Abstract
Purpose
It is well known that hypoxic milieu is the primary cancer environment. Therefore, tumor hypoxia is considered to be a potential therapeutic target. In the present study, we investigated the antitumor and antimetastatic effect of hypoxic cell radiosensitizer, TX-1877 on xenograft model of rectal cancer.
Methods
Nude mice bearing subcutaneously or orthotopically implanted human colon cancer cell lines HCT-116 and HT-29 were treated with TX-1877, irradiation or TX-1877 with irradiation. Tumor volume, survival, expression of matrix metalloproteinase (MMP)-2, MMP-7, MMP-9 and urokinase-type plasminogen activator (uPA) and incidence of lymph node metastasis were evaluated in treatment versus control group.
Results
In subcutaneous model, tumor treated with TX-1877 and irradiation showed significant reductions in volume (P < 0.05 vs. control, TX-1877 or irradiation group). Quantitative real-time reverse transcription-PCR and immunohistochemical analysis revealed that TX-1877 significantly inhibited expression of the MMP-9 and uPA. These treatments also inhibited the para-aortic lymph node metastasis, however, did not prolong the survival in orthotopic model.
Conclusions
These data show that the treatment of TX-1877 with irradiation decreased growth of human rectal cancer and, furthermore, suppressed lymph node metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rectal cancer is a common malignancy leading to high morbidity and considerable mortality. Five-year survival rate for rectal cancer patients have been ~65% in general. Despite the use of multimodality strategy, local recurrence alone or in combination with distant metastases causes severe and fatal outcome in patients who underwent excision of primary rectal cancer [1, 2]. In recent years, considerable progress has been made in the treatment of locally advanced rectal cancer, mainly due to improvements in the type and quality of surgery, better staging methods and regular use of chemoradiation (CRT) or radiation therapies. Although the use of preoperative CRT for resectable rectal cancer remains a controversial issue, preoperative CRT is clearly preferred when tumor shrinkage is required before surgery, i.e., in locally advanced T4 disease and low-lying tumors when sphincter preservation is attempted [3, 4]. To ensure maximum biological damage from the radiation therapy, oxygen need to be present in cancer cells and tissues, however, hypoxia, as a common feature of the tumor microenvironment, causes resistance to radiation in cancer cells at the time of radiation therapy.
Hypoxic cell radiosensitizers, as electron-affinic compounds, have oxygen-mimic effects to tumor hypoxic cells on fixing the radiation-induced damage in DNA or other molecules [5–7]. Tested in clinical trials, its development was discontinued because of its dose-limiting side effects, such as neurotoxicity [8]. Etanidazole [N1-(2-hydroxyethyl)-2-(2-nitro-1 H-imidazolyl)acetamide] was found to be 3–4 times less toxic than misonidasole, but to have radiosensitizing activity comparable to misonidasole. However, a phase III randomized trial of radiotherapy of head and neck cancer with or without etanidazole has failed [9], whereas, nimorazole has proven to be effective in carcinomas of the supraglottic larynx and pharynx [10].
Tirapazamine (TPZ, 1,2,4-benzotriazine 1,4-di-N-oxide, SR4233), that are specifically toxic to hypoxic cells, is the main compound in the class of bioreductive anticancer agents and is currently in phase II and III clinical trials for the treatment of some cancers [11]. We reported that hypoxic cytotoxins, such as the benzotriazine oxide derivative TX-1102 and TPZ and quinoxaline oxide TX-402, induced tumor cells to p53-independent apoptosis under hypoxic conditions selectively and inhibited angiogenesis [12].
Several attempts have been made to modify hypoxic milieu for the last several decades to overcome the hypoxic resistance and a number of hypoxia-targeting drugs have been developed and, in particular, hypoxic cell radiosensitizer were tested clinically to improve overall effects on radioresistant hypoxic tumor cells [9, 13–17]. However, the clinical usefulness of the sensitizers and bioreductive agents remain controversial.
In the present study, we investigated the antitumor effects of TX-1877 against rectal cancer xenograft model. Our results confirmed that TX-1877 promoted the antitumor activity and, furthermore, inhibited the lymph node metastasis.
