Abstract
Environmentally sustainable solutions for wastewater management including the improvement of water quality and water recycling are considered key priority areas globally. The challenges facing our water resources are unprecedented due to the presence of organic and inorganic pollutants derived from numerous anthropogenic activities. This situation has been further complicated by emerging persistent contaminants such as pharmaceuticals which possess low biodegradability and resistance to chemical and biological treatments. Excretion of pharmaceuticals and their metabolites via human waste, improper disposal and veterinary applications is recognized as the principal sources of pharmaceuticals ending up in various compartments of the environment. Heterogeneous photocatalysis using semiconductor titanium dioxide (TiO2) has proven to be a promising treatment method for the degradation of pharmaceuticals.
Here, we review recent research concerning the application of TiO2 photocatalysis for the removal of selected pharmaceuticals belonging to different therapeutic drug classes. These classes of pharmaceuticals were chosen based on their environmental prevalence and potential adverse effects. The highlighted conclusions from this review are that (1) TiO2 photocatalysis may play a major role in the degradation of pharmaceuticals; (2) various factors such as catalyst loading, initial concentrations, and water matrices significantly influence both the degradation rate and kinetics of degradation; (3) mineralization remains incomplete, despite complete abatement of the parent pharmaceutical due to the formation of stable by-products; (4) structures or number of intermediates formed differ due to the variation of photocatalytic experimental parameters, and (5) laboratory-scale experiments with artificial pharmaceutical solutions, in particular single compounds, are more common than pilot-scale or real wastewater samples. The main conclusion is that the use of heterogeneous photocatalysis can be considered a state-of-the-art pharmaceutical wastewater treatment. Further studies are needed to optimize the operating conditions for maximum degradation of wastewater containing multiple pharmaceuticals under realistic conditions and on industrial scales.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pharmaceutical pollutants
- TiO2 photocatalysis
- Wastewater
- Antibiotic
- Analgesic
- Drugs
- Advanced oxidation processes
3.1 Introduction: Advanced Oxidation Processes
Since water is essential to life, it is not surprising that there are a number of issues related to this essential resource. Poor sanitation, water scarcity, deteriorating water quality, waterborne diseases and lack of clean water supply are all posing global challenges due to the rising demand by an increasing world population. Further pressure on water resources has resulted from the introduction of emerging recalcitrant contaminants or xenobiotics, such as pharmaceuticals, endocrine disruptors, surfactants and personal care products. These substances display much more complex characteristics and resistance to degradation. Numerous studies highlighting the prevalence of pharmaceuticals in water environments has increased significantly since 2000, Heberer (2002a), Tixier et al. (2003), Fent et al. (2006), Choi et al. (2008) and Miège et al. (2009). This development has raised widespread concern of poorly known potential effects of pharmaceutical compounds and their metabolites on human and aquatic organisms, despite occurring in trace quantities ranging from ng/L to μg/L, Kümmerer (2009) and Mompelat et al. (2009).
Currently existing sewage treatment technologies can only be considered as a compromise for emerging micropollutants, although they have proven efficient for microbial, carbon, heavy metals and nitrogen removal. Complex pharmaceuticals with various physical and chemical properties in wastewater, lead to inadequate removal by existing wastewater treatment technologies which are not designed to handle this specific class of pollutants, Suárez et al. (2008). Incomplete removal of pharmaceutical residues clearly supports the urgent need for innovative technologies that can deal with their presence, either by improving existing or engaging in alternative technologies. Advanced Oxidation Processes (AOPs) are regarded to be appropriate to degrade pollutants which are known to be non-biodegradable or have low biodegradability, persistent, and possess high chemical stability. AOPs for water and wastewater treatment include photolysis and photocatalysis, ozonation, Fenton and photo-Fenton, ultrasound radiation, sonolysis, electrochemical oxidation, and wet air oxidation. These advanced technologies can be grouped into photochemical, non-photochemical and hybrid techniques (Fig. 3.1).
In principle, all AOPs are characterized by a common chemical feature known as reactive oxygen species which can react with non-biodegradable or recalcitrant compounds in water or wastewater, Dalrymple et al. (2007). Reactive oxygen or free radical species are strong oxidants required to initiate AOPs in order to mineralize pollutants to simpler and nontoxic molecules. These free radical species are based on atoms or molecules consisting of one or more unpaired electrons such as the hydroxyl radical (HO•), superoxide anion radical (O2 •−), hydroperoxyl radical (HO2 •) or alkoxyl radical (RO•). Of these, the HO• has attracted the most attention. The appealing characteristics of HO• radicals are its non-selective nature, high oxidation potential (2.8 V) when compared to other oxidants and its ability to react with a wide range of contaminants without any additives, with rate constants normally in the order of 106–109 mol L−1 s−1, Andreozzi et al. (1999). When HO• radicals reacts with organic molecules, they can either abstract a hydrogen atom or add to multiple bonds yielding oxidized intermediates or, in the case of complete mineralization, produce carbon dioxide, water and inorganic acids (3.1).
During an ideal treatment by AOPs, contaminants are structurally altered forming smaller and more biodegradable compounds until complete mineralization is achieved. For the application of AOPs in wastewater treatment, the objectives can be either to (i) increase the biodegradability of wastewater before applying conventional biological process, (ii) reduce the level of toxicity and micropollutants in the effluent or (iii) disinfect the wastewater instead of applying traditional disinfection methods such as chlorination, which is known to generate carcinogenic and mutagenic by-products such as trihalomethanes and haloacetic acids, Rizzo (2011). AOPs have been proven to be efficient for the treatment of industrial wastewater; however, due to the high chemical oxygen demand (COD) levels in industrial wastewater, AOPs are only considered efficient for a low COD level of <5 g/L. When dealing with high levels of COD, techniques such as incineration or wet oxidation are preferred, Malato et al. (2002). Semiconductor material mediated photocatalysis in particular with titanium dioxide (TiO2), is distinctive compared to other AOPs for the removal of persistent pollutants from wastewaters.
3.2 Heterogeneous Photocatalysis
The IUPAC defines photocatalysis as a “change in the rate of a chemical reaction or its initiation under the action of ultraviolet, visible or infrared radiation in the presence of a substance, the photocatalyst that absorbs light quanta and is involved in the chemical transformation of the reaction partners”, Braslavsky (2007). Heterogeneous photocatalysis may be also termed as a process which uses a semiconductor metal oxide as catalyst and oxygen as an oxidizing agent, Andreozzi et al. (1999).
In 1972, Fujishima and Honda discovered the possibility of water splitting by means of a photoelectrochemical cell consisting of a rutile TiO2 photoanode and a Pt counter electrode, Fujishima et al. (2008). This discovery opened ways for new applications such as air purification, self-cleaning surfaces application, organic synthesis, disinfection, and anti-cancer therapy. An interesting application of this breakthrough is in water purification. Water purification by means of illuminating TiO2 was first proposed by Frank and Bard for cyanide and sulfite removal, Fujishima et al. (2008). Since then, degradation of various organic target compounds such as dyes, pesticides and pharmaceuticals has been demonstrated.
A conventional heterogeneous photocatalysis process can be divided into five individual steps, Herrmann (1999):
-
i.
Diffusion of the reactants from the bulk phase to the surface of the catalyst;
-
ii.
Adsorption of at least one of the reactants;
-
iii.
Reaction in the adsorbed phase;
-
iv.
Desorption of the product(s);
-
v.
Removal of the product(s) from the interface region.
An ideal photocatalyst should be chemically and biologically inert, photoactive, photostable, inexpensive, non-toxic, and should be excited with visible and near/or ultraviolet (UV) light. Despite the existence of various chalcogenide semiconductor photocatalysts (oxides and sulfides) such as zinc oxide (ZnO), zinc sulfide (ZnS), ferric oxide (Fe2O3), cadmium sulfide (CdS), cerium dioxide (CeO2), tungsten trioxide (WO3), tin dioxide (SnO2) and titanium dioxide (TiO2), none of these fulfils all the characteristics of an ideal photocatalyst. The choice of catalyst in photocatalytic studies is frequently narrowed to TiO2, CdS and ZnO. Most studies involve TiO2 as a semiconductor candidate despite its limitations, such as a low efficiency and a narrow light response factor, Leary and Westwood (2011). CdS and ZnO are known to undergo self-oxidation, which leads to lower photoactivity, and also the release of potentially dangerous metals such as Cd2+, which makes these photocatalysts unattractive, Fox and Dulay (1993). Notably, ZnO has been reported to be a better photocatalyst in photocatalytic degradation than TiO2 due to its broader absorption within the solar spectrum, Elmolla and Chaudhuri (2010a).
3.2.1 Mechanisms of TiO2 Photocatalysis
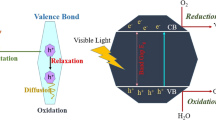
Mechanistic processes of TiO2 induced photocatalytic degradation of organic pollutants have been well described in the literature, Fox and Dulay (1993), Hoffmann et al. (1995), Gaya and Abdullah (2008), Chong et al. (2010) and Augugliaro et al. (2012). Photocatalysis occurs due to absorption of a photon with sufficient energy either equal or higher than the band gap energy (Eg) of the catalyst. The Eg represents the difference between the valence band (vB) and the conduction band (cB) of TiO2. The vB and cB energies of TiO2 are about +3.1 eV (vs. NHE) and −0.1 eV (vs. NHE), respectively. Consequently, the Eg between the vB and cB is 3.2 eV for anatase TiO2. When TiO2 is excited with UV light (λ < 380 nm for anatase TiO2 which is more common or λ < 400 nm for rutile TiO2), an electron (e−) is promoted from the vB to the cB of TiO2 generating a hole (h +) in the vB and an electron (e−) in the cB (3.2). The photogenerated e−-h + pair or charge carriers can either migrate to the surface of the TiO2, where they can actively perform their roles in oxidation-reduction reactions with the adsorbed pollutant (P) or recombine in the bulk or on the surface of the TiO2. The schematic representation of photogeneration of the e−-h + pair and of the subsequent oxidation-reduction is presented in Fig. 3.2.
A series of oxidation-reduction reactions may occur during TiO2 photocatalysis (3.3, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9, 3.10, and 3.11). A generated h + can react with an adsorbed water molecule, which is an essential process in TiO2 photocatalysis, or reacts with OH− anions to form powerful HO• radicals (3.3 and 3.4). The HO• radicals can subsequently oxidize water pollutants P to complete mineralization (3.5). When molecular oxygen is available, it is adsorbed onto the surface of TiO2 and can scavenge an electron to form the superoxide anion radical (O2 •−) (3.6).
Holes can also directly oxidize pollutants P by electron transfer (3.7 and 3.8).
The photogenerated e−-h + pairs can undergo rapid recombination within nano seconds in the absence of electron scavengers, such as oxygen, releasing heat without favouring any reactions (3.9, 3.10, and 3.11). The e− TR and h + TR (3.9, 3.10, and 3.11) represent the surface trap valence band electron and conduction band hole, respectively.
Wavelengths between 300 and 400 nm (near UV range) are of interest in TiO2 photocatalysis, which are provided either by artificial UV lamps or by a small section of the solar spectrum. Sunlight-induced photocatalytic processes have been the characteristic domain of TiO2 as a photocatalyst candidate. Demonstration-scale solar treatments of emerging contaminants using compound parabolic collectors (CPC) underpin this, Pérez-Estrada et al. (2005) and Miránda-García et al. (2011). However, a limitation of solar photocatalysis is the poor overlap of the solar spectrum with the absorption spectrum of TiO2 (<5 %). Thus, only 5 % of solar irradiation comprises the UV light that can be harvested by TiO2 photocatalysis. TiO2 doping with non-metals, metal-ion implantation, co-doping and sensitizing TiO2 with dyes has been applied to address this limitation and to improve the TiO2 photocatalytic efficiencies, Kumar and Devi (2011). The main objective of employing doping is to increase absorption in the visible region as it induces an optical response change i.e. decrease in band gap. One major drawback of doping is significant increase in the cost of the photocatalyst due to expense of ion implantation facilities, Zhang et al. (2009).
3.3 Titanium Dioxide: Structure and Properties
One most prevalent application of TiO2 is as a white pigment in paints, plastics, paper, fibres, foods, pharmaceuticals, and personal care products mainly due to its light scattering properties and high refractive index. Worldwide production of TiO2 powder has been reported as five million tons in 2005, with projections up to a 10 % increase by 2015, Skocaj et al. (2011). Additionally, production of nano-sized TiO2 which permits more applications such as anti-fogging and self-cleaning coatings has also increased from 2,000 t in 2005 to 5,000 t in 2010, Weir et al. (2012). However, the concern on safety and health aspects which arise from the usage of nano-TiO2 needs to be addressed. A recent study was conducted to assess the amount of TiO2 in various food, personal care products and pharmaceuticals and predict adverse effects of TiO2 nanoparticles on the environment, Weir et al. (2012).
TiO2 exists in three crystalline forms namely anatase, rutile and brookite with the anatase form more commonly used as an active photocatalyst than pure rutile phase. In contrast, brookite TiO2 is not commonly applied in photocatalysis. The anatase form tends to be the most photoactive and the most stable form at low temperatures (<700 °C). Anatase and brookite are however, thermodynamically metastable and they can be irreversibly converted to the rutile form, the most stable form at high temperatures, Silva and Faria (2009). Surface and structural properties such as crystal structure, surface area, particle size distribution, porosity, band gap and surface hydroxyl density control the efficiency of TiO2 as photocatalyst. Of these, crystallinity and specific surface area have great effect on the photocatalytic activity of TiO2.
The more common application of pure anatase compared to the pure rutile TiO2 is attributed to a higher density of superficial hydroxyl groups, which leads to an improved capacity of anatase to adsorb oxygen, a larger specific surface area compared to pure rutile samples, and a lower recombination velocity of e−-h + pairs in anatase, Hoffmann et al. (1995) and Achilleos et al. (2010a). Combinations of anatase and rutile have demonstrated better photocatalytic activity due to the promotion of charge pair separation and inhibition of e−-h + recombination, Pelaez et al. (2012).
The most prominent TiO2 photocatalyst, Degussa P25 (now known as AEROXIDE® TiO2 P25) contains 80:20 anatase to rutile weight ratio and has demonstrated good performance in photocatalytic applications due to its large surface area of 50 m2/g, with a particle size range of 20–30 nm. Although TiO2 P25 has been widely used as a benchmark photocatalyst, its effectiveness has been limited by poor light absorption in the visible region, as a result of its large band gap.
