Abstract
Plant-based environmental remediation has been widely pursued by academic and industrial scientists as a favorable low-cost clean-up technology. Phytoremediation is being developed as an alternative technology for removing or, more accurately, reducing the concentration of toxic pollutants to clean up the environment. In the present research, potential of green plants have been screened for phytoremediation of heavy metals both from aquatic and terrestrial environment. Indian mustard (Brassica juncea) has been found as a potential candidate for phytoremediation of heavy metals. B. juncea has been used for remediation of Cd, Pb and Zn at varying concentrations, viz., 0, 5, 10, 20 and 50 ppm. The depletion of heavy metals was observed at the intervals of 0, 1, 3, 7, 14 and 21 days and metal uptake was studied in the roots/shoots of the plants. The percentage removal of Cd, Pb and Zn was found 88.9%, 80% and 89.8%, respectively at the higher exposure concentration (50 ppm). Similarly B. juncea has also been used for phytoremediation of heavy metals (Cd, Pb and Zn) at varying concentrations, viz., 0, 5, 10, 20 and 50 mg/kg from mycorrhizal soil in pot culture technique and uptake was studied in the roots/shoots; after harvesting the plants. The uptake of metals in roots was found 25,000 μg g−1 – Cd, 32,750 μg g−1 -Pb and 30,550 μg g−1 –Zn; whereas uptake in shoots was found 4,596 μg g−1 Cd, 3,469 μg g−1 Pb and 15,878 μg g−1 Zn at higher exposure concentration (50 ppm). The research study has proved effective remediation of heavy metals (Cd, Pb and Zn) by B. juncea in water-soil environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
The industrialization and urbanization of the modern world has led to the proliferation of many different metals and compounds in our environment. Metals such as zinc, copper, nickel, manganese, magnesium, iron etc. are essential in very low concentrations for the survival of all forms of life, but are toxic in excess. Metal concentrations have a range of 1–100 mg kg−1 soil. If the concentration of a particular metal crosses the normal threshold, then it becomes potentially toxic and may lead to lethal changes. They build up in biological systems through food chain and become a significant health hazard. The most common heavy metal contaminants are: cadmium (Cd), chromium (Cr), copper (Cu), mercury (Hg), lead (Pb), nickel (Ni) and zinc (Zn) (USEPA 1997; Lasat 2002).
Natural contamination originates from either excessive weathering of mineral and metal ions from rocks or from displacement of certain contaminants from the groundwater or subsurface layers of the soil. The most common anthropogenic sources are human activities such as mining, smelting, electroplating, energy and fuel production, power transmission, sludge dumping, and melting operations, disposal of industrial effluents, deposition of air-borne industrial wastes, military operations, land-fill operations, industrial solid-waste disposal and the growing use of agricultural chemicals such as pesticides, herbicides and fertilizers (Okoronkwo et al. 2005; Jing et al. 2007; Igwe and Abia 2006; Lone et al. 2008). The threat of heavy metals to human and animal health is aggravated by their long-term persistence in the environment (Gisbert et al. 2003). There is an urgent need to develop multifaceted approaches for cleaning environment, which consume lesser resources and would be eco-friendly (Fulekar 2005a). Currently, conventional remediation methods of heavy metal contaminated soils include electrokinetical treatment, chemical oxidation or reduction, leaching, solidification, vitrification, excavation, and off-site treatment. These clean up processes of heavy metal pollution are expensive and environmentally destructive (Bio-Wise 2000; Aboulroos et al. 2006). In order to overcome some shortcomings of conventional methods, phytoremediation is introduced into remediation field (Meagher 2000; Wei et al. 2008).
Phytoremediation is defined as the use of plants to remove pollutants from the contaminated environment (Cunningham et al. 1995; Flathman and Lanza 1998; Salt et al. 1998; Weber et al. 2001). Actually, with some added advantages such as environmental beautification, easy acceptance by the public and potential application to a relatively large pollution area, phytoremediation of heavy metal contaminated soils is widely considered a promising remediation approach in the future (Chaney et al. 1997; Lewandowski et al. 2006; McGrath et al. 2006; Lai and Chen 2009). It utilizes the remarkable ability of the plants to concentrate or remove heavy metals from the environment. Plants are unique organisms equipped with remarkable metabolic and adsorption capabilities, as well as transport systems that can take up nutrients or contaminants selectively from the soil and water. Phytoremediation methods offer significant potential for certain application and permit a much larger site to be restored would generally be possible using more traditional remediation technologies (Fulekar 2005b). There are over 400 different plant species considered suitable to be used as phytoremediator. Indian mustard has been identified as a higher biomass producing plant with the capacity to accumulate zinc and cadmium at higher concentrations in plant cells (Kumar et al. 1995; Salt et al. 1995). After the completion of phytoremediation, the plants used for the study, can be harvested, incinerated and followed by recycling of the metals or as disposal in a landfill (Bennett et al. 2003; Angel and Linacre 2005). It results in reduction of metal contamination from the polluted site.
