Summary
Algae are characterized by the presence of plastids (chloroplasts), which are organelles of cyanobacterial origin. Plastids have their own genome, machineries for replication, transcription and translation, and are the site of photosynthesis (except in secondarily non-photosynthetic species) and a variety of other biological functions. Algae are subdivided into those whose plastids can be traced back to a common cyanobacterial endosymbiont (algae with primary plastids), and others in which plastids are second-hand acquisitions that were introduced by eukaryote-eukaryote endosymbioses.
Only a fraction of plastid components is encoded in plastid DNA; the majority of genes coding for plastid proteins are in the nucleus, many of which originated through transfers (in some cases still ongoing) from the organelle to the nuclear genome. Despite the broad phylogenetic affiliation of algae, most plastid genomes are fairly homogenous, coding for about 100–250 genes, except in non-photosynthetic algae that rapidly lose genes involved in photosynthesis. The most gene-rich and cyanobacteria-like plastid genomes are in red algae, followed by glaucophyte and green algae. Genomes in secondary or higher-order plastids usually have a reduced gene count, compared to their primary photosynthetic donors. In this chapter, we provide an overview on the evolutionary history, organization and coding properties of algal plastid genomes, for which complete (or almost complete) sequences are publicly available.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
The term ‘algae’ as used here includes all plastid-containing eukaryotes, except land plants and ‘blue-green algae’ (a popular misnomer for Cyanobacteria). Algae cover a large variety of about 20 taxonomic groups (among the best-known ones are green, red, brown and golden algae, diatoms, glaucophytes, raphidophytes, cryptophytes, haptophytes, chlorarachniophytes, dinoflagellates and euglenids). Some of these groups include both unicellular and multi-cellular species (e.g., the large-size brown algal kelp, various red and green algal taxa). In rare instances, algae are secondarily non-photosynthetic, carrying a plastid genome with reduced coding capacity; these include the colorless green algae Prototheca and Helicosporidium (Knauf and Hachtel 2002; Pombert and Keeling 2010); the euglenid Euglena (Astasia) longa (Knauf and Hachtel 2002); and Plasmodium and its apicomplexan relatives (McFadden and Waller 1997; Wilson and Williamson 1997).
Plastid genomes are best described and compared within an evolutionary framework (a phylogenetic tree based on plastid protein sequences is shown in Fig. 3.1), which is however more easily said than done. This is because phylogenetic placement of reduced or fast-evolving plastid sequences is challenging due to lack of phylogenetic signal. Another difficulty arises from the different evolutionary routes followed by plastids: vertical descent from a Cyanobacterium, and lateral acquisition from other eukaryotes. The latter entails the transfer of both the complete plastid DNA (ptDNA) plus an often undetermined number of nuclear genes from the symbiont to the host nucleus, leading to potential phylogenetic misinterpretations. For instance, the plastid tree in Fig. 3.1 groups dinoflagellates such as Kryptoperidinium and Durinskia with diatoms, apicomplexans with stramenopiles, cercozoans with green algae, and so on. One may indeed wonder which of the shown phylogenetic relationships represent vertical evolutionary descent at all. The only notable exception are primary photosynthetic eukaryotes (green, red, glaucophyte algae and land plants – collectively known as ‘Plantae’; (Cavalier-Smith 1981); see also Chap. 1), whose plastids derive directly from a cyanobacterial origin, and which are therefore expected to form a monophyletic group with nuclear, plastid and mitochondrial genes in phylogenetic analyses (Baurain et al. 2010).
Phylogeny based on ptDNA-encoded proteins. The phylogeny was inferred with a set of 76 derived ptDNA-encoded protein sequences that are most common across algae. Non-photosynthetic species such as Plasmodium or Helicosporidium were excluded, as their ptDNAs encode only small subsets of genes that evolve at elevated rates (i.e., problematic for phylogenetic inference). Sequences were aligned with software developed in-house. Briefly, derived protein sequences are pre-aligned with Muscle (Edgar 2004), and alignments are iteratively refined with HMMalign (S. Eddy; http://hmmer.janelia.org) using E-values obtained with respective HMM models as an optimization criterion. Sequence positions that are not aligned with a posterior probability value of 1.0 are discarded, and alignments are concatenated. The resulting dataset including 48 ptDNAs (plus a cyanobacterial outgroup) has 17,409 amino acid positions. It was analyzed using PhyloBayes that implements Bayesian inference and the CAT model, known to be least sensitive to LBA artifacts (Lartillot and Philippe 2004; Rodriguez-Ezpeleta et al. 2007b; Lartillot and Philippe 2008, and references therein). It is best suited for the placement of rapidly-evolving species. All branches are supported by posterior probability values of 1.0 except where indicated. Species are color-coded according to their plastid origins as green, red or magenta (glaucocystophytes). Plastids that were transferred by secondary or higher-order eukaryote-eukaryote endosymbiosis are marked with an asterisk. The branch length of Chromera (dotted broken line) is about three times as long as indicated. Its association with Alveolata could be due to LBA; if analyzed alone, Chromera tends to group weakly with stramenopiles.
A. Origin and Evolution of Primary Photosynthetic Algae and Their Plastids
The origin of primary plastids represents a relatively late step in eukaryotic evolution, well after the endosymbiosis with the α-Proteobacterium that evolved into the mitochondrion. Most plastid genomes retain many more features of their (cyano) bacterial ancestor than do mitochondrial genomes, such as large conserved bacterial operons and bacteria-like RNA polymerases (but see the notable exception in jakobid mitochondria; Lang et al. 1997). Although plastids came in relatively late, the exact nature of the eukaryotic group which acquired plastids remains vague, as primary (ancestrally) non-photosynthetic members belonging to Plantae are unknown. In fact, even phylogenomic evidence for the monophyly (i.e. divergence from a single, common origin) of Plantae varies with taxon and gene sampling, with significant statistical support in some cases (e.g., Rodriguez-Ezpeleta et al. 2005 and references therein) but not in others (e.g., Burki et al. 2009; Baurain et al. 2010; Parfrey et al. 2010; Chan et al. 2011). Likewise, the branching order of primary photosynthetic lineages has been elusive, depending much on the choice of genes and species included in phylogenies (Rodriguez-Ezpeleta et al. 2005; Reyes-Prieto and Bhattacharya 2007; Deschamps and Moreira 2009). Taken together, much remains to be done in terms of resolving the origin and the evolutionary divergence of Plantae. Apparently, the resolution of the deepest branches of the eukaryotic tree remains unsatisfying, as deep eukaryotic (protist) diversity continues to be poorly sampled at the genome level. Yet, for sake of simplicity, we will assume in the following that Plantae is a valid taxonomic grouping, and therefore discuss plastids in two major subdivisions, (1) those derived from a primary endosymbiotic event and (2) those that have been acquired by higher-order (secondary, tertiary …) endosymbioses among eukaryotes.
B. Algae with Second-Hand Plastids: Eukaryote-Eukaryote Endosymbioses
In contrast to Plantae – which are characterized by plastids with two surrounding membranes – there are three or four membranes in algae that have undergone eukaryote-eukaryote endosymbiosis, the focus of most reviews on plastid DNAs (ptDNAs; e.g., Douglas and Gray 1991; Wolfe et al. 1991; Douglas 1998; McFadden 1999; Moreira and Philippe 2001; Archibald and Keeling 2002; Stoebe and Maier 2002; Bhattacharya et al. 2004; Reyes-Prieto et al. 2007; Gould et al. 2008; Archibald 2009; Keeling 2009; Keeling 2010). These plastids are in most instances retraced to either a red or green algal origin (Fig. 3.1), but whether the endosymbiotic event is secondary, tertiary or higher-order often remains speculative. In particular, the source of the highly reduced ‘apicoplast’ plastids in alveolates (e.g., Plasmodium, Eimeria, etc.) remains uncertain, believed to be of either red (Williamson et al. 1994; Fast et al. 2001; Foth and McFadden 2003) or green algal origin (Kohler et al. 1997; Funes et al. 2002, 2004). Even gene transfer from mitochondrial DNA (mtDNA) to apicoplast DNA has been proposed (Obornik et al. 2002), and there is currently no convincing avenue for overcoming the massive phylogenetic artifacts (long-branch-attraction artifacts or LBA) that are the likely cause of the unsettled dispute. Phylogenetic analyses including these species are so questionable because of both the small number of remaining plastid genes and their extreme evolutionary rates.
Another confounding factor in these analyses is the number of symbiotic events that took place across eukaryotes. Plastids in cryptophytes, alveolates, stramenopiles plus haptophytes (collectively, CASH) likely arose from a single secondary endosymbiosis with a red alga because of a unique, shared feature of their plastids, the presence of chlorophyll c, and because phylogenies based on plastid sequences (plus a few nuclear genes involved in plastid function) clearly regroup CASH with red algae. However, the consensus in interpretation stops here. Based on the idea that eukaryote-eukaryote endosymbiosis is a very rare event, proponents of the ‘chromalveolate hypothesis’ (Cavalier-Smith 2002; Keeling 2009, 2010) postulate a single, ancient secondary endosymbiosis with a red alga. This supposition is contested by others predicting much more frequent (higher-order), serial plastid transfers (Sanchez-Puerta et al. 2007; Baurain et al. 2010; Gray 2010 and references therein). We share the interpretation of frequent transfers because of cumulating evidence in this direction. In dinoflagellates, for instance, there is compelling evidence for a number of subsequent plastid replacements (e.g., Minge et al. 2010). Another (contentious) example is in two presumed photosynthetic relatives of Apicomplexa, Chromera velia and Alveolata sp. (CCMP3115). Phylogenies with several concatenated nuclear genes confirm that they represent taxonomically deep divergences to Apicomplexa, and a 34 plastid gene phylogeny associates their plastids close to (but outside of) stramenopiles (heterokonts; Janouskovec et al. 2010). According to the authors’ interpretation, this represents support for the chromalveolate hypothesis. Yet, the plastid phylogeny that we performed for the purpose of this review, with an extended number of species (79) and proteins (76) comes to a different conclusion, placing Chromera and Alveolata plastids together within stramenopiles (Fig. 3.1), indicative of a higher-order endosymbiosis. This example indicates that phylogenetic analyses with data from fast-evolving genomes have to be interpreted with extreme prudence, in particular when these diverge deeply in a tree, an indicator for a potential phylogenetic reconstruction artifact (Philippe et al. 2005). In turn, when broader taxon sampling and/or the use of a superior (more realistic) evolutionary model, such as CAT (Lartillot and Philippe 2004; Lartillot et al. 2007), leads to an alternative tree topology favoring the regrouping of rapidly with slowly evolving species, even with limited statistical support (as in Fig. 3.1), it is more likely the correct one. Clearly, further investigation of the given example is needed, which falls outside the mission of this review.
Given the confusion in distinguishing secondary and higher-order eukaryote-eukaryote endosymbionts, we will only refer to the following five well established taxa: (1) golden, brown, diatom and raphidophyte algae (Stramenopila; Patterson 1989), (2) Alveolata plus Stramenopila and Rhizaria (SAR group; Burki et al. 2007; Hackett et al. 2007; Rodriguez-Ezpeleta et al. 2007a), (3) haptophytes, (4) cryptophytes, and (5) euglenids (belonging to the ‘JEH group’ uniting jakobids, Euglenozoa plus Heterolobosea; Rodriguez-Ezpeleta et al. 2007a). It is noteworthy that there is only one major eukaryotic supergroup without photosynthetic members (and without evident genetic remnants of eukaryote-eukaryote endosymbioses), the Unikonta. This group comprises Opisthokonta (animals, fungi and their protist relatives), Amoebozoa, and arguably, Apusozoa.
In the following, we will review plastid genome organization in the various groups of algae. The highly reduced alveolate ptDNAs (apicoplasts) will not be discussed in detail as they have been well described elsewhere (Wilson and Williamson 1997; McFadden 2011).
