Abstract
Pathogenic members of the genus Corynebacterium cause a wide range of serious infections in humans including diphtheria. Adhesion to host cells is a crucial step during infection. In Corynebacterium diphtheriae, adhesion is mediated primarily by filamentous structures called pili or fimbriae that are covalently attached to the bacterial cell wall. C. diphtheriae produces three distinct pilus structures, SpaA-, SpaD- and SpaH-type pili. Similar to other types, the prototype SpaA pilus consists of SpaA forming the pilus shaft and two minor pilins SpaB and SpaC located at the base and at the tip, respectively. The minor pilins SpaB/SpaC are critical for bacterial binding to human pharyngeal cells, and thus represent the major adhesins of corynebacteria. Like pili of many other gram-positive microbes, the assembly of corynebacterial pili occurs by a two-step mechanism, whereby pilins are covalently polymerized by a transpeptidase enzyme named pilin-specific sortase and the generated pilus polymer is subsequently anchored to the cell wall peptidoglycan via the base pilin by the housekeeping sortase or a non-polymerizing sortase. This chapter reviews the current knowledge of corynebacterial adhesion, with a specific focus on pilus structures, their assembly, and the mechanism of adhesion mediated by pili.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Diphtheria Toxin
- Cell Wall Peptidoglycan
- Pilus Length
- Corynebacterium Diphtheriae
- Corynebacterium Species
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 Introduction
Pathogenic corynebacteria cause a number of debilitating and life threatening diseases in humans (Coyle and Lipsky, 1990) (for a partial list, see Table 6.1). These organisms are from the genus Corynebacterium with more than 110 validated species, all of which are rod shaped, aerobic, non-motile, and non-spore forming gram-positive bacteria with high levels of mycolic acid in the cell wall. While most Corynebacterium species are innocuous (such as Corynebacterium glutamicum, which is used in the food industry), quite a few are medically relevant (Funke et al., 1997). The most well known among these is Corynebacterium diphtheriae, which secretes an extremely potent toxin and thereby causes cutaneous and pharyngeal diphtheria. Diphtheria is also linked to Corynebacterium ulcerans and Corynebacterium pseudotuberculosis, which share a zoonotic mode of transmission (Bonmarin et al., 2009). The other significant human diseases caused by pathogenic corynebacteria include endocarditis, septicemia, urinary tract infections, wound infections and infections on abiotic surfaces such as catheters and shunts (Table 6.1). Specific corynebacterial species are also associated with infections in immunocompromised patients; these include Corynebacterium jeikeium, Corynebacterium striatum, and Corynebacterium amycolatum (Adderson et al., 2008; Otsuka et al., 2006; Schiffl et al., 2004).
C. diphtheriae is one of the most extensively investigated bacterial pathogens and provided key evidence for Koch’s postulates on the germ theory. Likewise, the diphtheria toxin (DT) produced by toxigenic corynebacteria is an archetypal exotoxin. It not only served as a major paradigm for toxin research, but also helped to advance molecular immunology and the development of vaccines (Collier, 2001; Holmes, 2000; Popovic et al., 2000). Importantly, the genomic sequences of more and more corynebacterial species are becoming available, starting with the publication of the C. diphtheriae NCTC13129 genome in 2003 (Cerdeno-Tarraga et al., 2003), thus providing a facile new path of molecular investigations of these pathogens. In fact, a major outcome from genome sequencing was the discovery of the pilus gene clusters in C. diphtheriae (Ton-That and Schneewind, 2003) that now serve as a major experimental model for the assembly and function of the gram-positive pili (Mandlik et al., 2008b). In this chapter, we first outline the clinical and molecular aspects of C. diphtheriae and diphtheria, followed by an account of the relatively little that is known to date about the molecular mechanisms of corynebacterial adherence with an emphasis on pili.
6.2 Corynebacterium Diphtheriae and Clinical Disease
C. diphtheriae was designated as the type species of the genus Corynebacterium (Barksdale, 1970). A club-shaped bacillus, its cell wall contains arabinose, galactose, manose, and a muramyl peptide made of L-Ala-D-Glu-meso α, ɛ-diaminopimelic acid (DAP)-D-Ala. The peptide subunits of the murein are crosslinked via D-Ala-meso-DAP bridges. Mycolic and mycolenic acids are also part of corynebacterial cell walls. Based on the morphology of corynebacterial colonies and the severity of infections, three distinct types of C. diphtheriae have been identified: mitis (smooth colonies causing mild infections), gravis (semirough colonies causing severe disease) and intermedius (dwarf smooth colonies associated with infections of intermediate severity). The sequenced strain NCTC13129 belongs to the gravis biotype, and the genome of this strain contains an integrated prophage called corynephage beta (36.5 kb), which encodes the diphtheria toxin. C. diphtheriae is known to confer toxigenicity by lysogenic conversion (Barksdale, 1970).
