Abstract
The most salient feature of Streptococcus bovis (SB) is its clinical association with malignancy of the colon and rectum. The relationship between SB and colorectal cancer (CRC) was already recognized in the 1950s and many case reports and retrospective studies on this association have been published since then. SB is an opportunistic pathogen that normally resides asymptomatically in the human intestinal tract. In compromised individuals, however, this bacterium can cause systemic infections most often presenting as bacterial endocarditis. Investigators reported the presence of colorectal tumours in up to 60% of the cases in which a patient was diagnosed with SB endocarditis or bacteremia. Therefore, these infections are nowadays often regarded as indication for full bowel examination in clinical practice. Importantly, recent studies have indicated that the association between S. gallolyticus subsp gallolyticus (previously called SB biotype I) with CRC seems much more pronounced than that of other known SB biotypes. Nevertheless, the question whether SB has a causal or predominantly incidental involvement with cancer of the colon remains to be answered. Furthermore, still little is known about the precise molecular mechanisms that determine this specific relationship. This chapter aims to summarize the literature on this subject and to illustrate possible mechanisms behind the association of SB with CRC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Colon
- Colorectal cancer
- Tumour
- Polyp
- Carcinoma
- Adenoma
- Carcinogenesis
- Streptococcus bovis
- Streptococcus gallolyticus
- Endocarditis
- Bacteremia
- Inflammation
- Intestine
- Serology
- Diagnosis
- Biomarker
3.1 Colorectal Cancer and Microbial Agents
Colorectal cancer (CRC) is the third most common cancer for men and women in Western society. It is estimated that nearly 150,000 cases were newly diagnosed and 50,000 persons died of this disease in year 2009 in the USA (Horner et al. 2009). The temporal and geographic variations in CRC incidence in US whites and blacks (Horner et al. 2009) and among immigrants (Curado et al. 2007) are best explained by environmental factors rather than genetic predisposition. According to Dr. Parkin’s estimate (Parkin 2006), 17.8% of the worldwide cancer incidence is attributable to infectious agents, resulting in approximately 1.9 million cases per year. These include a variety of infectious agents: parasites such as Schistosoma haematobium and Opisthorchis viverrini, bacteria, such as Helicobacter pylori (HP), and, viruses, such as Epstein-Barr virus, Hepatitis virus, and Human herpes, papilloma (HPV), polyma and retro-viruses (IARC 1994; Persing and Prendergast 1999; Del Valle et al. 2002). Several mechanisms have been proposed, including direct effects on host cell proliferation and communication pathways, impairment of host immune system, induction of genomic instability and chronic inflammation (Herrera et al. 2005). Chronic inflammation often accompanies increased host cell turnover, which increases the probability of mutagenic events, and enhanced formation of reactive oxygen and nitrogen species that damage DNA and induce genomic instability (Coussens and Werb 2002; Blaser 2008; Hussain and Harris 2007; Terzić et al. 2010). Thus, inflammatory responses play decisive roles at different stages of tumour development, including initiation, promotion, malignant conversion, invasion, and metastasis (Grivennikov et al. 2007). Genomic instability may arise from inactivation of DNA mismatch repair (MMR) system (MSH1/2), which leads to the development of a specific molecular subtype of CRC termed microsatellite instability high (MSI-H) (Jass 2007). MSI has been observed frequently in long standing ulcerative colitis mucosa (Ishitsuka et al. 2001) as well as in HP-positive gastric cancer (Li et al. 2005) and MSH2 deficient mice are susceptible to inflammation associated colorectal tumours (Kohonen-Corish et al. 2002). In addition, overexpression of a COX-2 receptor protein has been characterized for MSI-H tumours (Baba et al. 2010). The large bowel is indeed the natural habitat for a large, dynamic and highly competitive bacterial community, which is essential for the control of intestinal epithelial homeostasis and human health. Strikingly, the increase in bacterial colonization from the ileum to the colon (six orders of magnitude; Stone and Papas 1997), is paralleled by a marked difference in cancer incidence (by at least a factor of 30) between the small and large intestines. Although bacterial etiologies in sporadic CRC have never been firmly established in humans, studies in germ free mice suggest that intestinal bacteria are indeed required for colorectal carcinogenesis in model systems (Hope et al. 2005; Sinicrope 2007). Finally, there is good evidence that aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) reduce the risk of CRC and its precursor (Rostom et al. 2007; Dubé et al. 2007).
3.2 Microbiological Characteristics of Streptococcus bovis
Streptococcus bovis (SB) is a gram-positive bacterium and lower-grade opportunistic pathogen that can cause systemic infections (endocarditis or bacteraemia) in humans. It is a group D streptococcus with the specific ability to grow in 40% bile (Moellering et al. 1974; Roberts 1992). The classification and identification of SB has been problematic for a long time. Based on phenotypic diversity, three SB biotypes (I, II/1, and II/2) have been reported. Recently, based on biochemical traits, DNA homology and divergence in 16S rRNA sequences, Schlegel et al. (2004) suggested to rename SB I into S. gallolyticus subsp. gallolyticus (SGG), to divide SB biotype II/1 strains into (i) S. infantarius subsp. coli (SIC) and (ii) S. infantarius subsp. infantarius (SII) and to rename SB II/2 into S. gallolyticus subsp. pasteurianus (SGP; see Table 3.1). In addition, the closely related non-pathogenic strain Streptococcus macedonicus was reclassified as S. gallolyticus subsp. macedonicus (SGM). Earlier studies suggest that SGG and SII are the most commonly isolated pathogens from the SB group, with the former being the more virulent in humans and more often associated with endocarditis (Corredoira et al. 2008a). In a recent reexamination of SB bacteremias in a 20-year period in France, the association of colon tumours with SGG was found to be ∼50% versus 11% for SII. Strikingly, however, for non-colonic cancer the association was 6% for SGG versus 57% for SII. Most of the non-colonic cancers associated with SII were of the pancreas and biliary tract (Corredoira et al. 2008b). Because of the lack of unified terminology information in literature, we refer to both SGG and SII as SB in the rest of this chapter.
