Abstract
The humungous load of pollutants added to the environment every day by the human activities is one of the major menaces facing by the world. Toxic substances released into the ecosystems are said to create imbalance to the equilibrium of the environment. Phytoremediation is a set of processes which have been considered as one of the most sustainable approaches to combat the problem of contaminants. Phytoremediation is considered to be more effective in comparison with traditional techniques because of the added benefits provided by the plants. The mechanisms adapted by the plants for extraction, accumulation, stabilization and degradation of contaminants from the polluted sites have been explored in this chapter. Various floral species which have been reported by several researchers that have the potential to remediate contaminated sites are listed in this report. The bioenergy crops, medicinal plants, trees and weeds have been found to be the best options for phytoremediation. Phytoremediation has proven to have a holistic approach which can help in restoration of contaminated sites with production timber, essential oils, energy, and employment to the rural peoples and with several other ecosystem services.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
The world population has exceeded seven billion and is rapidly approaching eight billion. This ever-increasing population has exerted tremendous chaos on the existing natural resources and has created immeasurable amount of wastes across the globe. When pollution is in manageable amount, the terrestrial, aquatic and atmospheric ecosystems can dilute, degrade or absorb the contaminants naturally. The rising burden of pollutants requires additional measures to curb the detrimental effects of pollution (Glick 2003; Glick 2010). Contaminants pose a threat to the environment because of their abundance and recalcitrant nature. Rampant industrialization and urbanization are the main culprits for the gradual degradation in environmental quality. The release of natural and anthropogenic contaminants is a major concern in the last few decades. There are numerous contaminants that continuously cause problems, some of which are easily curable but many are not. Plants act as Green Livers for the ecosystem clarifying any ill effects caused by contaminants and toxicants in the ambient environment (Sanderman 1994).
1.1.1 Contaminants: Sources, Types and Effects
A pollutant is anything that is present in the environment in excess to its original concentration. Waste generation by anthropogenic activities is so diverse in nature that it is difficult to categorize them effectively. Contaminants that create nuisance in soil and water are usually industrial wastes, municipal solid wastes, agricultural runoffs and leachates (organic pollutants) and radioactive wastes. The organic pollutants, heavy metals and radioactive wastes are dealt here as they are potentially the most problematic pollutants in terms of soil and water. They cause adverse effects directly to the plants as well as animals including human beings and sometimes indirectly by changing the natural composition of ecosystems (Fig. 1.1).
1.1.2 Heavy Metals
Heavy metals have been reported as one of the major nemeses for the environment. Apart from natural processes, maximum number of anthropogenic activities releases heavy metals (Tangahu et al. 2011). The problem lies when contaminants migrate to pristine areas in the form of metal dust or leachates as in the case of soil and also as sewage sludge (Gaur and Adholeya 2004). Heavy metals are those elements which have an atomic number more than 20. Metals are also present naturally in soil. Many of them are essential for growth and sustenance of soil flora and fauna. Zinc, copper, manganese, nickel and cobalt are imperative for survival of the plants. The importance of some metals such as cadmium, lead and mercury is unknown in respect to plants (Lasat 2000; Gaur and Adholeya 2004). Heavy metals are non-biodegradable, therefore creating problems in the overall biological systems. Heavy metals such as lead, cobalt and cadmium are more deleterious in nature because of their high bioaccumulation rate even at lower concentration (Pehlivan et al. 2009; Tangahu et al. 2011). Heavy metals may cause negative impact on plant growth and soil microflora (Roy et al. 2005). Arsenic is one major environmental pollutant which falls under the category of heavy metal having atomic number 33. Arsenic is found in the environment as organic arsenic species, inorganic arsenic compounds and arsine gas. Arsenic is a very toxic element, and its toxicity is usually dependents on the species. The inorganic compounds of arsenic are usually more toxic than its organic counterparts. Arsenites are more toxic in nature than arsenates as they are more prone to cause DNA breakdown (Ampiah-Bonney et al. 2007; Vaclavikova et al. 2008). Arsenates are found to be more stable thermodynamically than arsenites; therefore, they cause groundwater contamination (Chutia et al. 2009). Arsenic compounds are carcinogenic in nature and cause dermatitis where the groundwater is contaminated. Lead with atomic number 82 is a highly toxic element which is non-biodegradable and remains in the environment for a very long time and accumulates in the first 8 in. of the soil and remains immobile. Sources of lead include natural sources, industrial sites, leaded fuels and orchards where the use of lead arsenate takes place (Traunfeld and Clement 2001; Tangahu et al. 2011). The harmful effects of lead are spread across a wide range of organisms such as humans, animals, plants and microbes. In terms of human health, lead causes major adverse impacts such as mental retardation and brain damage (Cho-Ruk et al. 2006). Mercury is another heavy metal that is notoriously toxic and is available in soil in three soluble forms. It is a toxic element with a high bioaccumulation potential in living organisms such as human beings, fish and other animals. Mercury is found in naturally as well as by anthropogenic activities in the environment. Mercury pollution in the environment is caused by mining, petrochemical, painting industries, also from fertilizers, medical instruments, etc. (Resaee et al. 2005). Usually terrestrial plants are not very sensitive to the adverse impacts of mercury, but it has been found that mercury interferes with electron transport in mitochondria and chloroplasts and adversely affects oxidative metabolism and photosynthesis. Mercury acts as an inhibitor of aquaporin activities and causes reduction in water uptake in plant. In human beings, the toxic impacts of mercury include neurological and renal disorders (Resaee et al. 2005). As toxic metallic species cannot be degraded, there is a requirement of physical removal or transformation to lesser toxic or non-toxic compounds.
1.1.3 Organic Pollutants
Organic pollutants are synthetic and recalcitrant in nature. These organic xenobiotics are persistent in the environment and are highly toxic. They are known as persistent organic pollutants (POPs) as they are not easily degradable. Pesticides, petroleum products, pharmaceuticals, polyaromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) are some of the existing organic pollutants (Abhilash and Singh 2009). Twelve major POPs are known as the ‘dirty dozen’ which have been called for elimination and phasing out by the United Nations Environmental Program (US EPA 2005). Aldrin, dieldrin, chlordane, DDT, endrin, heptachlor, mirex, toxaphene, PCBs, HCBs, dibenzodioxins and dibenzofurans are the twelve most dangerous pollutants in respect to organic contaminants. Organic pollutants are a real menace for the ecosystem because of their persistence in the environment, lipophilic nature and high bioconcentration potential. These pollutants tend to get deposited in the adipose tissues of organisms (POPs, WHO Report 2008). Over a period of time, the pollutants reach a high level of toxicity because of their high bioconcentration potential, even though the exposure is limited. The pollutants move up the food chain as a result of biomagnification. Therefore, it is reported that the apex consumers reveal the maximum amount of organic pollutant concentration in their tissues. Marine mammals are known to have the highest concentration of these pollutants which caused reproductive disorders and higher susceptibility to infections resulting from microbes. The soil that is contaminated by organic pollutants causes death of soil microflora and reduction in plant growth and yield. Leaching of these pollutants causes groundwater contamination. Fertilizers when reaching the surface water bodies cause eutrophication by nutrient enrichment. The algal bloom caused by this nutrient enrichment reduces the dissolved oxygen level of the water bodies culminating in the death of aquatic flora and fauna. These are just few of the impacts of organic pollutants; there can be numerous direct and indirect effects of these contaminants. It is essential to remove these harmful toxicants from the environment to continue the balanced functioning of the ecosystems.
1.1.4 Radioactive Contaminants
Radioactive contaminants are introduced into the environment mostly by anthropogenic activities. Although radioactive elements are present in the environment naturally, they are not as harmful as contamination caused by anthropogenic causes because in nature, they are in a very low concentration. The environment is contaminated by radionuclides by nuclear weapon testing, disposal of nuclear wastes, emissions from nuclear power plants and also from spillage from plant operations such as nuclear fuel mining, milling and nuclear testing fallout, etc. In the process of oil drilling, sometimes radionuclides that occur naturally are brought up to the surface of the Earth (Fulekar et al. 2010). Chernobyl disaster in 1986 was one of the first nuclear power plant disasters which exposed the devastating effects of nuclear accidents to the world. The most recent nuclear accident occurred at Fukushima, Japan, during an earthquake at 2011; at Fukushima, an explosion was caused by failure of emergency cooling. Radionuclides are highly unstable nuclei possessing additional energy. There is a constant radioactive decay experienced by the radionuclides which forms alpha, beta and gamma particles as a result (Ghosh and Singh 2005; Fulekar et al. 2010). Consumption of food crops and water contaminated by radionuclides is one of the major causes of exposures to humans. The persistence of radiation in the environment can be over billions of years; therefore, it can cause irreparable damage to organisms as well as the ecosystem (Fig. 1.2) (Malhotra et al. 2014). Generally, the radiation released by the radionuclides can be carcinogenic and mutagenic in nature and is also known to cause birth defects and abnormalities in humans over a long period of exposure. Uranium-238 the most common natural isotope of uranium has a half-life of 4.46 billion years that is used in nuclear weapons and nuclear fuel. It is known to cause birth defects, cancer and mutations in the genes of humans (Jadia and Fulekar 2008). Thorium-232 is the most stable isotope with a half-life of 14 billion years, is used in nuclear fuel and alloying agent and is found to be carcinogenic in nature. Spinks and Woods (1990) state that radium-226 has a half-life of 1600 years and is used in an abundant fashion in our daily lives in the form of luminous paints and in dials of watches. An exposure for a long duration may cause fatal diseases like bone cancer, lymphoma, aplastic anaemia and leukaemia. During the Chernobyl accident, several radionuclides were released into the atmosphere; among them were isotopes of caesium-134 and caesium-137. These isotopes are retained by the soil and not washed away even by the heaviest rainfall. Isotopes of caesium are taken up by the plants, and they easily enter the food chain; also adverse effects are caused when there is an exposure to the contaminated soil surface (Westhoff 1999). The beta and gamma radiations of the radionuclides are highly dangerous and can cause ulcers, erythema or tissue necrosis in humans.
1.2 Contaminant Remediation Techniques
The above-mentioned problems are just the tip of the iceberg, and there are several underlying issues related to these contaminants that can cause direct or indirect impact on the environment. It is highly imperative to remediate the contaminated spheres of the environment. There are several conventional methods and techniques applied for the remediation of the contaminated areas. Some of the traditional methods to combat the problem of contaminated soil include:
-
1.
Soil excavation: Treatment or removal of contaminants in the case of soil is done by onsite management or by excavation of the contaminated soil and by its disposal at a landfill site. This method of disposal is not a real solution of the problem as it merely dislocates the contaminants from one area to another (Tangahu et al. 2011).
-
2.
