Abstract
Phytoremediation is an ecologically and economically feasible technique to remove heavy metal from soil. The aim of the study was to examine cadmium (Cd) toxicity and phytoremediation aptitude of Brassica juncea. In the present study, plants survived when exposed to different levels of Cd (0, 25, 50 and 100 mg/kg soil) and accumulated a large amount of Cd in its root and shoot. Translocation factor (TF) of Cd from root to shoot was > 1 at both 45 and 60‐day stage of growth suggesting that B. juncea is a hyperaccumulator and strong candidate for phytoextraction of Cd. Alongside, Cd impaired photolysis of water, PSII activity, nutrient uptake, photosynthesis and sugar accumulation in the plant. Cd-generated oxidative stress restricts the growth of B. juncea. The toxic effect of Cd was more pronounced at 45‐day stage of growth signifying the drifting of plant towards acquirement of exclusion strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Phytoremediation has emerged as an ecologically balanced and economically sound strategy to remove heavy metals (HM) from contaminated soils. This is pertinent in the context of intense augmentation of HM accumulation in soil during past two decades. Mining, application of chemical fertilizers, and discard of industrial waste in soil and water bodies are examples of anthropogenic activities that cause HM pollutants in soil (Faraz et al. 2019). Cadmium (Cd) is one of such pollutant that is usually found in air and soils exposed to industrial wastes, phosphatic fertilizers, cement factories, metal‐working industries, and disposal of Cd‐enriched wastewater treatment solids into water bodies and soil. In plants, Cd elicits a wide range of molecular mechanisms. It perturbs the normal functioning of various physiological processes such as photosynthesis, nitrogen‐metabolism, oxidative reactions, water relations, and nutrient uptake (Hasan et al. 2009). Cd toxicity results in growth retardation, leaf chlorosis and inhibition of different metabolic pathways. In Cd sensitive plants, Cd‐induced persistent inhibition of photosynthesis generates oxidative stress and reactive oxygen species (ROS)‐mediated destruction of photosynthetic apparatus (Hayat et al. 2014). Cd promotes ROS generation, for example superoxide anion (O2•–) and hydrogen peroxide (H2O2). Generation of ROS above a certain level results in membrane lipid peroxidation, thus impairing membrane fluidity and permeability (Tripathy and Oelmüller 2012). To counter the generation of ROS, plant defence system induces activation of antioxidant systems.
Brassica juncea could be used for the remediation of Cd from contaminated soils, because of its ability to translocate high Cd amounts into its stems and leaves (Goswami and Das 2015). Hence, this property makes B. juncea a proper candidate for crop rotation to control various toxic metal levels and curtail the load of toxic metal deposited in soils (Van Ginneken et al. 2007). The effects of Cd application on different physiological processes have been studied; however, a well-defined study having prime focus on photosynthesis, utilization of photosynthates, ROS localization and cell viability is still missing. The present study was designed to address these aforementioned loop holes and to elucidate the role of B. juncea as a phytoextraction species.
Materials and Methods
Seeds of Brassica juncea var. Varuna were procured from National Seed Corporation Ltd, New Delhi, India and an experiment was conducted in the Net House of Department of Botany, Aligarh Muslim University, India in the month of November-December. Seeds were sown in pots (12 inches) filled with soil and farmyard manure in the ratio of 6:1. Soil in the selected pots was mixed with different concentrations of Cd (0, 25, 50 or 100 mg Cd/kg of soil) and 5 replicates for each treatment were maintained. The sampling of plants was done at 45 and 60-day stages of growth to assess various growth and physiological parameters.
Growth characteristics, primary photochemistry, leaf gaseous exchange, elemental [carbon (C), nitrogen (N), potassium (K), magnesium (Mg), sulphur (S), manganese (Mn)] composition, carbohydrate content, histochemical studies (O2•– and H2O2 localization), malondialdehyde and proline content, and antioxidant enzymes were performed according to our previous study (Siddiqui et al. 2018a).
Methodology provided by Sadasivam and Manickam (1996) was followed to estimate glucose content. Phosphate buffer containing glucose oxidase and peroxidase was added to methanolic solution of o–dianisidine. The glucose oxidase‐peroxidase reagent (1 ml) and DDW (0.5 ml) were added to sugar extract and kept at 35°C for 40 min. After incubation, 6 N HCl was added to the mixture to terminate the reaction. The colour intensity was read at 540 nm.