3.3 Association of SB with Colorectal Disease
Although SB is a member of normal gastrointestinal flora in ruminants, e.g., cattle, sheep, horses, pigs, camels and deers (Ghali et al. 2004), it can also found in human feces as well as gastric biopsy materials (Schlegel et al. 2000; Ribeiro et al. 2004). Approximately 10% of healthy individuals have been estimated to carry this bacterium asymptomatically in their digestive tract (Schlegel et al. 2000). While fecal-oral or oral-oral is a possible transmission route between humans, it may be acquired through dietary intake of ruminant-derived foods, such as unpasteurized dairy products (Randazzo et al. 2006), red meat and animal organs (Schlegel et al. 2000). In fact SB is a frequently detected contaminant in commercially available meat (Knudtson and Hartman 1993; Thian and Hartman 1981). The correlation between SB and colonic disease has long been recognized. Besides case-reports for the patients who were diagnosed with asymptomatic colorectal neoplasia simultaneously with SB endocarditis or bacteremia (McMahon et al. 1991; Nielsen et al. 2007; Wentling et al. 2006; Gupta et al. 2010; Kahveci et al. 2010; Kim et al. 2010), investigators have reported increased prevalence of colorectal tumours (cancer and polyps) among patients diagnosed with SB endocarditis or bacteremia. As summarized in the Table 3.2, the prevalence of colorectal tumours ranges from 10% to 60% (Corredoira et al. 2005, 2008a; Murray and Roberts 1978; Klein et al. 1979; Reynolds et al. 1983; Pigrau et al. 1988; Ruoff et al. 1989; Clarridge et al. 2001; Gonzlez-Quintela et al. 2001; Gold et al. 2004; Lee et al. 2003; Zarkin et al. 1990; Jean et al. 2004; Alazmi et al. 2006; Giannitsioti et al. 2007; Beck et al. 2008; Vaska and Faoagali 2009), although these are based on diverse study populations in terms of patient demographics and colorectal surveillance methods. These variations may also be due to the heterogenous definition of the cases, as adenomas have been defined as diseased in some studies but not in others (Boleij et al. 2009b). None of these studies, however, have evaluated their results in comparison with expected frequencies in the general population. The second set of evidence is derived from studies comparing SB prevalence among various patient groups with or without colonic diseases (Table 3.3) (Klein et al. 1977; Burns et al. 1985; Darjee and Gibb 1993; Dubrow et al. 1991; Potter et al. 1998; Teitelbaum and Triantafyllopoulou 2006; Tjalsma et al. 2006; Abdulamir et al. 2009). While three small studies including 13–46 controls and corresponding 11 CRC, 47 pediatric inflammatory bowel disease (IBD) and 56 polyp patients failed to show any association (Dubrow et al. 1991; Potter et al. 1998; Teitelbaum and Triantafyllopoulou 2006), five other studies found SB carriage (either in stool or antibodies) rates were significantly higher in cancer patients than in controls. Interestingly, three studies also showed that patients with premalignant lesions (IBD or polyps) had intermediate SB carriage rate between cancer cases and controls (Klein et al. 1977; Teitelbaum and Triantafyllopoulou 2006; Tjalsma et al. 2006). For an update, see our recent literature-based meta-analysis on the association between S. bovis and CRC (Boleij et al. 2011).
3.4 Potential Mechanisms in Carcinogenesis
Despite observations discussed above, implications of SB infection on CRC remain largely elusive. There are several possible interpretations that are not necessarily mutually exclusive. First, it has been hypothesized that colorectal neoplastic sites provide a specific niche for SB resulting in sustained colonization, survival, and the establishment of a local tumour-associated (clinically silent) infection. Second, silent SB infection itself possibly promotes colorectal carcinogenesis, which has been supported by several experimental studies. Administration of SB or SB wall extracted antigens in rodents increases the formation of colorectal precursor lesions in a chemical carcinogenesis model (Ellmerich et al. 2000a). This was accompanied by increased expression of proliferative markers and enhanced interleukin IL-8 production in normal colonic mucosa of SB-injected animals. SB wall antigens are capable of adhering to various types of human cells, including GI-epithelial, endothelial and blood cells, as well as to extracellular matrix and induce IL-8 synthesis (Ellmerich et al. 2000b). In fact, increased IL-8 positive cells have been reported in SB seropositive human CRC cases compared with SB-seronegative cases (Abdulamir et al. 2009). IL-8 is a pro-inflammatory cytokine which also possesses mitogenic and angiogenic properties. It increases oxidative/ nitrosative stress and mediate the formation of carcinogenic compounds in gastrointestinal mucosa/lumen (Federico et al. 2007; Vermeer et al. 2004; Hussain and Harris 2007). IL-8 also leads to cyclooxygenase (COX)-2 overexpression (Biarc et al. 2004). COX-2 driven prostaglandin synthesis stimulates cell proliferation, motility and metastatic potential, promotes angiogenesis, and induces local immunosuppression (Harris 2007; Mutoh et al. 2006). On the other hand, selective and non-selective COX-2 inhibitors reduce the incidence and prevalence of colorectal polyps (Steinbach et al. 2000; Logan et al. 2008). Importantly, increased COX-2 expression has been demonstrated in rodent infectious colorectal carcinogenesis models (Skinn et al. 2006; Newman et al. 2001; Balish and Warner 2002; Wang and Huycke 2007). The induction of COX-2 by SB in colon tissue has been reported for a rat model (Biarc et al. 2004) and may also occur in humans (Fig. 3.1). These enhanced COX-2 activities may also exert synergistic effects with other enzymes sharing substrates (e.g., CYP1 family) in metabolic activation of diet-derived carcinogens, such as polycyclic aromatic hydrocarbons (PAH) found in cooked meat (Wiese et al. 2001; Almahmeed et al. 2004). Such an enzyme, CYP1A1/B1, is indeed overexpressed in CRC and its precursors (McKay