II. Plastid Genome Organization, Genes and Functions
We will start with a short introduction on the structure of plastid genomes and the type of genes they encode, across all eukaryotes. For sequence records we refer to the plastid genome section at GenBank, and two curated databases, GOBASE (O’Brien et al. 2009) and ChloroplastDB (Cui et al. 2006). Note that (1) the catalogue of complete ptDNAs in GenBank’s genome section is currently incomplete (e.g., most records of the reduced apicomplexan and several green algal ptDNAs are missing and have to be retrieved from the nucleotide section), and gene and intron information is only validated as to consistency; (2) information in GOBASE is no longer being updated as of 2010, and (3) ChloroplastDB’s last update (at the time of writing this review) was in 2007, lacks taxonomical grouping of species, and data on certain structural RNAs (RNase P, tmRNA and signal recognition particle RNAs).
A. Plastid Genome Structure
Generally, plastids contain a single type of chromosome in multiple copies. Restriction analysis and sequencing revealed that most ptDNAs are circular-mapping (not to be confused with truly circular DNA molecules), likely representing linear head-to-tail concatemers, plus subgenome-size fragments that tend to occur in genomes carrying repeat regions (Bendich 2004, 2007; Oldenburg and Bendich 2004). A similar genome structure is observed in mitochondria (Oldenburg and Bendich 2001; Ling and Shibata 2004). The mechanism of replication remains essentially an open question. Small organelle genomes might be replicated by a rolling circle mechanism, but the presence of a substantial fraction of subgenome-size fragments suggests a more complex mechanism, likely including recombination, or template switching in other instances. Experimental evidence for this or any other type of organization and replication is available for only a small fraction of known plastid genomes. A notable curiosity exists in dinoflagellates, where several genes are separately encoded on DNA minicircles (Zhang et al. 1999, 2002; Howe et al. 2008). However, whether these circles represent the principal genome organization or highly abundant subgenomic molecules (e.g., replicative rolling circle DNAs) remains to be demonstrated. For more information on dinoflagellate plastids, we refer to recent publications (e.g., Zhang et al. 1999, 2002; Stoebe and Maier 2002; Hackett et al. 2004; Laatsch et al. 2004; Howe et al. 2008; Keeling 2010).
A widespread feature of pt genomes is a large inverted repeat (IR) region that contains genes for rRNAs and a variable number of tRNAs and proteins (e.g., Gardner et al. 1993; Douglas and Penny 1999; Sanchez Puerta et al. 2005; Belanger et al. 2006; Cattolico et al. 2008; Tanaka et al. 2011). The biological role of the IR region is likely increased gene dosage for ribosomal components (ribosomes are among the most abundant sub-cellular structures). The IR region may be present or not in related species (e.g., Pedinomonas minor, Parachlorella kessleri and Oocystis solitaria have this trait, whereas Chlorella vulgaris does not; Turmel et al. 2009b). Similarly, the two ptDNAs of photosynthetic cryptomonads have large inverted repeat regions containing rDNA genes in contrast to the direct repeats in Porphyra species, and no repeat in Cyanidium (Glockner et al. 2000), Cyanidioschyzon (Ohta et al. 2003) and Gracilaria (Hagopian et al. 2004). The pt genome of the secondarily non-photosynthetic cryptomonad C. paramecium has single-copy rRNAs as most red algae, which is best explained as a secondary loss of the repeat. This comparison shows that, although repeat features are given high attention in many publications on complete plastid genomes, they are not well conserved across eukaryotes and are of undefined value for understanding the evolution of genome structure and function.
B. Plastid-Encoded Functions, Genes and Introns
Plastids perform numerous biological functions that rely to a large extent on nuclear genes, and that are translated in the cytoplasm and transported into plastids. For detailed information on protein import see (McFadden 1999; Wastl et al. 2000; Wastl and Maier 2000; van Dooren et al. 2001; Foth et al. 2003; Nassoury et al. 2003; Patron et al. 2005; Durnford and Gray 2006; Chaal and Green 2007; Patron and Waller 2007; Kessler and Schnell 2009; Ma et al. 2009; Felsner et al. 2010; Hempel et al. 2010; Kovacs-Bogdan et al. 2010; Li and Chiu 2010; Strittmatter et al. 2010).
Biological processes that involve at least some ptDNA-encoded genes are translation and photosynthesis. Only species that lost their photosynthetic capacity gradually eliminate the corresponding genes (Gockel and Hachtel 2000; de Koning and Keeling 2006). Additional biological processes that rely on pt-encoded genes involve transcription, protein transport and plastid division. Further, in a more restricted number of cases, ptDNAs code for components for tRNA processing (RNase P RNA), quality control of protein translation (tmRNAs; Gueneau de Novoa and Williams 2004), the signal recognition particle RNA (Rosenblad and Samuelsson 2004; Schunemann 2004), plus several other functions that are limited to the most gene-rich ptDNAs, in particular from red algae (Glockner et al. 2000; Ohta et al. 2003; Hagopian et al. 2004). Currently recognized pt genes and their functions are compiled in Table 3.1. This list is expected to extend as the functions of ycf genes and additional ORFs are being identified. All of the above processes are directly derived from the cyanobacterial ancestor of plastids (only a few genes/functions were acquired by lateral transfer). The pattern of genes and functions represented by ptDNA-encoded genes often does not correspond with phylogenetic affinities (i.e., gene presence/absence is an unreliable phylogenetic marker), as gene migration to the nucleus or complete gene loss has occurred numerous times independently across various eukaryotic lineages.
The most reduced ptDNAs of photosynthetically active species are those in dinoflagellates that are organized in minicircles (Howe et al. 2008), encoding a bit more than a dozen identified genes, followed by the apicomplexans Alveolata sp. (CCMP3115; 124 genes) and Chromera velia (112 genes; Janouskovec et al. 2010). At the other side of the spectrum, red algae have the most gene-rich, densely packed pt genomes (with up to ∼254 genes).
Intron counts for ptDNAs are most variable: none in almost all red algae and in plastids of red algal origin (e.g., Douglas and Penny 1999; Glockner et al. 2000; Hagopian et al. 2004; Sanchez Puerta et al. 2005; Oudot-Le Secq et al. 2007), but 26 in the green alga Floydiella terrestris, and contrary to expectations, more than 100 in the red alga Compsopogon caeruleus (B.F.L. unpublished). Introns in pt genomes belong to group I and II, and are sometimes difficult to classify, because distinct secondary structure features are highly derived; i.e. such introns are only detected because coding regions are discontinuous. The most derived introns (group III, some organized in ‘twintrons’) are present in Euglena gracilis and E. longa ptDNAs (Copertino and Hallick 1993; Hallick et al. 1993), and are likely derived from group II introns (Copertino and Hallick 1993). The >100 introns in Compsopogon sp. (staghorn alga) ptDNA are also group II-related, some typical but others barely recognizable (B.F.L., unpublished). Finally, in some instances of group II intron-mediated trans-splicing, exons are located in distant genomic regions, transcribed separately and ligated to give rise to functional mRNAs (e.g., Goldschmidt-Clermont et al. 1991; Rochaix 1996; Rivier et al. 2001; Turmel et al. 2002; Belanger et al. 2006; Brouard et al. 2008, 2010; Jacobs et al. 2010). In conclusion, identification of introns can be difficult and some may be missed, even when applying most sophisticated search algorithms.
Currently, only few tools are available for automated intron recognition plus classification (Eddy 2008; Beck and Lang 2009; Gardner et al. 2009), and ptDNA annotation in general (Wyman et al. 2004; Jansen et al. 2005; Beck and Lang 2010). Tools developed by us (MFannot, RNAweasel; Beck and Lang 2009, 2010), although not fine-tuned for ptDNAs, appear to be most effective and miss only a few genes, small exons, and complex gene structures due to trans-splicing. Identification of structured RNAs is an area that needs improvements, including precise delineation of rRNA gene extremities and intron/exon boundaries. RNAse P RNA can be identified with RNAweasel or MFannot, but search models for tmRNAs and signal recognition particle RNAs remain to be added, together with an update of structural models that allow prediction of the whole range of plastid introns. Given the rapidly increasing number of genome sequences produced by new sequencing technologies, we will have to develop increasingly effective, semi-automated ways of genome annotation and GenBank submission to keep pace with data production.
III. Plastids Derived from Primary Endosymbiosis with Cyanobacteria
Plantae is a potentially monophyletic assemblage of photosynthetic (and some secondarily non-photosynthetic) lineages with primary plastids, i.e. derived directly from an endosymbiotic cyanobacterium. This large and diverse group is divided into the glaucophytes, rhodophytes (red algae) and Viridiplantae (green algae and land plants). To date, plastid genomes are available for only two glaucophytes and seven red algae (two of which unpublished; B.F.L.), but a large and rapidly growing number of green algae. The reason for this bias may be related to the difficulty of growing sufficient quantities of cell material for red and glaucophyte algae, a difficulty that no longer exists with the new sequencing technologies that require only small quantities of total DNA.
A. Rhodophyta
Rhodophyta is a morphologically diverse group with several thousand described species, both unicellular and multicellular ones. Red algal cells are characterized by the lack of centrioles and a flagellar apparatus, and the presence of phycoerythrin-containing plastids with unstacked thylakoids. Resolution of phylogenetic relationships among red algal lineages is currently limited by taxon and gene sampling (e.g., Le Gall and Saunders 2007; Verbruggen et al. 2010) and references therein), and may also be due to unequal rates of sequence evolution among red algae.
Complete plastid genomes are available from only five species, These include the multicellular taxa Porphyra purpurea and Porphyra yeozensis (Bangiales; Reith and Munholland 1995), the unicellular Cyanidales Cyanidioschyzon merolae (Ohta et al. 2003) and Cyanidium caldarium (Glockner et al. 2000), and the florideophycean Gracilaria tenuistipitata (Hagopian et al. 2004). Two additional ptDNAs are currently being sequenced in our laboratory (Stylonema alsidii, UTEX LB1424 and Compsopogon caeruleus, UTEX LB1553).
The first sequenced red algal ptDNA (P. purpurea; Reith and Munholland 1995) turned out to be more cyanobacterial-like than any other alga, based on features such as gene count, a large tRNA set, genes encoding transcriptional regulators and bacteria-like operons. This conclusion also applies to other red algal pt genomes. Whereas land plant and green algal ptDNAs encode 88–138 genes (Lemieux et al. 2007; Turmel et al. 2007), this number is close to double in red algae (230–254). Many of these genes are unique to red algae or rare in other ptDNAs, and include RNase P RNA (present in all red algal ptDNAs including Cyandium; otherwise only present in a few green plastids including Nephroselmis, Pycnococcus, Monomastix, Ostreococcus and in cyanelles of the two glaucophytes; (Shevelev et al. 1995; Turmel et al. 2009a); our own analysis), tmRNA (http://www.indiana.edu/∼tmrna/; Andersen et al. 2006) and signal recognition particle RNA (Andersen et al. 2006). In contrast, genes for components of the NADPH dehydrogenase complex (in ptDNAs of some prasinophyte and most land plant lineages) are absent.
B. Glaucophyta
Glaucophytes (glaucocystophytes) are freshwater algae that are particularly important for understanding the origin and evolution of photosynthesis in eukaryotes. Plastids of these organisms are unique in having retained two cyanobacterial features: a true, bacterial-type peptidoglycan cell wall (Pfanzagl et al. 1996), and carboxysomes – polyhedral micro-compartments involved in CO2 fixation (Kaplan and Reinhold 1999). The presence of these unique features strongly suggests that glaucocystophyte plastids originated directly from a symbiosis with a Cyanobacterium, and there has been a perception that this algal group might therefore have emerged early in the evolution of photosynthetic eukaryotes. However, more recent phylogenetic analyses with broad species sampling and a large number of genes do not support this idea, placing the origin of the glaucophyte plastid close to the divergence point of green and red plastids (see for instance Fig. 3.1).
The only complete plastid genome sequence from glaucophytes is that of Cyanophora paradoxa (Löffelhardt and Bohnert 1994). Recently, we have sequenced most of the Glaucocystis nostochinearum ptDNA (Lang et al. unpublished). Despite their evolutionary distance (see the deep divergence in Fig. 3.1), the two genomes are similar in terms of genome organization and gene content (a potential inverted repeat region remains to be confirmed for Glaucocystis ptDNA). The number of genes in glaucophyte ptDNAs (a total of 191 in Cyanophora, including protein, tRNA and rRNA genes; Cui et al. 2006) is relatively low compared to that of red algae (between 230 and 254). This might seem unexpected when considering that glaucophyte plastids still have a bacterial cell wall and other ‘primitive’ cyanobacterial features. In addition, in phylogenetic analyses with plastid data, glaucophyte branches are amongst the shortest ones, whereas the red algal plastids are among the more rapidly evolving ones. Evidently, gene counts do not correlate with evolutionary rates in this example.
