, adsorption, electrodialysis, coagulation, Nalgonda technique, electrodialysis, coagulation, precipitation, etc. Even artificial recharging structures can also be built in suitable location for diluting fluorite concentration in groundwater. Rainwater harvesting is also found effective to reduce the fluoride concentration of groundwater in existing wells. A pilot study was carried out by construction of a dug well recharge system in Dharmapuri district, Tamil Nadu, India. The study successfully demonstrated the applicability of dug well recharge system at a carefully selected site based on the systematic long-term hydrogeochemical studies to solve the problem of fluoride contamination affecting millions of rural people.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
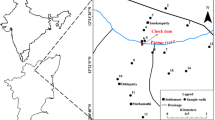
Geogenic and anthropogenic contamination may make groundwater unsuitable for various uses including its use as drinking water. Consumption of such water may cause many health problems. Presence of fluoride, arsenic, and iron above recommended limits is found in groundwater due to geogenic contamination in many parts of India and world (Mukherjee et al. 2015). Release of arsenic from arseno-pyrite minerals is responsible for higher concentration of arsenic in groundwater of Bengal basin (Farooq et al. 2011; Saha 2009). As about 80% of rural population of India depends on the use of groundwater for domestic use, the water needs to be treated before it is used for drinking purpose. The initial concentration of fluorite, occurrence and removal of co-contaminants, and disposal of sludge, etc., decide treatment methods of water to be treated (Brindha et al. 2016). Bhagavan and Raghu (2005) studied the effect of increase in recharge by check dams, and Pettanati et al. (2014) highlighted the use of percolation ponds in regions with fluoride problem in groundwater. Some studies have also indicated an increase in fluoride concentration in groundwater during the process of recharge when check dams are constructed (Bhagavan and Raghu 2005). Thus, contradicting findings are also observed from recharge for in situ mitigation of fluoride concentration (Brindha et al. 2016). Hence, it is essential to reveal the release mechanism of fluoride during the process of recharge based on a long-term temporal variation in fluorite concentration and in the level of groundwater in the area. The objective of this study is to understand the release processes of fluoride and identify a suitable location for construction of dug well recharge system in the area for reducing the concentration of fluoride in groundwater to an acceptable level. This pilot study was carried out in Dharmapuri district, Tamil Nadu (Fig. 1), where about 72,000 out of about 190,000 school students were found having dental fluorosis (UNICEF 2009).
2 Methodology
Systematic investigation during June 2011 helped in identifying 44 key monitoring well to represent the area (Fig. 1). These key wells were used to collect bimonthly groundwater level and water sample between June 2011 and August 2014. Largely, the key wells are >30 m in depth and are utilized for obtaining drinking and agricultural water. Water level indicators (Solinist) were used to collect water level data. Pre-washed and treated 1000-ml capacity clean polyethylene bottles were used to collect groundwater samples after rinsed with sample. Merck 0.22-μm filter paper (Merck 755004722) were used to filter the samples before collection. To know the in situ temperature, EC and pH of the groundwater in field portable digital meter (YSI 556MPS) were used. Total dissolved solids (TDS) were obtained using EC = TDS/0.64 (Carroll 1962). Aquamerck (1.11109.0001) test kit was used to measure CO3 2− and HCO3− in the field itself. Ion chromatograph (Metrohm 861) was used for measuring the concentrations of Na+, K+, Ca2+, Mg2+, Cl−, F−, NO3 −, and SO4 2− of samples in the laboratory. The concentration of silica was determined by using spectrophotometer (Systronics 201) for the groundwater samples collects in August 2014. Batch test experiment’s results were obtained to know the fluoride release processes from the rocks/soil, at laboratory.
