Abstract
The occurrence of pharmaceutical compounds in the natural water sources has been reported as early as in the year 1980. Until now, the presence of pharmaceutical compounds in the aquatic environment has been frequently reported in the literature. Moreover, increasing evidence suggests that these contaminants have posed a threat to both humans and ecosystems. In this regard, the present review paper seeks to offer an overview of this environmental issue of pharmaceutical pollution where the subject matters to be reviewed include the effects, sources and mitigation strategies of pharmaceuticals in the aquatic environment. Besides, a review of the fundamentals and mechanisms of heterogeneous photocatalysis technology is also presented in this paper. Heterogeneous photocatalysis is a rapidly expanding technology which has been extensively investigated and applied in wastewater treatment for the remediation of persistent pollutants such as pharmaceutical compounds during the last decade. Furthermore, the ideal photocatalyst titanium dioxide (TiO2), which can collaborate and perform well in the photocatalysis treatment process, is also discussed. The advantages and limitations associated with the application of this treatment method are summarized and discussed in details. Finally, this review paper focuses on the future trend of the photocatalysis technology and identifies the barriers and lacking parts which need to be resolved in the near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is one of the important resources on earth where human beings and ecological systems rely on it for survival. If there is no water, there will be no life on earth. Nowadays, the demand of water increases with the rapid growth of population and vigorous industrial development. High-quality water sources are necessary particularly in maintaining healthy ecosystems and assurance for safe drinking water.
Common water pollutants including pesticides, polychlorinated biphenyls (PCBs), poly aromatic hydrocarbons (PAHs), metals such as mercury, lead, cadmium and arsenic, to name a few, have been significantly reduced through the adoption of appropriate legal measures and the removal of numerous dominant pollution sources (Al-Odaini et al. 2013). Recently, the scientific community starts to focus on a new group of pollutants, which are pharmaceuticals (Choina et al. 2013; Doll and Frimmel 2005; Putschew et al. 2000; Ternes 1998). Pharmaceuticals are known as the “new emerging pollutants” since they are recently detected in the environment in increasing amount. Up to now, pharmaceuticals are not covered by regulations, and their effects on the environment and human health are still poorly understood (Quadra et al. 2016; Sangion and Gramatica 2016).
Pharmaceutical is one of the most indispensable elements with undeniable benefits in modern life (Blair 2016; Sangion and Gramatica 2016). They are extensively and increasingly used as an integral component to establish and maintain a healthy population of both humans and livestock (Choina et al. 2013; Mozia and Morawski 2012; Wilkinson et al. 2016). However, due to the widespread application of pharmaceuticals and their inadequate removal from wastewater, low levels of pharmaceuticals (ranging from the low ng/L to mg/L) have been ubiquitously detected (in both original and metabolized forms) in various aquatic compartments such as surface water, groundwater, effluents of sewage treatment plant, seawater (Daughton and Ternes 1999; Halling-Sørensen et al. 1998) and even in the drinking water (Doll and Frimmel 2005; Sacher et al. 2001; Vaizoğullar 2017).
The retained pharmaceuticals in different water sources may lead to some adverse effects on the biological balance and human health such as aquatic toxicity, resistance development in pathogenic bacteria, acute and chronic damage, hormonal and endocrine disruption (Emmanuel et al. 2005; K’oreje et al. 2016; Wu et al. 2012; Yang et al. 2008a). This situation is changing from bad to worse when these persistent pharmaceuticals are unable to be eliminated by using conventional wastewater treatment techniques due to the typical characteristics of the pharmaceuticals (Achilleos et al. 2010; Al-Odaini et al. 2013; Mozia and Morawski 2012). For example, pharmaceuticals are lipophilic to pass membranes and facilitate the absorption. Besides, they can escape from the biological treatment process since they are designed to be biologically active and persistent to maintain their therapeutic activity until the specific physiological function on the human and animals has been performed (Achilleos et al. 2010; Aguilar et al. 2011; Suarez et al. 2009; Zhang et al. 2008). Therefore, they have the properties to bioaccumulate and cause negative effects to aquatic or terrestrial ecosystems, such as immobilization, mortality, inhibition of growth and reproduction (Quadra et al. 2016).
The continuous input and persistence of pharmaceuticals in the aquatic ecosystem indicate an environmental challenge even only at low concentrations. Recently, studies are concerning on the removal of pharmaceuticals during treatment by using heterogeneous photocatalysis wastewater treatment technique (Bahnemann 2004; Lofrano et al. 2009; Sharma et al. 2012). The field of heterogeneous photocatalysis has been expanding rapidly within the last four decades (Ibhadon and Fitzpatrick 2013). Photocatalysis performs the redox function through the transformation, deactivation and, finally, the minimization of environmentally persistent compounds (Aziz et al. 2016).
This review paper aims to give an overview of the occurrence of pharmaceutical compounds in the aquatic environment, including the sources, effects and solutions to overcome the pharmaceutical pollution. Also, the understanding and fundamentals of heterogeneous photocatalytic wastewater treatment technology, including the advantages and limitations of the photocatalysis process, were discussed in details in the review. The last part of this review concerns on the lacking part of current studies and the areas of future research.
Pharmaceuticals in the aquatic environment
The most frequently detected pharmaceuticals in the water sources and their concentrations are summarized in Table 1 (Bagheri et al. 2016; Quadra et al. 2016; Wilkinson et al. 2016). Basically, they are divided into eight main classes based on their specific mode of therapeutic action, namely antibiotic, analgesic and antipyretic, central nervous system (CNS) stimulant, iodinated X-ray contrast media, antidepressant, beta blocker, hormone/steroid and lipid regulator. The presence of pharmaceutical compounds as the new emerging pollutants in the natural water resources is becoming an issue of global concern (Mozia and Morawski 2012; Nasuhoglu et al. 2011; Wu et al. 2012). The main factors concerned relating to this issue are the lack of the baseline data and the malfunctioning of the conventional wastewater treatment methods.
Pharmaceutical pollution has not been given a significant attention despite its increased and extensive application for different purposes throughout the world in nowadays (Choina et al. 2013; Mozia and Morawski 2012). Ignorance on this matter is due to the fact that pharmaceuticals are not regulated as environmental pollutants and not listed as pollutants in the WHO guidelines for drinking water quality (Al-Odaini et al. 2013). This, in turn, leads to the lack of baseline data regarding the occurrence of pharmaceutical compounds in the aquatic environment, thus prohibiting the evaluation of public exposure and the subsequent effects (Arpin-Pont et al. 2016; Sim et al. 2011; Taylor and Senac 2014). As the effects on human health are unknown and the findings on the aquatic organisms are limited, the severity of this issue may be covered up and turning to worse as day goes on (Bagheri et al. 2016). Most probably, the hidden effects may slowly accumulate and expose unexpectedly and irreversibly.
Besides, studies have proven that the conventional wastewater treatment plants are unable to eliminate the retained pharmaceutical compounds completely (Achilleos et al. 2010; Cai and Hu 2017; Mozia and Morawski 2012; Zhang et al. 2008). This is because the conventional wastewater treatment plants such as sedimentation and biological treatment process are not designed to remove the persistent pharmaceuticals which are polar, predominantly water soluble in nature, neither volatile nor biodegradable (Aguilar et al. 2011; Al-Odaini et al. 2013; Bagheri et al. 2016; Luo et al. 2015; Maroga Mboula et al. 2012; Palominos et al. 2009). Other treatment methods such as activated carbon adsorption, air stripping and reverse osmosis have also been investigated for the elimination of pharmaceuticals. However, these processes are just transferring the pharmaceutical compounds from one phase to another without eliminating them (Elmolla and Chaudhuri 2010a).
Effects of retained pharmaceuticals
Pharmaceuticals are one of the vital substances which are beneficial to our daily life, particularly in terms of therapy and healthcare facilities. However, when the pharmaceuticals are exposed to the nontarget organisms, the beneficial function may not be performed. In contrary, its biological activity may cause abundant of acute and chronic effects which can adversely affect the wildlife and ecosystem health (Sangion and Gramatica 2016; Wilkinson et al. 2016). In the last three decades, the presence and impact of pharmaceuticals in the environment have gained an increasing concern. Pharmaceuticals, being a large, diverse and persistent group of emerging organic micropollutants, are found to be poorly removed by conventional wastewater treatment processes and thus have been ubiquitously detected in the natural water sources and even drinking water. So far, the retained pharmaceutical compounds in the environment are reported at concentrations ranging from the low ng/L to mg/L (K’oreje et al. 2016). However, it is important to highlight that these pollutants can pose a risk for aquatic life by various mechanisms even at low concentration (Blair 2016; Wu et al. 2012).
