Abstract
In this chapter, biosorption, applied in removing heavy metal ions from wastewater, will be introduced and discussed. A vast array of biological materials, especially bacteria, fungi, algae, and plant leaves and their extraction, have received increasing attention for heavy metal removal and recovery due to their perfect performance in both experimental research and field treatment, low cost, and large available quantities. Through summarizing the published literatures and reports, these progresses in this field involving biosorbents will be briefly reviewed and discussed. Also in this field, recycling economy is a hot and rising topic, for example, to synthesize metallic nanoparticles in the process of wastewater treatment. Although these explorations are still in their infancy of theoretic and experimental phase, the trend leads irresistibly the future of this field to a green and economical one. That is, for the concrete example, a controlled synthesis method will be developed of metallic nanoparticles of well-defined size and shape while treating waste effluents.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Heavy Metal
- Cetyl Trimethyl Ammonium Bromide
- Biosorption Capacity
- Biosorption Process
- Heavy Metal Removal
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 Introduction
Rapid industrialization and urbanization have resulted in elevated levels of toxic heavy metals. This has been entangled, almost everywhere, in most industrial activities involving leakage and redistribution of heavy metals, such as mining and metalliferous smelting, metallurgy, iron and steel, electroplating, electrolysis, electro-osmosis, leatherworking, photography etc. Subsequently, these courses result in gradual exhaustion of metal land mineral resources. Especially, wastewaters produced from these industrial activities not only bring about serious environmental effect, but also threaten human health and ecosystem. These heavy metals, for the convenience of analyses, reportedly fall into three families, including toxic metals (such as Hg, Cr, Pb, Zn, Cu, Ni, Cd, As, Co, Sn, etc.), precious metals (such as Pd, Pt, Ag, Au, Ru etc.), and radionuclides (such as U, Th, Ra, Am, etc.) [1].
Methods for removing metal ions from aqueous solution mainly consist of physical, chemical, and biological technologies. Traditional physiochemical methods include chemical precipitation, oxidation or reduction, filtration, ion exchange, electrochemical treatment, reverse osmosis, membrane technology, and evaporation recovery. Most of these are ineffective or excessively expensive when the metal concentrations are less than 100 mg/L [2, 3]. For example, usual physical pathways like ion exchange, activated carbon, and membrane adsorption belong to the latter costly group, especially when treating large amounts of wastewater. In recent years, in a way of financial convenience, applying biotechnology in controlling and removing metal pollution has been paid much attention, and gradually becomes hot topic, for potential application, in the field of metal pollution control. Further alternative process is biosorption, which utilizes various certain natural materials of biological origin, including bacteria, fungi, yeast, algae, etc. These biosorbents possess instinctive metal-sequestering features to decrease the concentration of heavy metal ions in solution from ppm to ppb level. It can effectively capture dissolved metal ions and carry them out of dilute complex solutions with high efficiency and celerity; sound features as an ideal candidate for treating high volume and low concentration complex wastewaters [1].
This metabolism-independent mechanism is called biosorption [4]. According to Volesky and Holan [5], who presented an extensive review on biosorption, the strong biosorbent behavior of certain types of microbial biomass toward metallic ions is a function of the chemical makeup of microbial cells. In fact, the biomass in practice is nonliving and all cells are metabolically inactive. Compared with conventional treatment methods, biosorption has the following advantages [6–9]:
-
High efficiency and selectivity for heavy metals in low concentrations
-
Energy-saving
-
Broad operational range of pH and temperature
-
Easy reclamation of heavy metal
-
Easy recycling of the biosorbent
Heavy metal removal by biosorption has been investigated during the last several decades [10]. The capability of some living microorganisms to accumulate metallic elements has first been observed from toxicological point of view [10]. However, further researches revealed that inactive/dead microbial biomass can passively bind metal ions via various physicochemical mechanisms. This discovery then led biosorption to the frontier of researches and an active field for the removal of metal ions. A large quantity of materials has been investigated as biosorbents for extensive removal of metals or organics. The tested biosorbents can be basically classified into the following categories: Bacteria (e.g., Bacillus subtillis), fungi (e.g., Rhizopus arrhizus), yeast (e.g., Saccharomyces cerevisiae), algae, industrial wastes (e.g., S. cerevisiae waste biomass from fermentation and food industry), agricultural wastes (e.g., corn core), and other polysaccharide materials, etc. [11]. The role of some groups of microorganisms has been given enough concern, such as bacteria, fungal, yeast, algae, etc.
Some potential biomaterials with high metal-binding capacity have been identified, such as Sargassum natans, Bacillus subtillis, Rhizopus arrhizus, Saccharomyces cerevisae, and waste microbial biomass from the fermentation and food industries [5, 8, 9, 12–17]. Among these materials or biosorbents, some types bind and collect the majority of heavy metals with no specific priority, while others can even be specific for certain types of metals [5].
Researches on biosorption focus on the biosorbents, the biosorption mechanism, and large-scale experiments. Although many biological materials can bind heavy metals, only those with sufficiently high metal-binding capacity and selectivity for heavy metals are suitable for use in a full-scale biosorption process. Mechanisms responsible for biosorption are understood to a limited extent, and commercial application of microbial biomass as a biosorbent suffered from problems associated with the physical characteristics of this material [18]. Attempts to apply biosorption process into practice have proved to be futile [19]. So, the biosorption process is basically at the stage of laboratory-scale study. Also, great efforts have to be made to improve biosorption process, including immobilization of biomaterials, improvement of regeneration and reuse, optimization of biosorption process, etc. In sum, theoretical and practical development about biosorption should be allowed to forge ahead.
The aim of this chapter is to present the state-of-the-art biosorbent investigation and to compare results found in the literature. The effects of environmental factors on biosorption capacities, biosorption mechanisms, advantages of biotechnology, regeneration/reuse of biosorbents, as well as proposals about improving biotechnology are presented and discussed. What is more, current hot topics, trends, and outlook of this field are also touched upon.
5.2 Brief Review of Physicochemical Removal Technology
Before the introduction, this brief review offers a control to the biological pathways that will be discussed in the next paragraph.
Conventional physicochemical methods, such as activated carbon, precipitation, reverse osmosis, membrane separation, chemical oxidation, and ionic exchange and so on, have been commonly used. However, most of those are less effective, excessively expensive, and eco-unfriendly when initial metal concentrations are in the range of 10–100 mg/L [20]. For instance, chemical precipitation and electrochemical treatment are not very effective, especially when metal ion concentration in aqueous solution is among 1–100 mg/L, and also produce large quantity of sludge required to treat with still more great difficulty [21].
The advantages and limitations in application of these traditional methods are given below in detail as follows:
5.2.1 Activated Carbon
It is a crude form of graphite with a random or amorphous structure, which is highly porous, exhibiting abroad range of pore sizes, from visible cracks, crevices, and slits of molecular dimensions [22]. And with its specific high surface area, microporous character, and chemical nature of their surface, activated carbons have made them potential adsorbents for the removal of heavy metals from industrial wastewater [23, 24]. In spite of its advantages, it also shows many shortcomings such as high cost to prepare, reactivation resulting in a loss of the carbon, performance type dependent of carbon, being nonselective, and so on. Although modified activated carbon [25] can improve the selectivity, its preparation cost roars up subsequently.
5.2.2 Chemical Precipitation
This pathway is widely used for heavy metal removal from inorganic effluent [26, 27]. Typically, the metal precipitates from the solution in the form of hydroxide [28]. Since lime or calcium hydroxide is the most commonly employed precipitant agent, this method gains availability at most corners of the world. Lime precipitation, another option, can be trusted to effectively treat inorganic effluent with a metal concentration of higher than 1,000 mg/L with a simple process. For instance, it helped to remove heavy metals such as Zn(II), Cd(II), and Mn(II) cations with initial metal concentrations of 450, 150, and 1,085 mg/L, respectively, in a batch continuous system [29]. In spite of its advantages, chemical precipitation requires a large amount of chemicals to reduce metals to an acceptable level for discharge [30]. And generally it can hardly be used to handle low concentration of metal wastewater, which is below 100 mg/L [21]. Other drawbacks are its excessive sludge production that requires further treatment, the increasing cost of sludge disposal, slow metal precipitation, and the long-term environmental impacts of sludge disposal [31–33].
5.2.3 Chemical Oxidation and Reduction
Adding oxidizing or reducing agent makes toxic substances in wastewater being oxidized or reduced to nontoxic or low toxic substances. It has been reported that heavy metals such as Mn2+, Cu2+, Pb2+, Cd2+, Cr3+, and Hg2+ could be effectively removed using potassium ferrate(VI) as oxidation and co-precipitation [34, 35]. However, currently chemical oxidation and reduction was generally used as a pretreatment for wastewater before more powerful procedures.
5.2.4 Membrane Separation
Membrane separation methods like electrodialysis (ED), nanofiltration (NF), ultrafiltration (UF), and reverse osmosis (RO) [36] have received considerable attention for the treatment of inorganic effluent, since they are capable of removing not only suspended solid and organic compounds, but also inorganic contaminants such as heavy metals [37–39]. Although it can be applied in occasions of low metal ion concentrations, for example, Tzanetakis’s [40] removal efficiency of Co(II) and Ni(II) were 90% and 69%, at initial metal concentrations of 0.84 and 11.72 mg/L, respectively. The main disadvantages of these processes are inadequate selectivity, high energy consumption, and immature technologies, which limit the popularization of this technology.
5.2.5 Ion Exchange
This is a method that uses ion-exchange resin or agent to exchange ions in dilute solution to achieve the purpose of extraction or removal of certain ions in solution. And it is widely used for the recovery and removal of metals from process and waste streams in chemical process industries [41–43].
In general, ion exchange is reportedly effective to treat inorganic effluent within a relatively wide range of metal concentrations from 10 mg/L [44, 45] to 10–100 mg/L [46–48], or even up to higher than 100 mg/L [49, 50]. Furthermore, unlike chemical precipitation, ion exchange does not present any sludge disposal problems [51]. Despite these advantages, it also has some limitations in treating wastewater laden with heavy metals. Suitable ion exchange resins are not available for all heavy metals, and the capital and operational cost is high [52].
5.3 Biological Removal Technology
In recent decades, more and more attention have been paid to biotechnological removal of heavy metal and application of natural biological origin, including bacteria, fungi, yeast, algae, etc. These methods have the advantage over low operating cost, minimization of the pollutant concentration, and high efficiency in detoxifying the dilute effluents.
5.3.1 Heavy Metals Removal by Bacteria
5.3.1.1 Introduction
Novel methods of metal removal and recovery based on biological materials have been considered and widely used. Bacteria are the most abundant and versatile of microorganisms and constitute a significant fraction of the entire living terrestrial biomass of ∼1018 g [53]. In addition, because of their small size, ubiquity, ability to grow under controlled conditions, and resilience to a wide range of environmental situations, bacteria were often used to remove heavy metal ions [54]. Certain types of microbial biomass can retain relatively high quantities of metals on their cell wall due to its microstructure. Bacteria species such as Bacillus, Pseudomonas, Streptomyces, Escherichia, and Micrococcus have been tested for uptake metals or organics. Moreover, many factors such as biomass type and concentration, metal concentration, pH, and biomass–metal contact time affect removal ratios of heavy metals.
Table 5.1 summarizes some of the important results of metal biosorption using bacterial biomasses, according to published references [2, 11]. Metal uptake capacity does not necessarily reach the maximum values in the field application. Some uptake values were experimentally observed, and some were predicted by the Langmuir model. Table 5.1 also provides the basic information to evaluate the possibility of using bacterial biomass for the removal of metal ions.
Large quantities of metals can be accumulated by a variety of processes dependent or independent on metabolism. Both living and dead biomass as well as cellular products such as polysaccharides can be used for this purpose. The basic principle of biosorption lies in biomass which are metabolically inactive [87].
Investigations from Ziagova et al. [75] proved that nonliving cells are advantageous over living counterparts for operating without an auxiliary cultivation system, immunity to toxic wastes around, and low storage requirement. A study [88] demonstrated that living and dead cells of Bacillus sphaericus OT4b31 showed a biosorption of 25% and 44.5% of Cr, respectively, while B. sphaericus IV(4)10 showed a biosorption of 32% and 45%, respectively. Practically biosorption experiments usually use heat-killed cells or bacterial biomass through heat treatment to break cell wall, exposing more functional groups, thus achieving maximum binding capacity [79, 89]. Biosorption is responsible for metal concentration by nonliving biomass owing to the absence of metabolic activity necessary for intracellular metal accumulation [90, 91].
5.3.1.2 Effect of Environmental Factors on Biosorption
Many researchers found that some environmental factors, such as pH, biomass concentration, metal concentration, and biomass-metal contact time, affected biosorption process, result in different removal effects. Further, the kinetics of adsorption has been examined and isotherms models, Langmuir and Freundlich, have been overwhelmingly used to analyze the equilibrium data.
5.3.1.2.1 Effect of pH
For biosorption of heavy metal ions, pH is one of the most important environmental factors. This value of solution strongly influences not only the site dissociation of the biomass surface, but also the solution chemistry of the heavy metals: hydrolysis, complexation by organic and/or inorganic ligands, redox reactions, precipitation, speciation, and biosorption availability of the heavy metals [92]. Generally, at low pH values, several cytomembrane functional groups such as amine, phosphonate, sulfonate, carboxyl, and hydroxyl groups are probably associated with the hydrogen ions, making the overall surface charge on the microorganisms positive [93]. On the contrary, rising pH increases the negative charge at the surface of the cells until all relevant functional groups are deprotonated, which favors electrochemical attraction and adsorption of cations. However, when pH climbs to a certain value, for example, as high as 7.0, precipitation of the metal can occur by the formation of metal hydroxides [94]. The difference in optimum pH values may be attributed to the different components of the cell wall of these species. Reportedly, this optimum is organism dependent, due to different adsorptive sites of microorganisms [95]. For example, Ziagova et al. [75] observed that optimum pH values were estimated to be 1.0 for Staphylococcus xylosus with 36% biosorption yield and between 4.0 and 5.0 for Pseudomonas sp. with 42% biosorption yield in the case of chromium which exists in the solution as anionic group. They revealed that S. xylosus is a Gram positive bacterium with its surface consisting of peptidoglycans, teichoic, and teichuronic acids. These substances provide most of the anionic groups of the cell and are protonated at low pH and facilitate the interaction of chromium. Also, the study of Hancock [96] indicated that the optimum pH value for chromium biosorption was estimated to be 4.0. However, Gialamouidis [87] demonstrated that this value for Mn(II) biosorption was 6.0 for both of Pseudomonas sp. and S. xylosus, which suggested that the adsorption of metals onto the biomass could be ruled by ionic attraction.
5.3.1.2.2 Effect of Biomass Concentration
Most studies reported that high biomass concentrations result in low metal sorption due to electrostatic interactions between cells, which protect binding sites from metal occupation [97]. However, increase of the biomass concentration results in reducing the equilibrium concentration of metal solution owing to the fact that there are more available sites for interaction on biomass. The study of Gialamouidis et al. [87] showed that the biosorption capacity of metal ions is inversely proportional to biomass concentration, when the initial concentration of metal ions is kept constant with Pseudomonas sp. concentrations over 4.0 g/L. Ziagova et al. [75] observed that chromium removal with Pseudomonas sp. does not change significantly at higher than 1.0 g/L biomass concentrations. However, in the case of chromium, there is a significant increase in the metal uptake around 40% by altering biomass concentration of S. xylosus from 1.0 to 8.0 g/L. This may be attributed to the higher number of available binding sites, which interact with chromium ions of the solution, as it has been reported before [98, 99]. At higher than 8.0 g/L biomass concentration, there is no further positive effect on chromium removal, which probably results from limitations in metal ion mobility.
5.3.1.2.3 Effect of Contact Time
The kinetics of metal uptake, assumed to be a passive physical adsorption at the cell surface, is very rapid and occurs in a very short time after the microorganisms have come into contact with metal ions [100]. The adsorption speed of uranium by Citrobacter freudii was very fast in the first 20 min and then slowed down gradually and reached equilibrium after 50 min [101]. In the study of Ziagova et al. [75], the amount of adsorbed Cd(II) reached the highest value, 50%, within the first 30 min with Pseudomonas sp. and 55% after 1.0 h with S. xylosus., respectively. Gialamouidis et al. [87] indicated that the biosorption capacities of Pseudomonas sp. and S. xylosus for Mn2+ increased with contact time rapidly and thereafter it proceeded at a lower rate and finally attained equilibrium within 10 min. This result is important because equilibrium time is one of the parameters for economical wastewater treatment plant application [24]. This behavior suggests that in the initial stage adsorption takes place rapidly on the external surface of the adsorbent followed by a slower internal diffusion process, which may be the rate-determining step.