Materials and methods
Materials
The hypoxic cell radiosensitizer, TX-1877 (Fig. 1), was synthesized as described previously [18]. Antibodies purchased were as follows: goat polyclonal anti-human matrix metalloproteinase 2 (MMP-2) (Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal anti-human MMP-7 (Fuji Chemical, Toyama, Japan); rabbit polyclonal anti-human MMP-9 (Oncogene Research Products, Cambridge, MA); mouse monoclonal anti-urokinase plasminogen activator (uPA) (Greenwich, CT). Dako LSAB kit (DAKO, Carpinteria, CA) was used according to the manufacturer’s instructions.
Cell line
The colon cancer cell lines HCT-116 and HT-29 were obtained from the American Type Culture Collection (Manassas, VA). All cell lines were cultured in RPMI 1640 supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Irradiation
X-ray irradiation was carried out by using an X-ray unit (Hitachi X-ray unit, model MBR-1505R3 with 0.5 mm Al/1.0 mm Cu filter, 150 kV, 1.08 Gy/min). In vivo assay, mice whole-body were irradiated with whole-body irradiation of 2 Gy every 4 days. Mice were given 0.2 ml of physiologic saline or 0.4 mg/g of TX-1877 intraperitoneally (i.p.) 30 min before the irradiation. This dose is less than 10% of the detected LD50 of TX-1877 (4.6 mg/g).
Hypoxic condition
The hypoxic cultures were placed within a modular incubator chamber (Billups-Rothenberg, Inc., Del Mar, CA, USA) that was flushed with a gas mixture of 5% CO2, 94% N2, and 1% O2. The whole modular incubator chamber was then placed in a CO2 incubator and the culture was continued for 72 h.
Cell proliferation assay
The effect of TX-1877 and irradiation on cell proliferation was determined by WST-8(2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) assay as described previously [19]. Cell proliferation was measured using a Cell Counting Kit-8. Experiments were carried out in triplicate in duplicate plates.
Animal model
HCT-116 cells were harvested from subconfluent cultures by a brief exposure to 0.25% trypsin and 0.02% EDTA. Female Balb/c nude mice (CLEA Japan, Tokyo, Japan) were inoculated with 5 × 106 HCT-116 cells in 200 μl PBS subcutaneously into the left flank. When the tumor grew approximately 100 mm3 in volume, the mice were divided into four groups, and the mice were given 0.2 ml of physiologic saline or 0.4 mg/g of TX-1877 i.p. and monitored every 4 days. Tumor volume was estimated by measuring tumor size and using the following formula: tumor volume = 0.5 × L × W 2, where L and W represent the largest diameter and the smallest diameter, respectively. At day 27, the animals were sacrificed, and tumors were removed and fixed with formalin for hematoxylin and eosin (H&E) staining and for immunohistochemical staining; total RNA were isolated from the removed tumors for real-time RT-PCR analysis.
To evaluate whether TX-1877 treatment have an effect on lymph node metastasis and survival, rectal orthotopic xenograft models were made as described previously [20]. On the seventh day after inoculation of 2 × 106 HT-29 cell, the mice were given 0.2 ml of physiologic saline or 0.4 mg/kg of TX-1877 i.p. every 4 days. The mice were killed when moribund. Survival and incidence of lymph node metastasis around the abdominal aorta from the lower margin of the renal vein to the aortic bifurcation were recorded. All experimental protocols were approved by the Animal Investigation Committee of Tokushima University, Tokushima, Japan.
Quantitative real-time reverse transcription-PCR
Tissue samples were homogenized with a Multi-Beads Shocker (Yasui-kikai, Osaka, Japan). RNA was extracted using RNeasy Mini kit (Qiagen, Valencia, CA). RNA was reverse transcribed with oligo-dT primers at 42°C for 50 min using the SuperScript First Strand System (Invitrogen, Carlsbad, CA).
Quantitative real-time RT-PCR was done on the ABI Prism 7500 using the commercially available gene expression assay for MMP-2, MMP-7, MMP-9 and uPA (Hs00234422, Hs00159163, Hs00234579 and Hs00170182, respectively). A 25 μL final reaction volume containing 1× TaqMan Universal PCR Master Mix (Applied Biosystems), 1× Multiscribe with RNase inhibitors, and 1× gene expression assay was used to amplify 25 ng of total RNA with the following cycling conditions: 30 min at 48°C, 10 min at 95°C, then 50 cycles of 95°C for 15 s and 60°C for 1 min. The 7500 Sequence Detection System 1.3.1 software automatically determined fold change for each gene in each sample using the ΔΔCT method [21]. Calculations were also done for each gene in tumors relative to their corresponding matched normal tissue.