C. diphtheriae is transmitted by direct contact with infected persons or bodily fluids. The pathogen causes two forms of diphtheria: pharyngeal diphtheria (infection of the upper respiratory tract), which is common, and cutaneous diphtheria (infection of skin), which is much less frequent. Diphtheria is characterized by the formation of a pseudomembrane at the site of infection due to bacterial colonization and growth, toxin production, tissue damage and host immune response (Love and Murphy, 2006). Respiratory diphtheria presents as a sore throat with a low-grade fever. Left untreated, the developing pseudomembrane can cause complete airway obstruction, leading to suffocation and death (Hadfield et al., 2000).
Diphtheria toxin (DT) is a prototype A-B toxin composed of two subunits. The A subunit is the active toxin, whose uptake and release in the host cell cytoplasm is aided by the B subunit. The B subunit binds to the heparin-binding EGF-like growth factor (HB-EGF) on human epithelial cells (Naglich et al., 1992), and facilitates toxin uptake by endocytosis. As the pH of the endocytic vesicle drops, the A subunit is released in the cytoplasm. There, the toxin obliterates protein synthesis by ADP-ribosylating translation elongation factor 2 (EF-2), ultimately causing host cell death (Mitamura et al., 1995; Naglich et al., 1992). The systemic effects of DT are most commonly manifested in the heart and nerves, leading to myocarditis, acute cardiac failure, and severe neuropathy (Hadfield et al., 2000).
6.3 History of Diphtheria
The earliest clinical description of diphtheria is found in writings of the ancient Greek physician Hippocrates during the fourth century c.e. (Shulman, 2004). During the modern era, the first recorded outbreak of diphtheria occurred in 1659 in Roxbury, MA. Episodic outbreaks occurred in 1686 in Virginia and in 1689 in Connecticut (Shulman, 2004). Decades later, between 1735 and 1740, a severe outbreak in New England killed nearly 40% of children under the age of 10. Records reveal that this epidemic took an estimated 5000 lives (2.5% of the total population at that time), sometimes wiping out entire families (English, 1985).
Bretonneau, who described the disease in great detail in 1826 (Semple, 1859), is credited with naming the bacterium diphtherite from a “Greek root [meaning] skin or hide, because the pharyngeal membrane in diphtheria often looked like a piece of leather” (English, 1985). Bretonneau also made the astonishing association of the organism releasing a “blood poison,” similar to that found in jequirity seeds (Pappenheimer, 1984). This diphtheria-associated toxin was eventually isolated by Emile Roux and Alexander Yersin (Roux and Yersin, 1888), soon after Friedrich Loeffler demonstrated the etiological agent of diphtheria by growing it in pure culture (Kwantes, 1984; Loeffler, 1884). Interestingly, the diphtheria toxin works by a mechanism similar to the toxin found in jequirity seeds, abrin. Both toxins are AB-type toxins that block protein synthesis in human cells.
During this remarkable era in the history of modern medicine, Emil Adolf von Behring used antitoxin from a horse to successfully treat a human case of diphtheria. von Behring received the first ever Nobel prize in Medicine in 1901 “for his work on serum therapy and especially for its use against diphtheria, with which he opened up a new path in the field of medical science and gave the physician a powerful weapon with which to combat disease and death” (Winau and Winau, 2002), In 1913, von Behring developed the vaccine against DT, with widespread vaccinations beginning in 1920.
6.4 Epidemiology of Diphtheria
Before the development of the antitoxin and vaccination, there were more than 40 deaths per 100,000 people in the United States every year (Linder and Grove, 1947). By 1920, when vaccination began, that number had already been reduced to around 15 deaths per 100,000 with the use of the antitoxin alone. By 1950, the combination of vaccination and antitoxin therapy had reduced the number of deaths to less than 1 per 100,000 (Grove and Hetzel, 1968). By 1980, there were less than 1 in 50 million cases, and since 2004, there have been no reported cases of diphtheria in the US (Wagner et al., 2009).
However, diphtheria outbreaks still occur in other regions of the world. The largest recent outbreak was in the independent states of the former Soviet Union, with more than 140,000 cases and a large number of deaths between 1991 and 1997 (Mattos-Guaraldi et al., 2003; Vitek and Wharton, 1998). More recently, the World Health Organization (WHO) reported an outbreak in a refugee camp in Afghanistan in 2003 where 6% of the infected population died.