The sol–gel method has been one of the widely used synthetic methods for the preparation of TiO2 nanocrystalline forms. Several synthetic pathways are available for the preparation of the photocatalyst from precursor chemicals, for example, titanium alkoxide or titanium halogenide. The precursors are normally calcined at very high temperatures to obtain the desired crystal form and strong adhesion to surface materials or solid support such as activated carbon, zeolite, silica and glass. Hydroxyl groups from the catalyst surface and the support can react upon loss of a molecule of water, creating an oxygen bond thus increasing the adherence of the catalyst to the support during calcinations, Shan et al. (2010). The rate of hydrolysis is difficult to control due to the affinity of the TiO2 precursors for water leading to poor modification of TiO2’s intrinsic properties such as surface structure, structural properties and porosity. Nitric acid, hydrochloric acid and complexing reagents such as oxalate and citrate have also been commonly used in the sol–gel synthesis. Weak acids such as acetic acid also exhibit excellent control of the hydrolysis rate of TiO2, as they maintain pH due to the chelating effects of anions formed (e.g. acetate ion), generation of pH buffer, and stabilization of the sol, Shamaila et al. (2010).
3.4 Pharmaceuticals in the Environment
The United States Environmental Protection Agency’s (USEPA) priority pollutants such as volatile organics, semivolatile, pesticides and polyvinylchloride biphenyls, metals and inorganics have been the main focus of water pollution over the past 30 years. However, in recent years, concerns in water pollution have shifted towards emerging contaminants. In particular, pharmaceutical and personal care products have become pollutants of interest due to their notable detection and potential detrimental impact on aquatic environments, Khetan and Collins (2007). Pharmaceuticals, classified as environmental emerging contaminants because of their endocrine-disrupting properties are either of natural origin or are produced synthetically, Oller et al. (2011).
The term ‘pharmaceutical’ or active pharmaceutical ingredient (API) for the purpose of this review refers to chemicals used for diagnosis, treatment, alteration, and prevention of disease, health conditions or functions of the human body and also includes veterinary pharmaceuticals and illicit or recreational drugs, Daughton and Ternes (1999). APIs are complex molecules with various functionalities and physicochemical and biological properties, balanced in terms of hydrophilicity and lipophilicity with molecular weights ranging from 200 to 1,000 Da, Kümmerer (2009). Pharmaceuticals are generally designed with high stability for their intended effects on humans and are eventually metabolised in the body by biochemical processes. They can be eliminated from the human body either after being partially or completely converted to water soluble metabolites or, in some cases, without being metabolized, Khan and Ongerth (2004). Their classifications are generally based on their therapeutic uses and those of environmental interest can be categorized into eight groups, namely non-steroidal anti-inflammatory drugs, antibiotics, beta-blockers, antiepileptics, blood lipid-lowering agents, antidepressants, hormones and antihistamines, Khetan and Collins (2007).
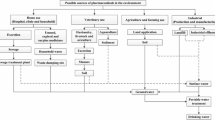
Inappropriate disposal and excretion of consumed medications by households, pharmaceutical manufacturers, hospitals, pharmacies and animal farming or aquaculture have been identified as the major sources of pharmaceuticals either as the parent drug or their metabolites in water courses (Fig. 3.3). Wastewater treatment plants (WWTPs) are thus a major source of the presence of this class of micropollutants in the environment. Various studies have reported on the discrepancies in efficiency and variation of WWTPs in the removal of pharmaceuticals due to their stability and differences in physicochemical properties. In fact, studies have also confirmed the detection of pharmaceuticals in effluents and influents from WWTP samples collected, in trace concentrations, Rodil et al. (2012).
A significant contribution of pharmaceuticals in surface water also comes from inappropriate disposal of expired or unused medications from households. Occurrence of pharmaceuticals in groundwater is related to a leaching process from landfills and soil and from sludge or manure spreading. Groundwater, being the extraction source of drinking water, has also been affected by pharmaceutical pollution, which can cause long term accumulation of trace amounts in humans. Finally, the accumulation of pharmaceuticals can be also expected in aquatic and terrestrial organisms via the food chain, Hoeger et al. (2005) and Deegan et al. (2011).
Monitoring studies conducted thus far have verified the occurrence of pharmaceutical in trace amounts in surface waters, groundwater and also sewage effluents, Andreozzi et al. (2003), Carballa et al. (2004), Lishman et al. (2006) and Moldovan (2006). Moreover, concentrations of pharmaceuticals in the water environment have also been established based on their therapeutic use, Nikolaou et al. (2007), Pal et al. (2010) and Ziylan and Ince (2011). The occurrence of pharmaceutical residues in drinking and tap water has also been reported, Ternes et al. (2002) and Rodil et al. (2012). A study reported levels of numerous pharmaceuticals and endocrine disruptors in the finished water of 19 full-scale water utilities in the United States, Benotti et al. (2009).
Once released into the environment, APIs are exposed to different natural conditions and resulting transformations, which is not only affected by their physicochemical properties, but also their structure. These changes create further complexities and concerns regarding the identity, toxicity and potential risk of any new products, which may be formed.
Although studies are generally focused on a wide spectrum of pharmaceuticals, those frequently prescribed have gained importance amongst researchers. For example, acidic pharmaceuticals such as ibuprofen, ketoprofen, bezafibrate, and naproxen have been frequently monitored in receiving waters and WWTPs, Lindqvist et al. (2005) and Wang et al. (2010). Selected therapeutic classes such as antibiotics, NSAIDs and analgesics have also been reviewed, Kümmerer (2009) and Ziylan and Ince (2011). Accurate statistics on sales and consumption of pharmaceuticals present a challenge, due to difficulty in monitoring of prescription medication, in addition to non-prescription medicines, which can be obtained over-the counter or through the internet. Statistics on global consumptions and production of pharmaceuticals are not available in the literature. In addition, about 3,000 new APIs are introduced each year as a result of advancements in medicine, Beausse (2004).
The annual per capita consumption of selected pharmaceuticals in some European countries and Australia are presented in Table 3.1. The consumption patterns vary according to countries and demand or popularity of a drug. In Australia, 262 million prescriptions were dispensed via community pharmacies in 2008 which accounts for about 12 prescriptions per person, AIHW (2011). This figure represents an increase of 24 % over prescriptions dispensed in 2001. Among the mostly prescribed medicines are cholesterol-lowering drugs, antibiotics and drugs for the treatment of high blood pressure. Amoxicillin (3.9 %), an antibiotic, emerged in the top 10 most commonly prescribed medicines in 2009–2010 followed by paracetamol (3.2 %), an analgesic and cephalexin (3.2 %), a cephalosporin antibiotic. The presences of amoxicillin and cephalexin were confirmed in WWTP influents and surface water (ng/L) in a monitoring study of 28 antibiotics in watersheds of south-east Queensland. None of these drugs was, however, detected in drinking water, Watkinson et al. (2009).
Although the fate of many pharmaceuticals released into the environment remains unknown, three possible scenarios have been proposed: (i) the pharmaceutical is mineralized to carbon dioxide, water, and other inorganic ions or acids such nitrate, sulfate and phosphate ions, (ii) the pharmaceutical is not readily degradable and will be retained in sludge due to its lipophilic nature or (iii) the pharmaceutical will be metabolized to a more hydrophilic form than the parent compound, Halling-Sørensen et al. (1998) and Klavarioti et al. (2009).
The main concern from the presence of pharmaceuticals and their metabolites is the potential adverse effects that they may pose on human health and to other living organisms, which thus far remains unknown and unclear. Due to their complex physicochemical properties as a result of mixtures of various pharmaceuticals and other pollutants in the environment, advanced treatment such as TiO2 photocatalysis undoubtedly can be applied for their degradation and a substantial amount of work has been published in this field, Abellán et al. (2007), Yurdakal et al. (2007), Giraldo et al. (2010) and Tong et al. (2012).
3.5 Degradation, Mineralization and Transformation Pathways of Pharmaceuticals Through Heterogeneous Photocatalysis
Positive developments in the photooxidation efficiency of TiO2 based photocatalysis (UV/TiO2) of pharmaceuticals have lead to an increasing interest in its application for the removal of micropollutants in spiked wastewaters and also real wastewater such as wastewater effluents, river water and drinking water. Determination of optimal operating conditions to yield the highest performance in terms of degradation and mineralization has been investigated in various heterogeneous photocatalytic studies on pharmaceuticals. The disappearance of the parent API over the treatment time has been the primary focus. However, studies have reported that kinetic assessment of APIs during UV/TiO2 degradation can often be complex, due to the formation of various transformation products. Interferences between the disappearance of the parent API and newly formed transformation products may also occur. The non-selective nature of hydroxyl radicals contributes to this complexity, Oller et al. (2011).
Another general observation is that while high removal rates of the parent API can be accomplished by UV/TiO2, mineralization remains incomplete in most cases. The degree of mineralization also tends to vary according to the nature of the water matrices. In general, a solution of the API prepared in the laboratory with distilled water or deionized water generally yields higher mineralization compared to that of doped effluent or influent samples from WWTPs, groundwater or river water.
The interference provided by the presence of organic matter such as humic and fulvic acids and radical scavengers such as carbonate species, HCO3 − and CO3 2− cannot be ruled out, as these species retard photocatalysis by competing for active sites and radiation attenuation. Organic matter present in water can be classified into two major classes namely dissolved organic matter and particulate organic matter. The existence of organic matter can be naturally occurring organic matter, such as humic and fulvic acids, or that derived from anthropogenic activities, Oppenländer (2003).
The ultimate aim of TiO2 photocatalysis (and also for other AOPs) is threefold, to degrade pollutants, to reduce toxicity and to enhance the biodegradability of the treated wastewater. Global parameters such as total organic carbon (TOC), COD, biochemical oxygen demand (BOD), dissolved organic carbon (DOC) and BOD/COD ratio are also often measured. The ratio of BOD5/COD is commonly used in this context and ratio values of above 0.4 are regarded as highly biodegradable in wastewater, Abellán et al. (2007). Toxicity assays are used to indicate the toxicity of photocatalytically treated wastewater and various types of bioassays based on microorganisms, plants and algae, invertebrates and fish have been applied to measure the response, Rizzo (2011).
In terms of the API selection, it is either driven by their consumption which can correlate to the high probability of detection in the environment or due to an existing gap of information on the degradation rate of the particular API. Frequently investigated operational parameters include catalyst load, initial concentration, type of photocatalysts, pH of the solution, wavelength and light intensity. Other operational parameters for enhancement of the degradation rate by the addition of co-oxidants and the effect of the concentration of dissolved oxygen (DO) have also been incorporated in various TiO2 photocatalysis studies. These variables are chosen in order to establish their relationship with respect to the API degradation. However, it is also widely accepted that the design and geometry of the photoreactor dictates the optimal degradation rate of APIs, Malato et al. (2009) and Friedmann et al. (2010).
3.5.1 Non-steroidal Anti-inflammatory and Analgesic Drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) primarily reduce inflammation, while analgesic (AN) drugs are widely used to relieve pain, Ziylan and Ince (2011). Examples of NSAIDs include naproxen, ketoprofen, diclofenac, fenoprofen, indomethacin, and ibuprofen, whereas ANs are acetaminophen (paracetamol), acetylsalicylic acid (aspirin) and the opioid analgesics such as morphine. Chemical structures of selected NSAIDs and ANs are presented in Table 3.2. One of the most prominent characteristics of NSAIDs is the carboxylic acid moiety which contributes to both its activity and side effects.
Photocatalytic degradation of NSAIDs and ANs has been conducted by various researchers primarily aiming to explore the degradation kinetics and degradation efficiency of APIs under selected experimental conditions. Among the most frequently studied NSAIDs and ANs are diclofenac, ketoprofen, ibuprofen, naproxen and paracetamol. Photocatalytic studies of NSAIDs and ANs are summarized in Table 3.3 and detailed discussions of selected studies are provided to highlight both the significant findings and differences in the experimental conditions.
3.5.1.1 Diclofenac
Diclofenac (DCF) has been identified as one of the most recognized pharmaceuticals in the aquatic environment as a result of its elevated use worldwide, Heberer (2002b). The global consumption of DCF was reported to be 940 t per year with a daily dose of 100 mg. These numbers clearly demonstrate its significance as an environmentally important pharmaceutical, Zhang et al. (2008a). Threats on fish such as rainbow trout, and birds, specifically the vulture populations, have been reported, Oaks et al. (2004). NSAIDs such as DCF (and NPX) with a partition coefficient (log Kow) greater than 3 are generally capable of bioaccumulating in the tissues of organisms, Rizzo et al. (2009a). The concentration of DCF has been reported to be less than 10 ng/L in a drinking water sample from tap water in Berlin and ranged between 0.14 and 1.48 μg/L in WWTPs effluents and surface water, Zhang et al. (2008a). As for the removal efficiency by WWTPs, there has been a wide range varying from 0 % to 80 % with typical ranges between 21 % and 40 %, which clearly indicates insufficient removal of this pharmaceutical, Zhang et al. (2008a).
One of the most comprehensive studies of the effect of UV/TiO2 on DCF to date was performed by Calza and his group (2006). The study included the identification of intermediates formed, mineralization and also degradation rate controlling parameters. The photocatalytic efficiency increased with the amount of TiO2, but high loadings (0.8–0.9 g/L) showed no positive effect on the degradation due to scattering of light. The optimum combination of TiO2 loading/initial concentration, 0.6 g/L/8.17 mg/L yielded the highest degradation rate with a residual DCF percentage of 0.4 % after 30 min of irradiation. Longer irradiation times of up to 2 h were required to achieve complete mineralization. The toxicity of irradiated solutions was monitored with a microtox bioassay (Vibrio fischeri) and showed a decrease in the inhibition percentage to less than 1 % in 2 h, thus demonstrating the efficiency of photocatalytic detoxification. A total of 11 transformation products with four products with similar m/z ratios of 312 were generated from hydroxylation of DCF. This was attributed to the non-selective nature of the HO• radical. Hydroxy and bishydroxy-DCF derivatives were formed with subsequent formation of other chloro and hydroxy-phenol derivatives.