In the present study, Indian mustard plant has been screened and selected for the phytoremediation of heavy metals such as cadmium, lead and zinc in aquatic and terrestrial environment. Studies are conducted to determine the potential and uptake capacity of cadmium, lead and zinc by Brassica juncea from aquatic and terrestrial environment under controlled environmental conditions.
6.2 Screening of Plants
The success of phytoremediation depends on screening and selection of an ideal plant. A plant suitable for phytoremediation possesses the characteristics: ability to tolerate/accumulate metals, fast growth, effective accumulating capacity, high biomass and easily harvestable. Researchers have reported that plant species that are long-term competitors and survivors under adverse conditions normally have an advantage for phytoremediation. They adapt self operative defensive mechanism to survive in adverse environmental conditions. A screening for phytotoxicity and effectiveness of plant’s cultivars/varieties is required on a site-specific basis as an initial step in plant selection (Pivetz 2001; Schnoor 2002). The plants which have potential to survive and grow in the contaminated environment are screened and reported by the Scientists for phytoremediation of heavy metals and radionuclides. The green plants which survive in such adverse conditions, but not exploited for phytoremediation of heavy metals have been studied and screened for the present research work. After studying the toxicity of heavy metals at varying concentrations, Brassica juncea has been screened as suitable plant for phytoremediation of heavy metals. Indian mustard (Brassica juncea), also known as mustard greens, and leaf mustard, is a species of mustard plants and belongs to the family Brassicaceae. The plant was selected for the phytoremediation study based on their fast growth rate/ high biomass along with the tolerance potential to thrive in heavy metals contaminated environment. This plant is used to remove heavy metals from the soil in the hazardous waste sites because it has a higher tolerance towards most of the toxic metals and very high capability of storing them in its parts. Brassica juncea is a good candidate for efficient phytoextraction of heavy metals such as Cd from polluted soils (Schneider et al. 1999). The plant is then harvested and disposed off properly. This method is easier and less expensive than traditional methods for the removal of heavy metals. It also prevents erosion of soil from these sites preventing further contamination.
6.3 Methodology Adapted
6.3.1 Plant Materials
Healthy seeds of Indian mustard (Brassica juncea L. Czern and Coss) var. TM4 (Trombay Mustard 4) were selected for the research studies. Mustard seeds were obtained from gamma field, Bhabha Atomic Research Centre, Mumbai. Seeds were pre-soaked in soap and dettol solution for 15 min and thoroughly washed in running tap water until the soap solution was completely removed. Then the seeds were sterilized with 70% ethanol for 30 s followed by sterilization with 0.1% mercuric chloride for 3–5 min. The seeds were thoroughly rinsed 5 times with sterile distilled water. These sterilized seeds were inoculated in test tubes containing MS (Murashige and Skoog 1962) basal medium supplemented with 3% sucrose. Seedlings were allowed to grow for 1 month under in vitro condition.
6.3.2 Hydroponic Experimental Setup
Uniform plants were selected for the uptake study. MS medium was drained off and replaced with hydroponics media i.e. Hoagland solution (Hoagland and Arnon 1938) containing nutrient solution for the acclimatization for 1 week prior to the experiment. They are then transferred to another Hoagland solution which contained each of the following heavy metal in a separate set up: Cadmium supplied as Cd (NO3)2. 4H2O; lead supplied as Pb (NO3)2; and zinc supplied as ZnSO4. The different concentrations of metals used in these studies were 5, 10, 20, and 50 μg ml−1 with control. Each experiment was carried out in triplicates. Plants grown in nutrient solution without metals served as control. The sampling from aqueous solution containing metals has been carried at an interval of 0, 1st, 3rd 7th, 14th and 21st days in vitro condition to ensure that uptake of each metal is being taking place (Anamika et al. 2009). 500 μl of aliquots were withdrawn from each concentration with increasing period. These samples were used for the analysis of cadmium, lead and zinc content. The reduction in concentration of these metals in the medium was attributed to their uptake by the plants. At the end of the experiment the plant samples were collected and washed with de-ionized water twice and rinsed with distilled water. Each sample was divided into root and shoot and oven-dried at 60°C until completely dry. Dry weights of roots and shoots were determined and noted.