5.3.1.3 Biosorption Models
5.3.1.3.1 Kinetics Model
In order to examine the mechanism of biosorption process such as mass transfer and chemical reaction, a suitable kinetic model is needed to describe the data. The linear pseudo-first-order equation is given as follows [102]:
where q t and q eq are the amounts of metal ions adsorbed at time t and equilibrium (mg/g), respectively, and k 1 is the rate constant of pseudo-first-order adsorption process (min−1).
The linear pseudo-second-order equation is given [102]:
where k 2 is the equilibrium rate constant of pseudo-second-order biosorption (g/mg min).
These results of Gialamouidis et al. [87] strongly suggest that the biosorption of Mn(II) onto Pseudomonas sp. and S. xylosus cells is most appropriately represented by a pseudo-second-order rate process. In the study of Xie et al. [101], the adsorption kinetics of uranium by C. freudii showed that the pseudo-second-order model fitted the absorption curve much better than the pseudo-first-order model, which indicated the adsorption rate was proportional to the number of unoccupied sites. Song et al. [103] demonstrated that the equilibrium of Au(III) biosorption was achieved within 5 min and the adsorption yield reached 100%, whereas that of Cu(II) was only 66.61% after 30 min in the binary system of Au(III) and Cu(II) by magnetotactic bacteria. They suggested that the first-order equation of Lagergren in most cases does not fit well for the whole range of contact time [104]. The pseudo-second-order equation can be used in this case assuming that the measured concentrations are equal to cell surface concentrations, and it is more likely to predict behavior over the whole range of adsorption and is in agreement with an adsorption mechanism being the rate controlling step. Consequently, they demonstrated that the experimental data were fitted well into the pseudo-second-order kinetic model in the single and binary component situations.
5.3.1.3.2 Isotherm Models
Langmuir and Freundlich isotherm equations were frequently used to describe the equilibrium state for single-ion adsorption experiments. The theoretical basis of Langmuir equation relies on the assumption. That is, there is a finite number of binding sites which are homogeneously distributed over the adsorbent surface of the cells, having the same affinity for adsorption of a single molecular layer and there is no interaction between adsorbed molecules. The mathematical description of the equation is considered.
where \( C_i \) is the metal residual concentration in solution (mg/L); \( {Q_{\max }} \) is the maximum specific uptake corresponding to sites saturation (mg/g), and b is the biomass-metal binding affinity (mg/L) [105].
The Freundlich equation is the empirical relationship whereby it is assumed that the adsorption energy of a metal binding to a site on an adsorbent depends on whether or not the adjacent sites are already occupied. This empirical equation is presented.
where \( K_f \) and n are constants indicating adsorption capacity and adsorption intensity, respectively [106]. The results of some studies about Langmuir and Freundlich isotherm models are presented in Table 5.2.
5.3.1.4 Selective Adsorption in the Case of Multicomponent System
Competitive biosorption takes place in the multicomponent system. Under most conditions, aqueous sources of precious metals contain not only one but also several kinds of metal ions. Although many published reports are available on the single-metal biosorption, limited attention has been paid to the biosorption of multimetal ion system [108]. Summarization of these present literatures concerning biosorption of this more complicated system revealed different biosorptive features from those of single counterparts [103]. To make this reasonable, competition among the different metal ions for the cellular surface binding sites is believed to certainly occur and depend to some extent on the ionic characteristics for certain microbe. The examination of the effects of binary metal ions in various combinations, discussed in this section, is more representative of the actual environmental problems as it has been also reported [109], since real wastewaters are polluted with more than one toxic metals.
Song et al. [103] observed that the maximum adsorption capacity for Au(III) by magnetotactic bacteria in Au-Cu binary system was nearly doubled as that in the single system. The results demonstrated that Cu(II) has a “catalyzing” effect on Au(III) sorption during competitive biosorption process, which also indicates that the MTB cell walls have high selectivity toward Au(III) ions in binary-solute biosorption process.
Besides, Langmuir model and Freundlich model have been also introduced to describe both single and binary system biosorption. The Freundlich and Langmuir constants evaluated from linear regression analysis are listed in Tables 5.3 and 5.4, respectively. Within the metal concentration range of 80–500 mg/L, both models were suitable for describing the adsorption isotherms of Au(III) or Cu(II) ions; that of Au(III) was more fitted by Langmuir model and that of Cu(II) by Freundlich in binary system.
In the study of Wang et al. [108], simultaneous biosorptions of Ag(I) and Cu(II) ions by Magnetospirillum gryphiswaldense (MSR-1) were evaluated using several mediums that contained 2 mmol/L each of the two metal ions. In each medium, the molar ratios of Ag(I) and Cu(II) were different, which were 5:1, 4:1, 3:1, 2:1, 1:1, 1:2, 1:3, 1:4, 1:5. The results were compared with that of single systems, which included the same molar concentration of Ag(I) and Cu(II).
As shown in Fig. 5.1, the biosorption capacities of Ag(I) on MSR-1 in the presence of Cu(II) were higher than that of noncompetitive conditions. Interestingly, when the molar ratios of Ag(I) and Cu(II) were less than 4:1, the biosorption capacities of Cu(II) on MSR-1 were higher than that of noncompetitive cases, whereas those were lower under the molar ratios of Ag(I) to Cu(II) of both 5:1 and 4:1. They deduced that the most possible factor which affected the phenomenon of selective biosorption was the ions themselves.
The results of Ag(I)–Cu(II) binary system adsorption experiments (Reprinted from Ref. [108]. With kind permission of © Elsevier BV (2011))
In addition, they demonstrated that the adsorption isotherm of Ag(I) can also be perfectly expressed by the Langmuir model just as that in the single ion solution. Nevertheless, both models are mathematically unsuitable for describing Cu(II) adsorption isotherms in binary system, with the regression coefficients (R 2) less than 0.95. So, a new mathematically consistent Eq. (5) was selected for accurate description of Cu(II).
The constants in this case evaluated from regression analysis were a = 3.50917, b = 7.38993, k = 0.11474, with a high regression coefficient (R 2) 0.99343. This model, not fit for the single metal system in this study, is under further checking among vast analogous competitive adsorptive systems to find universality before it is introduced to mathematically describe the prohibited one(s). Undoubtedly, it has an important guiding significance for the future research.
The results indicated that biosorption in binary mixtures is a rather complicated adsorption mechanism affected by a lot of factors, including surface charge, functional groups, properties of the solution (e.g., pH), and the adsorbates (e.g., concentration, ionic strength, and weight). In addition, there are many interactions not only between the metal ions but also between the metal ions and the surface of the biomass [75]. If highly selective biosorbents for a certain metal ion were created, great progress would be made in the removal of heavy metal ions.
5.3.1.5 Regeneration of Biosorbents
The entire biosorption process for metal removal include sorption followed by desorption, i.e., to concentrate the solute. Biotechnological exploitation of biosorption technology for removal of heavy metal(s) depends on the efficiency of the regeneration of biosorbent after metal desorption. It is important to reclaim the biosorbents especially, since the biomass preparation is costly. Therefore, nondestructive recovery by mild and cheap desorbing agents is desirable for regeneration of biomass and reuse in multiple cycles. Appropriate eluants are necessary to attain the above-mentioned objective, which strongly depends on the type of biosorbent and the mechanism of biosorption [21]. Also, the eluant must meet the following requirements: (1) Non-damaging to the biomass, (2) less costly, (3) environmental friendly, and (4) effective [11]. Acidic and alkaline condition were used for desorption. The eluants such as CaCl2 with HCl, HCl with EDTA, NaOH were reported [11]. According to the study of Chen et al. [110], approximately 74.8% of the adsorbed Cu was desorbed from nonliving Pseudomonas putida CZ1 by 1.0 M CH3COOK, and more than 80% Zn. Gialamouidis et al. [87] observed that more than 88% of Mn(II) adsorbed by Pseudomonas sp. was released into the HNO3 solution at the first cycle. After three cycles, biosorption ability of Pseudomonas sp. reduced by 77.4%, which may be due to the fact that HNO3 solution modifies the cell wall, while the desorption ability remains to high levels.
The efficiency of desorption is often expressed by the S/L ratio, i.e. solid to liquid ratio. The solid represents the solid sorbent (in mg dry wt) and the liquid represents the amount of eluant applied (in mL). High values of S/L are desirable for complete elution and to make the process more economical. Sometimes, metal-selective elution is desirable and it is dependent on metal sequestration mechanism. Dilute mineral acids, EDTA, carbonates and bicarbonates, NH4OH, KHCO3, and KCN have been used to remove metal(s) from the loaded biomass [111].
To date, less attention has been paid to investigate the regeneration ability of the biosorbent, more relevant work is necessary for future field biosorption application.
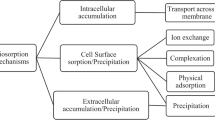
5.3.1.6 Biosorption Mechanism Discussion
One of them is bioaccumulation, based on the incorporation of metals inside the living biomass. Another process is biosorption, in which metallic ions remain at the cellular surface by different mechanisms [11]. Elucidation of mechanisms active in metal biosorption is essential for successful exploitation of the phenomenon and for regeneration of biosorbent materials in multiple reuse cycles. The complex nature of biosorbent materials makes this task particularly challenging [5]. These results show that in binary mixtures, biosorption is a rather complicated adsorption mechanism affected by a lot of factors, including surface charge, functional groups, properties of the solution (e.g., pH), and the adsorbates (e.g., concentration, ionic strength, and weight). In addition, there are many interactions, not only between the metal ions, but also between the metal ions and the surface of the biomass. Velásquez and Dussan [88] suggested that in dead cells metals could have been adhered to surface molecules such as the S-layer which is a porous one that can have saturation velocity. Metallic ions must find a union target and pass through other cellular components before this happens. What is more, in living cells cellular density increases as time elapses; therefore, there are more available binding sites for metals. Additionally, if the metal gets inside the cell, it first needs to join surface molecules and then gets in through different mechanisms. So, S-layer proteins might execute a trapping role of metallic ions in both living and dead cells, being a potential alternative for bioremediation processes of heavy metals in field. Algae, fungi, yeast, and bacteria can remove heavy metals from aqueous solutions by binding the cationic metals onto negatively charged functional groups distributed on their cell walls, such as carboxyl and phosphoryl groups [112, 113]. According to Volesky and Holan [5], who presented an extensive review on biosorption, the strong biosorbent behavior of certain types of microbial biomass toward metallic ions is a function of the chemical makeup of microbial cells. Beveridge [114] reported that bacteria are excellent biosorbents due to their high surface-to-volume ratios and high content of potentially active chemisorptions sites such as teichoic acid in their cell walls. The study of Gialamouidis et al. [87] is very interesting. They calculated thermodynamic parameters such as enthalpy change (ΔHo), entropy change (ΔSo), and free energy change of the sorption (ΔGo), demonstrating that the heats of biosorption of Mn(II) on Pseudomonas sp. and S. xylosus cells were found to be of the same order as the heat of chemisorptions whereas the heat of biosorption on Blakeslea trispora cells is lower and in the range of physical adsorption. Besides, equilibrium between the cell surface and the metal ions is usually rapidly attained and easily reversible, because the energy requirements are limited. Studies of copper or zinc interactions with microorganisms showed that Cu or Zn may be associated with the functional groups on the cellular surface [115–118], intercellular accumulation, and storage via active cationic transport systems [114, 119], or precipitation of Cu sulfides at the biofilm surface [120]. In terms of biosorption, polysaccharides, proteins, and lipids on bacterial cell walls offer many functional groups such as carboxylate, hydroxyl, phosphate, amine, and sulphate groups which can bind metal ions. This natural affinity of biological compounds for metallic elements could contribute to the purification of metal-contaminated wastewater [18]. Vullo et al. [18] thought that metal biosorption depends upon the available surface negative charges. Metal ions biosorption could affect these surface negative charges and thus bacterial electric properties. Electrophoretic mobility measurements are generally done to understand bacterial cell electric properties [121–123], determining Zeta potential values. Moreover, in their study, for concentrations greater than 0.05 mM, no changes are observed in Z potential, which could be explained by the saturation of the cell surface sites available for Cd. Xie et al. [101] observed that the adsorption capacity of uranium by C. freudii increased after the strain was pretreated with 0.5 mol/L NaOH. Proteins, lipids, and some dissolved polysaccharides on the surface of the bacteria could be removed by NaOH. The macromolecular configuration of the cell wall was destroyed and became relaxed, which could improve the diffusion capability and facilitate the entry of metal ions into the cell. In addition, acetyl in testa could be removed by alkali, and amidocyanogen could be exposed to form Louis alkali, which increased the active sites and the adsorption capacity eventually. By sequential pretreatment with NaOH and methanol-thick hydrochloric acid, the carboxyl on the C. freudii walls was locked and the adsorption capacity of uranium decreased by 45.66% compared with raw bacterium strain, which confirmed that carboxyl, as one of the active sites, played an important role in adsorption.
It is necessary to continue searching for the most promising biosorbents from an extremely large pool of readily available and inexpensive biomaterials [8, 9]. The mechanism involved in metal biosorption is far from well understood up to date. It is our mission to investigate the microbe-metal interactions and obtain the mechanism of metal uptake by biosorbents.
Bacteria may either possess the capacity for biosorption of many elements or, alternatively, depending on the species, may be element specific. It is likely that, in the future, microorganisms will be tailored for a specific element or a group of elements, using recombinant DNA technology which is based on genetic modification using endorestrictive nucleases [53].
5.3.2 Heavy Metals Removal by Fungi
5.3.2.1 Introduction
Powered by another mechanism, fungi also reveal the feature of metal capturers. There are three groups of fungi that have major practical importance: molds, yeasts, and mushrooms. Filamentous fungi and yeasts have been investigated for biosorption of heavy metal ions in many instances.
It is known for a long time that metallic ions are important to fungal metabolism. The presence of heavy metals had a significant impact on not only the metabolic activities of fungal cultures, but also the commercial fermentation processes. The results from these studies led to a notion of using fungi for the removal of toxic metals from wastewater and recovery of precious metals from process waters [124]. Fungal cell walls are mainly 80–90% polysaccharide, with proteins, lipids, polyphosphates, and inorganic ions, making up the wall-cementing matrix [21]. It is because of these components that both living and dead fungal cells have an extraordinary ability for taking up toxic and precious metals.
The biosorption of heavy metal by filamentous fungi and yeast have been reported and reviewed in many articles. The fungal organisms are widely used in a variety of large-scale industrial fermentation processes. For example, strains of Aspergillus are used in the production of ferrichrome, kojic acid, gallic acid, itaconic acid, citric acid, and enzymes like amylases, glucose isomerase, pectinase, lipases, and glucanases while Saccharomyces cerevisiae in the food and beverage industries. The use of fungi biomass as an adsorbent for heavy-metal pollution control not only can generate economic benefits by recycling industries wastes, but also reduce the burden of disposal costs associated with the waste biomass produced. Alternatively, the biomass can also be grown using unsophisticated fermentation techniques and inexpensive growth media [125].
This section will review and summarize the removal of heavy metals by yeast (Saccharomyces spp.) and filamentous fungi (such as Penicillium sp., Aspergillus sp., Mucor sp., Rhizopus sp.) from aqueous solutions.
5.3.2.2 Fungal Biosorbents
Research has proved that yeast and filamentous fungi can remove toxic metals, recover precious metals, and clean radionuclides from aqueous solutions to various extents. Yeasts of genera Saccharomyces, Candida, different species of Penicillium, under some circumstances, as well as Aspergillus are efficient biosorbents for heavy metal ions. Table 5.5 summarizes some of the important results of metal biosorption using fungal biomass.
Except for these four kinds of fungal biomass widely covered by the published reports, there are also a lot of other fungi employed for the same purpose.
The cell wall of R. arrhizus involves a high content of chitin. The ability of chitin to complex metal ions has been confirmed [148]. Viable R. arrhizus could remove Cu(II) with the maximum specific uptake capacity of 10.76 mg/g at 75 mg/L, the initial Cu(II) concentration [148]. Of the six species of inactivated fungal mycelia, R. arrhizus, Mucor racemosus, Mycotypha africana, Aspergillus nidulans, A. niger, and Schizosaccharomyces pombe, R. arrhizus exhibited the highest capacity (q max = 213 μmol/g). Further experiments with different cellular fractions of R. arrhizus showed that Zn was predominantly bound to cell-wall chitin and chitosan (q max = 312 μmol/g). R. arrhizus were reported to adsorb Th(IV) [152], Pb(II) [153], Cd(II), Ni(II), and Cr(III) [154]. Brady and Tobin [155] investigated the freeze-dried R. arrhizus biomass for its potential to absorb the hard metal ion Sr2+ and the borderline metal ions Mn2+, Zn2+, Cd2+, Cu2+, and Pb2+ from aqueous solutions.