UV/TiO2 degradation of DCF alone and in mixture solutions was examined in two studies by Rizzo et al. (2009a, b). For the investigation of DCF as a single compound in Milli-Q water, the authors investigated two operational parameters under oxygen supply, the initial concentration (5–80 mg/L) and loading of TiO2 Aeroxide P25 (0.2–1.6 g/L) with a batch reactor. All degradation data followed pseudo-first order kinetics, but at higher initial concentrations, 40 mg/L and 80 mg/L and 1.6 g TiO2/L, DCF degradation was reported to follow second-order kinetics. COD removal increased from 78 % to 85 % proportionally with TiO2 loading from 0.4 to 0.8 g/L and a DCF initial concentration of 15 mg/L, but declined when DCF concentrations were increased to 40–80 mg/L, Rizzo et al. (2009a).
The degradation of DCF in the presence of two other APIs, namely amoxicillin (AMX) and carbamazepine (CBZ), was investigated in a subsequent study by the same group, Rizzo et al. (2009b). In this study, spiked samples in Milli-Q water and wastewater samples collected from a WWTP in Italy downstream of the biological process were used. The experimental set-up was similar to that in their previous study. Photocatalytic oxidation displayed higher removal from the sample mixture prepared using pure water after 30 min of irradiation, than the sample mixture in real wastewater. This difference is attributed to the existence of other oxidizable species and radical scavengers such as carbonates in the wastewater samples, which are able to compete with the APIs during the photocatalytic treatment. The following Eqs. (3.12) and (3.13) show the scavenging effects of carbonate species on hydroxyl radicals:
TOC removal of up to 80 % after 2 h and a rapid increase after 30 min in the sample mixtures in pure water was observed. However, TOC t1/2 (half-life time) was found to be significantly higher in the sample mixtures in both water matrices compared to that of the single compound samples investigated.
UV-A photocatalytic irradiation was applied to the decomposition of DCF with different types of TiO2, water matrices, H2O2 concentrations, TiO2 loadings and initial concentrations investigated, Achilleos et al. (2010a). An immersion-well type reactor equipped with a UV-A lamp (350–400 nm, 9 W) was used in this study. All experiments were conducted at a nominal pH of 6 with continuous oxygen flow. Among six TiO2 samples tested, the TiO2 Degussa P25 experiment showed the highest conversion of 85 % (initial concentration 10 mg/L and TiO2 250 mg/L) after 4 h of irradiation due to the better photocatalytic activity of anatase. Nevertheless, the P25 TiO2 loading resulted in no improvement of DCF conversion and in all cases only 85 % conversion was attained. The results demonstrated that UV-A/TiO2 treatment is effective for DCF abatement as well as mineralization. Another major finding was that photocatalytically treated 10 mg/L DCF (500 mg/L TiO2) samples taken after 120 min were highly toxic to the freshwater species Daphnia magna.
An attempt was made to compare the effect of the irradiation source (near UV–vis and UV) on the photocatalytic degradation of DCF with commercial TiO2 P25 and synthesized TiO2, Martínez et al. (2011). This study highlighted that UV radiation (254 nm) was found to be superior to near UV–vis (366 nm) for DCF photocatalytic degradation. The optimum conditions for DCF removal included 0.5 g/L of synthesized anatase in the presence of 50 % (v/v) DO. In terms of mineralization, irradiation of aqueous DCF in the presence of 1 g/L TiO2 P25 with near UV–vis caused about 40 % reduction in TOC in 60 min where the concentration declined from 112 mg/L to 68.6 mg/L. Eight photoproducts were identified regardless of reaction conditions. The main photoproduct formed was identified as a monohalogenated carbazole with a m/z of 259 g/mol as a result of photocyclization of DCF which was also in accordance with a DCF photolysis study, Agüera et al. (2005). Another important pathway, decarboxylation generated two other intermediates corresponding to a m/z of 251 g/mol and 215 g/mol (Scheme 3.1), respectively.
Photocatalytic degradation pathways and degradants of diclofenac (DCF), Martínez et al. (2011)
More recently, the combination of heterogeneous photocatalysis with other AOPs for DCF degradation has been studied. The effect of photocatalytic ozonation (O3/UV-A/TiO2) on DCF was assessed, Aguinaco et al. (2012). Irradiation was performed using a high pressure Hg lamp which was placed in the centre of a borosilicate-type glass photoreactor. Commercial TiO2 Degussa P25 and ozone generated from pure oxygen were used. One interesting finding was that the wastewater sample of DCF showed no differences in terms of degradation rate and TOC removal for a DCF concentration of 30 mg/L, whereas differences were observed when Milli-Q water was used under the same conditions indicating a significant effect of the water matrix. The study reported negligible differences when investigating TiO2 loadings (0.5–2.5 g/L) on 30 mg/L DCF and in all cases complete degradation was achieved in 5.5 min.
From the results above on the UV/TiO2 degradation of DCF, it is evident that attempts have been made to correlate selected operational parameters with DCF degradation in pure water and also wastewater. It can be concluded that the choice of initial concentration of DCF, TiO2 loading and irradiation time differs between the studies. However, there is no single study which exclusively covers all the operating parameters such as pH (from acidic to basic) and TiO2 loading (low to high range). The effects of radiant flux and wavelength are not clearly understood despite the fact that UV-A type of irradiation has been chosen for the majority of the studies reviewed.
3.5.1.2 Naproxen
The occurrence of naproxen (NPX) has been reported both in water and wastewater. A recent review has also indicated its continuous presence in the water bodies, Ziylan and Ince (2011). Concentrations of NPX in WWTP effluents and surface water ranged between 0.1–2.6 μg/L and 0.01–0.1 μg/L respectively, Boyd et al. (2005). However, there is still a considerable lack of information on its photocatalytic degradation compared to its direct photolysis which has thus far been performed with artificial UV light and direct sunlight, Felis et al. (2007) and Packer et al. (2003). NPX photodegradation products were found to be more toxic than the parent compound based on a toxicity studies conducted using Daphnia magna and Vibrio fischeri, Isidori et al. (2005).
Only one study has been conducted on the photocatalytic and photolytic degradation of NPX, Méndez-Arriaga et al. (2008a). In this study, 3 h of photolysis and photocatalysis with a solar simulator produced 90 % and 40 % NPX removal, respectively. Lower percentage removal during the photocatalytic process resulted from either low adsorption of NPX onto the TiO2 surface or possible recombination and deactivation of the HO• radical. A higher mineralization (20 %) level was however achieved with photocatalysis compared to photolysis (5 %). Initial degradation rate of NPX increased with TiO2 loading (0.1–1 g/L) for 0.8 mM/L NPX. The by-products formed from the 180 min of treatment were identified by means of liquid chromatography-electrospray ionization-time of flight mass spectrometry (LC/ESI-TOF-MS). Two major pathways including demethylation and decarboxylation were thus suggested to occur on the photocatalytic treatment of NPX (Scheme 3.2).
The proposed degradation pathway of naproxen (NPX) by TiO2 photocatalysis, Méndez-Arriaga et al. (2008a)
In another study, the photocatalytic degradation of NPX was evaluated together with two other NSAIDs, namely DCF and ibuprofen (IBP), Méndez-Arriaga et al. (2008b). A Duran tubular photoreactor was placed in a solar simulator and irradiated with a Xe-OP lamp (290–400 nm; photon flux 6.9 μEinstein/s). A maximum degradation of NPX was obtained at a TiO2 loading of 0.1 g/L for 200 mg/L NPX (30 °C and flow rate of 0.2 mL/min) with a kinetic rate constant of 7.0 × 10−3 min−1. An increase in TiO2 (0.1–1 g/L) did not improve the degradation rate. NPX degradation and TOC conversion however showed a slight increase at 40 °C. The reaction rate for each NSAID did not vary significantly. Biodegradability (BOD5/COD) showed no improvement after photocatalytic treatment of NPX, implying that the by-products formed affect post-biological treatment.
Another study was carried out to evaluate the feasibility of commercial nanofiltration membranes and TiO2 P25 Degussa for NPX and six other pharmaceuticals (furosemide, rantinide, ofloxacine, phenazone, carbamazepine and clofibric acid) degradation, Molinari et al. (2006). For irradiation purposes, a medium pressure Hg lamp (125 W) was used under continuous oxygen bubbling. NPX showed a 95 % adsorption at pH 3 and only 3 % at pH 11 due to high solubility. Degradation of NPX was only performed in a batch photoreactor without any membrane. A comparison with the membrane photoreactor for NPX removal was thus not possible. In a batch photoreactor, the concentration of NPX decreased with increasing irradiation time following pseudo-first order kinetics. The rate constants for 5 mg/L NPX at pH 3 and pH 11 were 7.86 × 10−2 and 4.91 × 10−1 min−1, respectively.
Although NPX is known to undergo direct photolysis under natural sunlight, Packer et al. (2003), various aspects of the efficiency of photocatalytic NPX degradation remain to be investigated. Further research needs to be undertaken in order to gain sufficient knowledge on the degradation kinetics and toxicity outcome of photocatalytically treated samples, in addition to the by-products generated.
3.5.1.3 Ibuprofen
As for the other NSAIDs, ibuprofen (IBP) has been found in water, wastewater and wastewater effluents due to only partial removal in WWTPs. For example, the concentrations of IBP and one of its metabolites was recorded to be more than 500 ng/L in the effluent of a primary WWTP, Sabri et al. (2012). Elimination of IBP in WWTPs varies between 75 % and 90 %, Tixier et al. (2003). The most prominent metabolites of IBP, namely carboxy and/or hydroxy IBP, have been reported to be present after biological treatment as toxic by-products, Mozia and Morawski (2012). Despite being one of the most consumed pharmaceuticals, there has been little attention devoted to the photocatalytic oxidation of IBP thus far.
A systematic study of IBP (and CBZ) photocatalytic degradation and mineralization spiked in Milli-Q and wastewater samples was performed under UV-A and simulated solar irradiation, Achilleos et al. (2010b). The study concluded that heterogeneous photocatalysis with both, UV-A and solar irradiation, was effective for IBP removal in the presence of TiO2 in particular Degussa P25. The study proposed that this treatment can be employed as a post-secondary treatment in WWTPs.
UV/TiO2 degradation of IBP and two other NSAIDs, DCF and NPX, revealed that IBP can be completely removed, Méndez-Arriaga et al. (2008b). A maximum conversion of 200 mg/L IBP was achieved in the presence of 1 g/L TiO2 after 240 min of irradiation with a first-order kinetic rate constant of 9.1 × 10−3 min−1. A low initial concentration of IBP (25 ppm) resulted in maximum degradation within 60 min and 50 % mineralization. Further investigation showed that biodegradability (BOD5/COD index) of IBP was enhanced in the presence of excess DO. The study thus proposed the feasibility of post-biological treatment. Electrospray ionization/mass spectrometry (ESI/MS) analysis at the end of 4 h of photocatalytic treatment revealed that hydroxylation or HO• attack on the propanoic moiety or/and isobutyl side chain is the dominant pathway for IBP degradation. Demethylation and decarboxylation processes resulted in the formation of other organic acids such as propionic, formic or hydropropionic acid or their sodium salts (Scheme 3.3).
Photocatalytic degradation pathways of ibuprofen (IBP), Méndez-Arriaga et al. (2008b)
Solar photocatalysis has proven to be an effective method for IBP degradation based on a study conducted in three solar pilot plants, Méndez-Arriaga et al. (2009a). TOC and IBP removal were enhanced in the presence of H2O2. In contrast, no improvement was found on inclusion of Na2S2O8 as the pH decreased up to 4.5 which correlates to the pKa of IBP (4.5) and thus leads to precipitation. Improvement in the biodegradability (BOD5/COD) of the treated solution suggested coupling potential with biological treatment.
Effective photocatalytic degradation rates have also been reported when hybrid AOP methods were applied to IBP, Méndez-Arriaga et al. (2009b) and Madhavan et al. (2010). One study confirmed that AOP hybrid processes (sonophotocatalysis with TiO2, sonophoto-Fenton and sonophotobicatalysis with TiO2 and Fe2+) improved the degree of mineralization of IBP in parallel with IBP removal. The combination of TiO2/Fe2+/sonolysis turned out to be the most beneficial application, owing to its highest mineralization (98 %), Méndez-Arriaga et al. (2009b). The combination of UV and ultrasound with TiO2 (UV/TiO2/ultrasound) enhanced the degradation of 18.5 mg/L IBP (in 1 g/L TiO2 P25) from 61 % (photocatalysis only) to 85 % after 15 min of irradiation, Madhavan et al. (2010).
3.5.1.4 Acetaminophen
Acetaminophen commonly known as paracetamol is the API in a variety of over-the-counter analgesic and antipyretic drugs. This drug is extensively used around the globe. In England, it was used in more than 400 t in 2000 while the export quantity from China was estimated to be 15,348 t in 2001, Zhang et al. (2012). Its occurrence in levels up to 6 ppb and 10 ppb, respectively, in natural waters, in European sewage treatment plant effluents and in the United States has been reported. However, only few studies deal with the removal of paracetamol by means of UV/TiO2.
A study dedicated to explore the effect of various operating parameters on photocatalytic degradation for paracetamol in Milli-Q water was reported in 2008, Yang et al. (2008). Comparing UV-A/TiO2 and UV-C/TiO2 irradiation, the latter was found to be more effective to degrade 4.0 mM (0.4 g/L TiO2) of the parent compound as well as reducing TOC to about 60 % in 300 min. Increasing TiO2 loading from 0.04 g/L to 5.0 g/L caused the degradation rate to increase from 4.9 ± 0.5 to 14.7 ± 1.7 × 10−3 min−1 until it levelled off to 13.7 ± 1.2 × 10−3 min−1 at 0.8 g/L. Insignificant effects were found at 7.0 g/L due to scattering phenomena. Increase in pH from 3.5 to 9.5 also increased the degradation rates from 11.0 to 16.5 × 10−3 min−1. However, a higher pH of 11 significantly slowed down the degradation rate due to repulsion between negatively charged TiO2 and paracetamol. A similar pH effect was also observed by Zhang et al. (2008b). Addition of oxygen showed a significant impact on paracetamol degradation compared to argon as the former inhibits e−-h + recombination.
In order to establish the nature of the most active species (ecb −, hvb +, HO•, HO2 •, O2 •− and H2O2) for paracetamol degradation, a subsequent study was undertaken by Yang et al. (2009) using UV-A irradiation. It was proposed that the HO• radical plays a prominent role in the photocatalytic degradation of paracetamol.