et al. 1993; Kumarakulasingham et al. 2005; Chang et al. 2005). This interplay is potentially important because meat consumption is one of the SB acquisition routes and because meat-derived PAH can induce intestinal CYP1A1/B1 (Lampen et al. 2004). In addition, SB can induce matrix metalloproteinases (MMPs), e.g., MMP2 and MMP9 (Mungall et al. 2001), that play crucial roles in CRC growth and progression (Paduch et al. 2010; Sinnamon et al. 2008; Kim et al. 2009; Miyake et al. 2009). Furthermore, SB may also contribute to intra-colonic formation of potential carcinogens, e.g., nitroso-compounds (McKnight et al. 1999). The human large intestine contains a large amount of nitrogenous residues and nitrosating agents from dietary protein, and enzymatic activities of intestinal bacteria, e.g., streptococci, mediate these reactions (Hughes et al. 2001; Calmels et al. 1996, 1988). Intriguingly, consumption of red meat, a presumed route of SB acquisition, promotes colonic N-nitrosation via increasing supplies of colonic amine, nitrite and arginine (Hughes et al. 2001; Bingham et al. 1996, 2002; Silvester et al. 1997). Notably, large intestinal N-nitrosation does not occur in germ-free animals (Rowland et al. 1991).
COX-2 induction by SB. (a) The induction of COX-2 by SB was measured in HT-29 colorectal tumour cells in vitro. SB and HT-29 cells were co-incubated for 0.5, 1, 2 or 4 h. Subsequently RNA was extracted for real-time PCR procedures. The relative expression of COX-2 was determined by real time PCR using GAPDH as endogenous internal control and considered to be induced at values greater than 1.5. The bar graph shows that SB induces the expression of COX-2 after 2 and 4 h which is consistent with previously results in literature (Biarc et al. 2004). (b) The correlation of COX-2 expression and the presence of SB in tumour tissue from 4 CRC patients was determined in parallel. The presence of SB was monitored by a nested PCR on the SB sodA gene. The results are suggestive for a correlation of SB and COX-2 expression in vivo
3.5 SB Serology in CRC Patients
Although infections have been recognized as a major preventable cause of human cancer (Kuper et al. 2000), bacterial etiologies in sporadic CRC have not been established in humans. Notably, SB has indeed been recognized as an infectious agent that fulfills the criteria for inferring causality to the highest extent among the four agents evaluated as a potential cause of CRC in a recent review (Burnett-Hartman et al. 2008). However, to our knowledge there have been no epidemiologic studies properly designed to address this issue. The lack of good serological assays for SB infection may have been one of the reasons for scarcity of epidemiologic data. Darjee and Gibb (1993) were the first to monitor increased SB antibody responses in CRC patients by an ELISA approach. After that, Tjalsma et al. (2006, 2007) established an SB antibody profiling assay exploiting immunocapture time-of-flight mass spectrometry (IC-TOF MS) (Tjalsma et al. 2008), Abdulamir et al. (2009) also developed an ELISA to monitor SB antibodies in CRC patients and controls. As shown in Table 3.3, stronger associations observed by these approaches suggest that antibody assays may be a more powerful tool than fecal culture in assessing the associations between this bacterial infection and colorectal disease. Furthermore, as infectious agents in general induce a more pronounced immune response compared to tumour “self”-antigens, SB antigens could become instrumental in the immunodiagnosis of CRC (Tjalsma 2010).
3.6 SB and CRC Risk
To further investigate the exposure to SB in CRC patients, Boleij et al. (2010), developed an ELISA based on SB antigen RpL7/L12, previously assigned as a diagnostic antigen (Tjalsma et al. 2007). This assay was exploited for serological evaluation in Dutch (n = 209) and American (n = 112) populations. These analyses showed that an immune response against this bacterial antigen was increased in polyp patients and stage I/II CRC patients as compared to controls (Odds ratio (OR ) 1.50, 95% Confidence Interval (CI) 0.48–4.62 in the Netherlands; OR 2.75, 95%CI 0.96–7.88 in the US). Notably, increased anti-RpL7/L12 levels were not or only mildly detected in late stage colorectal cancer patients having lymph node or distant metastasis (Fig. 3.2). Increased anti-RpL7/L12 levels were not paralleled by increased antibody production to endotoxin, an intrinsic cell wall component of the majority of intestinal bacteria, which implicates that the humoral immune response against RpL7/L12 is not a general phenomenon induced by the loss of colonic barrier function. The age-adjusted OR for all colorectal tumours combined was very similar in the US (2.30 95% CI 1.06–5.00) and Netherlands (1.90, 95% CI 0.49–2.84). Even a relatively modest increase should be relevant for the progression of colon adenomas to carcinomas (accumulation of mutations), a process which can take over a decade to take place. In this respect, it is interesting to note that the ORs of 1.5 and 2.8 for early stage CRC were within the range of those calculated for the serological response to a panel of Helicobacter pylori antigens in patients with early stage gastric precancerous lesions (ORs ranging from 1 to 9) (Gao et al. 2009). Unfortunately, no data are yet available (August 2010) that correlate SB colonization of tumour tissue with the humoral immune response to SB antigens. Nevertheless, our preliminary studies suggest that tumour tissue provides a niche that allows increased SB colonization (Fig. 3.3). Altogether, these findings suggest that SB constitutes a risk factor for the development and/or progression of pre-malignant lesions into carcinomas. Importantly, cross-sectional and retrospective studies, including the current study and others, are not able to address the temporal relationship between an exposure and a disease outcome directly. Thus, future prospective studies are essential to elucidate the etiological roles of SB in colorectal carcinogenesis.