Biosorption of metal ions such as Li+, Ag+, Pb2+, Cd2+, Ni2+, Zn2+, Cu2+, Sr2+, Fe2+, Fe3+, and Al3+ by Rhizopus nigricans biomass was studied, with the maximum biosorption capacity for the individual metal ions ranging from 160 to 460 μmol/g [156].
The live and dead white rot fungus Trametes versicolor entrapped in Ca-alginate beads were able to adsorb Cd(II). The maximum experimental biosorption capacity was 102.3 ± 3.2 and 120.6 ± 3.8 mg/g, respectively [157].
A white rot fungus species Lentinus sajorcaju biomass was entrapped into alginate gel via a liquid curing method in the presence of Ca(II) ions. The maximum experimental biosorption capacity for entrapped live and dead fungal mycelia of L. sajorcaju were found to be 104.8 ± 2.7 mg/g and 123.5 ± 4.3 mg/g, individually [158].
5.3.2.3 Influencing Factors of Removal of Heavy Metals by Fungi
5.3.2.3.1 Pretreatment
The study of Yunsong Zhang et al. [159] showed that ethanol/caustic pretreatment could increase the biosorption capabilities of Cu2+ on Saccharomyces cerevisiae. In addition, SEM and Zeta potential of the three samples account for that caustic and ethanol-pretreatment resulted in the change of baker’s yeast surface structure and charge which is relative to adsorption. These results demonstrate that the increase of biosorption capacity for Cu2+ by ethanol and caustic-baker’s yeast was attributed to the increase and exposure of carboxyl and amino groups on the surface of biomass sample.
Biosorption of chromium ions was found to vary with the type of pretreatment. All pretreatment methods (acid (0.1N H2SO4); alkali (0.01N NaOH); acetone (50%, v/v); formaldehyde (10%, v/v); cetyl trimethyl ammonium bromide (CTAB) (5%, w/v); polyethylemine (PEI) (1%, w/v); and 3-(2-amino ethyl amino) propyl trimethoxy silane (APTS) (3%, v/v)) increased the biosorption of chromium compared to the autoclaved A. niger biomass [160]. Park et al. [161] also reported approximately 30% removal of chromium using A. niger biomass. The acetone pretreated biomass showed a biosorption capacity of 1.8 mg/g, which was slightly higher compared to autoclaved biomass.
5.3.2.3.2 pH
Solution’s initial pH is a critical parameter for adsorption experiments [162]. This parameter strongly influences the solution chemistry of the metals, the activity of functional groups (carboxylate, phosphate and amino groups) on the cell wall, as well as the competition of metallic ions for the binding site [1]. At low pH (003C1.0), the biosorption capacity for metal ions is very low, because large quantity of hydrogen ions competes with metal ions at sorption sites. As the pH increases, more negatively charged cell surface become available thus facilitating great metal uptake [163]. However, metal precipitates at high pH values (003E7.0) thus inhibiting the contact of metal with the most fungal biomass.
Ting Fan et al. [143] investigated the effects of the initial pH on biosorption of Cd(II), Zn(II), and Pb(II) ions in aqueous solution by Penicillium simplicissimum. Maximum biosorption capacities were obtained at pH 4.0, 6.0, and 5.0 for Cd(II), Zn(II), and Pb(II), respectively.
The biosorption of nickel(II) ions by deactivated protonated yeast was observed with respect to the initial pH by V. Padmavathy et al. [164]. The adsorption capacity was pH dependent with a maximum value of 11.4 mg/g at a pH of 6.75. The cadmium(II) ion adsorption capacity of Baker’s yeast increased with increasing pH, and was maximum at a pH of 6.5 [165].
5.3.2.3.3 Contact Time
It is reported that biosorption of chromium consisted of two phases [161]: a primary rapid phase and a secondary slow phase. The rapid phase lasted for about 15 min and accounted for a major portion in the total metal biosorption. Chromium removal of 72% was observed in this rapid phase. A maximum chromium removal of 76% was achieved after 2 h. From 2 h to the end of the kinetic study (12 h), biosorption was slow and chromium removal varied by ±2%. This rapid initial uptake is consistent with reports on the biosorption of Cr(VI) by other researchers [166–170].
This is because the kinetics of metal adsorption is usually rapid during the early period of contact between the adsorbent and adsorbate possibly by reason of electrostatic attraction. The active adsorption sites of the adsorbent become involved in metal complexation as soon as the adsorbent is introduced into the system. It has been suggested that the slow second phase of metal ions uptake may be due to the reduced availability of active sites [171].
5.3.2.3.4 Initial Metal Ion Concentration
As a rule, increasing the initial metal concentration results in an increase in the biosorption capacity because the initial metal concentration provides a driving force to overcome mass transfer resistances between the biosorbent and biosorption medium so that higher sorption capacities were obtained at higher initial concentrations. The adsorption yields are determined at different initial metal ion concentrations. Increasing the metal ion concentration generally caused a decrease in the biosorption yield. In the case of lower concentrations, the ratio of initial number of metal ions to the available sorption sites was low and higher biosorption yields were obtained. At higher concentrations, the available sites of biosorption became fewer and the saturation of the sorption sites was observed. So biosorption yields decreased.
The effect of initial metal ion concentration on the biosorption capacity of Aspergillus flavus was studied by Tamer Akar and Sibel Tunali [172]. The biosorption of Pb(II) and Cu(II) ions on A. flavus increased with increasing initial concentration of metal ions, becoming saturated at 200 and 150 mg/L for Pb(II) and Cu(II) ions, respectively.
5.3.2.4 Selectivity and Competitive Biosorption by Fungi
Competitive biosorption of Cd2+ and Pb2+ together with Cu2+ ions with ethanol treated yeast cells were conducted for solution containing 25 mg/L of each metal ions at pH 4 and 5 [130]. In their study [130], the highest biosorption capacity of Cu2+ ions was determined with caustic treated waste baker’s yeast cells; but for comparison, ethanol treated yeast biomass was also used for biosorption of Cu2+ ions in competitive biosorption studies. The competitive metal uptake values are shown in Fig. 5.2.
Competitive metal uptake values (Reprinted from Ref. [103]. With kind permission of © Elsevier (2007))
The competitive biosorption capacities of the yeast biomass for most metal ions were lower than non-competitive conditions. The decreased metal uptake in competitive conditions was thought to be a response to increased competition between like charged species for binding sites of the ethanol treated yeast cells. The order of the sorption capacity was found as Pb 003E Cu 003E Cd for both pH values.
P. canescens at competitive or noncompetitive cases exhibited the same preferential order: Pb(II) 003E Cd(II) 003E Hg(II) 003E As(III). Under noncompetitive conditions, the sorption capacity was 26.4 mg/g for As(III), 54.8 mg/g for Hg(II), 102.7 mg/g for Cd(II), and 213.2 mg/g for Pb(II) respectively. The competitive adsorption capacity for the heavy metal ions were 2.0 mg/g for As(III), 5.8 mg/g for Hg(II), 11.7 mg/g for Cd(II), and 32.1 mg/g for Pb(II), when the initial concentration of the metal ions was 50 mg/L [146].
The biosorption capacities of Penicillium simplicissimum in the binary and trinary metal mixture of Cd(II) + Zn(II), Zn(II) + Pb(II), and Cd(II) + Zn(II) + Pb(II) system were lower than noncompetitive conditions that of unitary system of Cd(II), Zn(II), Pb(II), and Cd(II) [143]. The results clearly demonstrated that the combined action of multiple ions was antagonistic and that Cd(II) exerted the inhibitoriest effect on the biosorption of other metals, followed by Pb(II) and Zn(II).
A similar phenomenon had been observed in the binary adsorption Pb(II) and Cu(II) biosorption onto Aspergillus flavus, where it was shown that Pb(II) and Cu(II) strongly competed with each other; the biosorption capacities of the binary metal mixture were lower than that of noncompetitive conditions; and the uptake capacities of Pb(II) ions were smaller influence than Cu(II) ions [172].
All these published experiments illustrated prohibited competitive biosorptive capacities, in comparison with the non-competitive cases, for the biomass to uptake metal ions. The most likely reason for this antagonistic effect is the competition for adsorption sites on the cell surfaces and/or the screening effect by the competing metal ions.
5.3.2.5 Mechanism of Metal Uptake
DAI Shu-juan et al. [173] provided that cadmium can be adsorbed from electroplating wastewater by waste Saccharomyces cerevisiae and accumulated mainly in form of chemical chelating. Electrostatic attraction, hydrogen bonding, and van der Waals force all function in adsorption process. And –NH2–, –C=O–, –C=O–NH–, –CH3, –OH are the main adsorption groups.
Fourier transform, infrared, and X-ray photoelectron spectroscopies were used to discover that carboxyl, amide, and hydroxyl groups on the biomass surface were involved in the sorption of copper and cadmium by Penicillium chrysogenum and ion exchange and complexation dominated the sorption process [174].
The understanding of the mechanism by which microorganisms accumulate metals is crucial to the development of microbial processes for concentration, removal, and recovery of metals from aqueous solution. Metabolism-independent metal binding to the cell walls and external surfaces is the only mechanism present in the case of non-living biomass. Metabolism-independent uptake essentially involves adsorption process such as ionic, chemical, and physical adsorption. A variety of ligands located on the fungal walls are known to be involved in metal chelation. These include carboxyl, amine, hydroxyl, phosphate, and sulfhydryl groups. Metal ions could be absorbed by complexing with negatively charged reaction sites on the cell surface [175, 176]. The relative importance of each functional group is often difficult to resolve [177]. Microbial cell wall is rich in polysaccharide and glycoproteins such as glucans, chitin, mannans, and phospho-mannans. These polymers form abundant source of metal binding ligands. Cell walls of fungi present a multi-laminate architecture where up to 90% of their dry mass consists of amino or non-amino polysaccharides [178]. In general, the fungal cell wall can be regarded as a two-phase system consisting of the chitin skeleton framework embedded in an amorphous polysaccharide matrix [178]. Up to 30% of Aspergillus niger biomass is comprised of an association of chitin and glucan [179]. Chitin and chitosan components of the cell wall are suggested to be important for metal uptake [180, 181].
5.3.2.6 Modeling of Biosorption: Isotherm and Kinetic Models
Analysis of equilibrium data is important for developing an equation that can be used for design purposes. There are several isotherm and kinetic models to describe the sorption phenomena.
5.3.2.6.1 Isotherm Modeling
The biosorption isotherms by biomasses are usually described by the well-known adsorption isotherm models of Freundlich and Langmuir (see Sect. 5.3.1.3).
The parameters of Langmuir and Freundlich isotherm model are presented in Table 5.6. Broadly speaking, most of the data were fit better to Langmuir isotherm model than Freundlich isotherm model.
Except for these two models, Redlich–Peterson and Temkin adsorption isotherms were also used to describe the sorption phenomena. Arzu Y. Dursun [183] used Langmuir, Freundlich, and Redlich–Peterson adsorption models to describe the sorption phenomena of Cu(II) and Pb(II) to dried A. niger. In view of the results, the isotherms appeared to follow the Langmuir model more closely than the other models at all the temperatures studied. However, the Freundlich and Redlich–Peterson adsorption models also seemed to agree well with the experimental data considering that percentage error values were lower than 11.5. Jing-song Wang [184] used Langmuir, Freundlich, and Temkin isotherms models to describe the sorption phenomena of U(VI) to A. fumigatus. Based on the correlation coefficient R 2, the Langmuir isotherm model was the best model to describe the experimental data, but the negative value of qmax indicated that this model is not suitable for this case. Compared with the other two models, it is clear that both the Freundlich and Temkin isotherm models fitted with the experimental data well, and the former model was a better fit than the latter.
5.3.2.6.2 Kinetic Modeling
The biosorption kinetics by biomasses is usually described by first-order rate equation and the second-order rate equation (see Sect. 5.3.1.3).
In most cases, the first-order equation of Lagergren does not fit well over the entire adsorption period and is generally applicable for the initial 30–50 min of the sorption process [185]. Different to the pseudo first-order model, the pseudo-second-order model predicts the behavior over the whole duration of adsorption and is in agreement with the feature of the adsorption mechanism in the rate-controlling step.
Kuber C. Bhainsa et al. [186] investigated the kinetic of biosorption for Th by Aspergillus fumigatus. Data obtained from the kinetic of uptake when modeled with pseudo-second-order equation showed excellent fitting and the kinetic rate constant and R 2 values are 003E0.99. This suggests that the kinetics of Th uptake followed Lagergren’s pseudo-second-order equation. This was also further confirmed by calculating the qe based on pseudo-second-order reaction that showed very good fit between the experimental data and the predicted curve obtained by modeling the uptake value.
Removal of cadmium and zinc ions from aqueous solution by living Aspergillus niger was investigated by LIU Yun-guo et al. [182]. The parameters of pseudo-first and pseudo-second order rate kinetics are presented. The theoretical Q eq values estimated from the first-order kinetic give significantly different values compared with the experimental values, and the correlation coefficients are also found to be slightly lower. The correlation coefficients for the second-order kinetic model and the theoretical values of Q, also agree well with the experimental ones.
Almost all of the kinetic data of sorption by fungi biomass kinetic were more fitted to pseudo-second-order equation.
5.3.3 Heavy Metals Removal by Algae
5.3.3.1 Introduction
Algae, primarily marine microalgae, are of special interest in search for and the development of new biosorbents materials due to their high sorption capacity and their ready availability in practically unlimited quantities in the seas and oceans [187, 188]. Actually a vast array of biological materials, especially bacteria, yeasts, fungi, and algae have received increasing attention for heavy metal removal and recovery due to their good performance, low cost, and large available quantities. In general, the heavy metal uptake capacities varied significantly for different types of biomass studied. For divalent heavy metal ions, the reported values for bacterium biomass typically ranged from 0.05 to 0.2 mmol/g, fungi and yeast 0.2–0.5 mmol/g, fresh water algae 0.5–1.0 mmol/g, and marine algae 1.0–1.5 mmol/g [189].
From the published literature, among the three groups of algae (red, green, brown algae) brown algae received the most attention. Higher uptake capacity has been found for brown algae than for red and green algae [190]. Table 5.7 summarized the results achieved with brown algae, green algae, and red algae. We know that brown algae have since proven to be the most effective and promising substrates among other biosorbents.
The development of new biosorbent materials is a research area full of challenge, as algae biomass is an accessible, economically competitive, and renewable source. Therefore, its potential value in the treatment of heavy metal wastewater is obvious.
5.3.3.2 Influencing Factors on Biosorption
5.3.3.2.1 Effect of pH
This parameter plays an important role for the biosorption process of heavy metal ions from aqueous solution, according to a variety of earlier studies [192–194]. Whereas the calculation from the solubility product equilibrium constant (K sp) [195] demonstrated that the suitable pH ranges for the various metal ions were slightly different, i.e., experiments for Cu2+ sorption was performed for the pH range of 1–6, Cd2+ at pH of 1–8, Zn2+ at pH of 1–7, and Pb2+ at pH of 1–7.5. For the algae biosorption, pH is also a key factor. Algal biomasses contain high content of carboxyl groups from mannuronic and guluronic acids on the cell wall polysaccharides, which suggests that the biosorption process could be affected by changes in the solution pH [196]. Some examples are cited of algae biosorption on different metal ions.
Experimental biosorption on green algae Spirogyra species studied by V. K. Gupta [197, 198] showed that the percent adsorption of Cr(VI) increases with pH from pH 1.0 to 2.0 and thereafter decreases with further increase in pH. And the maximum adsorption at all the concentrations takes place at pH 2.0. As to lead sorption, when the pH of the lead solution (100 and 200 mg/L) increased from 2.99 to 7.04, the adsorption capacity of lead was changed, i.e., it first increased from pH 2.99 to pH 5.0 and then dramatically decreased up to pH 7.04. The results stated strong pH dependence of biosorption. This is consistent with the results obtained for the other adsorbent systems [199, 200].
Additionally, cadmium (II) uptake by C. vulgaris studied by Z. Aksu [185] is also a function of solution pH. The results showed that the biosorption of cadmium (II) increased with pH up to 4.0 and then declined with further increase in pH. And the maximum equilibrium uptake value was found to be 62.3 mg/g at pH 4.0.
Summarizing, almost all the relative publications revealed similar information that a good control of pH is of great significance for metal biosorption by algae.