Both studies identified 11 common intermediates, including aromatic compounds, carboxylic acids, and nitrogen containing aliphatic compounds from photocatalytic degradation of paracetamol (Scheme 3.4). Two additional inorganic intermediates, namely ammonium and nitrate, were proposed as a result of successive mineralization of the intermediates. The degradation is initiated by hydroxylation through HO• addition onto the aromatic ring, which subsequently results in further oxidation to form carboxylic acid derivatives, Yang et al. (2009).
Recent advances in the photocatalytic degradation of paracetamol revealed that UV-A/LEDs (Light Emitting Diode) can be used instead of the traditional Hg lamps. Conventional lamps are known for their high energy cost and the incorporation of toxic mercury, Xiong and Hu (2012). Negligible amounts of paracetamol were removed with UV-A/LED photolysis. In contrast, TiO2 P25 caused a significant level of degradation with complete degradation within 20 min. An increase in light intensity from 1 to 4 mW/cm2 significantly enhanced the degradation rate of paracetamol. One important advantage demonstrated in this study is the possibility to employ low average amount of TiO2 (0.01 g/L) due to the high light intensity from LED lamps. This finding has important implications for pursuing the applications of UV-A/LED light sources in water and wastewater treatment.
3.5.2 Antibiotics
In recent years, there has been an increasing amount of literature on the photocatalytic treatment of antibiotics, due to their extensive use in both humans and animals as agents to prevent and treat microbial infections. Classifications of the major antibiotics are presented in Table 3.4, according to importance attached to their chemical structure and mechanism of action.
The annual consumption of antibiotics for human and veterinary usage is estimated to be about 100,000–200,000 t, Xu et al. (2007). The first detection of antibiotics in river water was reported in 1982 in England, where macrolides, tetracycline (TC) and sulphonamides at levels of 1 μg/L were found, Homem and Santos (2011). Since then, numerous studies have reported the presence of antibiotics in water courses around the world, Watkinson et al. (2009). The polar and non-volatile nature of antibiotics contributes significantly to their accumulation in the environment due to their persistence either as the parent compound or metabolites after conventional water treatment. One of the major effects of the continuous accumulation is the emergence of antibiotic resistance strains of bacteria, Elmolla and Chaudhuri (2010a). Accumulation of small quantities of antibiotics in receiving water over a long term has been the main concern, as they may have adverse effects on both humans and aquatic organisms.
Studies conducted to assess the potential of UV/TiO2 degradation on four important classes of antibiotics namely β-lactam antibiotics, quinolones, tetracyclines and sulphonamides are summarized in Table 3.5.
3.5.2.1 β-Lactam Antibiotics
Amoxicillin (AMX) (C16H19N3O5S) has been one of the most popular candidates amongst the β-lactam antibiotics selected for UV/TiO2 treatment. This API has three pK as namely 2.7, 7.5 and 9.6, Dimitrakopoulou et al. (2012). In water, AMX shows absorption up to 290 nm (Fig. 3.4). Oral consumption of 500 mg of AMX has resulted in 86 ± 8 % of AMX unchanged in human urine within 2 h of consumption, Martins et al. (2009). Excretion of unchanged AMX due to a slow rate of metabolism in humans has led to its discharge into the environment. AMX has also been detected in effluents from drug manufacturing facilities with levels reaching several hundreds of milligrams per liter, Mavronikola et al. (2009). The possibilities of AMX elimination by means of UV/TiO2 have been investigated by several research groups, Martins et al. (2009) and Dimitrakopoulou et al. (2012).
AMX spiked ultrapure water and secondary effluent water were used in a UV-A/TiO2 degradation comparative study, Dimitrakopoulou et al. (2012). Of the various catalysts tested, TiO2 Degussa P25 (250 mg/L) was found to be the most efficient TiO2 catalyst, yielding complete degradation after 25 min and 93 % mineralization within 90 min of irradiation for 10 mg/L of AMX. Under the same conditions, TiO2 (Anatase) required at least 45 min to cause a complete removal. The other six TiO2 catalysts needed much longer reaction times and in most cases lead to incomplete degradation. In addition, the degrees of mineralization failed to rise above 75 % after 90 min of irradiation. Direct photolysis with a UV-A light source had little effect on AMX degradation (only 4 %) due to its poor absorbance within the emission spectrum of the irradiation source. The comparison between ultrapure water and secondary effluent showed that the latter hindered the degradation due to the presence of organic matter and scavenging effects of bicarbonates and chlorides.
AMX spiked hospital wastewater samples yielded complete degradation with TiO2 Degussa P25 photocatalysis (800 mg/L at pH 4 and 30 °C) after 30 min of irradiation while only 85 % degradation was achieved using the photo-Fenton process after an extended period of 60 min, Martins et al. (2009). The lower degradation in the latter case was explained by the pH conditions as it has a significant effect on the treatment. At pH 4 and beyond, the formation of Fe2+ hydroxide complexes and Fe3+ species retard the production of HO•. In contrast, the percentage COD removal was higher for photo-Fenton than for photocatalysis with 64.6 % versus 44 %, respectively. Toxicity inhibition studies with Artemia salina showed that both treatments were efficient in removing toxicity and reductions of 43.5 % and 46.3 % were obtained in photo-Fenton and UV/TiO2 processes, respectively.
Another study compared the efficiency of four AOPs, namely Fenton, photo-Fenton, TiO2 photocatalysis (UV/TiO2 and UV/TiO2/H2O2) and ZnO photocatalysis (UV/ZnO) on AMX (104 mg/L) in distilled water in combination with two other antibiotics, ampicillin (105 mg/L) and cloxacillin (103 mg/L) under UV-A irradiation (365 nm), Elmolla and Chaudhuri (2010b). Based on the pseudo-first order rate constants under optimum operating conditions, photo-Fenton demonstrated the highest rate constant of 0.029 min−1, followed by Fenton with 0.0144 min−1 with t1/2 of 49.5 min and 69.3 min, respectively. Although the performances of UV-A/TiO2/H2O2 and UV/ZnO were similar in terms of the reported rate constants of 0.0005 min−1 and 0.00056 min−1, respectively, the rate constant for UV/TiO2 was not reported therefore preventing a meaningful comparison. With the exception of UV-A/ZnO, biodegradability (BOD5/COD) was improved for all treatment methods investigated.
A separate study reported the efficacy of TiO2 (Anatase with purity >99 %) on AMX (and ampicillin and cloxacillin) degradation in distilled water, irradiated with UV-A lamp (365 nm, 6 W) which was further enhanced by the addition of H2O2, Elmolla and Chaudhuri (2010c). These results, somewhat contradictory with those reported by Martins et al. (2009), may be due to differences in the photocatalytic activity of the different TiO2 used, the irradiation source, addition of H2O2 and also the water matrix. None of these studies described the degradation products formed during the photocatalytic treatment.
A comparison between photocatalysis using doped and undoped titania with artificial UV-A and sunlight showed that that latter produced a three times faster degradation for AMX than artificial UV, Klauson et al. (2010). The improved efficiency of 2 h of solar (16 mW/cm2) photocatalytic illumination with TiO2 Degussa P25 over 6 h irradiation with artificial UV-A light (365 nm, 0.5 mW/cm2) for AMX was apparent. The faster degradation occurred at pH 6 (20 °C) and a concentration of 50 mg/L and yielded the highest photocatalytic efficiency of 80 %. Doped catalysts with Fe and C also showed comparable efficiencies to that of Degussa P25. With artificial UV light, COD was removed to a percentage between 10 % and 40 % in all experiments. This study revealed the superiority of solar photocatalysis for AMX conversion. Photocatalytic degradation pathways were proposed to occur for different AMX concentrations, 10, 25 and 100 mg/L (pH 6, 20 °C) at three different reaction rates of control. The majority of the degradation products differed under these three reaction conditions, with only a few common products identified. Only small amounts of ammonia, nitrate and sulphate were detected, which implies that the heteroatoms N and S remained in the organic by-products. p-Hydroxybenzoic acid (m/z 139) was a common degradation product found under all photocatalytic conditions. It is proposed to form through the fragmentation of the peptide bond, which is in close proximity to the aromatic ring in AMX. Scheme 3.5 shows the proposed degradation pathway for AMX.
Proposed photocatalytic degradation pathway for amoxicillin (AMX) (10 mg/L AMX; pH 6; 20 °C), Klauson et al. (2010)
It can be concluded that artificial UV and solar photocatalysis with TiO2 facilitated efficient AMX degradation. ZnO was also effective, albeit only one study used this photocatalyst. As a result of this, more research is needed to evaluate the potential of ZnO for AMX degradation by optimizing the parameters influencing its degradation as this catalyst has higher photocatalytic activity than TiO2. There are also similarities expressed between studies where the UV-A type of irradiation was sufficient to cause AMX degradation.
3.5.2.2 Sulfonamide and Other Sulfa Antibiotics
Sulfonamides and sulfanilamides are derived from sulfanilic acid and their common features are the sulfanilamide group and a five or six member heterocyclic ring (shown as R in the general structure) and are represented in Table 3.4. Selected sulphonamides, with their common names and the nature of their R groups are shown in Table 3.6. Sulfonamides and sulfanilamide derivatives are antibacterial agents used both in animals and humans for the treatment of infections. Almost 80 % of all sulfonamides have been detected in the environment, which could easily infiltrate groundwater, and have shown high toxicity to microorganisms, algae and plants, Baran et al. (2011).
Sulfamethoxazole (SMX) (C10H11N3O3S), being the most commonly used member of the group of sulfonamide antibiotics, is prescribed for urinary tract infections and has been detected in the environment. This synthetic antibiotic is used either alone or in combination with trimethoprim. SMX has been detected in plant effluents at concentrations ranging between 0.01 and 2.0 μg/L in countries such as Germany, Spain and Italy and in surface waters between 0.03 and 0.48 ug/L, Trovó et al. (2009). SMX has two pKa values, pKa1 = 1.6 and pKa2 = 5.7, and thus exists predominantly in the cationic form at pH < 1.6 and in the anionic form at pH > 5.7. There have been reports on UV/TiO2 treatment of this pharmaceutical using various lamp sources, Abellán et al. 2009 and Nasuhoglu et al. (2011).
SMX has been reported to be susceptible to direct photolysis with a Xe lamp resulting in significant degradation, but poor mineralization, Abellán et al. (2007, 2009). In water, SMX is capable of absorbing light up to 310 nm. When irradiated without the use of a filter and photocatalyst, SMX degraded up to 80 % but only resulted in a reduction of 14 % of TOC, Abellán et al. (2007). In contrast, the photocatalytic degradation of 100 ppm SMX (irradiation of 6 h) in the presence of 1.0 g/L TiO2 yielded 88 % reduction in SMX. With 2.0 g/L TiO2, the degradation marginally increased to 91 %. The optimum TiO2 P25 concentration was found between 0.5 and 1.0 g/L. Variations in pH (2–11) resulted in very minor changes and the degradation remained almost constant at 85 %, with a very small increase at lower pHs. A total of five intermediates were identified using mass spectrometry. Of these, the intermediate with a molecular weight of m/z = 397 was found to be the major product and was assigned to a dimer product of the parent SMX. Other intermediates possessed molecular weights of 222 in ES positive ion mode and 197, 269 and 287 in ES negative ion mode, Abellán et al. (2007) (Scheme 3.6).
Degradation pathways of sulfamethoxazole (SMX) with TiO2 photocatalysis, Abellán et al. (2007)
UV-C irradiation (254 nm) was employed to study the photolytic and photocatalytic degradation of SMX in pure water, Nasuhoglu et al. (2011). Irradiation was carried out in a cylindrical acrylic photoreactor equipped with an Hg-Ar lamp source. The results suggest that photolysis is an important removal mode for SMX (12 mg/L) which corroborates the findings of Abellán et al. (2007). The high tendency for direct photolysis was explained based on the matching of the SMX absorbance with the highest intensity emission of the UV-C lamp. UV-C radiation yielded complete degradation of 12 mg/L SMX within 10 min, while UV/TiO2 required 30 min to achieve a similar level of degradation. However, mineralization measured as COD was more efficient on photocatalysis treatment with 87 % removal, compared to only 24 % removal upon photolysis. Photoproducts generated as a result of both treatments were reported to be more toxic than the parent SMX, as demonstrated by the acute toxicity Daphnia magna testing.
SMX was reported to undergo UV-A (350–400 nm) induced photocatalytic degradation with TiO2 Degussa P25, Xekoukoulotakis et al. (2011). A 9 W lamp (photon flux 2.81 × 10−4 Einstein/min) in an immersion well batch photoreactor was used under continuous oxygen sparging. Complete degradation of 10 mg/L SMX with 250 mg/L TiO2 P25 was achieved in 30 min. Likewise, a TOC reduction of up to 90 % was observed. The photocatalytic degradation at different initial concentrations (2.5–30 mg/L) followed the Langmuir-Hinshelwood (L-H) kinetic model.
The behaviour of three sulfa pharmaceuticals, namely sulfachlorpyridazine, sulfapyridine and sulfisoxazole with similar structures towards UV/TiO2 treatment were investigated by Yang et al. (2010). A Pyrex reactor with a high pressure Hg lamp (λmax = 365 nm) was chosen. Photocatalytic treatment proved generally effective on these sulfa drugs, with removal efficiencies varying between 85.2 % and 92.5 % after 60 min of irradiation. TOC was concomitantly removed in high percentages (81.5–90.8 %) within 240 min with all three drugs showing complete mineralization to CO2, H2O and inorganic ions. Photocatalytic degradation rate constants of these three sulfa drugs also increased with the amount of TiO2 from 0.25 to 3 g/L. It was proposed that h + and HO• play the most important role in the disappearance of sulfa pharmaceuticals. Remarkably, different photocatalytic degradation rate constants for the three drugs were obtained at different pH values despite their similarity in structure. This was attributed to changes in their zwitterionic nature in different pH environments. Various intermediates formed rapidly within 15 or 20 min of irradiation and then slowly disappeared. The two major pathways for photocatalytic degradation of the sulfa drugs were hydroxylation and cleavage of the S-N bond by the photohole (Scheme 3.7).