Soil washing: As an alternative to the dislocation of contamination from the source to a landfill area, an onsite management method is applied. Soil washing is carried out by two processes: first of them is by dissolution or suspension of contaminated soil in a wash solution which is chemical in nature and the second process concentrates the contaminants into a smaller volume of soil by techniques such as gravity separation, particle size separation and attrition scrubbing. Heavy metals, organic xenobiotics and radionuclides can be removed by this process. This method is not cost-effective, and residues rich in contaminants require additional treatment. Therefore, this process is not extensively used (Tangahu et al. 2011).
-
3.
Stabilization/solidification: In this process, the contaminants present in the soil are stabilized or solidified either by physical or chemical interactions between the contaminant and a stabilizing agent (Gomes 2012).
-
4.
Vitrification: In the process of vitrification, heat is used for melting and subsequently solidifying the contaminants in a solid material which is glasslike in nature. Vitrification can be carried out onsite (in situ vitrification) and also aboveground in a separate treatment unit (ex situ vitrification).
-
5.
Electrokinetic treatment: The electrokinetic remediation technique is solely in situ-based where direct electric potential is applied using cathodes and anodes. According to Cameselle et al. (2013), various reactions take place in the contaminated soil due to the electric potential; as a result, the contaminants move towards the cathode or anode. The mobilization or transport mechanisms in electrokinetic treatment are of two types, electro-osmosis and electromigration. When there is a combined effect of electric charge and electric field on soil particle surface, it results in an electro-osmotic flux which causes the movement of negatively charged particles towards the cathode. In the electromigration mechanism, movement of ionic species takes place in the electric field towards the oppositely charged electrode (Cameselle and Reddy 2012).
Some other methods such as incineration and chemical oxidation/reduction are also used for the remediation of contaminated soil, but most of these traditional methods are not feasible because of high cost and problems regarding disposal of contamination-rich residues. Some of these techniques also destroy the soil biota causing the area to become devoid of life. Hence, it is essential for the sake of the environment to find alternative technologies that are environment-friendly and green in approach. These technologies must be cost-effective and reduce the pollutant load in the environment, and at the same time, the technique should have features which help them to resolve other major concerns like fuel crisis, emission of greenhouse gases, etc.
1.3 Phytoremediation: A Successful and Environment-Friendly Approach
Everyday new technologies are being developed by humans to vanquish the evil effects of pollution created by humans themselves. The solution lies in the hands of nature itself; plants are the nature’s best defence against all man-made pollution. The word phytoremediation originates by combining two words Phyto (Greek) meaning plants and remedium (Latin) meaning removal or correction of evil. In general words, phytoremediation means removal, degradation or stabilization of pollutants using plants. At current time, plants have regained their former status of importance because of their multifaceted applications. The contaminants are removed from soil, water and sediments using plants. Certain plant root systems have special uptake capabilities, and also the shoot systems are capable in translocation, accumulation and degradation of the contaminants. These features allow efficient uptake and removal of harmful toxicants from the environment. Phytoremediation is a solar energy-driven process and does not require external energy, so it is cost-effective and less (zero) polluting in comparison with traditional methods. There are several definitions of phytoremediation given by various researchers; few have been compiled in Table 1.1.
1.3.1 Types of Phytoremediation
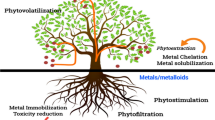
1.3.1.1 Phytoextraction
In terms of economic opportunities, phytoextraction presents the largest benefits (Raskin et al. 1997; Ismail 2012). Phytoextraction is considered as the most efficient method for removal of an isolation of contaminants from the polluted medium that is the soil where the fertility and structure of the soil is retained (EPA 2000). In the process of phytoextraction, the plant absorbs contaminants from the soil/water through roots and transfers or translocates them to the aerial parts of the plants. The aerial parts can be burnt to gain energy, and the metal can be recycled from the ash (Liu et al. 2000; Prasad and Freitas 2003 Erakhrumen and Agbontalor 2007; Moreno et al. 2008). Phytoextraction is most effective in large areas which have a contamination level of low to medium range, and the depth is also shallow (Kumar et al. 1995a, b; Blaylock and Huang 2000). The plant must possess some special characteristics to be efficient in the process of phytoextraction. These characteristics include tolerance towards the specific contaminant, efficient translocation of contaminants to aerial and harvestable parts of the plant and ability of plant to survive in stress conditions like soil pH, salinity, soil structure, water content and resistance to pests (Brooks 1994; Ismail 2012).
1.3.1.2 Phytostabilization
There are certain plant species that specialize in immobilizing contaminants in the soil or groundwater itself. These plants absorb and accumulate the contaminants in plant tissues, adsorb on the root surface or precipitate them within the root zone thereby preventing migration of contaminants in the soil and their movement by erosion (Liu et al. 2000; Prasad and Freitas 2003; Erakhrumen and Agbontalor 2007; Moreno et al. 2008). This method of phytoremediation is also known as phytorestoration. The plants used for phytostabilization must be weak in translocating the contaminants from the root to the aerial parts; must grow fast, having developed root systems and canopies, and must be tolerant towards abiotic and biotic stresses (Ismail 2012).
1.3.1.3 Phytofiltration
The process of phytofiltration can be of two types, one through the roots that is known as rhizofiltration and another one by seedlings that is known as blastofiltration. The roots or seedlings of the plant accumulate the contaminants from the effluents when grown in water that is aerated (Raskin et al. 1997). In this technique, plants are grown hydroponically; then they are transplanted in polluted water where they accumulate the contaminants (Dushenkov et al. 1995; Salt et al. 1995; Flathman and Hannza 1998). The phytoremediation of effluent or domestic wastewater is carried out using rhizofiltration. The contaminants are adsorbed or precipitated onto the plant roots and also in some cases absorbed and sequestered in the roots of plants present in constructed wetland for purification of effluent and wastewater (Liu et al. 2000; Prasad and Freitas 2003 Erakhrumen and Agbontalor 2007; Moreno et al. 2008). Ideally for rhizofiltration, plants must have roots that are fast growing and have higher efficiency in accumulation of contaminants over a longer time period. The toxic contaminants form a precipitate over the root surface which is then harvested and disposed (Flathman and Hannza 1998). The process of blastofiltration belongs to the second generation of water treatment technology which is plant based. After germination as there is an immense increase in the surface and volume ratio, the seedlings more effectively absorb or adsorb larger amounts of contaminants in ionic form making it more efficient than rhizofiltration (Raskin et al. 1997).
1.3.1.4 Phytovolatilization
In the process the contaminant is taken up by the plant and released by the process of transpiration either in the same form or in a modified form. In the process of phytovolatilization, the plant uptakes water which includes the contaminants, and the contaminants when reaching the aerial parts of the plants move out by transpiration (Liu et al. 2000; Prasad and Freitas 2003 Erakhrumen and Agbontalor 2007; Moreno et al. 2008). Some toxic contaminants exist in the atmosphere in gaseous form, for example, metallic species-like arsenic, mercury and selenium. In case of heavy metals, the plants adsorb metals in their elemental form, and then they are biologically converted into gaseous species which is known as biomethylation to create volatile molecules that are released into the atmosphere. There is a major disadvantage of this process in that volatile gaseous species may return to the ecosystem by precipitation thus creating havoc by spreading the toxic metals to a wider range of area (Henry 2000).
1.3.2 Mechanism of Phytoremediation
The basic steps involved in metal detoxification include metal ion binding on the cell wall of roots, metal ion transportation to the shoots and chelation of contaminants in cytosol (Fig. 1.5). The first step of mechanism of contaminant accumulation is the adsorption of metals on the root surface of the plants. Numerous metal transporters are located in the cell wall which allows metal ions to move inside the cell. Metal transporters can be grouped into ZIP family, NRAMP family and CTR family. IRT1 was found in Arabidopsis thaliana that belongs to the ZIP family expressed to accumulate higher amount of Fe at the time of Fe deficiency (Eide et al. 1996; Zaal et al. 1999; Guerinot 2000; Vert et al. 2002). This element has also been found to be characterized in A. thaliana and responsible for the accumulation and transport of Mn, Zn and Cd (Cohen et al. 1998; Korshunova et al. 1999; Zaal et al. 1999). Nishida et al. (2011) reported that expression of AtIRT1 enhances Ni accumulation in Saccharomyces cerevisiae. NRAMP is another metal transporter family which helps the plants to transport a number of metals like Cd, Ni, Zn, Fe, Cu, etc. (Nevo and Nelson 2006; Krämer et al. 2007).
In metal accumulator and hyperaccumulator plants, there are several defence mechanisms involved like (1) production of antioxidative components, e.g. ascorbate peroxidase (ASP), catalase (CAT), superoxide dismutase (SOD), glutathione S-transferase (GST), glutathione reductase (GR), proline, etc. (Ni et al. 2013; Shanmugaraj et al. 2013 ; Yu et al. 2013 ; Bauddh and Singh 2012a, b, 2015a, b), (2) production of phytochelatins (Cobbett 2000; Lee et al. 2003; Manara 2012), (3) production of metallothioneins (Nordberg 2004; Zimeri et al. 2005; Zhigang et al. 2006), (4) production of ferritins (Ravet et al. 2009; Liu et al. 2010; Yin et al. 2008; Rastgoo and Alemzadeh 2011), etc. These systems make a plant tolerant and enhance the metal-accumulating ability of plants at an even higher contamination level.
The production of metallothioneins in metal accumulator plants has been reported, and it is found that this component has the ability to detoxify the metal ion (Cobbett and Goldsbrough 2002; Papoyan and Kochian 2004; Zhigang et al. 2006; Mijovilovich et al. 2009). Many studies showed a substantial role of MTs in detoxification of Cu in many plants like Nicotiana tobacum, N. caerulescens, Thlaspi caerulescens, etc. (Kägi 1991; Maiti et al. 1991; Roosens et al. 2004; Papoyan and Kochian 2004; Mijovilovich et al. 2009; Leitenmaier and Küpper 2013).
It has been observed that during exposure to a biotic stresses like heavy metals, drought, salinity, etc. plants experience the overproduction of reactive oxygen species (ROS), e.g. superoxide radical (O2 −), hydroxyl radical (OH•), hydrogen peroxide (H2O2), singlet oxygen (1O2), etc. (Fig. 1.3) which can lead to a number of abnormalities like peroxidation of lipids and damage of proteins, enzymes, cell wall, etc. (Mittler 2002; Sharma and Dubey 2005; Asada 2006; Vanderauwera et al. 2011; Sharma et al. 2012; Noctor et al. 2014; Arora et al. 2016).
To overcome these adverse changes caused by ROS, plants produce antioxidative defence system which comprises of both enzymatic components like superoxide dismutase (SOD), catalase (CAT), peroxidase, ascorbate peroxidase (APX), glutathione reductase (GR), guaiacol peroxidase (GPX), etc. and several non-enzymatic components like ascorbate, carotenoids, glutathione (GSH), phenolics, tocopherols, etc. (Fig. 1.4) (Asada 2006; Slater et al. 2008; Sharma et al. 2012; Sewelam et al. 2016).