5.3.3.2.2 Effect of Temperature
The binding of most metals to microorganisms by biosorption observed to enhance as temperature rising [201–203]. Also, increasing temperature is known to stir up the diffusion rate of adsorbate molecules within pores as a result of decreasing solution viscosity and will also modify the equilibrium capacity of the adsorbent for a particular adsorbate [204]. Thus temperature is also an important factor affecting the adsorption.
Research of cadmium (II) and nickel (II) biosorption with C. vulgaris carried out by Z. Aksu [185, 208] manifested that the equilibrium uptake of cadmium (II) and nickel (II) ions to the dried green alga was significantly affected by the temperature. The uptake of cadmium (II) decreased with an increasing temperature, while nickel (II) increased. And their optimum adsorption temperature was 20°C and 45°C, respectively. That is, cadmium (II) adsorption is normally exothermic; thus, the extent of adsorption generally increases with decreasing temperature. This result is typical for biosorption of metals involving no energy-mediated reactions, where metal removal from solution is due to purely physical/chemical interactions between the biomass and metal in solution. Increasing the temperature reduced the biosorption capacity of biomass. Nevertheless, nickel (II) adsorption was endothermic; thus, the extent of adsorption increased with increasing temperature. Besides, the sorption of nickel (II) ions by dried C. vulgaris may involve not only physical but also chemical sorption. So at higher temperatures, an increase in active sites occurs due to bond rupture.
There are many studies that show the information on the effect of temperature in recent years, which are associated with the adsorption mechanism [196, 198, 206].
5.3.3.2.3 Effect of Contact Time
The contact time has also been evaluated as one of the most important factors affecting the biosorption efficiency. The alga Chlorella vulgaris was reported to exhibit the feature of rapid uptake of nickel and then reach the equilibrium in 30–60 min [205]. Also, the study of Sari A. [196] showed the biosorption efficiency of Cd (II) ions by C. virgatum. The biosorption efficiency increases with rising contact time up to 60 min, after which it is almost constant. Another biosorption of lead from aqueous solutions by green algae (Spirogyra sp.) [198] demonstrated that maximum adsorption took place within first 100 min. In fact, the equilibrium time needed for the different metal–biomass systems reportedly range from 1 to 3 h, that is, about 90% of the total metal ion sorption was achieved within 60 min [207].
Overall, reported experiments on contact time reveal, without exception, that the sorption took place in two stages: A very rapid surface adsorption followed by a long period of equilibrium. Adsorption got slowed down in the later stage because initially a large number of vacant surface sites may be available for adsorption and after a certain point of time, the remaining vacant surface sites may be difficult to occupy due to forces between the solute molecules of the solid and bulk phase [208–210]. The diminishing removal with increasing time may also attribute to intraparticle diffusion process dominating over adsorption [211].
5.3.3.2.4 Effect of Biomass Dosage
The amount of biosorbent used for the treatment of heavy metals is also an important parameter which influences the biosorption capacity. The biosorption of Cu(II) in the work of Karthikeyan [212] showed an increased uptake of Cu(II) with the alga quantity which can be accounted for the higher dose of adsorbent in the solution and the greater availability of exchangeable sites for the ions. But the maximum uptake was attained at about 100 mg. And he analyzed that this trend was caused by the formation of biosorbent aggregates at higher biomass concentration, which in turn could reduce the effective surface area available for the biosorption. In fact, many studies [196, 198, 213] showed the similar phenomenon that with increasing biomass dosage, the absorption efficiency experienced an inverted-U curve arousal.
5.3.3.3 Adsorption Mechanism
Metal ion binding during biosorption processes has been found to involve complex mechanisms, such as ion-exchange, complexation, electrostatic attraction, and microprecipitation [5]. In fact, for many years, it had been thought that in the process of biosorption, metal ions bind to highly developed surface of a biosorbent via the mechanism of physical adsorption [214]. Whereas the biosorption of Cr3+, Cd2+, and Cu2+ ions by blue–green algae Spirulina sp. studied by Chojnacka [215] showed that the mechanism of biosorption is rather chemisorptions than physical adsorption (ion-exchange), which was further confirmed by the low surface area associated with physical adsorption and the presence of cations that appeared in the solution after biosorption. The maximum contribution of physical adsorption in the overall biosorption process was evaluated to be 3.7%. Study of the mechanisms of biosorption by Raize [216] manifested that the main cadmium cation sequestration mechanism by the algal biomass was apparently chelation, while that of nickel was mainly ion exchange. Lead cations exhibit higher affinity to the algal biomass, and their binding mechanism includes a combination of ion exchange, chelation, and reduction reactions, accompanied by metallic lead precipitation on the cell wall matrix. The SEM method was used by Han et al. [217] to probe that the surface complexation was the exact mechanism in Cr(III) biosorption by Chlorella miniata. In fact, among various proposed mechanisms, ion exchange was thought to be the most important process for the algal biomass [214]. Ion exchange mechanism was thought to be reversible, and more than 90% of the biosorbed metal could be recovered through acid washing [9].
5.3.3.4 Biosorption Models
5.3.3.4.1 Isotherm Modeling
The equilibrium data, commonly known as adsorption isotherms, are the basic requirements for the design of the adsorption systems. Several isotherm equations, such as Langmuir, Freundlich, Redlich–Peterson, D-R isotherms, and so on, have been used for the equilibrium modeling of biosorption systems. Langmuir and Freundlich isotherms (see Sect. 5.3.1.3) were widely used to quantitatively describe metal sorption by algae [196, 198, 205, 206, 218–220].
Additionally, some authors explain that Langmuir isotherm corresponds to a dominant ion-exchange mechanism while the Freundlich isotherm shows adsorption–complexation reactions taking place in the adsorption process [221, 222]. Furthermore, most of the equilibrium data were fit better to Langmuir isotherm model than Freundlich isotherm model. The parameters of Langmuir and Freundlich isotherm model are presented in Table 5.8 [223].
5.3.3.4.2 Kinetic Models
In order to investigate the mechanism of biosorption and potential rate-controlling step such as mass transport and chemical reaction processes, kinetic models are used to test the experimental data. Numerous models (e.g., Elovich, diffusion, pseudo-first-order, and pseudo-second-order equations) have been suggested to analyze the kinetics of sorption process. Kinetic models based on the capacity of the adsorbent such as the first-order equation and second-order expression were commonly used (see Sect. 5.3.1.3).
In the work of O.M. Freitas [224], experimental data suggested that the pseudo-first-order model successfully describes the kinetics of the biosorption of Zn on L. hyperborea and Cd on B. bifurcate. The results of Aravindhan Rathinam [28] also suggest that kinetics of cadmium biosorption by H. valentiae biomass followed pseudo-first-order kinetic model very well.
The pseudo-second-order kinetic model has been applied successfully to metal biosorption by several algae [185, 198, 200, 206, 225–228]. Additionally, these parameters in equations can change depending on experimental conditions as it was found by P. Lodeiro [225].
The Weber and Morris sorption kinetic model [229], a kind of diffusion model, was employed to investigate the sorption mechanism in the study of Prasert Pavasant [195].
To sum up, kinetic model is an important part of the study of heavy metal biosorption, from which we can get the information of the control steps and mechanism of the process.
5.3.4 Plant Leaves and Their Extraction
5.3.4.1 Introduction
There is a considerable potential for adopting a natural, abundant, and economical metal adsorption system, and plant leaves and their extraction, which are cheap and easily available in a great supply, could be used as an adsorbent for the removal of heavy metals from aqueous solution. In fact, tree leaves and their extraction are frequently employed in heavy metal removal [230–236]. Studies show that adsorption capability also relies on leaves used. Examples of efficient types of plant leaves for removal of metal ions are reed [237] for cadmium, poplar for lead and copper [238, 239], cinchona for copper [239], pine for nickel [240], and cypress for aluminum [241]. Moreover, R. Salim [232] conducted cadmium removal experiments from aqueous solutions by 20 species of plant leaves and their combinations. The results showed that most efficient types of plant leaves for cadmium removal were those of styrax, plum, pomegranate, and walnut.
5.3.4.2 Effects of Environmental Factors on Biosorption
5.3.4.2.1 Effect of pH
The pH of the sorbate solution is considered one of the most important environmental factors affecting the biosorption process. This factor is capable of influencing not only the binding site dissociation state, but also the solution chemistry of the target metal [242]. Successful biosorption of base metal cations usually takes place in the range of pH 3–7, and is extremely pH dependent. Özer A. and Özer D. [243] reported an optimal pH value for lead and nickel uptake of 5.0, while Vianna LNL [244] found that maximal copper, cadmium, and zinc uptakes happened at pH 4.5, and decreased significantly when the pH was dropped to 3.5 or 2.5.
H. Benaissa [231] studied the removal of copper by dried sunflower leaves finding the maximum copper sorption occurs at around initial pH 5–6. A. Sharma and K.G. Bhattacharyya [230] used leaf powder as biosorbent to reclaim Cd (II) obtaining continuous adsorption increases from 8.8% at pH 4.0 to 70.0% at pH 7.0, and 93.6% at pH 9.5. Similarly, R. Salim [232] found that the optimum experimental condition reached at pH 4.1. Rice bran was also used for the removal of Cr (VI) from wastewater [245] and the maximum removal yield was 99.4% also at a low pH of 2.0. Even grape stalks wastes came in handy to absorb copper and nickel ions and I. Villaescusa [246] found a pH-dependent profile for the course. Maximum sorption for both metals was found to occur at around pH 5.5–6.0. Y. Bulut and Z. Baysal [247] used wheat bran as a sorbate on biosorption of Pb (II) and found that the Pb (II) adsorption by wheat bran increases with pH. It can be observed that the removal of Cu (II) and Pb (II) exhibits similar trend, i.e., it increases with increasing pH, climbing to maximum at a certain pH range. Bin Yu and Y. Zhang [248] reported that the maximum removal of Pb (II) by sawdust sorption occurs around pH 5.0, while that of Cu (II) 7.0. Furthermore, the greatest increases in the sorption rate of metal ions on sawdust were observed in a range of pH from 2 to 8 for copper and 2–5 for lead.
5.3.4.2.2 Effect of Temperature
As one of the factors affecting the biosorption efficiency, temperature was studied in many papers [249–253]. S. Qaiser [254] studied the biosorption of lead from aqueous solution by Ficus religiosa leaves and found that the temperature change in the range of 20–40 ◦C affected the biosorption capacity and the maximum removal was observed at 25°C. In M.S. Al-Masri’s [255] study, the influence of temperature on U, Pb, and Cd removal capacities using different parts of poplar trees is different. The maximum U and Cd removal capacity using leaves occurred at a temperature of 25°C while the maximum U, Pb, and Cd removal capacities using branches occurred at a temperature of 35°C.
5.3.4.3 Mechanism Analysis
The mechanism of metal biosorption varies with the metal species and type of biosorbent [217]. To bind and accumulate these pollutants, different previously mentioned mechanisms reportedly also empower the course, solo or ensemble, such as physical adsorption, complexation, ion exchange, and surface microprecipitation [203, 256–258]. For example, S. Qaiser [254] reported the release of Ca, Mg, and Na ions during lead biosorption revealing ion exchange as the suitable major removal mechanism. Javad Zolgharnein [259] in his study of Cr (VI) adsorption onto Elaeagnus tree leaves found chemisorption as the predominant mechanism of this special biosorption.
5.3.5 Summary
In fact, in recent years, applying biotechnology in controlling and removing metal pollution has been paid much attention, and gradually becomes hot topic in the field of metal pollution control because of its potential application. And biosorption is an alternative process, which provides a variety of sorption materials, including bacteria, fungi, yeast, algae, plant leaves, etc. stated above. These biosorbents with relatively high metal-binding capacity and selectivity possess metal-sequestering property and are suitable for the extraction of metal ions from large volumes of water. Therefore it is an ideal candidate for the treatment of high-volume and low concentration complex wastewaters.
5.4 Combination and Improvement of Different Pathways
Commercial application of microbial biomass as a biosorbent may suffer from the problem of how to remove or condense the metal ions-loaded microorganisms from aqueous solution to facilitate reclaiming in the next step. Generally, millipore filtration and evaporation methods have been carried out, but the high cost of membrane and high energy consumption impede them from wide application. Song et al. [103] studied competitive biosorption of Au(III) and Cu(II) ions by magnetotactic bacteria, which may be significant for developing promising biosorbents. Besides, Wang et al. [108] observed that Cu (II) promoted the adsorption of Ag (I) in the competitive biosorption of Ag (I) and Cu (II) on Magnetospirillum gryphiswaldense (MSR-1), which was confirmed magnetotactic bacterium [260], was reported to be sensitive to magnetic field and might be one of the promising options for solving this problem by means of external field. These bacteria were observed to be able to move along the local magnetic field lines, which is a very useful feature to facilitate them to be easily separated from the solutions. This unique finding indicates the high possibility to recover heavy metal ions from wastewater using the method of “MTB biosorption and magnetic separation”, which was simple, effective, and environmentally friendly. However, it is still faced with another problem. For example, Song et al. [261] applied a magnetotactic bacterium, Stenotrophomonas sp., to remove Au(III) from contaminated wastewater. The analyses from FTIR and XRD confirmed that the reduction of Au(III) to Au(0) by the reductants on the MTB biomass occurred, and the deposition of nanocrystal Au(0) particles, ranging from 24.7 to 31.4 nm, could be estimated on the biomass surface. Although easy to remove the MTB-nano Au(0) group by the external magnetic field, it may be a great challenge to separate MTB from nano Au(0), because both nano Au(0) and nanometer-scale magnetosomes own magnetism.
Also, according to combination of abiotic and biotic components, Chen et al. [110] used the bacteria-mineral composite as a geochemically reactive solid and quantified its trace metal ion scavenging ability. In their study, the first quantitative comparison of the metal-binding capacities of P. putida CZ1–goethite composite to its individual components was evaluated. As a consequence, the nonliving cells–goethite composite retained approximately 82% more Zn than that predicted by its solo counterpart.
What is more, some attention was focused on immobilization technology for heavy metal removal biomaterial [262–265]. Entrapment, cellular aggregation, and surface fixation are the most common methods to support this technology [266–270]. The choice of an adequate matrix for cell immobilization affects the process performance, since the metal biosorption efficiency can be affected by these heterogeneous systems [271, 272]. Microorganisms loaded natural and synthetic adsorbents have also been used for separation and preconcentration of heavy metals at trace levels. S. cerevisiae loaded on sepiolite was utilized as biosorbent for copper(II), zinc(II), and cadmium(II) in natural waters [264]. Bag et al. [91] proposed a biosorptive enrichment procedure for Cr (III) and Cr (VI) ions by Saccharomyces cerevisiae-loaded sepiolite. S. cerevisiae and Chlorella vulgaris loaded on silica gel has been used for the separation–preconcentration of Pt2+ and Pd2+ [273]. Vullo et al. [18] made another progress by successfully immobilizing P. veronii 2E on inert surfaces such as Teflon membranes, silicone rubber, and polyurethane foams. In addition, P. veronii 2E was found to be able to grow on all three surfaces and develop a film over the matrix surfaces, form aggregates, and adhere to glass during batch cultures. Removal rate of Pb(II) reaches the summit 97.7% at pH 4, at 200 mg/L of initial Pb(II) concentration with 10 g/L of P. sanguineus beads, prepared by dropping a mixture of 1.5% (w/v) sodium alginate solution and P. sanguineus mycelial mat into a 2% (w/v) CaCl2 solution [274].
Furthermore, the idea about combination of abiotic and biotic components coupled with the immobilization theory and magnetic separation technology. Magnetic particles have been applied as a new sorbent to adsorb metal ions, in which the difficulty of separation was resolved in an external magnetic field [275]. Based on these researches, the biofunctional magnetic bead was synthesized and utilized in the wastewater treatment [276]. Li et al. [276] removed heavy metal in wastewater by bio-functional magnetic beads constituted by the powder of Rhizopus cohnii and Fe3O4 particles coated with alginate and polyvinyl alcohol (PVA). Then magnetic separation technology could make the separation of solid and liquid phase easier. The combined technique of biosorption and magnetic separation holds the advantages of flexibility, eco-friendly characteristics, and economic in operational cost. Thus, the current investigation into this technique is very important and inspiring.
5.5 Comparative Conclusions of the Methods
Every before-mentioned method for heavy metal removal, especially biosorption has actually its advantages and disadvantages for recovering heavy metals. This means in some cases, two or more methods may be chose to treat the target solution to work their way through drastically. Please refer to Table 5.9 for detail.