Humoral immune response against SB antigen RpL7/L12. Serum anti-RpL7/L12 were determined in healthy control subjects (n = 60), “early stage CRC” (polyp and local tumours; n = 70) and “advanced CRC” (tumours with regional and distant metastases; n = 50) by an ELISA (Tjalsma et al. 2007). The results are indicative for a moderate, but significant (*), increased exposure to this antigen during the early stages of CRC. Median levels, second and third quartile (boxes), ad ranges (lines) are indicated. Relative anti-RpL7/L12 IgG levels were expressed as arbitrary optical density units
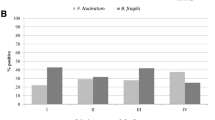
SB detection in human colonic biopsy samples. The presence of SB in human biopsies from tumour tissue (T) and adjacent non-malignant mucosa (N) was monitored by a nested PCR on the SB sodA gene using biopsy-extracted DNA from 8 CRC patients as a template. CRC disease staging is indicated. A broad range 16S rRNA PCR was run in parallel to control for the presence of bacterial DNA and PCR inhibiting substances. The results are suggestive for a preferred colonization of tumour tissue by SB. The identity of the sodA PCR fragments was confirmed by nucleotide sequencing, which showed that all products had the highest degree of similarity with SGG (SB biotype I)
3.7 Model for the Association of SB with CRC
Based on the current knowledge the following model for the association of SB with CRC can be envisaged (Fig. 3.4). Pre-malignant lesions are initiated by carcinogenic (dietary) factors that diffuse through the colonic mucus layer and induce mutations within the APC or B-catenin genes (Cho and Vogelstein 1992). These thereby immortalized epithelial cells are prone to the accumulation of other mutations and, as a side effect, the aberrant epithelial physiology disturbs the mucus layer covering the epithelial cells (Corfield et al. 2000) and makes it susceptible to bacterial infiltration. Such (pre-) malignant epithelial sites may also provide a selective bacterial microenvironment, for instance by the excretion of specific metabolites, recruitment of immune cells and/or production of selective anti-microbial substances. Bacteria, such as SB, which are unable to effectively colonize the healthy colon may have a competitive advantage in this microenvironment and survive for prolonged periods of time. Tumour infiltration of SB may exert inflammatory factors such as IL-8 and COX-2 and/or lead to increased levels of genotoxins and thereby promote intestinal carcinogenesis. These (pre-) malignant lesions also provide a portal of entry for SB which explains the increased anti-SB antibody titers and increased incidence of SB endocarditis in CRC patients. Late stage tumours entering the metastatic phase may change in such a way that bacterial survival on the tumour surface is diminished or antibody expression due to bacterial interaction is reduced. The possibility that tumour progression may drive bacteria out of the cancerous tissue is similar to what has been reported for H. pylori during gastric cancer progression (Kang et al. 2006; Brenner et al. 2007). If true, this phenomenon may partly account for a wide range of the prevalence of SB reported for CRC patients that is comprised of various stages of the disease.
Model for a temporal association between SB and CRC. The development of colorectal tumours is schematically depicted from left (healthy) to right (invasive and metastasizing carcinomas). Initiation of carcinogenesis is a multi-factorial process in which dietary factor play an important role. It may be envisaged that adenomas and early carcinomas provide a preferred niche for SB, which leads to subclinical infection and an increased exposure to SB which can be measured by serological assays. Moreover, this could explain the increased incidence of SB bacteremia and endocarditis in CRC patients as these (pre-) malignant lesions can form a portal of entry into the human body. In addition, SB may interfere with colon carcinogenesis for instance by the induction of IL-8 and COX-2, whereas tumour progression may drive SB out of advanced cancerous tissue (see text for details)
3.8 Conclusion
The clinical association between SB and CRC is widely acknowledged, and an SB infection is often regarded as an indication for full bowel examination in clinical practice. However, still little is known about the molecular mechanisms behind this association (Boleij et al. 2009a, b). The recent deciphering of the SGG (SB biotype I) genome revealed unique features among streptococci, probably related to its adaptation to the intestinal environment (Rusniok et al. 2010). For instance, SGG has the capacity to use a broad range of carbohydrates of plant origin, in particular to degrade polysaccharides derived from the plant cell wall. Its genome encodes a large repertoire of transporters and catalytic activities, like tannase, phenolic compounds decarboxylase, and bile salt hydrolase, which should contribute to the detoxification of the gut environment. Furthermore, SGG has the potential to synthesize all 20 amino acids and more vitamins than any other sequenced Streptococcus species (Rusniok et al. 2010). The surface properties (Fig. 3.5) of this bacterium might be implicated in resistance to innate immunity defenses, and glucan mucopolysaccharides, three types of pili, and collagen binding proteins may play a role in adhesion to tissues in the course of endocarditis. Recent in vitro studies revealed that SGG has a unique repertoire of virulence factors that may facilitate infection through (pre-)malignant colonic lesions and subsequently can provide SGG with a competitive advantage to evade the innate immune system and to form resistant vegetations at collagen-rich sites in susceptible CRC patients (Boleij et al. 2011a). However, many questions on the relationship between SGG and CRC remain to be answered. Therefore, future studies should answer to which extent polyps and tumours actually provide a niche for SB colonization, and if so, which factors are involved in the adherence to, and/or survival in, the tumour microenvironment and how this increased colonization promotes carcinogenesis. In addition, improved (ELISA) assays are desirable to address the relationship between SB exposure and CRC directly in prospective and retrospective studies. Together, these molecular and epidemiological studies are essential for the full elucidation of the etiological roles of SB in colorectal carcinogenesis.