Globally, there were over 7,000 cases of diphtheria reported to the WHO in 2008 (Papaioannou et al., 2009). Importantly, some of these have occurred in regions where greater than 95% of the population has been vaccinated. Cases are being reported worldwide where the infecting strains were non-toxigenic, i.e. those lacking the corynephage that encodes the diphtheria toxin. The fact that the vaccine is against the toxin and that the vaccinated population is at risk for infection by non-toxigenic strains of C. diphtheriae has led to a renewed interest in the mechanisms of pathogenesis of C. diphtheriae, as well as a systematic investigation of the virulence factors that mediate adherence, colonization and invasion (Mandlik et al., 2008b; Mattos-Guaraldi et al., 2000). A better understanding of the pathogenic mechanisms and the underlying virulence factors of corynebacterial infection would certainly provide the new therapeutic strategies required to combat not only diphtheria but also many other important diseases caused by Corynebacterium species (Table 6.1).
6.5 Adherence Factors of Corynebacteria
Compared to our knowledge of some bacterial pathogens, relatively little is known about corynebacterial adherence. Early studies of adhesion used various strains of Corynebacterium to test for autoagglutination, haemagglutination, neuraminidase activity, and trans-sialidase activity (reviewed by Mattos-Guaraldi et al., 2000); however, the mechanisms or proteins responsible for these phenotypes were never established. Similarly, many studies have examined the ability of various clinical isolates to adhere to epithelial cells (Colombo et al., 2001; Deacock et al., 1983; Hirata, et al., 2004; Honda and Yanagawa, 1975; Marty et al., 1991; Moreira et al., 2003; Silva De Souza et al., 2003), but the surface molecule involved remained unknown. One specific surface component of C. diphtheriae that may be involved in adhesion is a lipoglycan called CdiLAM. A recent study found that the C. diphtheriae cell surface contains CdiLAM, and that preincubation of bacteria with anti-CdiLAM significantly inhibited adherence to HEp-2 cells (Moreira et al., 2008). Another adhesive component identified in C. diphtheriae is a cell surface protein, DIP1281. Mutants lacking DIP1281 show over 3-fold reduction in adherence to human pharyngeal cells compared to their isogenic parental strains (Bensing and Sullam, 2002). Importantly, the DIP1281 mutant was also defective in invasion.
6.5.1 Cell-Wall-Anchored Surface Proteins
Surface proteins are key virulence factors of most bacterial pathogens (Isberg, 1991). Gram-positive pathogens use a variety of surface proteins to bind to host tissues, evade the innate and acquired immune systems, and invade host epithelial and immune cells (Navarre and Schneewind, 1999). Many, but not all, surface proteins in gram-positive bacteria are covalently anchored to the cell wall peptidoglycan by a novel mechanism that is catalyzed by a sortase enzyme (Cossart and Jonquieres, 2000). The cell-wall-linked surface proteins contain a cell wall sorting signal (CWSS) with a conserved LPXTG motif at the carboxyl terminus. Sortases are transpeptidase enzymes that cleave the surface protein LPXTG motif between the threonine and glycine residues and tether the threonine-carboxyl group to the amino group of cell wall cross bridge within the lipid II peptidoglycan precursor (Mazmanian et al., 2001; Ton-That et al., 1999). First discovered in Staphylococcus aureus and named SrtA for surface protein sorting A (Mazmanian et al., 1999), sortases are ubiquitous in gram-positive bacteria. By comparative genomic analysis (Comfort and Clubb, 2004; Dramsi et al., 2005), sortase homologs have been classified into four groups based on primary sequence and putative function: class A (with S. aureus SrtA as the prototype), class B (S. aureus SrtB for iron acquisition), class C (pilin-specific sortases; see below) and class D (includes the housekeeping sortase SrtF of C. diphtheriae).
A homolog of the class A sortase is found in all corynebacterial genomes sequenced to date. This sortase catalyzes cell wall anchoring of surface proteins that contain the cell wall sorting signal with the LPXTG motif (see Ton-That et al., 2004a for the detailed mechanism of cell wall sorting). In C. diphtheriae strain NCTC13129, there are 17 genes encoding proteins with the LPXTG motif (Boekhorst et al., 2005) nine clustered at three genetic loci that are now known to encode the various pilus proteins (see below). The other LPXTG-containing proteins, scattered throughout the chromosome, are likely anchored to the cell wall as monomers and involved in host interactions based on their sequence features. This remains to be investigated.