3 Sources of Fluoride in Groundwater
Fluoride-bearing minerals are common in nature; therefore, contamination of fluorite is vast. Sometime it is intensive and became alarming in many places. The permissible range for fluoride in drinking water as per the Bureau of Indian Standards (BIS 2012) is from 0.6 to 1.5 mg/l. A desired concentration of fluoride in drinking water is favorable to determine the physiological activities in human bodies, whereas the consumption of fluoride above or below the permissible limits may cause health problems such as fluorosis of dental and skeletal structure. In India, northwestern and southern states are severely affected with fluorosis (Agarwal et al. 1997; Ali et al. 2016). Weathering of rocks rich in fluoride is the common natural cause for the fluoride in groundwater. The marine sediments and sediments of foothill areas (WHO 2002; Fawell et al. 2006), as well as marginal alluvial terrain of the Gangetic Plains (Saha et al. 2008; Saha and Alam 2014), are more vulnerable for water with high concentration of fluoride. Fluorine occurs naturally in igneous and sedimentary rocks. Common fluoride minerals are sellaite, fluorite or fluorspar, cryolite, fluorapatite, apatite, topaz, phlogopite, biotite, epidote, tremolite and hornblende, mica, clays, villuanite, and phosphorite (Brindha and Elango 2011; Haidouti 1991; Gaumat et al. 1992; Gaciri and Davies 1993; Kundu et al. 2001). Nalgonda district, Andhra Pradesh, known for its fluorite contamination is due to the inherent fluoride-rich granitic rocks. The fluoride content in granitic rocks in Nalgonda district varies from 325 to 3200 mg/kg with a mean of 1440 mg/kg. Thus, the Nalgonda granites having higher fluoride content than world granite, where it occurs average 810 mg/kg (Brindha and Elango 2011),whereas in Hyderabad granites, it is estimated 910 mg/kg (Brindha and Elango 2011; Ramamohana Rao et al. 1993). However, groundwater in northeastern districts of Tamil Nadu has high fluoride because of their fluoride-rich mineral such as epidote, hornblende, biotite, apatite, carbonatite, mica, and fluorapatite (Jagadeshan 2015; Jagadeshan et al. 2015a, b; Brindha et al. 2016). Jagadeshan et al. (2015b) carried out a study of rocks of Vaniyar river basin in Dharmapuri district, Tamil Nadu, to understand the mineralogical composition. He made thin sections of the charnockite and epidote hornblende biotite gneiss rocks (Fig. 2), where plagioclase, hypersthene, biotite, quartz, hornblende, orthoclase, and muscovite opaque (probably iron oxide) were the major minerals. Biotite which consists of fluorine was also identified in charnockite rock.
Geological map of the study area and thin section photographs (X5 magnification, field of view 2500 µm wide, plan polarized illumination) showing quartz (Qt), plagioclase (PI), epidote (Ep), biotite (Bi), hypersthene (Op), hornblende (Hb), pyroxene, and other opaque minerals (Op) in epidote hornblende gneiss (left) and charnockite (right)
4 Geochemical Analysis of Groundwater
Largely, the elevated amount of fluorite in groundwater is due to its release from host rock. The climatic conditions, pH, host rock mineralogy, and hydrogeological condition of an area are generally governing the release of fluoride into groundwater (Shan et al. 2013; Raju et al. 2009). The phosphatic fertilizers use may also lead to rise in fluoride concentration in groundwater anthropogenically.
Utilization of fertilizers and industrial activities such as brick kilning are some of the other causes for elevated fluoride concentration in groundwater (Selvam 2015). The weathering of these rocks results in increased fluoride content in groundwater. Longer residence time in aquifers with fractured fluoride-rich rocks enhances fluoride levels in the groundwater. Granite and granitic gneisses in Nalgonda, India, contain fluoride-rich minerals such as fluorite (0–3.3%), biotite (0.1–1.7%), and hornblende (0.1–1.1%) (Brindha and Elango 2011; Brindha et al. 2016; Ramamohana Rao et al. 1993). A strong positive correlation is found among sodium, potassium, silica, bicarbonate + fluoride versus sodium + potassium and bicarbonate versus fluoride (Fig. 3).
However, negative correlation was obtained for fluoride versus calcium and magnesium (Fig. 4) (Jagadeshan et al. 2015b). These diagrams support the leaching of fluoride from minerals.
During laboratory experiments Jagadeshan et al. (2015b) found leaching of fluoride from charnockite and epidote hornblende biotite gneiss rocks resulted in enhanced fluoride in water from 1.41 to 3.54 mg/l, respectively, after 2000 hours (Fig. 5).
For reducing or removal of fluoride concentration from drinking water, there exist various methods at domestic or community level. The various techniques of fluoride removal are ion exchange method, precipitation method, adsorption method, Nalgonda method, and reverse osmosis. Thus, the existing treatment methods of water to remove fluoride for domestic use have several limitations. Further the people’s mind-set is also not to use treatment methods as it is time consuming, expensive, and also requires trained personal. To overcome the problems and limitation of fluoride removal methods from the water, induced recharge may be adopted. David et al. (2014) reported in tropical countries such as India, rainwater harvesting could be useful for dilution of high-fluoride groundwater. The recharge from the storage tank (Gupta and Deshpande 1998), subsurface barrier (Rolland et al. 2011), and check dams (Bhagavan and Raghu 2005) has reduced the concentration of fluoride in groundwater by dilution.