Methodology of Roe (1934) was employed to estimate sucrose content however with slight modifications. To the test extract, 6% KOH was added and heated on boiling water bath for 20 min to destroy free fructose. After cooling the solution, 0.1% resorcinol and 30% hydrogen chloride were added. The solution was again incubated at 80°C for 10 min. Absorbance was read at 490 nm.
Protocol given by Ashwell (1957) was followed to estimate fructose content. Recorcinol reagent comprising thiourea and glacial acetic acid was added to the test extract. HCl was added and the test solution was heated at 80°C. After cooling the mixture, the absorbance was read at 520 nm.
For estimation of starch content, fresh leaves were homogenized in 80% ethanol to remove sugars followed by centrifugation. Residue was collected and washed with ethanol till it gives a colourless solution upon reacting with anthrone reagent. After dehydrating, water and 52% perchloric acid was added to the residue. After centrifugation, supernatant was stored for starch estimation using anthrone reagent. Intensity of green colour was observed at 630 nm.
NATIVE-PAGE was performed to visualize the activity of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) proteins in gel and the bands obtained were quantified using ImageJ software.
Root and shoot samples were immersed in 5 mM CaCl2 solution for 10 min to dislodge extracellular Cd and washed with DDW. Biological samples were oven dried (Meuwly and Rauser 1992). Samples were digested in nitric acid:perchloric acid (3:1, v/v). Cd concentration was determined by atomic absorption spectrophotometer (Perkin-Elmer A, Analyst, 300).
TF was calculated using formula (Das et al. 2014): cadmium in shoot (µg g−1)/cadmium in root (µg g−1).
SPSS ver. 17 for windows (IBM Corporation, 1 New Orchard Road, Armonk, New York 10504‐1722, United States 914‐499‐1900) was used for statistical analysis. Analysis of variance (ANOVA) and standard error was performed using 5 replicates (n = 5) to determine the least significance difference (LSD) between treatment means with the level of significance at p ≤ 0.05.
Results and Discussion
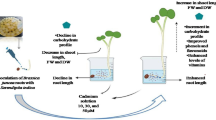
Presence of 3–8 mg/kg of Cd in the soil is considered toxic to plants (Kabata‐Pendias and Pendias2001). In the present study, the plants survived at a concentration of 100 mg/kg Cd suggesting high tolerance of Brassica juncea. Though, there was a reduction in plant height and biomass in presence of Cd (Fig. 1, A–G). Maximum reduction was observed in plants growing in 100 mg/kg soil of Cd. Root length was decreased by 54% and 42.4%; shoot length by 53% and 32.5%; root fresh mass by 62.9% and 45%; shoot fresh mass by 45% and 32.5%; shoot dry mass by 48.2% and 39% and root dry mass by 56.7% and 30%, respectively at 45% and 60% DAS. Similar trend was observed for reduction in leaf area in Cd-grown plants (Fig. 2b, A). The Cd‐induced toxicity was easily distinguished from mild to lethal crop failure. Reduction in growth and biomass is considered the most general symptom of Cd toxicity (Jinadasa et al. 2016; Hayat et al. 2007,2014).
Evaluation of chl is considered as an important indicator of photosynthesis (Dalio 2011). As shown in Fig. 2b, SPAD value decreased with increasing Cd concentration. 100 mg/kg soil Cd proved most lethal and lowered the SPAD value (55% and 37%). The Cd‐mediated decrease in SPAD value might depend on its target PSII system (Song et al. 2019) and Mg uptake (Fig. 4b, C) which is an integral part of chl pyrole ring. There is a transport of electrons away from PSII to calvin cycle and stomatal opening upon illumination. And this consumption of electrons for photosynthetic activity is called photochemical quenching (qP) (Guidi et al. 2019) that defines the fraction of open PSII reaction centres. Whereas the excitation energy that is lost as heat and this is called non‐photochemical quenching (NPQ). ɸPSII determines the amount of light absorbed by PSII allied chl molecule that is used in photochemistry. It is directly correlated with ETR and indicative of comprehensive photosynthetic rate. Fv/Fo representing water splitting activity (Siddiqui et al. 2018a) is fundamentally similar to Fv/Fm however more subtle to even a slight vicissitudes in efficiency (Pessarakli 2016).