The mechanism of formation of reactive oxygen species and their removal by antioxidants and antioxidative enzymes. AA ascorbic acid, DHA dehydroascorbic acid, GHS glutathione, GSSG oxidized glutathione, SOD superoxide dismutase (Adopted from Slater et al. 2008; Page No. 230)
Phytochelatins are low molecular weight cysteine-rich proteins synthesized from glutathione by an enzyme phytochelatin synthase during prolonged exposure of heavy metals (Tommasini et al. 1998; Cobbett 2000; Clemens 2001; Schützendübel and Polle 2002; Harada et al. 2002; Gao et al. 2013). Phytochelatins contain gamma glutamylcystein and glycine in its structure (γ-Glu-Cys)n-Gly) (Kondo et al. 1984; Grill et al. 1986). An enhanced transcription of genes which synthesizes the precursor (glutathione reductase) of PCs was reported by Xiang and Oliver (1998) which confirmed the role of PCs as metal detoxifier (Hartley-Whitaker et al. 2001; Andresen et al. 2013). Further, Gao et al. (2013) demonstrated that the synthesis of PCs in plant Phytolacca americana is Cd dose dependent.
Ferritins are the proteins which have the ability to bind excess content of Fe in plants (Briat 1996; Fabisiak et al. 1999; Briat et al. 2006; Ravet et al. 2009; Briat et al. 2010). Phytoferritins are basically found in the mitochondria (Zancani et al. 2004, 2007) and non-photosynthetic plastids such as chromoplasts, proplastids, etioplasts, etc. (Seckback 1982; Ragland et al. 1990). Deák et al. (1999) proposed that ferritin can protect the plant from oxidative damage persuaded by a number of abiotic as well as biotic stresses (Fig. 1.5).
On the other hand, many plants secrete exudates from their roots which can chelate the metals and in soil only and prevent metal uptake inside the cell (Fig. 1.6) (Marschner 1995; Salt et al. 2000; Jung et al. 2003; Liao and Xie 2004, Schwab et al. 2005; Bais et al. 2006; Dong et al. 2007). The production of several organic acids as root secretion like malate, citrate, succinic, malonic, oxalate, etc. have been also reported to serve as a line of defence against toxic metals (Bidwell et al. 2002; Hall 2002; Pittman 2005; Hinsinger et al. 2006; Sun et al. 2006; Verbruggen et al. 2009; Gao et al. 2013). Verbruggen et al. (2009) suggested that these organic acids help in vacuolar transportation of heavy metals especially for Cd. Phytosiderophores have been reported by many authors that they produced specially by roots of leguminous crops during exposure of several heavy metals like Cu, Zn, Cd, etc. and play an important role in restricting the entry of metal ions inside the cell (Awad and Römheld, 2000; Shenker et al. 2001 Chaignon et al. 2002; Xu et al. 2005; Phytotechnology Mechanism 2005). Active metal efflux system in metal excluder plants also helps to restrict the entry of toxic metals (Baker 1981; van Hoof et al. 2001; Tong et al. 2004; Yang et al. 2005; Kushwaha et al. 2016).
1.3.2.1 Factors That Affect Uptake Mechanisms
The uptake mechanisms of plants used in phytoremediation are affected by several factors. The knowledge of these factors can be used to increase the efficiency of the phytoremediation potential of the plants.
1.3.2.1.1 Plant Species
Certain species of plants have superior remediation properties than other species; therefore, more efficient species must be selected for phytoremediation of contaminants. The plants that are most suitable must be hyperaccumulators and must produce more amounts of biomass (Rodriguez et al. 2005).
1.3.2.1.2 Properties of Growing Medium
Development of agronomical practices is carried out for enhancement of phytoremediation; factors such as pH, chelators and fertilizers are adjusted to increase the phytoremediation efficiency (Prasad and Freitas 2003).
1.3.2.1.3 Root Zone
Root zone is the main site for extraction, accumulation and stabilization of the contaminants. Therefore, the root zone must be well developed with high extraction, accumulation and stabilization efficiency. Sometimes, the degradation of contaminants takes place by enzymes that are exuded by the plant roots (Merkl et al. 2005).
1.3.2.1.4 Uptake Mechanism by Vegetative Parts
The environmental factors play a critical role in the uptake mechanism by vegetative parts. The growth enzymes are affected by the temperature which in turn affects the root length. The fate of metabolic activities of the contaminants inside the plants is very important in deciding the phytoremediation potential and efficacy (Mwegoha 2008).
1.3.2.1.5 Chelating Agents
The addition of chelating agents can enhance the capacity of the plants to extract and accumulate contaminants from the soil. Even micronutrients can be added along with the chelators to increase uptake. Chelating agents like EDTA are added in case of heavy metal contaminants. There is a chance of leaching in case of addition of chelators which are synthetic in nature (Van Ginneken et al. 2007; Tangahu 2011).
1.3.3 Indices Used for Assessment of Phytoremediation Potential
The suitability of plant for the purpose of phytoremediation depends on several factors: some of them are intrinsic plant characteristics; others are dependent on the environment or the contaminants. It is of utmost importance for the plants to accumulate a large amount of contaminants from the site. Also the ability of the plant to translocate the contaminants from the roots to shoots is of concern. Enrichment coefficient and translocation factor are two methods to measure the amount of contaminant accumulated and translocated by the plant. The amount or degree of heavy metal concentration/accumulation in the plants which are grown on contaminated sites is determined by enrichment coefficient (Kisku et al. 2000).
Translocation factor (TF) is the ratio which defines the movement or mobilization of metal from roots to shoots of any plant. Equation 1.2 gives the formula for calculation of TF (Barman et al. 2000; Gupta et al. 2008; Shi et al. 2011).
Tolerance index is another major index which determines the suitability of any plant for the purpose of phytoremediation. It is imperative for a plant to exhibit healthy growth for its own survival and for extraction and accumulation of toxicants. The TI of any plant is based on the biomass produced by the plant. Equation 1.3 states the formula for the calculation of tolerance index (de Souza et al. 2012).
These indices are used by the researchers to test the potential of the desired plants for phytoremediation.
1.3.4 Different Aspects of Phytoremediation
1.3.4.1 Application of Edible Crops
In the present world, the availability of land as a resource is a major cause of concern due to the exponential population rise. It is imperative that land usage should be judicious and serve multidimensional benefits. Therefore, researchers have tried hitting two birds with one stone and have developed phytoremediation techniques using edible crops. Application of edible crops for remediation will serve several benefits such as decontamination of the land, food production, and efficient land usage. The edible crops studied for phytoremediation potential by various researchers include wheat (Khan et al. 2011), maize (Mojiri 2011), sunflower (Liphadzi et al. 2003), Indian mustard (Sainger et al. 2014), Amaranthus (Shevyakova et al. (2011), tobacco (Chitra et al. 2011), tomato (Uera et al. 2007), Trapa (Sweta et al. 2015), etc. Tabulation of these examples has been done in Table 1.2. These are just few examples of the edible crop plants utilized for phytoremediation; there are plenty of literatures available on many other plants as well. Albeit, numerous studies have been carried out testing the phytoremediation potential of edible crops; there are some major demerits associated with them. According to Bauddh et al. (2015a, b), the first obvious demerit is the bioaccumulation of toxicants in the edible plant which can further lead to biomagnification and move up the food chain causing toxicity to animals and humans. Other negative traits of edible crops regarding phytoremediation include short life span, low biomass production and high palatability. For efficient remediation of contaminants, the plants ideally must have a long life span, should be unpalatable and must produce larger amount of biomass for higher accumulation of contaminants (Pandey and Singh 2011). The above-mentioned problems of edible crops reduce the overall feasibility of phytoremediation by using these crops. If these problems can be solved like containing the contaminants in the unpalatable portions of the plant and increasing the biomass by technological interventions (biotechnological), only then edible crops may also be effectively used for the remediation purposes.
1.3.4.2 Application of Weeds
Phytoremediation can effectively curb the toxic impacts of the environmental contaminants. Researchers have tried developing methods of phytoremediation using weeds. Terrestrial as well as aquatic weeds have been experimented with, and encouraging results have been recorded. If aquatic weeds efficiently remove contaminants from effluents, it will prove to be a boon as they are fast growing, and surface water can be easily treated by using them. Several researchers have used aquatic weeds such as alligator weed, duckweed, water lettuce, water hyacinth and Azolla spp. for the remediation of several toxicants from water (Cho-Ruk et al. 2006; Skinner et al. 2007; Zhang et al. 2008; Rahman et al. 2008). Terrestrial weeds such as Parthenium hysterophorus, Tridax procumbens, Cyperus procera, Euphorbia hirta and Datura stramonium are just few of the examples which have been studied for their phytoremediation potential against heavy metals (Kumar et al. 2013). Table 1.3 describes some of the successful experiments on remediation of contaminants by terrestrial as well as aquatic weeds.
In the studies conducted by Kumar et al. (2012 and 2013), emphasis has been given on EC and TF of the contaminants in the plant bodies. Enrichment coefficient gives an accurate estimation of the total contaminant (heavy metal accumulated by the plants from a contaminated site). If the EC is high, the plant is considered to be suitable for phytoremediation. Eichhornia crassipes showed high values of EC and TF when tested with heavy metals such as Cr, Pb, Ni and Cd making this aquatic weed most suitable among other weeds for phytoremediation of heavy metals (Kumar et al. 2012). Among the terrestrial weeds, Tridax procumbens, Cyperus procera, Euphorbia hirta, Parthenium hysterophorus and Datura stramonium exhibited higher EC in that order and were found suitable for phytoremediation purpose by Kumar et al. (2013). According to Baker (1981) if the translocation factor is more than one, then the plant is termed as metal accumulator, and if it is below one, the plant is known as metal excluder. Kumar et al. (2013) in their study found that TF of the terrestrial weeds ranged between 0.119 for Cd in T. procumbens and 3.86 for lead in S. oleracea (described in Fig. 1.7). P. hysterophorus and S. oleracea exhibited TF more than one for all the heavy metals studied (Cu, Pb, Cd and Ni) which made them ideal metal accumulators. The Cyprus spp. (C. procera and C. rotundus) recorded all the TF values less than one making the weeds unsuitable for phytoremediation.
Translocation factor (TF) of the terrestrial weeds grown naturally in the metal-contaminated sites (Kumar et al. 2013)
The aquatic weeds studied by Kumar et al. (2012) presented impressive results regarding TF. It was found that most of the aquatic weeds had TF above one. The study of the average TF (Fig. 1.8) for all these aquatic weeds disclosed the fact that Marsilea minuta (2.82), Bacopa monnieri (1.84) and Hydrilla verticillata (1.69) were most efficient in translocation of heavy metals from the roots to shoots and thus can be used for remediation of heavy metal-contaminated sites.