Immunohistochemical analysis of xenograft
Immunohistochemical staining was performed by the avidin–biotin–peroxidase complex (ABC) method. Briefly, sections were deparaffinized, incubated in a 0.3% H2O2 solution for 30 min, immersed sequentially in phosphate-buffered saline (PBS) and 10% normal goat serum for 60 min at room temperature, then incubated with each antibody described above for 60 min at room temperature. After washing, they were overlaid with biotinylated goat antirabbit or antimouse antibody (Vector Laboratories, Burlingame, CA) for 30 min at room temperature, washed in PBS, then labeled with streptavidin–peroxidase complex (Vector Laboratories). The peroxidase reaction was developed with 3,3′-diaminobenzidine as chromogen. The sections were counterstained with hematoxylin, dehydrated with ethanol, treated with xylene and enclosed in synthetic resin.
Statistical analysis
Statistical comparisons of mean values were done by one-way ANOVA. Survival analysis was computed by the Kaplan–Meier method and compared by the log-rank test. Statistical analysis was performed using Stat View 5.0 J software (SAS Institute, Inc., Cary, NC, USA). A P value of less than 0.05 was considered to be statistically significant.
Results
Radiosensitive effect of TX-1877 on colon cancer cell
To examine whether TX-1877 inhibits the proliferation and whether enhances the radiosensitivity of HCT-116, we first performed cell proliferation assay. HCT-116 was incubated with 0, 0.01, 0.1, 1, or 10 mM of TX-1877 for 72 h under normoxic or hypoxic condition. As shown in Fig. 2, TX-1877 of 0.1, 1, or 10 mM enhanced the radiosensitivity of HCT-116 under hypoxic condition compared with normoxic condition.
Inhibition of cell proliferation by TX-1877 with irradiation for HCT-116 cell. At 72 h after the treatment with indicated dose of TX-1877 with irradiation under normoxic or hypoxic condition (1% O2), viable cells were counted by the cell proliferation assay. Experiments were performed in triplicate; data are the mean ± SE. * P < 0.05 in comparison with normoxic condition
Antitumor effect of TX-1877 in xenograft
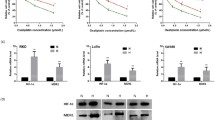
Next, we examined the effects of 0.4 mg/g TX-1877 or irradiation, on the growth of subcutaneously (s.c.) implanted rectal tumor. HCT-116 cell were implanted into the left flank of nude mice, and animals were vehicle-treated as control group, treated with TX-1877, irradiation, or TX-1877 and irradiation. As shown in Fig. 3a, the mean tumor volume was 761 ± 304 mm3 in control group, 558 ± 286 mm3 in TX-1877 group, 226 ± 111 mm3 in irradiation group, 123 ± 46 mm3 in TX-1877 with irradiation group. The tumor volume in TX-1877 with irradiation group was significantly lower compared with control group, TX-1877 group, and irradiation group at day 27 (P < 0.05 vs. other groups). The tumor volume in irradiation groups were also statistically significant lower compared with control group and TX-1877 group (P < 0.05). There was not statistically significant difference of tumor volume in TX-1877 group compared with control group. As shown in Fig. 3b, there were no differences in body weight between the four respective treatment groups caused by the medication.
TX-1877 and irradiation therapy delay tumor growth in HCT-116 xenograft. a Five injections of TX-1877 (0.4 mg/g per injection, i.p.) in HCT-116 tumor with or without five fractions of 2 Gy irradiation (on days 7, 11, 15, 19, 23). After the tumor volume reached 100 mm3, the volume was plotted against the time after the initiation of the treatment. Points means of 7 mice; bars ±SD. *P < 0.05 versus control group; **P < 0.05 versus TX-1877 group; ***P < 0.05 versus irradiation group. b During the 27 days, there was no difference in body weights between mice treated with vehicle, TX-1877, irradiatioin, or TX-1877 with irradiation
Expression of MMP-2, MMP-7, MMP-9 and uPA
To elucidate whether TX-1877 inhibit the mRNA expression of MMP-2, MMP-7, MMP-9 and uPA, we then performed quantitative real-time RT-PCR analysis of HCT-116 xenograft tumor. As shown in Fig. 4a, quantitative real-time RT-PCR analysis revealed a significant decrease of mRNA expression of MMP-9 and uPA in the TX-1877 group or TX-1877 with irradiation group, compared with the control group or irradiation group (P < 0.05), while there were no significant differences in mRNA expression of MMP-2 and MMP-7.