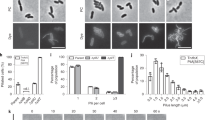
During colonization, tissue-specific adhesion may involve bacterial cell surface carbohydrates that bind specific host cell surface lectins, and conversely, bacterial surface proteins that bind specific host cell surface carbohydrates or proteins. However, prior to colonization, the initial bacterial attachment is likely mediated by proteinaceous fibers (fimbriae or pili) that extend from the cell surface. Although these structures were first observed on the surface of Corynebacterium renale in 1968 by Yanagawa et al (Fig. 6.1a), the nature and mechanism of pilus assembly in corynebacteria, and in gram-positive bacteria in general, was not elucidated until 2003 (Ton-That and Schneewind, 2003). As described below, these pili are covalent polymers of a distinct set of pilin proteins that are joined by pilus-specific sortases and ultimately attached to the cell wall by a non-polymerizing sortase.
Electron microscopy of corynebacterial pili. Panel A shows the first published image of corynebacterial pilus structures, reported by Yanagawa and colleagues in Corynebacterium renale (Yanagawa et al., 1968) (reprint with permission). Panel B shows Corynebacterium diphtheriae pili by immuno-electron microscopy. C. diphtheriae cells were incubated with a specific antiserum against SpaA, followed by 18 nm gold particles conjugated with IgG and 1% uranyl acetate (Courtesy of Chungyu Chang)
6.5.2 Sortase-Mediated Pilus Assembly in Corynebacterium Diphtheriae
As mentioned, C. diphtheriae harbours three pilus gene clusters that encode a total of nine pilus proteins, named SpaA through SpaI (Spa for sortase-mediated pilus assembly), each containing the LPXTG motif. The three pilus loci also encode a total of five class C sortases, named SrtA through SrtE (Ton-That and Schneewind, 2004). The sixth sortase of C. diphtheriae (SrtF) is a class D sortase homolog (Dramsi et al., 2005), located at a different region of the chromosome, and is now referred to as the housekeeping sortase. The evidence that each pilus gene cluster encodes a distinct pilus structure was obtained by generating antibodies against individual pilins and using the antibodies in immunogold labeling and electron microscopy experiments (Fig. 6.1b), which revealed three distinct types of pili on the surface of C. diphtheriae. Consequently, these pili were named the SpaA-, SpaD-, and SpaH-type pili after the major pilin subunit from each pilus type (Gaspar and Ton-That, 2006; Swierczynski and Ton-That, 2006; Ton-That and Schneewind, 2003). Electron microscopy and biochemical experiments established that each pilus is composed of three proteins, a major pilin subunit, which forms the shaft of the pilus, and two minor pilin subunits, which are located at the pilus tip and base. In the case of the SpaA-type pilus, SpaA forms the shaft, SpaC is the tip protein, and SpaB is found along the shaft and at the base (Mandlik et al., 2008a; Ton-That and Schneewind, 2003). Corynebacterial pili, as well as other gram-positive pili, remain intact upon treatment with formic acid or boiling in SDS, indicative of covalent linkage of pilus polymers (Mandlik et al., 2008b; Scott and Zahner, 2006; Telford et al., 2006).
The mechanism of sortase-mediated polymerization of pilus polymers was elucidated largely from an investigation of the SpaA-type pilus as the prototype. The SpaA pilus is produced from the gene cluster spaA-srtA-spaB-spaC (Fig. 6.2). In addition to the cell wall sorting signal, SpaA (and the other major pilin subunits, SpaD and SpaH) contains a pilin motif with the consensus sequence of WxxxVxVYPKN (Ton-That et al., 2004b). It was proposed that the pilin motif lends the conserved lysine residue to join two pilin subunits through a threonine-lysine isopeptide bond. Indeed, mutation of either the lysine or the threonine residue obliterates pilus assembly. According to the current model, the pilin-specific sortase, SrtA, cleaves the SpaA LPXTG motif between threonine and glycine and forms an acyl-enzyme intermediate with the substrate (Fig. 6.2). An acyl-enzyme SrtA-SpaC intermediate is also formed this way. Incorporation of SpaC at the tip occurs when the lysine residue of the SpaA pilin motif resolves the SrtA-SpaC intermediate by nucleophilic attack, leading to the formation of a SpaC-SpaA linkage (Fig. 6.2). Pilus extension or polymerization begins when additional SrtA-SpaA intermediates feed additional SpaA monomers to the growing SpaC-SpaA pilus via a lysine-mediated transpeptidation reaction. The existence of SrtA-Spa intermediates with varying polymer lengths has now been documented (Guttilla et al., 2009), and overproduction of the major pilin subunit has been shown to increase pilus length (Swierczynski and Ton-That, 2006). Pilus length is also increased when the minor pilin SpaB is absent; however, the resulting polymers are largely secreted into the culture medium (Mandlik et al., 2008a). Conversely, pilus length is shortened when SpaB is overproduced. These results led to the realisation of the biphasic mechanism of pilus assembly: pilus polymerization ends when SpaB is incorporated to the pilus base by a transpeptidation reaction that utilizes a lysine residue of SpaB (Mandlik et al., 2008a) and transfers the pilus from SrtA to SrtF (Fig. 6.2). SrtF then catalyzes the joining of the SpaB-terminated pilus polymer to the cell wall peptidoglycan via the lipid II molecule, similar to that of monomeric cell surface proteins (Swaminathan et al., 2007). There is evidence that SpaC and SpaB pilins are also anchored to the cell wall as monomers, especially if SpaA is absent (Mandlik et al., 2007) (Fig. 6.2).