Translocation factor (TF) of the aquatic weeds (macrophytes) naturally growing in the drain receiving tannery effluent (Kumar et al. 2012)
1.3.4.3 Application of Trees
Trees are considered as one of the most important entities in terms of phytoremediation. Trees have higher biomass and extensive root system which enable them to accumulate more contaminants from the surrounding soil. Many authors have studied the phytoremediation potential of the trees extensively, and few of the studies have been compiled in Table 1.4. The species of trees from the Salicaceae family (willow, poplar) were found to be most appropriate for the phytoremediation purpose of contaminants. More research needs to be carried out using multipurpose trees that would help in remediation of contaminants in addition to carbon sequestration and employment generation.
De Souza et al. (2012) studied three species of leguminous plants Erythrina speciosa, Schizolobium parahyba and Mimosa caesalpiniaefolia for their lead tolerance at seedling stage. The indices studied by the author were TF, BCF and TI. The tolerance index is calculated on the basis of the biomass yield of the plant (Shi et al. 2011). The biomass yield of the controlled plant and plants grown in a contaminated site are compared. The author found that the tolerance index of Mimosa caesalpiniaefolia recorded the highest readings of 1.20, 1.28 and 1.29 for 250, 500 and 1000 mg Kg−1 of lead; Erythrina speciosa recorded 0.71, 0.78 and 0.65 for 250, 500 and 1000 mg Kg−1 of lead; and Schizolobium parahyba recorded 0.84, 0.76and 0.67 for the similar Pb concentrations. Therefore, it can be concluded that Mimosa caesalpiniaefolia was the most tolerant species followed by Schizolobium parahyba and Erythrina speciosa. The TI of the three species is represented in Fig. 1.9 .
Tolerance index (TI) of some tree species growing in lead-contaminated soil (Source: de Souza et al. 2012)
1.3.4.4 Application of Bioenergy Crops
Holistic approach should be applied for remediation of toxicants from the environment. It is of utmost importance to detoxify the contaminants using sustainable means. Amalgamation of phytoremediation techniques with sustainable approach would provide multidimensional benefits for the entire Earth. Using bioenergy crops or trees is one such measure that is sustainable in approach and can be effectively tapped for phytoremediation. Several bioenergy crops have been tested for phytoremediation potential by the researchers in the recent past. If bioenergy crops are used for phytoremediation, it would save contaminated sites from being discarded; also it would generate employment and increase the interest of the people in plantation of such crops. Both edible and nonedible energy crops have been tested for their phytoremediation potential by researchers with encouraging results (Rowe et al. 2009; Shi and Cai 2009; Meers et al. 2010; Bauddh and Singh 2012a, b, 2015a, b; Bauddh et al. 2015a, b, 2016a, b). The use of edible crops for phytoremediation poses a bit of a concern because it is assumed that toxicants might enter the food chain. The study conducted by Meers et al. (2010) showed that the grains, the edible part of maize, accumulated the lowest amount of heavy metals. The researcher attributed this result to the defence mechanism of the plant to restrict toxicity from reaching the reproductive parts and seeds and constraining them within the vegetative parts of the plants. More research needs to be carried out to test the phytoremediation potential of the bioenergy crops as it would help in detoxifying the environment along with the generation of clean fuel and lower the carbon emission into the atmosphere. Using bioenergy crops would provide the most wholesome results in comparison with all other plants combined (Table 1.5).
1.3.4.5 Aromatic Plants Used in Phytoremediation
It is of preference to use nonedible crops for the purpose of phytoremediation because of the obvious reasons of avoiding bioaccumulation and biomagnifications of toxicants. Very recently few aromatic plants have been tested for their potential to remediate contaminants. This will serve the dual purpose of providing essential oils derived from the plant along with cleansing the environment. The plants such as Ocimum basilicum (basil), Cymbopogon martinii (palmarosa), Vetiveria zizanioides (vetiver), Cymbopogon flexuosus (lemon grass), Mentha sp. (geranium mint) (citronella) and Cymbopogon winterianus have been considered for their phytoremediation potential. Gupta et al. (2013) suggest that the likes of basil are viable and feasible for phytoremediation, and other aromatic grasses (lemon grass, citronella, palmarosa and vetiver) are perennial in nature as well as stress tolerant. These qualities make them appropriate for removal of toxicants from the environment. These perennial herbs can be planted at the contaminated sites, and they can accumulate contaminants in the biomass. The plants can be harvested, and their essential oil can be extracted by steam distillation. In this process, the essential oil forms a separate layer on the top, and the water containing the contaminants is left in the lower layer; the essential oil can be separated and used after its quality assessment (Pandey et al. 2015).
1.3.4.6 Plants as Hyperaccumulators
Certain plants have the tendency to accumulate larger amount of contaminants from the environment without showing adverse effects. These plants can be considered to be most ideal in terms of suitability for removal of toxicants. Different researchers have put forth several definitions for hyperaccumulator plant species; the plant species that can accumulate contaminants (metals, metalloids, etc.) at levels 50–500 times higher than their concentrations in soil are considered as hyperaccumulators (Clemens 2006, Kotrba et al. 2009). Another variation is mentioned by Brooks et al. (1998) who state that hyperaccumulators are those plant species which accumulate any element from the substrate at concentration 100 times higher than the substrate or medium. There are certain standards set for considering any plant as a hyperaccumulator; specifically for metals the concentration must be 0.1 weight % as dry weight; for Cd it is variable up to 0.01 weight % and 1 % for Zn (Reeves and Baker 2000). More than 45 families of plants are known to belong to hyperaccumulating species and over 450 plants. The number of hyperaccumulating plants is less in context to the problem of pollution because of their biomass which is low, their slower growth rate and being specific in contaminant accumulation (Chaney et al. 2005). Few examples of hyperaccumulating plant species have been tabulated in Table 1.5. It is seen that the plant family Brassicaceae is dominant in producing hyperaccumulators; other families such as Fabaceae and Crassulaceae also contain hyperaccumulators. Certain plants that are hyperaccumulators can be made more efficient with genetic engineering and with biological amendments (Table 1.6).
1.3.5 Application of Chemical and Biological Amendments to Enhance Phytoremediation
Although phytoremediation is an excellent option for the effective removal of contaminants from the environment, there are few drawbacks of this technique too. One of the major drawbacks is the time taken for complete remediation of a particular site which could be as long as 15–20 years, even if hyperaccumulating species are used. At the recent past, certain amendments in the process of phytoremediation are applied to make it more effective in terms of time and efficiency. Even highly efficient plants exhibit deleterious effects of heavy dosage of contaminants. There is usually a reduction in growth and yield of plants due to over accumulation of the contaminants. The phytoremediation potential of plant species as well as other organisms is being thoroughly studied to find methods to eliminate the risk of ever-increasing contaminant load. Algae, fungi and bacteria are few organisms which have the ability to speed up the process of phytoremediation. Since the past few years, researchers are working on making biological amendments to plant species to increase their efficiency in remediation of toxicants. The importance of bacteria and fungi in increasing plant efficiency for phytoremediation has been dealt in this chapter.
1.3.6 Role of Bacteria in Enhancement of Phytoremediation Potential of Plants
According to Glick (2010), there are rich population of bacteria near the rhizosphere because of the release of nutrient-rich exudates; these bacteria can degrade organic contaminants by phytostimulation or rhizodegradation (Kuiper et al. 2004). In context to phytoremediation, the biodegradative bacteria and bacteria that promote plant growth are very useful. Bacterial species such as Pseudomonas spp. are capable of degrading organic xenobiotics with the help of several enzymes produced on its plasmids (Cork and Krueger 1991; Glick 2010). The bacteria that are degradative in nature are capable of converting nonhalogenated compounds in easily metabolizable compounds catechol or protocatechuate. Halogen-based aromatic compounds which are the main constituents of biocides are very slowly degraded by plasmid-encoded enzymes (Glick 2010). Growth-promoting bacterial species releases phytohormones such as auxin which have a direct effect on the plant (Brown 1974; Patten and Glick 1996). A higher concentration of the heavy metals in the plant body causes synthesis of stress ethylene and deficiency in iron content (Glick 2010). A few bacteria release an enzyme ACC deaminase that is capable of lowering the phytohormone ethylene in a plant that is subjected to stress (Glick 2010). Another such enzyme IAA is released by IAA bacteria which helps in adventitious and lateral root elongation and prevent environmental stress-related adverse effects (Lindberg et al. 1985; Frankenberger and Arshad 1995). Table 1.5 represents few examples of bacteria and associated plants used for phytoremediation (Table 1.7).
1.3.7 Role of Fungi in Enhancement of Phytoremediation Potential of Plants
According to Glick (2010) almost 90 % of plants that are terrestrial have mycorrhizal association. Therefore it is prudently suggested that the beneficial impacts of fungi in regard to phytoremediation must also be taken into account to increase the efficiency of the plants for the remediation of harmful toxicants. The species of fungi that form mycorrhizal association with the plants have proven to increase the accumulation and tolerance of contaminants from the soil or water. Few examples of fungi and plant association that remediates contaminants have been listed in Tables 1.7 and 1.8.
1.3.8 Technological Interventions in Plants Used for Phytoremediation
It is said that plants have intrinsic qualities that enable them to detoxify contaminants, but there is a lacuna in terms of catabolic pathway which they lack, inhibiting complete degradation of the contaminants. Microbes are efficient in this matter and can completely degrade xenobiotics (Abhilash et al. 2009). Genetic engineering plays a pivotal role in enhancement of the plants’ ability to accumulate and detoxify contaminants. Transgenic plants as well as electrokinetic techniques have been employed to enhance the phytoremediation potential, and it has been successfully implemented. The role of transgenic crops and electrokinetic process in enhancement of phytoremediation potential has been briefly described in this section. For the enhancement of phytoremediation potential, another approach has been followed by Bauddh and Singh (2015a). The authors have used inorganic fertilizers, biofertilizers (Bacillus subtilis and Azotobacter chrocoocum), slow-release fertilizers and vermicompost to study their effects on accumulation and partitioning capacity of Brassica juncea and Ricinus communis for cadmium. It was found that protein content that decreased due to Cd stress was recovered by using biofertilizers. The use of biofertilizers increased metal accumulation, whereas vermicompost decreased bioaccumulation by the plants. The biofertilizers and vermicompost increased the overall health of the plants. Ricinus communis was found to be more tolerant and accumulated more Cd than Brassica juncea.
1.3.8.1 Transgenic Plants and Phytoremediation
Earlier applied only for inorganic pollutants; gradually, transgenic plants have progressed towards remediation of organic pollutants such as explosives, chlorinated solvents and hydrocarbons (Salt et al. 1998; Pilon-Smits 2005). Heavy metals were the first contaminants to be remediated by transgenic plants using tobacco plant which expressed a metallothionein gene to create higher tolerance for cadmium and Arabidopsis thaliana plant which overexpressed a reductase gene mercuric ion for creating more tolerance to Hg ( Misra and Gedamu 1989; Rugh et al. 1996). The plants that have been developed with transgenes are used in two ways for phytoremediation purpose: first is the use of transgenes for metabolizing the contaminants and second is the use of transgenes to increase the resistance of the plants towards the toxicants (Abhilash et al. 2009). Some examples of transgenic plants used for remediation of contaminants have been listed below in Tables 1.7 and 1.9.