5 Pilot Study
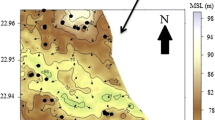
A pilot study was carried out by the construction of a dug well recharge system in Dharmapuri district, Tamil Nadu. A few photographs taken during the construction of this structure are shown in Fig. 6. The schematic diagram of the constructed recharge structure is shown in Fig. 7. The site was chosen on the basis of three-year temporal variation in the fluoride concentration and fluctuation in groundwater level (Jagadeshan et al. 2015b). The groundwater level in induced recharge well (Well no. 44) raised to 9.1 m from 14.5 m (bgl), and the electrical conductivity has decreased from 1342 to 945 μS/cm (Jagadeshan et al. 2015b). Geochemical results indicate that fluoride concentration has decreased from 3.1 to 1.41 mg/l in well no. 44 due to dilution by the rainwater passing through induced recharge structure into dug well. This clearly indicates that the raise in groundwater level reduces the electrical conductivity and fluoride concentration of groundwater. The induced recharge from the structure constructed benefited an area of about 1 km2 (Fig. 8). This study promises that the concentration of fluoride in groundwater can be decreased in other fluoride-affected areas if such low-cost induced recharge structures are constructed.
6 Conclusion
Groundwater with high fluoride is a major problem as evident from studies carried out by numerous researchers in different parts of the world. The host rock rich in fluorine is the major source of fluoride in groundwater. Concentration of fluoride in groundwater increases due to host rocks weathering and prolonged residence time of water. Treatment of the high-fluoride groundwater is possible by several methods, but they have limitations and cost intensive. Technique such as rainwater harvesting, constructing check dams and percolation ponds, and facilitating artificial recharge of rainwater through existing wells are suitable on-site treatment available. A pilot study carried out by a dug well recharge system in Dharmapuri district, Tamil Nadu, demonstrated the potential of this scheme in reducing the concentration of fluoride in groundwater over an area of about 1 km2. The recharge from the structure has reduced concentration of fluoride in groundwater from 3.1 to 1.44 mg/l due to dilution. The study successfully demonstrated the applicability of dug well recharge system at a carefully selected site based on the systematic long-term hydrogeochemical studies to solve the problem of fluoride contamination affecting millions of rural people.
References
Agarwal V, Vaish AK, Vaish P (1997) Ground water quality: focus on fluoride and fluorosis in Rajasthan. Curr Sci 73(9):743–746
Ali S, Thakur SK, Sarkar S, Shekhar S (2016) Worldwide contamination of water by fluoride. Environ Chem Lett. doi:10.1007/s10311-016-0563-5
Bhagavan SV, Raghu V (2005) Utility of check dams in dilution of fluoride concentration in ground water and the resultant analysis of blood serum and urine of villagers, Anantapur District, Andhra Pradesh, India. Environ Geochem Health 27:97–108
BIS (2012) Bureau of Indian standards drinking water-specification. IS: 10500, New Delhi
Brindha K, Elango L (2011) Fluoride in groundwater: causes, implications and mitigation measures fluoride properties. Appl Environ Manag 111–136
Brindha K, Jagadeshan G, Kalpana L, Elango L (2016) Fluoride in weathered rock aquifers of southern India: managed aquifer recharge for mitigation. Environ Sci Pollut Res. doi:10.1007/s11356-016-6069-7
Carroll D (1962) Rainwater as a chemical agent of geological processes—a review. U.S. Geological Survey Water-Supply Paper 1535-G, p 18
David E, Reisner T, Pradeep (2014) Aquananotechnology. Global Prospects CRC Press, Science, p 887
Farooq SH, Chandrasekharam D, Norra S, Berner Z, Eiche E, Thambidurai P, Stüben D (2011) Temporal variations in arsenic concentration in the groundwater of Murshidabad District, West Bengal, India. Water Res 44:5575–5578
Fawell J, Bailey K, Chilton J, Dahi E, Fewtrell L, Magara Y (2006) Fluoride in drinking water. WHO, IWA Publishing, pp 1–144
Gaciri SJ, Davies TC (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143:395–412
Gaumat MM, Rastogi R, Misra MM (1992) Fluoride level in shallow groundwater in central part of Uttar Pradesh. Bhu-Jal News 7(2 & 3):17–19