Expression of MMP-2, MMP-7, MMP-9 and uPA in HCT-116 xenograft tumor. a Effects of TX-1877 detected by quantitative real-time RT-PCR analysis. Tumors were harvested from control mice and mice treated with 0.4 mg/g TX-1877, irradiation, or 0.4 mg/g TX-1877 with irradiation. b Immunohistochemical analysis of MMP-2, MMP-7, MMP-9 and uPA protein in HCT-116 xenograft tumor. The sections were immunostained for expression of MMP-2, MMP-7, MMP-9 and uPA. Representative samples are shown (×200 magnification). Columns mean percentage of mRNA expression, bars ±SE, *P < 0.05 versus control or irradiation group
Protein expression was investigated by immunohistochemical staining of HCT-116 xenograft tumor. As shown in Fig. 4b, consistent with quantitative real-time RT-PCR analysis, immunohistochemical staining revealed a significant decrease in protein expression of MMP-9 and uPA in the TX-1877 group or TX-1877 with irradiation group, compared with the control group or irradiation group, while there were no significant differences in protein expression of MMP-2 and MMP-7.
Effect of TX-1877 on lymph node metastasis and survival in orthotopic xenograft
TX-1877 has not only direct toxicity and radiosensitizing activity against cancer cell, but also inhibits the metastasis. To evaluate whether TX-1877 or irradiation treatment have an effect on lymph node metastasis and survival, HT-29 cell were injected into the posterior rectal wall submucosally of nude mice. Seven days later, the mice were randomized into four groups of ten mice each. When moribund, mice were killed. Incidence of lymph node metastasis around the abdominal aorta from the lower margin of the renal vein to the aortic bifurcation and survival were recorded. As shown in Fig. 5, visible lymph node metastasis were present in 90% (9 of 10) of the control group, 30% (3 of 10) of the TX-1877 group, 70% (7 of 10) of the irradiation group and 20% (2 of 10) of the TX-1877 with irradiation group.
Antimetastatic effect by TX-1877 and irradiation treatment in orthotopic HT-29 xenograft. Incidence of lymph node metastasis around the abdominal aorta from the lower margin of the renal vein to the aortic bifurcation and survival were recorded when sacrificed. TX-1877 decreases the incidence of lymph node metastasis (n = 10). Visible lymph node metastasis were present in 90% of the control group, 30% of 0.4 mg/g TX-1877 group, 70% of the irradiation group, and 20% of 0.4 mg/g TX-1877 with irradiation group
As shown in Fig. 6, median survival time for the control group was 48 days. After treatment with TX-1877, irradiation and TX-1877 with irradiation, the median survival time was 58, 44, and 46 days, respectively (P = NS).
Antitumor effect by TX-1877 and irradiation treatment in orthotopic HT-29 xenograft. HT-29 cell were slowly injected in the posterior rectal wall submucosally with a 29-gauge needle. Seven days later, the mice were randomized into four treatment groups (n = 10). Mice were killed when moribund. Survival was analyzed by the Kaplan–Meier method and compared by the log-rank test. Filled square control group, filled triangle 0.4 mg/g TX-1877 group, filled inverted triangle irradiation group, filled circle 0.4 mg/g TX-1877 with irradiation group
Discussion
Regions of acute/chronic hypoxia are present in the majority of solid human tumors [22–24]. The level of hypoxia in tumor has a profound influence on the outcome of cancer chemotherapy and radiation therapy and is a strong prognostic factor for disease progression and survival. Furthermore, hypoxic condition in solid tumors accelerates malignant progression and increases metastasis [25–29]. Therefore, hypoxic cells are important targets for cancer therapy.
Colorectal cancer display significant regions of hypoxia that are often resistant to cell killing by radiation and certain chemotherapeutics [30–33]. In this present study, we showed for the first time that TX–1877 undergoes dose-dependent and hypoxic tumor selective activation in vivo, which results in significant inhibition of tumor growth and progression in xenograft model of rectal cancer. In the orthotopic model, treatment with TX-1877 with irradiation inhibited the para-aortic lymph node metastasis of tumor-bearing mice in a manner comparable with other groups.