Cd impaired the photolysis of water (Fig. 2a, A) and declined the photochemical quenching (Fig. 2a, B) of electrons, moreover, it promoted the NPQ (Fig. 2a, E) which collectively resulted in the reduction of PSII efficiency and ETR (Fig. 2a, D and F). The maximum dip in Fv/Fo (57% and 32%), qP, (20.2% and 15.0%), ɸPSII (20.0% and 14.8%), Fv/Fm (23.9% and 7.6%) and ETR (20.0% and 14.8%) was observed at 45 and 60‐day stage samplings, respectively at a concentration of 100 mg/kg of Cd in comparison with their respective control plants. The findings are supported by the study of Sharmila et al. (2017) where PSII activity declined in a concentration dependent manner under Cd stress. The reduction in PSII activity and ETR affect the rate of generation of reducing power (NADPH) and ATP which are essential for carbon and nitrate assimilation (Baker and Oxborough 2004). There was a progressive decrease in leaf gaseous exchange parameters (net photosynthetic rate, PN stomatal conductance, gs internal CO2 concentration, Ci transpiration rate, E) with increasing Cd concentration (0 < 25 < 50 < 100 mg/kg soil Cd) where 100 mg/kg soil Cd proved most toxic and reduced the PN (51.2% and 45.28%), Ci (25.5% and 16.2%), gs (72.7% and 57.1%) and E (26.9% and 20.2%) at 45 and 60 DAS, respectively. It has been reported that low gs (Fig. 2b) reduces photosynthesis (Haworth et al. 2016). The decrease in leaf gaseous exchange parameters i.e. PN, E, gs, and Ci are an outcome of stomatal abnormality such as stomatal closure and density (Ying et al. 2010).
Sucrose, glucose and fructose are few sugars that play signalling role in plant stress (Sami et al. 2016). These are important compatible solutes (Couée et al. 2006) and active participants during removal of free radicals under abiotic stress conditions (Kaur et al. 2017). Thus, sugars provide resistance against stress and maintain osmotic potential, redox reactions and structure of membrane and other macromolecules (Wang et al. 2011). In this study, sugar accumulation increased mildly at lowest dose of Cd, however, with subsequent rise in Cd concentration there was a steady decrease in sugar level (Fig. 3A–E). Similar trend was observed by Kapoor et al. (2016) in Brassica juncea growing under Cd stress, wherein the qualitative analysis of sugars revealed that glucose and fructose level increase at lower dose of Cd but decrease at higher. The increase in sugars despite of low photosynthetic rate might be a result of starch breakdown (Fig. 3F). This result finds support from the study conducted by Sun et al. (2017) wherein hexose and sucrose content of cucumber plants increased in presence of Cd whereas starch accumulation declined swiftly.
a SEM–EDX mapping: Effect of cadmium (0 and 100 mg/kg soil) on elemental composition of dried Brassica juncea leaves at 45 day stage of growth. Each element is mapped with a particular color. Elements are denoted with their symbols (C = carbon, N = nitrogen, Mg = magnesium, P = phoshphorus, S = sulphur and K = potassium). b Effect of different concentrations of cadmium (0, 25, 50 and 100 mg/kg soil) on the percent weight of different elements asterisk indicates significant difference between the control and different treatments (p ≤ 0.05)
Cd is known to inhibit various physiological functions in plants. It accelerates the cellular energy deficiency and oxidative stress which results in ROS production such as O2− and H2O2. H2O2 increased by 102.3% and 87% whereas O2− by 62.5% and 51.2%, respectively at 45 and 60 DAS under Cd (100 mg/kg) as compared to their respective controls (Fig. 5a, B and D). They damage the cell via peroxidation of polyunsaturated fatty acids of membrane, protein oxidation and DNA damage, thus, generating oxidative stress (Lannig et al. 2006; Kumar et al. 2012; Gill and Tuteja 2011). In this study, ROS outburst was observed in presence of Cd (Fig. 5a, A–D). The findings are similar to those of Faraz et al. (2019) where Cd promoted ROS production. ROS outburst was clearly visualized during histochemical studies where number of blue (O2−) and brown (H2O2) spots on leaves (Fig. 5a, A and C). Cd‐induced damage was evidently visible both in root and shoot (Fig. 5a, E). The increase in pink spots in leaves and roots of plants, growing in presence of Cd (100 mg/kg soil) was suggestive of enhanced lipid peroxidation. As a result of increased lipid peroxidation (5a, F), there was an increase in cell death or decreased cell viability (Fig. 7, A and B) with Cd (100 mg/kg soil) treatment showing large number of stained nuclei as compared to control.