Pharmaceutical pollution has been verified to induce the endocrine or hormonal disruption problem, causing the abnormal reproduction of fish (Khataee et al. 2013; Yang et al. 2008a). Kidd et al. (2007), who conducted the experiment in a pristine lake at the Experimental Lakes Area in Canada, has found out that the addition of steroid estrogen ethinyl estradiol (EE2) can lead to the feminization of males as well as the collapse of a fish population. Recently, Sangion and Gramatica (2016) have also reported on the same endocrine disruption problem where the EE2 caused the feminization of male fish in river and water bodies, even at concentrations of few ng/l. In addition, fish with intersex gonads and feminization of males or vice versa have been found in some rivers in the UK. The similar incident occurred in the city of Boulder, Colorado, where the white suckers (a type of fish) also encountered the endocrine disruption problems such as gonadal intersex, disrupted ovarian, reduced gonad size and testicular histopathology. These endocrine disruption problems are caused by the long-term exposure of the aquatic organisms to the estrogenic compounds which are retained in the water sources (Stoner and Kosinski 2008).
Furthermore, pharmaceutical residues in the aquatic environment are reported to have some ecotoxic effects. For example, enrofloxacin and ciprofloxacin (two different types of fluoroquinolone antibiotics) which are frequently detected in wastewater and surface water are toxic to algae (Ebert et al. 2011). Besides, the antibiotic of tetracycline has been proven to bring the toxic effects to the plants and the early stage of aquatic organisms. It also causes the negative impact to the sewage sludge bacteria by inhibiting their protein synthesized process (Yahiat et al. 2011). Moreover, diclofenac is known as the most toxic analgesics in acute exposure studies with the growth inhibition (EC50) below 100 mg/L (Fent et al. 2006). Again, diclofenac and hormones have been shown to bring fatal effects on fish, crustaceans and algae at very low doses (Bagheri et al. 2016). The developed antibiotic resistance by the pathogenic bacteria is also one of the verified adverse effects identified in the previous studies (Elmolla and Chaudhuri 2010b; Maroga Mboula et al. 2012). These developed antibiotic resistance genes which could cause the genotoxic effects to the microorganisms will then spread in the environment and lead to the occurrence of aquatic toxicity (Khataee et al. 2013; Yang et al. 2008a).
Further instances of the effects of different pharmaceutical compounds on the aquatic organisms are summarized in Table 2. Based on Table 2, it is noticeable that the pharmaceuticals can induce some negative impacts to the flora, fauna and microorganisms in the aquatic environment. Although most of the reported half-maximal effective concentrations (EC50) and half-maximal inhibitory concentration (IC50) are found to be higher than those detected in the environment, these results are still concerned due to the possibility of synergistic effects with other pharmaceuticals and more intense effects at long-term exposure (Quadra et al. 2016). For example, research by Cleuvers (2003) has indicated that, in comparison to the singly measured toxicities, mixture of ibuprofen and diclofenac can cause more immobilization in Daphnia magna (microcrustacean) and inhibition of the average growth rate for algae, which clearly demonstrate the synergistic effect of the mixture pharmaceuticals.
Up to now, concerns still remain regarding the harm of pharmaceutical residues to human health, particularly to a developing fetus (Blair 2016). Somehow, it is suggested that long-term exposure to the retained pharmaceuticals may bring unintended effects to the human health since pharmaceuticals are designed to be highly interactive with the receptors of human and animals (Abellán et al. 2007; Jelić et al. 2012). It is important to highlight that the continuous discharge of pharmaceutical compounds will lead to a higher retained concentration in the future time associated with unpredictable adverse effects on human, aquatic and terrestrial organisms. Hence, further studies are required to acquire more verified adverse effects of the retained pharmaceutical compounds on the living organisms, especially on the human being, so that it can become a convincing fact to alert the society regarding the seriousness of this pharmaceutical pollution.
Major sources of pharmaceuticals
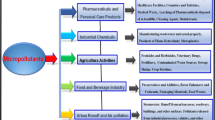
Tons of drugs are produced every year and broadly applied in both human and veterinary medicine (Quadra et al. 2016). More than 200 types of pharmaceutical compounds (mainly painkillers, vascular drugs, antibiotics and antidepressants) are frequently detected in aquatic and terrestrial compartments with the concentration ranging from few ng/l to thousand μg/l (Sangion and Gramatica 2016). Therefore, understanding the sources and fates of pharmaceuticals in the environment is vital for the determination of suitable approaches to prevent them from entering the water supply (Bagheri et al. 2016; Helwig et al. 2016). Pharmaceuticals may reach aquatic ecosystems through many pathways (Quadra et al. 2016). Figure 1 illustrates the possible sources and fates of pharmaceuticals in the environment. Basically, there are four major sources which contribute to the occurrence of pharmaceuticals in the aquatic environment: human use, veterinary use, agriculture and farming use, as well as the industrial production.
As shown in Fig. 1, pharmaceuticals can reach the water sources via the emission from healthcare facilities (hospitals and clinics) and households (such as private residences, dormitories, hotels, residential care facilities) (Abellán et al. 2007; Chang et al. 2010; LA Ioannou et al. 2011). Among all, hospitals act as the main source of pharmaceuticals released to the environment particularly through the patients’ excretions (Bagheri et al. 2016; Straub 2016). Following human consumption, excreted residue (feces and urines) may enter the sewer in either original parent compound or metabolite (Helwig et al. 2016). Depending on the properties of pharmaceuticals, only part of them is removed by the ordinary microbiological treatments in wastewater treatment plants (WWTPs), whereas the remaining residue is discharged and dispersed in the aquatic environment (Abellán et al. 2007; Sangion and Gramatica 2016). Due to that, WWTPs are often recognized as the main route of entry for pharmaceuticals in the environment, leading to a ubiquitous and continuous contamination (Quadra et al. 2016; Sangion and Gramatica 2016). Besides, the improper disposal of unused, expired and surplus pharmaceuticals into the drains or toilets is another important pathway to contaminate the environment (Al-Odaini et al. 2013; Moctezuma et al. 2012; Wu et al. 2012). These pharmaceuticals are most probably infiltrated into the groundwater depending on the hydrology system (Quadra et al. 2016).
In addition, veterinary use is also an important source of drugs to the environment. Abundant of veterinary pharmaceuticals is applied in husbandry sector as a growth promoter, for breeding and therapeutic purposes (Bundschuh et al. 2016; LA Ioannou et al. 2011; Kümmerer 2009; Sim et al. 2011). After the consumption of pharmaceutical by the livestock, part of the pharmaceutical is excreted through the urine and feces, in either metabolized or parent form. Without any treatment, the excreted pharmaceuticals get into the soil and groundwater directly (Kümmerer 2001; Quadra et al. 2016). Often, livestock’s manure is used as fertilizer in the agricultural field. The manure which contains the pharmaceutical residues can be either mobilized into surface water during heavy precipitation or infiltrated into the groundwater (Abellán et al. 2007). Other than livestock, fish farming is also an important source of pharmaceuticals (Quadra et al. 2016). The direct application of pharmaceuticals in the aquaculture for the prevention and treatment of microbial infections has caused the increment of retained pharmaceuticals in the aquatic environment (Kümmerer 2009).
Also, pharmaceuticals are extensively applied in the agricultural and farming fields to control the bacterial disease (Fukahori et al. 2012; Kümmerer 2009; Mozia and Morawski 2012). These pharmaceuticals can accumulate in farmland and eventually reach the natural water sources through leaching and runoff (Abellán et al. 2009; Wilkinson et al. 2016). Last but not least, production and manufacturing are among the industries which have highly contribute to the occurrence of pharmaceutical pollution through the inappropriate disposal, accidental spillage during the manufacturing and distribution processes (Al-Odaini et al. 2013; Yang et al. 2008a), as well as the direct discharge of pharmaceutical waste into the water sources (Abellán et al. 2007; Emmanuel et al. 2005; Gautam et al. 2007; LA Ioannou et al. 2011; Kümmerer 2001).