Many studies have shown that enhanced production of members of the plasminogen activator pathway and MMP family contributes to tumor invasion, angiogenesis, and metastasis [34]. Components of the plasminogen system include the plasminogen activators (PAs), uPA, and tissue-type plasminogen activator (tPA). Inhibition of uPA and/or of the uPA/uPAR interaction prevents or reduces metastasis in [35]. uPA–uPAR binding initiates interaction between a number of cell surface proteins, e.g., vitronectin, integrin receptors, CK2, nucleolin, and caveolin, at focal adhesion sites under certain physiological conditions [36]. In addition to the cell surface activation of many proteins and the degradation of extracellular matrix (ECM) proteins, the uPA–uPAR interaction leads to the intracellular phosphorylation of kinases, activation of the Jak–Stat pathway and subsequently, kinases of the mitogen-activated protein kinase signaling pathways [37]. Furthermore, evidence demonstrates that uPA binding to uPAR-mediated signaling events results in the expression of cathepsin B and Mr 92,000 gelatinase (MMP-9) in monocytic cells [38]. Our data showed that TX-1877 suppressed the expression of MMP-9 and uPA and reduced the lymph node metastasis, however, failed to prolong the survival. Though the mechanisms remain unclear, the decrease of lymph node metastasis may be attributed to the inhibition of metastatic potential by TX-1877 partially.
In conclusion, multifunctional hypoxic radiosensitizer, TX-1877 showed not only hypoxic radiosensitizing effect but also the inhibitory effect of lymph node metastasis on xenograft model of rectal cancer. Considering the pleiotropic activities of TX-1877 in cancer growth and progression, we propose that TX-1877 has significant potential for an effective radiotherapy of hypovasculer rectal cancer that can overcome radioresistance. Further clinical studies are necessary to confirm our findings in patients with rectal cancer.
References
Bakx R, van Tinteren H, van Lanschot JJ et al (2004) Surgical treatment of locally recurrent rectal cancer. Eur J Surg Oncol 30:857–863
Law WL, Chu KW (2000) Resection of local recurrence of rectal cancer: results. World J Surg 24:486–490 (discussion 490)
Bosset JF, Collette L, Calais G et al (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123
Gerard JP, Conroy T, Bonnetain F et al (2006) Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol 24:4620–4625
Coleman CN, Bump EA, Kramer RA (1988) Chemical modifiers of cancer treatment. J Clin Oncol 6:709–733
Suzuki K, Nagasawa H, Uto Y et al (2005) Napthalimidobenzamide DB-51630: a novel DNA binding agent inducing p300 gene expression and exerting a potent anti-cancer activity. Bioorg Med Chem 13:4014–4021
Wardman P (2007) Chemical radiosensitizers for use in radiotherapy. Clin Oncol (R Coll Radiol) 19:397–417
Lee DJ, Pajak TF, Stetz J et al (1989) A phase I/II study of the hypoxic cell sensitizer misonidazole as an adjunct to high fractional dose radiotherapy in patients with unresectable squamous cell carcinoma of the head and neck: a RTOG randomized study (#79-04). Int J Radiat Oncol Biol Phys 16:465–470
Lee DJ, Cosmatos D, Marcial VA et al (1995) Results of an RTOG phase III trial (RTOG 85–27) comparing radiotherapy plus etanidazole with radiotherapy alone for locally advanced head and neck carcinomas. Int J Radiat Oncol Biol Phys 32:567–576
Overgaard J, Hansen HS, Overgaard M et al (1998) A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish head and neck cancer study (DAHANCA) Protocol 5–85. Radiother Oncol 46:135–146
Brown JM, Wang LH (1998) Tirapazamine: laboratory data relevant to clinical activity. Anticancer Drug Des 13:529–539
Nagasawa H, Yamashita M, Mikamo N et al (2002) Design, synthesis and biological activities of antiangiogenic hypoxic cytotoxin, triazine-N-oxide derivatives. Comp Biochem Physiol A Mol Integr Physiol 132:33–40
Cottrill CP, Bishop K, Walton MI et al (1998) Pilot study of nimorazole as a hypoxic-cell sensitizer with the “chart” regimen in head and neck cancer. Int J Radiat Oncol Biol Phys 42:807–810
Huilgol NG, Chatterjee N, Mehta AR (1996) An overview of the initial experience with AK-2123 as a hypoxic cell sensitizer with radiation in the treatment of advanced head and neck cancers. Int J Radiat Oncol Biol Phys 34:1121–1124