SB surface structure. Electron microscopy picture of SGG cells (strain UCN34 (Rusniok et al. 2010)) showing the capsule of glucan mucopolysaccharides after polycationic ferritin labelling (Vanrobaeys et al. 1999), which may be important for immune evasion in the course of endocarditis (The picture was kindly provided by Philippe Glaser and Nadège Cayet, Unité de Génomique des Microorganismes Pathogènes, Institute Pasteur, Paris, France)
Abbreviations
- CRC:
-
Colorectal cancer
- ELISA:
-
Enzyme-linked immunosorbent assay
- HP:
-
Helicobacter pylori
- IBD:
-
Inflammatory bowel disease
- IC-TOF MS:
-
Immunocapture time-of-flight mass spectrometry
- MMP:
-
Matrix metalloproteinases
- NSAID:
-
Non steroidal anti inflammatory drugs
- OR:
-
Odds ratio
- PAH:
-
Polycyclic aromatic hydrocarbons
- SB:
-
Streptococcus bovis
- SIC:
-
S. infantarius subsp. coli
- SGG:
-
S. gallolyticus subsp. gallolyticus
- SGM:
-
S. gallolyticus subsp. macedonicus
- SGP:
-
S. gallolyticus subsp. pasteurianus
- SII:
-
S. infantarius subsp. infantarius
References
Abdulamir AS, Hafidh RR, Mahdi LK et al (2009) Investigation into the controversial association of Streptococcus gallolyticus with colorectal cancer and adenoma. BMC Cancer 9:403
Alazmi W, Bustamante M, O’Loughlin C et al (2006) The association of Streptococcus bovis bacteremia and gastrointestinal diseases: a retrospective analysis. Dig Dis Sci 51:732–736
Almahmeed T, Boyle JO, Cohen EG et al (2004) Benzo[a]pyrene phenols are more potent inducers of CYP1A1, CYP1B1 and COX-2 than benzo[a]pyrene glucuronides in cell lines derived from the human aerodigestive tract. Carcinogenesis 25:793–799
Baba Y, Nosho K, Shima K et al (2010) PTGER2 overexpression in colorectal cancer is associated with microsatellite instability, independent of CpG island methylator phenotype. Cancer Epidemiol Biomarkers Prev 19:822–831
Balish E, Warner T (2002) Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol 160:2253–2257
Beck M, Frodl R, Funke G (2008) Comprehensive study of strains previously designated Streptococcus bovis consecutively isolated from human blood cultures and emended description of Streptococcus gallolyticus and Streptococcus infantarius subsp. coli. J Clin Microbiol 46:2966–2972
Biarc J, Nguyen IS, Pini A et al (2004) Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S. bovis). Carcinogenesis 25:1477–1484
Bingham SA, Pignatelli B, Pollock JR et al (1996) Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis 17:515–523
Bingham SA, Hughes R, Cross AJ (2002) Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J Nutr 132:3522S–3525S
Blaser NJ (2008) Understanding microbe-induced cancers. Cancer Prev Res 1:15–20
Boleij A, Schaeps RM, de Kleijn S et al (2009a) Surface-exposed histone-like protein a modulates adherence of Streptococcus gallolyticus to colon adenocarcinoma cells. Infect Immun 77:5519–5527
Boleij A, Schaeps RM, Tjalsma H (2009b) Association between Streptococcus bovis and colon cancer. J Clin Microbiol 47:516
Boleij A, Roelofs R, Schaeps RMJ et al (2010) Increased exposure to bacterial antigen RpL7/L12 in early stage colorectal cancer patients. Cancer 116:4014–4022
Boleij A, Muytjens CMJ, Bukhari CI, Cayet N, Glaser P, Hermans PW, Swinkels DS, Bolhuis A, Tjalsma H (2011a) Novel clues on the specific association of Streptococcus gallolyticus subsp gallolyticus with colorectal cancer. J Infect Dis 203:1101–1109
Boleij A, van Gelder MM, Swinkels DW, Tjalsma H (2011b) Clinical Importance of Streptococcus gallolyticus Infection Among Colorectal Cancer Patients: Systematic Review and Meta-analysis. Clin Infect Dis 53:870–878
Brenner H, Rothenbacher D, Weck MN (2007) Epidemiologic findings on serologically defined chronic atrophic gastritis strongly depend on the choice of the cutoff-value. Int J Cancer 121:2782–2786
Burnett-Hartman AN, Newcomb PA, Potter JD (2008) Infectious agents and colorectal cancer: a review of Helicobacter pylori, Streptococcus bovis, JC virus, and human papillomavirus. Cancer Epidemiol Biomarkers Prev 17:2970–2979
Burns CA, McCaughey R, Lauter CB (1985) The association of Streptococcus bovis fecal carriage and colon neoplasia: possible relationship with polyps and their premalignant potential. Am J Gastroenterol 80:42–46
Calmels S, Ohshima H, Bartsch H (1988) Nitrosamine formation by denitrifying and non-denitrifying bacteria: implication of nitrite reductase and nitrate reductase in nitrosation catalysis. J Gen Microbiol 134:221–226
Calmels S, Ohshima H, Henry Y et al (1996) Characterization of bacterial cytochrome cd(1)-nitrite reductase as one enzyme responsible for catalysis of nitrosation of secondary amines. Carcinogenesis 17:533–536
Chang H, Su JM, Huang CC et al (2005) Using a combination of cytochrome P450 1B1 and beta-catenin for early diagnosis and prevention of colorectal cancer. Cancer Detect Prev 29:562–569
Cho KR, Vogelstein B (1992) Genetic alterations in the adenoma–carcinoma sequence. Cancer 15:1727–1731