Once the pharmaceutical residue in the environment, their behavior can be significantly affected by some factors such as solubility, chemical structure, persistence, sorption behavior, climatic factors (temperature, precipitation), water body properties (pH, redox potential, organic carbon content), composition of sediments and environmental degradation. Due to this immense complexity, more research in this field is required to identify the behaviors of drugs in the environment (Quadra et al. 2016). Furthermore, strategies are required for the removal of numerous dominant pollution sources. Besides the enactment of appropriate legal measures, a standard or guideline of pharmaceuticals wastewater effluent is also required to be invented.
Pharmaceuticals mitigation strategies
Pharmaceuticals are often poorly removed by conventional wastewater treatment techniques (K’oreje et al. 2016). The continual emission of pharmaceuticals (either in parent form or metabolites) into the surface water sources not only causes the ubiquitous contamination of freshwater supplied, but it is also proved to induce some adverse effects to the aquatic organisms particularly in terms of endocrine disruptions (Khataee et al. 2013; Talib and Randhir 2016). Reported strategies in this regard are thus reviewed as tabulated in Table 3 to evaluate and compare the different solutions which have been suggested.
Besides depending on the wastewater treatment process such as the most promising advanced oxidation processes (AOPs) for the mitigation of pharmaceuticals in the aquatic environment, there are some other solutions which have been suggested and discussed as presented in Table 3. Among the suggested pharmaceuticals abatement strategies, take-back program is the only strategy which currently exists and being the most recognized upstream strategies (Blair 2016). This program provides plenty of benefits by retrieving the unused pharmaceuticals, but at the same time, its limitations are capable of restricting it from the worldwide application. Next, source treatment such as urine separating toilets can be an effective way of discarding the retained pharmaceuticals in the urine, but the pharmaceutical residues in the feces have been sadly neglected. In this case, this treatment will be unable to provide a thorough elimination of pharmaceuticals and can most probably increase the complexity of the treatment process and cost.
On the other hand, it can be observed that the trash disposal is the simplest and cost-saving technique to be implemented. Yet, it may not be the most secure solution since it can induce the same environmental problem through leaching from landfill and accidental exposure if there is incautious handling. As for the invention of green pharmaceuticals, although it may be costly in terms of pharmaceuticals research and development, but its ease to degrade and less harmful properties contribute to the sustainable protection of aquatic resources. There are some newly discovered strategies like starting new prescriptions at lower dosages (Daughton 2003). In this regard, patient is advised to start with the lowest effective dose and adjusting the dosage as necessary. This prescript way not only reduces the patient costs and unused pharmaceuticals disposal cost, but it also improves the therapeutic efficacy and prevents the unnecessary side effects to the patient, as well as avoids the accidental exposures and emission of pharmaceuticals to the aquatic environment. Last but not least, the mitigation technique which is known as “eco-directed sustainable prescribing” is mainly about the selection of pharmaceuticals with lower excretion rate (Daughton 2014). Unlike the former strategy (lower prescription dose), this solution emphasizes on the metabolism and excretion of pharmaceutical drugs rather than the initially ingested dose by the patient, because the emission of pharmaceuticals into the environment via sewers is dictated by the excretion profile and pharmacokinetics of the different types of pharmaceutical compounds. This solution involves the reduction in dose of medications with high excretion profile and replaces with another suitable medication which is poorly excreted, if possible. Nevertheless, the understanding on the effectiveness, usage, participation and feasibility of this strategy is still incomplete.
In summary, an ideal solution for the pharmaceuticals mitigation in the aquatic environment is yet to be met. The above-discussed strategies which equip with both limitation and strength may require further investigation to compensate the scarcity of the information and subsequently identifying the most effective strategy for real application.
Heterogeneous photocatalyst
During the last decade, AOPs appears to be a promising wastewater treatment technology which could successfully degrade the soluble organic pollutants and the volatile organic compounds retained in the water and air, respectively. The only chemical property which unites the different types of AOPs is the generation of reactive oxygen species (ROSs), particularly the hydroxyl radical (·OH), which is a powerful oxidizing agent that possesses the second highest oxidizing potential (2.8 eV). It can be generated in situ to oxidize and further mineralize the recalcitrant pollutants into the harmless end products such as carbon dioxide and water (De la Cruz et al. 2013; Kaur et al. 2016; Xekoukoulotakis et al. 2011; Yahiat et al. 2011).
AOPs such as Fenton, photo-Fenton, ozonation, ultrasound radiation, photolysis and photocatalysis are applied for the treatment of pharmaceutical wastewater. However, these treatment processes are found to be less effective with the fluctuating degree of removal efficiency. The tertiary treatment methods such as electrodialysis, activated carbon, chemical removal, coagulation, filtration and membrane filtration are also examined for their removal efficiency of pharmaceutical compounds retained in the wastewater after secondary treatment. Yet, the efficiencies of these methods are observed to be fluctuated critically (Kaur et al. 2016).
To date, heterogeneous photocatalysis has been known as the most distinctive, popular, effective, interesting and promising wastewater treatment technique for the removal of recalcitrant and persistent contaminants (Aguilar et al. 2011; Maroga Mboula et al. 2012; Yahiat et al. 2011; Yang et al. 2008a). This treatment process is initiated by the interaction between the UV and catalyst, where it can be simplified as “the speeding up of photoreaction in the presence of a catalyst” (Al-Rasheed 2005; De la Cruz et al. 2013). It utilizes the near ultraviolet band of the solar spectrum (<400 nm) to excite the catalyst integrated with the presence of oxygen and water (LA Ioannou et al. 2011). Then, the generated ROSs are applied for the destruction of contaminants appeared in the water body. Among the photosemiconductors, titanium dioxide (TiO2) with anatase phase appears to be the most extensively used photocatalyst due to its idealizing properties such as high stability, cost-effective and favorable performance (Kaur et al. 2016).
Mechanisms of heterogeneous photocatalysis
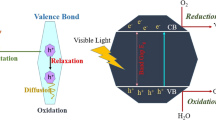
Numerous studies have been published on the reaction mechanism of photocatalytic process using TiO2 (Al-Rasheed 2005; Elmolla and Chaudhuri 2010b; Ibhadon and Fitzpatrick 2013; Jaeger and Bard 1979; Moctezuma et al. 2012; Yang et al. 2008a, b). Heterogeneous photocatalysis can be defined as the acceleration of photoreaction in the presence of semiconductor photocatalyst. Among the variety of catalyst, TiO2 is mostly preferred in the photocatalysis process for features such as chemical stability, acceptable band gap, ability to adsorb electrons and low cost (Gani and Kazmi 2016). Figure 2 illustrates the simplified mechanisms for the photoactivation of a semiconductor photocatalyst, TiO2. As shown in Fig. 2, the valence and conduction bands of a TiO2 are separated by a band gap or energy gap. In order to initiate the degradation process, TiO2 requires photoexcitation with light at a wavelength of <387 nm, to exceed the band gap of the active anatase phase of 3.2 eV and rutile phase of 3.0 eV (Ibhadon and Fitzpatrick 2013). In other words, photocatalysis over a TiO2 is initiated by the absorption of photons with energy equal to or greater than its band gap. This promotes the movement of electron from the valence band to the conduction band of TiO2. Subsequently, it generates the positive valence band hole \(\left( {{\text{h}}_{\text{vb}}^{ + } } \right)\) and negative conduction band electron \(\left( {{\text{e}}_{\text{cb}}^{ - } } \right)\):
Simplified mechanisms for the photoactivation of a semiconductor catalyst (Al-Rasheed 2005)
The electron and hole can recombine when there is a lack of oxygen to react with the electron for the formation of \({\text{O}}_{2}^{ - \cdot }\). During the recombination, the absorbed light energy released as heat with no chemical reaction takes place (Ibhadon and Fitzpatrick 2013).