Photocatalytic degradation pathways of sulfa pharmaceuticals, Yang et al. (2010)
Another study employed a UV-A light source to determine the degradation of SMX and a few other sulphonamides, namely sulfamethizole, sulfathiazole and sulfisoxazole, Hu et al. (2007). The effects of natural organic matter (NOM) and bicarbonate ion on the degradation of SMX were investigated. The occurrences of NOM and bicarbonate ions in water are known to impede the degradation of compounds by scavenging HO•. However, the results showed that an increase in bicarbonate concentrations generated a higher degradation rate for SMX. This was attributed to considerably higher HCO3 − and CO3 2− concentrations compared to SMX, which is likely to induce a stronger interaction with Ti (IV) than between SMX and Ti (IV). As a result, these ions efficiently scavenged the adsorbed HO• radicals, which in turn reduced the recombination of the charge carriers. The CO3 −• radicals generated were proposed to react with SMX in the bulk rather than on the surface because they are more stable and posses longer lifetimes. In contrast, an increase in NOM concentrations (2–20 mg/L) inhibited the SMX degradation, in particular at higher concentrations of NOM.
3.5.2.3 Quinolones and Fluoroquinolones
Quinolones are synthetic antibacterial agents which are widely used in both humans and livestock. The basic structure of quinolones contains two fused rings with a carboxylic acid and a ketone group (Table 3.4). First and second generation quinolones are active against gram-negative bacteria, while the third and fourth generations have extended activity against gram-positive bacteria as well, Nasuhoglu et al. (2012). Fluoroquinolones (FQs) have attracted substantial attention due to their incomplete metabolism and frequent detection in the environment. A FQ compound contains a fluorine substituent in position R4 of the quionolone ring as shown in Table 3.4.
FQs are excreted to between 20 % and 80 % and their persistence has led to detection in surface waters, municipal wastewaters and tertiary treated effluents, Michael et al. (2010). As with other synthetic antibiotics, FQs are not fully metabolized and thus excreted unchanged. The persistent nature of FQs is associated with the quinolone ring, which results in high chemical stability and contributes additionally to their resistance towards hydrolysis, Sturini et al. (2010). Only a few studies dealing with the degradation of FQs by TiO2 mediated photocatalytic treatment have been reported thus far. In general, incomplete mineralization of FQs results in the generation of a range of intermediates which still contain the quinolone ring structure and as a result continue to exhibit antibacterial activity.
The performance of immobilized TiO2 integrated into an annular reactor was tested with black light (360 nm) using 18 ppm oxolinic acid (OX) at pH 9 under oxygenated conditions. A comparison was made with a 1 g/L titania suspension, using the same reactor. The coated titania was capable of removing OX acid to over 90 % after 60 min, while TOC was reduced by 50 % after 100 min of irradiation. In suspension, 90 % removal was achieved after 20 min whereas total removal took 40 min. The superior performance of the suspended TiO2 was explained by the superior adsorption, which subsequently increased the reaction rate, Palominos et al. (2008).
A systematic evaluation of OX acid removal in ultrapure water was conducted by Giraldo et al. (2010). The optimum conditions for complete degradation of 20 mg/L OX acid after 60 min of irradiation were with 1.0 g/L TiO2 and pH 7.5. COD was reduced by 50 % and the degradation followed pseudo-first order kinetics. The study also revealed that OX acid intermediates, which were identified by high performance liquid chromatography-mass spectrometry (HPLC/MS) exhibited lower toxicity than the parent compound in addition to a loss of antimicrobial activity based on an Escherichia coli assay.
UV-A/TiO2 is also an effective technology for oxidation of ciprofloxacin (CIP) in pure water, An et al. (2010) and Paul et al. (2010). UV-A and Vis light sources were employed to study the photolytic and photocatalytic treatment of CIP, Paul et al. (2010). Based on the pseudo-first order rate constants, the efficiency of CIP degradation increased in the order UV-A/TiO2 > Vis/TiO2 > UV-A only. Degradation of CIP by UV-A/TiO2 is proposed to occur via three mechanisms: direct UV-A photolysis, charge transfer and charge separation involving the semiconductor. Various piperazine ring transformation products were formed upon UV-A/TiO2 and Vis/TiO2 treatment and their proposed structures are shown in Scheme 3.8. Energy efficiencies for the three treatment protocols were compared and the UV-A/TiO2 turned out to be the most energy efficient process.
Possible degradation products of ciprofloxacin (CIP) from photolysis and TiO2 photocatalysis, Paul et al. (2010)
Degradation of 100 μM CIP and a short t1/2 of 1.9–10.9 min were furthermore achieved at different pH ranges (3.0–11.0) using 1.5 g TiO2/L Degussa P25 and UV-A irradiation (365 nm) emitted from a high pressure Hg lamp. The degradations followed pseudo-first order kinetics and the rate constant increased from 0.06 ± 0.01 min−1 at pH 3 to 0.38 ± 0.01 min−1 at pH 9. At a higher pH value of 11, the degradation rate decreased to 0.07 ± 0.01 min−1 due to the repulsive effect between the negatively charged compound and the negatively charged TiO2 surface. Addition of hydroxyl radicals to CIP and photohole attack were established as the two predominant reaction pathways, An et al. (2010).
Photocatalytic degradation of four FQs, namely ofloxacin, norfloxacin, ciprofloxacin and enrofloxacin, was examined simultaneously using a solar simulator, Li et al. (2012). Alkaline conditions (pH 9) resulted in almost complete degradation (98–100%) for all four FQs after 150 min of irradiation. Likewise, TOC was reduced by 58 %. The threshold TiO2 load was found to be 0.5 g/L at pH 9 to achieve 96–100% of degradation of the four FQs. An increase in the TiO2 load from 0.1 g/L to 1.5 g/L did not produce a direct correlation with TOC removal. The effects of pH changes on these four FQs were rather complex, due to different ionization states of the catalyst and the substrates (two pKa values for each). In particular, these FQs can either exist in their cationic (pH < pK a1), or anionic (pH > pK a2) or in zwitterionic form (pK a1 < pH < pK a2) (Table 3.7) due to the presence of a carboxylic acid group in the quinolone structure and an additional amine group from the piperazinyl ring.
A third generation FQ, moxifloxacin (MOX) was subjected to UV/TiO2 and the influence of various parameters was examined using a batch photoreactor equipped with a UV-A lamp (300–440 nm, 485 μW/cm2), Van Doorslaer et al. (2012). A catalyst loading of 5 g/L, temperature of 25 °C and air sparging at 60 mL/min were found optimal for removing 37.4 μM of MOX. The initial degradation rate and the t1/2 were determined to be 16.2 ± 0.3 μM/min and 1.6 min, respectively. A stirring speed of 13.2 rps produced a higher degradation rate. The TiO2 P25 loading ranged from 0.1 to 5.0 g/L.
An independent study highlighted that TiO2 Degussa P25 was more effective than two other common photocatalysts, Hombikat UV 100 and PC 500 for the degradation of 0.25 mM of norfloxacin (NOR) (pH 6.3) with 1 g/L of photocatalyst, Haque and Muneer (2007). Almost complete degradation was achieved in 80 min of irradiation from a medium pressure Hg lamp (125 W) under oxygen purging. Likewise, the TOC content was depleted as a function of irradiation time. The efficiency of the photocatalytic degradation of NOR was subsequently correlated with pH, substrate concentration, catalyst concentration, and the addition of electron acceptor, H2O2. The results revealed that addition of 10 mM of H2O2 significantly enhanced the degradation rate and mineralization of 0.25 mM NOR (pH 6.3 and 1 g/L TiO2 P25).
A recent study was conducted with unfiltered river water to explore the photocatalytic decomposition of six FQs, namely ciprofloxacin, danofloxacin, enrofloxacin, levofloxacin, marbofloxacin and moxifloxacin, under natural sunlight. Compared to direct photolysis under natural sunlight, all of the tested drugs except CIP underwent faster degradation with degradation rate constants ranging from 0.22 to 2.78 min−1 in the presence of TiO2 (0.5 g/L). The results demonstrated the effectiveness of solar TiO2 photocatalysis for the removal of FQs from river water matrix despite the presence of other non-target matrix components, Sturini et al. (2012). CIP in hospital wastewater was also satisfactorily removed with UV/TiO2 using a medium pressure Hg lamp. For comparison, ozonation was used as an alternative AOP. Both processes generated similar degradation products and oxidation of the piperazine group was found to be the major degradation pathway, Vasconcelos et al. (2009).
The results presented for quinolones and FQs are significant and demonstrate the potential removal from wastewaters not only for single compounds but also for mixtures.
3.5.2.4 Tetracyclines
A comparison of photocatalytic degradation and mineralization of oxytetracycline (OTC) in a laboratory and at pilot-scale was reported by Pereira et al. (2011). Laboratory scale experiments with a solar simulator showed that photocatalysis of 20 mg/L OTC with 0.5 g/L TiO2 at free initial pH produced 95 % degradation. In contrast, only 36 % degradation was achieved upon direct photolysis after 60 min of irradiation. Solar photocatalysis in a CPC pilot plant reactor showed complete removal of OTC with energy consumption 10 times less than solar photolysis which was also performed by means of CPC reactor. Solar photocatalysis in the pilot plant reactor yielded 80 % mineralization. All experiments followed pseudo-first order kinetics, but the rate constants increased from the photolytic to photocatalytic operation modes. Solar photocatalysis in a CPC reactor produced the highest pseudo-first order rate constant of 2.63 ± 0.03 L/kJ, indicating the efficiency of such a photoreactor to harvest and utilize solar photons.
Several studies involving tetracycline (TC) have been carried out to determine its photocatalytic degradation, Reyes et al. (2006), Palominos et al. (2009) and Mboula et al. (2012). Catalyst performance and biodegradability or antibacterial efficiency arising from the treated solutions differed for all studies reported. Using a multivariate and response surface method approach, the optimum degradation rate for each catalyst was investigated. ZnO exhibited a slightly higher oxidation rate than TiO2 P25. The optimum oxidation conditions for TiO2 were established as 1.5 g/L and at pH 8.7. For ZnO, the most appropriate conditions were 1.0 g/L and pH 11, Palominos et al. (2009).
The TC disappearance over the irradiation time, at different pH values and with three different light sources, namely UV lamp (λ > 254 nm), UV-A lamp (λ = 365 nm) and a solarium device (λ = 300–400 nm), was carried out by Reyes et al. (2006). Based on the pseudo-first order rate constants, direct photolysis was regarded negligible for TC. In contrast, faster degradation rates were achieved with the solarium and UV light in the presence of TiO2 (Fig. 3.5). These findings were supported by the high irradiance intensities measured from the UV lamp (1,210 μW/cm2 at 365 nm) and the solarium lamp (1,980 μW/cm2 at 365 nm), respectively. Rapid disappearance of TC and subsequent complete mineralization was achieved after 2 h of irradiation.
Comparison of pseudo-first order constant for tetracycline (TC) degradation (TC: 40 mg/L; TiO2 P25: 0.5 g/L) (Data taken from Reyes et al. (2006))
3.5.3 Antiepileptics
The most commonly investigated antiepileptic is the dibenzazepine derivative, carbamazepine (CBZ) (Fig. 3.6). CBZ is also used as sedative for numerous mental disorders, can cause toxic effects in the liver and is a haematopoietic. Only 72 % of CBZ is absorbed when orally administered, while 28 % remains unchanged and is finally excreted through faeces. CBZ has been known to persist in the environment and studies have reported the removal efficiency of CBZ in WWTPs as below 10 %, Andreozzi et al. (2002) and Zhang et al. (2008a). Thus far, CBZ has been detected in various water compartments around the world. In Germany, CBZ was detected in surface water samples at a concentration up to 1,075 ng/L, Heberer (2002b) and also in groundwater and rivers in the USA, Canada and Germany, Khetan and Collins (2007). These observations clearly confirm the CBZ presence in the aquatic environment. Although the effects of CBZ are not clearly known or confirmed, its toxic effect on aquatic organisms has been already published. There is also evidence of specific chronic effects of CBZ on the oligochaete Chiromus, Oetken et al. (2005). These facts show that this antiepileptic needs to be removed with effective remediation technology. In this context, photocatalysis has been shown to be effective for CBZ degradation. A summary of UV/TiO2 studies of CBZ is tabulated in Table 3.8.
There has been an insufficient amount of information available pertaining to photocatalytic degradation of CBZ. Photocatalytic degradation of CBZ has however been examined in deionized water, Dai et al. (2012) and Im et al. (2012), real wastewater samples from WWTPs, Achilleos et al. (2010b) and formulated hospital wastewater, Chong and Jin (2012). In general, there are some discrepancies in the photocatalytic efficiency on CBZ.
A comparative study on the effect of different on CBZ (4.2 μM) degradation efficiencies showed the following trend: UV/Fenton (86.9 %) > UV/TiO2 (70.4 %) > Fenton (67.8 %) > UV/H2O2 (40.6 %) > UV (14.2 %). On the basis of operating costs, the Fenton process was the most cost-effective in comparison with the other four AOPs (Table 3.9), Dai et al. (2012). As shown in Table 3.9, UV/TiO2 and UV can be considered as costly approaches for wastewater treatment. However the authors reported that this statement can be challenged on the grounds that the cost of photocatalytic treatment can be reduced by applying sunlight as the irradiation source.
The removal efficiency by UV-A (350–400 nm) and solar irradiation provided by a solar simulator on CBZ was investigated along with another API, IBP, Achilleos et al. (2010b). Greater degradation was attained with UV-A irradiation than with artificial solar radiation for both APIs. Degradation of CBZ in pure water was found to be more dependent on changes in the loading of Degussa P25 TiO2 compared to IBP. Conversion of 74 % CBZ was achieved after 120 min of reaction with 100 mg/L P25 under UV-A irradiation, but it decreased to 35 % upon solar irradiation, which was attributed to completely different reactor set-ups. Wastewater samples spiked with 10 mg/L CBZ had a detrimental effect on DOC removal, due to the presence of scavengers of HO• radicals and naturally occurring DOC in the raw wastewater sample. Nevertheless, the study proved that UV-A and solar irradiation can be applied to CBZ removal (and also for IBP). Direct photolysis with a UV-A lamp produced negligible degradation and DOC removal for CBZ.