Clarridge JE 3rd, Attorri SM, Zhang Q et al (2001) 16 S ribosomal DNA sequence analysis distinguishes biotypes of Streptococcus bovis: Streptococcus bovis Biotype II/2 is a separate genospecies and the predominant clinical isolate in adult males. J Clin Microbiol 39:1549–1552
Corfield AP, Myerscough N, Longman R et al (2000) Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut 47:589–594
Corredoira JC, Alonso MP, García JF et al (2005) Clinical characteristics and significance of Streptococcus salivarius bacteremia and Streptococcus bovis bacteremia: a prospective 16-year study. Eur J Clin Microbiol Infect Dis 24:250–255
Corredoira J, Alonso MP, Coira A et al (2008a) Characteristics of Streptococcus bovis endocarditis and its differences with Streptococcus viridans endocarditis. Eur J Clin Microbiol Infect Dis 27:285–291
Corredoira J, Alonso MP, Coira A et al (2008b) Association between Streptococcus infantarius (formerly S. bovis II/1) bacteremia and noncolonic cancer. J Clin Microbiol 46:1570
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Curado MP, Edwards B, Shin HR et al (eds) (2007) Cancer incidence in five continents, vol IX, IARC Scientific Publications No. 160. IARC, Lyon
Darjee R, Gibb AP (1993) Serological investigation into the association between Streptococcus bovis and colonic cancer. J Clin Pathol 46:1116–1119
Del Valle L, Gordon J, Enam S et al (2002) Expression of human neurotropic polyomavirus JCV late gene product agnoprotein in human medulloblastoma. J Natl Cancer Inst 94:267–273
Dubé C, Rostom A, Lewin G et al (2007) U.S. Preventive Services Task Force. The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med 146:365–375
Dubrow R, Edberg S, Wikfors E et al (1991) Fecal carriage of Streptococcus bovis and colorectal adenomas. Gastroenterology 101:721–725
Ellmerich S, Djouder N, Schöller M et al (2000a) Production of cytokines by monocytes, epithelial and endothelial cells activated by Streptococcus bovis. Cytokine 12:26–31
Ellmerich S, Schöller M, Duranton B et al (2000b) Promotion of intestinal carcinogenesis by Streptococcus bovis. Carcinogenesis 21:753–756
Federico A, Morgillo F, Tuccillo C et al (2007) Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer 121:2381–2386
Gao L, Weck MN, Michel A et al (2009) Association between chronic atrophic gastritis and serum antibodies to 15 Helicobacter pylori proteins measured by multiplex serology. Cancer Res 69:2973–2980
Ghali MB, Scott PT, Al Jassim RA (2004) Characterization of Streptococcus bovis from the rumen of the dromedary camel and Rusa deer. Lett Appl Microbiol 39:341–346
Giannitsioti E, Chirouze C, Bouvet A et al (2007) AEPEI Study Group. Characteristics and regional variations of group D streptococcal endocarditis in France. Clin Microbiol Infect 13:770–776
Gold JS, Bayar S, Salem RR (2004) Association of Streptococcus bovis bacteremia with colonic neoplasia and extracolonic malignancy. Arch Surg 139:760–765
Gonzlez-Quintela A, Martínez-Rey C, Castroagudín JF et al (2001) Prevalence of liver disease in patients with Streptococcus bovis bacteraemia. J Infect 42:116–119
Grivennikov SI, Greten FR, Karin M (2007) Immunity, inflammation, and cancer. Cell 140:883–899
Gupta A, Madani R, Mukhtar H (2010) Streptococcus bovis endocarditis, a silent sign for colonic tumour. Colorectal Dis 12:164–171
Harris RE (2007) Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem 42:93–126
Herrera LA, Benítez-Bribiesca L, Mohar A et al (2005) Role of infectious diseases in human carcinogenesis. Environ Mol Mutagen 45:284–303
Hope ME, Hold GL, Kain R et al (2005) Sporadic colorectal cancer–role of the commensal microbiota. FEMS Microbiol Lett 244:1–7
Horner MJ, Ries LAG, Krapcho M et al (eds) (2009) SEER Cancer Statistics Review, 1975–2006. National Cancer Institute, Bethesda. http://seer.cancer.gov/csr/1975_2006/. Based on November 2008 SEER data submission, posted to the SEER web site, 2009
Hughes R, Cross AJ, Pollock JR et al (2001) Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis 22:199–202
Hussain SP, Harris CC (2007) Inflammation and cancer: An ancient link with novel potentials. Int J Cancer 121:2373–2380
IARC (1994) IARC monographs on the evaluation of carcinogenic risks to humans: vol 61. Schistosomes, liver flukes and Helicobacter pylori. IARC, Lyon
Ishitsuka T, Kashiwagi H, Konishi F (2001) Microsatellite instability in inflamed and neoplastic epithelium in ulcerative colitis. J Clin Pathol 54:526–532
Jass JR (2007) Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 50:113–130
Jean SS, Teng LJ, Hsueh PR et al (2004) Bacteremic Streptococcus bovis infections at a university hospital, 1992–2001. J Formos Med Assoc 103:118–123
Kahveci A, Ari E, Arikan H et al (2010) Streptococcus bovis bacteremia related to colon adenoma in a chronic hemodialysis patient. Hemodial Int 14:91–93
Kang HY, Kim N, Park YS et al (2006) Progression of atrophic gastritis and intestinal metaplasia drives Helicobacter pylori out of the gastric mucosa. Dig Dis Sci 51:2310–2315
Kim YH, Kim MH, Kim BJ et al (2009) Inhibition of cell proliferation and invasion in a human colon cancer cell line by 5-aminosalicylic acid. Dig Liver Dis 41:328–337