5.6 Recovery and Reuse of Heavy Metal
Heavy metal ions from the contaminated water could be reduced into metal nanoparticles by physical, chemical, and biological technology [277, 278]. Most of these physical and chemical methods need extreme conditions like temperature, pressure etc. Chemical reducing agents are believed to be associated with environmental toxicity or biological hazards, whereas comparatively safer reluctant like citrate, ascerbate, simple sugars like glucose, fructose etc. are not efficient in productivity. It has been, therefore, of increasing interest to develop efficient green biological reduction [278]. What is more, current chemical methods of metal nanoparticle synthesis have shown limited success and is expected that the use of a biological approach may overcome many of these obstacles. The exploitation of microorganisms for the biosynthesis of metal nanoparticles is an area of research that has received increasing interest over the last decade. The use of living microbes as a tool for nanoparticle biosynthesis has been researched extensively [279]. The size and shape-dependent physicochemical and optoelectronic properties of metal nanoparticles have important applications in catalysis, biosensing, recording media, and optics and so on [280]. For example, gold nanoparticles (Au NPs) have potentially exciting applications in hyperthermia of tumors, optical coatings, and scanning tunneling microscopes as conductive tips [281]. Especially, biological method available for low metal concentration, less than 100 mg/L, could exert the advantage of simple course, easy operation, low cost, little pollution, and high recovery rate of heavy metal etc. [18]. If all of the removal technologies could be applied in to commercial production, not only effluents are purified but also the toxic heavy metal ions are effectively reused to make a green and perfect recycle between the nature and our industrial society. In sum, researches on heavy metal removal give a wide vision and a promising future to development of all the fields.
5.7 Current Hot Topics, Trends, and Outlook of This Field
Nowadays, more researche has been focused on the search for alternative and innovative wastewater treatment techniques. The interesting one was the utilization of biological materials such as algae, fungi, and bacteria for the metal removal and recovery among these techniques [21]. In other studies, biomasses have exhibited economic, rapid adsorption, and eco-friendly characteristics [2]. However, it is still necessary to continue the steps towards the world of these promising biosorbents from an extremely large pool of readily available and inexpensive biomaterials [8, 9]. The mechanism involved in metal biosorption is far from well understood up to date. It is the mission of this field to investigate the microbe-metal interactions and obtain the mechanism of metal uptake by biosorbents and then grasp this tool to change the world.
Also in the field, it is a hot and rising topic to synthesize metallic nanoparticles using biological technology by simultaneously reducing heavy metal ions level in the wastewater. The development of these green techniques for the controlled synthesis of metallic nanoparticles of well-defined size and shape is a big challenge and numerous chemical methods, aimed at controlling the physical properties of the particles, are still in the development stage and problems are often experienced with stability of the nanoparticle preparations, control of the crystal growth, and aggregation of the particles [115, 117, 118]. Although, these researches are still in their infancy of theoretic and experimental phase, the coming fruits are sure to be of excitingly wide perspective and practicability in the near future.
References
Wang JL, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24:427–451. doi:10.1016/j.biotechadv.2006.03.001
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98:2243–2257. doi:10.1016/j.biortech.2005.12.006
Patterson JW, Minear R, Gasca E, Petropoulou C (1998) Industrial discharges of metals to water. In: Allen HE, Garrison AW, Luther GW (eds) Metals in surface waters, 3rd edn. Ann Arbor Press, Michigan
Alexander M (1999) Biodegradation and bioremediation, 2nd edn. Academic, San Diego
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Progress 11:235–250. doi:10.1021/bp00033a001
Chen YS, Sun QJ, Chen J, Zhang YY (1997) Research on technology of biosorption of heavy metals. Adv Environ Sci 5:34–43. doi:10.1021/bp00033a001
Chen M, Gan YR (1999) Biosorption of heavy metal. Chem Ind Eng 16:19–25. doi:cnki:ISSN:1004%979533.0.1999%9701%97004
Kratochvil D, Volesky B (1998) Biosorption of Cu from ferruginous wastewater by algae biomass. Water Res 32:2760–2768. doi:10.1016/S0043-1354(98),%2000015-3
Kratochvil D, Volesky B (1998) Advances in the biosorption of heavy metals. Trends Biotechnol 16:291–300. doi:10.1016/S0167-7799(98),%2001218-9
Volesky B (1990) Biosorption and biosorbents. In: Volesky B (ed) Biosorption of heavy metals. CRC Press, Boca Raton
Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291. doi:10.1016/j.biotechadv.2008.02.002
Gavrilesca M (2004) Removal of heavy metals from the environmental by biosorption. Eng Life Sci 4:219–232. doi:10.1002/elsc.200420026
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330. doi:10.1016/S0043-1354(03),%2000293-8
Volesky B (2001) Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy 59:203–216. doi:10.1016/S0304-386X(00),%2000160-2
Veglio F, Beolchini F (1997) Removal of metals by biosorption: a review. Hydrometallurgy 44:301–316. doi:10.1016/S0304-386X(96)00059-X
Kapoor A, Viraraghavan T (1995) Fungi biosorptiondan alternative treatment option for heavy metal bearing wastewaters: a review. Bioresour Technol 53:195–206. doi:10.1016/0960-8524(95)00072-M
White C, Wilkinson SC, Gadd GM (1995) The role of microorganisms in biosorption of toxic metals and radionuclides. Int Biodeter Biodegr 35:17–40. doi:10.1016/0964-8305(95),%2000036-5
Vullo DL, Ceretti HM, Daniel MA, Ramırez SAM, Zalts A (2008) Cadmium, zinc and copper biosorption mediated by Pseudomonas veronii 2E. Bioresour Technol 99:5574–5581. doi:10.1016/j.biortech.2007.10.060
Tsezos M (2001) Biosorption of metals. The experience accumulated and the outlook for technology development. RefDoc. http://cat.inist.fr/?aModele=afficheN%26cpsidt=898441. Accessed 2001
Aksu Z (1998) Biosorption of heavy metals by micro algae in batch and continuous systems. In: Wong YS, Tam NFY (eds) Wastewater treatment with algae. Springer, Berlin
Wang JL, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226. doi:10.1016/j.biotechadv.2008.11.002
Hamerlinck Y, Mertens DH (1994) In: Vansant EF (ed) Activated carbon principles in separation technology. Elsevier, New York
Dobrowolski R, Stefaniak E (2000) Study of chromium (VI) adsorption from aqueous solution on to activated carbon. Adsorpt Sci Technol 18:97–106. doi:10.1260/0263617001493314
Kadirvelu K, Namasivayam C (2003) Activated carbon from coconut coirpith as metal adsorbent: adsorption of Cd (II) from aqueous solution. Adv Environ Res 7:471–478. doi:10.1016/S1093-0191(02),%2000018-7
Gomez-Serrano V, Macias-Garcia A, Espinosa-Mansilla A, Valenzuela-Calahorro A (1998) Adsorption of mercury, cadmium and lead from aqueous solution on heat-treated and sulphurized activated carbon. Water Res 32:1–4. doi:10.1016/S0043-1354(97),%2000203-0
Benefield LD, Morgan JM (1999) Chemical precipitation. In: Letterman RD (ed) Water quality and treatment. McGraw-Hill, New York
US Environmental Protection Agency (EPA) (2000) Chemical precipitation. US EPA, Washington, DC, EPA832-F-00–018
Tunay O (2003) Developments in the application of chemical technologies to wastewater treatment. Water Sci Technol 48:43–52
Charerntanyarak L (1999) Heavy metals removal by chemical coagulation and precipitation. Water Sci Technol 39:135–138. doi:10.1016/S0273-1223(99),%2000304-2
Juttner K, Galla U, Schmieder H (2000) Electrochemical approaches to environmental problems in the process industry. Electrochim Acta 45:2575–2594. doi:10.1016/S0013-4686(00)00339-X
Yang XJ, Fane AG, Mac Naughton S (2001) Removal and recovery of heavy metals from wastewater by supported liquid membranes. Water Sci Technol 43:341–348
Bose P, Bose MA, Kumar S (2002) Critical evaluation of treatment strategies involving adsorption and chelation for wastewater containing copper, zinc, and cyanide. Adv Environ Res 7:179–195. doi:10.1016/S1093-0191(01),%2000125-3
Wingenfelder U, Hansen C, Furrer G, Schulin R (2005) Removal of heavy metals from mine water by natural zeolites. Environ Sci Technol 39:4606–4613. doi:10.1021/es048482s
Jain A, Sharma VK, Mbuya OS (2009) Removal of arsenic by Fe (VI), Fe (VI)/Fe (III), and Fe (VI)/Al (III) salts: effect of pH and anions. J Hazard Mater 169:339–344. doi:10.1016/j.jhazmat.2009.03.101
Jiang JQ (2007) Research progress in the use of ferrate (VI) for the environmental remediation. J Hazard Mater 146:617–623. doi:10.1016/j.jhazmat.2007.04.075
Kurniawan TA, Chan GYS, Lo WH, Babel S (2006) Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J 118:83–98. doi:10.1016/j.cej.2006.01.015
Ahn KH, Song KG, Cha HY, Yeom IT (1999) Removal of ions in nickel electroplating rinse water using low-pressure nanofiltration. Desalination 122:77–84. doi:10.1016/S0011-9164(99),%2000029-6
Juang RS, Shiau RC (2000) Metal removal from aqueous solutions using chitosan-enhanced membrane filtration. J Membr Sci 165:159–167. doi:10.1016/S0376-7388(99),%2000235-5
Ujang Z, Anderson GK (1996) Application of low-pressure reverse osmosismembranefor Zn2+ and Cu2+ removal from wastewater. Water Sci Technol 34:247–253. doi:10.1016/S0273-1223(96),%2000811-6
Tzanetakis N, Taama WM, Scott K, Jachuck RJJ, Slade RS, Varcoe J (2003) Comparative performance of ion exchange membrane for electrodialysis of nickel and cobalt. Sep Purif Technol 30:113–127. doi:10.1016/S1383-5866(02),%2000139-9
Fernández Y, Maraón E, Castrillón L, Vázquez I (2005) Removal of Cd and Zn from inorganic industrial waste leachate by ion exchange. J Hazard Mater 126:169–175. doi:10.1016/j.jhazmat.2005.06.016
Lee IH, Kuan YC, Chern JM (2006) Factorial experimental design for recovering heavy metals from sludge with ion-exchange resin. J Hazard Mater 138:549–559. doi:10.1016/j.jhazmat.2006.05.090
Juang RS, Kuo HC, Liu FY (2006) Ion exchange recovery of Ni (II) from simulated electroplating waste solutions containing anionic ligands. J Hazard Mater 128:53–59. doi:10.1016/j.jhazmat.2005.07.027
Sapari N, Idris A, Hisham N (1996) Total removal of heavy metal from mixed plating rinse wastewater. Desalination 106:419–422. doi:10.1016/S0011-9164(96),%2000139-7
Gode F, Pehlivan E (2003) A comparative study of two chelating ion exchange resins for the removal of chromium (III) from aqueous solution. J Hazard Mater 100:231–243. doi:10.1016/S0304-3894(03),%2000110-9
lvarez-Ayuso EA, Garcia-Sanchez A, Querol X (2003) Purification of metal electroplating wastewaters using zeolites. Water Res 37:4855–4862. doi:10.1016/j.watres.2003.08.009
Papadopoulos A, Fatta D, Parperis K, Mentzis A, Harambous KJ, Loizidou M (2004) Nickel uptake from a wastewater stream produced in a metal finishing industry by combination of ion-exchange and precipitation methods. Sep Purif Technol 39:181–188. doi:10.1016/j.seppur.2003.10.010
Keane MA (1998) The removal of copper and nickel from aqueous solution using Y zeolite ion exchangers. Colloid Surf A 138:11–20. doi:10.1016/S0927-7757(97),%2000078-2
Ali AA, Bishtawi RE (1997) Removal of lead and nickel ions using zeolite tuff. J Chem Technol Biotechnol 69:27–34. doi:10.1002/(SICI)1097-4660(199705
Lin SH, Kiang CD (2003) Chromic acid recovery from waste acid solution by an ion exchange process: equilibrium and column ion exchange modeling. Chem Eng J 92:193–199. doi:10.1016/S1385-8947(02),%2000140-7
Dobrevsky I, Todorova-Dimova M, Panayotova T (1996) Electroplating rinse wastewater treatment by ion exchange. Desalination 108:277–280. doi:10.1016/S0011-9164(97),%2000036-2
Ahmed S, Chughtai S, Keane MA (1998) The removal of cadmium and lead from aqueous solution by ion exchange with Na–Y zeolite. Sep Purif Technol 13:57–64. doi:10.1016/S1383-5866(97),%2000063-4
Mann H (1990) Removal and recovery of heavy metals by biosorption. In: Volesky B (ed) Biosorption of heavy metals. CRC press, Boca Raton
Urrutia MM (1997) General bacterial sorption processes. In: Wase J, Forster C (eds) Biosorbents for metal ions. CRC Press, London
Tunali S, Cabuk A, Akar T (2006) Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem Eng J 115:203–211. doi:10.1016/j.cej.2005.09.023
Salehizadeh H, Shojaosadati SA (2003) Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res 37:4231–4235. doi:10.1016/S0043-1354(03),%2000418-4
Choi SB, Yun YS (2004) Lead biosorption by waste biomass of Corynebacterium glutamicum generated from lysine fermentation process. Biotechnol Lett 26:331–336. doi:10.1023/B:BILE.0000015453.20708.fc
Lu WB, Shi JJ, Wang CH, Chang JS (2006) Biosorption of lead, copper and cadmium by an indigenous isolate Enterobacter sp J1 possessing high heavy-metal resistance. J Hazard Mater 134:80–86. doi:10.1016/j.jhazmat.2005.10.036
Chang JS, Law R, Chang CC (1997) Biosorption of lead, copper and cadmium by biomass of Pseudomonas aeruginosa PU21. Water Res 31:1651–1658. doi:10.1016/S0043-1354(97),%2000008-0
Lin CC, Lai YT (2006) Adsorption and recovery of lead(II) from aqueous solutions by immobilized Pseudomonas aeruginosa PU21 beads. J Hazard Mater 137:99–105. doi:10.1016/j.jhazmat.2006.02.071
Uslu G, Tanyol M (2006) Equilibrium and thermodynamic parameters of single and binary mixture biosorption of lead(II) and copper(II) ions onto Pseudomonas putida: effect of temperature. J Hazard Mater 135:87–93. doi:10.1016/j.jhazmat.2005.11.029
Pardo R, Herguedas M, Barrado E, Vega M (2003) Biosorption of cadmium, copper, lead and zinc by inactive biomass of Pseudomonas putida. Anal Bioanal Chem 376:26–32. doi:10.1007/s00216-003-1843-z
Selatnia A, Boukazoula A, Kechid N, Bakhti MZ, Chergui A, Kerchich Y (2004) Biosorption of lead (II) from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Biochem Eng J 19:127–135. doi:10.1016/j.bej.2003.12.007