The results from another study confirmed that direct photolysis supplied by a UV-C irradiation source is not effective for CBZ elimination, Im et al. (2012). An important contribution of the study is that UV-C irradiation increased the removal efficiency compared to that of UV-A irradiation employed on the 0.021 mM CBZ in the presence of 0.5 g/L TiO2 P25. Addition of radical scavengers decreased the removal rate, implying that HO• has a crucial role to play in the photocatalytic degradation of this compound. Addition of oxygen also enhanced the mineralization.
A TiO2 nanofiber was evaluated as a pre-treatment option and for improvement of biodegradability of 5,000 μg/L CBZ in synthetic hospital wastewater, Chong and Jin (2012). This application was found to be efficient for CBZ abatement in synthetic hospital wastewater and also for COD removal. High CBZ degradation rate of 48.33 μg/L min was obtained, which also resulted in 40 % COD reduction after 4 h of treatment. The study highlighted the fact that the TiO2 based system has a good potential as a sustainable pre-treatment system for hospital wastewater.
Doll and Frimmel (2004, 2005a, b) conducted multiple photocatalytic studies on CBZ under various conditions, which revealed that TiO2 photocatalysis is a promising abatement method for CBZ degradation. When a comparison was made with Hombikat UV100, CBZ was degraded more efficiently with TiO2 P25 under simulated solar irradiation (1,000 W), Doll and Frimmel (2004). In the subsequent study, photocatalytic degradation of CBZ was investigated with two types of TiO2 photocatalysts in the presence and absence of NOM under simulated solar UV light, Doll and Frimmel (2005a). It was noted that different concentrations of NOM had different effects on the kinetics of CBZ degradation. In general, NOM retarded the photocatalytic degradation rate of CBZ. This was attributed to competitive inhibition as NOM scavenged the holes. The research group also suggested possible photocatalytic degradation products for CBZ, Doll and Frimmel (2005b) (Scheme 3.9).
Photocatalytic degradation pathways of carbamazepine (CBZ), Doll and Frimmel (2005b)
3.6 Conclusion
A typical UV/TiO2 investigation is shown in Fig. 3.7. After one or more model compounds have been chosen, the next step is the photocatalytic experimental set-up or the choice of the photoreactor and light source, which will have a significant effect on the outcome of the degradation rate, Legrini et al. (1993). There are various factors that need to be taken into consideration with respect to photoreactors, namely the material it is made of, shape, geometry, radiation path lengths, and the cooling apparatus. Similar issues have been highlighted in a review which has commented on this lack of information, when explaining photoproducts formed from the photocatalytic and photolytic studies, Fatta-Kassinos et al. (2011a).
When considering light sources, artificial light sources have been employed in most studies. These can be grouped into monochromatic light sources such as low pressure Hg lamps and polychromatic light source namely medium pressure Hg lamps and high pressure Hg lamps. Other irradiation devices include Xe, Xe-arc, Hg and Ra lamps. Solar simulators have been commonly applied to simulate solar irradiation. LED based light sources are also emerging as an alternative light source, due to advantages which include no mercury waste, longer life time compared to Hg based lamps and improved flexibility in terms of reactor design, Landgraf (2001) and Chen et al. (2007). Very few studies have made a comparison between the lamp's spectral domain and the absorption spectrum of the reactant. Photon flux, intensity and irradiance emitted from the light source also need to be clearly stated as these values determine the rate of photons emitted, which has a direct correlation to the degradation rate. Selection of a radiation source (shape and dimension) is also greatly influenced by the type of photoreactor.
Reaction rates are known to be dependent on operation conditions. Optimization of these variables thus allows for maximum degradation. Statistical approaches such as multivariate analysis and response surface methodology have also been applied to establish the optimal degradation rate. One of the most commonly investigated and important factors is the TiO2 loading. It has been shown that the degradation rate is not always proportional to the catalyst load, but an optimum load needs to be determined. Difficulty arises when comparing studies for a particular compound of interest. In general, optimal catalyst load is influenced by the photoreactor design including its diameter and source of irradiation. Commercially available TiO2 such as Degussa P25, Hombikat UV 100, PC 500 and Aldrich have been used in various photocatalytic degradation studies of pharmaceuticals. Among them, TiO2 Degussa P25 has demonstrated its superiority in removing various APIs. A concentration below the optimum catalyst loading is typically chosen for further investigation, due to the fact that catalyst deposition might occur during the experiment. For example, 0.4 g/L TiO2 was chosen instead of 0.5 g/L which gave the best results for metoprolol and propranolol degradation, Romero et al. (2011).
Initial concentrations of APIs applied in photocatalytic studies tend to be higher than the environmental level. Furthermore, in most cases single pharmaceuticals are investigated despite the fact that real wastewaters contain complex mixtures of pharmaceuticals. Although most studies have demonstrated efficiency for API in spiked distilled water or ultrapure water, real wastewater samples are rarely used. The presence of radical scavengers such as HCO3 −, CO3 2− ions and NOM in real wastewater typically impacts on degradation efficiencies. Radical scavengers or inhibitors are frequently studied to determine the role of reactive intermediates, e.g. the HO• radical, O2 •− anion and holes in the photocatalytic degradation. Isopropanol, benzoquinone and iodide ions are commonly used for this purpose.
In general, laboratory scale experiments are more common than pilot scale operations due to a more controllable environment. There is usually no direct correlation between the effectiveness of photodegradation in the laboratory with that under real environmental conditions. Conducting photodegradation studies on pilot scales will provide more realistic environmental conditions such as season, latitude, and hardness of water. These are generally not encountered when working in the laboratory. Trials using solar energy have been reported for pharmaceutical degradation, although the results thus far vary between compounds of interest. Commercial and industrial scale applications of solar energy have been established in a few European countries, the USA and Canada.
In the subsequent step, degradation monitoring of pharmaceuticals is generally carried out by using HPLC detection or UV–vis spectrophotometry. The latter has been reported to be a fast and useful tool of monitoring although it is not the state-of-the-art technique for API detection. The kinetics of degradation in terms of percentage API removed or API disappearances versus irradiation time or concentration change versus irradiation time (C/Co vs time) are commonly reported. The L-H model has been often applied to describe photocatalytic mineralization of pharmaceutical degradation.
Apart from determining the removal rate of APIs with photocatalytic treatment, the degree of mineralization is a critical parameter in heterogeneous photocatalysis. DOC has been frequently measured as an indication of the formation of CO2. In most cases, complete degradation of the pharmaceutical does not correspond directly with the mineralization rate mainly due to due to the formation of more stable compounds or transformation products during the degradation. A non-biodegradable fraction frequently remains in the treated solution. Mineralization can be also determined by measuring the formation of inorganic ions such as chlorine, sulphate and nitrate.
Although the ultimate goal of heterogeneous photocatalysis is to achieve complete mineralization of parent compounds, it is also important to evaluate the by-products generated to ensure that there are no toxic or more persistent compounds produced during the treatment. This will contradict the ultimate goal of the application of such an AOP. However, photodegradation products are not commonly studied. A few possible reasons for this are: (i) difficulties in separating and identifying a large number of these transformation products formed; (ii) lack of or non-existent analytical standards to determine the identity of these transformation products; and (iii) requirement for more than one analytical technique or sample preparation technique due to their diverse physicochemical properties, Agüera et al. (2005) and Fatta-Kassinos et al. (2011b). Moreover, studies have also assessed the toxicity of the photocatalytically treated samples by applying toxicity assays on various microorganisms and invertebrates.
In conclusion, despite most promising findings reported, the lack of compatibility in terms of degradation schemes and photoproducts formed, removal rates and degree of mineralization of APIs highlights the complex behaviour of the various APIs towards UV/TiO2. Meaningful comparison between studies for scaling-up and application to real wastewater treatment at a pilot scale is limited by all these factors. Additional studies are thus urgently required to eliminate these shortcomings.
Abbreviations
- AAS:
-
Atomic absorption spectrometry
- AMX:
-
Amoxicillin
- AN:
-
Analgesic
- AOP:
-
Advanced oxidation processes
- API:
-
Active pharmaceutical ingredient
- BOD:
-
Biochemical oxygen demand
- CBZ:
-
Carbamazepine
- CIP:
-
Ciprofloxacin
- COD:
-
Chemical oxygen demand
- CPC:
-
Compound parabolic collector
- DCF:
-
Diclofenac
- DO:
-
Dissolved oxygen
- DOC:
-
Dissolved organic carbon
- DOM:
-
Dissolved organic matter
- Eg :
-
Band gap energy
- ESI-MS:
-
Electrospray ionization mass spectrometry
- FIA:
-
Flow injection analysis
- FQ:
-
Fluoroquinolone
- GC-MS:
-
Gas chromatography mass spectrometry
- HPLC:
-
High performance liquid chromatography
- IBP:
-
Ibuprofen
- IC:
-
Ion chromatography
- ICP-AES:
-
Inductively coupled plasma atomic emission spectroscopy
- IUPAC:
-
International union of pure and applied chemistry
- LC/ESI-MS-MS:
-
Liquid chromatography-electrospray ionization tandem mass spectrometry
- LC/ESI-TOF-MS:
-
Liquid chromatography-electrospray ionization time-of-flight mass spectrometry
- LC-MS:
-
Liquid chromatography mass spectrometry
- LED:
-
Light emitting diode
- L-H:
-
Langmuir-Hinshelwood
- Log Kow :
-
Octanol/water partition coefficient
- MOX:
-
Moxifloxacin
- NHE:
-
Normal hydrogen electrode
- NOM:
-
Natural organic matter
- NOR:
-
Norfloxacin
- NPX:
-
Naproxen
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- OTC:
-
Oxytetracycline
- OX:
-
Oxolinic acid
- QTOF-MS:
-
Quadrupole time of light-mass spectrometry
- SMX:
-
Sulfamethoxazole
- t1/2 :
-
Half-life time
- TC:
-
Tetracycline
- TOC:
-
Total organic carbon
- TOF-MS:
-
Time-of-flight mass spectrometry
- UPLC-ESI/MS:
-
Ultra performance liquid chromatography-electrospray mass spectrometry
- USEPA:
-
United States environmental protection agency
- UV:
-
Ultraviolet
- WWTP:
-
Wastewater treatment plant
References
Abellán MN, Bayarri B, Giménez J, Costa J (2007) Photocatalytic degradation of sulfamethoxazole in aqueous suspension of TiO2. Appl Catal B Environ 74(3–4):233–241. doi:10.1016/j.apcatb.2007.02.017
Abellán MN, Giménez J, Esplugas S (2009) Photocatalytic degradation of antibiotics: the case of sulfamethoxazole and trimethoprim. Catal Today 144(1–2):131–136. doi:10.1016/j.cattod.2009.01.051
Achilleos A, Hapeshi E, Xekoukoulotakis NP, Mantzavinos D, Fatta-Kassinos D (2010a) Factors affecting diclofenac decomposition in water by UV-A/TiO2 photocatalysis. Chem Eng J 161(1–2):53–59. doi:10.1016/j.cej.2010.04.020
Achilleos A, Hapeshi E, Xekoukoulotakis NP, Mantzavinos D, Fatta-Kassinos D (2010b) UV-A and solar photodegradation of ibuprofen and carbamazepine catalyzed by TiO2. Sep Sci Technol 45(11):1564–1570. doi:10.1080/01496395.2010.487463
Agüera A, Estrada LAP, Ferrer I, Thurman EM, Malato S, Fernández-Alba AR (2005) Application of time-of-flight mass spectrometry to the analysis of phototransformation products of diclofenac in water under natural sunlight. J Mass Spectrom 40(7):908–915. doi:10.1002/jms.867
Aguinaco A, Beltrán FJ, García-Araya JF, Oropesa A (2012) Photocatalytic ozonation to remove the pharmaceutical diclofenac from water: influence of variables. Chem Eng J 189–190:275–282. doi:10.1016/j.cej.2012.02.072
AIHW Australian Institute of Health and Welfare (2011) Drugs in Australia 2010: tobacco, alcohol and other drugs, vol 27, Drug statistics series. Australian Institute of Health and Welfare, Canberra, Cat. no. PHE 154. ISBN 978-1-74249-230-8
An TC, Yang H, Li GY, Song WH, Cooper WJ, Nie XP (2010) Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl Catal B Environ 94(3–4):288–294. doi:10.1016/j.apcatb.2009.12.002
Andreozzi R, Caprio V, Insola A, Marotta R (1999) Advanced oxidation processes (AOP) for water purification and recovery. Catal Today 53(1):51–59. doi:10.1016/s0920-5861(99)00102-9