6.3.3 Soil Sampling and Characterization
The alluvial soil used in the pot experiment, was collected from a depth of about 0–15 cm along the banks of Surya River, Palghar (located 100 km away from Mumbai). The soil was air-dried and then passed through 2 mm sieve, and large stones and plant root debris were removed. This prepared soil was stored in a plastic bag at room temperature (27–30°C) until the use. The physico-chemical characteristics of the soil were measured by standard methods (Table 6.1). The content of heavy metals (Cd, Pb and Zn) in soil was estimated by atomic absorption spectrophotometer (APHA 1998).
6.3.4 Mycorrhizal Inoculum
Soil based mycorrhizal inoculum was developed by pot-culture technique at laboratory scale with the help of starter inoculums and using sorghum as a host plant. A starter culture of mycorrhizal fungi (VAM) was procured from Division of Microbiology, IARI, New Delhi. The physico-chemical parameters (Table 6.1) and microbial characterization (Table 6.2) of soil were done after the development of mycorrhizal soil. The developed mycorrhizosphere provide a direct link between soil and roots, and are renowned for their ability to increase plant mineral nutrients, notably P and enhance phytoremediation (Leyval et al. 1997; Gaur and Adholeya 2004; Bush 2008; Anamika and Fulekar 2010).
6.3.5 Pot Culture Experiment
Pot culture experiments were conducted in the green house. The growth medium in the pots consisted of alluvial soil and mycorrhizal inoculum (5:1) and treated as Mycorrhizal soil (MS). The alluvial soil without the mycorrhizal inoculum was treated as control. Mycorrhizal soil filled in each 1 kg capacity of pot (having perforated base for proper aeration and drain connecting system) and amended with heavy metals: Cd as Cd (NO3)2. 4H2O; Pb as Pb (NO3)2 and Zn as ZnSO4, separately. The various concentrations applied for each heavy metal was; 0, 5, 10, 20, 50 mg kg−1.
The healthy seeds of Indian mustard (Brassica juncea L. Czern and Coss) var. TM4 (Trombay Mustard 4) were surface-sterilized with 0.1% mercuric chloride for 5 min and subsequently washed several times with distilled water to avoid fungal contamination. Six seeds were sown into each pot and the pots were randomly placed in a green house at an average diurnal temperature of 25–27°C, and a relative humidity 40–60%. Plants were watered to maintain soil moisture at 60–70% of water holding capacity by adding water during the experiment. One hundred milli liter of Hoagland solution (Hoagland and Arnon 1938) was given to growing plants once in a week. The drainage collected at the bottom of the pot was also added in the pots to avoid the loss of metals through leachate. The plants were grown for a period of two and half months.
6.3.6 Analytical Methods
Each sample (dried root and shoots) was digested with 10 ml of perchloric acid: nitric acid (HClO4: HNO3- 1:5 v/v) mixture separately. Acid digestion was carried out on hot plate at 70–100°C until yellow fumes of HNO3 and white fumes HClO4 were evolved. The digestion process was continued until a clear solution remains after volatilization of acids. The digestion was stopped when the residue in the flask was clear and white. The digested sample was dissolved in distilled water, filtered for the removal of impurities (APHA 1998) and made up to the desired volume. The samples were analyzed by GBC 932 B+ Atomic Absorption Spectrophotometer (Australia) using air-acetylene flame to estimate cadmium, lead and zinc contents in the plant samples.
6.3.7 Data Analysis
The heavy metals are taken up by the plants through their roots from the solution. Experimental data were analyzed for uptake of cadmium, lead and zinc by Indian mustard plants. The experiment was carried out in triplicates and average values are reported. Data were analyzed for mean and standard deviation (X ± S.D.) using standard statistical methods (Mahajan 1997).