C. Viridiplantae
Viridiplantae (green plants) is a morphologically and ecologically diverse group including the Streptophyta (land plants and their closest green algal relatives, the charophytes) and Chlorophyta (i.e., the rest of the green algae; Lewis and McCourt 2004; Sluiman 1985). Based on flagellar apparatus ultrastructure and features related to cytokinesis, Chlorophyta is further divided into four classes: Prasinophyceae (a paraphyletic group of unicellular species thought to be descendants of the ancestral flagellates from which the main green algal lineages evolved), Trebouxiophyceae, Chlorophyceae and Ulvophyceae (Lewis and McCourt 2004; Mattox and Stewart 1984). Although molecular data support the early divergence of prasinophytes (e.g., Guillou et al. 2004), the branching order of Trebouxiophyceae, Ulvophyceae and Chlorophyceae within Chlorophyta remains uncertain (see Pombert et al. 2004, 2006 for discussion and references), which is also consistent with our analysis (Fig. 3.1).
To date, 28 green algal plastid genomes (22 from Chlorophyta and 6 from Streptophyta) have been fully sequenced (Table 3.2), and they revealed an unexpected diversity both within and between algal groups. Overall, green algal ptDNAs differ in many respects from the well characterized plastid genomes of land plants (see Chaps. 4, 5). The latter typically share the same quadripartite structure (characterized by the presence of two copies of a large inverted repeat sequence separating a small single-copy and a large single-copy region) and have the same gene partitioning pattern between the two single copies. Their genes are densely packed and most of them are organized in conserved clusters. In contrast, green algal ptDNAs are “hotbeds” for chloroplast genome evolution (Belanger et al. 2006), exhibiting great diversity in genome and gene organization, including loss or inversion of the inverted repeat, gene rearrangements, intergenic expansions, invasion by repeat elements and introns, gene loss, gene expansion and gene fragmentation.
Prasinophytes
Prasinophytes are primarily marine unicellular algae that show great variation in terms of cell size and shape, flagella number, membrane covering (i.e., with our without scales) and biochemical features (Graham and Wilcox 2000). Seven prasinophyte clades are currently recognized; however, the exact relationships between these lineages and their affiliation with other green algal groups remain unresolved (Marin and Melkonian 2010).
The six currently available plastid genome sequences belong to: (1) Nephroselmis olivacea (Pseudoscourfieldiales) – a flagellate unicellular alga; (2) Pycnococcus provasolli (Pseudoscourfieldiales, Pycnococcaceae) – a coccoid picoplanktonic alga; (3) Ostreococcus tauri (Mamiellales) – the smallest known eukaryotic organism; (4) Pyramimonas (Pyramimonadales) – a scaly quadriflagellate alga; (5) Monomastix – a scaly flagellate of unknown affiliation; and (6) Pedinomonas minor (Pedinomonadales) – a small naked uniflagellate with no clear affiliation to the other prasinophyte clades probably related to, or ancestral to, Trebouxiophyceae (Turmel et al. 2009b; see also Fig. 3.1). Overall, prasinophyte ptDNAs show extreme diversity in size (an almost 3-fold variation), gene repertoire and genome organization. On the other hand, these genomes are similar in base composition and harbor no or just a few introns (Table 3.2).
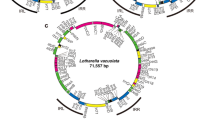
Interestingly, both ancestral and derived types of genome organization (relative to the presumed plastid genome in the most recent common ancestor of green plants; Turmel et al. 1999) have been reported among the plastid genomes described in this group. Ancestral types are characterized by large gene complements, ancestral gene clusters and a quadripartite genome structure (i.e., two identical copies of a large inverted repeat (IR), separated by single-copy (SC) regions), whereas derived types have reduced and re-arranged genomes. With 128 conserved genes, the 200.8 Kbp plastid genome of Nephroselmis has the largest gene complement yet reported for a chlorophyte alga and has retained many ancestral gene clusters (Turmel et al. 1999). Its quadripartite architecture resembles that of streptophyte counterparts in displaying (1) unequal SC regions – a large and a small one – that contain highly conserved sets of genes and (2) IR-encoded rRNA operons transcribed towards the small SC region. The ptDNA of Nephroselmis codes for several genes with limited phylogenetic distribution; for instance, ftsI (involved in peptidoglycan synthesis) has not been reported in other ptDNAs, and ndh genes (coding for subunits of the NADH:ubiquinone oxidoreductase) are absent from chlorophyte ptDNAs, but are present in other prasinophytes and land plants. At the other extreme is the plastid genome of Ostreococcus, with 88 genes highly scrambled over 71.6 Kbp, representing the smallest genome with the most reduced gene complement among photosynthetic green plants (Robbens et al. 2007). Both the small size and overall low proportion of intergenic spacers (representing 15% of the genome and varying from 1 to 476 nt length) as well as the presence of three cases of overlapping genes make this genome one of the most compact green plant ptDNAs (Table 3.2). Moreover, in contrast to Nephroselmis, its SC regions – although different in size – have the same number of genes, and the rRNA operons are transcribed away from the SC regions.
Reductions in plastid genome size and gene complement as well as the loss of the inverted repeat took place independently in several other prasinophyte lineages, leading to a variety of distinct genome configurations. For instance, the Pycnococcus plastid genome resembles the Ostreococcus counterpart in being small and highly compact (with two cases of overlapping genes and only ∼11% intergenic regions). However, it lacks the IR, and its gene complement is more similar to that of chlorophycean plastid genomes (Turmel et al. 2009a). On the other hand, the plastid genome of Monomastix has a larger size but a slightly lower number of genes (Table 3.2; Turmel et al. 2009a). The ptDNA of Pyramimonas displays intermediate genome size, compactness and gene repertoire (including six ndh genes present only in Nephroselmis and land plants, and two other genes – rpl22 and ycf65 – not reported in other chlorophytes; Turmel et al. 2009a). Lastly, the ptDNA of Pedinomonas, although very small, compact, and with a low gene count (Table 3.2), has retained the highest degree of ancestral gene linkages among all chlorophyte algae (i.e., linkages that predate the divergence of chlorophytes and streptophytes; Turmel et al. 2009b).
Trebouxiophyceae
Trebouxiophyceae (sensu Friedl 1995) are a group of morphologically heterogeneous algae (unicellular non-flagellated or filamentous) that inhabit mostly soil and freshwaters. Most phycobionts of lichens, ciliates and animals are also included in this class (Booton et al. 1998; Graham and Wilcox 2000; Lewis and McCourt 2004). To date, five plastid genomes from four photosynthetic species (Chlorella vulgaris and Oocystis solitaria – Chlorellales; Parachlorella kessleri and Leptosira terrestris – Ctenocladales) and one non-photosynthetic relative (Helicosporidium sp. – Chlorellales) have been published (Wakasugi et al. 1997; de Cambiaire et al. 2007; Turmel et al. 2009a). In addition, nearly complete ptDNAs are available from Coccomyxa sp C-169 (Coccomyxaceae; GenBank accession number HQ693844), Chlorella ellipsoidea and the colorless Prototheca wickerhamii (Knauf and Hachtel 2002; Yamada 1991). All trebouxiophyte ptDNAs sequenced so far are rather AT-rich, with Helicosporidium and Leptosira being among the most AT-rich green algal genomes (Table 3.2).
Although the plastid genomes from the four fully characterized photosynthetic species have similar gene contents, they vary significantly in size (a twofold variation). Most of this variation is accounted for by size differences in intergenic regions (Table 3.2). Gene order also varies considerably. For instance, the Chlorella plastid genome has retained many of the gene clusters present in streptophytes and prasinophytes. On the other hand, Leptosira shares little similarity in gene order with other plastid genomes and exhibits derived traits reminiscent of evolutionary patterns described for the ulvophyte and chlorophycean lineages (Turmel et al. 2009b).
The IR is missing in both Chlorella and Leptosira pt genomes, which is a feature also shared with the non-photosynthetic Helicosporidium (Table 3.2). Nevertheless, it is believed that the last common ancestor of trebouxiophytes possessed a plastid genome with a quadripartite structure (very similar to that of Nephroselmis and streptophytes) and that the IR was lost independently on at least two occasions. These suggestions are based on the finding of IRs in other trebouxiophyte plastid genomes (including that of Chlorella ellipsoidea, which has a large IR with a split rRNA operon; Yamada and Shimaji 1987) and on the presence of an IR remnant in Chlorella vulgaris (de Cambiaire et al. 2007).
The ptDNAs of the non-photosynthetic trebouxiophytes Helicosporidium and Prototheca are both highly reduced in size (partially sequenced; ∼37.5 and 45 Kbp, respectively). Based on its structure and compactness, the Helicosporidium genome is more similar to that described in the non-photosynthetic plastids of apicomplexan parasites. As expected, it lacks all genes for photosynthesis (de Koning and Keeling 2006), but its size reduction is due to both gene loss and reduced non-coding regions, overlapping genes, and the loss of the IR. Notable is the loss of the rRNA operon structure – an event that is thought to have taken place independently in several other lineages (including the trebouxiophyte C. ellipsoidea, several ulvophytes and charopytes as well as other non-photosynthetic algae; de Koning and Keeling 2006).
Chlorophyceae
The Chlorophyceae (sensu Mattox and Stewart 1984) comprise mostly freshwaters species, but several marine species are also known. Species in this group show diverse morphologies – from unicellular (flagellated or coccoid) to complex multicellular (colonial or filamentous) forms – and distinct configurations of their flagellar apparatus. Based on the arrangement of the flagellar basal bodies in their motile cells, two sister clades are generally described in this group. They are commonly referred to as CW (“clockwise”; Chlamydomonadales) and DO (“directly opposed”; Sphaeropleales) groups (Booton et al. 1998). Three additional lineages (Oedogoniales, Chaetopeltidales and Chaetophorales) are basal to these clades, but their divergence order is not well understood (Brouard et al. 2010; Buchheim et al. 2001; Shoup and Lewis 2003; Turmel et al. 2008). To date, seven plastid genomes from representatives of the five main chlorophycean lineages have been completely sequenced: (1) Chlamydomonadales – Chlamydomonas reinhardtii (Maul et al. 2002), Volvox carteri (Smith and Lee 2009, 2010), and Dunaliella salina (Smith et al. 2010); (2) Sphaeropleales – Scenedesmus obliquus (de Cambiaire et al. 2006); (3) Chaetophorales – Stigeoclonium helveticum (Belanger et al. 2006); (4) Oedogoniales – Oedogonium cardiacum (Brouard et al. 2008); and (5) Chaetopeltidales – Floydiella terrestris (Brouard et al. 2010).
Overall, plastid genomes in this group show tremendous variation in terms of genome size, intergenic spacers and intron numbers (Table 3.2). At the same time, the number of genes encoded in these genomes has been kept remarkably constant, within the range of derived prasinophyte pt genomes (Pycnococcus and Monomastix; Table 3.2). In terms of general genome organization, both types – with or without inverted repeats – are found among chlorophycean ptDNAs.
In cases where ptDNAs maintained the quadripartite structure, the organization of the IR and SC regions as well as the gene distribution within these regions differ among lineages. For instance, in Chlamydomonas, the two SC regions have similar sizes and differ radically in both gene content and gene organization from their counterparts in ancestral prasinophyte plastid genomes (Maul et al. 2002). Interestingly, although the Scenedesmus ptDNA shares with its Chlamydomonas counterpart a similar quadripartite structure, the sets of genes in the SC regions are very different between the two species, which indicates that genes were shuffled since the divergence of the DO and CW lineages (de Cambiaire et al. 2006). On the other hand, the Oedogonium plastid genome revealed an atypical structure with an IR significantly larger than in most of its green algal counterparts (with the notable exception of Nephroselmis) and two SC regions of vastly unequal size. Furthermore, the partitioning of genes among the two SC regions is distinctly different from that in Chlamydomonas and Scenedesmus (de Cambiaire et al. 2006).