Andreozzi R, Marotta R, Pinto G, Pollio A (2002) Carbamazepine in water: persistence in the environment, ozonation treatment and preliminary assessment on algal toxicity. Water Res 36(11):2869–2877. doi:10.1016/s0043-1354(01)00500-0
Andreozzi R, Marotta R, Paxeus N (2003) Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 50(10):1319–1330. doi:10.1016/s0045-6535(02)00769-5
Augugliaro V, Bellardita M, Loddo V, Palmisano G, Plamisano L, Yurdakal S (2012) Overview of oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J Photochem Photobiol C Photochem Rev 13(3):224–245. doi:10.1016/j.jphotochemrev.2012.04.003
Baran W, Adamek E, Ziemiańska J, Sobczak A (2011) Effects of the presence of sulfonamides in the environment and their influence on human health. J Hazard Mater 196:1–15. doi:10.1016/j.jhazmat.2011.08.082
Beausse J (2004) Selected drugs in solid matrices: a review of environmental determination, occurrence and properties of principal substances. Trac Trends Anal Chem 23(10–11):753–761. doi:10.1016/j.trac.2004.08.005
Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD, Snyder SA (2009) Pharmaceuticals and endocrine disrupting compounds in US drinking water. Environ Sci Technol 43(3):597–603. doi:10.1021/es801845a
Boyd GR, Zhang SY, Grimm DA (2005) Naproxen removal from water by chlorination and biofilm processes. Water Res 39(4):668–676. doi:10.1016/j.watres.2004.11.013
Braslavsky SE (2007) Glossary of terms used in photochemistry, 3rd edition. Pure Appl Chem 79(3):293–465. doi:10.1351/pac200779030293
Calza P, Sakkas VA, Medana C, Baiocchi C, Dimou A, Pelizzetti E, Albanis T (2006) Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl Catal B Environ 67(3–4):197–205. doi:10.1016/j.apcatb.2006.04.021
Carballa M, Omil F, Lema JM, Llompart M, Garcia-Jares C, Rodriguez I, Gomez M, Ternes T (2004) Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res 38(12):2918–2926. doi:10.1016/j.watres.2004.03.029
Carballa M, Omil F, Lema JM (2008) Comparison of predicted and measured concentrations of selected pharmaceuticals, fragrances and hormones in Spanish sewage. Chemosphere 72(8):1118–1123. doi:10.1016/j.chemosphere.2008.04.034
Chen HW, Ku Y, Irawan A (2007) Photodecomposition of o-cresol by UV-LED/TiO2 process with controlled periodic illumination. Chemosphere 69(2):184–190. doi:10.1016/j.chemosphere.2007.04.051
Choi K, Kim Y, Park J, Park CK, Kim M, Kim HS, Kim P (2008) Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea. Sci Total Environ 405(1–3):120–128. doi:10.1016/j.scitotenv.2008.06.038
Chong MN, Jin B (2012) Photocatalytic treatment of high concentration carbamazepine in synthetic hospital wastewater. J Hazard Mater 199:135–142. doi:10.1016/j.jhazmat.2011.10.067
Chong MN, Jin B, Chow CWK, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Res 44(10):2997–3027. doi:10.1016/j.watres.2010.02.039
Dai CM, Zhou XF, Zhang YL, Duan YP, Qiang ZM, Zhang TC (2012) Comparative study of the degradation of carbamazepine in water by advanced oxidation processes. Environ Technol 33(10):1101–1109. doi:10.1080/09593330.2011.610359
Dalrymple OK, Yeh DH, Trotz MA (2007) Removing pharmaceuticals and endocrine-disrupting compounds from wastewater by photocatalysis. J Chem Technol Biotechnol 82(2):121–134. doi:10.1002/jctb.1657
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107(6):907–938. doi:10.2307/3434573
Deegan AM, Shaik B, Nolan K, Urell K, Oelgemöller M, Tobin J, Morrissey A (2011) Treatment options for wastewater effluents from pharmaceutical companies. Int J Environ Sci Technol 8(3):649–666
Dimitrakopoulou D, Rethemiotaki I, Frontistis Z, Xekoukoulotakis NP, Venieri D, Mantzavinos D (2012) Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J Environ Manage 98:168–174. doi:10.1016/j.jenvman.2012.01.010
Doll TE, Frimmel FH (2004) Kinetic study of photocatalytic degradation of carbamazepine, clofibric acid, iomeprol and iopromide assisted by different TiO2 materials – determination of intermediates and reaction pathways. Water Res 38(4):955–964. doi:10.1016/j.watres.2003.11.009
Doll TE, Frimmel FH (2005a) Photocatalytic degradation of carbamazepine, clofibric acid and iomeprol with P25 and Hombikat UV100 in the presence of natural organic matter (NOM) and other organic water constituents. Water Res 39(2–3):403–411. doi:10.1016/j.watres.2004.09.016
Doll TE, Frimmel FH (2005b) Removal of selected persistent organic pollutants by heterogeneous photocatalysis in water. Catal Today 101(3–4):195–202. doi:10.1016/j.cattod.2005.03.005
Elmolla ES, Chaudhuri M (2010a) Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J Hazard Mater 173(1–3):445–449. doi:10.1016/j.jhazmat.2009.08.104
Elmolla ES, Chaudhuri M (2010b) Comparison of different advanced oxidation processes for treatment of antibiotic aqueous solution. Desalination 256(1–3):43–47. doi:10.1016/j.desal.2010.02.019
Elmolla ES, Chaudhuri M (2010c) Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 252(1–3):46–52. doi:10.1016/j.desal.2009.11.003
Fatta-Kassinos D, Vasquez MI, Kummerer K (2011a) Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes – degradation, elucidation of byproducts and assessment of their biological potency. Chemosphere 85(5):693–709. doi:10.1016/j.chemosphere.2011.06.082
Fatta-Kassinos D, Meric S, Nikolaou A (2011b) Pharmaceutical residues in environmental waters and wastewater: current state of knowledge and future research. Anal Bioanal Chem 399(1):251–275. doi:10.1007/s00216-010-4300-9
Felis E, Marciocha D, Surmacz-Gorska J, Miksch K (2007) Photochemical degradation of naproxen in the aquatic environment. Water Sci Technol 55(12):281–286. doi:10.2166/wsat.2007.417
Fent K, Weston AA, Caminada D (2006) Ecotoxicology of human pharmaceuticals. Aquat Toxicol 76(2):122–159. doi:10.1016/j.aquatox.2005.09.009
Fox MA, Dulay MT (1993) Heterogeneous photocatalysis. Chem Rev 93(1):341–357. doi:10.1021/cr00017a016
Friedmann D, Mendive C, Bahnemann D (2010) TiO2 for water treatment: parameters affecting the kinetics and mechanisms of photocatalysis. Appl Catal B Environ 99(3–4):398–406. doi:10.1016/j.apcatb.2010.05.014
Fujishima A, Zhang XT, Tryk DA (2008) TiO2 photocatalysis and related surface phenomena. Surf Sci Rep 63(12):515–582. doi:10.1016/j.surfrep.2008.10.001
Garciá-Araya JF, Beltran FJ, Aguinaco A (2010) Diclofenac removal from water by ozone and photolytic TiO2 catalysed processes. J Chem Technol Biotechnol 85(6):798–804. doi:10.1002/jctb.2363
Gaya UI, Abdullah AH (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem Photobiol C Photochem Rev 9(1):1–12. doi:10.1016/j.jphotochemrev.2007.12.003
Giraldo AL, Penuela GA, Torres-Palma RA, Pino NJ, Palominos RA, Mansilla HD (2010) Degradation of the antibiotic oxolinic acid by photocatalysis with TiO2 in suspension. Water Res 44(18):5158–5167. doi:10.1016/j.watres.2010.05.011
Halling-Sørensen B, Nielsen SN, Lanzky PF, Ingerslev F, Lützhøft HCH, Jørgensen SE (1998) Occurrence, fate and effects of pharmaceutical substances in the environment – a review. Chemosphere 36(2):357–394. doi:10.1016/S0045-6535(97)00354-8, DOI:10.1016/S0045-6535%2897%2900354-8
Haque MM, Muneer M (2007) Photodegradation of norfloxacin in aqueous suspensions of titanium dioxide. J Hazard Mater 145(1–2):51–57. doi:10.1016/j.jhazmat.2006.10.086
Heberer T (2002a) Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J Hydrol 266(3–4):175–189. doi:10.1016/s0022-1694(02)00165-8
Heberer T (2002b) Occurrence, fate and removal of pharmaceutical residues in aquatic environment: a review of recent research data. Toxicol Lett 131(1–2):5–17. doi:10.1016/s0378-4274(02)00041-3
Herrmann JM (1999) Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal Today 53(1):115–129. doi:10.1016/S0920-5861(99)00107-8
Hoeger B, Kollner B, Dietrich DR, Hitzfeld B (2005) Water-borne diclofenac affects kidney and gill integrity and selected immune parameters in brown trout (Salmo trutta f. fario). Aquat Toxicol 75(1):53–64. doi:10.1016/j.aquatox.2005.07.006
Hoffmann MR, Martin ST, Choi WY, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95(1):69–96. doi:10.1021/cr00033a004
Homem V, Santos L (2011) Degradation and removal methods of antibiotics from aqueous matrices – a review. J Environ Manage 92(10):2304–2347. doi:10.1016/j.jenvman.2011.05.023
Hu LH, Flanders PM, Miller PL, Strathmann TJ (2007) Oxidation of sulfamethoxazole and related antimicrobial agents by TiO2 photocatalysis. Water Res 41(12):2612–2626. doi:10.1016/j.watres.2007.02.026
Im JK, Son HS, Kang YM, Zoh KD (2012) Carbamazepine degradation by photolysis and titanium dioxide photocatalysis. Water Environ Res 84(7):554–561. doi:10.2175/106143012x13373550427273
Isidori M, Lavorgna M, Nardelli A, Parrella A, Previtera L, Rubino M (2005) Ecotoxicity of naproxen and its phototransformation products. Sci Total Environ 348(1–3):93–101. doi:10.1016/j.scitotenv.2004.12.068
Khan SJ, Ongerth JE (2004) Modelling of pharmaceutical residues in Australian sewage by quantities of use and fugacity calculations. Chemosphere 54(3):355–367. doi:10.1016/j.chemosphere.2003.07.001
Khetan SK, Collins TJ (2007) Human pharmaceuticals in the aquatic environment: a challenge to green chemistry. Chem Rev 107(6):2319–2364. doi:10.1021/cr020441w
Klauson D, Babkina J, Stepanova K, Krichevskaya M, Preis S (2010) Aqueous photocatalytic oxidation of amoxicillin. Catal Today 151(1–2):39–45. doi:10.1016/j.cattod.2010.01.015
Klavarioti M, Mantzavinos D, Kassinos D (2009) Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Int 35(2):402–417. doi:10.1016/j.envint.2008.07.009
Kockler J, Kanakaraju D, Glass B, Oelgemöller M (2012) Photochemical and photocatalytic degradation of diclofenac and amoxicillin using natural and simulated sunlight. J Sustain Sci Manage 7(1):23–29, ISSN 1823–8556
Kumar SG, Devi LG (2011) Review on modified TiO2 photocatalysis under UV/visible light: selected results and related mechanisms on interfacial charge carrier transfer dynamics. J Phys Chem A 115(46):13211–13241. doi:10.1021/jp204364a
Kümmerer K (2009) The presence of pharmaceuticals in the environment due to human use-present knowledge and future challenges. J Environ Manage 90(8):2354–2366. doi:10.1016/j.jenvman.2009.01.023
Landgraf S (2001) Application of semiconductor light sources for investigations of photochemical reactions. Spectrochim Acta A Mol Biomol Spectrosc 57(10):2029–2048. doi:10.1016/s1386-1425(01)00502-9
Leary R, Westwood A (2011) Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 49(3):741–772. doi:10.1016/j.carbon.2010.10.010
Legrini O, Oliveros E, Braun AM (1993) Photochemical processes for water treatment. Chem Rev 93(2):671–698. doi:10.1021/cr00018a003
Li WH, Guo CS, Su S, Xu J (2012) Photodegradation of four fluoroquinolone compounds by titanium dioxide under simulated solar light irradiation. J Chem Technol Biotechnol 87(5):643–650. doi:10.1002/jctb.2759
Lindqvist N, Tuhkanen T, Kronberg L (2005) Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters. Water Res 39(11):2219–2228. doi:10.1016/j.watres.2005.04.003
Lishman L, Smyth SA, Sarafin K, Kleywegt S, Toito J, Peart T, Lee B, Servos M, Beland M, Seto P (2006) Occurrence and reductions of pharmaceuticals and personal care products and estrogen by municipal wastewater treatment plants in Ontario, Canada. Sci Total Environ 367(2–3):544–558. doi:10.1016/j.scitotenv.2006.03.021
Madhavan J, Grieser F, Ashokkumar M (2010) Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J Hazard Mater 178(1–3):202–208. doi:10.1016/j.jhazmat.2010.01.064
Malato S, Blanco J, Vidal A, Richter C (2002) Photocatalysis with solar energy at a pilot-plant scale: an overview. Appl Catal B Environ 37(1):1–15. doi:10.1016/S0926-3373(01)00315-0
Malato S, Fernandez-Ibanez P, Maldonado MI, Blanco J, Gernjak W (2009) Decontamination and disinfection of water by solar photocatalysis: recent overview and trends. Catal Today 147(1):1–59. doi:10.1016/j.cattod.2009.06.018
Martínez C, Canle M, Fernandez MI, Santaballa JA, Faria J (2011) Aqueous degradation of diclofenac by heterogeneous photocatalysis using nanostructured materials. Appl Catal B Environ 107(1–2):110–118. doi:10.1016/j.apcatb.2011.07.003
Martins AF, Mayer F, Confortin EC, Frank CD (2009) A study of photocatalytic processes involving the degradation of the organic load and amoxicillin in hospital wastewater. Clean Soil Air Water 37(4–5):365–371. doi:10.1002/clen.200900022
Mavronikola C, Demetriou M, Hapeshi E, Partassides D, Michael C, Mantzavinos D, Kassinos D (2009) Mineralisation of the antibiotic amoxicillin in pure and surface waters by artificial UVA and sunlight-induced Fenton oxidation. J Chem Technol Biotechnol 84(8):1211–1217. doi:10.1002/jctb.2159
Mboula VM, Hequet V, Gru Y, Colin R, Andres Y (2012) Assessment of the efficiency of photocatalysis on tetracycline biodegradation. J Hazard Mater 209:355–364. doi:10.1016/j.jhazmat.2012.01.032
Méndez-Arriaga F, Giménez J, Esplugas S (2008a) Photolysis and TiO2 photocatalytic treatment of naproxen: degradation, mineralization, intermediates and toxicity. J Adv Oxid Technol 11(3):435–444
Méndez-Arriaga F, Esplugas S, Giménez J (2008b) Photocatalytic degradation of non-steroidal anti-inflammatory drugs with TiO2 and simulated solar irradiation. Water Res 42(3):585–594. doi:10.1016/j.watres.2007.08.002
Méndez-Arriaga F, Maldonado MI, Giménez J, Esplugas S, Malato S (2009a) Abatement of ibuprofen by solar photocatalysis process: enhancement and scale up. Catal Today 144(1–2):112–116. doi:10.1016/j.cattod.2009.01.028