Mameri N, Boudries N, Addour L, Belhocine D, Lounici H, Grib H (1999) Batch zinc biosorption by a bacterial nonliving Streptomyces rimosus biomass. Water Res 33:1347–1354. doi:10.1016/S0043-1354(98),%2000349-2
Incharoensakdi A, Kitjaharn P (2002) Zinc biosorption from aqueous solution by a halotolerant cyanobacterium Aphanothece halophytica. Curr Microbiol 45:261–264. doi:10.1007/s00284-002-3747-0
Chen XC, Wang YP, Lin Q, Shi JY, Wu WX, Chen YX (2005) Biosorption of copper(II) and zinc(II) from aqueous solution by Pseudomonas putida CZ1. Colloids Surf B Biointerfaces 46:101–107. doi:10.1016/j.colsurfb.2005.10.003
Puranik PR, Paknikar KM (1997) Biosorption of lead and zinc from solutions using Streptoverticillium cinnamoneum waste biomass. J Biotechnol 55:113–124. doi:10.1016/S0168-1656(97),%2000067-9
Celaya RJ, Noriega JA, Yeomans JH, Ortega LJ, Ruiz-Manriquez A (2000) A biosorption of Zn(II) by Thiobacillus ferrooxidans. Bioprocess Eng 22:539–542. doi:10.1007/s004499900106
Liu HL, Chen BY, Lan YW, Cheng YC (2004) Biosorption of Zn(II) and Cu(II) by the indigenous Thiobacillus thiooxidans. Chem Eng J 97:195–201. doi:10.1016/S1385-8947(03),%2000210-9
Nakajima A, Yasuda M, Yokoyama H, Ohya-Nishiguchi H, Kamada H (2001) Copper biosorption by chemically treated Micrococcus luteus cells. World J Microbiol Biotechnol 17:343–347. doi:10.1023/A:1016638230043
Savvaidis I, Hughes MN, Poole RK (2003) Copper biosorption by Pseudomonas cepacia and other strains. World J Microbiol Biotechnol 19:117–121. doi:10.1023/A:1023284723636
Beolchini F, Pagnanelli R, Toro L, Veglio F (2006) Ionic strength effect on copper biosorption by Sphaerotilus natans: equilibrium study and dynamic modeling in membrane reactor. Water Res 40:144–152. doi:10.1016/j.watres.2005.10.031
Ozturk A, Artan T, Ayar A (2004) Biosorption of nickel(II) and copper(II) ions from aqueous solution by Streptomyces coelicolor A3(2). Colloids Surf B Biointerfaces 34:105–111. doi:10.1016/j.colsurfb.2003.11.008
Loukidou MX, Karapantsios TD, Zouboulis AI, Matis KA (2004) Diffusion kinetic study of cadmiurn (II) biosorption by Aeromonas caviae. J Chem Technol Biotechnol 79:711–719. doi:10.1002/jctb.1043
Ziagova M, Dimitriadis G, Aslanidou D, Papaioannou X, Tzannetaki EL, Liakopoulou-Kyriakides M (2007) Comparative study of Cd(II) and Cr(VI) biosorption on Staphylococcus xylosus and Pseudomonas sp. in single and binary mixtures. Bioresour Technol 98:2859–2865. doi:10.1016/j.biortech.2006.09.043
Puranik PR, Chabukswar NS, Paknikar KM (1995) Cadmium biosorption by Streptomyce pimprina waste biomass. Appl Microbiol Biotechnol 43:1118–1121. doi:10.1007/BF00166935
Selatnia A, Bakhti MZ, Madani A, Kertous L, Mansouri Y (2004) Biosorption of Cd2+ from aqueous solution by a NaOH-treated bacterial dead Streptomyces rimosus biomass. Hydrometallurgy 75:11–24. doi:10.1016/j.hydromet.2004.06.005
Selatnia A, Boukazoula A, Kechid N, Bakhti MZ, Chergui A (2004) Biosorption of Fe3+ from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Process Biochem 39:1643–1651. doi:10.1016/S0032-9592(03),%2000305-4
Srinath T, Verma T, Ramteke PW, Garg SK (2002) Chromium (VI) biosorptionand bioaccumulation by chromate resistant bacteria. Chemosphere 48:427–435. doi:10.1016/S0045-6535(02),%2000089-9
Nourbakhsh M, Sag Y, Ozer D, Aksu Z, Kutsal T, Caglar A (1994) A comparative study of various biosorbents for removal of chromium(VI) ions from industrial wastewaters. Process Biochem 29:1–5. doi:10.1016/0032-9592(94),%2080052-9
Zhou M, Liu YG, Zeng GM, Li X, Xu WH, Fan T (2007) Kinetic and equilibrium studies of Cr(VI) biosorption by dead Bacillus licheniformis biomass. World J Microbiol Biotechnol 23:43–48. doi:10.1007/s11274-006-9191-8
Sahin Y, Ozturk A (2005) Biosorption of chromium(VI) ions from aqueoussolutionby the bacterium Bacillus thuringiensis. Process Biochem 40:1895–1901. doi:10.1016/j.procbio.2004.07.002
Ozturk A (2007) Removal of nickel from aqueous solution by the bacterium Bacillus thuringiensis. J Hazard Mater 147:518–523. doi:10.1016/j.jhazmat.2007.01.047
Selatnia A, Madani A, Bakhti MZ, Kertous L, Mansouri Y, Yous R (2004) Biosorption of Ni2+ from aqueous solution by a NaOH-treated bacterial dead Streptomyces rimosus biomass. Miner Eng 17:903–911. doi:10.1016/j.mineng.2004.04.002
de Vargas I, Macaskie LE, Guibal E (2004) Biosorption of palladium and platinum by sulfatereducing bacteria. J Chem Technol Biotechnol 79:49–56. doi:10.1002/jctb.928
Nakajima A, Tsuruta T (2004) Competitive biosorption of thorium and uranium by Micrococcus luteus. J Radioanal Nucl Chem 260:13–18. doi:10.1023/B:JRNC.0000027055.16768.1e
Gialamouidis D, Mitrakas M, Liakopoulou-Kyriakides M (2010) Equilibrium, thermodynamic and kinetic studies on biosorption of Mn(II) from aqueous solution by Pseudomonas sp., Staphylococcus xylosus and Blakeslea trispora cells. J Hazard Mater 182:672–680. doi:10.1016/j.jhazmat.2010.06.084
Velasquez L, Dussan J (2009) Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J Hazard Mater 167:713–716. doi:10.1016/j.jhazmat.2009.01.044
Fehrmann C, Pohl P (1993) Cadmium adsorption by the non-living biomass of microalgae grown in axenic mass culture. J Appl Phycol 5:555–562. doi:10.1007/BF02184634
Godlewska-Zyłkiewicz B (2004) Preconcentration and separation procedures for the spectrochemical determination of platinum and palladium. Microchim Acta 147:189–210. doi:10.1007/s00604-004-0234-2
Bag H, Turker AR, Lale M, Tunceli A (2000) Separation and speciation of Cr (III) and Cr (VI) with Saccharomyces cerevisiae immobilized on sepiolite and determination of both species in water by FAAS. Talanta 51:895–902. doi:10.1016/S0039-9140(99),%2000354-9
Esposito A, Pagnanelli F, Veglio F (2002) pH-related equilibria models for biosorption in singlemetal systems. Chem Eng Sci 57:307–313. doi:10.1016/S0009-2509(01),%2000399-2
Duran C, Bulut VN, Gundogdu A, Soylak M, Belduz AO, Beris FS (2009) Biosorption of heavy metals by Anoxybacillus gonensis immobilized on Diaion HP-2MG. Sep Sci Technol 44:335–358. doi:10.1080/01496390802437131
Klimmek S, Stan HJ, Wilke A, Bunke G, Buchholz R (2001) Comparative analysis of the biosorption of cadmium, lead, nickel and zinc by algae. Environ Sci Technol 35:4283–4288. doi:10.1021/es010063x
Aksu Z, Akpinar D (2001) Competitive biosorption of phenol and Cr(VI) from binary mixtures onto dried anaerobic activated sludge. Biochem Eng J 7:183–193. doi:10.1016/S1369-703X(00),%2000126-1
Hancock IC (1986) The use of Gram-positive bacteria for the removal of metals from aqueous solutions. In: Thompson R (ed) Trace metal removal from aqueous solution. Royal Chemistry Society, London
Sekhar KC, Subramanian S, Modak JM, Natarajan KA (1998) Removal of metal ions using an industrial biomass with reference to environmental control. Int J Miner Process 53:107–120. doi:10.1016/S0301-7516(97),%2000061-6
Basci N, Kocadagistan E, Kocadagistan B (2004) Biosorption of copper(II) from aqueous solutions by wheat shells. Desalination 164:135–140. doi:10.1016/S0011-9164(04),%2000172-9
Valdman E, Erijman L, Pessoa FLP, Leite SGF (2001) Continuous biosorption of Cu and Zn by immobilised waste biomass Sargassum sp. Process Biochem 36:869–873. doi:10.1016/S0032-9592(00),%2000288-0
Singh S, Rai BN, Rai LC (2001) Ni(II) and Cr(VI) sorption kinetics by Microcystis in single and multimetallic systems. Process Biochem 36:1205–1213. doi:10.1016/S0032-9592(01),%2000160-1
Xie SB, Yang J, Chen C, Zhang XJ, Wang QL, Zhang C (2008) Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. J Environ Radioact 99:126–133. doi:10.1016/j.jenvrad.2007.07.003
Ozacar M, Sengil IA (2003) Adsorption of reactive dyes on calcined alunite from aqueous solutions. J Hazard Mater 98:211–224. doi:10.1016/S0304-3894(02),%2000358-8
Song HP, Li XG, Sun JS, Yin XH, Wang YH, Wu ZH (2007) Biosorption equilibrium and kinetics of Au(II1) and Cu(I1) on magnetotactic bacteria. Chin J Chem Eng 15:847–854. doi:10.1016/S1004-9541(08),%2060013-0
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. doi:10.1016/S0032-9592(98),%2000112-5
Donmez G, Aksu Z (2002) Removal of chromium(VI) from saline wastewaters by Dunaliella species. Process Biochem 38:751–762. doi:10.1016/S0032-9592(02),%2000204-2
Bayramoglou G, Celik G, Yalcin E, Yilmaz M, Arica MY (2005) Modification of surface properties of Lentinus sajor-caju mycelia by physical and chemical methods: evaluation of their Cr6+ removal efficiencies from aqueous medium. J Hazard Mater 119:219–229. doi:10.1016/j.jhazmat.2004.12.022
Ozdemir G, Ozturk T, Ceyhan N, Isler R, Cosar T (2003) Heavy metal biosorption by biomass of Ochrobactrum anthropi producing exopolysaccharide in activated sludge. Bioresour T10. 10.1016/S0960-8524(03),%2000088-9
Wang YH, Gao H, Sun JS, Li J, Su YX, Ji YL, Gong CM (2011) Selective reinforced competitive biosorption of Ag (I) and Cu (II) on Magnetospirillum gryphiswaldense. Desalination 270:258–263. doi:10.1016/j.desal.2010.11.053
Sag Y, Akcael B, Kutsal T (2003) Application of multicomponent adsorption models to the biosorption of Cr(VI), Cu(II), and Cd(II) ions on Rhizopus arrhizus from ternary metal mixtures. Chem Eng Commun 190:797–812. doi:10.1080/00986440302119
Chen XC, Chen LT, Shi JY, Wu WX, Chen YX (2008) Immobilization of heavy metals by Pseudomonas putida CZ1/goethite composites from solution. Colloids Surf B Biointerfaces 61:170–175. doi:10.1016/j.colsurfb.2007.08.002
Vieira R, Volesky B (2000) Biosorption: a solution to pollution. Int Microbiol 3:17–24
Ehrlich HL (1997) Microbes and metals. Appl Microbiol Biotechnol 48:687–692. doi:10.1007/s002530051116
Gadd GM (1990) Biosorption. Chem Ind 13: 421–426. RefDoc. http://cat.inist.fr/?aModele=afficheN%26cpsidt=19367157. Accessed 1990
Beveridge TJ (1989) Role of cellular design in bacterial metal accumulation and mineralization. Annu Rev Microbiol 43:147–171. doi:10.1146/annurev.mi.43.100189.001051
BeveridgeTJ HMN, Lee H, Leung KT, Poole RK, Savvaidis I, Silver S, Trevors JT (1997) Metal–microbe interactions: contemporary approaches. Adv Microb Physiol 38:177–243. doi:10.1016/S0065-2911(08),%2060158-7
Kretschmer KC, Gardea-Torresdey J, Chianelli R, Webb R (2002) Determination of copper binding in Anabaena flos-aquae purified cell walls and whole cells by X-ray absorption spectroscopy. Microchem J 71:295–304. doi:10.1016/S0026-265X(02)00022-X
YeeN BLG, Phoenix VR, Ferris FG (2004) Characterization of metal-cyanobacteria sorption reactions: a combined macroscopic and infrared spectroscopic investigation. Environ Sci Technol 38:775–782. doi:10.1021/es0346680
Toner B, Manceau A, Marcus MA, Millet DB, Sposito G (2005) Zinc sorption by a bacterial biofilm. Environ Sci Technol 39:8288–8294. doi:10.1021/es050528+
Chen XC, Shi JY, Chen YX, Xu XH, Xu SY, Wang YP (2006) Tolerance and biosorption of copper and zinc by Pseudomonas putida CZ1 isolated from metal-polluted soil. Can J Microbiol 52:308–316
White C, Gadd GM (2000) Copper accumulation by sulfate-reducing bacterial biofilms. FEMS Microbiol Lett 183:313–318. doi:10.1111/j.1574-6968.2000.tb08977.x
Borrok DM, Fein JB (2005) The impact of ionic strength on the adsorption of protons, Pb, Cd and Sr onto surfaces of Gram negative bacteria: testing non-electrostatic diffuse and triple monolayer models. J Colloid Interface Sci 286:110–126. doi:10.1016/j.jcis.2005.01.015
Hayashi H, Seiki H, Tsuneda S, Hirata A, Sasaki H (2003) Influence of growth phase on bacterial cell electrokinetic characteristics examined by soft particle electrophoresis theory. J Colloid Interface Sci 264:565–568. doi:10.1016/S0021-9797(03),%2000418-1
Hetzer A, Daughney CJ, Morgan HW (2006) Cadmium ion biosorption by the thermophilic bacteria Geobacillus stearothermophilus and G. thermocatenulatus. Appl Environ Microbiol 72:4020–4027. doi:10.1128/AEM.00295%9706
Kapoor A, Viraraghavan T (1997) Fungi as biosorbents. In: Wase J, Forster C (eds) Biosorbents for metal ions. CRC Press, London
Kapoor A, Viraraghavan T (1995) Fungal biosorption—an alternative treatment option for heavy metal bearing wastewaters: a review. Bioresour Technol 53:195–206. doi:10.1016/0960-8524(95)00072-M
Cabuk A, Akar T, Tunali S, Gedikli S (2007) Biosorption of Pb(II) by industrial strain of Saccharomyces cerevisiae immobilized on the biomatrix of cone biomass of Pinus nigra: equilibrium and mechanism analysis. Chem Eng J 131:293–300. doi:10.1016/j.cej.2006.12.011
Özer A, Özer D (2003) Comparative study of the biosorption of Pb (II), Ni (II) and Cr (VI) ions onto S. cerevisiae: determination of biosorption heats. J Hazard Mater 100:219–229. doi:10.1016/S0304%973894(03)00109%972
Mapolelo M, Torto N (2004) Trace enrichment of metal ions in aquatic environments by Saccharomyces cerevisiae. Talanta 64:39–47. doi:10.1016/j.talanta.2003.10.058
Al-Saraj M, Abdel-Latif MS, El-Nahal I, Baraka R (1999) Bioaccumulation of some hazardous metals by sol-gel entrapped microorganisms. J Non-Cryst Solids 248:137–140. doi:10.1016/S0022-3093(99),%2000306-3
Goksungur Y, Uren S, Guvenc U (2005) Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour Technol 96:103–109. doi:10.1016/j.biortech.2003.04.002
Bustard M, McHale AP (1998) Biosorption of heavy metals by distillery-derived biomass. Bioprocess Eng 19:351–353. doi:10.1007/s004490050531
Chen C, Wang JL (2006) Review on biosorption of heavy metal by Saccharomyces cerevisiae. China Biotechnol 26:69–76. doi:cnki:ISSN:1671-8135.0.2006-01-014
Dhankhara R, Hoodaa A (2011) Fungal biosorption – an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ Technol 32:467–491. doi:10.1080/09593330.2011.572922
Tsezos M (1997) Biosorption of lanthanides, actinides and related materials in biosorbents for metal ions. In: Wase J, Forster C (eds) Biosorbents for metal ions. CRC Press, London
Baysal Z, Çinar E, Bulut Y, Alkan H, Dogru M (2009) Equilibrium and thermodynamic studies on biosorption of Pb(II) onto Candida albicans biomass. J Hazard Mater 161:62–67. doi:10.1016/j.jhazmat.2008.02.122
Zu YG, Zhao XH, Hu MS, Ren Y, Xiao P, Zhu L, Cao YJ, Zhang Y (2006) Biosorption effects of copper ions on Candida utilis under negative pressure cavitation. J Environ Sci 18:1254–1259. doi:10.1016/S1001-0742(06),%2060071-5
Yin H, He BY, Lu XY, Peng H, Ye JS, Yang F (2008) Improvement of chromium biosorption by UV–HNO2 cooperative mutagenesis in Candida utilis. Water Res 42:3981–3989. doi:10.1016/j.watres.2008.07.005