Overgaard J, Eriksen JG, Nordsmark M et al (2005) Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol 6:757–764
Shibamoto Y, Sugie C, Ito M et al (2004) The Japanese experiences with hypoxia-targeting pharmacoradiotherapy: from hypoxic cell sensitisers to radiation-activated prodrugs. Expert Opin Pharmacother 5:2459–2467
Urtasun RC, Palmer M, Kinney B et al (1998) Intervention with the hypoxic tumor cell sensitizer etanidazole in the combined modality treatment of limited stage small-cell lung cancer: a one-institution study. Int J Radiat Oncol Biol Phys 40:337–342
Kasai S, Nagasawa H, Yamashita M et al (2001) New antimetastatic hypoxic cell radiosensitizers: design, synthesis, and biological activities of 2-nitroimidazole-acetamide, TX-1877, and its analogues. Bioorg Med Chem 9:453–464
Miyake K, Shimada M, Nishioka M et al (2008) The novel hypoxic cell radiosensitizer, TX-1877 has antitumor activity through suppression of angiogenesis and inhibits liver metastasis on xenograft model of pancreatic cancer. Cancer Lett 272:325–335
Tsutsumi S, Kuwano H, Morinaga N et al (2001) Animal model of para-aortic lymph node metastasis. Cancer Lett 169:77–85
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Hockel M, Schlenger K, Knoop C et al (1991) Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res 51:6098–6102
Vaupel P, Harrison L (2004) Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist 9(Suppl 5):4–9
Vaupel P, Schlenger K, Knoop C et al (1991) Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res 51:3316–3322
Brizel DM, Dodge RK, Clough RW et al (1999) Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol 53:113–117
Brizel DM, Sibley GS, Prosnitz LR et al (1997) Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 38:285–289
Cairns R, Papandreou I, Denko N (2006) Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol Cancer Res 4:61–70
Hockel M, Schlenger K, Aral B et al (1996) Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 56:4509–4515
Vaupel P, Mayer A, Hockel M (2004) Tumor hypoxia and malignant progression. Methods Enzymol 381:335–354
Koong AC, Mehta VK, Le QT et al (2000) Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys 48:919–922
Megibow AJ (1992) Pancreatic adenocarcinoma: designing the examination to evaluate the clinical questions. Radiology 183:297–303
Ranniger K, Saldino RM (1966) Arteriographic diagnosis of pancreatic lesions. Radiology 86:470–474
Yassa NA, Yang J, Stein S et al (1997) Gray-scale and color flow sonography of pancreatic ductal adenocarcinoma. J Clin Ultrasound 25:473–480
Mackay AR, Corbitt RH, Hartzler JL et al (1990) Basement membrane type IV collagen degradation: evidence for the involvement of a proteolytic cascade independent of metalloproteinases. Cancer Res 50:5997–6001
Subramanian R, Gondi CS, Lakka SS et al (2006) siRNA-mediated simultaneous downregulation of uPA and its receptor inhibits angiogenesis and invasiveness triggering apoptosis in breast cancer cells. Int J Oncol 28:831–839
Chapman HA, Wei Y, Simon DI et al (1999) Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb Haemost 82:291–297
Irigoyen JP, Munoz-Canoves P, Montero L et al (1999) The plasminogen activator system: biology and regulation. Cell Mol Life Sci 56:104–132
Rao NK, Shi GP, Chapman HA (1995) Urokinase receptor is a multifunctional protein: influence of receptor occupancy on macrophage gene expression. J Clin Invest 96:465–474
Acknowledgments
Grant support: Grants in aid 18591466 (M. Nishioka), 19591590 (K. Miyake) from the Ministry of Education, Culture, Sports, Science and Technology, Japan and Cancer Research Project Cooperated by TAIHO Pharmaceutical Co., Ltd and The University of Tokushima.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyake, K., Shimada, M., Nishioka, M. et al. Downregulation of matrix metalloprotease-9 and urokinase plasminogen activator by TX-1877 results in decreased tumor growth and metastasis on xenograft model of rectal cancer. Cancer Chemother Pharmacol 64, 885–892 (2009). https://doi.org/10.1007/s00280-009-0937-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-0937-5