Gupta SK, Deshpande RD (1998) Depleting Groundwater levels and increasing fluoride concentration in villages of Mehsana District, Gujarat, India. Cost to Economy and Health, Water Resource and Research Foundation, Ahmadabad, p 74
Haidouti C (1991) Fluoride distribution in soils in the vicinity of a point emission source in Greece. Geoderma 49:129–138
Jagadeshan G (2015) Geochemical reaction responsible for fluoride rich groundwater and remediation by induced recharge in Vaniyar River Basin, Tamil Nadu. Ph.D. thesis, Anna University, Chennai, India
Jagadeshan G, Kalpana L, Elango L (2015a) Major ion signatures for identification of geochemical reactions responsible for release of fluoride from geogenic sources to groundwater and associated risk in Vaniyar River basin, Dharmapuri district, Tamil Nadu, India. Environ Earth Sci 73(7):67–80
Jagadeshan G, Kalpana L, Elango L (2015b) Hydrogeochemistry of high fluoride groundwater in hard rock aquifer in a part of Vaniyar River basin, Tamil Nadu, India. Geochem Int 53(6):554–564
Kundu N, Panigrahi MK, Tripathy S, Munshi S, Powell MA, Hart BR (2001) Geochemical appraisal of fluoride contamination of groundwater in the Nayagarh District of Orissa, India. Environ Geol 41:451–460
Mukherjee A, Saha D, Harvey CF, Taylor RG, Ahmed KM, Bhanja SN (2015) Groundwater systems of the Indian Sub-Continent. J Hydrol Reg Stud (New online journal of Journal of Hydrology, whose Impact Factor is 3.053). http://dx.doi.org/10.1016/j.ejrh.2015.03.005
Pettenati M, Picot CG, Thiery D, Boisson A, Alazard M, Perrin J, Dewandel B, Maréchal JC, Ahmed S, Kloppmann W (2014) Water quality evolution during managed aquifer recharge (MAR) in Indian crystalline basement aquifers: reactive transport modeling in the critical zone. Procedia Earth Planet Sci 10:82–87
Raju NJ, Dey S, Das K (2009) Fluoride contamination in ground waters of Sonbhadra district, Uttar Pradesh, India. Curr Sci 96:979–985
Ramamohana Rao NV, Suryaprakasa Rao K, Schuiling RD (1993) Fluorine distribution in waters of Nalgonda District, Andhra Pradesh, India. Environ Geol 21:84–89
Rolland A, Muralidharan D, Rangarajan R, Sathyanarayana U, Deshmukh SD (2011) Mitigation of fluoride problem through artificial recharge strategies, case study from Nalgonda District, Andhra Pradesh. J Geol Soc India 2:45–53
Saha D (2009) Arsenic groundwater contamination in parts of middle Ganga plain, Bihar. Curr Sci 97(6)
Saha D, Alam F (2014) Groundwater vulnerability assessment using DRASTIC and pesticide DRASTIC models in intense agriculture area of the gangetic plains. Environ Monit Assess, India. doi:10.1007/s10661-014-4041-x
Saha D, Dhar YR, Sikdar PK (2008) Geochemical evolution of groundwater in the pleistocene aquifers of South Ganga Plain, Bihar. J Geol Soc India 71:473–482
Selvam S (2015) A preliminary investigation of lithogenic and anthropogenic influence over fluoride ion chemistry in the groundwater of the southern coastal city, Tamil Nadu, India
Shan H, Ting L, Chuanyong J (2013) Principal component analysis of fluoride geochemistry of groundwater in Shanxi and Inner Mongolia, China. J Geochem Explor 135:124–129
UNICEF (2009) Position on water fluoridation. Fluoride in water: an overview. http://www.nofluoride.com/Unicef_fluor.cfm
WHO (2002) Fluorides, Geneva, World Health Organization. Environmental Health Criteria, pp 227, 268
Acknowledgements
The authors would like to thank the University of Grant Commission, New Delhi, for the financial support under Centre with Potential for Excellence in Environmental Sciences scheme (CPEPA Grant no. F. No. 1-9/2002 NS/PE). Authors also thank the Jai-Kranti Abhiyan scheme of the Ministry of Water Resources, Government of India and Dipankar Saha, Member, CGWB, for motivating them to prepare this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Elango, L., Jagadeshan, G. (2018). Fluoride Contamination in Groundwater: A Pilot Study on Dug Well Recharge System for In situ Mitigation. In: Saha, D., Marwaha, S., Mukherjee, A. (eds) Clean and Sustainable Groundwater in India. Springer Hydrogeology. Springer, Singapore. https://doi.org/10.1007/978-981-10-4552-3_2
Download citation
DOI: https://doi.org/10.1007/978-981-10-4552-3_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4551-6
Online ISBN: 978-981-10-4552-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)