6.4 Results
6.4.1 Phytoremediation of Heavy Metals in Aquatic Environment
The remediation of heavy metals (Cd, Pb and Zn) were carried out using Brassica juncea (Indian Mustard) from aquatic environment at the concentrations ranging from 0, 5, 10, 20 and 50 ppm for a period of 21 days. The healthy plants of B. juncea were grown in Hoagland solution spiked with various concentrations of Cd, Pb and Zn, separately. After the growth, the plants were harvested and analyzed for biomass and metal uptake of heavy metals in the roots/ shoots.
6.4.1.1 Depletion of Metals from the Solution
Metal depletion was studied to understand the potential of plant- B. juncea at various exposure levels. Figure 6.1a–c demonstrates the depletion of Cd, Pb and Zn from aquatic environment after 21 days of exposure period. B. juncea has remediated 35.2–88.9% Cd, 26–80.1% Pb and 30–89.8% Zn from lower to higher concentration of heavy metals. Pb uptake was found lower than Cd and Zn by B. juncea.
6.4.2 Dry Biomass Analysis
Brassica juncea was harvested after 21 days of phytoremediation of heavy metals from aquatic environment. Biomass of plant was found to be 0.009 g in roots and 0.063 g in shoots for Cd, 0.028 g in roots and 0.107 g in shoots for Pb and 0.015 g in roots and 0.061 g in shoots for Zn at 50 ppm exposure; after 21 days period. The significant difference in biomass was found for each metal exposure with increasing period as demonstrated in Table 6.3. The statistical analysis has been given for each heavy metal (Cd, Pb and Zn) for minimum 0–50 ppm concentrations. Result shows that B. juncea has tolerance potential to grow in contaminated environment and effectively phytoremediated metals.
6.4.3 Bioaccumulation of Metals in the Roots and Shoots of Plants
Bioaccumulation of metals has been studied in root/shoots of plant- B. juncea. Bioaccumulation of Pb, Cd and Zn in roots was found to be 12,264, 18,419 and 26,517 μg g−1, respectively at higher exposure concentration (50 ppm), after 21 days exposure period. Whereas bioaccumulation of Pb, Cd and Zn in shoots of B. juncea was found 2,477, 3,349 and 2,585 μg g−1, respectively at 50 ppm after phytoremediation of heavy metals in aquatic environment. Result shows that all the metals studied have bioaccumulated more in roots as compared to shoots (Fig. 6.2a–c) of B. juncea.
Phytoremediation studies show that Pb- 1,258 μg g−1, Cd- 2,230 μg g−1 and Zn- 2,250 μg g−1 in roots of the plants at lower exposure level (5 ppm); whereas bioaccumulation of metals in shoots was recorded 254 μg g−1 Pb, 344 μg g−1 Cd and 471 μg g−1 Zn. The bioaccumulation of metals increased with increasing concentrations and found 12,517 μg g−1 Pb, 18, 419 μg g−1 Cd and 26,517 μg g−1 Zn in the roots of B. juncea; whereas 2,477 μg g−1 Pb, 3,349 μg g−1 Cd and 2,585 μg g−1 Zn in shoots at higher exposure level (50 ppm). The comparison of data analysis revealed that Cd was more accumulated in shoots than roots as compared to Zn and Pb. Metal accumulation in B. juncea was found to be 5.4, 4.9 and 5.96 times higher in roots as compared to the shoots in case of cadmium, lead and zinc respectively. The data showed that the heavy metals accumulation by B. juncea were found in order of Zn > Cd > Pb. The comparison has been made to demonstrate concentration of Pb, Cd and Zn in roots and shoots of B. juncea at varying concentrations. The results of the present study have shown that B. juncea has the potential for the uptake and accumulation of cadmium and lead. This plant may be of practical use for the decontamination of polluted water and soil containing these metals.
Brassica juncea – an identified potential green plant has been used for phytoremediation of heavy metals from aquatic environment. The depletion of heavy metals in aquatic solution and uptake by plants with increasing exposure period have been observed. After the proper growth, the plant biomass, uptake of each selected heavy metals in roots/shoots of the plants including their translocation factor to assess bioaccumulation potential of plants have been studied. The biomass of B. juncea was found higher upto the concentration of 10 ppm in case of Cd and Pb, and thereafter decreased as the exposure levels of Cd and Pb increased i.e. 20 and 50 ppm. Zn has shown positive effect on plant biomass. Heavy metals were found efficiently taken up mainly by the roots of B. juncea plants at all the evaluated concentrations. Similar findings were reported by Jadia and Fulekar (2008) for uptake of heavy metals (Cd, Cu, Ni, Pb and Zn) by fibrous root grass. Once metal ions are absorbed, they can be accumulated in the roots or be exported to the shoots via the transpiration stream (Ximenez-Embun et al. 2001).