Biphasic model of pilus assembly in Corynebacterium diphtheriae. This figure depicts the biphasic mechanism of pilus assembly in gram-positive bacteria, derived from studies of the archetype SpaA-type pili, encoded by the gene locus spaA-srtA-spaB-spaC (top). The model outlines the basic steps of pilus polymerization followed by the cell wall anchoring step. As shown, Spa pilin precursors are synthesized in the cytoplasm, translocated across the cytoplasmic membrane by the secretion system (Sec), and inserted into the membrane by the cell wall sorting signal. Membrane-bound sortase enzymes cleave the LPXTG motif of the CWSS and form acyl-enzyme intermediates. Pilus polymerization occurs via lysine-mediated transpeptidation reactions catalyzed by the pilin-specific sortase, SrtA (black). Polymerization ends by the incorporation of the base pilin SpaB via lysine-mediated transpeptidation. The housekeeping sortase, SrtF (teal), catalyzes anchoring of the resulting polymer to the cell wall peptidoglycan. Curved arrows indicate direction of nucleophilic attack. Modified from (Mandlik et al., 2008a) with permission
6.5.3 Corynebacterial Pili as Adhesins
The first observation that corynebacterial pili may mediate host cell interactions was proposed in early studies with C. renale pili, which were shown to agglutinate trypsinized sheep red blood cells (Honda and Yanagawa, 1974). Over 30 years later, Mandlik et al. identified the minor pilins SpaB and SpaC of C. diphtheriae as specific adhesins that mediate efficient corynebacterial adherence to host pharyngeal cells (Mandlik et al., 2007). Wild type C. diphtheriae cells were shown to bind to human lung epithelial, laryngeal, and pharyngeal cells. However, mutants that lacked SrtA (and thus did not polymerize SpaA-type pili) showed over a 90% reduction in the ability to adhere to human pharyngeal cells. Surprisingly, mutants that lacked only the major pilin subunit, SpaA, showed a modest 10% reduction in adherence to these cells. This is in contrast to mutants that lacked either of the minor pilin subunits, SpaB or SpaC, which showed a 70–75% reduction in adherence. Additionally, latex beads coated with only SpaB or SpaC were sufficient to adhere to host pharyngeal cells, while beads coated with SpaA showed no binding. As mentioned above, SpaB and SpaC anchor to the cell wall as monomers independent of pilus structures. It is speculated that the long pili mediate initial attachment, whereas monomeric pilins on the bacterial surface may help to create an intimate zone of adhesion that allows for efficient delivery of toxin and other virulence factors, and may even play a significant role in host cell signaling.
Importantly, the host cell receptor(s) targeted by SpaB and SpaC have yet to be identified as well as the specific functions of the SpaD- and SpaH-type pili. Such studies are likely to be rewarding because some important corynebacterial pathogens do not contain the SpaA-type pilus but harbor only the SpaD-type or the SpaH-type pilus (Fig. 6.3).