Consistent with the situation among trebouxiophytes, the IR-lacking ptDNAs of Stigeoclonium and Floydiella also have loosely packed genes and intergenic regions rich in short repeats (Brouard et al. 2010). The most re-arranged chlorophycean plastid genome appears to be that of Stigeoclonium, which completely lacks the ancestral gene partitioning pattern displayed by Nephroselmis and streptophytes, and overall, exhibits the fewest ancestral features among all plastid genomes completely sequenced to date (Belanger et al. 2006).
Chlorophycean ptDNAs differ substantially in the amount of short repeated sequences. At one extreme, there are Oedogonium and Scenedesmus, in which such sequences occupy only 1.3% and 3% of genomes, respectively. At the other extreme, there are the ptDNAs of Chlamydomonas, Stigeoclonium, Volvox, and Floydiella, which are extremely rich in repeated sequences. For instance, short palindromic repeats (potentially acquired via mitochondria-to-plastid transfers involving mobile introns) constitute ∼64% of the Volvox plastid genome. Repeats larger than 30 bp account for half of the Floydiella pt genome (almost three times more than in Chlamydomonas and Stigeoclonium; Brouard et al. 2010; Smith and Lee 2009).
Several atypical features have also been described in this group, including: (1) strong bias in the distribution of genes between the two DNA strands (in Stigeoclonium and Scenedesmus), (2) breakup of protein-coding genes by putatively trans-spliced group II introns (rbcL, psaC, petD, psaA) (in Stigeoclonium and Floydiella); (3) fragmentation of protein-coding genes into distinct open reading frames (contiguous or distant from each other) that are not associated with any introns (rpoC1, rps2, rpoB); (4) the substantial expansion (over fivefold increase) of many protein-coding genes (e.g., cemA, clpP, ftsH, rpoB, rpoC1, rpoC2, rps3, rps4, and ycf1) due to the presence of insertions whose post-transcriptional fate (i.e., excised or not) or biological significance are mostly unknown; (5) intergenic intron-like sequences of unknown origin and function in Dunaliella; and (6) genes (int and dpoB, coding for a tyrosine recombinase and a DNA-dependent DNA polymerase, respectively) potentially acquired via horizontal gene transfer from a mitochondrial genome donor in Oedogonium (Belanger et al. 2006; Brouard et al. 2008, 2010; Smith et al. 2010).
Overall, the plastid genome in this group of algae has experienced major changes, and it displays the lowest degree of ancestral traits relative to other chlorophytes. Some of the most eccentric ptDNAs among all Viridiplantae are also found in this group: over 520 Kbp and over 77% intergenic spacers in Floydiella and Volvox; 73% AT-content in Scenedesmus; and 43 introns in Dunaliella (Table 3.2).
Ulvophyceae
Ulvophyceae are unicellular (including macroscopic forms composed of a single, large multinucleate cell) and multicellular species that are common in rocky intertidal coasts of temperate regions, but secondarily freshwater species are also known. The flagellar basal bodies in their motile cells are arranged in a counterclockwise (CCW) orientation (Floyd and Okelly 1984). To date, complete plastid genome sequences are available from three unicellular ulvophyte species: Oltmannsiellopsis viridis (Oltmannsiellopsidales; Pombert et al. 2006), Pseudendoclonium akinetum (Ulotrichales; Pombert et al. 2005) and Bryopsis hypnoides (Bryopsidales; Lu et al. 2010). The first two species belong to lineages believed to occupy a basal position within the group, whereas the phylogenetic position of the latter is uncertain (Lu et al. 2010 and Fig. 3.1). Partial sequence information is also available from Codium fragile (Ulvales; Manhart et al. 1989) and Caulerpa sertularoides (Bryopsidales; Lehman and Manhart 1997).
Although different in size, the Oltmannsiellopsis and Pseudendoclonium plastid genomes share a similar number of genes and coding density (Table 3.2). The difference in genome size is mostly accounted for by a difference in intron numbers (Table 3.2). The 27 introns in Pseudendoclonium make up for 14.8% of the genome and are thought to have arisen from the intragenomic proliferation of a few founding introns in this lineage (Pombert et al. 2005). Both genomes share a quadripartite structure that deviates from the ancestral type. Nevertheless, the IR sequences in the two genomes differ in size (with that of Oltmannsiellopsis being ∼12 Kbp larger) and gene content (the Pseudendoclonium IR encodes only the rRNA operon, while the Oltmannsiellopsis IR contains five additional genes). Also, Pseudendoclonium shows evidence of inter-organellar lateral transfer (involving some dispersed repeats and one intron) between its plastid and mitochondrial genomes (Pombert et al. 2005).
The plastid genome of Bryopsis differs significantly from those of Oltmannsiellopsis and Pseudendoclonium in several important ways. These include the absence of IRs (also lacking in the two other ulvophytes for which partial information is available; Caulerpa and Codium) and the presence of multimeric forms of ptDNA (including monomer, dimer, trimer, tetramer, and even higher-order multimers), which is a trait that has only been reported in land plants (Lu et al. 2010). Furthermore, this genome is unique in possessing 10 tRNA genes that have not been found in other completely sequenced chlorophyte ptDNAs. Note that while five of them are known in embryophytes the other five have only been reported in some bacterial genomes. Also, its rRNA locus consists of five (rrn23, rrn16, rrn7, rrn5, and rrn3) instead of the usual four coding regions; a similar situation is only found in C. reinhardtii ptDNA (Maul et al. 2002). The number of genes reported for this ptDNA is similar to that of the other two ulvophytes (Table 3.2). However, our preliminary analyses indicate a larger gene complement for this genome; likewise, the number of introns in this genome might prove to be different than listed in Table 3.2. Overall, although ulvophyte ptDNAs feature an atypical quadripartite structure, they maintained a relatively large gene complement and the degree of remodeling is intermediate relative to those seen in their trebouxiophyte and chlorophycean counterparts.
Charophyceae
Charophytes comprise thousands of mainly freshwater algal species exhibiting great variability in morphology and reproduction. They are subdivided into six monophyletic lineages: (1) Mesostigmatales represented by the scaly biflagellate Mesostigma viride (previously regarded as a member of the Prasinophyceae), (2) Chlorokybales also represented by a single species (the sarcinoid Chlorokybus atmophyticus), (3) Klebsormidiales, (4) Zygnematales, (5) Coleochaetales and (6) Charales. Phylogenetic analyses indicate Mesostigmatales and Chlorokybales as the earliest-diverging charophycean lineages (forming a distinct clade; Turmel et al. 2007). The branching order among the other groups remains debatable. Charales are the closest relatives of plants in some studies, while other analyses favor that Charales diverged prior to Coleochaetales and Zygnematales (see Turmel et al. 2006 for discussion and references; see also Fig. 3.1).
Complete plastid genome sequences are available from six species belonging to five of the six main charophycean lineages: Mesostigma viride (Mesostigmatales), Chlorokybus atmophyticus (Chlorokybales), Staurastrum punctulatum and Zygnema circumcarinatum (Zygnematales), Chaetosphaeridium globosum (Coleochaetales) and Chara vulgaris (Charales; Lemieux et al. 2007; Turmel et al. 2002, 2005, 2006). In addition, the almost complete ptDNA of Klebsormidium flaccidum has been sequenced (Fig. 3.1; BFL unpublished). Overall, charophycean ptDNAs vary in size, gene content, intron content, gene order and include the most gene-rich green plastid genomes (Table 3.2).
Consistent with their basal position among charophytes, the plastid genomes of Mesostigma and Chlorokybus are gene-rich and feature a typical quadripartite structure (Turmel et al. 2007). The two genomes are similar in gene content and gene order, with the notable presence in each of the two genomes of genes that have not been identified in other green algal and land plant pt genomes. Genes are loosely packed in Chlorokybus (the average size of intergenic spacers in Chlorokybus is twice that of Mesostigma), which also reflects in the larger genome size (Table 3.2; Turmel et al. 2007). Nevertheless, relative to the gene order in Nephroselmis and Streptophyta ptDNAs, the Chlorokybus plastid genome is more rearranged than its Mesostigma counterpart. Both genomes are intron-poor, with none in Mesostigma and a single intron in Chlorokybus (Table 3.2).
Relative to Mesostigma and Chlorokybus, the plastid genomes of the two zygnematalean lineages, Staurastrum and Zygnema, have a slightly reduced gene repertoire (Table 3.2) and lack the rRNA-encoding IR typical of other charophytes and streptophytes. Notably, the lack of IR is also shared with Spirogyra maxima – another zygnematalean species for which partial genome information is available (Manhart et al. 1990). Furthermore, both these genomes are loosely packed with genes (due to the expansion of their intergenic spacers), and feature a larger number of introns (which have also expanded in size). However, the two genomes differ extensively from one another in gene order. Also, many intergenic regions in the Staurastrum ptDNA harbour tandem repeats while such sequences are virtually absent in the Zygnema counterpart (Turmel et al. 2005).
On the other hand, the pt genomes of Chaetosphaeridium globosum (Coleochaetales) and Chara vulgaris (Charales) exhibit the typical quadripartite structure found in streptophytes, and resemble their land plant counterparts more closely than do other charophycean relatives. Although the two genomes have similar coding capacities (Table 3.2), Chara features four genes (rpl12, trnL(gag), rpl19, and ycf20) that are entirely missing from other charophycean and land plant ptDNAs. Furthermore, despite similarities in genome organization, gene content and intron composition, the two genomes differ in size, gene density and AT content, with the Chara genome representing the largest and most AT-rich streptophyte ptDNA (Table 3.2). Notably, Chara’s increased genome size and AT-content is mainly accounted for by increased AT-rich intergenic spacers and introns, which represent 38.8% and 13.4% of the total genome, respectively (Turmel et al. 2006). Overall, among streptophyte green algae, the ptDNAs of the charophytes Mesostigma and Chlorokybus exhibit the most ancestral features (including the largest gene complement among Viridiplantae; 137–138 genes), while the genomes of Chara and Chaetosphaeridium resemble most their land plant counterparts.
IV. Plastids Acquired via Eukaryote-Eukaryote Endosymbiosis
According to the chromalveolate hypothesis, chlorophyll c-containing plastids originated from a single photosynthetic ancestor, which acquired its plastids only once by secondary endosymbiosis with a red alga (Cavalier-Smith 2002; Keeling 2009, 2010). However, phylogenetic studies suggest a much higher incidence of plastid transfer among eukaryotes, favoring complex evolutionary scenarios involving multiple eukaryote-eukaryote endosymbioses (Sanchez-Puerta et al. 2007; Archibald 2009). The arguably most rigorous analysis in this sense is by Baurain and co-workers (Baurain et al. 2010), who find that monophyly of Cryptophytes, Alveolates, Stramenopiles, and Haptophytes (CASH) is seen neither with mitochondrial nor nuclear sequence data. This means that the very strongly supported phylogenetic relationships in trees constructed with plastid proteins (plastid-encoded as in Fig. 3.1; as well as nucleus-encoded genes of cyanobacterial origin) do not represent the evolution of CASH species but more likely multiple plastid transfers. In some instances, higher-order eukaryote-eukaryote endosymbioses are in fact evident, for instance, the grouping of plastids from the dinoflagellates (Durinskia and Kryptoperidinium; Imanian et al. 2010) with diatoms (Fig. 3.1), and the (weak) association of Alveolata sp. (Apicomplexa) plastids with stramenopiles.
A shared characteristic of ‘second hand’ plastid genomes is their reduced coding capacity relative to that of the plastid donor, which is in most instances a red and only in rare cases a green alga (i.e., in the rhizarian Bigelowiella and relatives, and the euglenozoan Euglena). Plastids of red origin are in general remarkably similar in gene content, despite their turbulent evolutionary past. In the following we will focus on the few main differences, and refer the reader otherwise to the corresponding original publications. It should be noted that gene counts and identifications differ slightly across different papers and database compilations (Cui et al. 2006; O’Brien et al. 2009). Although minor (up to about ten), these differences need to be resolved in the future, by establishing gene identification based on the same criteria. Eventually, all ptDNAs should be reannotated by using the same tools, a task that was unfortunately out of reach for this review.