1.3.8.2 Role of Electrokinesis for Enhanced Phytoremediation
In situ treatment of contaminated sites can be done by the techniques associated with electrokinetic remediation (Reddy and Cameselle 2009). In this technique the contaminated soil is subjected to electric potential directly by inserting electrodes into the ground. Various transport processes and reactions are induced by the electric potential; this causes the movement of contaminants towards the oppositely charged electrodes. The mobilization of the toxicants occurs by two processes: (a) electromigration is a process in which the contaminants move towards the electrodes of opposite charge and (b) electro-osmosis is a process in which the net flux of water is induced by electric field through structure of soil that is porous in nature. Usually, the particles of soil are charged negatively; thus they move towards the cathode (Cameselle and Reddy 2012). Phytoremediation coupled with electrokinetic techniques have a promising future and need to be researched further for contaminants like heavy metals and others as well. Several researchers imply electrokinetics during cultivation of plants in contaminated sites and have been found that the application of electrokinetics enhanced the bioaccumulation of contaminants (Tables 1.8 and 1.10).
1.3.9 Multitasking Approach of Phytoremediation
It is known that all plants provide innumerable benefits to the ecosystem. We are aware of only a small fraction of ecosystem services that is provided by the plants. Hence, the preference of plants over traditional techniques for remediation of contaminants is understandable. The traditional methods would only address the problem of the contaminants, but when plants are applied for the same purpose, several added advantages would be achieved (Fig. 1.10). The first and the foremost advantage of phytoremediation is the release of oxygen by the plant which would be a major boon. The second merit would be the carbon sequestration by the plants. It is well known that plants are the major storehouses of carbon. If trees are used for phytoremediation, a large amount of CO2 can be fixed by the plants which would help in curbing the greenhouse effect. The use of bioenergy crops for phytoremediation would remove the contaminant along with energy generation; this would be a very major advantage for the people as well as the environment. As phytoremediation is a solar energy-driven process, using plants, the energy may be used up in application of the traditional methods. If the plants used for the remediation of contaminants are cash crops, they would provide employment for the masses. This is the most important merit for the humans especially the ones living in the developing countries. Employment generation would boost the application of plants for phytoremediation as the plants can be harvested for their parts, and the pollutants can be removed at the same time. It would help in the overall societal development and improve the ambient environment.
1.3.10 Economic Feasibility of Phytoremediation Over Conventional Methods
Any technology or process needs to be economically feasible to be practically applied. It is same in the case of the phytoremediation also as the process needs to be beneficial in terms of monetary gains as well. It has been found that using plants for remediating pollutants has indeed been superior to traditional techniques in monetary terms. Table 1.9 is a compilation of comparison of the cost between phytoremediation and traditional techniques of studies conducted by several authors like Black (1995), Jipson (1996), Plummer (1997), Wang and Wan (2013), etc. Traditional techniques have cost more than the phytoremediation processes making phytoremediation feasible for implementation. Phytoremediation is more economically beneficial than traditional techniques because of the additional merits such as energy generation, food production, essential oil production, timber production and several other ecosystem and societal services (Table 1.11).
1.3.11 Constraints of Phytoremediation
All technologies and processes comprise of some pros and cons, and this is also applicable in the case of phytoremediation. Phytoremediation is a time-taking process as a long time taken for maximum removal of contaminants from the site; even then complete removal of the contaminants is not guaranteed. After excavation, incineration or disposal might take maximum time in months to accomplish the task, whereas phytodegradation or phytoextraction might take several years (Mwegoha 2008). The phytoremediation process is dependent on edaphic factors and soil chemistry where the soil pH, conductivity, porosity, nutrient levels and presence of soil microbes are instrumental in deciding the uptake mechanisms of the plants. Climatic factors are also very essential in determining the uptake mechanisms, and climatic stress can cause lower phytoremediation potential of the plants. Toxicants are known to have detrimental effects on the plant bodies; even hyperaccumulators exhibit negative impacts after prolonged exposure to the toxicants. Therefore, over a period of time, the efficiency of the plants for phytoremediation reduces making the process unfeasible. Another factor that might hamper the phytoremediation potential of the plant is the age of the plant. Younger plants are said to accumulate more contaminants that the older plants. Some studies suggest that older plant having more biomass accumulates more toxicants in total which can compensate for their lower physiological activities (Tu et al. 2004). Overall despite several constraints, phytoremediation proves to be an environment-friendly and sustainable approach which can be implemented effectively.
1.4 Conclusions
At present era, phytoremediation provides a solution to the most disastrous problem of pollution that is faced by mankind. Phytoremediation not only addresses the problem of pollution but also provides several ecosystem services along with making it a viable and feasible approach. Especially the use of bioenergy crops, aromatic plants and tree species can result in a holistic development of the ecosystem and its population. Being economically feasible, it can be encouraged to be adapted by the masses for decontamination of the sites. A wide range of contaminants can be remediated by plants at a lower cost which is a commendable feat. Technological and biological amendments can be made to increase the efficiency of the plants for the remediation of the contaminants. It is of immense importance for the sake of our environment to promote phytoremediation.
References
Abhilash PC, Singh N (2009) Pesticide use and application: an Indian scenario. J Hazard Mater 165:1–12
Abhilash PC, Jamil S, Singh N (2009) Transgenic plants for enhanced biodegradation and phytoremediation of organic xenobiotics. Biotechnol Adv 27:474–488
Aboughalma H, Bi R, Schlaak M (2008) Electrokinetic enhancement on phytoremediation in Zn, Pb, Cu and Cd contaminated soil using potato plants. J Environ Sci Health Part A 43:926–933
Ampiah-Bonney RJ, Tyson JF, Lanza GR (2007) Phytoextraction of arsenic from soil by Leersia oryzoides. Int J Phytoremed 9(1):31–40
Andresen E, Mattusch J, Wellenreuther G, Thomas G, Arroyo Abad U, Küpper H (2013) Different strategies of cadmium detoxification in the submerged macrophytes Ceratophyllum demersum L. Metallomics 5(10):1377–1386
Antonious GF, Snyder JC (2007) Accumulation of heavy metals in plants and potential phytoremediation of lead by potato, Solanum tuberosum L. J Environ Sci Health A Tox Hazard Subst Environ Eng 42(6):811–816
Arora D, Jain P, Singh N, Kaur HC (2016) Mechanisms of nitric oxide crosstalk with reactive oxygen species scavenging enzymes during abiotic stress tolerance in plants. Free Radic Res 50(3):291–303
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141(2):391–396
Ashry NA, Mohamed HI (2012) Impact of secondary metabolites and related enzymes in flax resistance and/or susceptibility to powdery mildew. Afr J Biotechnol 11(5):1073–1077
Awad F, Römheld V (2000) Mobilization of heavy metals from contaminated calcareous soils by plant born, microbial and synthetic chelators and their uptake by wheat plants. J Plant Nutr 23:1847–1855
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Baker AJM (1981) Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr 3:643–654
Barman SC, Sahu RK, Bhargava SK, Chatterjee C (2000) Distribution of heavy metals in wheat, mustard and weed grains irrigated with industrial effluents. Bull Environ Contam Toxicol 64:489–496
Bauddh K, Singh RP (2012a) Cadmium tolerance and its phytoremediation by two oil yielding plants Ricinus communis (L.) and Brassica juncea (L.) from the contaminated soil. Int J Phytoremed 14:772–785
Bauddh K, Singh RP (2012b) Growth, tolerance efficiency and phytoremediation potential of Ricinus communis (L.) and Brassica juncea (L.) in salinity and drought affected cadmium contaminated soil. Ecotoxicol Environ Saf 85:13–22
Bauddh K, Singh RP (2015a) Effects of organic and inorganic amendments on bio-accumulation and partitioning of Cd in Brassica juncea and Ricinus communis. Ecol Eng 74:93–100
Bauddh K, Singh RP (2015b) Assessment of metal uptake capacity of castor bean and mustard for phytoremediation of nickel from contaminated soil. Biom J 19(2):124–138
Bauddh K, Singh K, Singh B, Singh RP (2015a) Ricinus communis: a robust plant for bio-energy and phytoremediation of toxic metals from contaminated soil. Ecol Eng 84:640–652
Bauddh K, Singh K, Singh RP (2015b) Ricinus communis L. a value added crop for remediation of cadmium contaminated soil. Bull Environ Contam Toxicol 96(2):265–269
Bauddh K, Kumar A, Srivastava S, Singh RP, Tripathi RD (2016a) A study on the effect of cadmium on the antioxidative defense system and alteration in different functional groups in castor bean and Indian mustard. Arch Agron Soil Sci 62(6):877–891
Bauddh K, Singh B, Singh RP (2016b) Ricinus communis L. as a value added alternative for restoration of cadmium contaminated and degraded agricultural ecosystem. Bull Environ Contam Toxicol 96(2):265–269
Bi R, Schlaak M, Siefert E, Lord R, Connolly H (2010) Alternating current electrical field effects on lettuce (Lactuca sativa) growing in hydroponic culture with and without cadmium contamination. J Appl Electrochem 40:1217–1223