The exposure of plant to high Cd concentration results in ROS generation which rapidly causes necrosis of cells exposed to stress (Garnier et al. 2006; De Michele et al. 2009). To counter the ROS production, plants generate antioxidant enzymes such as SOD, CAT and POX as well as non‐enzymatic antioxidant such as proline (Siddiqui et al. 2018b; Kaur et al. 2017). The observed increase antioxidant enzymes (Fig. 5b, A, D and E) along with proline content (Fig. 5b; 3G) are expected to be ROS dependent response (Fig. 6,A–D). The increase in ROS level (Fig. 5a, A–D) triggered the accumulation of antioxidants. Similar response upon encountering Cd stress was reported by Irfan et al. (2014). The in‐gel activity of SOD, CAT and POX visualized at 45‐day stage of growth (Fig. 5b, B, C and F) showed the increase in band width and intensity in Cd (100 mg/kg soil) treated plants as compared to the water treated control. Moreover, protein quantification peaks confirmed the enhancement in the protein and its activity (Fig. 5b, B, C and F).
Cd inhibits the growth of root and shoot moreover, it alters the nutrient uptake and gets incorporated in plant parts (Rizwan et al. 2018; Di Toppi and Gabrielli 1999). In this study, there was a steep decline in the nutrient status (C, N, Mg, S, P and K) of plant (Fig. 4). A dip of 60% and 53.7% for N; 39.0% and 32.6% for C; 57.6% and 48.1% for Mg; 72.2% and 52.5% for K; 52.% and 40.8% for S; 39.5% and 33.9% for P was observed at 45 and 60 DAS, respectively in presence of highest Cd concentration used (100 mg/kg) over their respective control (Fig. 4b). SEM–EDX mapping of dried leaf provided visual evidence supporting reduction in colour intensity of various elements (C, N, Mg, S, P and K) in Cd treated (100 mg/kg soil) plants as compared to the non‐treated control at 45 DAS (Fig. 4a). E is considered indispensible for transport of mineral nutrients to different parts of plants (Campbell et al. 1999). Mg, S, P and K are involved in photosynthesis and activation of respiratory enzymes (Taiz and Zeiger 1998). Being cationic in nature Cd competes with mineral elements in their uptake and transport across the cell membrane (Ismael et al. 2019). Hence, the decrease in growth (Fig. 1) could be a consequence of imbalance in nutrient uptake (Fig. 4) due to competition between toxic Cd ions and mineral elements.
Cd‐induced toxicity seemed to be more pronounced 45‐day stage of growth when compared to 60 DAS (Figs. 1, 2, 3, 4, 5, 6, 7, 8). This effect might be an outcome of comparatively higher expression of Ferrochelatase‐1 (FC1) gene in presence of Cd (Song et al. 2017) at 60 DAS. FC1 codes for an important enzyme of heme biosynthesis and for insertion of ferrous iron into protoporphyrin IX (Proto IX) to produce protoheme. During heme and chl biosynthesis, Proto IX forms the branch point of the tetrapyrrole pathway. Heme works as a cofactor of several important proteins required for different biological processes for example electron transfer, oxygen and secondary metabolism (Heinemann et al. 2008). Moreover, FC1‐produced heme is also required for the up–regulation of genes involved in synthesis of ROS scavengers to confer tolerance against abiotic stress (Singh et al. 2002; Nagai et al. 2007; Cao et al. 2011; Phung et al. 2011; Shen et al. 2011).