Méndez-Arriaga F, Torres-Palma RA, Petrier C, Esplugas S, Giménez J, Pulgarin C (2009b) Mineralization enhancement of a recalcitrant pharmaceutical pollutant in water by advanced oxidation hybrid processes. Water Res 43(16):3984–3991. doi:10.1016/j.watres.2009.06.059
Michael I, Hapeshi E, Michael C, Fatta-Kassinos D (2010) Solar Fenton and solar TiO2 catalytic treatment of ofloxacin in secondary treated effluents: evaluation of operational and kinetic parameters. Water Res 44(18):5450–5462. doi:10.1016/j.watres.2010.06.053
Miège C, Choubert JM, Ribeiro L, Eusèbe M, Coquery M (2009) Fate of pharmaceuticals and personal care products in wastewater treatment plants – conception of a database and first results. Environ Pollut 157(5):1721–1726. doi:10.1016/j.envpol.2008.11.045
Miránda-García N, Suarez S, Sanchez B, Coronado JM, Malato S, Maldonado MI (2011) Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl Catal B Environ 103(3–4):294–301. doi:10.1016/j.apcatb.2011.01.030
Moldovan Z (2006) Occurrences of pharmaceuticals and personal care products as micropollutants in rivers from Romania. Chemosphere 64(11):1808–1817. doi:10.1016/j.chemosphere.2006.02.003
Molinari R, Pirillo F, Loddo V, Palmisano L (2006) Heterogeneous photocatalytic degradation of pharmaceuticals in water by using polycrystalline TiO2 and a nanofiltration membrane reactor. Catal Today 118(1–2):205–213. doi:10.1016/j.cattod.2005.11.091
Mompelat S, Le Bot B, Thomas O (2009) Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ Int 35(5):803–814. doi:10.1016/j.envint.2008.10.008
Mozia S, Morawski AW (2012) The performance of a hybrid photocatalysis-MD system for the treatment of tap water contaminated with ibuprofen. Catal Today 193(1):213–220. doi:10.1016/j.cattod.2012.03.016
Nasuhoglu D, Yargeau V, Berk D (2011) Photo-removal of sulfamethoxazole (SMX) by photolytic and photocatalytic processes in a batch reactor under UV-C radiation (lambda (max) = 254 nm). J Hazard Mater 186(1):67–75. doi:10.1016/j.jhazmat.2010.10.080
Nasuhoglu D, Rodayan A, Berk D, Yargeau V (2012) Removal of the antibiotic levofloxacin (LEVO) in water by ozonation and TiO2 photocatalysis. Chem Eng J 189–190:41–48. doi:10.1016/j.cej.2012.02.016
Nikolaou A, Meric S, Fatta D (2007) Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal Bioanal Chem 387(4):1225–1234. doi:10.1007/s00216-006-1035-8
Oaks JL, Gilbert M, Virani MZ, Watson RT, Meteyer CU, Rideout BA, Shivaprasad HL, Ahmed S, Chaudhry MJI, Arshad M, Mahmood S, Ali A, Khan AA (2004) Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427(6975):630–633. doi:10.1038/nature02317
Oetken M, Nentwig G, Löffler D, Ternes D, Oehlmann J (2005) Effects of pharmaceuticals on aquatic vertebrates. Part I. The antiepileptic drug carbamazepine. Arch Environ Contam Toxicol 49:353–361. doi:10.1007/s00244-004-0211-0
Oller I, Malato S, Sanchez-Perez JA (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination – a review. Sci Total Environ 409(20):4141–4166. doi:10.1016/j.scitotenv.2010.08.061
Oppenländer T (2003) Photochemical purification of water and air. Advanced oxidation processes (AOPs): principles, reaction mechanisms, reactor concepts. Wiley-VCH, Weinheim, pp 101–121. ISBN 3-527-30563-7
Packer JL, Werner JJ, Latch DE, McNeill K, Arnold WA (2003) Photochemical fate of pharmaceuticals in the environment: naproxen, diclofenac, clofibric acid and ibuprofen. Aquat Sci 65(4):342–351. doi:10.1007/s00027-003-0671-8
Pal A, Gin KYH, Lin AYC, Reinhard M (2010) Impacts of emerging organic contaminants on freshwater resources: review of recent occurrences, sources, fate and effects. Sci Total Environ 408(24):6062–6069. doi:10.1016/j.scitotenv.2010.09.026
Palominos RA, Mora A, Mondaca MA, Perez-Moya M, Mansilla HD (2008) Oxolinic acid photo-oxidation using immobilized TiO2. J Hazard Mater 158(2–3):460–464. doi:10.1016/j.jhazmat.2008.01.117
Palominos RA, Mondaca MA, Giraldo A, Penuela G, Perez-Moya M, Mansilla HD (2009) Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal Today 144(1–2):100–105. doi:10.1016/j.cattod.2008.12.031
Paul T, Dodd MC, Strathmann TJ (2010) Photolytic and photocatalytic decomposition of aqueous ciprofloxacin: transformation products and residual antibacterial activity. Water Res 44(10):3121–3132. doi:10.1016/j.watres.2010.03.002
Pelaez M, Nolan NT, Pillai SC, Seery MK, Falaras P, Kontos AG, Dunlop PSM, Hamilton JWJ, Byrne JA, O’Shea K, Entezari MH, Dionysiou DD (2012) A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Catal B Environ 125:331–349. doi:10.1016/j.apcatb.2012.05.036
Pereira JHOS, Vilar VJP, Borges MT, Gonzalez O, Esplugas S, Boaventura RAR (2011) Photocatalytic degradation of oxytetracycline using TiO2 under natural and simulated solar radiation. Sol Energ 85(11):2732–2740. doi:10.1016/j.solener.2011.08.012
Pérez-Estrada LA, Maldonado MI, Gernjak W, Agϋera A, Fernández-Alba AR, Ballesteros MM, Malato S (2005) Decomposition of diclofenac by solar driven photocatalysis at pilot plant scale. Catal Today 101(3–4):219–226. doi:10.1016/j.cattod.2005.03.013
Reyes C, Fernandez J, Freer J, Mondaca MA, Zaror C, Malato S, Mansilla HD (2006) Degradation and inactivation of tetracycline by TiO2 photocatalysis. J Photochem Photobiol A Chem 184(1–2):141–146. doi:10.1016/j.jphotochem.2006.04.007
Rizzo L (2011) Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res 45(15):4311–4340. doi:10.1016/j.watres.2011.05.035
Rizzo L, Meric S, Kassinos D, Guida M, Russo F, Belgiorno V (2009a) Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res 43(4):979–988. doi:10.1016/j.watres.2008.11.040
Rizzo L, Meric S, Guida M, Kassinos D, Belgiorno V (2009b) Heterogeneous photocatalytic degradation kinetics and detoxification of an urban wastewater treatment plant effluent contaminated with pharmaceuticals. Water Res 43(16):4070–4078. doi:10.1016/j.watres.2009.06.046
Rodil R, Quintana JB, Concha-Grana E, Lopez-Mahia P, Muniategui-Lorenzo S, Prada-Rodriguez D (2012) Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 86(10):1040–1049. doi:10.1016/j.chemosphere.2011.11.053
Romero V, De la Cruz N, Dantas RF, Gimenez PMJ, Esplugas S (2011) Photocatalytic treatment of metoprolol and propranolol. Catal Today 161(1):115–120. doi:10.1016/j.cattod.2010.09.026
Sabri N, Hanna K, Yargeau G (2012) Chemical oxidation of ibuprofen in the presence of iron species at near neutral pH. Sci Total Environ 427–428:382–389. doi:10.1016/j.scitotenv.2012.04.034
Shamaila S, Sajjad AKL, Chen F, Zhang JL (2010) Synthesis and characterization of mesoporous-TiO2 with enhanced photocatalytic activity for the degradation of chloro-phenol. Mater Res Bull 45(10):1375–1382. doi:10.1016/j.materresbull.2010.06.047
Shan AY, Ghazi TIM, Rashid SA (2010) Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: a review. Appl Catal A Gen 389(1–2):1–8. doi:10.1016/j.apcata.2010.08.053
Silva CG, Faria JL (2009) Anatase vs. rutile efficiency on the photocatalytic degradation of clofibric acid under near UV to visible irradiation. Photochem Photobiol Sci 8(5):705–711. doi:10.1039/b817364h
Skocaj M, Filipic M, Petkovic J, Novak S (2011) Titanium dioxide in our everyday life; is it safe? Radiol Oncol 45(4):227–247. doi:10.2478/v10019-011-0037-0
Sturini M, Speltini A, Maraschi F, Profumo A, Pretali L, Fasani E, Albini A (2010) Photochemical degradation of marbofloxacin and enrofloxacin in natural waters. Environ Sci Technol 44(12):4564–4569. doi:10.1021/es100278n
Sturini M, Speltini A, Maraschi F, Profumo A, Pretali L, Irastorza EA, Fasani E, Albini A (2012) Photolytic and photocatalytic degradation of fluoroquinolones in untreated river water under natural sunlight. Appl Catal B Environ 119:32–39. doi:10.1016/j.apcatb.2012.02.008
Suárez S, Carballa M, Omil F, Lema JM (2008) How are pharmaceutical and personal care products (PPCPs) removed from urban wastewaters? Rev Environ Sci Biotechnol 7:125–138. doi:10.1007/s11157-008-9130-2
Ternes TA, Meisenheimer M, McDowell D, Sacher F, Brauch HJ, Gulde BH, Preuss G, Wilme U, Seibert NZ (2002) Removal of pharmaceuticals during drinking water treatment. Environ Sci Technol 36(17):3855–3863. doi:10.1021/es015757k
Tixier C, Singer HP, Oellers S, Muller SR (2003) Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ Sci Technol 37(6):1061–1068. doi:10.1021/es025834r
Tong AYC, Braund R, Warren DS, Peake BM (2012) TiO2-assisted photodegradation of pharmaceuticals – a review. Cent Eur J Chem 10(4):989–1027. doi:10.2478/s11532-012-0049-7
Trovó AG, Nogueira RFP, Aguera A, Fernandez-Alba AR, Sirtori C, Malato S (2009) Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Res 43(16):3922–3931. doi:10.1016/j.watres.2009.04.006
Van Doorslaer X, Demeestere K, Heynderickx PM, Van Langenhove H, Dewulf J (2011) UVA and UVC induced photolytic and photocatalytic degradation of aqueous ciprofloxacin and moxifloxacin: reaction kinetics and role of adsorption. Appl Catal B Environ 101(3–4):540–547. doi:10.1016/j.apcatb.2010.10.027
Van Doorslaer X, Heynderickx PM, Demeestere K, Debevere K, Van Langenhove H, Dewulf J (2012) TiO2 mediated heterogeneous photocatalytic degradation of moxifloxacin: operational variables and scavenger study. Appl Catal B Environ 111–112:150–156. doi:10.1016/j.apcatb.2011.09.029
Vasconcelos TG, Kummerer K, Henriques DM, Martins AF (2009) Ciprofloxacin in hospital effluent: degradation by ozone and photoprocesses. J Hazard Mater 169(1–3):1154–1158. doi:10.1016/j.jhazmat.2009.03.143
Wang L, Ying GG, Zhao JL, Yang XB, Chen F, Tao R, Liu S, Zhou LJ (2010) Occurrence and risk assessment of acidic pharmaceuticals in the Yellow River, Hai River and Liao River of North China. Sci Total Environ 408(16):3139–3147. doi:10.1016/j.scitotenv.2010.04.047
Watkinson AJ, Murby EJ, Kolpin DW, Constanzo SD (2009) The occurrence of antibiotics in an urban watershed: from wastewater to drinking water. Sci Total Environ 407(8):2711–2723. doi:10.1016/j.scitotenv.2008.11.059
Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N (2012) Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 46(4):2242–2250. doi:10.1021/es204168d
Xekoukoulotakis NP, Drosou C, Brebou C, Chatzisymeon E, Hapeshi E, Fatta-Kassinos D, Mantzavinos D (2011) Kinetics of UV-A/TiO2 photocatalytic degradation and mineralization of the antibiotic sulfamethoxazole in aqueous matrices. Catal Today 161(1):163–168. doi:10.1016/j.cattod.2010.09.027
Xiong P, Hu JY (2012) Degradation of acetaminophen by UV-A/LED/TiO2 process. Sep Purif Technol 91(SI):89–95. doi:10.1016/j.seppur.2011.11.012
Xu WH, Zhang G, Zou SC, Li XD, Liu YC (2007) Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high performance liquid chromatography electrospray ionization tandem mass spectrometry. Environ Pollut 145(3):672–679. doi:10.1016/j.envpol.2006.05.038
Yang L, Yu LE, Ray MB (2008) Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res 42(13):3480–3488. doi:10.1016/j.watres.2008.04.023
Yang LM, Yu LE, Ray MB (2009) Photocatalytic oxidation of paracetamol: dominant reactants, intermediates, and reaction mechanisms. Environ Sci Technol 43(2):460–465. doi:10.1021/es8020099
Yang H, Li GY, An TC, Gao YP, Fu JM (2010) Photocatalytic degradation kinetics and mechanism of environmental pharmaceuticals in aqueous suspension of TiO2: a case of sulfa drugs. Catal Today 153(3–4):200–207. doi:10.1016/j.cattod.2010.02.068
Yurdakal S, Loddo V, Augugliaro V, Berber H, Palmisano G, Palmisano L (2007) Photodegradation of pharmaceutical drugs in aqueous TiO2 suspensions: mechanism and kinetics. Catal Today 129(1–2):9–15. doi:10.1016/j.cattod.2007.06.044
Zhang YJ, Geissen SU, Gal C (2008a) Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 73(8):1151–1161. doi:10.1016/j.chemosphere.2008.07.086
Zhang X, Wu F, Wu XW, Chen PY, Deng NS (2008b) Photodegradation of acetaminophen in TiO2 suspended solution. J Hazard Mater 157(2–3):300–307. doi:10.1016/j.jhazmat.2007.12.098
Zhang W, Zou LD, Wang LZ (2009) Photocatalytic TiO2/adsorbent nanocomposites prepared via wet chemical impregnation for wastewater treatment: a review. Appl Catal A Gen 371(1–2):1–9. doi:10.1016/j.apcata.2009.09.038
Zhang HH, Cao BP, Liu WP, Lin KD, Feng J (2012) Oxidative removal of acetaminophen using zero valent aluminum-acid system: efficacy, influencing factors, and reaction mechanism. J Environ Sci 24(2):314–319. doi:10.1016/s1001-0742(11)60769-9
Ziylan A, Ince NH (2011) The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: treatability by conventional and non-conventional processes. J Hazard Mater 187(1–3):24–36. doi:10.1016/j.jhazmat.2011.01.057
Acknowledgements
The authors thank James Cook University for financial support (FAIG award 2009, GRS awards 2011 and 2012). DK thanks the Malaysian Government for a University Doctorate Training Award.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Kanakaraju, D., Glass, B.D., Oelgemöller, M. (2013). Heterogeneous Photocatalysis for Pharmaceutical Wastewater Treatment. In: Lichtfouse, E., Schwarzbauer, J., Robert, D. (eds) Green Materials for Energy, Products and Depollution. Environmental Chemistry for a Sustainable World, vol 3. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6836-9_3
Download citation
DOI: https://doi.org/10.1007/978-94-007-6836-9_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6835-2
Online ISBN: 978-94-007-6836-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)