Research has shown that metal concentration in plant tissues is a function of the heavy metals content in the growing environment (Cui et al. 2004), and that the uptake and accumulation of different metals may vary from plant to plant species. Kim et al. (2003) suggested that such discrepancies arise due to variation in type of heavy metals, its concentration, form of metal present and plant species. Different metals are differently mobile within a plant; cadmium and zinc are more mobile than lead and copper (Greger 2004). Cd is one the most toxic heavy metals due to its high mobility and the small concentration at which its effects on the plants begin to show (Vázquez et al. 1992).
Our results further showed that Pb is accumulated more in roots as compared to the other two metals (Zn and Cd). Pb uptake studies in plants have demonstrated that roots have an ability to take up significant quantities of lead whilst simultaneously greatly restricting its translocation to above ground parts (Lane and Martin 1977). Liu et al. (2000) have reported that B. juncea has considerable ability to remove lead from solutions and accumulate it in roots. Kumar et al. (1995) have also reported the higher accumulation of lead in roots of sorghum species, with indications that lead can be found on the outer surface of plant roots, as crystalline or amorphous deposits, and could be deposited in the cell walls or in vesicles.
The Cd and Zn uptake were found to be higher in shoot as compared to Pb. Zn and Cd have many physical and chemical similarities as they both belong to Group II of the periodic table. They are usually found together in the ores and compete with each other for various ligands. Thus the interaction between Zn and Cd in the biological system is likely to be similar. The fact that cadmium is a toxic metal and Zn is an essential element makes this association interesting as it raises the possibility that the toxic effects of cadmium may be preventable or treatable by Zn (Chowdhury and Chandra 1987).
6.4.4 Phytoremediation of Heavy Metals in Mycorrhizosphere
The research work carried out for phytoremediation of heavy metals by alfalfa in mycorrhizal soil as discussed above has been followed for Brassica juncea. Phytoremediation of cadmium, lead and zinc at varying concentrations viz. 0, 5, 10, 20 and 50 ppm using B. juncea in the mycorrhizospheric soil for a period of two and half months has been studied.
6.4.4.1 Dry Biomass Analysis
The dry biomass of B. juncea was recorded and presented in Table 6.4 for Cd, Pb and Zn metals at varying concentrations ranging from 5 to 50 ppm. Biomass of B. juncea was found to be 0.021 g in roots and 0.297 g in shoots for Cd, 0.027 g in roots and 0.288 g in shoots for Pb and 0.037 g in roots and 0.486 g in shoots for Zn at 50 ppm of exposure level over a period of two and half months in mycorrhizosphere pot culture experiment. B. juncea has produced high biomass in case of Zn as compared to Cd and Pb. Biomass decreased gradually as the concentration of Cd and Pb increased (i.e. 50 ppm) in mycorrhizal soil. The plant’s biomass yield affected at the higher ppm levels exposure of Cd. Lead showed low effect on plant biomass. The positive effect has been seen in Zn concentrations exposure from lower to higher, resulting into high biomass yield.
6.4.4.2 Bioaccumulation of Metals in the Roots and Shoots of Plants
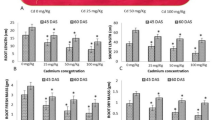
The study shows that heavy metals were efficiently taken at all concentrations using high biomass producing plant B. juncea grown in mycorrhizal soil. The mean uptake of metals Cd, Pb and Zn by B. juncea increased as the concentrations of these metals in mycorrhizal soil increased. In plant, shoots and roots were observed to have a characteristic uptake capacity for different metals. The research findings demonstrated that uptake of Cd from mycorrhizosphere by B. juncea was 2,783 μg g−1 at 5 ppm and 25,000 μg g−1 at 50 ppm in roots and 838 and 4,596 μg g−1 at minimum to maximum concentrations (0–50 ppm) in shoot, respectively. The uptake of Pb in roots was recorded 2,938 and 32,750 μg g−1 at 5 and 50 ppm, while in shoots it was found 486 μg g−1 at 5 ppm and 3,469 μg g−1 at 50 ppm. However, the uptake of Zn was found more as compared to Cd and Pb, reached highest accumulation, i.e. 30,550 μg g−1 in roots and 15,878 μg g−1 in shoots at highest concentration (50 ppm). The heavy metals were taken by the B. juncea in the following order: Zn > Cd > Pb. These results could be seen in Fig. 6.3a–c.