Akhtar K, Akhtar MW, Khalid AM (2008) Removal and recovery of zirconium from its aqueous solution by Candida tropicalis. J Hazard Mater 156:108–117. doi:10.1016/j.jhazmat.2007.12.002
Muter O, Lubinya I, Millers D, Grigorjeva L, Ventinya E, Rapoport A (2002) Cr(VI) sorption by intact and dehydrated Candida utilis cells in the presence of other metals. Process Biochem 38:123–131. doi:10.1016/S0032-9592(02),%2000065-1
Deng SB, Ting YP (2005) Characterization of PEI-modified biomass and biosorption of Cu(II), Pb(II) and Ni(II). Water Res 39:2167–2177. doi:10.1016/j.watres.2005.03.033
Holan ZR, Volesky B (1995) Accumulation of cadmium, lead, and nickel by fungal and wood biosorbents. Appl Biochem Biotechnol 53:133–146. doi:10.1007/BF02788603
Tan TW, Hu B, Su HJ (2004) Adsorption of Ni2+ on amine-modified mycelium of Penicillium chrysogenum. Enzyme Microb Technol 35:508–513. doi:10.1016/j.enzmictec.2004.08.035
Fan T, Liu YG, Feng BY, Zeng GM, Yang CP, Zhou M, Zhou HZ, Tan ZF, Wang X (2008) Biosorption of cadmium (II), zinc (II) and lead (II) by Penicillium simplicissimum: isotherms, kinetics and thermodynamics. J Hazard Mater 160:655–661. doi:10.1016/j.jhazmat.2008.03.038
Li XM, Liao DX, Xu XQ, Yang Q, Zeng GM, Zheng W, Guo L (2008) Kinetic studies for the biosorption of lead and copper ions by Penicillium simplicissimum immobilized within loofa sponge. J Hazard Mater 159:610–615. doi:10.1016/j.jhazmat.2008.02.068
Say R, Yılmaz N, Denizli A (2003) Biosorption of cadmium, lead, mercury, and arsenic ions by the fungus Penicillium purpurogenum. Sep Sci Technol 38:2039–2053. doi:10.1081/SS-120020133
Say R, Yilmaz N, Denizli A (2003) Removal of heavy metal ions using the fungus Penicillium canescens. Adsorpt Sci Technol 21:643–650. doi:10.1260/026361703772776420
Shah MP, Vora SB, Dave SR (1999) Evaluation of potential use of immobilized Penicillium griseofulvum in bioremoval of copper. Process Metall 9:227–235. doi:10.1016/S1572-4409(99),%2080112-6
Dursun AY, Uslu G, Tepea O, Cuci Y, Ekiz HI (2003) A comparative investigation on the bioaccumulation of heavy metal ions by growing Rhizopus arrhizus and Aspergillus niger. Biochem Eng J 15:87–92. doi:10.1016/S1369-703X(02),%2000187-0
Kapoor A, Viraraghavan T, Cullimore DR (1999) Removal of heavy metals using the fungus Aspergillus niger. Bioresour Technol 70:95–104. doi:10.1016/S0960-8524(98),%2000192-8
Gulati R, Saxena RK, Gupta R (2002) Fermentation waste of Aspergillus terreus: a potential copper biosorbent. World J Microbiol Biotechnol 18:397–401. doi:10.1023/A:1015540921432
Dias MA, Lacerda ICA, Pimentel PF, de Castro HF, Rosa CA (2002) Removal of heavy metals by an Aspergillus terreus strain immobilized in a polyurethane matrix. Lett Appl Microbiol 34:46–50. doi:10.1046/j.1472-765x.2002.01040.x
Gadd GM, White C (1992) Removal of thorium from simulated acid process streams by fungal biomass—potential for thorium desorption and reuse of biomass and desorbent. J Chem Technol Biotechnol 55:39–44. doi:10.1002/jctb.280550107
Naja G, Mustin C, Berthelin J, Volesky B (2005) Lead biosorption study with Rhizopus arrhizus using a metal-based titration technique. J Colloid Interface Sci 292:537–543. doi:10.1016/j.jcis.2005.05.098
Bhattacharyya S, Pal TK, Basumajumdar A, Banik AK (2002) Biosorption of heavy metals by Rhizopus arrhizus and Aspergillus niger. J Indian Chem Soc 79:747–750
Brady JM, Tobin JM (1995) Binding of hard and soft metal ions to Rhizopus arrhizus biomass. Enzyme Microb Technol 17:791–796. doi:10.1016/0141-0229(95)00142-R
Kogej A, Pavko A (2001) Comparison of Rhizopus nigricans in a pelleted growth form with some other types of waste microbial biomass as biosorbents for metal ions. World J Microbiol Biotechnol 17:677–685. doi:10.1023/A:1012901224684
Arica MY, Kacar Y, Genc O (2001) Entrapment of white-rot fungus Trametes versicolor in Caalginate beads: preparation and biosorption kinetic analysis for cadmium removal from an aqueous solution. Bioresour Technol 80:121–129. doi:10.1016/S0960-8524(01),%2000084-0
Bayramoglu G, Denizli A, Bektas S, Arica MY (2002) Entrapment of Lentinus sajorcaju into Caalginate gel beads for removal of Cd (II) ions from aqueous solution: preparation and biosorption kinetics analysis. Microchem J 72:63–76. doi:10.1016/S0026-265X(01),%2000151-5
Zhang YS, Liu WG, Xu M, Zheng F, Zhao MJ (2010) Study of the mechanisms of Cu2+ biosorption by ethanol/caustic-pretreated baker’s yeast biomass. J Hazard Mater 178:1085–1093. doi:10.1016/j.jhazmat.2010.02.051
Mungasavalli DP, Viraraghavan T, Jin YC (2007) Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: batch and column studies. Colloids Surf A Physicochem Eng Asp 301:214–223. doi:10.1016/j.colsurfa.2006.12.060
Park D, Yun YS, Park JM (2005) Use of dead fungal biomass for the detoxification of hexavalent chromium: screening and kinetics. Process Biochem 40:59–2565. doi:10.1016/j.procbio.2004.12.002
Saygideger S, Gulnaz O, Istifli ES, Yucel N (2005) Adsorption of Cd (II), Cu (II) and Ni (II) ions by Lemna minor L.: effect of physicochemical environment. J Hazard Mater 126:96–104. doi:10.1016/j.jhazmat.2005.06.012
Ahuja P, Gupta R, Saxena RK (1999) Zn2+ biosorption by Oscillatroria anguistissima. Process Biochem 34:77–85. doi:10.1016/S0032-9592(98),%2000072-7
Padmavathy V, Vasudevan P, Dhingra SC (2003) Biosorption of nickel(II) ions on baker’s yeast. Process Biochem 38:1389–1395. doi:10.1016/S0032-9592(02),%2000168-1
Vasudevan P, Padmavathy V, Dhingra SC (2003) Kinetics of biosorption of cadmium on baker’s yeast. Bioresour Technol 89:281–287. doi:10.1016/S0960-8524(03),%2000067-1
Tewari N, Vasudevan P, Guha BK (2005) Study of biosorption of Cr(VI) by Mucor hiemalis. Biochem Eng J 23:185–192. doi:10.1016/j.bej.2005.01.011
Tunali S, Kiran I, Akar T (2005) Chromium(VI) biosorption characteristics of Neurospora crassa fungal biomass. Miner Eng 18:681–689. doi:10.1016/j.mineng.2004.11.002
Prakasham RS, Merrie JS, Sheela R, Saswathi N, Ramakrishna SV (1999) Biosorption of chromium VI by free and immobilized Rhizopus arrhizus. Environ Pollut 104:421–427. doi:10.1016/S0269-7491(98),%2000174-2
Bai RS, Abraham TE (2001) Biosorption of Cr (VI) from aqueous solution by Rhizopus nigrican. Bioresour Technol 79:73–81. doi:10.1016/S0960-8524(00),%2000107-3
Sag Y, Aktay Y (2002) Kinetic studies on sorption of Cr(VI) and Cu(II) ions by chitin, chitosan and Rhizopus arrhizus. Biochem Eng J 12:143–153. doi:10.1016/S1369-703X(02),%2000068-2
Sag Y, Aktay Y (2000) Mass transfer and equilibrium studies for the sorption of chromium ions onto chitin. Process Biochem 36:157–173. doi:10.1016/S0032-9592(00),%2000200-4
Akar T, Tunali S (2006) Biosorption characteristics of Aspergillus flavus biomass for removal of Pb(II) and Cu(II) ions from an aqueous solution. Bioresour Technol 97:1780–1787. doi:10.1016/j.biortech.2005.09.009
Dai SH, Wei DZ, Zhou DQ, Jia CY, Wang YG, Liu WG (2008) Removing cadmium from electroplating wastewater by waste Saccharomyces cerevisiae. Trans Nonferr Metal Soc China 18:1008–10013. doi:10.1016/S1003-6326(08),%2060173-9
Deng SB, Ting YP (2005) Fungal biomass with grafted poly(acrylic acid) for enhancement of Cu(II) and Cd(II) biosorption. Langmuir 21:5940–5948. doi:10.1021/la047349a
Beveridge TJ, Murray RGE (1980) Sites of metal deposition in the cell walls of Bacillus subtilis. J Bacteriol 141:876–887
Gupta R, Ahuja P, Khan S, Saxena RK, Mohapatra M (2000) Microbial biosorbents: meetings challenges of heavy metals pollution in aqueous solution. Curr Sci 78:967–973. doi:10.1517/13543784.13.4.373
Strandberg GW, Shumate SE, Parrott JR (1981) Microbial cells as biosorbents for heavy metals: accumulation of uranium by Saccharomyces cerevisiae and Pseudomonas aeruginosa. Appl Environ Microbiol 41:237–245, PMCID:PMC243671
Farkas V (1980) Biosynthesis of cell wall of fungi. Microbiol Rev 44:117–141, PMCID: PMC281469
Muzzarelli RA, Tanfari F (1982) The chelating ability of chitinous material from Aspergillus niger, Streptomyces, Mucor rouxii, Phycomyces blakeseanus and Choamephora curcurnitium. In: Mirano S, Tokura S (eds) Chitin and Chitiosan. Japanese Society of Chitin and Chitiosan, Sapporo
Tsezos M, Volesky B (1981) Biosorption of uranium and thorium. Biotechnol Bioeng 23:583–604. doi:10.1002/bit.260230309
Fourest E, Roux JC (1992) Heavy metal biosorption by fungal mycelial by-products: mechanism and influence of pH. Appl Microbiol Biotechnol 37:399–403. doi:10.1007/BF00211001
Liu YG, Fan T, Zeng GM, Li X (2006) Removal of cadmium and zinc ions from aqueous solution by living Aspergillus niger. Trans Nonferr Metal Soc China 16:681–686. doi:10.1016/S1003-6326(06),%2060121-0
Dursun AY (2006) A comparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper(II) and lead(II) ions onto pretreated Aspergillus niger. Biochem Eng J 28:187–195. doi:10.1016/j.bej.2005.11.003
Wang JS, Hu XJ, Xie SB, Bao ZL (2010) Biosorption of uranium (VI) by immobilized Aspergillus fumigatus beads. J Environ Radioact 101:504–508, 10.1016/j.jenvrad.2010.03.002
Aksu Z (2001) Equilibrium and kinetic modelling of cadmium (II) biosorption by C. Vulgaris in a batch system: effect of temperature. Sep Purif Technol 21:285–294. doi:10.1016/S1383-5866(00),%2000212-4
Bhainsa KC, D’Souza SF (2009) Thorium biosorption by Aspergillus fumigatus, a filamentous fungal biomass. J Hazard Mater 165:670–676. doi:10.1016/j.jhazmat.2008.10.033
Kuyicak N, Volesky B (1990) Biosorption by fungal biomass. In: Volesky B (ed) Biosorption of heavy metals. CRC press, Boca Raton
Rincon J, Gonzalez F, Ballester A, Blazquez ML, Munoz JA (2005) Biosorption of heavy metals by chemically-activated alga Fucus vesiculosus. J Chem Technol Biotechnol 80:1403–1407. doi:10.1002/jctb.1342
Yu Q, Matheickal JT, Yin P (1999) Heavy metal uptake capacities of common marine macro algal biomass. Water Res 33:1534–1537. doi:10.1016/S0043-1354(98),%2000363-7
Brinza L, Dring MJ, Gavrilescu M (2007) Marine micro- and macro-algal species as biosorbents for heavy metals. Environ Eng Manag J 6:237–251
Romera E, Gonzalez F, Ballester A, Blazquez ML, Munoz JA (2006) Biosorption with algae: a statistical review. Crit Rev Biotechnol 26:223–235. doi:10.1080/07388550600972153
Matheickal JT, Iyengar L, Venkobachar C (1991) Sorption and desorption of Cu (II) by Ganoderma lucidum. Water Qual Res J Can 26:187–200
Fourest E, Canal C, Roux JC (1994) Improvement of heavy metal biosorption by mycelial dead biomasses (Rhizopus arrhizus, Muchor miehei, and Pencillium chrysogenum): pH control and cationic activation. FEMS Microbiol Rev 14:325–332. doi:10.1016/0168-6445(94),%2090050-7
Matheickal JT, Yu Q (1996) Biosorption of lead from aqueous solutions by marine alga Ecklonia radiate. Water Sci Technol 34:1–7. doi:10.1016/S0273-1223(96),%2000780-9
Pavasant P, Apiratikul R, Sungkhum V, Suthiparinyanont P, Wattanachira S, Marhaba TF (2006) Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour Technol 97:2321–2329. doi:10.1016/j.biortech.2005.10.032
Sari A, Tuzen M (2008) Biosorption of cadmium (II) from aqueous solution by red algae (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies. J Hazard Mater 157:448–454. doi:10.1016/j.jhazmat.2008.01.008
Gupta VK, Shrivastava AK, Jain N (2001) Biosorption of chromium (VI) from aqueous solutions by green algae Spirogyra species. Water Res 35:4079–4085. doi:10.1016/S0043-1354(01),%2000138-5
Gupta VK, Rastogi A (2008) Biosorption of lead from aqueous solutions by green algae Spirogyra species: kinetics and equilibrium studies. J Hazard Mater 152:407–414. doi:10.1016/j.jhazmat.2007.07.028
Tuzun I, Bayramoglu G, Alcin YE, Basaran G, Celik G, Arica MY (2005) Equilibrium and kinetic studies on biosorption of Hg(II), Cd(II) and Pb(II)ions onto microalgae Chlamydomonas reinhardtii. J Environ Manage 77:85–92. doi:10.1016/j.jenvman.2005.01.028
Deng LP, Su YY, Su H, Wang XX, Zhu XB (2007) Sorption and desorption of lead(II) from wastewater by green algae Cladophora fascicularis. J Hazard Mater 143:220–225. doi:10.1016/j.jhazmat.2006.09.009
Ajmal M, Rao RAK, Ahmad R, Ahmad J (2000) Adsorption studies on Citrus reticulata (fruit peel of orange): removal and recovery of Ni (II) from electroplating wastewater. J Hazard Mater B 79:117–131. doi:10.1016/S0304-3894(00)00234-X
Aksu Z, Kutsal T (1990) A comparative study for biosorption characteristics of heavy metal ions with C. vulgaris. Environ Technol 11:979–987. doi:10.1080/09593339009384950
Donmez GC, Aksu Z, Ozturk A, Kutsal T (1999) A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem 34:885–892. doi:10.1016/S0032-9592(99),%2000005-9
Khezami L, Capart R (2005) Removal of chromium (VI) from aqueous solution by activated carbons: kinetic and equilibrium studies. J Hazard Mater 123:223–231. doi:10.1016/j.jhazmat.2005.04.012
Aksu Z (2002) Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel (II) ions onto Chlorella vulgaris. Process Biochem 38:89–99. doi:10.1016/S0032-9592(02),%2000051-1
Pahlavanzadeh H, Keshtkar AR, Safdari J, Abadi Z (2010) Biosorption of nickel (II) from aqueous solution by brown algae: equilibrium, dynamic and thermodynamic studies. J Hazard Mater 175:304–310. doi:10.1016/j.jhazmat.2009.10.004
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci 275:131–141. doi:10.1016/j.jcis.2004.01.036
Bishnoi NR, Pant A, Garima (2004) Biosorption of copper from aqueous solution using algal biomass. J Sci Ind Res 63:813–816, ISSN 0022–4456
Chand S, Agarwal VK, Kumar P (1994) Removal of hexavalent Cr from wastewater by adsorption. Indian J Environ Health 36:151–158, ISSN 0367–827X
Saravanane R, Sundararajan T, Sivamurthyreddy S (2002) Efficiency of chemically modified low cost adsorbents for the removal of heavy metals from wastewater: a comparative study. Indian J Environ Health 44:78–87
Deo N, Ali M (1992) Optimization of a new low cost adsorbent in removal of Cr(VI) from wastewater. Indian J Environ Protect 12:828–834
Karthikeyan S, Balasubramanian R, Iyer CSP (2007) Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresour Technol 98:452–455. doi:10.1016/j.biortech.2006.01.010
Aravindhan R, Maharshi B, Sreeram KJ (2010) Biosorption of cadmium metal ion from simulated wastewaters using Hypnea valentiae biomass: a kinetic and thermodynamic study. Bioresour Technol 101:1466–1470. doi:10.1016/j.biortech.2009.08.008