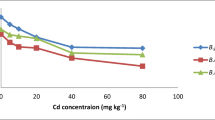
Uptake of Cd ions uses the same transporters that are involved in the uptake Fe2+, Ca2+, Mg2+, Zn2+ and Cu2+ (Clemens 2006). BjCdR15 (bZIP transcription factor) plays important role in the regulation of Cd uptake by roots and its transport from root to shoot (Farinati et al. 2010). The toxic effects of plant Cd on plant physiology were outcome of increased translocation of Cd from root to shoot. Cd reduced the growth (Fig. 1) of the plant and got accumulated in plant tissues. There was a rise in the uptake of Cd by roots (Fig. 8C) and its transport to shoot (Fig. 8D). B. juncea is considered a model plant for phytoremediation because of its ability to accumulate Cd more than 400 µg/g dry weight in shoot (Minglin et al. 2005). In the present study, B. juncea accumulated a much higher than 100 µg/g dry weight of Cd which is the minimum requirement for a plant to be recommended as hyperaccumulator (Baker and Brooks 1989) in both root and shoot. Moreover, Cd hyperaccumulator possess the ability to accumulate around 104 mg Kg−1 Cd in shoot dry weight (Mahajan and Kaushal 2018). Highest dose of Cd (100 mg/kg of soil) resulted in accumulation of 9969 and 10,011 µg/g dry weight at 45 and 60 DAS, respectively in shoots whereas, in root the content was 8410 and 9630 µg/g dry weight at 45 and 60 DAS, respectively. Cd accumulation in plant was manifold higher than its usual concentration in plant which is 0.21 µg/g (FAO/WHO 1984). For a plant to be considered as a hyperaccumulator, the value of translocation factor should be more than 1 (Garbisu and Alkorta 2001). Figure 8 clearly indicates that TF for B. juncea in this study was more than 1 at all concentrations as well as growth stages. TF increased in the order of Cd concentration 100 < 25 < 50 mg/kg soil 45 and 60 DAS. The comparatively lower value of TF (Fig. 8) at highest Cd concentration (100 mg/kg soil) is suggestive of drifting of the plant towards acquirement of exclusion strategy (Goswami and Das 2015).
Exposure of B. juncea plants to Cd altered the physiology which resulted in reduced growth of the plant. Cd impaired the photolysis of water and PSII activity which resulted in the inhibition of ETR. Furthermore, the reduction in gs restricted the entry of CO2 inside the leaf thus, lowering Ci values and ultimately low PN. Reduced E as well as competition posed by Cd resulted in limited absorption of nutrients such as Mg, P, S and K from soil which hampered with chlorophyll biosynthesis and normal functioning of various physiological processes. Sugars accumulated in response to lower level of stress (25 mg/kg soil) and acted as an osmolyte, this rise in sugar level despite of low PN is expected to be an outcome of starch breakdown. However, higher dose of Cd proved highly toxic, and reduced the sugar as well as starch level. Cd perturbed the balance between ROS and antioxidants and induced lipid peroxidation which resulted in cell death. Low photosynthesis, nutrient scarcity and ROS outbreak affected the normal growth of the plant. Though Cd accumulation was a bit higher at 60 DAS than 45 DAS but the toxicity symptoms were more pronounced at 45 DAS suggesting an adaptive response of B. juncea towards Cd stress. The toxicity was an outcome of enhanced translocation of HM from root to shoots. The TF > 1 at all the stages of growth and doses suggests that B. juncea is a hyperaccumulator. Despite of toxicity, altered physiology and stunted growth the plant managed to survive and adapt to the stressful environment hence, B. juncea could be recommended as an ideal plant for phytoextraction purpose.
References
Ashwell G (1957) Colorimetric analysis of sugars. In: Colowick SJ, Kaplan NO (eds) Methods in Enzymology, vol 3. Academic Press, New York, pp 373–105
Baker AJM, Brooks R (1989) Terrestrial higher plants which hyperaccumulate metallic elements A review of their distribution, ecology and phytochemistry. Biorecov. 1(2):81–126
Baker NR, Oxborough K (2004) Chlorophyll fluorescence as a probe of photosynthetic productivity. Chlorophyll a Fluorescence. Springer, Dordrecht, pp 65–82
Campbell NA, Reece JB, Mitchell LG (1999) Biology, 5th edn. Addison Wesley Longman, Boston p, p 703
Cao Z, Geng B, Xu S, Xuan W et al (2011) BnHO1, a haem oxygenase–1 gene from Brassica napus, is required for salinity and osmotic stress–induced lateral root formation. J Exp Bot 62(13):4675–4689
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimica 88(11):1707–1719
Couée I, Sulmon C, Gouesbet G, El Amrani A (2006) Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot 57(3):449–459
Dalio RJD, Pinheiro HP, Sodek L, Haddad CRB (2011) The effect of 24-epibrassinolide and clotrimazole on the adaptation of Cajanus cajan (L.) Millsp. to salinity. Acta Physiol Plant 33(5):1887–1896.
Das S, Goswami S, Talukdar AD (2014) A study on cadmium phytoremediation potential of water lettuce, Pistia stratiotes L. Bull Environ Contam Toxic 92(2):169–174
De Michele R, Vurro E, Rigo C, Costa A et al (2009) Nitric oxide is involved in cadmium–induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol 150(1):217–228
Di Toppi LS, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41(2):105–130
FAO/WHO (1984) In: Contaminants. Codex Alimentarius. Vol. XVII, 1st ed. Rome (Italy): FAO/WHO, Codex Alimentarius Commission.