A. Stramenopila
Stramenopiles is the largest group among CASH protists whose monophyly is well supported (e.g., (Baurain et al. 2010). A sizable portion of stramenopile taxa are non-photosynthetic and without plastid relicts, such as oomycetes (Phytophthora) and bicosoecids (Cafeteria). Whether or not the stramenopile ancestor had plastids, and of which origin, has been the subject of heated debates. The controversy is in part due to over-interpretation of BLAST analyses and lack of resolution in single-gene phylogenies (Stiller et al. 2009) and references therein). The few clear examples pointing to a plastid origin of genes in plastid-less stramenopiles may in fact be explained by transfer of individual genes, rather than endosymbiotic events.
PtDNA sequences are available from bacillariophytes (diatoms), phaeophytes (brown algae), raphidophytes, pelagophytes, xanthophytes, but curiously not from chrysophytes (golden algae).
Diatoms
Bacillariophyta are most diverse (>250 genera), unicellular, silica-walled algae that live either attached to surfaces or are planktonic. Complete ptDNAs have been sequenced from four phylogenetically relatively distant species: Phaeodactylum tricornutum, Thalassiosira pseudonana (Oudot-Le Secq et al. 2007), Odontella sinensis (Kowallik et al. 1995) and Fistulifera sp. (Tanaka et al. 2011).
These ptDNAs are relatively uniform, coding for a similar set of 160–170 genes. A putative serine recombinase gene (serC2) is potentially of plasmid origin. It also occurs in the diatom plastids residing in certain dinoflagellates (Imanian et al. 2010).
Phaeophytes
Brown algae are a large group of multicellular organisms (∼250 genera) that occur mostly in marine habitats and grow attached to surfaces. Complete ptDNAs are published from two representatives of distinct orders, Ectocarpus siliculosus and Fucus vesiculosus (Le Corguille et al. 2009). Their gene counts are similar to those of diatoms, with only minor differences.
Raphidophytes
Raphidophytes is a small group (four genera) of flagellated unicellular organisms that occur in both marine and fresh water habitats, and that lack a rigid cell wall. A complete ptDNA sequence is available for two strains of Heterosigma akashiwo (Cattolico et al. 2008). The number of ptDNA-encoded genes (197) is relatively high compared to other algae with plastids from secondary or higher-order endosymbioses, and a putative serine recombinase gene is present as in diatoms. Another unusual ORF codes for a potential G-protein-coupled receptor. Again, the functionality and biological role of these extra genes remain to be demonstrated. Several protein-coding genes and their mRNAs contain large, in-frame inserts, when compared to orthologs in other plastids. These inserts likely represent derived forms of protein introns (inteins; Liu 2000; Gogarten and Hilario 2006) that may have lost their capacity for splicing. In fact, one typical bona fide intein has been identified in the dnaB gene of H. akashiwo ptDNA (Cattolico et al. 2008).
Pelagophytes
This group of algae known for causing algal blooms was originally included in the Chrysophyceae, but based on biochemical, physiological and phylogenetic criteria it now forms its own class Pelagophyceae. Complete ptDNAs are available from Aureococcus anophagefferens and Aureoumbra lagunensis (Ong et al. 2010). The large inverted repeat, otherwise common in other second-hand red plastids is missing, and the two genomes code for only 137 and 141 genes, respectively. About 20 genes that are usually present in stramenopile ptDNAs are absent from both pelagophytes. According to our phylogenetic analysis with plastid data, pelagophytes branch deeply within stramenopiles, but their placement relative to the raphidophytes and xanthophytes is unresolved (Fig. 3.1).
Xanthophytes
The Vaucheria litorea plastid genome has been characterized during the course of a most unusual investigation of the green sea slug Elysia chlorotica. This animal acquires plastids (“kleptoplasts”, see Chap. 2) by ingesting Vaucheria litorea as food, and sequestrating the organelles into the digestive epithelium, where photosynthesis occurs for several months (Rumpho et al. 2008). As it turns out, the plastid genome sequence is typical for stramenopiles (167 genes), and contains the common inverted repeat. According to the authors, some nuclear gene products that have to be imported and are required for plastid function are likely encoded in the animal’s nuclear genome (the algal nucleus is digested during the organelle sequestration process). So far, horizontal gene transfer from the algal genome to the mollusk genome has been demonstrated only for a few nuclear genes. Evidently, nuclear genome sequences of the sea slug and of Vaucheria are required to substantiate this unusual case of horizontal gene transfer (see Chap. 2).
B. Alveolata
Alveolates comprise ciliates, apicomplexans and dinoflagellates, but only the two latter ones contain photosynthetic plastids.
Dinoflagellata
In most dinoflagellates, the ptDNA consists of multiple minicircles that code for a total of about a dozen genes. Here we will only discuss the pt genomes of Kryptoperidinium foliaceum and Peridinium quinquecorne that possess a conventional genome organization, since their ptDNAs derive from a higher-order endosymbiosis with diatoms (Imanian et al. 2010; see also Fig. 3.1). These dinoflagellate ptDNAs possess IR regions similar to those in diatoms, and K. foliaceum has as a putative serine recombinase gene that is characteristic for diatom and raphidophyte ptDNAs. According to the authors’ interpretation (Imanian et al. 2010), the larger size of the K. foliaceum ptDNAs may be due to the insertion of numerous plasmid-derived genes that are dispensable for plastid function.
Apicomplexa
As already mentioned in the introduction, ptDNAs have been sequenced from two photosynthetic relatives of Apicomplexa, Chromera velia and Alveolata sp. (CCMP3115; Janouskovec et al. 2010). The Chromera plastid DNA is very rapidly evolving, and therefore difficult to place in phylogenetic analyses. Its genome is larger than that of Alveolata sp., and translates UGA stop codons as tryptophan as is otherwise common for (in most cases also rapidly evolving) mtDNAs.
The gene count of both ptDNAs is modest (124 and 112 genes, respectively) compared to other second-hand red algal ptDNAs. A gene for a horizontally transferred phosphonopyruvate decarboxylase is inserted into the rRNA operon of Alveolata. According to our phylogenetic analysis (Fig. 3.1), plastids of the two species could have a common origin by vertical descent, yet the positioning of the Chromera ptDNA alone is unresolved, somewhere close to stramenopiles. According to our phylogenetic results with Alveolata, its plastids may stem from a tertiary endosymbiosis with a photosynthetic stramenopile rather than from a unique secondary acquisition, as proposed by the chromalveolate hypothesis. In fact, the authors of the original genome paper state that ‘comparing gene content among alveolate plastids reveals the nearly mutually-exclusive gene sets of apicomplexans and dinoflagellates’, which can be interpreted as further evidence against their common origin.
C. Cercozoa (Rhizaria)
Chlorarachniophytes are a small group of photosynthetic marine flagellates with two recognized genera Chlorarachnion and Bigelowiella. Similar to cryptophytes (for details on cryptomonads see below) they carry a second reduced nucleus (nucleomorph), but of green algal origin (not precisely identified according to our analyses presented in Fig. 3.1 and those published by others; Rogers et al. 2007). A complete ptDNA sequence is available for Bigelowiella natans. The genome has a small size (69.2 Kbp), a highly compact gene organization, and a nearly full complement of photosynthesis-related genes that is similar to those in some of the less gene-rich green algae such as Chlamydomonas (Rogers et al. 2007). Most of the reduction in gene content comes from the loss of ycf and tRNA genes.
D. Cryptomonada
Cryptomonads are unicellular flagellates that are mostly photosynthetic, containing chlorophyll c and phycobilins as photosynthetic pigments. They carry direct physical evidence for eukaryote-eukaryote endosymbiosis in form of a second, remnant eukaryotic nucleus, the ‘nucleomorph’ (for a recent review see Moore and Archibald 2009) of evidently red algal origin. Non-photosynthetic cryptomonad species include Cryptomonas paramecium that contains plastids with a secondarily reduced plastid genome (Donaher et al. 2009), and heterotrophic Goniomonas species that have no plastids. Whether Goniomonas is indeed primarily without plastids (e.g., Keeling et al. 1999) and may thus represent the ancestral group that engulfed an alga with red plastids, remains to be demonstrated with nuclear genome sequence data.
The three completely sequenced cryptomonad pt DNAs are from Guillardia theta (Douglas and Penny 1999), Rhodomonas (Pyrenomonas) salina (Khan et al. 2007) and the non-photosynthetic C. parasiticum (Donaher et al. 2009). The gene count of cryptomonad ptDNAs is >180, more than in green algae but about a quarter less than in red algae. The non-photosynthetic C. parasiticum has about 70 genes less in its plastid genome\, including only a few remaining members of the pet, psa and psb photosynthetic gene families (Donaher et al. 2009). An interesting acquisition in R. salina ptDNA is a gene for the tau/gamma subunit of DNA polymerase III (dnaX) that was likely acquired by lateral gene transfer from a firmicute bacterium (Khan et al. 2007). Whether or not this gene is transcribed, translated, and functional in plastids, remains to be shown.
E. Haptophyta
Haptophytes (prymnesiophytes) are unicellular photosynthetic flagellates (some are colonial), and unlike in cryptophytes, heterotrophic taxa are unknown in this clade. Currently, pt genomes of only two species are available, those of Emiliana huxleyi (Sanchez Puerta et al. 2005) and Pavlova lutheri (Burger et al. unpublished). Their genomes have about the same size and gene content (105 Kbp and 155 genes in E. huxleyi), and carry few notable features. Phylogenetic analyses based on pt data sometimes (but not always) unite haptophytes and cryptophytes (Bachvaroff et al. 2005; Keeling 2009; Le Corguille et al. 2009; Fig. 3.1).
F. Euglenids
Euglenids are unicellular flagellates, some of which contain plastids (chlorophyll a and b, β-carotene and xanthophylls), which were acquired via secondary endosymbiosis with a green alga. Euglenid ptDNA sequences are available from two species, Euglena gracilis (Copertino and Hallick 1993; Hallick et al. 1993) and the non-photosynthetic Euglena (Astasia) longa (Knauf and Hachtel 2002). In both instances, plastid genes are loaded with a large number of unusual introns (see above). At only 73 Kbp, the A. longa ptDNA has about half the size of its photosynthetic relatives, with all photosynthesis-related protein genes missing except for rbcL. According to published phylogenetic analyses based on pt sequences (Turmel et al. 2009a), Euglena plastids derive from a relative of the green alga Pyramimonas, which is clearly corroborated by our phylogenetic analysis (Fig. 3.1).
V. Conclusions
The availability of information on plastid genomes has increased over the last few years at an almost disquieting pace, in particular in green algae (as well as in land plants that are not covered in this chapter). Unfortunately, from the standpoint of evolutionary biology, the traditional bias in attention to green algae and plants remains. In particular, we have sequence data from just a handful of red algal pt genomes, a skimpy two from glaucophytes, and similarly low coverage for the numerous groups of algae with second-hand plastids. In fact, we are surprised that sequencing of almost identical flowering plant ptDNAs appears to be more important than sequencing those for which we know so little.
During the course of writing this review, we have come across several issues that touch on data production and analysis. For most pt genome projects underway, sequencing is performed with new technologies, some of which are fraught with systematic error (e.g., pyrosequencing technology suffers from frameshifts in homopolymer stretches among other, less well understood sequencing artifacts). This shortcoming may lead to mistaking genes for pseudogenes with great confidence (based on high coverage of systematic error). In a few cases, we have seen omission of gene annotation that may be due to such frameshifts. Further, as new genome data are pouring in at an unprecedented rate, detailed genome annotation by the end user (typically manual intervention) becomes increasingly challenging. The best solution to both issues, detecting erroneous gene features and potential sequencing error, and keeping up with high standards of genome annotation, is the development of automated genome annotation pipelines. We are aware of only one published tool for organelle genome annotation (DOGMA; (Wyman et al. 2004), and the currently unpublished but freely available tools developed by ourselves (MFannot, RNAweasel; Lang et al. 2007; Beck and Lang 2009, 2010). These are still far from perfect, justifying a continued time investment that should ideally be integrated with ongoing large scale sequencing projects. In this context we noticed that plastid gene identification is relatively straightforward, based on a wide consensus on gene names and functions (which cannot be said for mitochondrial genes). Yet, it seems that renaming ycf genes with now known functions would be timely, so would be a systematic identification and renaming of conserved ORFs as ycf, as long as they are present in distant species. Identification of weakly conserved genes is best achieved by HMM searches (http://hmmer.janelia.org; Eddy 1996, 1998) that are as fast and by far more sensitive and reliable than BLAST.