The accumulation of Cd, Pb and Zn was compared in roots and shoots of B. juncea. The metal uptake in roots was found 2,783 μg g−1 (Cd), 2,938 μg g−1 (Pb) and 3,175 μg g−1 (Zn) at lower doses (5 ppm). The uptake of metals in roots was increased upto 25,000 μg g−1 – Cd, 32,750 μg g−1 -Pb and 30,550 μg g−1 -Zn at higher concentration- 50 ppm. Similarly, the uptake of metal in shoots was found to be 838 μg g−1 Cd, 486 μg g−1 Pb and 705 μg g−1 Zn at lower doses (5 ppm); whereas uptake increased to 4,596 μg g−1 Cd, 3,469 μg g−1 Pb and 15,878 μg g−1 Zn at higher doses (50 ppm). The comparison of data analysis shows that B. juncea was found to accumulate 5.4 times more Cd, 9.4 times more Pb and 1.9 times more Zn in roots as compared to the shoots. The research findings show that Pb was bioaccumulated more in roots than shoots of plants as compared to Cd and Zn.
Phytoremediation of heavy metals using B. juncea has been studied in mycorrhizal soil using pot culture technique. The results showed an increasing trend for biomass production (Cd and Pb) as the concentrations increased from 5 to 10 mg/kg. The positive effects have been seen in case of Zn concentrations. However, the biomass yield was found only affected at the higher concentrations, i.e. 20 and 50 mg/kg of Cd and Pb that shows inhibitory effect on plant growth. Higher doses of heavy metal can affect physiology, reduced plant growth and dry biomass. It is reported that Mycorrhizal fungi helps/ protects the plants against metal toxicity, however restricts the translocation of metals from root to shoot. Therefore, in the present research there were no toxicity symptoms like chlorosis, retarded growth etc. observed in case of B. juncea plants. In the present research, the significant effect on plant growth in mycorrhizosphere was observed. M. sativa plants have produced better biomass which results in higher uptake of heavy metals in mycorrhizosphere. The results showed the enhanced nutrients availability as well microbial communities in mycorrhizal soil, which favour the uptake of heavy metals by green plants. Higher levels of organic matter and nutrients content in the mycorrhizosphere had beneficial influence on soil chemical and biochemical properties and plant growth, thus increasing biomass yields. Various researchers have reported the similar findings where the mycorrhizosphere was shown to change soil structure by stabilizing aggregates (Miller and Jastrow 1990; Bearden and Petersen 2000; Augé et al. 2001), thereby enhancing soil-HM retention capacity in the plants.
The results showed that B. juncea efficiently bioaccumulated heavy metals at the varying concentrations range of 5, 10, 20 and 50 mg/kg. The accumulation of Cd, Pb and Zn was found increased with increasing concentrations of metals in the mycorrhizosphere. Root of the plants is the primary source of metals accumulation followed by shoot. The root of plants is being in the direct contact with mycorrhizal soil able to take up the heavy metals, along with their nutrients uptake ability. Once the metal is taken by the root of plants, it translocated to shoot via vascular tissues. In the soil-mycorrhizosphere, the mycorrhizal hyphae also help to take up the heavy metals from contaminated environment.
6.5 Conclusions
The green plant Brassica juncea has been identified as potential candidate for remediation of heavy metals – Cd, Pb and Zn from aquatic/ terrestrial environment. The research study shows that the plant has grown in contaminated environment and produced significant biomass (roots/shoots) which has found directly proportionate to uptake of heavy metals. The bioaccumulation of heavy metals was found higher in roots than the shoots of B. juncea for each of the heavy metal studied. The present study has proved the effective remediation of heavy metals by the green plant which could be buried or disposed off at safe environment. The higher concentrations of heavy metals bioaccumulated in plants can also be recovered and reused. Therefore, phytoremediation study will be beneficial for decontamination of heavy metals contaminated environment and/or extraction of metals for beneficial purpose.