Pilus gene clusters in pathogenic Corynebacterium species. The genome of C. diphtheriae NCTC13129 contains three pilus gene clusters, which encode for the SpaA-type pili (spaA-srtA-spaB-spaC), the SpaD-type pili (srtB-spaD-srtC-spaE-spaF) and the SpaH-type pili (spaG-spaH-srtD-srtE-spaI). The housekeeping sortase, srtF, is located elsewhere on the chromosome. Similar pilus gene clusters and the housekeeping sortase gene are found in many pathogenic species of the genus Corynebacterium. Shown in dots, hatches, and grey are genes encoding pilus shaft, tip protein and pilus base, respectively, based on C. diphtheriae pilus systems. Sortase genes are shown in black and dark grey, and other unknown genes are shown in light grey. All arrows are drawn to scale
6.6 Concluding Remarks
Pili serve as major adhesins in bacterial pathogens. Corynebacteria will continue to serve as a major paradigm to understand the mechanism of gram-positive pilus assembly and function in pathogenesis. One or more pilus gene clusters with specific types of pilins and pilus-specific sortases are found in the genomes of many different Corynebacterium species sequenced to date. It will be important to determine whether these gene clusters encode pilus structures, like the ones described in C. diphtheriae, and whether they are instrumental to bacterial interactions with specific host tissues. The well-developed genetics and biochemistry of the C. diphtheriae pilus system should provide the key methodology and experimental approaches to seed studies of the various pili in the different Corynebacterium species.
References
Adderson EE, Boudreaux JW, Hayden RT (2008) Infections caused by coryneform bacteria in pediatric oncology patients. Pediatr Infect Dis J 27:136–141
Barksdale L (1970) Corynebacterium diphtheriae and its relatives. Bacteriol Rev 34:378–422
Bensing BA, Sullam PM (2002) An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol Microbiol 44:1081–1094
Boekhorst J, de Been MW, Kleerebezem M, Siezen RJ (2005) Genome-wide detection and analysis of cell wall-bound proteins with LPxTG-like sorting motifs. J Bacteriol 187:4928–4934
Bonmarin I, Guiso N, Le Fleche-Mateos A, Patey O, Patrick AD, Levy-Bruhl D (2009) Diphtheria: a zoonotic disease in France? Vaccine 27:4196–4200
Cerdeno-Tarraga AM, Efstratiou A, Dover LG, Holden MT, Pallen M, Bentley SD, Besra GS, Churcher C, James KD, De Zoysa A, Chillingworth T, Cronin A, Dowd L, Feltwell T, Hamlin N, Holroyd S, Jagels K, Moule S, Quail MA, Rabbinowitsch E, Rutherford KM, Thomson NR, Unwin L, Whitehead S, Barrell BG, Parkhill J (2003) The complete genome sequence and analysis of Corynebacterium diphtheriae NCTC13129. Nucleic Acids Res 31:6516–6523
Collier RJ (2001) Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon 39:1793–1803
Colombo AV, Hirata Júnior R, Rocha de Souza CM, Monteiro-Leal LH, Previato JO, Formiga LC, Andrade AF, Mattos-Guaraldi AL (2001) Corynebacterium diphtheriae surface proteins as adhesins to human erythrocytes. FEMS Microbiol Lett 197:235–239
Comfort D, Clubb RT (2004) A comparative genome analysis identifies distinct sorting pathways in gram-positive bacteria. Infect Immun 72:2710–2722
Cossart P, Jonquieres R (2000) Sortase, a universal target for therapeutic agents against gram-positive bacteria? Proc Natl Acad Sci USA 97:5013–5015
Coyle MB, Lipsky BA (1990) Coryneform bacteria in infectious diseases: clinical and laboratory aspects. Clin Microbiol Rev 3:227–246
Deacock SJ, Steward KA, Carne HR (1983) The role of adherence in determining the site of infection by Corynebacterium diphtheriae. J Hyg (Lond) 90:415–424
Dramsi S, Trieu-Cuot P, Bierne H (2005) Sorting sortases: a nomenclature proposal for the various sortases of Gram-positive bacteria. Res Microbiol 156:289–297
English PC (1985) Diphtheria and theories of infectious disease: centennial appreciation of the critical role of diphtheria in the history of medicine. Pediatrics 76:1–9
Funke G, von Graevenitz A, Clarridge JE 3rd, Bernard KA (1997) Clinical microbiology of coryneform bacteria. Clin Microbiol Rev 10:125–159