Abbreviations
- aa –:
-
Amino acid;
- CASH –:
-
Cryptophyta Alveolata, Stramenopila plus Haptophyta their plastids are of red algal origin and pt genomes are closely related (which is incompatible with respective nuclear genome phylogenies).
- CW –:
-
‘Clockwise’ arrangement of flagellar basal bodies in Chlamydomonadales;
- DO –:
-
‘Directly opposed’ arrangement of flagellar basal bodies in Sphaeropleales;
- IR –:
-
Inverted genomic repeat region occurs in a large number of ptDNAs;
- LBA –:
-
Long Branch Attraction phylogenetic artifact that leads to the incorrect grouping of fast-evolving species or attraction to distant outgroups, due to evolutionary model violations and under-estimation of repeated sequence change;
- mtDNA –:
-
Mitochondrial DNA protists – eukaryotes other than fungi animals and plants;
- pt –:
-
Plastid (chloroplast);
- ptDNA –:
-
Plastid DNA;
- SC –:
-
Single-copy regions separating large inverted repeats in ptDNAs;
- tmRNA –:
-
Transfer mRNA occurs in bacterial some plastid and jakobid mitochondrial genomes typically contains a tRNA-like and a protein-coding domain involved in releasing ribosomes that are stalled by degraded mRNAs without in-frame stop codons
References
Andersen ES, Rosenblad MA, Larsen N, Westergaard JC, Burks J, Wower IK, Wower J, Gorodkin J, Samuelsson T, Zwieb C (2006) The tmRDB and SRPDB resources. Nucleic Acids Res 34:D163–D168
Archibald JM (2009) The puzzle of plastid evolution. Curr Biol 19:R81–R88
Archibald JM, Keeling PJ (2002) Recycled plastids: a ‘green movement’ in eukaryotic evolution. Trends Genet 18:577–584
Bachvaroff TR, Sanchez Puerta MV, Delwiche CF (2005) Chlorophyll c-containing plastid relationships based on analyses of a multigene data set with all four chromalveolate lineages. Mol Biol Evol 22:1772–1782
Baurain D, Brinkmann H, Petersen J, Rodriguez-Ezpeleta N, Stechmann A, Demoulin V, Roger AJ, Burger G, Lang BF, Philippe H (2010) Phylogenomic evidence for separate acquisition of plastids in cryptophytes, haptophytes, and stramenopiles. Mol Biol Evol 27:1698–1709
Beck N, Lang BF (2009) RNAweasel, a webserver for identification of mitochondrial, structured RNAs. http://megasun.bch.umontreal.ca/RNAweasel
Beck N, Lang BF (2010) MFannot, organelle genome annotation websever. http://megasun.bch.umontreal.ca/papers/MFannot
Belanger AS, Brouard JS, Charlebois P, Otis C, Lemieux C, Turmel M (2006) Distinctive architecture of the chloroplast genome in the chlorophycean green alga Stigeoclonium helveticum. Mol Genet Genomics 276:464–477
Bendich AJ (2004) Circular chloroplast chromosomes: the grand illusion. Plant Cell 16:1661–1666
Bendich AJ (2007) The size and form of chromosomes are constant in the nucleus, but highly variable in bacteria, mitochondria and chloroplasts. Bioessays 29:474–483
Bhattacharya D, Yoon HS, Hackett JD (2004) Photosynthetic eukaryotes unite: endosymbiosis connects the dots. Bioessays 26:50–60
Booton AS, Floyd GL, Fuerst PA (1998) Polyphyly of tetrasporalean green algae inferred from nuclear small subunit rDNA. J Phycol 34:306–311
Brouard JS, Otis C, Lemieux C, Turmel M (2008) Chloroplast DNA sequence of the green alga Oedogonium cardiacum (Chlorophyceae): unique genome architecture, derived characters shared with the Chaetophorales and novel genes acquired through horizontal transfer. BMC Genomics 9:290
Brouard JS, Otis C, Lemieux C, Turmel M (2010) The exceptionally large chloroplast genome of the green alga Floydiella terrestris illuminates the evolutionary history of the Chlorophyceae. Genome Biol Evol 2:240–256
Buchheim MA, Michalopulos EA, Buchheim JA (2001) Phylogeny of the Chlorophyceae with special references to the Sphaeropleales. J Phycol 37:819–835
Burki F, Shalchian-Tabrizi K, Minge M, Skjaeveland A, Nikolaev SI, Jakobsen KS, Pawlowski J (2007) Phylogenomics reshuffles the eukaryotic supergroups. PLoS One 2:e790
Burki F, Inagaki Y, Brate J, Archibald JM, Keeling PJ, Cavalier-Smith T, Sakaguchi M, Hashimoto T, Horak A, Kumar S, Klaveness D, Jakobsen KS, Pawlowski J, Shalchian-Tabrizi K (2009) Large-scale phylogenomic analyses reveal that two enigmatic protist lineages, telonemia and centroheliozoa, are related to photosynthetic chromalveolates. Genome Biol Evol 1:231–238
Cattolico RA, Jacobs MA, Zhou Y, Chang J, Duplessis M, Lybrand T, McKay J, Ong HC, Sims E, Rocap G (2008) Chloroplast genome sequencing analysis of Heterosigma akashiwo CCMP452 (West Atlantic) and NIES293 (West Pacific) strains. BMC Genomics 9:211
Cavalier-Smith T (1981) Eukaryote kingdoms: seven or nine? Biosystems 14:461–481
Cavalier-Smith T (2002) The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int J Syst Evol Microbiol 52:297–354
Chaal BK, Green BR (2007) Protein targeting in “secondary” or “complex” chloroplasts. Methods Mol Biol 390:207–217
Chan CX, Yang EC, Banerjee T, Yoon HS, Martone PT, Estevez JM, Bhattacharya D (2011) Red and green algal monophyly and extensive gene sharing found in a rich repertoire of red algal genes. Curr Biol 21:328–333
Copertino DW, Hallick RB (1993) Group II and group III introns of twintrons: potential relationships with nuclear pre-mRNA introns. Trends Biochem Sci 18:467–471
Cui L, Veeraraghavan N, Richter A, Wall K, Jansen RK, Leebens-Mack J, Makalowska I, dePamphilis CW (2006) ChloroplastDB: the chloroplast genome database. Nucleic Acids Res 34:D692–D696
de Cambiaire JC, Otis C, Lemieux C, Turmel M (2006) The complete chloroplast genome sequence of the chlorophycean green alga Scenedesmus obliquus reveals a compact gene organization and a biased distribution of genes on the two DNA strands. BMC Evol Biol 6:37
de Cambiaire JC, Otis C, Turmel M, Lemieux C (2007) The chloroplast genome sequence of the green alga Leptosira terrestris: multiple losses of the inverted repeat and extensive genome rearrangements within the Trebouxiophyceae. BMC Genomics 8:213
de Koning A, Keeling P (2006) The complete plastid genome sequence of the parasitic green alga Helicosporidium sp. is highly reduced and structured. BMC Biol 4:12
Deschamps P, Moreira D (2009) Signal conflicts in the phylogeny of the primary photosynthetic eukaryotes. Mol Biol Evol 26:2745–2753
Donaher N, Tanifuji G, Onodera NT, Malfatti SA, Chain PS, Hara Y, Archibald JM (2009) The complete plastid genome sequence of the secondarily nonphotosynthetic alga Cryptomonas paramecium: reduction, compaction, and accelerated evolutionary rate. Genome Biol Evol 1:439–448
Douglas SE (1998) Plastid evolution: origins, diversity, trends. Curr Opin Genet Dev 8:655–661
Douglas SE, Gray MW (1991) Plastid origins. Nature 352:290
Douglas SE, Penny SL (1999) The plastid genome of the cryptophyte alga, Guillardia theta: complete sequence and conserved synteny groups confirm its common ancestry with red algae. J Mol Evol 48:236–244
Durnford DG, Gray MW (2006) Analysis of Euglena gracilis plastid-targeted proteins reveals different classes of transit sequences. Eukaryot Cell 5:2079–2091
Eddy SR (1996) Hidden Markov models. Curr Opin Struct Biol 6:361–365
Eddy SR (1998) Profile hidden Markov models. Bioinformatics 14:755–763
Eddy S (2008) Infernal website. http://infernal.janelia.org
Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:113
Fast NM, Kissinger JC, Roos DS, Keeling PJ (2001) Nuclear-encoded, plastid-targeted genes suggest a single common origin for apicomplexan and dinoflagellate plastids. Mol Biol Evol 18:418–426
Felsner G, Sommer MS, Maier UG (2010) The physical and functional borders of transit peptide-like sequences in secondary endosymbionts. BMC Plant Biol 10:223
Floyd GL, Okelly CJ (1984) Motile cell ultrastructure and the circumscription of the orders Ulotrichales and Ulvales (Ulvophyceae, Chlorophyta). Am J Bot 71:111–120
Foth BJ, McFadden GI (2003) The apicoplast: a plastid in Plasmodium falciparum and other Apicomplexan parasites. Int Rev Cytol 224:57–110
Foth BJ, Ralph SA, Tonkin CJ, Struck NS, Fraunholz M, Roos DS, Cowman AF, McFadden GI (2003) Dissecting apicoplast targeting in the malaria parasite Plasmodium falciparum. Science 299:705–708
Friedl T (1995) Inferring taxonomic positions and testing genus level assignments in coccoid green lichen algae – a phylogenetic analysis of 18S ribosomal RNA sequences from Dictyochloropsis reticulata and from members of the genus Myrmecia (Chlorophyta, Trebouxiohyceae Cl-Nov). J Phycol 31:632–639
Funes S, Davidson E, Reyes-Prieto A, Magallón S, Herion P, King MP, González-Halphen D (2002) A green algal apicoplast ancestor. Science 298:2155
Funes S, Reyes-Prieto A, Pérez-Martínez X, González-Halphen D (2004) On the evolutionary origins of apicoplasts: revisiting the rhodophyte vs. chlorophyte controversy. Microbes Infect 6:305–311
Gardner MJ, Feagin JE, Moore DJ, Rangachari K, Williamson DH, Wilson RJ (1993) Sequence and organization of large subunit rRNA genes from the extrachromosomal 35 kb circular DNA of the malaria parasite Plasmodium falciparum. Nucleic Acids Res 21:1067–1071
Gardner PP, Daub J, Tate JG, Nawrocki EP, Kolbe DL, Lindgreen S, Wilkinson AC, Finn RD, Griffiths-Jones S, Eddy SR, Bateman A (2009) Rfam: updates to the RNA families database. Nucleic Acids Res 37:D136–D140
Glockner G, Rosenthal A, Valentin K (2000) The structure and gene repertoire of an ancient red algal plastid genome. J Mol Evol 51:382–390
Gockel G, Hachtel W (2000) Complete gene map of the plastid genome of the nonphotosynthetic euglenoid flagellate Astasia longa. Protist 151:347–351
Gogarten JP, Hilario E (2006) Inteins, introns, and homing endonucleases: recent revelations about the life cycle of parasitic genetic elements. BMC Evol Biol 6:94
Goldschmidt-Clermont M, Choquet Y, Girard-Bascou J, Michel F, Schirmer-Rahire M, Rochaix JD (1991) A small chloroplast RNA may be required for trans-splicing in Chlamydomonas reinhardtii. Cell 65:135–143
Gould SB, Waller RF, McFadden GI (2008) Plastid evolution. Annu Rev Plant Biol 59:491–517
Graham LE, Wilcox LW (2000) Algae. Prentice-Hall, Upper Saddle River
Gray MW (2010) Rethinking plastid evolution. EMBO Rep 11:562–563
Gueneau de Novoa P, Williams KP (2004) The tmRNA website: reductive evolution of tmRNA in plastids and other endosymbionts. Nucleic Acids Res 32:D104–D108
Guillou L et al (2004) Diversity of picoplanktonic prasinophytes assessed by direct nuclear SSU rDNA sequencing of environmental samples and novel isolates retrieved from oceanic and coastal marine ecosystems. Protist 155:193–214