Kim SY, Joo SI, Yi J et al (2010) A case of Streptococcus gallolyticus subsp. gallolyticus infective endocarditis with colon cancer: identification by 16 S ribosomal DNA sequencing. Korean J Lab Med 30:160–165
Klein RS, Recco RA, Catalano MT et al (1977) Association of Streptococcus bovis with carcinoma of the colon. N Engl J Med 297:800–802
Klein RS, Catalano MT, Edberg SC et al (1979) Streptococcus bovis septicemia and carcinoma of the colon. Ann Intern Med 91:560–562
Knudtson LM, Hartman PA (1993) Comparison of fluorescent gentamicin-thallous-carbonate and KF streptococcal agars to enumerate enterococci and fecal streptococci in meats. Appl Environ Microbiol 59:936–938
Kohonen-Corish MR, Daniel JJ, te Riele H et al (2002) Susceptibility of Msh2-deficient mice to inflammation-associated colorectal tumors. Cancer Res 62:2092–2097
Kumarakulasingham M, Rooney PH, Dundas SR et al (2005) Cytochrome p450 profile of colorectal cancer: identification of markers of prognosis. Clin Cancer Res 11:3758–3765
Kuper H, Adami H-O, Trichopoulos D (2000) Infections as a major preventable cause of human cancer. J Intern Med 248:171–183
Lampen A, Ebert B, Stumkat L et al (2004) Induction of gene expression of xenobiotic metabolism enzymes and ABC-transport proteins by PAH and a reconstituted PAH mixture in human Caco-2 cells. Biochim Biophys Acta 1681:38–46
Lee RA, Woo PC, To AP et al (2003) Geographical difference of disease association in Streptococcus bovis bacteraemia. J Med Microbiol 52:903–908
Li JH, Shi XZ, Lv S et al (2005) Effect of Helicobacter pylori infection on p53 expression of gastric mucosa and adenocarcinoma with microsatellite instability. World J Gastroenterol 11:4363–4366
Logan RF, Grainge MJ, Shepherd VC et al (2008) ukCAP Trial Group. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology 134:29–38
McKay JA, Murray GI, Weaver RJ et al (1993) Xenobiotic metabolising enzyme expression in colonic neoplasia. Gut 34:1234–1239
McKnight GM, Duncan CW, Leifert C et al (1999) Dietary nitrate in man: friend or foe? Br J Nutr 81:349–358
McMahon AJ, Auld CD, Dale BA et al (1991) Streptococcus bovis septicaemia associated with uncomplicated colonic carcinoma. Br J Surg 78:883–885
Miyake K, Shimada M, Nishioka M et al (2009) Downregulation of matrix metalloprotease-9 and urokinase plasminogen activator by TX-1877 results in decreased tumor growth and metastasis on xenograft model of rectal cancer. Cancer Chemother Pharmacol 64:885–892
Moellering RC Jr, Watson BK, Kunz LJ (1974) Endocarditis due to group D streptococci. Comparison of disease caused by Streptococcus bovis with that produced by the enterococci. Am J Med 57:239
Mungall BA, Kyaw-Tanner M, Pollitt CC (2001) In vitro evidence for a bacterial pathogenesis of equine laminitis. Vet Microbiol 79(3):209–223
Murray HW, Roberts RB (1978) Streptococcus bovis bacteremia and underlying gastrointestinal disease. Arch Intern Med 138:1097–1099
Mutoh M, Takahashi M, Wakabayashi K (2006) Roles of prostanoids in colon carcinogenesis and their potential targeting for cancer chemoprevention. Curr Pharm Des 12:2375–2382
Newman JV, Kosaka T, Sheppard BJ et al (2001) Bacterial infection promotes colon tumorigenesis in Apc(Min/+) mice. J Infect Dis 184:227–230
Nielsen SD, Christensen JJ, Laerkeborg A et al (2007) Molecular-biological methods of diagnosing colon-related Streptococcus bovis endocarditis. Ugeskr Laeger 169:610–611
Paduch R, Kandefer-Szerszeń M, Szuster-Ciesielska A et al (2010) Transforming growth factor-beta1 modulates metalloproteinase-2 and −9, nitric oxide, RhoA and alpha-smooth muscle actin expression in colon adenocarcinoma cells. Cell Biol Int 34(2):213–223
Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118:3030–3044
Persing DH, Prendergast FG (1999) Infection, immunity, and cancer. Arch Pathol Lab Med 123:1015–1022
Pigrau C, Lorente A, Pahissa A et al (1988) Streptococcus bovis bacteremia and digestive system neoplasms. Scand J Infect Dis 20:459–460
Potter MA, Cunliffe NA, Smith M et al (1998) A prospective controlled study of the association of Streptococcus bovis with colorectal carcinoma. J Clin Pathol 51:473–474
Randazzo CL, Vaughan EE, Caggia C (2006) Artisanal and experimental Pecorino Siciliano cheese: microbial dynamics during manufacture assessed by culturing and PCR-DGGE analyses. Int J Food Microbiol 109:1–8
Reynolds JG, Silva E, McCormack WM (1983) Association of Streptococcus bovis bacteremia with bowel disease. J Clin Microbiol 17:696–697
Ribeiro ML, Godoy AP, Benvengo YH et al (2004) The influence of endoscopic procedures upon the contamination of Helicobacter pylori cultures. Arq Gastroenterol 41:100–103
Roberts RB (1992) Streptococcal endocarditis: the viridans and beta hemolytic streptococci. In: Kaye D (ed) Infective endocarditis. Raven Press, Ltd, New York, p 191
Rostom A, Dubé C, Lewin G et al (2007) U.S. Preventive Services Task Force. Nonsteroidal anti-inflammatorydrugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med 146:376–389