As a result of irradiation, TiO2 can act as an electron donor or molecules acceptor. The powerful oxidant, \({\text{h}}_{\text{vb}}^{ + }\), oxidizes the water and hydroxide ion (\({\text{OH}}^{ - }\)) to produce the ·OH, whereby the reductant, \({\text{e}}_{\text{cb}}^{ - }\), reduces the oxygen to form superoxide radical anion (\({\text{O}}_{2}^{ - \cdot }\)). \({\text{O}}_{2}^{ - \cdot }\) then reacts with hydrogen ion (\({\text{H}}^{ + }\)) to form hydrogen peroxide radical (\({\text{HO}}_{2}^{ \cdot }\)) and further protonated by \({\text{H}}^{ + }\) can produce hydrogen peroxide (H2O2). These oxidation and reduction processes which take place on the surface of the photoexcited semiconductor not only generate abundant of ROSs such as ·OH, \({\text{O}}_{2}^{ - \cdot }\), \({\text{HO}}_{2}^{ \cdot }\) and H2O2 which takes part in the contaminant degradation process, but also prevent the recombination of \({\text{h}}_{\text{vb}}^{ + }\) and \({\text{e}}_{\text{cb}}^{ - }\). The following chain reactions have been widely postulated (Al-Rasheed 2005):
On the other hand, the cleavage of H2O2 by one of the reactions below can also generate ·OH:
The ROSs (·OH,\({\text{O}}_{2}^{ - \cdot }\), \({\text{HO}}_{2}^{ \cdot }\) and H2O2) including \({\text{h}}_{\text{vb}}^{ + }\) and \({\text{e}}_{\text{cb}}^{ - }\) then degrade the organic compounds adsorbed on the surface of photocatalyst and suspended in the solution. Although the degradation commences with a partial degradation, the term photocatalytic degradation usually refers to the complete photo mineralization or oxidation, essentially to the harmless end products of water and carbon dioxide (Saadati et al. 2016). ·OH is verified to be the most abundant and dominant oxidizing species since it degrades the organic compounds directly in a TiO2 aqueous suspension (Moctezuma et al. 2012; Yang et al. 2008a).
Titanium dioxide (TiO2) as photocatalyst
Among the photosemiconductors (ZnO, ZrO2, MgO, MoO3, CdS, SnO2, WO3, etc.), TiO2 is the most extensively used photocatalyst due to its biological and chemical inertness, non-toxicity, low cost, high catalytic efficiency and photochemical stability (Abellán et al. 2009; Aguilar et al. 2011; Ji et al. 2013; Laoufi et al. 2013). Besides, with the ideal properties such as excellent pigmentary properties, high UV absorption and resistance to photocorrosion in aqueous environment, eco-friendly, low energy band gap and can be applied individually without additional chemicals, TiO2 remains a benchmark among other semiconductors (Abellán et al. 2007; Al-Rasheed 2005; Belgiorno et al. 2007; Choina et al. 2013; Lofrano et al. 2009; Yang et al. 2008a).
Basically, TiO2 has three different crystalline forms. Anatase and rutile are the most common forms. Anatase is more efficient than rutile in the photocatalysis application since the former crystalline form has an open structure and exhibits higher photocatalytic activity, whereby brookite is the third crystalline form which is uncommon and unstable to be used in the photocatalysis treatment process. The commercial TiO2 powder, such as anatase crystalline, and the combinations of anatase and rutile (Degussa P25, 25:75 or 80:20 anatase/rutile) have been the most extensively used heterogeneous catalysts in the photocatalysis treatment process due to their chemical stability, reproducibility, highest detoxification efficiencies and readily available as a catalyst for oxidation processes (Al-Rasheed 2005; Spasiano et al. 2015).
Suspended TiO2 powder and immobilized TiO2 are the two different modes of TiO2 photocatalyst which are typically used in the photocatalytic degradation processes. Both of them offer their respective advantages and disadvantages as tabulated in Table 4. The slurry or suspended TiO2 has been the most commonly used system because of its simplicity in terms of preparation and application. The suspended TiO2 system can provide a higher efficiency than the immobilized system, mainly due to a higher ratio of catalyst active site to reaction volume. Nevertheless, an additional separation process is required to recover the TiO2 powder at the end of the treatment process, by either filtration or centrifugation. This separation process not only increases the complexity and cost of the overall process, but it is also time-consuming (Spasiano et al. 2015). Furthermore, the suspended TiO2 powder tends to agglomerate into larger particles at high concentration, which could reduce the catalytic activity (Hao et al. 2012; Lalhriatpuia et al. 2015). On the other hand, the immobilized TiO2 system allows the direct discharges of decontaminated water effluent without the recovery of TiO2 since the catalyst remains confined to the packed-bed (Borges et al. 2015). Consequently, the post-processing treatment can be avoided, thereby saving time and costs (Hashim et al. 2017). However, as compared to the suspended catalyst, it is reported that 60–70% of reduction in performance has been observed in the aqueous system for immobilized TiO2 since the latter system offers a lower reaction surface area for the photocatalysis reaction to take place (Bautista et al. 2015; Gaya and Abdullah 2008; Jiménez et al. 2015). Besides, the immobilized catalyst is more difficult to be synthesized and it requires the inert support materials such as glass or ceramic fibers, alumina pellets or molecular sieves (Spasiano et al. 2015). Despite aforementioned disadvantages, currently, coated photocatalyst and immobilization techniques are still investigated intensively due to the assumption that this system is more effective in the removal of organic compound as compared to the suspended TiO2 (Gaya and Abdullah 2008).
Besides being a promising photocatalyst in the wastewater treatment process, TiO2 is also an important material in many commercial products, ranging from drugs to foods, paints to pharmaceuticals, cosmetics to catalysts and sunscreens to solar cells, where TiO2 is applied as a desiccant, brightener and reactive mediator. TiO2 is used as the inactive ingredients in the food products up to 1%, as permitted by the U.S. Food and Drug Administration. The health effects associated with the consumption and application of TiO2 are still not clearly known (Ibhadon and Fitzpatrick 2013). So far, only Gaya and Abdullah (2008) has mentioned on the toxic effect of the TiO2 suspension on the E. coli. Also, the increased antibacterial inhibition is observed with the increment of TiO2 loading during the photocatalytic experiment. Therefore, more studies are required to further investigate the effect of TiO2 on the environment and organisms. Meanwhile, the regulatory framework for the use of TiO2 in the food products should be established in the near future, particularly in the developing nations (Ibhadon and Fitzpatrick 2013).
Advantages and limitations of heterogeneous photocatalysis
Apparently, current conventional water and wastewater treatment processes have failed to overcome the pharmaceutical pollution as they are not designed to remove the suspended pharmaceuticals (Blair 2016). To date, heterogeneous photocatalytic degradation using TiO2 photocatalyst remains as a viable alternative in the elimination of organic contaminants persistent in both aquatic and atmospheric (Gaya and Abdullah 2008). Since the last decade, this wastewater treatment technique has been extensively applied for the elimination of different types of pharmaceuticals. Table 5 summarizes previous studies on the heterogeneous photocatalysis treatment technique for the degradation of pharmaceuticals. It is noticeable in Table 5 that the initial concentration of pharmaceutical employed in the previous studies was usually higher than that typically detected in the natural water sources (µg/L–ng/L). This is to allow the accurate determination of residual concentration and the assessment of treatment efficiency within a measurable time scale (Achilleos et al. 2010). As shown in Table 5, most of the TiO2 photocatalytic studies focused on the determination of optimal operating conditions to attain the best performance in terms of degradation and mineralization. Markedly, the degradation rate of pharmaceutical is greatly affected by the operational parameters such as the type and loading of photocatalyst, initial concentration of the pharmaceutical under investigation, pH of the solution and light (source, wavelength and intensity). Other operational parameters which can enhance the degradation rate include the addition of co-oxidants and the concentration of electron acceptors. Moreover, the design and geometry of the photoreactor also influence the optimal degradation rate of pharmaceutical (Kanakaraju et al. 2014). Due to this, different optimum operating conditions are normally documented by the different studies for the highest pharmaceutical removal efficiency (regardless of the studied pharmaceuticals are different or similar), since the degradation rate of pharmaceutical is dictated by a great deal of aspects and parameters. Here, there are three main advantages associated with this treatment method and could be explained from the aspects of health and safety, sustainability as well as the simplicity of technology.