Gaspar AH, Ton-That H (2006) Assembly of distinct pilus structures on the surface of Corynebacterium diphtheriae. J Bacteriol 188:1526–1533
Grove RD, Hetzel AM (1968) Vital statistics rates in the United States 1940–1960. In: E. U.S. Department of Health E, and Welfare (ed) National Center for Health Statistics, Washington, DC, Amo Press, New York, NY, pp 587–596
Guttilla IK, Gaspar AH, Swierczynski A, Swaminathan A, Dwivedi P, Das A, Ton-That H (2009) Acyl enzyme intermediates in sortase-catalyzed pilus morphogenesis in gram-positive bacteria. J Bacteriol 191:5603–5612
Hadfield TL, McEvoy P, Polotsky Y, Tzinserling VA, Yakovlev AA (2000) The pathology of diphtheria. J Infect Dis 181(Suppl 1):S116–S120
Hirata Júnior R, Souza SMS, Rocha desouza CM, Andrade AF, Monteiro-Leal LH, Formiga LC, Mattos-Guaraldi AL (2004) Patterns of adherence to HEp-2 cells and actin polymerisation by toxigenic Corynebacterium diphtheriae strains. Microb Pathog 36:125–130
Holmes RK (2000) Biology and molecular epidemiology of diphtheria toxin and the tox gene. J Infect Dis 181(Suppl 1):S156–S167
Honda E, Yanagawa R (1974) Agglutination of trypsinized sheep erythrocytes by the pili of Corynebacterium renale. Infect Immun 10:1426–1432
Honda E, Yanagawa R (1975) Attachment of Corynebacterium renale to tissue culture cells by the pili. Am J Vet Res 36:1663–1666
Isberg RR (1991) Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science 252:934–938
Kwantes W (1984) Diphtheria in Europe. J Hyg (Lond) 93:433–437
Linder FE, Grove RD (1947) Vital statistics rates in the United States 1900–1940. In: E. U.S. Department of Health E, and Welfare (ed). National Center for Health Statistics, Washington, DC, Amo Press, New York, NY, pp 559–578
Loeffler F (1884) Untersuchungen über die Bedeutung der Mikroorganismen für die Enstehung der Diphtherie beim Menschen, bei der Taube und beim Kalbe. Mitt Klin Gesundheitsamte Berlin 2:421–499
Love JF, Murphy JR (2006) Corynebacterium diphtheriae: iron-mediated activation of DtxR and regulation of diphtheria toxin expression. In: Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI (eds) Gram-positive pathogens. ASM Press, Washington, DC, pp 726–737
Mandlik A, Das A, Ton-That H (2008a) The molecular switch that activates the cell wall anchoring step of pilus assembly in gram-positive bacteria. Proc Natl Acad Sci USA 105:14147–14152
Mandlik A, Swierczynski A, Das A, Ton-That H (2007) Corynebacterium diphtheriae employs specific minor pilins to target human pharyngeal epithelial cells. Mol Microbiol 64:111–124
Mandlik A, Swierczynski A, Das A, Ton-That H (2008b) Pili in gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends Microbiol 16:33–40
Marty N, Agueda L, Lapchine L, Clave D, Henry-Ferry S, Chabanon G (1991) Adherence and hemagglutination of Corynebacterium group D2. Eur J Clin Microbiol Infect Dis 10:20–24
Mattos-Guaraldi AL, Duarte Formiga LC, Pereira GA (2000) Cell surface components and adhesion in Corynebacterium diphtheriae. Microbes Infect 2:1507–1512
Mattos-Guaraldi AL, Moreira LO, Damasco PV, Hirata Júnior R (2003) Diphtheria remains a threat to health in the developing world–an overview. Mem Inst Oswaldo Cruz 98:987–993
Mazmanian SK, Liu G, Ton-That H, Schneewind O (1999) Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285:760–763
Mazmanian SK, Ton-That H, Schneewind O (2001) Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol Microbiol 40:1049–1057
Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E (1995) Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem 270:1015–1019
Moreira LO, Andrade AF, Vale MD, Souza SMS, Hirata Júnior R, Asad LM, Asad NR, Monteiro-Leal LH, Previato JO, Mattos-Guaraldi AL (2003) Effects of iron limitation on adherence and cell surface carbohydrates of Corynebacterium diphtheriae strains. Appl Environ Microbiol 69:5907–5913
Moreira LO, Mattos-Guaraldi AL, Andrade AF (2008) Novel lipoarabinomannan-like lipoglycan (CdiLAM) contributes to the adherence of Corynebacterium diphtheriae to epithelial cells. Arch Microbiol 190:521–530
Naglich JG, Metherall JE, Russell DW, Eidels L (1992) Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell 69:1051–1061