Crist DR, Crist RH, Martin JR, Watson JR (1994) Ion exchange systems in proton–metal reaction with algal cell walls. FEMS Microbiol Rev 14:309–314. doi:10.1111/j.1574-6976.1994.tb00104.x
Chojnacka K, Chojnacki A, Gorecka H (2005) Biosorption of Cr3+, Cd2+and Cu2+ ions by blue–green algae Spirulina sp.: kinetics, equilibrium and the mechanism of the process. Chemosphere 59:75–84. doi:10.1016/j.chemosphere.2004.10.005
Raize O, Argaman Y, Yannail S (2004) Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol Bioeng 87:451–458. doi:10.1002/bit.20136
Han X, Wong YS, Tam NFY (2006) Surface complexation mechanism and modeling in Cr (III) biosorption by a microalgal isolate, Chlorella miniata. J Colloid Interface Sci 303:365–371. doi:10.1016/j.jcis.2006.08.028
Kalyani S, Rao PS (2004) Removal of nickel (II) from aqueous solutions using marine macroalgae as the sorbing biomass. Chemosphere 57:1225–1229. doi:10.1016/j.chemosphere.2004.08.057
Ajjabiv LC, Chouba L (2009) Biosorption of Cu2+ and Zn2+ from aqueous solutions by dried marine green macroalga Chaetomorpha linum. J Environ Manage 90:3485–3489. doi:10.1016/j.jenvman.2009.06.001
Sahmurova A, Türkmenler H (2010) Biosorption kinetics and isotherm studies of Cd(II) by dried Enteromorpha compress macroalgae cells from aqueous solutions. Clean Soil Air Water 38:936–941. doi:10.1002/clen.201000108
Murugesan GS, Sathishirkumar M, Suaminathan K (2006) Arsenic removal from groundwater by pretreated waste tea fungal biomass. Bioresour Technol 97:483–487. doi:10.1016/j.biortech.2005.03.008
Fiol N, Villascusa I, Martinez M, Mirralles N, Poch J, Seralos J (2006) Sorpton of Pb (II), Ni (II), Cu (II) and Cd (II) from aqueous solutions by olive stone waste. Sep Purif Technol 50:132–140. doi:10.1016/j.seppur.2005.11.016
Singh A, Mehta SK, Gaur JP (2007) Removal of heavy metals from aqueous solution by common freshwater filamentous algae. World J Microbiol Biotechnol 23:1115–1120. doi:10.1007/s11274-006-9341-z
Freitas OM, Martins RJE, Delerue-Matos CM, Boaventura RAR (2008) Removal of Cd (II), Zn (II) and Pb (II) from aqueous solutions by brown marine macro algae: kinetic modeling. J Hazard Mater 153:493–501. doi:10.1016/j.jhazmat.2007.08.081
Lodeiro P, Cordero B, Grille Z, Herrero R, Sastre de Vicente ME (2004) Physicochemical studies of cadmium (II) biosorption by the invasive alga in Europe, Sargassum muticum. Biotechnol Bioeng 88:237–247. doi:10.1002/bit.20229
Cruz CCV, da Costa ACA, Henriques CA, Luna AS (2004) Kinetic modeling and equilibrium studies during cadmium biosorption by dead Sargassum sp. biomass. Bioresour Technol 91:249–257. doi:10.1016/S0960%978524(03)00194%979
Martins BL, Cruz CCV, Luna AS, Henriques CA (2006) Sorption and desorption of Pb2+ ions by dead Sargassum sp. biomass. Biochem Eng J 27:310–314. doi:10.1016/j.bej.2005.08.007
Kumar YP, King P, Prasad VSRK (2006) Removal of copper from aqueous solution using Ulva fasciata sp., a marine green algae. J Hazard Mater 137:367–373. doi:10.1016/j.jhazmat.2006.02.010
Weber WJ, Morris JC (1962) Advance in water pollution research: removal of biological resistant pollutions from wastewater by adsorption. In: Proceedings of the international conference on water pollution symposium. Pergamon Press, Oxford
Sharma A, Bhattacharyya KG (2005) Azadirachta indica (neem) leaf power as a biosorbent for removal of Cd (II) from aqueous medium. J Hazard Mater B 125:102–112. doi:10.1016/j.jhazmat.2005.05.012
Benaissa H, Elouchdi MA (2007) Removal of copper ions from aqueous solutions by dried sunflower leaves. Chem Eng Process 46:614–622. doi:10.1016/j.cep.%202006.08.006
Salim R, Al-Subu M (2008) Efficiency of removal of cadmium from aqueous solutions by plant leaves and the effects of interaction of combinations of leaves on their removal efficiency. J Environ Manage 87:521–532. doi:10.1016/j.jenvman.2007.01.028
Al Rmalli SW, Dahmani AA, Abuein MM, Gleza AA (2008) Biosorption of mercury from aqueous solutions by powdered leaves of castor tree (Ricinus communis L.). J Hazard Mater 152:955–959. doi:10.1016/j.jhazmat.2007.07.111
Sangi M, Shahmoradi A, Zolgharnein J, Azimi GH, Ghorbandoost M (2008) Removal and recovery of heavy metals from aqueous solution using Ulmus carpinifolia and Fraxinus excelsior tree leaves. J Hazard Mater 155:513–522. doi:10.1016/j.jhazmat.2007.11.110
Zolgharnein J, Shamoradi A, Sangi MR (2008) Optimization of Pb (II) biosorption by Robinia tree leaves using statistical design of experiments. Talanta 76:528–532. doi:10.1016/j.talanta.2008.03.039
Zolgharnein J, Shahmoradi A (2010) Characterization of sorption isotherms, kinetic models, and multivariate approach for optimization of Hg (II) adsorption onto Fraxinus tree leaves. J Chem Eng Data 55:5040–5049. doi:10.1021/je1006218
Sayrafi O, Salim R, Sayrafi SA (1996) Removal of cadmium from polluted water using decaying leaves: effects of type of leaves and of concentration of cadmium. J Environ Sci Health A 31:2503–2513. doi:10.1080/10934529609376506
Salim R, Al-Subu M, Qashoa S (1994) Removal of lead from polluted water using decaying plant leaves. J Environ Sci Health A 29:2087–2114. doi:10.1080/10934529409376166
Al-Subu M, Salim R, Abu-Shqair I, Swaileh K (2001) Removal of dissolved copper from polluted water using plant leaves: effects of acidity and plant species. Rev Int Contam Ambient 17:91–96
Salim R (1988) Removal of nickel (II) from polluted water using decaying leaves-effects of pH and type of leaves. J Environ Sci Health A 23:183–197, 321–334. doi: 10.1080/10934528809375403
Salim R, Robinson JW (1985) Removal of dissolved aluminum released by acid rain using decaying leaves. J Environ Sci Health A 20:701–734. doi:10.1080/10934528509375253
Fiol N, Villaescusa I, Martínez M, Miralles N, Poch J, Serarols J (2006) Sorption of Pb (II), Ni (II), Cu (II) and Cd (II) from aqueous solution by olive stone waste. Sep Purif Technol 50:132–140. doi:10.1016/j.seppur.2005.11.016
Özer A, Özer D (2003) Comparative study of the biosorption of Pb (II), Ni (II) and Cr (VI) ion onto S. cerevisiae: determination of biosorption heats. J Hazard Mater B 100:219–228. doi:10.1016/S0304%973894(03)00109%972
Vianna LNL, Andrade MC, Nicoli JR (2000) Screening of waste biomass from Saccharomyces cerevisiae, Aspergillus oryzae and Bacillus lentus fermentations forremoval of Cu, Zn and Cd by biosorption. World J Microbiol Biotechnol 16:437–440. doi:10.1023/A:1008953922144
Singh KK, Rastogi R, Hasan SH (2005) Removal of Cr (VI) from wastewater using rice bran. J Colloid Interface Sci 290:61–68. doi:10.1016/j.jcis.2005.04.011
Villaescusa I, Fiol N, Martinez M, Miralles N, Poch J, Serarols J (2004) Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Res 38:992–1002. doi:10.1016/j.watres.2003.10.040
Bulut Y, Baysal Z (2006) Removal of Pb (II) from wastewater using wheat bran. J Environ Manage 78:107–113. doi:10.1016/j.jenvman.2005.03.010
Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL (2001) The removal of heavy metals from aqueous solutions by sawdust adsorption: removal of lead and comparison of its adsorption with copper. J Hazard Mater 84:83–94. doi:10.1016/S0304-3894(01),%2000198-4
Ho YS, Huang CT, Huang HW (2002) Equilibrium sorption isotherm for metal ions on tree fern. Process Biochem 37:1421–1430. doi:10.1016/S0032-9592(02),%2000036-5
Wang R, Liao X, Shi B (2005) Adsorption behaviors of Pt(II) and Pd(II) on collagen fibre immobilized bayberry tannin. Ind Eng Chem Res 44:4221–4226. doi:10.1021/ie049069w
Ahluwalia SS, Goyal D (2005) Removal of heavy metals from waste tea leaves from aqueous solution. Eng Life Sci 5:158–162. doi:10.1002/elsc.200420066
Singh KK, Talat M, Hasan SH (2006) Removal of lead from aqueous solutions by agricultural waste maize bran. Bioresour Technol 97:2124–2130. doi:10.1016/j.biortech.2005.09.016
Reddya D, Harinatha Y, Seshaiah K (2010) Biosorption of Pb (II) from aqueous solutions using chemically modified Moringa oleifera tree leaves. Chem Eng J 162:626–634. doi:10.1016/j.cej.2010.06.010
Qaiser S, Saleemi AR, Umar M (2009) Biosorption of lead from aqueous solution by Ficus religiosa leaves: batch and column study. J Hazard Mater 166:998–1005. doi:10.1016/j.jhazmat.2008.12.003
Al-Masri MS, Amin Y (2010) Biosorption of cadmium, lead, and uranium by powder of poplar leaves and branches. Appl Biochem Biotechnol 160:976–987. doi:10.1007/s12010-009-8568-1
Aksu Z, Donmez G (2001) Comparison of copper (II) biosorptive properties of live and treated Candida sp. J Environ Sci Health A 36:367–381. doi:10.1081/ESE-100102928
Yang J, Volesky B (1999) Sorption of copper on algae and fungi. Environ Sci Technol 33:751–757
Aksu Z, Kutsal T, Gun S, Haciosmanoglu N, Gholaminejad M (1991) Investigation of biosorption of Cu(II), Ni(II) and Cr(VI) ions to activated sludge bacteria. Environ Technol 12:915–921. doi:10.1080/09593339109385086
Zolgharnein J, Shahmoradi A (2010) Adsorption of Cr (VI) onto elaeagnus tree leaves: statistical optimization, equilibrium modeling, and kinetic studies. J Chem Eng Data 55:3428–3437. doi:10.1021/je100157y
Tian JS, Li Y, Li JL (2008) High-yield growth and magnetosome formation by Magnetospirillum gryphiswaldense MSR-1 in an oxygen-controlled fermentor supplied solely with air. Appl Microbiol Biotechnol 9:389–397. doi:10.1007/s00253-008-1453-y
Song HP, Li XG, Sun JS, Xu XM, Han X (2008) Application of a magnetotactic bacterium, Stenotrophomonas sp. to the removal of Au(III) from contaminated wastewater with a magnetic separator. Chemosphere 72:616–621. doi:10.1016/j.chemosphere.2008.02.064
Krishna MVB, Chandrasekaran K, Rao SV, Karunasagar D, Arunachalam J (2005) Speciation of Cr(III) and Cr(VI) in waters using immobilized moss and determination by ICP-MS and FAAS. Talanta 65:135–142. doi:10.1016/j.talanta.2004.05.051
Godlewska-Zyłkiewicz B, Kozłowska M (2005) Solid phase extraction using immobilized yeast Saccharomyces cerevisiae for determination of palladium in road dust. Anal Chim Acta 539:61–67. doi:10.1016/j.aca.2005.02.051
Bag H, Lale M, Turker AR (1999) Determination of Cu, Zn and Cd in water by FAAS after preconcentration by baker’s yeast (Saccharomyces cerevisiae) immobilized on sepiolite. Fresenius J Anal Chem 363:224–230. doi:10.1007/s002160051178
Menegario AA, Smichowski P, Polla G (2005) On-line preconcentration and speciation analysis of Cr(III) and Cr(VI) using baker’s yeast cells immobilised on controlled pore glass. Anal Chim Acta 546:244–250. doi:10.1016/j.aca.2005.05.030
Akhtar N, Iqbal J, Iqbal M (2004) Removal and recovery of nickel (II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiana: characterization studies. J Hazard Mat B 108:85–94. doi:10.1016/j.jhazmat.2004.01.002
Bender J, Phillips P (2004) Microbial mats for multiple applications in aquaculture and bioremediation. Bioresour Technol 94:229–238. doi:10.1016/j.biortech.2003.12.016
Chang WC, Hsu GS, Chiang SM, Su MC (2006) Heavy metal removal from aqueous solution by wasted biomass from a combined AS-biofilm process. Bioresour Technol 97:1503–1508. doi:10.1016/j.biortech.2005.06.011
Gadd GM (2000) Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr Opin Biotechnol 11:271–279. doi:10.1016/S0958-1669(00),%2000095-1
Liu Y, Xu H, Yang S, Tay J (2003) A general model for biosorption of Cd, Cu, and Zn by aerobic granules. J Biotechnol 102:233–239. doi:10.1016/S0168-1656(03),%2000030-0
Han R, Zhang J, Zou W, Xiao H, Shi J, Liu H (2006) Biosorption of copper(II) and lead(II) from aqueous solution by chaff in a fixed-bed column. J Hazard Mater 133:262–268. doi:10.1016/j.jhazmat.2005.10.019
Vijayaraghavan K, Jegan J, Palanivelu K, Velan M (2005) Biosorption of copper, cobalt and nickel by marine green alga Ulva reticulate in a packed column. Chemosphere 60:419–426. doi:10.1016/j.chemosphere.2004.12.016
Godlewska-Zylkiewicz B (2003) Biosorption of platinum and palladium for their separation preconcentration prior to graphite furnace atomic absorption spectrometric determination. Spectrochim Acta B Atom Spectrosc 58:1531–1540. doi:10.1016/S0584-8547(03),%2000076-4
Azila YY, Mashitah MD, Bhatia S (2008) Process optimization studies of lead (Pb(II)) biosorption onto immobilized cells of Pycnoporus sanguineus using response surface methodology. Bioresour Technol 99:8549–8552. doi:10.1016/j.biortech.2008.03.056
Hu J, Chen GH, Irene MCL (2005) Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res 39:4528–4536. doi:10.1016/j.watres.2005.05.051
Li HD, Li Z, Liu T, Xiao X, Peng ZH, Deng L (2008) A novel technology for biosorption and recovery hexavalent chromium in wastewater by bio-functional magnetic beads. Bioresour Technol 99:6271–6279. doi:10.1016/j.biortech.2007.12.002
Sau TK, Murphy CJ (2004) Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. Am Chem Soc 126:8648–8649. doi:10.1021/ja047846d
Binupriya AR, Sathishkumar M, Vijayaraghavan K, Yun SI (2010) Bioreduction of trivalent aurum to nano-crystalline gold particles by active and inactive cells and cell-free extract of Aspergillus oryzae var. viridis. J Hazard Mater 177:539–545. doi:10.1016/j.jhazmat.2009.12.066
Riddin T, Gericke M, Whiteley CG (2010) Biological synthesis of platinum nanoparticles: effect of initial metal concentration. Enzyme Microb Technol 46:501–505. doi:10.1016/j.enzmictec.2010.02.006
Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A (2004) Biological synthesis of triangular gold nanoprisms. Nat Mater 3:482–488. doi:10.1038/nmat1152
Shankar SS, Rai A, Ahmad A, Sastry M (2005) Controlling the optical properties of lemongrass extract synthesized gold nanotriangles and potential application in infrared-absorbing optical coatings. Chem Mater 17:566–572. doi:10.1021/cm048292g
Acknowledgments
The authors greatly acknowledge the financial support from the National Natural Science Foundation, P.R. China (Project No. 21076155). We herein express our thankfulness to the Analysis Center of Tianjin University for Product Purity testing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Sun, J., Ji, Y., Cai, F., Li, J. (2012). Heavy Metal Removal Through Biosorptive Pathways. In: Sharma, S., Sanghi, R. (eds) Advances in Water Treatment and Pollution Prevention. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4204-8_5
Download citation
DOI: https://doi.org/10.1007/978-94-007-4204-8_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4203-1
Online ISBN: 978-94-007-4204-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)