Bidwell SD, Batianoff GN, Sommer-Knudsen J, Woodrow IE (2002) Hyperaccumulation of manganese in the rainforest tree Austromyrtus bidwillii (Myrtaceae) from Queensland, Australia. Funct Plant Biol 29:899–905
Black H (1995) Absorbing possibilities: phytoremediation. Environ. Health Perspect 103:1106–1108
Blaylock MJ, Huang JW (2000) Phytoextraction of metals. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York, pp 53–70
Briat JF (1996) Roles of ferritin in plants. J Plant Nutr 19:8-9
Briat JF, Cellier F, Gaymard F (2006) Ferritins and iron accumulation in plant tissues. In: Barton LL, Abadía J (eds) Iron nutrition in plants and rhizospheric microorganisms, Chapter 17. Springer, New York, pp 341–357
Briat J, Ravet K, Arnaud N, Duc C, Boucherez J, Touraine B, Cellier F, Gaymard F (2010) New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot 105(5):811–822
Brooks RR (1994) In: Gargo ME (ed) VCH Verlagsgesellsschaft. Plants and chemical elements: biochemistry, uptake, tolerance and toxicity. Weinheim, 88–105
Brooks RR, Chambers MF, Nicks LJ, Robinson BH (1998) Phytomining. Trends Plant Sci 3:359–362
Brown ME (1974) Seed and root Bacterization. Annu Rev Phytopathol 12:181–197
Cameselle C, Reddy KR (2012) Development and enhancement of electro-osmotic flow for the removal of contaminants from soils. Electrochim Acta 86:10–22
Cameselle C, Chirakkara RA, Reddy KR (2013) Electrokinetic-enhanced phytoremediation of soils: status and opportunities. Chemosphere 93(4):626–636
Chaignon V, Di Malta D, Hinsinger P (2002) Fe-deficiency increases Cu acquisition by wheat cropped in a Cu-contaminated, vineyard soil. New Phytol 154:121–130
Chandra R, Bharagava RN, Yadav S, Mohan D (2009) Accumulation and distribution of toxic metals in wheat (Triticum aestivum L.) and Indian mustard (Brassica campestris L.) irrigated with distillery and tannery effluents. J Hazard Mater 162(2–3):1514–1521
Chaney RL, Angle JS, McIntosh MS, Reeves RD, Li YM, Brewer EP, Chen KY, Roseberg RJ, Perner H, Synkowski EC, Broadhurst CL, Wang S, Baker AJM (2005) Using hyperaccumulator plants to phytoextract soil Ni and Cd. Z Naturforsch C 60(3–4):190–198
Chitra K, Sharavanan S, Vijayaragavan M (2011) Tobacco, corn and wheat for phytoremediation of cadmium polluted soil. Recent Res Sci Technol 3(2):148–151
Cho-Ruk K, Kurukote J, Supprung P, Vetayasuporn S (2006) Perennial plants in the phytoremediation of lead contaminated soils. Biotechnol 5(1):1–4
Chutia P, Kato S, Kojima T, Satokawa S (2009) Arsenic adsorption from aqueous solution on synthetic zeolites. J Hazard Mater 162(1):440–447
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Cobbett CS (2000) Phytochelatin biosynthesis and function in heavy metal detoxification. Curr Opin Plant Biol 3:211–216
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Cohen CK, Fox TC, Garvin DF, Kochian LV (1998) The role of Iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol 116:1063–1072
Cork DJ, Krueger JP (1991) Microbial transformations of herbicides and pesticides. Adv Appl Microbiol 36:1–66
de Souza SCR, de Andrade SAL, de Souza LA, Schiavinato MA (2012) Lead tolerance and phytoremediation potential of Brazilian leguminous tree species at the seedling stage. J Environ Manag 110:299–307
Deák M, Horváth GV, Davletova S, Török K, Sass L, Vass I, Barna B, Király Z, Dudits D (1999) Plants ectopically expressing the ironbinding protein, ferritin, are tolerant to oxidative damage and pathogens. Nat Biotechnol 17:192–196
Dimkpa CO, Merten D, Svatos A, Büchel G, Kothe E (2009) Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J Appl Microbiol 107:1687–1696
Dong J, Mao WH, Zhang GP, Wu FB, Cai Y (2007) Root excretion and plant tolerance to cadmium toxicity – a review. Plant Soil Environ 53(5):193–200
Dushenkov V, Kumar P, Motto H, Raskin I (1995) Rhizofiltration: the use of plants to remove heavy metals from arduous streams. Environ Sci Technol 29:1239–1245
Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci U S A 93:5624–5628
EPA (2000) (U. S. Environmental Protection Agency), “Introduction to Phytoremediation,” National Risk Management Research Laboratory, EPA/600/R-99/107, 2000, http://www.clu-in.org/download/remed/introphyto.pdf
Erakhrumen A, Agbontalor A (2007) Review Phytoremediation: an environmentally sound technology for pollution prevention, control and remediation in developing countries. Educ Res Rev 2(7):151–156
Fabisiak JP, Pearce LL, Borisenko GG, Tyhurina YY, Tyurin VA, Razzack J, Lazo JS, Pitt BR, Kagan VE (1999) Bifunctional anti/pro-oxidant potential of metallothionein: redox signaling of copper binding and release. Antioxid Redox Signal 1:349–364
Flathman PE, Hannza GR (1998) Phytoremediation: current view on an emerging green technology. Soil Contamin 7(4):415–432
Flocco CG, Lindblom SD, Smits E (2004) Over expression of enzymes involved in glutathione synthesis enhances tolerance to organic pollutants in Brassica juncea. Int J Phytoremed 6:289–304
Frankenberger JWT, Arshad M (1995) Microbial synthesis of auxins. In: Frankenberger WT, Arshad M (eds) Phytohormones in soils. Marcel Dekker, New York, pp 35–71
Fulekar MH, Singh A, Thorat V, Kaushik CP, Eapen S (2010) Phytoremediation of 137Cs from low level nuclear waste using Catharanthus roseus. Indian J Pure Appl Phys 48(7):516–519
Gao L, Peng K, Xia Y, Wang G, Niu L, Lian C, Shen Z (2013) Cadmium and manganese accumulation in Phytolacca americana L and the roles of non-protein thiols and organic acids. Int J Phytoremed 15(4):307–319
Garg N, Aggarwal N (2011) Effects of interactions between cadmium and lead on growth, nitrogen fixation, phytochelatin, and glutathione production in mycorrhizal Cajanus cajan (L.) Millsp. J Plant Growth Regul 30(3):286–300
Gaur A, Adholeya A (2004) Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Curr Sci 86(4):528–534
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of its by products. Asian J Energy Environ 6(4):18
Glick BR (2003) Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv 21(5):383–393
Glick BR (2010) Using soil bacteria to facilitate phytoremediation bacteria. Biotechnol Adv 28:367–374
Gomes HI (2012) Phytoremediation for bioenergy: challenges and opportunities. Environ Technol Rev 1(1):59–66
Grill E, Gekeler W, Winnacker EL, Zenk HH (1986) Homo-phytochelatins are heavy metal-binding peptides of homo-glutathione containing Fabales. FEBS Lett 205(1):47–50
Guerinot ML (2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465(1–2):190–198
Gupta S, Nayek S, Saha RN, Satpati S (2008) Assessment of heavy metal accumulation in macrophyte, agricultural soil and crop plants adjacent to discharge zone of sponge iron factory. Environ Geol 55:731–739
Gupta AK, Verma SK, Khan K, Verma RK (2013) Phytoremediation using aromatic plants: a sustainable approach for remediation of heavy metals polluted sites. Environ Sci Technol 47(18):10115–10116
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53(366):1–11
Hammer D, Kayser A, Keller C (2003) Phytoextraction of Cd and Zn with Salix viminalis in field trial. Soil Use Manag 19(3):187–192
Hamon RE, Holm PE, Lorenz SE, McGrath SP, Christensen TH (1999) Metal uptake by plants from sludge-amended soils: caution is required in the plateau interpretation. Plant Soil 216(1–2):53–64
Hannink N, Rosser SJ, French CE, Basran A, Murray JAH, Nicklin S, Bruce NC (2001) Phytodetoxification of TNT by transgenic plants expressing a bacterial nitroreductase. Nat Biotechnol 19:1168–1172
Hannink NK, Subramanian M, Rosser SJ, Basran A, Murray JA, Shanks JV, Bruce NC (2007) Enhanced transformation of TNT by tobacco plants expressing a bacterial nitroreductase. Int J Phytorem 9(5):385–401
Harada E, Yamaguchi Y, Koizumi N, Sano H (2002) Cadmium stress induces production of thiol compounds and transcripts for enzymes involved in sulfur assimilation pathways in Arabidopsis. J Plant Physiol 159:445–448
Hartley-Whitaker J, Ainsworth G, Vooijs R, Ten Bookum W, Schat H, Meharg AA (2001) Phytochelatins are involved in differential arsenate tolerance in Holcus lanatus. Plant Physiol 126:299–306
He LY, Chen ZJ, Ren GD, Zhang YF, Qian M, Sheng XF (2009) Increased cadmium and lead uptake of a cadmium hyperaccumulator tomato by cadmium resistant bacteria. Exotoxicol Environ Safety 72:1343–1348
Henry JR (2000) Overview of the phytoremediation of lead and mercury. In: Overview of the phytoremediation of lead and mercury. EPA, Washington, DC
Hinsinger P, Plassard C, Jaillard B (2006) Rhizosphere: a new frontier for soil biogeochemistry. J Geochem Explor 88:210–213
Inui H, Ohkawa H (2005) Herbicide resistance in transgenic plants with mammalian P450 monooxygenase genes. Pest Manag Sci 61:286–291
Ismail S (2012) Phytoremediation: a green technology. Iranian J Plant Physiol 3(1):567–576
Jadia CD, Fulekar MH (2008) Phytoremediation: the application of vermicompost to remove zinc, cadmium, copper, nickel and lead by sunflower plant. Environ Eng Manag J 7(5):547–558
Jipson E (1996) Chevron grows a new remediation technology: Alfalfa and Poplars. Envirobiz News and Press Release Archive, Available from http://www.envirobiz.com
Jung C, Maeder V, Funk F, Frey B, Sticher H, Frossard E (2003) Release of phenols from Lupinus albus L. roots exposed to Cu and their possible role in Cu detoxification. Plant Soil 252:301–312
Kägi JHR (1991) Overview of metalothioneins. Methods Enzymol 205:613–623
Kapoor R, Bhatnagar AK (2007) Attenuation of cadmium toxicity in mycorrhizal celery (Apium graveolens L.). World J Microbiol Biotechnol 23:1083–1089
Kawahigashi H, Hirose S, Inui H, Ohkawa H, Ohkawa Y (2005) Enhanced herbicide cross-tolerance in transgenic rice plants co-expressing human CYP1A1, CYP2B6, and CYP2C19. Plant Sci 168:773–781
Khan NU, Varma B, Imrana N, Shetty PK (2011) Phytoremediation using an indigenous crop plant (wheat): the uptake of methyl parathion and metabolism of p-nitrophenol. Ind J Sci Technol 4(12):1661–1667
Kisku GC, Barman SC, Bhargava SK (2000) Contamination of soil and plants with potentially toxic elements irrigated with mixed industrial effluent and its impact on the environment. Water Air Soil Pollut 120(1–2):121–137
Kondo N, Imani K, Isobe M, Goto T, Murasugi A, Wada-Nakagawa C, Hayashi Y (1984) Cadystin A and B, major unit peptides comprising cadmium binding peptides induced in a fission yeast separation, revision of structures and synthesis. Tetrahedron Lett 25:3869–3872
Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB (1999) The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol 40:37–44