Various types of advanced treatment methods such as activated carbon adsorption, air stripping and reverse osmosis have been applied for the elimination of retained pharmaceuticals. Yet, studies have found out that these processes are less effective for the overall mineralization, which only transfer the pharmaceutical compounds from one phase to another or just collecting the pharmaceutical compounds without eliminating them (Elmolla and Chaudhuri 2010b; Gültekin and Ince 2007). Besides, the purification techniques like ozonization, chlorination and filtration too have their own limitations in terms of the energy sources consumption and the generation of harmful waste (Sharma et al. 2012). However, in contrast with the aforementioned treatment methods, heterogeneous photocatalyst which relies on the generation of ROSs is able to render the complete mineralization of pharmaceuticals (Kaan et al. 2012). Among the variety of ROSs, ·OH has attracted the most attention. The positive characteristics of ·OH radicals are including non-selective nature, high oxidation potential (2.8 eV) when compared to other oxidants, ability to react with a wide range of contaminants without any additives, as well as rate constants normally in the order of 106–109 mol/L/s (Kanakaraju et al. 2014). During the photocatalysis treatment process, ·OH appears to be a strong oxidant that can react and further break down the non-biodegradable or recalcitrant compounds (such as pharmaceuticals) in water or wastewater, from complex molecules into the simplest and non-hazardous end products such as water and carbon dioxide (Elmolla and Chaudhuri 2010b; Khataee et al. 2013; Lofrano et al. 2009). Therefore, heterogeneous photocatalysis degradation process can provide promising clean water sources that ensure the health and safety of human, living organisms and the environment.
The growth and development of the world have induced several environmental issues, mainly regarding the depletion of natural resources (Spasiano et al. 2015). It has been circulated worldwide that the depletion rate of these resources is increasing year by year, causing it to become one of the major concern among humans globally. Due to this issue, priority is given to the sustainability concept regardless of sectors. Heterogeneous photocatalyst is one of the wastewater treatment methods which in line with the concept of sustainability. The recent studies start to utilize the natural UV radiation (from sunlight) instead of the synthetic UV radiation (from UV lamp and solar simulator) as the source of photocatalyst excitation. Meanwhile, the ideal photocatalyst, TiO2, is found to be water insoluble under most of the environmental conditions and remains unchanged throughout the treatment process (Belgiorno et al. 2007; Choina et al. 2013; Ibhadon and Fitzpatrick 2013). As the main elements of UV radiation and oxygen are unlimited supplied by the solar and atmosphere, respectively, as well as the photocatalyst can be recovered and reused after the treatment process, the photocatalytic technology is considered to be low cost, environmentally friendly, low energy consumption with no consumable chemicals required (Aziz et al. 2016; LA Ioannou et al. 2011).
Heterogeneous photocatalyst treatment which involves a simple operating system with limited equipment required could potentially be used as a standalone treatment. This treatment method is able to attain the complete mineralization with no sludge generated. Therefore, the post-treatment and disposal of sludge can be avoided (Al-Rasheed 2005; Chong et al. 2015; Ibhadon and Fitzpatrick 2013). Additionally, the achieved photocatalysis removal efficiencies of pharmaceuticals in the previous studies are believed to be sufficient for the actual treatment process. In other words, the photocatalytic degradation of pharmaceuticals in the real situation is likely to occur rapidly since the concentrations of pharmaceuticals detected in the natural water sources (µg/L–ng/L) are far lower than that employed in the previous studies. Hence, this treatment method is able to perform effectively for a technical use in the wastewater treatment plant. Besides, it is a totally safe treatment method which is conducted under the ambient temperature and ordinary pressure (Aziz et al. 2016).
Despite the aforementioned advantages, there are also some limitations associated with the application of photocatalysis. The main limitation is that this method is unable to perform well in turbid water. This is because the reduction of light transmissivity, the adsorption of contaminants onto the surface of catalyst or the absorption of available photons by the contaminants will eventually induce a lower intensity of photons to excite the catalyst surface (Abellán et al. 2009; LA Ioannou et al. 2011; Kümmerer 2009; Yang et al. 2008a). Therefore, heterogeneous photocatalysis treatment is unable to integrate with primary and secondary treatment process, but it is suggested to be used as a tertiary treatment process, particularly for the elimination of pharmaceutical compounds which are still retained in the treated wastewater (Naddeo et al. 2009).
Another major limitation is that TiO2 can only be photoexcited by the light at a wavelength of <388 nm due to its wide band gap (3–3.2 eV) (Achilleos et al. 2010; Daghrir and Drogui 2013). Therefore, it is important to avoid any loss of solar radiation during the treatment process. For better results, this treatment process can be assisted by the artificial illumination of catalyst to achieve the degradation of contaminants in time that is practical for the real wastewater treatment processing (Ibhadon and Fitzpatrick 2013). Besides, it is suggested that the nanomaterial should be modified so that it can utilize the visible light in solar irradiation (Kaur et al. 2016).
Last but not least, heterogeneous photocatalysis process must be operated cautiously to avoid the incomplete mineralized organic contaminants to be released into the water sources. It is important to highlight that the ultimate goal of the treatment process should be emphasized on the complete mineralization of pollutants, since the previous studies have reported that the metabolites and intermediates generated during the treatment process can be more toxic than the parent compounds (Kümmerer 2009; Maroga Mboula et al. 2012; Nasuhoglu et al. 2011). For instance, Szabo and his co-researchers have reported that the intermediates of paracetamol (e.g., acetamide, hydroquinone) have posed a higher toxicity level as compared to paracetamol itself (Szabó et al. 2012). Besides, the phenolic intermediates of paracetamol which are classified as the persistent organic pollutants are found to be toxic for both environment and human beings (Torun et al. 2015).
Future trends
Maroga Mboula et al. (2012) indicated that the complete mineralization of contaminants under the heterogeneous photocatalyst treatment method is associated with a high operating cost caused by the excessive consumption of energy and TiO2. In order to minimize the overall cost of photocatalysis treatment process and to overcome the depletion of non-renewable energy resources, scientists are currently searching for a new energy resource that is clean, renewable and alternative to the fossil fuels. Among the alternative sources, solar, which is derived from the sunlight, was recognized as the most promising natural energy source. This potential energy can be converted into chemical energy by natural solar photoassisted reactions (Spasiano et al. 2015). Table 6 shows the recent studies applying the solar radiation for the photocatalysis degradation of pharmaceuticals. It can be observed that the solar heterogeneous photocatalysis has been an efficient technique for the elimination of pollutants from aqueous. As photocatalysis makes use of sunlight for UV radiation, the technology is inexpensive, environmentally friendly and can be applied worldwide. Also, it requires only minimal equipment, which is highly deployable and appropriate for developing countries and remote sites with no access to electricity (Ibhadon and Fitzpatrick 2013).
TiO2 remains as a benchmark and excellent photocatalyst among other semiconductors due to its ideal properties (Kaan et al. 2012). The main drawback which currently restricts its wider commercial application is the limited solar spectrum to be used (limited to ultraviolet region), which is only about 3–4% of the solar energy reaching the terrestrial surface. Therefore, much effort has been devoted to the development of visible-light-harvesting photocatalysts through the reduction in catalyst’s band gap to increase the amount of visible sunlight which TiO2 can absorb (Spasiano et al. 2015). Other inventions include the increment of catalyst surface area, active site and photon energy absorption (Al-Rasheed 2005; Ibhadon and Fitzpatrick 2013). Additionally, the retrieval and reuse of TiO2 is also an essential issue to be resolved. There are a few TiO2 retrieval methods which have been suggested for the recovery of TiO2 suspended in the reaction mixture in aqueous slurry systems. Ultracentrifugation and membrane filtration separation methods are efficient in the separation of nanomaterials, but both of them have their own limitation of high energy consumption and easily to be blocked, respectively. Compared to the aforementioned methods, the magnetic separation method is considered a rapid and effective technique for the recovery of nanoparticles (Bagheri et al. 2016). Nevertheless, Saadati et al. (2016) have mentioned that future studies should focus on the immobilized system, where the photocatalyst is immobilized on suitable support matrices to avoid the post-separation and recovery of the catalyst particles at the end of the treatment process. Overall, each of the recovery methods equipped with advantages and disadvantages. The selection of method should be based on the quality standards which have to be met, efficiency and cost (Bagheri et al. 2016).
Besides the replacement of artificially generated photons with the natural solar irradiation and the modification of TiO2, there are some barriers and lacking parts which still need to be overcome in the near future:
-
Although solar photocatalysis has produced significant interest in research on nowadays, so far, there is a lack of “in situ” experiments on the long-term reliability of solar operation (Spasiano et al. 2015).