Faraz A, Faizan M, Sami F, Siddiqui H, Hayat S (2019) Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. J Plant Growth Regul 9:1–15
Farinati S, DalCorso G, Varotto S, Furini A (2010) The Brassica juncea BjCdR15, an ortholog of Arabidopsis TGA3, is a regulator of cadmium uptake, transport and accumulation in shoots and confers cadmium tolerance in transgenic plants. New Phytol 185(4):964–978
Garbisu C, Alkorta I (2001) Phytoextraction: a cost–effective plant–based technology for the removal of metals from the environment. Biores Technol 77(3):229–236
Garnier L, Simon-Plas F, Thuleau P, Agnel JP et al (2006) Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ 29(10):1956–1969
Gill SS, Tuteja N (2011) Cadmium stress tolerance in crop plants: probing the role of sulfur. Plant Signal Behave 6(2):215–222
Goswami S, Das S (2015) A study on cadmium phytoremediation potential of Indian mustard Brassica juncea. Int J Phytoremed 17(6):583–588
Guidi L, Landi M, Lo Piccolo E (2019) Chlorophyll fluorescence, photoinhibition and abiotic stress: does it make any difference the fact to be a C3 or C4 species? Front Plant Sci 10:174
Hasan SA, Fariduddin Q, Ali B, Hayat S, Ahmad A (2009) Cadmium: toxicity and tolerance in plants. J Environ Biol 30(2):165–174
Haworth M, Killi D, Materassi A, Raschi A, Centritto M (2016) Impaired stomatal control is associated with reduced photosynthetic physiology in crop species grown at elevated [CO2]. Front Plant Sci 7:1568
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60(1):33–41
Hayat S, Khalique G, Wani AS, Alyemeni MN, Ahmad A (2014) Protection of growth in response to 28–homobrassinolide under the stress of cadmium and salinity in wheat. Int J Biol Mac 64:130–136
Heinemann IU, Jahn M, Jahn D (2008) The biochemistry of heme biosynthesis. Arch Biochem Biophys 474(2):238–251
Irfan M, Ahmad A, Hayat S (2014) Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J Biol Sci 21(2):125–131
Ismael MA, Elyamine AM, Moussa MG, Cai M, Zhao X, Hu C (2019) Cadmium in plants: uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 11(2):255–277
Jinadasa N, Collins D, Holford P, Milham PJ, Conroy JP (2016) Reactions to cadmium stress in a cadmium-tolerant variety of cabbage (Brassica oleracea L.): is cadmium tolerance necessarily desirable in food crops? Environ Sci Pollut Res 23:5296–5306
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants. CRC Press, Boca Raton
Kapoor D, Rattan A, Bhardwaj R, Kaur S (2016) Photosynthetic efficiency, ion analysis and carbohydrate metabolism in Brassica juncea plants under cadmium stress. J Pharmacog Phytochem 5(3):279
Kaur R, Yadav P, Thukral AK, Walia A, Bhardwaj R (2017) Co-application of 6-ketone type brassinosteroid and metal chelator alleviates cadmium toxicity in B. juncea L. Environ Sci Pollut Res 24:685–700
Kumar A, Prasad MNV, Sytar O (2012) Lead toxicity, defense strategies and associated indicative biomarkers in Talinum triangulare grown hydroponically. Chemosphere 89(9):1056–1065
Lannig G, Cherkasov AS, Sokolova IM (2006) Temperature–dependent effects of cadmium on mitochondrial and whole–organism bioenergetics of oysters (Crassostrea virginica). Mar Environ Res 62:S79–S82
Mahajan P, Kaushal J (2018) Role of phytoremediation in reducing cadmium toxicity in soil and water. J Toxicol 2018:16. https://doi.org/10.1155/2018/4864365
Meuwly P, Rauser WE (1992) Alteration of thiol pools in roots and shoots of maize seedlings exposed to cadmium: adaptation and developmental cost. Plant Physiol 99(1):8–15
Minglin L, Yuxiu Z, Tuanyao C (2005) Identification of genes up–regulated in response to Cd exposure in Brassica juncea L. Gene 363:151–158
Nagai S, Koide M, Takahashi S, Kikuta A et al (2007) Induction of isoforms of tetrapyrrole biosynthetic enzymes, AtHEMA2 and AtFC1, under stress conditions and their physiological functions in Arabidopsis. Plant physiol 144(2):1039–1051