Kotrba P, Najmanova J, Macek T, Ruml T, Mackova M (2009) Genetically modified plants in phytoremediation of heavy metal and metalloid soil and sediment pollution. Biotechnol Adv 27:799–810
Krämer U, Talke IN, Hanikenne M (2007) Transition metal transport. FEBS Lett 581:2263–2272
Kuiper I, Lagendijk EL, Bloemberg GV, Lugtenberg BJ (2004) Rhizoremediation: a beneficial plant-microbe interaction. Mol Plant-Microbe Interact 17(1):6–15
Kumar PB, Dushenkov ANV, Motto H, Raskin I (1995a) Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol 29(5):1232–1238
Kumar PBAN, Dushenkov V, Motto H, Raskin I (1995b) Phytoextration-the use of plants to remove heavy metals from soils. Environ Sci Technol 29:1232–1238
Kumar N, Bauddh K, Barman SC, Singh DP (2012) Accumulation of metals in selected macrophytes grown in mixture of drain water and tannery effluent and their phytoremediation potential. J Environ Biol 33:323–327
Kumar N, Bauddh K, Kumar S, Dwivedi N, Singh DP, Barman SC (2013) Heavy metal uptake by plants naturally grown on industrially contaminated soil and their phytoremediation potential. Ecol Eng 61:491–495
Kushwaha A, Rani R, Kumar S, Gautam A (2016) Heavy metal detoxification and tolerance mechanisms in plants: implications for phytoremediation. Environ Rev 24(1):39–51
Lasat MM (2000) Phytoextraction of metals from contaminated soil: a review of plant/soil/metal interaction and assessment of pertinent agronomic issues. J Hazard Sub Res 2(5):1–25
Lee S, Moon JS, Ko TS, Petros D, Goldsbrough PB, Korban SS (2003) Over expression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol 131:656–663
Leitenmaier B, Küpper H (2013) Compartmentation and complexation of metals in hyperaccumulator plants. Front Plant Sci 4:374
Leung HM, Wu FY, Cheung KC, Ye ZH, Wong MH (2010) The effect of arbuscular mycorrhizal fungi and phosphate amendement on arsenic uptake, accumulation and growth of Pteris vittatain as-contaminated soil. Int J Phytoremed 12(4):384–403
Li WC, Ye ZH, Wong MH (2007) Effects of bacteria on enhanced metal uptake of the Cd/Zn hyperaccumulating plant, Sedum alfredii. J Exp Bot 58:4173–4182
Liao M, Xie XM (2004) Cadmium release in contaminated soils due to organic acids. Pedosphere 14:223–228
Lim JM, Salido AL, Butcher DJ (2004) Phytoremediation of lead using Indian mustard (Brassica juncea) with EDTA and electrodics. Microchem J 76:3–9
Lindberg T, Granhall U, Tomenius H (1985) Infectivity and acetylene reduction of diazotrophic rhizosphere bacteria in wheat (Triticum aestivum) seedlings under gnotobiotic conditions. Biol Fertil Soils 1:123–129
Liphadzi MS, Kirkham MB, Mankin KR, Paulsen GM (2003) EDTA-assisted heavy metal uptake by poplar and sunflower grown at a long-term sewage sludge farm. Plant Soil 257:171–182
Liu D, Jiang W, Liu C, Xin C, Hou W (2000) Uptake and accumulation of lead by roots, hypocotyls and shoots of Indian mustard [Brassica juncea (L.)]. Bioresour Technol 71(3):273–277
Liu WX, Shen LF, Liu JW, Wang YW, Li SR (2007) Uptake of toxic heavy metals by rice (Oryza sativa L.) cultivated in the agricultural soil near Zhengzhou city, People’s Republic of China. Bull Environ Contam Toxicol 79(2):209–213
Liu X, Peng K, Wang A, Lian C, Shen Z (2010) Cadmium accumulation and distribution in populations of Phytolacca americana L. and the role of transpiration. Chemosphere 78:1136–1141
Maiti IB, Wagner GJ, Hunt AG (1991) Light inducible and tissue specific expression of a chimeric mouse metallothionein cDNA gene in tobacco. Plant Sci 76:99–107
Malekzadeh E, Alikhani HA, Savaghebi-Firoozabadi GR, Zarei M (2011) Influence of arbuscular mycorrhizal fungi and an improving growth bacterium on Cd uptake and maize growth in Cd-polluted soils. Span J Agric Res 9(4):1213–1223
Malhotra R, Agarwal S, Gupta P (2014) Phytoremediation of radioactive metals. J Civil Eng Environ Technol 1:75–79
Manara A (2012) Plant responses to heavy metal toxicity. In: Furini A (ed) Plants and heavy metals, Springer briefs in biometals. Springer, Dordrecht
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Meers E, Van Slycken S, Adriaensen K, Ruttens A, Vangronsveld J, Du Laing G, Witters N, Thewys T, Tack FMG (2010) The use of bio-energy crops (Zea mays) for ‘phytoattenuation’of heavy metals on moderately contaminated soils: a field experiment. Chemosphere 78(1):35–41
Merkl N, Schultze-Kraft R, Infante C (2005) Phytoremediation in the tropics—influence of heavy crude oil on root morphological characteristics of graminoids. Environ Pollut 138(1):86–91
Mijovilovich A, Leitenmaier B, Meyer-Klaucke W, Kroneck PM, Götz B, Küpper H (2009) Complexation and toxicity of copper in higher plants. II. Different mechanisms for copper versus cadmium detoxification in the copper-sensitive cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype). Plant Physiol 151:715–731
Milner MJ, Kochian LV (2008) Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann Bot 102:3–13
Misra S, Gedamu L (1989) Heavy metal tolerant transgenic Brassica napus L. and Nicotiana tabacum L. plants. Theor Appl Genet 78:161–168
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Mojiri A (2011) The potential of corn (Zea mays) for phytoremediation of soil contaminated with cadmium and lead. J Biol Environ Sci 5(13):17–22
Moreno FN, Anderson CWN, Stewart RB, Robinson BH (2008) Phytofiltration of mercury-contaminated water: volatilisation and plant-accumulation aspects. Environ Exp Bot 62(1):78–85
Mwegoha WJS (2008) The use of phytoremediation technology for abatement soil and groundwater pollution in Tanzania: opportunities and challenges. J Sust Dev Africa 10(1):140–156
Nevo Y, Nelson N (2006) The NRAMP family of metal-ion transporters. Biochim Biophys Acta 1763:609–620
Ni LX, Acharya K, Hao XY, Li SY (2013) Antioxidant and metabolism responses to polyphenol stress in cyanobacterium Microcystis aeruginosa. J Environ Sci Health, Part B 48:153–161
Nishida S, Tsuzuki C, Kato A, Aisu A, Yoshida J, Mizuno T (2011) AtIRT1, the primary iron uptake transporter in the root, mediates excess nickel accumulation in Arabidopsis thaliana. Plant Cell Physiol 52:1433–1442
Noctor G, Mhamdi A, Foyer CH (2014) The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol 164:1636–1648
Nordberg GF (2004) Cadmium and health in the 21st century—historical remarks and trends for the future. Biometals 17:485–489
O’Connor CS, Lepp NW, Edwards R, Sunderland G (2003) The combined use of electrokinetic remediation and phytoremediation to decontaminate metal polluted soils: a laboratory-scale feasibility study. Environ Monit Assess 84:141–158
Pandey VC, Singh K (2011) Is Vigna radiata suitable for the re-vegetation of fly ashlandfills? Ecol Eng 37:2105–2106
Pandey VC, Pandey DN, Singh N (2015) Sustainable phytoremediation based on naturally colonizing and economically valuable plants. J Clean Prod 86:37–39
Papoyan A, Kochian LV (2004) Identification of Thlaspi caerulescens genes that may be involved in heavy metal hyperaccumulation and tolerance. Characterization of a novel heavy metal transporting ATPase. Plant Physiol 136(3):3814–38223
Patten C, Glick BR (1996) Bacterial biosynthesis of indole-3- acetic acid. Can J Microbiol 42:207–220
Pehlivan E, Özkan AM, Dinç S, Parlayici S (2009) Adsorption of Cu2+ and Pb2+ ion on dolomite powder. J Hazard Mater 167(1–3):1044–1049
Persistent Organic Pollutants (POPs) (2008) Children’s health and the environment WHO training package for the health sector world health organization www.who.int/ceh
Phytotechnology Mechanism (2005) Solid waste and emergency response (5102G), EPA 542-R- 05-002, 2005, http://www.clu-in.org/download/remed/542-r-05-002.pdf
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39
Pittman JK (2005) Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytol 167:733–742
Plummer C (1997) Interest increases in using plants for environmental remediation. In: Glaser L (ed) Industrial uses of agricultural materials situation and outlook. Economic Research Service, US Department of Agriculture, Washington, DC, USA, Available from http://www.ers.usda.gov/publications/IUS6/ius6g%200002.pdf
Prasad MNV, Freitas De HMO (2003) Metal hyperaccumulation in plants biodiversity prospecting for phytoremediation technology. Electron J Biotechnol 6(3):110–146
Putra RS, Ohkawa Y, Tanaka S (2013) Application of EAPR system on the removal of lead from sandy soil and uptake by Kentucky bluegrass (Poa pratensis L.). Sep Purif Technol 102:34–42
Ragland M, Briat JF, Gagnon J, Laulhere JP, Massenet O, Theil EC (1990) Evidence for conservation of ferritin sequences among plants and animals and for a transit peptide in soybean. J Biol Chem 265:18339–18344
Rahman MA, Hasegawa H, Ueda K, Maki T, Rahman MM (2008) Arsenic uptake by aquatic macrophyte Spirodela polyrhiza L.: interactions with phosphate and iron. J Hazard Mater 160(2–3):356–361
Rajkumar M, Freitas H (2008) Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere 71:834–842
Rajkumar M, Nagendran R, Lee KJ, Lee WH, Kim SZ (2006) Influence of plant growth promoting bacteria and Cr6þ on the growth of Indian mustard. Chemosphere 62:741–748
Raskin I, Smith RD, Salt DE (1997) Phytoremediation of metals using plants to remove pollutants from the environment. Curr Opin Biotechnol 8:221–226
Rastgoo L, Alemzadeh A (2011) Biochemical responses of Gouan (Aeluropus littoralis) to heavy metals stress. AJCS 5:375–383
Ravet K, Touraine B, Boucherez J, Briat J-F, Gaymard F, Cellier F (2009) Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J 57:400–412
Reddy KR, Cameselle C (2009) Electrochemical remediation technologies for polluted soils, sediments and groundwater. Wiley, Hoboken
Reeves RD, Baker AJM (2000) Metal-accumulating plants. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals, using plants to clean up the environment. Wiley-Interscience, New York, pp 193–229
Resaee A, Derayat J, Mortazavi SB, Yamini Y, Jafarzadeh MT (2005) Removal of mercury from chlor-alkali industry wastewater using Acetobacter xylinum cellulose. American J Environ Sci 1(2):102–105
Rodriguez L, Lopez-Bellido FJ, Carnicer A, Recreo F, Tallos A, Monteagudo JM (2005) Mercury recovery from soils by phytoremediation. In: Book of environmental chemistry. Springer, Berlin, pp 197–204
Roosens NH, Bernard C, Leplae R, Verbruggen N (2004) Evidence for copper homeostasis function of metallothionein (MT3) in the hyperaccumulator Thlaspi caerulescens. FEBS Lett 577:9–16
Rowe RL, Street NR, Taylor G (2009) Identifying potential environmental impacts of large- scale deployment of dedicated bioenergy crops in the UK. Renew Sust Energ Rev 13(1):271–290