-
Photoreactor is a specific device which is used to convey the solar photons and chemical reagents to in contact with the photocatalyst efficiently. Unlike the classic chemical reactors, solar photoreactors more concern on the physical geometry to ensure the effective collection of solar radiation for the optimal photocatalytic treatment process, as well as the uniform distribution of sunlight inside the photoreactor, as the poor distribution may reduce the overall efficiencies of the photochemical process. However, studies have found out that the design and scale-up of a solar photoreactor is one of the major problems associated with the application heterogeneous photocatalyst treatment method, where it is very difficult to reproduce the same ratio of irradiated surface to total volume during scale-up (Aziz et al. 2016; Spasiano et al. 2015).
-
Future research should prioritize on the investigation of AOPs under real treatment conditions since the literature about real field applications of AOPs is very scarce, which may limit the wider application of so far obtained data (Gani and Kazmi 2016).
-
More investigation should be conducted to acquire more data on the environmental occurrence of human and veterinary pharmaceuticals in use today, so that a proper estimation of exposure to pharmaceuticals in environmental and drinking water, and their potential risks to the ecosystem and human health can be assessed (K’oreje et al. 2016).
-
So far, there is a notable lack of evidence regarding the harmfulness of the retained pharmaceuticals, particularly on the human health. Although information on the potential environmental risks of pharmaceuticals is available, this effect cannot be easily extrapolated to the human harm. Therefore, extra findings on the potential biota and human health effects are required, in both low-level and long-term exposure. Besides, information regarding the impact of pharmaceuticals in drinking water (often in the ng/L range) is also incomplete (Blair 2016; Quadra et al. 2016).
-
Abundant of studies deals with the removal of pharmaceutical individually instead of pharmaceuticals mixture which simulates the real wastewater matrix (Rizzo et al. 2009). In this regard, the extra concern should be given to the synergistic effects of the multiple compounds on both ecological and human health (Ji et al. 2013). In addition, Nasuhoglu et al. (2011) have indicated that the intermediates generated during the pharmaceuticals treatment process are found to be more toxic and higher retained concentrations as compared to the parent compounds. Therefore, the pharmaceutical metabolites should also be given the similar attention to identify their hidden toxic potential on both ecological and human health. Besides the endocrine disruption, further research should focus on the possible disruption to other biological systems (such as nervous and immune systems), especially in system development during pre-/perinatal exposure periods (Wilkinson et al. 2016).
Conclusion
Water pollution by persistent pharmaceutical compounds is an ever-increasing environmental problem concerned globally. In this regard, more research is crucially required to obtain more information about the effects of pharmaceuticals in both parent and metabolites forms, in single and mixture conditions, as well as in low-level and long-term exposure. These findings are particularly important to human being as it can become a convincing fact to alert the society regarding the seriousness of the pharmaceutical pollution. On the other hand, while the solar photocatalysis treatment process has emerged as a reliable, sustainable, environmentally friendly and economical treatment technology, its application is constrained by several major technical issues mainly related to the main elements in the photocatalysis process such as photocatalyst, solar UV source and photoreactor. Further investigation, modifications and improvements on these important elements can be implemented to increase the industrial acceptance as well as the effectiveness and practical application of this treatment technique in the real wastewater treatment system.
References
Abellán M, Bayarri B, Giménez J, Costa J (2007) Photocatalytic degradation of sulfamethoxazole in aqueous suspension of TiO2. Appl Catal B Environ 74:233–241
Abellán M, Giménez J, Esplugas S (2009) Photocatalytic degradation of antibiotics: the case of sulfamethoxazole and trimethoprim. Catal Today 144:131–136
Achilleos A, Hapeshi E, Xekoukoulotakis N, Mantzavinos D, Fatta-Kassinos D (2010) UV-A and solar photodegradation of ibuprofen and carbamazepine catalyzed by TiO2. Sep Sci Technol 45:1564–1570
Aguilar C, Montalvo C, Ceron J, Moctezuma E (2011) Photocatalytic degradation of acetaminophen. Int J Environ Res 5:1071–1078
Ai C, Zhou D, Wang Q, Shao X, Lei Y (2015) Optimization of operating parameters for photocatalytic degradation of tetracycline using In2S3 under natural solar radiation. Solar Energy 113:34–42
Akbarzadeh R, Umbarkar SB, Sonawane RS, Takle S, Dongare MK (2010) Vanadia–titania thin films for photocatalytic degradation of formaldehyde in sunlight. Appl Catal A 374:103–109
Al-Odaini NA, Zakaria MP, Yaziz MI, Surif S, Abdulghani M (2013) The occurrence of human pharmaceuticals in wastewater effluents and surface water of Langat River and its tributaries, Malaysia. Int J Environ Anal Chem 93:245–264
Al-Rasheed RA (2005) Water treatment by heterogeneous photocatalysis an overview. In: 4th SWCC acquired experience symposium, Jeddah, pp 1–14
An J, Zhou Q, Sun F, Zhang L (2009) Ecotoxicological effects of paracetamol on seed germination and seedling development of wheat (Triticum aestivum L.). J Hazard Mater 169:751–757
Arpin-Pont L, Bueno MJM, Gomez E, Fenet H (2016) Occurrence of PPCPs in the marine environment: a review. Environ Sci Pollut Res 23:4978–4991
Aziz NAA, Palaniandy P, Aziz HA, Dahlan I (2016) Review of the mechanism and operational factors influencing the degradation process of contaminants in heterogenous photocatalysis. J Chem Res 40:704–712
Bagheri H, Afkhami A, Noroozi A (2016) Removal of pharmaceutical compounds from hospital wastewaters using nanomaterials: a review. Anal Bioanal Chem Res 3:1–18
Bahnemann D (2004) Photocatalytic water treatment: solar energy applications. Sol Energy 77:445–459
Bautista R, Anderson W, Pagsuyoin S, Munoz J (2015) Degradation of tetracycline in synthesized wastewater using immobilized TiO2 on rotating corrugated aluminum drum. In: Systems and information engineering design symposium (SIEDS), pp 115–119
Becker JA, Ortner P, Susan T-M (2010) Don’t rush to flush: safer pharmaceutical practices for hospice home care and home health nurses. Home Health Care Manage Pract 22(3):202–206
Belgiorno V et al (2007) Review on endocrine disrupting-emerging compounds in urban wastewater: occurrence and removal by photocatalysis and ultrasonic irradiation for wastewater reuse. Desalination 215:166–176
Blair BD (2016) Potential upstream strategies for the mitigation of pharmaceuticals in the aquatic environment: a brief review. Curr Environ Health Rep 3:153–160
Borghi AA, Palma MSA (2014) Tetracycline: production, waste treatment and environmental impact assessment. Braz J Pharm Sci 50:25–40
Borsuk ME, Maurer M, Lienert J, Larsen TA (2008) Charting a path for innovative toilet technology using multicriteria decision analysis. Environ Sci Technol 42:1855–1862
Borges M, García DM, Hernández T, Ruiz-Morales JC, Esparza P (2015) Supported photocatalyst for removal of emerging contaminants from wastewater in a continuous packed-bed photoreactor configuration. Catalysts 5:77–87
Bundschuh M, Hahn T, Ehrlich B, Höltge S, Kreuzig R, Schulz R (2016) Acute toxicity and environmental risks of five veterinary pharmaceuticals for aquatic macroinvertebrates. Bull Environ Contam Toxicol 96:139–143
Cai Q, Hu J (2017) Decomposition of sulfamethoxazole and trimethoprim by continuous UVA/LED/TiO2 photocatalysis: decomposition pathways, residual antibacterial activity and toxicity. J Hazard Mater 323:527–536