Hackett JD, Yoon HS, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Nosenko T, Bhattacharya D (2004) Migration of the plastid genome to the nucleus in a peridinin dinoflagellate. Curr Biol 14:213–218
Hackett JD, Yoon HS, Li S, Reyes-Prieto A, Rummele SE, Bhattacharya D (2007) Phylogenomic analysis supports the monophyly of cryptophytes and haptophytes and the association of rhizaria with chromalveolates. Mol Biol Evol 24:1702–1713
Hagopian JC, Reis M, Kitajima JP, Bhattacharya D, de Oliveira MC (2004) Comparative analysis of the complete plastid genome sequence of the red alga Gracilaria tenuistipitata var. liui provides insights into the evolution of rhodoplasts and their relationship to other plastids. J Mol Evol 59:464–477
Hallick RB, Hong L, Drager RG, Favreau MR, Monfort A, Orsat B, Spielmann A, Stutz E (1993) Complete sequence of Euglena gracilis chloroplast DNA. Nucleic Acids Res 21:3537–3544
Hempel F, Felsner G, Maier UG (2010) New mechanistic insights into pre-protein transport across the second outermost plastid membrane of diatoms. Mol Microbiol 76:793–801
Howe CJ, Nisbet RE, Barbrook AC (2008) The remarkable chloroplast genome of dinoflagellates. J Exp Bot 59:1035–1045
Imanian B, Pombert JF, Keeling PJ (2010) The complete plastid genomes of the two ‘dinotoms’ Durinskia baltica and Kryptoperidinium foliaceum. PLoS One 5:e10711
Jacobs J, Glanz S, Bunse-Grassmann A, Kruse O, Kuck U (2010) RNA trans-splicing: identification of components of a putative chloroplast spliceosome. Eur J Cell Biol 89:932–939
Janouskovec J, Horak A, Obornik M, Lukes J, Keeling PJ (2010) A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc Natl Acad Sci USA 107:10949–10954
Jansen RK, Raubeson LA, Boore JL, de Pamphilis CW, Chumley TW, Haberle RC, Wyman SK, Alverson AJ, Peery R, Herman SJ, Fourcade HM, Kuehl JV, McNeal JR, Leebens-Mack J, Cui L (2005) Methods for obtaining and analyzing whole chloroplast genome sequences. Methods Enzymol 395:348–384
Kaplan A, Reinhold L (1999) CO2 concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Physiol Plant Mol Biol 50:539–570
Keeling PJ (2009) Chromalveolates and the evolution of plastids by secondary endosymbiosis. J Eukaryot Microbiol 56:1–8
Keeling PJ (2010) The endosymbiotic origin, diversification and fate of plastids. Philos Trans R Soc Lond B Biol Sci 365:729–748
Keeling PJ, Deane JA, Hink-Schauer C, Douglas SE, Maier UG, McFadden GI (1999) The secondary endosymbiont of the cryptomonad Guillardia theta contains alpha-, beta-, and gamma-tubulin genes. Mol Biol Evol 16:1308–1313
Kessler F, Schnell D (2009) Chloroplast biogenesis: diversity and regulation of the protein import apparatus. Curr Opin Cell Biol 21:494–500
Khan H, Parks N, Kozera C, Curtis BA, Parsons BJ, Bowman S, Archibald JM (2007) Plastid genome sequence of the cryptophyte alga Rhodomonas salina CCMP1319: lateral transfer of putative DNA replication machinery and a test of chromist plastid phylogeny. Mol Biol Evol 24:1832–1842
Knauf U, Hachtel W (2002) The genes encoding subunits of ATP synthase are conserved in the reduced plastid genome of the heterotrophic alga Prototheca wickerhamii. Mol Genet Genomics 267:492–497
Kohler S, Delwiche CF, Denny PW, Tilney LG, Webster P, Wilson RJ, Palmer JD, Roos DS (1997) A plastid of probable green algal origin in Apicomplexan parasites. Science 275:1485–1489
Kovacs-Bogdan E, Soll J, Bolter B (2010) Protein import into chloroplasts: the Tic complex and its regulation. Biochim Biophys Acta 1803:740–747
Kowallik KV, Stoeb B, Schaffran I, Kroth-Pancic P, Freier U (1995) The chloroplast genome of a chlorophyll a+c-containing alga, Odontella sinenesis. Plant Mol Biol Rep 13:336–342
Laatsch T, Zauner S, Stoebe-Maier B, Kowallik KV, Maier UG (2004) Plastid-derived single gene minicircles of the dinoflagellate Ceratium horridum are localized in the nucleus. Mol Biol Evol 21:1318–1322
Lang BF, Burger G, O’Kelly CJ, Cedergren R, Golding GB, Lemieux C, Sankoff D, Turmel M, Gray MW (1997) An ancestral mitochondrial DNA resembling a eubacterial genome in miniature. Nature 387:493–497
Lang BF, Laforest MJ, Burger G (2007) Mitochondrial introns: a critical view. Trends Genet 23:119–125
Lartillot N, Philippe H (2004) A Bayesian mixture model for across-site heterogeneities in the amino-acid replacement process. Mol Biol Evol 21:1095–1109
Lartillot N, Philippe H (2008) Improvement of molecular phylogenetic inference and the phylogeny of Bilateria. Philos Trans R Soc Lond B Biol Sci 363:1463–1472
Lartillot N, Brinkmann H, Philippe H (2007) Suppression of long-branch attraction artefacts in the animal phylogeny using a site-heterogeneous model. BMC Evol Biol 7(Suppl 1):S4
Le Corguille G, Pearson G, Valente M, Viegas C, Gschloessl B, Corre E, Bailly X, Peters AF, Jubin C, Vacherie B, Cock JM, Leblanc C (2009) Plastid genomes of two brown algae, Ectocarpus siliculosus and Fucus vesiculosus: further insights on the evolution of red-algal derived plastids. BMC Evol Biol 9:253
Le Gall L, Saunders GW (2007) A nuclear phylogeny of the Florideophyceae (Rhodophyta) inferred from combined EF2, small subunit and large subunit ribosomal DNA: establishing the new red algal subclass Corallinophycidae. Mol Phylogenet Evol 43:1118–1130
Lehman RL, Manhart JR (1997) A preliminary comparison of restriction fragment patterns in the genus Caulerpa (Chlorophyta) and the unique structure of the chloroplast genome of Caulerpa sertularioides. J Phycol 33:1055–1062
Lemieux C, Otis C, Turmel M (2007) A clade uniting the green algae Mesostigma viride and Chlorokybus atmophyticus represents the deepest branch of the Streptophyta in chloroplast genome-based phylogenies. BMC Biol 5:2
Lewis LA, McCourt RM (2004) Green algae and the origin of land plants. Am J Bot 91:1535–1556
Li HM, Chiu CC (2010) Protein transport into chloroplasts. Annu Rev Plant Biol 61:157–180
Ling F, Shibata T (2004) Mhr1p-dependent concatemeric mitochondrial DNA formation for generating yeast mitochondrial homoplasmic cells. Mol Biol Cell 15:310–322
Liu XQ (2000) Protein-splicing intein: genetic mobility, origin, and evolution. Annu Rev Genet 34:61–76
Löffelhardt W, Bohnert HJ (1994) Structure and function of the cyanelle genome. Int Rev Cytol 151:29–65
Lu F, Xu W, Tian C, Wang G, Niu J, Pan G, Hu S (2010) The Bryopsis hypnoides plastid genome: multimeric forms and complete nucleotide sequence. PLoS One 6:e14663
Ma Y, Jakowitsch J, Deusch O, Henze K, Martin W, Löffelhardt W (2009) Transketolase from Cyanophora paradoxa: in vitro import into cyanelles and pea chloroplasts and a complex history of a gene often, but not always, transferred in the context of secondary endosymbiosis. J Eukaryot Microbiol 56:568–576
Manhart JR, Hoshaw RW, Palmer JD (1990) Unique chloroplast genome in Spirogyra maxima (Chlorophyta) revealed by physical and gene mapping. J Phycol 26:490–494
Manhart JR, Kelly K, Dudock BS, Palmer JD (1989) Unusual characteristics of Codium fragile chloroplast DNA revealed by physical and gene mapping. Mol Gen Genet 216:417–421
Marin B, Melkonina M (2010) Molecular phylogeny and classification of the Mamiellophyceae class. nov (Chlorophyta) based on sequence comparisons of the nuclear- and plastid-encoded rRNA operons. Protist 161:304–336
Mattox KR, Stewart KD (1984) Classification of the green algae: a concept based on comparative ecology. In: Irvine DEG, John DM (eds) The systematics of the green algae. Academic Press, London, pp 29–72
Maul JE et al. (2002) The Chlamydomonas reinhardtti plastid chromosome: islands of genes in a sea of repeats. Plant Cell 14:2659–2679
McFadden GI (1999) Plastids and protein targeting. J Eukaryot Microbiol 46:339–346
McFadden GI (2010) The apicoplast. Protoplasma. 248:641–650
McFadden GI, Waller RF (1997) Plastids in parasites of humans. Bioessays 19:1033–1040
Minge MA, Shalchian-Tabrizi K, Torresen OK, Takishita K, Probert I, Inagaki Y, Klaveness D, Jakobsen KS (2010) A phylogenetic mosaic plastid proteome and unusual plastid-targeting signals in the green-colored dinoflagellate Lepidodinium chlorophorum. BMC Evol Biol 10:191
Moore CE, Archibald JM (2009) Nucleomorph genomes. Annu Rev Genet 43:251–264
Moreira D, Philippe H (2001) Sure facts and open questions about the origin and evolution of photosynthetic plastids. Res Microbiol 152:771–780
Nassoury N, Cappadocia M, Morse D (2003) Plastid ultrastructure defines the protein import pathway in dinoflagellates. J Cell Sci 116:2867–2874
Obornik M, Van de Peer Y, Hypsa V, Frickey T, Slapeta JR, Meyer A, Lukes J (2002) Phylogenetic analyses suggest lateral gene transfer from the mitochondrion to the apicoplast. Gene 285:109–118
O’Brien EA, Zhang Y, Wang E, Marie V, Badejoko W, Lang BF, Burger G (2009) GOBASE: an organelle genome database. Nucleic Acids Res 37:D946–D950
Ohta N, Matsuzaki M, Misumi O, Miyagishima SY, Nozaki H, Tanaka K, Shin IT, Kohara Y, Kuroiwa T (2003) Complete sequence and analysis of the plastid genome of the unicellular red alga Cyanidioschyzon merolae. DNA Res 10:67–77
Oldenburg DJ, Bendich AJ (2001) Mitochondrial DNA from the liverwort Marchantia polymorpha: circularly permuted linear molecules, head-to-tail concatemers, and a 5’ protein. J Mol Biol 310:549–562
Oldenburg DJ, Bendich AJ (2004) Most chloroplast DNA of maize seedlings in linear molecules with defined ends and branched forms. J Mol Biol 335:953–970
Ong HC, Wilhelm SW, Gobler CJ, Bullerjahn G, Jacobs MA, McKay J, Sims EH, Gillett WG, Zhou Y, Haugen E, Rocap G, Cattolico RA (2010) Analysis of the complete chloroplast genome sequences of two members of the Pelagophyceae: Aureococcus anophagefferens and Aureoumbra lagunensis. J Phycol 46:602–615
Oudot-Le Secq MP, Grimwood J, Shapiro H, Armbrust EV, Bowler C, Green BR (2007) Chloroplast genomes of the diatoms Phaeodactylum tricornutum and Thalassiosira pseudonana: comparison with other plastid genomes of the red lineage. Mol Genet Genomics 277:427–439
Parfrey LW, Grant J, Tekle YI, Lasek-Nesselquist E, Morrison HG, Sogin ML, Patterson DJ, Katz LA (2010) Broadly sampled multigene analyses yield a well-resolved eukaryotic tree of life. Syst Biol 59:518–533
Patron NJ, Waller RF (2007) Transit peptide diversity and divergence: a global analysis of plastid targeting signals. Bioessays 29:1048–1058
Patron NJ, Waller RF, Archibald JM, Keeling PJ (2005) Complex protein targeting to dinoflagellate plastids. J Mol Biol 348:1015–1024
Patterson DJ (1989) Stramenopiles: chromophyte from a protistan perspective. In: Green JC, Leadbeater ESC, Diver WL (eds) The chromophyte algae: problems and perspectives. Clarendon, Oxford, pp 357–379