Roy S, Labelle S, Mehta P et al (2005) Phytoremediation of heavy metal and PAH-contaminated brown field sites. Plant Soil 272(1–2):277–290
Rugh CL, Wilde D, Stack NM, Thompson DM, Summer AO, Meagher RB (1996) Mercuric ion reduction and resistance in transgenic Arabidopsis thaliana plants expressing a modified bacterial merA gene. Proc Natl Acad Sci U S A 93:3182–3187
Sainger M, Sharma A, Bauddh K, Sainger PA, Singh RP (2014) Remediation of nickel-contaminated soil by Brassica juncea L. cv. T-59 and effect of the metal on some metabolic aspects of the plant. Biom J 18:100–110
Salt DE, Blaylock M, Kumar NPBA, Duskenkov V, Eustry D, Chet D, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Nat Biotechnol 13:468–474
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49:643–668
Salt DE, Kato N, Krämer U, Smith RD, Raskin I (2000) The role of root exudates in nickel hyperaccumulation and tolerance in accumulator and nonaccumulator species of Thlaspi. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. CRC Press LLC, Boca Raton
Sanderman H (1994) Higher plant metabolism of xenobiotics: The ‘green liver’ concept. Pharmacogenetics 4:225–241
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53(372):1351–1365
Schwab AP, He YH, Banks MK (2005) The influence of organic ligands on the retention of lead in soil. Chemosphere 61:856–866
Seckback JJ (1982) Ferreting out the secret of plant ferritin—a review. J Plant Nutr 5:369–394
Sewelam N, Kazan K, Schenk PM (2016) Global plant stress signaling: Reactive oxygen species at the cross-road. Front Plant Sci, 23 February 2016 http://dx.doi.org/10.3389/fpls.2016.00187
Shanmugaraj BM, Chandra HM, Srinivasan B, Ramalingam S (2013) Cadmium induced physio-biochemical and molecular response in Brassica juncea. Int J Phytoremed 15:206–218
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46(3):209–221
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26 Article ID 217037, 26 pages
Shenker M, Fan TWM, Crowley DE (2001) Phytosiderophores influence on cadmium mobilization and uptake by wheat and barley plants. J Environ Qual 30:2091–2098
Shevyakova N, Cheremisina A, Kuznetsov V (2011) Phytoremediation potential of Amaranthus hybrids: antagonism between nickel and iron and chelating role of polyamines. Russ J Plant Physiol 58(4):634–642
Shi G, Cai Q (2009) Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol Adv 27(5):555–561
Shi X, Zhang X, Chen G, Chen Y, Wang L, Shan X (2011) Seedling growth and metal accumulation of selected woody species in copper and lead/zinc mine tailings. J Environ Sci 23:266–274
Skinner K, Wright N, Porter-Goff E (2007) Mercury uptake and accumulation by four species of aquatic plants. Environ Pollut 145(1):234–237
Slater A, Scott NW, Fowler MR (2008) Plant Biotechnology; the genetic manipulation of plants 2ndnd edn; Oxford University Press, Oxford
Sonoki T, Kajita S, Ikeda S, Uesugi M, Tatsumi K, Katayama Y, Iimura Y (2005) Transgenic tobacco expressing fungal laccase promotes the detoxification of environmental pollutants. App Microbiol Biotechnol 67:138–142
Spinks JWT, Woods RJ (1990) An introduction to radiation chemistry, 3rd edn. Wiley-Interscience, New York
Sun R, Zhou Q, Jin C (2006) Cadmium accumulation in relation to organic acids in leaves of Solanum nigrum L. as a newly found cadmium hyperaccumulator. Plant Soil 285:125–134
Sweta BK, Singh R, Singh RP (2015) Trapa natans cultivated in contaminated water is found hazardous and not suitable for phytoremediation of heavy metals. Ecol Eng 83:39–42
Tangahu BV, Sheikh Abdullah SR, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng: http://dx.doi.org/10.1155/2011/939161
Tommasini R, Vogt E, Fromenteau M, Hoertensteiner S, Matile P, Amrhein N, Martinoia E (1998) An ABC transporter of Arabidopsis thaliana has both glutathione conjugate and chlorophyll catabolite transport activity. Plant J 13:773–780
Tong Y, Kneer R, Zhu Y (2004) Vacuolar compartmentalization: a second-generation approach to engineering plants for phytoremediation. Trends Plant Sci 9(1):7–9
Traunfeld JH and Clement DL (2001) Lead in garden soils. Home and garden. Maryland Cooperative Extention, University of Maryland, 2001, http://www.hgic.umd.edu/media/documents/hg18.pdf
Tu S, Ma LQ, Fayiga AO, Zillioux EJ (2004) Phytoremediation of arsenic-contaminated groundwater by the arsenic hyperaccumulating fern Pteris vittata L. Int J Phytoremed 6(1):35–47
U. S. Environmental Protection Agency (2005) Use of field-scale phytotechnology, for chlorinated solvents, metals, explosives, and propellants, and pesticides, vol 9. U.S. Environmental Protection Agency, Solid Waste and Emergency Response, Washington, DC, pp. 7–9
Uera RB, Paz-Alberto AM, Sigua GC (2007) Phytoremediation potentials ofselected tropical plants for ethidium bromide. Environ Sci Pollut Res 14(7):505–509
Vaclavikova M, Gallios GP, Hredzak S, Jakabsky S (2008) Removal of arsenic from water streams: an overview of available techniques. Clean Techn Environ Policy 10(1):89–95
Vamerali T, Bandiera M, Coletto L, Zanetti F, Dickinson NM, Mosca G (2009) Phytoremediation trials on metal- and arsenic-contaminated pyrite wastes (Torviscosa, Italy). Environ Pollut 157(3):887–894
Van Ginneken L, Meers E, Guisson R, Ruttens A, Elst K, Tack FM, Vangronsveld J, Diels L, Dejonghe W (2007) Phytoremediation for heavy metal-contaminated soils combined with bioenergy production. J Environ Eng Landsc 15(4):227–236
Van Hoof NA, Koevoets PL, Hakvoort HW, Ten Bookum WM, Schat H, Verkleij JA, Ernst WH (2001) Enhanced ATP dependent copper efflux across the root cell plasma membrane in copper tolerant Silene vulgaris. Physiol Plant 113(2):225–232
Vandecasteele B, Meers E, Vervaeke P, Vos BD, Quataert P, Tack FMG (2005) Growth and trace metal accumulation of two Salix clones on sediment-derived soils with increasing contamination levels. Chemosphere 58(8):995–1002
Vanderauwera S, Suzuki N, Miller G, van de Cotte B, Morsa S, Ravanat JL (2011) Extranuclear protection of chromosomal DNA from oxidative stress. Proc Natl Acad Sci U S A 108:1711–1716
Verbruggen N, Hermans C, Schat H (2009) Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 181:759–776
Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat J-F, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233
Wang Z, Wan J (2013) An economic analysis of the use of water hyacinth for phytoremediation and biogas production in Dianchi Lake, China, No. rr2013025. Economy and Environment Program for Southeast Asia (EEPSEA)
Wani PA, Khan MS, Zaidi A (2007) Effect of metal tolerant plant growth promoting Bradyrhizobium sp. (vigna) on growth, symbiosis, seed yield and metal uptake by greengram plants. Chemosphere 70:36–45
Westhoff Alex (1999) Mycorrhizal plants for phytoremediation of soils contaminated with radionuclides. University of Minnesota, Department of Horticultural Science. Retrieved from the University of Minnesota Digital Conservancy, http://hdl.handle.net/11299/59501
Whiting SN, de Souza SP, Terry N (2001) Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens. Environ Sci Technol 35:3144–3150
Wu SC, Cheung KC, Luo YM, Wong MH (2006) Effects of inoculation of plant growth-promoting rhizobacteria on metal uptake by Brassica juncea. Environ Pollut 140:124–135
Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10:1539–1550
Xu JK, Yang LX, Wang YL, Wang ZQ (2005) Advances in the study uptake and accumulation of heavy metal in rice (Oryza sativa) and its mechanisms. Chinese Bull Bot 22:614–622
Yang X, Feng Y, He Z, Stoffella P (2005) Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J Trace Elem Med Biol 18:339–353
Yang XE, Li TQ, Yang JC, He ZL, Lu LL, Meng FH (2006) Zinc compartmentation in root, transport into xylem, and absorption into leaf cells in the hyperaccumulating species of Sedum alfredii Hance. Planta 224(1):185–195
Yin H, Chen Q, Yi M (2008) Effects of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regul 54:45–54
Yu F, Liu K, Li M, Zhou Z, Deng H, Chen B (2013) Effects of cadmium on enzymatic and non-enzymatic antioxidative defences of rice (Oryza sativa L.). Int J Phytoremed 15(6):513–521
Zaal BJ, Neuteboom LW, Pinas JE, Chardonnes AN, Scjat H, Verleij JAC, Hooykass PJJ (1999) Over expression of a novel Arabidopsis gene related to putative Zn transporter genes from animals lead to enhanced zinc resistance and accumulation. Plant Physiol 19:1047–1055
Zancani M, Peresson C, Biroccio A, Federici G, Urbani A, Murgia I, Soave C, Micali F, Vianello A, Macri F (2004) Evidence for the presence of ferritin in plant mitochondria. Eur J Biochem 271:3657–3664
Zancani M, Peresson C, Patui S, Tubaro F, Vianello A, Macri F (2007) Mitochondrial ferritin distribution among plant organs and its involvement in ascorbate-mediated iron uptake and release. Plant Sci 173:182–189
Zhang XH, Zhu YG, Chen BD, Lin AJ, Smith SE, Smith FA (2005) Arbuscular mycorrhizal fungi contribute to resistance of upland rice to combined metal. J Plant Nutr 28:2065–2077
Zhang X, Lin AJ, Zhao FJ, Xu GZ, Duan GL, Zhu YG (2008) Arsenic accumulation by the aquatic fern Azolla: comparison of arsenate uptake, speciation and efflux by A. caroliniana and A. filiculoides. Environ Pollut 156(3):1149–1155
Zhigang A, Cuijie L, Yuangang Z, Yejie D, Wachter A, Gromes R, Rausch T (2006) Expression of BjMT2, a metallothionein 2 from Brassica juncea, increases copper and cadmium tolerance in Escherichia coli and Arabidopsis thaliana, but inhibits root elongation in Arabidopsis thaliana seedlings. J Exp Bot 57:3575–3582
Zimeri AM, Dhankher OP, McCaig B, Meagher RB (2005) The plant MT1 metallothioneins are stabilized by binding cadmium and are required for cadmium tolerance and accumulation. Plant Mol Biol 58:839–855
Acknowledgement
Dr. Kuldeep Bauddh is thankful to UGC for the award of UGC Start-up grant (3(B):2202.03.789.03.01.31).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chakravarty, P., Bauddh, K., Kumar, M. (2017). Phytoremediation: A Multidimensional and Ecologically Viable Practice for the Cleanup of Environmental Contaminants. In: Bauddh, K., Singh, B., Korstad, J. (eds) Phytoremediation Potential of Bioenergy Plants. Springer, Singapore. https://doi.org/10.1007/978-981-10-3084-0_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-3084-0_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3083-3
Online ISBN: 978-981-10-3084-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)