Pessarakli M (2016) Handbook of photosynthesis. CRC Press, Taylor and Francis group, Boca Raton
Phung TH, Jung HI, Park JH, Kim JG et al (2011) Porphyrin biosynthesis control under water stress: sustained porphyrin status correlates with drought tolerance in transgenic rice. Plant physiol 157(4):1746–1764
Rizwan M, Ali S, ur Rehman MZ, et al (2018) Cadmium phytoremediation potential of Brassica crop species: a review. Sci Total Environ 631:1175–1191
Roe JH (1934) A colorimetric method for the determination of fructose in blood and urine. J Biol Chem 107(1):15–22
Sadasivam S, Manickam A (1996) Biochemical methods. New Age International, New Delhi
Sami F, Yusuf M, Faizan M, Faraz A, Hayat S (2016) Role of sugars under abiotic stress. Plant Physiol Biochem 109:54–61
Sharmila P, Kumari PK, Singh K, Prasad NVSRK, Pardha-Saradhi P (2017) Cadmium toxicity-induced proline accumulation is coupled to iron depletion. Protoplasma 254:763–770
Shen QI, Jiang M, Li HUA, Che LL, Yang ZM (2011) Expression of a Brassica napus heme oxygenase confers plant tolerance to mercury toxicity. Plant Cell Environ 34(5):752–763
Siddiqui H, Ahmed KBM, Hayat S (2018a) Comparative effect of 28–homobrassinolide and 24–epibrassinolide on the performance of different components influencing the photosynthetic machinery in Brassica juncea L. Plant Physiol Biochem 129:198–212
Siddiqui H, Yusuf M, Faraz A et al (2018b) 24-Epibrassinolide supplemented with silicon enhances the photosynthetic efficiency of Brassica juncea under salt stress. South African J Bot 118:120–128
Singh DP, Cornah JE, Hadingham S, Smith AG (2002) Expression analysis of the two ferrochelatase genes in Arabidopsis in different tissues and under stress conditions reveals their different roles in haem biosynthesis. Plant Mol Biol 50(4–5):773–788
Song J, Feng SJ, Chen J, Zhao WT, Yang ZM (2017) A cadmium stress-responsive gene AtFC1 confers plant tolerance to cadmium toxicity. BMC Plant Biol 17(1):187
Song X, Yue X, Chen W, Jiang H, Han Y, Li X (2019) Detection of cadmium risk to the photosynthetic performance of Hybrid Pennisetum. Front Plant Sci 10:798
Sun H, Wang X, Wang Y, Wei Y, Wang G (2017) Alleviation of cadmium toxicity in cucumber (Cucumis sativus) seedlings by the application of selenium. Span J Agric Res 14(4):1105
Taiz L, Zeiger E (1998) Plant Physiology, 2nd edn. Sinaeur Associates Inc, Saunderland, pp 109–110
Tripathy BC, Oelmüller R (2012) Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7(12):1621–1633
Van Ginneken L, Meers E, Guisson R, Ruttens A et al (2007) Phytoremediation for heavy metal-contaminated soils combined with bioenergy production. J Environ Eng Landsc Manage 15(4):227–236
Wang RS, Pandey S, Li S, Gookin TE, Zhao Z, Albert R, Assmann SM (2011) Common and unique elements of the ABA-regulated transcriptome of Arabidopsis guard cells. BMC genomics 12(1):216
Ying RR, Qiu RL, Tang YT, Hu PJ et al (2010) Cadmium tolerance of carbon assimilation enzymes and chloroplast in Zn/Cd hyperaccumulator Picris divaricata. J Plant Physiol 167(2):81–87
Acknowledgements
Authors gratefully acknowledge the Chairman, Department of Botany, Aligarh Muslim University, India for providing the required facilities to carry out this experimental work and UGC under Grant No. 15PHDBTM003 for providing funds in the form of non‐net fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siddiqui, H., Ahmed, K.B.M., Sami, F. et al. Phytoremediation of Cadmium Contaminated Soil Using Brassica juncea: Influence on PSII Activity, Leaf Gaseous Exchange, Carbohydrate Metabolism, Redox and Elemental Status. Bull Environ Contam Toxicol 105, 411–421 (2020). https://doi.org/10.1007/s00128-020-02929-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02929-3