Pfanzagl B, Zenker A, Pittenauer E, Allmaier G, Martinez-Torrecuadrada J, Schmid ER, De Pedro MA, Löffelhardt W (1996) Primary structure of cyanelle peptidoglycan of Cyanophora paradoxa: a prokaryotic cell wall as part of an organelle envelope. J Bacteriol 178:332–339
Philippe H, Delsuc F, Brinkmann H, Lartillot N (2005) Phylogenomics. Annu Rev Ecol Evol Syst 36:541–562
Pombert JF, Keeling PJ (2010) The mitochondrial genome of the entomoparasitic green alga Helicosporidium. PLoS One 5:e8954
Pombert JF, Lemieux C, Turmel M (2006) The complete chloroplast DNA sequence of the green alga Oltmannsiellopsis viridis reveals a distinctive quadripartite architecture in the chloroplast genome of early diverging ulvophytes. BMC Biol 4:3
Pombert JF, Otis C, Lemieux C, Turmel M (2004) The complete mitochondrial DNA sequence of the green alga Pseudendoclonium akinetum (Ulvophyceae) highlights distinctive evolutionary trends in the chlorophyta and suggests a sister-group relationship between the Ulvophyceae and Chlorophyceae. Mol Biol Evol 21:922–935
Pombert JF, Otis C, Lemieux C, Turmel M (2005) The chloroplast genome sequence of the green alga Pseudendoclonium akinetum (Ulvophyceae) reveals unusual structural features and new insights into the branching order of chlorophyte lineages. Mol Biol Evol 22:1903–1918
Reith ME, Munholland J (1995) Complete nucleotide sequence of the Porphyra pupurea chloroplast. Plant Mol Biol Rep 13:333–335
Reyes-Prieto A, Bhattacharya D (2007) Phylogeny of nuclear-encoded plastid-targeted proteins supports an early divergence of glaucophytes within Plantae. Mol Biol Evol 24:2358–2361
Reyes-Prieto A, Weber AP, Bhattacharya D (2007) The origin and establishment of the plastid in algae and plants. Annu Rev Genet 41:147–168
Rivier C, Goldschmidt-Clermont M, Rochaix JD (2001) Identification of an RNA-protein complex involved in chloroplast group II intron trans-splicing in Chlamydomonas reinhardtii. EMBO J 20:1765–1773
Robbens S, Derelle E, Ferraz C, Wuyts J, Moreau H, Van de Peer Y (2007) The complete chloroplast and mitochondrial DNA sequence of Ostreococcus tauri: organelle genomes of the smallest eukaryote are examples of compaction. Mol Biol Evol 24:956–968
Rochaix JD (1996) Post-transcriptional regulation of chloroplast gene expression in Chlamydomonas reinhardtii. Plant Mol Biol 32:327–341
Rodriguez-Ezpeleta N, Brinkmann H, Burey SC, Roure B, Burger G, Löffelhardt W, Bohnert HJ, Philippe H, Lang BF (2005) Monophyly of primary photosynthetic eukaryotes: green plants, red algae, and glaucophytes. Curr Biol 15:1325–1330
Rodriguez-Ezpeleta N, Brinkmann H, Burger G, Roger AJ, Gray MW, Philippe H, Lang BF (2007a) Toward resolving the eukaryotic tree: the phylogenetic positions of jakobids and cercozoans. Curr Biol 17:1420–1425
Rodriguez-Ezpeleta N, Brinkmann H, Roure B, Lartillot N, Lang BF, Philippe H (2007b) Detecting and overcoming systematic errors in genome-scale phylogenies. Syst Biol 56:389–399
Rogers MB, Gilson PR, Su V, McFadden GI, Keeling PJ (2007) The complete chloroplast genome of the chlorarachniophyte Bigelowiella natans: evidence for independent origins of chlorarachniophyte and euglenid secondary endosymbionts. Mol Biol Evol 24:54–62
Rosenblad MA, Samuelsson T (2004) Identification of chloroplast signal recognition particle RNA genes. Plant Cell Physiol 45:1633–1639
Rumpho ME, Worful JM, Lee J, Kannan K, Tyler MS, Bhattacharya D, Moustafa A, Manhart JR (2008) Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica. Proc Natl Acad Sci USA 105:17867–17871
Sanchez Puerta MV, Bachvaroff TR, Delwiche CF (2005) The complete plastid genome sequence of the haptophyte Emiliania huxleyi: a comparison to other plastid genomes. DNA Res 12:151–156
Sanchez-Puerta MV, Bachvaroff TR, Delwiche CF (2007) Sorting wheat from chaff in multi-gene analyses of chlorophyll c-containing plastids. Mol Phylogenet Evol 44:885–897
Schunemann D (2004) Structure and function of the chloroplast signal recognition particle. Curr Genet 44:295–304
Shevelev EL, Bryant DA, Löffelhardt W, Bohnert HJ (1995) Ribonuclease-P RNA gene of the plastid chromosome from Cyanophora paradoxa. DNA Res 2:231–234
Shoup S, Lewis LA (2003) Polyphyletic origin of parallel basal bodies in swimming cells of chlorophycean green algae (Chlorophyta). J Phycol 39:789–796
Sluiman HJ (1985) A cladistic evaluation of the lower and higher green plants (Viridiplantae). Plant Syst Evol 149:217–232
Smith DR, Lee RW (2009) The mitochondrial and plastid genomes of Volvox carteri: bloated molecules rich in repetitive DNA. BMC Genomics 10:132
Smith DR, Lee RW (2010) Low nucleotide diversity for the expanded organelle and nuclear genomes of Volvox carteri supports the mutational-hazard hypothesis. Mol Biol Evol 27:2244–2256
Smith DR, Lee RW, Cushman JC, Magnuson JK, Tran D, Polle JE (2010) The Dunaliella salina organelle genomes: large sequences, inflated with intronic and intergenic DNA. BMC Plant Biol 10:83
Stiller JW, Huang J, Ding Q, Tian J, Goodwillie C (2009) Are algal genes in nonphotosynthetic protists evidence of historical plastid endosymbioses? BMC Genomics 10:484
Stoebe B, Maier UG (2002) One, two, three: nature’s tool box for building plastids. Protoplasma 219:123–130
Strittmatter P, Soll J, Bolter B (2010) The chloroplast protein import machinery: a review. Methods Mol Biol 619:307–321
Tanaka T, Fukuda Y, Yoshino T, Maeda Y, Muto M, Matsumoto M, Mayama S, Matsunaga T (2011) High-throughput pyrosequencing of the chloroplast genome of a highly neutral-lipid-producing marine pennate diatom, Fistulifera sp. strain JPCC DA0580. Photosynth Res 109:223–229
Turmel M, Otis C, Lemieux C (1999) The complete chloroplast DNA sequence of the green alga Nephroselmis olivacea: insights into the architecture of ancestral chloroplast genomes. Proc Natl Acad Sci USA 96:10248–10253
Turmel M, Otis C, Lemieux C (2002) The complete mitochondrial DNA sequence of Mesostigma viride identifies this green alga as the earliest green plant divergence and predicts a highly compact mitochondrial genome in the ancestor of all green plants. Mol Biol Evol 19:24–38
Turmel M, Otis C, Lemieux C (2005) The complete chloroplast DNA sequences of the charophycean green algae Staurastrum and Zygnema reveal that the chloroplast genome underwent extensive changes during the evolution of the Zygnematales. BMC Biol 3:22
Turmel M, Otis C, Lemieux C (2006) The chloroplast genome sequence of Chara vulgaris sheds new light into the closest green algal relatives of land plants. Mol Biol Evol 23:1324–1338
Turmel M, Otis C, Lemieux C (2007) An unexpectedly large and loosely packed mitochondrial genome in the charophycean green alga Chlorokybus atmophyticus. BMC Genomics 8:137
Turmel M, Brouard JS, Gagnon C, Otis C, Lemieux C (2008) Deep division in the Chlorophyceae (Chlorophyta) revealed by chloroplast phylogenomic analyses. J Phycol 44:739–750
Turmel M, Gagnon MC, O’Kelly CJ, Otis C, Lemieux C (2009a) The chloroplast genomes of the green algae Pyramimonas, Monomastix, and Pycnococcus shed new light on the evolutionary history of prasinophytes and the origin of the secondary chloroplasts of euglenids. Mol Biol Evol 26:631–648
Turmel M, Otis C, Lemieux C (2009b) The chloroplast genomes of the green algae Pedinomonas minor, Parachlorella kessleri, and Oocystis solitaria reveal a shared ancestry between the Pedinomonadales and Chlorellales. Mol Biol Evol 26:2317–2331
van Dooren GG, Schwartzbach SD, Osafune T, McFadden GI (2001) Translocation of proteins across the multiple membranes of complex plastids. Biochim Biophys Acta 1541:34–53
Verbruggen H, Maggs CA, Saunders GW, Le Gall L, Yoon HS, De Clerck O (2010) Data mining approach identifies research priorities and data requirements for resolving the red algal tree of life. BMC Evol Biol 10:16
Wakasugi T, Nagai T, Kapoor M, Sugita M, Ito M, Ito S, Tsudzuki J, Nakashima K, Tsudzuki T, Suzuki Y, Hamada A, Ohta T, Inamura A, Yoshinaga K, Sugiura M (1997) Complete nucleotide sequence of the chloroplast genome from the green alga Chlorella vulgaris: the existence of genes possibly involved in chloroplast division. Proc Natl Acad Sci USA 94:5967–5972
Wastl J, Maier UG (2000) Transport of proteins into cryptomonads complex plastids. J Biol Chem 275:23194–23198
Wastl J, Duin EC, Iuzzolino L, Dorner W, Link T, Hoffmann S, Sticht H, Dau H, Lingelbach K, Maier UG (2000) Eukaryotically encoded and chloroplast-located rubredoxin is associated with photosystem II. J Biol Chem 275:30058–30063
Williamson DH, Gardner MJ, Preiser P, Moore DJ, Rangachari K, Wilson RJ (1994) The evolutionary origin of the 35 kb circular DNA of Plasmodium falciparum: new evidence supports a possible rhodophyte ancestry. Mol Gen Genet 243:249–252
Wilson RJ, Williamson DH (1997) Extrachromosomal DNA in the Apicomplexa. Microbiol Mol Biol Rev 61:1–16
Wolfe KH, Morden CW, Palmer JD (1991) Ins and outs of plastid genome evolution. Curr Opin Genet Dev 1:523–529
Wyman SK, Jansen RK, Boore JL (2004) Automatic annotation of organellar genomes with DOGMA. Bioinformatics 20:3252–3255
Yamada T (1991) Repetitive sequence-mediated rearrangements in Chlorella ellipsoidea chloroplast DNA – completion of nucleotide-sequence of the large inverted repeat. Curr Genet 19:139–147
Yamada T, Shimaji M (1987) Splitting of the ribosomal-RNA operon on chloroplast DNA from Chlorella ellipsoidea. Mol Gen Genet 208:377–383
Zhang Z, Green BR, Cavalier-Smith T (1999) Single gene circles in dinoflagellate chloroplast genomes. Nature 400:155–159
Zhang Z, Cavalier-Smith T, Green BR (2002) Evolution of dinoflagellate unigenic minicircles and the partially concerted divergence of their putative replicon origins. Mol Biol Evol 19:489–500
Acknowledgments
We are grateful to Dr. G. Burger (Université de Montréal, Montreal, Canada) for insightful discussion and comments on the manuscript. This work was supported by the Canadian Research Chair Program and the Natural Sciences and Engineering Research Council (NSERC; 194560–2011) of Canada.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Lang, B.F., Nedelcu, A.M. (2012). Plastid Genomes of Algae. In: Bock, R., Knoop, V. (eds) Genomics of Chloroplasts and Mitochondria. Advances in Photosynthesis and Respiration, vol 35. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-2920-9_3
Download citation
DOI: https://doi.org/10.1007/978-94-007-2920-9_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-2919-3
Online ISBN: 978-94-007-2920-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)