Chang X et al (2010) Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of three Gorge Reservoir in China. Environ Pollut 158:1444–1450
Choina J, Kosslick H, Fischer C, Flechsig G-U, Frunza L, Schulz A (2013) Photocatalytic decomposition of pharmaceutical ibuprofen pollutions in water over titania catalyst. Appl Catal B Environ 129:589–598
Chong MN, Cho YJ, Poh PE, Jin B (2015) Evaluation of Titanium dioxide photocatalytic technology for the treatment of reactive Black 5 dye in synthetic and real greywater effluents. J Clean Prod 89:196–202
Cleuvers M (2003) Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol Lett 142:185–194
Daghrir R, Drogui P (2013) Tetracycline antibiotics in the environment: a review. Environ Chem Lett 11:209–227
Daughton CG (2003) Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. II. Drug disposal, waste reduction, and future directions. Environ Health Perspect 111:775
Daughton CG (2014) Eco-directed sustainable prescribing: feasibility for reducing water contamination by drugs. Sci Total Environ 493:392–404
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107:907
David A, Pancharatna K (2009) Effects of acetaminophen (paracetamol) in the embryonic development of zebrafish, Danio rerio. J Appl Toxicol 29:597–602
De la Cruz N, Dantas R, Giménez J, Esplugas S (2013) Photolysis and TiO2 photocatalysis of the pharmaceutical propranolol: solar and artificial light. Appl Catal B Environ 130:249–256
Doll TE, Frimmel FH (2005) Cross-flow microfiltration with periodical back-washing for photocatalytic degradation of pharmaceutical and diagnostic residues—evaluation of the long-term stability of the photocatalytic activity of TiO2. Water Res 39:847–854
Ebert I, Bachmann J, Kühnen U, Küster A, Kussatz C, Maletzki D, Schlüter C (2011) Toxicity of the fluoroquinolone antibiotics enrofloxacin and ciprofloxacin to photoautotrophic aquatic organisms. Environ Toxicol Chem 30:2786–2792
Elmolla ES, Chaudhuri M (2010a) Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J Hazard Mater 173:445–449
Elmolla ES, Chaudhuri M (2010b) Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2 TiO2 photocatalysis. Desalination 252:46–52
Emmanuel E, Perrodin Y, Keck G, Blanchard J-M, Vermande P (2005) Ecotoxicological risk assessment of hospital wastewater: a proposed framework for raw effluents discharging into urban sewer network. J Hazard Mater 117:1–11
Fent K, Weston AA, Caminada D (2006) Ecotoxicology of human pharmaceuticals. Aquat Toxicol 76:122–159
Fukahori S, Fujiwara T, Ito R, Funamizu N (2012) Photocatalytic decomposition of crotamiton over aqueous TiO2 suspensions: determination of intermediates and the reaction pathway. Chemosphere 89:213–220
Gani KM, Kazmi AA (2016) Phthalate contamination in aquatic environment: a critical review of the process factors that influence their removal in conventional and advanced wastewater treatment. Crit Rev Environ Sci Technol 46:1402–1439
Gautam AK, Kumar S, Sabumon P (2007) Preliminary study of physico-chemical treatment options for hospital wastewater. J Environ Manag 83:298–306
Gaya UI, Abdullah AH (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem Photobiol C Photochem Rev 9:1–12
Gibson K (2010) Pharmaceuticals and personal care products (PPCPs): disposal, scientific, and regulatory challenges. Environ Qual Manage 20:39–48
Gültekin I, Ince NH (2007) Synthetic endocrine disruptors in the environment and water remediation by advanced oxidation processes. J Environ Manag 85:816–832
Halling-Sørensen B, Nors Nielsen S, Lanzky P, Ingerslev F, Holten Lützhøft H, Jørgensen S (1998) Occurrence, fate and effects of pharmaceutical substances in the environment-a review. Chemosphere 36:357–393
Hao R, Xiao X, Zuo X, Nan J, Zhang W (2012) Efficient adsorption and visible-light photocatalytic degradation of tetracycline hydrochloride using mesoporous BiOI microspheres. J Hazard Mater 209:137–145
Hashim N, Thakur S, Patang M, Crapulli F, Ray AK (2017) Solar degradation of diclofenac using Eosin-Y-activated TiO2: cost estimation, process optimization and parameter interaction study. Environ Technol 38:933–944
Helwig K, Hunter C, McNaughtan M, Roberts J, Pahl O (2016) Ranking prescribed pharmaceuticals in terms of environmental risk: inclusion of hospital data and the importance of regular review. Environ Toxicol Chem 35:1043–1050
Ibhadon AO, Fitzpatrick P (2013) Heterogeneous photocatalysis: recent advances and applications. Catalysts 3:189–218
Jaeger CD, Bard AJ (1979) Spin trapping and electron spin resonance detection of radical intermediates in the photodecomposition of water at titanium dioxide particulate systems. J Phys Chem 83:3146–3152
Jallouli N, Elghniji K, Trabelsi H, Ksibi M (2014) Photocatalytic degradation of paracetamol on TiO2 nanoparticles and TiO2/cellulosic fiber under UV and sunlight irradiation. Arab J Chem. doi:10.1016/j.arabjc.2014.03.014
Jelić A, Gros M, Petrović M, Ginebreda A, Barceló D (2012) Occurrence and elimination of pharmaceuticals during conventional wastewater treatment. Emerging and priority pollutants in Rivers. Springer, Berlin Heidelberg, pp 1–23
Ji Y, Zhou L, Ferronato C, Yang X, Salvador A, Zeng C, Chovelon J-M (2013) Photocatalytic degradation of atenolol in aqueous titanium dioxide suspensions: kinetics, intermediates and degradation pathways. J Photochem Photobiol A Chem 254:35–44
Jiménez M, Ignacio Maldonado M, Rodríguez EM, Hernández-Ramírez A, Saggioro E, Carra I, Sánchez Pérez JA (2015) Supported TiO2 solar photocatalysis at semi-pilot scale: degradation of pesticides found in citrus processing industry wastewater, reactivity and influence of photogenerated species. J Chem Technol Biotechnol 90:149–157
Kaan CC, Aziz AA, Matheswaran M, Saravanan P, Ibrahim S (2012) Heterogeneous photocatalytic oxidation an effective tool for wastewater treatment—a review. In: Studies on water management issues. INTECH, pp 219–236
Kanakaraju D, Glass BD, Oelgemöller M (2014) Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ Chem Lett 12:27–47
Kaur A, Umar A, Kansal SK (2016) Heterogeneous photocatalytic studies of analgesic and non-steroidal anti-inflammatory drugs. Appl Catal A Gen 510:134–155
Khetan SK, Collins TJ (2007) Human pharmaceuticals in the aquatic environment: a challenge to green chemistry. Chem Rev 107:2319–2364
Khataee A, Fathinia M, Joo S (2013) Simultaneous monitoring of photocatalysis of three pharmaceuticals by immobilized TiO2 nanoparticles: chemometric assessment, intermediates identification and ecotoxicological evaluation. Spectrochim Acta Part A Mol Biomol Spectrosc 112:33–45
Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW (2007) Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci 104:8897–8901
K’oreje K, Vergeynst L, Ombaka D, De Wispelaere P, Okoth M, Van Langenhove H, Demeestere K (2016) Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere 149:238–244
Kümmerer K (2001) Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—a review. Chemosphere 45:957–969
Kümmerer K (2009) Antibiotics in the aquatic environment—a review—part I. Chemosphere 75:417–434
LA Ioannou HE, Vasquez MI, Mantzavinos D, Fatta-Kassinos D (2011) Solar/TiO2 photocatalytic decomposition of beta-blockers atenolol and propranolol in water and wastewater. Sol Energy 85:1915–1926
Lalhriatpuia C, Tiwari D, Tiwari A, Lee SM (2015) Immobilized Nanopillars-TiO2 in the efficient removal of micro-pollutants from aqueous solutions: physico-chemical studies. Chem Eng J 281:782–792
Lamichhane K, Babcock R (2012) An economic appraisal of using source separation of human urine to contain and treat endocrine disrupters in the USA. J Environ Monit 14:2557–2565
Lange A, Paull GC, Coe TS, Katsu Y, Urushitani H, Iguchi T, Tyler CR (2009) Sexual reprogramming and estrogenic sensitization in wild fish exposed to ethinylestradiol. Environ Sci Technol 43:1219–1225