Abbreviations
- Cd:
-
Cadmium
- cm:
-
Centimeter
- kg:
-
Kilogram
- μg:
-
Micro gram
- μl:
-
Micro liter
- mg:
-
Milli gram
- ml:
-
Milli liter
- MS:
-
Mycorrhizal soil
- Pb:
-
Lead
- ppm:
-
Parts per million
- VAM:
-
Vesicular arbuscular mycorrhiza
- Zn:
-
Zinc
References
Aboulroos SA, Helal MID, Kamel MM (2006) Remediation of Pb and Cd polluted soils using in situ immobilization and phytoextraction techniques. Soil Sediment Contam 15:199–215
Anamika S, Fulekar MH (2010) Impact of heavy metals in mycorrhizosphere: strategy for phytoremediation. Can J Pure Appl Sci 4:1293–1302
Anamika S, Eapen S, Fulekar MH (2009) Potential of Medicago sativa for uptake of cadmium from contaminated environment. Roman Biotechnol Lett 14:4164–4169
Angle JS, Linacre NA (2005) Metal phytoextraction – a survey of potential risks. Int J Phytoremediation 7:241–254
APHA, AWWA, WEF (1998) Standard methods for the examination of water and wastewater. The Association, Washington, DC
Augé RM, Stodola AJW, Tims JE, Saxton AM (2001) Moisture retention properties of a mycorrhizal soil. Plant Soil 230:87–97
Bearden BN, Petersen L (2000) Influence of arbuscular mycorrhizal fungi on soil structure and aggregate stability of a vertisol. Plant Soil 218:173–183
Bennett LE, Burkhead JL, Hale KL, Terry N, Pilon M, Pilon-smits EAH (2003) Analysis of transgenic Indian mustard plants for phytoremediation of metals-contaminated mine tailings. J Environ Qual 32:432–440
BIO-WISE (2000) Contaminated land remediation: a review of biological technology. DTI, London
Bush JK (2008) The potential role of mycorrhizae in the growth and establishment of Juniperus seedlings. In: Van Auken OW (ed) Western north American Juniperus communities. Springer, New York, pp 111–130
Chaney RL, Malik M, Li YM, Brown SL, Brewer EP, Angel JS, Baker AJM (1997) Phytoremediation of soil metals. Curr Opin Biotechnol 8:279–284
Chowdhury BA, Chandra RK (1987) Biological and health implications of toxic heavy metals and essential trace element interactions. Prog Food Nutr Sci 11:55–113
Cui Y, Wang Q, Christie P (2004) Effect of elemental sulphur on uptake of cadmium, zinc and sulphur by oilseed rape growing in soil contaminated with zinc and cadmium. Commun Soil Sci Plant Anal 35:2905–2916
Cunningham SD, Berti WR, Huang JW (1995) Phytoremediation of contaminated soils. Trends Biotechnol 13:393–397
Flathman PE, Lanza GR (1998) Phytoremediation: current views on an emerging green technology. J Soil Contam 7:415–432
Fulekar MH (2005a) Bioremediation technologies for environment. India J Environ Prot 25:358–364
Fulekar MH (2005b) Environmental biotechnology. Oxford & IBH, New Delhi
Gaur A, Adholeya A (2004) Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Curr Sci 86:528–534
Gisbert C, Ros R, De Haro A, Walker DJ, Pilar BM, Serrano R, Avino JN (2003) A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochem Biophys Res Commun 303:440–445
Greger M (2004) Metal availability, uptake, transport and accumulation in plants. In: Prasad MN (ed) Heavy metal stress in plants: from biomolecules to ecosystems, 2nd edn. Springer, Berlin
Hoagland DR, Arnon DI (1938) The water culture method for growing plants without soil. Calif Agric Exp Stn Circ 3:346–347
Igwe JC, Abia AA (2006) A bio-separation process for removing heavy metals from waste water using biosorbents. Afr J Biotechnol 5:1167–1179
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India, New Delhi
Jadia CD, Fulekar MH (2008) Phytotoxicity and remediation of heavy metals by fibrous root grass in soil – vermicompost media. J Appl Biosci 10:491–499
Jing Y, He Z, Yang X (2007) Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J Zhejiang Univ Sci B 8:192–207
Kim IS, Kang KH, Green PJ, Lee EJ (2003) Investigation of heavy metal accumulation in Polygonum thunbergii for phytoextraction. Environ Pollut 126:235–243
Kumar PBAN, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol 29:1232–1238