Navarre WW, Schneewind O (1999) Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev 63:174–229
Otsuka Y, Ohkusu K, Kawamura Y, Baba S, Ezaki T, Kimura S (2006) Emergence of multidrug-resistant Corynebacterium striatum as a nosocomial pathogen in long-term hospitalized patients with underlying diseases. Diagn Microbiol Infect Dis 54:109–114
Papaioannou W, Gizani S, Haffajee AD, Quirynen M, Mamai-Homata E, Papagiannoulis L (2009) The microbiota on different oral surfaces in healthy children. Oral Microbiol Immunol 24:183–189
Pappenheimer AM Jr (1984) The diphtheria bacillus and its toxin: a model system. J Hyg (Lond) 93:397–404
Popovic T, Mazurova IK, Efstratiou A, Vuopio-Varkila J, Reeves MW, De Zoysa A, Glushkevich T, Grimont P (2000) Molecular epidemiology of diphtheria. J Infect Dis 181(Suppl 1):S168–S177
Roux E, Yersin A (1888) Contribution à l’étude de la diphtérie. Annales de l’Institut Pasteur 2:629–661
Schiffl H, Mucke C, Lang SM (2004) Exit-site infections by non-diphtheria corynebacteria in CAPD. Perit Dial Int 24:454–459
Scott JR, Zahner D (2006) Pili with strong attachments: gram-positive bacteria do it differently. Mol Microbiol 62:320–330
Semple RH (1859) Memoirs on Diphtheria: from the writings of Bretonneau, P., Guersant, Trousseau, Bouchut, Empis, and Daviot. The New Sydenham Society, London
Shulman ST (2004) The history of pediatric infectious diseases. Pediatr Res 55:163–176
Silva De Souza SM, Hirata Júnior R, Moreira LO, Gomes ML, Braga De Andrade AF, Bernardo-Filho M, Mattos-Guaraldi AL (2003) Influence of stannous chloride on the adhesive properties of Corynebacterium diphtheriae strains. Int J Mol Med 12:657–661
Swaminathan A, Mandlik A, Swierczynski A, Gaspar A, Das A, Ton-That H (2007) Housekeeping sortase facilitates the cell wall anchoring of pilus polymers in Corynebacterium diphtheriae. Mol Microbiol 66:961–974
Swierczynski A, Ton-That H (2006) Type III pilus of corynebacteria: Pilus length is determined by the level of its major pilin subunit. J Bacteriol 188:6318–6325
Telford JL, Barocchi MA, Margarit I, Rappuoli R, Grandi G (2006) Pili in Gram-positive pathogens. Nat Rev Microbiol 4:509–519
Ton-That H, Liu G, Mazmanian SK, Faull KF, Schneewind O (1999) Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc Natl Acad Sci USA 96:12424–12429
Ton-That H, Marraffini LA, Schneewind O (2004a) Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim Biophys Acta 1694:269–278
Ton-That H, Marraffini LA, Schneewind O (2004b) Sortases and pilin elements involved in pilus assembly of Corynebacterium diphtheriae. Mol Microbiol 53:251–261
Ton-That H, Schneewind O (2003) Assembly of pili on the surface of Corynebacterium diphtheriae. Mol Microbiol 50:1429–1438
Ton-That H, Schneewind O (2004) Assembly of pili in Gram-positive bacteria. Trends Microbiol 12:228–234
Vitek CR, Wharton M (1998) Diphtheria in the former Soviet Union: reemergence of a pandemic disease. Emerg Infect Dis 4:539–550
Wagner KS, Stickings P, White JM, Neal S, Crowcroft NS, Sesardic D, Efstratiou A (2009) A review of the international issues surrounding the availability and demand for diphtheria antitoxin for therapeutic use. Vaccine 28:14–20
Winau F, Winau R (2002) Emil von Behring and serum therapy. Microbes Infect 4:185–188
Yanagawa R, Otsuki K, Tokui, T (1968) Electron microscopy of fine structure of Corynebacterium renale with special reference to pili. Jpn J Vet Res 16:31–37
Acknowledgments
We thank Anjali Mandlik, Anu Swaminathan, Andrew Gasper and Arlene Swierczynski, Arunima Mishra, Chenggang Wu and Chungyu Chang for their invaluable contributions to the studies of pili; supported by grants AI061381 and DE017382 from the NIH to HTT.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Rogers, E.A., Das, A., Ton-That, H. (2011). Adhesion by Pathogenic Corynebacteria. In: Linke, D., Goldman, A. (eds) Bacterial Adhesion. Advances in Experimental Medicine and Biology, vol 715. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0940-9_6
Download citation
DOI: https://doi.org/10.1007/978-94-007-0940-9_6
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0939-3
Online ISBN: 978-94-007-0940-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)