Rowland IR, Granli T, Bøckman OC et al (1991) Endogenous N-nitrosation in man assessed by measurement of apparent total N-nitroso compounds in faeces. Carcinogenesis 12:1395–1401
Ruoff KL, Miller SI, Garner CV et al (1989) Bacteremia with Streptococcus bovis and Streptococcus salivarius: clinical correlates of more accurate identification of isolates. J Clin Microbiol 27:305–308
Rusniok C, Couvé E, Da Cunha V et al (2010) Genome sequence of Streptococcus gallolyticus: insights into its adaptation to the bovine rumen and its ability to cause endocarditis. J Bacteriol 192:2266–2276
Schlegel L, Grimont F, Collins MD et al (2000) Streptococcus infantarius sp. nov., Streptococcus infantarius subsp. infantarius subsp. nov. and Streptococcus infantarius subsp. coli subsp. nov., isolated from humans and food. Int J Syst Evol Microbiol 50:1425–1434
Schlegel L, Grimont F, Ageron E et al (2004) Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Indian J Med Res 119:252–256
Silvester KR, Bingham SA, Pollock JR et al (1997) Effect of meat and resistant starch on fecal excretion of apparent N-nitroso compounds and ammonia from the human large bowel. Nutr Cancer 29:13–23
Sinicrope FA (2007) Sporadic colorectal cancer: an infectious disease? Gastroenterology 132:797–801
Sinnamon MJ, Carter KJ, Fingleton B et al (2008) Matrix metalloproteinase-9 contributes to intestinal tumourigenesis in the adenomatous polyposis coli multiple intestinal neoplasia mouse. Int J Exp Pathol 89:466–475
Skinn AC, Vergnolle N, Zamuner SR et al (2006) Citrobacter rodentium infection causes iNOS-independent intestinal epithelial dysfunction in mice. Can J Physiol Pharmacol 84:1301–1312
Steinbach G, Lynch PM, Phillips RK et al (2000) The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 342:1946–1952
Stone WL, Papas AM (1997) Tocopherols and the etiology of colon cancer. J Natl Cancer Inst 89:1006–1014
Teitelbaum JE, Triantafyllopoulou M (2006) Inflammatory bowel disease and Streptococcus bovis. Dig Dis Sci 51:1439–1442
Terzić J, Grivennikov S, Karin E et al (2010) Inflammation and colon cancer. Gastroenterology 138:2101–2114
Thian TS, Hartman PA (1981) Gentamicin-thallous-carbonate medium for isolation of fecal streptococci from foods. Appl Environ Microbiol 41:724–728
Tjalsma H (2010) Identification of biomarkers for colorectal cancer through proteomics-based approaches. Exp Rev Proteomic 7:879–895
Tjalsma H, Schöller-Guinard M, Lasonder E et al (2006) Profiling the humoral immune response in colon cancer patients: diagnostic antigens from Streptococcus bovis. Int J Cancer 119:2127–2135
Tjalsma H, Lasonder E, Schöller-Guinard M et al (2007) Shotgun immunoproteomics to identify disease-related bacterial antigens: application to human colon cancer. Proteomic Clin Appl 1:429–434
Tjalsma H, Schaeps RMJ, Swinkels DW (2008) Immunoproteomics: from biomarker discovery to diagnostic applications. Proteomic Clin Appl 2:167–180
Vanrobaeys M, De Herdt P, Charlier G et al (1999) Ultrastructure of surface components of Streptococcus gallolytics (S. bovis) strains of differing virulence isolated from pigeons. Microbiology 145:335–342
Vaska VL, Faoagali JL (2009) Streptococcus bovis bacteraemia: identification within organism complex and association with endocarditis and colonic malignancy. Pathology 41:183–186
Vermeer IT, Henderson LY, Moonen EJ et al (2004) Neutrophil-mediated formation of carcinogenic N-nitroso compounds in an in vitro model for intestinal inflammation. Toxicol Lett 154:175–182
Wang X, Huycke MM (2007) Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology 132:551–561
Wentling GK, Metzger PP, Dozois EJ et al (2006) Unusual bacterial infections and colorectal carcinoma–Streptococcus bovis and Clostridium septicum: report of three cases. Dis Colon Rectum 49:1223–1227
Wiese FW, Thompson PA, Kadlubar FF (2001) Carcinogen substrate specificity of human COX-1 and COX-2. Carcinogenesis 22:5–10
Zarkin BA, Lillemoe KD, Cameron JL et al (1990) The triad of Streptococcus bovis bacteremia, colonic pathology, and liver disease. Ann Surg 211:786–791
Acknowledgements
We thank all researchers for their valuable contributions to unravel the role of SB in CRC and we apologize for the fact that we could not mention all SB-related studies in this chapter. We especially thank Philippe Glaser, Albert Bolhuis, Julian Marchesi, Bas Dutilh, Dorine Swinkels and Rian Roelofs for their inspiring discussions on this subject. AB was supported by the Dutch Cancer Society (KWF; project KUN 2006–3591) and IK was supported in part by the US National Institutes of Health (Research Grant R01-CA93817).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Tjalsma, H., Boleij, A., Kato, I. (2012). Streptococcus bovis and Colorectal Cancer. In: Khan, A. (eds) Bacteria and Cancer. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-2585-0_3
Download citation
DOI: https://doi.org/10.1007/978-94-007-2585-0_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-2584-3
Online ISBN: 978-94-007-2585-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)