Laoufi NA, Hout S, Tassalit D, Ounnar A, Djouadi A, Chekir N, Bentahar F (2013) Removal of a persistent pharmaceutical micropollutant by UV/TiO2 process using an immobilized titanium dioxide catalyst: parametric study. Chem Eng 32:1951–1958
Länge R et al (2001) Effects of the synthetic estrogen 17α-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ Toxicol Chem 20:1216–1227
Lee CM, Palaniandy P, Zaman NQ, Adlan MN (2015) Pharmaceutical removal from synthetic wastewater using heterogeneous-photocatalyst. Appl Mech Mater 802:507–512
Lienert J, Haller M, Berner A, Stauffacher M, Larsen TA (2003) How farmers in Switzerland perceive fertilizers from recycled anthropogenic nutrients (urine). Water Sci Technol 48:47–56
Lofrano G, Rizzo L, Grassi M, Belgiorno V (2009) Advanced oxidation of catechol: a comparison among photocatalysis, Fenton and photo-Fenton processes. Desalination 249:878–883
Luo Z, Li L, Wei C, Li H, Chen D (2015) Role of active oxidative species on TiO2 photocatalysis of tetracycline and optimization of photocatalytic degradation conditions. J Environ Biol 36:837
Maroga Mboula V, Hequet V, Gru Y, Colin R, Andres Y (2012) Assessment of the efficiency of photocatalysis on tetracycline biodegradation. J Hazard Mater 209:355–364
Moctezuma E, Leyva E, Aguilar CA, Luna RA, Montalvo C (2012) Photocatalytic degradation of paracetamol: intermediates and total reaction mechanism. J Hazard Mater 243:130–138
Mozia S, Morawski AW (2012) The performance of a hybrid photocatalysis–MD system for the treatment of tap water contaminated with ibuprofen. Catal Today 193:213–220
Moore M, Greenway S, Farris J, Guerra B (2008) Assessing caffeine as an emerging environmental concern using conventional approaches. Arch Environ Contam Toxicol 54:31–35
Naddeo V, Meriç S, Kassinos D, Belgiorno V, Guida M (2009) Fate of pharmaceuticals in contaminated urban wastewater effluent under ultrasonic irradiation. Water Res 43:4019–4027
Nasuhoglu D, Yargeau V, Berk D (2011) Photo-removal of sulfamethoxazole (SMX) by photolytic and photocatalytic processes in a batch reactor under UV-C radiation (λ max = 254 nm). J Hazard Mater 186:67–75
Oggier DM, Weisbrod CJ, Stoller AM, Zenker AK, Fent K (2010) Effects of diazepam on gene expression and link to physiological effects in different life stages in zebrafish Danio rerio. Environ Sci Technol 44:7685–7691
Palominos RA, Mondaca MA, Giraldo A, Penuela G, Pérez-Moya M, Mansilla HD (2009) Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal Today 144:100–105
Putschew A, Wischnack S, Jekel M (2000) Occurrence of triiodinated X-ray contrast agents in the aquatic environment. Sci Total Environ 255:129–134
Quadra GR, de Souza HO, dos Santos Costa R, dos Santos Fernandez MA (2016) Do pharmaceuticals reach and affect the aquatic ecosystems in Brazil? A critical review of current studies in a developing country. Environ Sci Pollut Res 24:1200–1218
Rizzo L, Meric S, Guida M, Kassinos D, Belgiorno V (2009) Heterogenous photocatalytic degradation kinetics and detoxification of an urban wastewater treatment plant effluent contaminated with pharmaceuticals. Water Res 43:4070–4078
Rosa G (2008) Study the effects of propranolol drug for Ceriodaphnia silvestrii (Cladocera, Crustacea) with emphasis on effects on populations (In Portuguese). Master Dissertation, University of São Paulo
Runnalls TJ, Hala DN, Sumpter JP (2007) Preliminary studies into the effects of the human pharmaceutical clofibric acid on sperm parameters in adult Fathead minnow. Aquat Toxicol 84:111–118
Saadati F, Keramati N, Ghazi MM (2016) Influence of parameters on the photocatalytic degradation of tetracycline in wastewater: a review. Crit Rev Environ Sci Technol 46:757–782
Sacher F, Lange FT, Brauch H-J, Blankenhorn I (2001) Pharmaceuticals in groundwaters: analytical methods and results of a monitoring program in Baden-Württemberg, Germany. J Chromatogr A 938:199–210
Sangion A, Gramatica P (2016) Hazard of pharmaceuticals for aquatic environment: prioritization by structural approaches and prediction of ecotoxicity. Environ Int 95:131–143
Sharma M, Jain T, Singh S, Pandey O (2012) Photocatalytic degradation of organic dyes under UV–visible light using capped ZnS nanoparticles. Sol Energy 86:626–633
Sim W-J, Lee J-W, Lee E-S, Shin S-K, Hwang S-R, Oh J-E (2011) Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere 82:179–186
Spasiano D, Marotta R, Malato S, Fernandez-Ibanez P, Di Somma I (2015) Solar photocatalysis: materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl Catal B Environ 170:90–123
Stoner S, Kosinski K (2008) Pharmaceuticals as emerging contaminants: a rationale for reduction in New York State’s waters. http://www.dec.ny.gov/docs/water_pdf/drugbkgrd_.pdf. Accessed 24 March 2015
Straub JO (2016) Reduction in the environmental exposure of pharmaceuticals through diagnostics, Personalised Healthcare and other approaches. A mini review and discussion paper. Sustain Chem Pharm 3:1–7
Suarez S, Lema JM, Omil F (2009) Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresour Technol 100:2138–2146
Szabó L, Tóth T, Homlok R, Takács E, Wojnárovits L (2012) Radiolysis of paracetamol in dilute aqueous solution. Radiat Phys Chem 81:1503–1507
Talib A, Randhir TO (2016) Managing emerging contaminants in watersheds: need for comprehensive, systems-based strategies. Exposure Health 8:143–158
Taylor D, Senac T (2014) Human pharmaceutical products in the environment–The “problem” in perspective. Chemosphere 115:95–99
Ternes TA (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res 32:3245–3260
Torun M, Gültekin Ö, Şolpan D, Güven O (2015) Mineralization of paracetamol in aqueous solution with advanced oxidation processes. Environ Technol 36:970–982
Vaizoğullar Aİ (2017) TiO2/ZnO Supported on sepiolite: preparation, structural characterization and photocatalytic degradation of flumequine antibiotic in aqueous solution. Chem Eng Commun 204:689–697
Wilkinson JL, Hooda PS, Barker J, Barton S, Swinden J (2016) Ecotoxic pharmaceuticals, personal care products, and other emerging contaminants: a review of environmental, receptor-mediated, developmental, and epigenetic toxicity with discussion of proposed toxicity to humans. Crit Rev Environ Sci Technol 46:336–381
Wolff M (2011) Ecotoxicological assessment of antidepressant fluoxetine hydrochloride (In Portuguese). Master Degree Dissertation, State University of Campinas
Wu S, Zhang L, Chen J (2012) Paracetamol in the environment and its degradation by microorganisms. Appl Microbiol Biotechnol 96:875–884
Xekoukoulotakis NP, Drosou C, Brebou C, Chatzisymeon E, Hapeshi E, Fatta-Kassinos D, Mantzavinos D (2011) Kinetics of UV-A/TiO2 photocatalytic degradation and mineralization of the antibiotic sulfamethoxazole in aqueous matrices. Catal Today 161:163–168
Yahiat S, Fourcade F, Brosillon S, Amrane A (2011) Removal of antibiotics by an integrated process coupling photocatalysis and biological treatment–case of tetracycline and tylosin. Int Biodeterior Biodegrad 65:997–1003
Yang L, Liya EY, Ray MB (2008a) Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res 42:3480–3488
Yang L, Yu LE, Ray MB (2008b) Photocatalytic oxidation of paracetamol: dominant reactants, intermediates, and reaction mechanisms. Environ Sci Technol 43:460–465
Zhang X, Wu F, Wu X, Chen P, Deng N (2008) Photodegradation of acetaminophen in TiO2 suspended solution. J Hazard Mater 157:300–307
Acknowledgements
The authors would like to acknowledge Ministry of Higher Education (MOHE) for funding this project under grant Fundamental Research Grant Scheme (FRGS); Grant number: 203/PAWAM/6071256 as well as the support of Universiti Sains Malaysia (USM) for providing the Intensif Grant and USM Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, C.M., Palaniandy, P. & Dahlan, I. Pharmaceutical residues in aquatic environment and water remediation by TiO2 heterogeneous photocatalysis: a review. Environ Earth Sci 76, 611 (2017). https://doi.org/10.1007/s12665-017-6924-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6924-y