Lai H-Y, Chen Z-S (2009) In-situ selection of suitable plants for the phytoremediation of multi-metals-contaminated sites in central Taiwan. Int J Phytoremediation 11:235–250
Lane SD, Martin ES (1977) A histochemical investigation of lead uptake in Raphanus sativus. New Phytol 79:281–286
Lasat MM (2002) Phytoremediation of toxic metals: a review of biological mechanisms. J Environ Qual 31:109–120
Lewandowski I, Schmidt U, Londo M, Faaij A (2006) The economic value of the phytoremediation function- assessed by the example of cadmium remediation by willows (Salix sp.). Agric Syst 89:68–89
Leyval C, Turnau K, Haselwandter K (1997) Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza 7:139–153
Liu JR, Suh M, Ch Choi D (2000) Phytoremediation of cadmium contamination: overexpression of metallothionein in transgenic tobacco plants. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 43:126–130
Lone MI, He Z, Stoffella PJ, Yang X (2008) Phytoremediation of heavy metal polluted soils and water: progress and perspectives. J Zhejiang Univ Sci B 9:210–220
Mahajan BK (1997) Methods in biostatistics for medical students and research workers, 6th edn. Jaypee Brothers, New Delhi
McGrath SP, Lombi E, Gray CW, Caille N, Dunham SJ, Zhao FJ (2006) Field evaluation of Cd and Zn phytoextraction potential by the hyperaccumulators Thlaspi caerulescens and Arabdopsis halleri. Environ Pollut 141:115–125
Meagher RB (2000) Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol 3:153–162
Miller RM, Jastrow JD (1990) Hierarchy of root and mycorrhizal fungal interactions with soil aggregation. Soil Biol Biochem 22:579–584
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Okoronkwo NE, Igwe JC, Onwuchekwa EC (2005) Risk and health implication of polluted soils for crop production. Afr J Biotechnol 4:1521–1524
Pivetz BE (2001) Phytoremediation of contaminated soil and ground water at hazardous waste sites. EPA ORD Ground Water Issue, EPA/540/S–01/500
Salt DE, Blaylock M, Kumar PBAN, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnol 13:468–475
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49:643–668
Schneider T, Haag-Kerwer A, Maetz M, Niecke M, Povh B, Rausch T (1999) Micro-PIXE studies of elemental distribution in Cd-accumulating Brassica juncea L. Nucl Instrum Methods Phys Res B: Interact Mater Atoms 158:329–334
Schnoor JL (2002) Technology evaluation report: phytoremediation of soil and groundwater. GWRTAC Series TE-02-01
USEPA (1997) Cleaning Up the Nation’s waste sites: markets and technology trends, united states environmental protection agency EPA/542/R-96/005. Office of Solid Waste and Emergency Response, Washington, DC
Vázquez MD, Barceló J, Poschenrieder C, Mádico J, Hatton P, Baker AJM, Cope GH (1992) Localization of zinc and cadmium in Thlaspi caerulescens (Brassicaceae), a metallophyte that can hyperaccumulate both metals. J Plant Physiol 140:350–355
Weber O, Scholz RW, Bühlmann R, Grasmück D (2001) Risk perception of heavy metals soil contamination and attitudes toward decontamination strategies. Risk Anal 21:967–977
Wei S, Jaime A, Da Silva T, Zhou Q (2008) Agro-improving method of phytoextracting heavy metal contaminated environment. J Hazard Mater 150:662–668
Ximenez-Embun P, Madrid-Albarran Y, Camara C, Cuadrado C, Burbano C, Muzquiz M (2001) Evaluation of lupines species to accumulate heavy metals from waste waters. Int J Phytoremediation 3:369–379
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Singh, A., Fulekar, M.H. (2012). Phytoremediation of Heavy Metals by Brassica juncea in Aquatic and Terrestrial Environment. In: Anjum, N., Ahmad, I., Pereira, M., Duarte, A., Umar, S., Khan, N. (eds) The Plant Family Brassicaceae. Environmental Pollution, vol 21. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-3913-0_6
Download citation
DOI: https://doi.org/10.1007/978-94-007-3913-0_6
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-3912-3
Online ISBN: 978-94-007-3913-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)