Abstract
Plant-parasitic nematodes are pests of agricultural crops and cause considerable economic loss and, especially in developing countries, adverse social impact. They exhibit a variety of parasitic modes, with the endoparasitic cyst and root-knot nematodes attracting the most research interest. As a group, plant-parasitic nematodes display a variety of adaptations to the parasitic lifestyle and some species show remarkable abilities to survive and disperse in the absence of a host. The urgent need to find environmentally acceptable methods of control has provided the impetus for molecular studies to increase understanding of plant-nematode interactions. The burgeoning interest in comparative genomics will be a vital component in understanding the parasitic life style and identifying novel targets for control options. This introductory chapter provides background information about the life-cycle biology of plant-parasitic nematodes, their different feeding strategies and aspects of the host-parasitic interactions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cyst nematodes
- Development
- Evolution
- Hatching
- Host location
- Invasion
- Plant parasitism
- Root-knot nematodes
- Survival
1 Introduction to Nematodes
Nematodes are astonishing organisms. Despite their deceptively simple morphology and the fact that they are essentially aquatic, requiring at least a film of liquid for active life, they have been successful in colonising an enormous range of environments. Irrespective of their habitat, nematodes have a similar external morphology, with a worm shaped, bilaterally symmetrical, unsegmented body. The phylum Nematoda comprises >25,000 described species and the importance of nematodes should never be underestimated. Species parasitic on plants and animals have a massive deleterious social and economic impact on man. As will be discussed below, one major attribute that contributes to the undoubted success of nematodes is the amazing ability of some of the life cycle stages to survive adverse environmental conditions. Free-living nematodes of the species Caenorhabditis elegans, carried as part of the experimental payload on the Columbia spacecraft, even survived when the spacecraft broke up on re-entry in 2003 (Szewczyk and Lamb 2005).
Free-living species, which make up the bulk of the phylum Nematoda, feed primarily on bacteria and fungi, and are found in soil, marine and freshwater habitats; species have been thawed out of Antarctic ice (Cobb 1914) and others have been found in hot water springs in New Zealand (Rahm 1937). The free-living forms in the soil are beneficial as they are involved in nutrient turnover; in addition, they may be of use as indicator species for pollution monitoring (Wilson and Khakouli-Duarte 2009). The parasitic forms have devastating effects on man, his crops and his livestock as well as infecting wild plants and animals. Nematodes infecting plants are also known as ‘eelworms’ and some species infecting animals are colloquially called ‘roundworms’.
Entomopathogenic nematodes of the genera Steinernema and Heterorhabditis have been commercialised as environmentally acceptable control agents for several insect pests (Ehlers 2001; Gaugler and Han 2002). Like many free-living nematodes, individuals of these genera feed on bacteria but they have become specialised in using insect larval stages as a basis for culturing their food supply. The infective juvenile nematodes invade the target insects and release symbiotic bacteria in the haemocoel of the host. The bacteria multiply and kill the insect by septicaemia, usually within 48h, and the nematodes feed on the proliferating bacteria, develop and reproduce. The research information on these nematodes is extensive (Gaugler 2002).
Although the majority of nematodes are microscopic in size (less than 1 mm in length and between 15 and 20 µm in diameter; Fig. 1.1), the animal-parasitic species are often considerably larger. The largest nematode is Placentonema gigantisma, discovered in the placenta of a sperm whale; the adult nematode can grow to 8 m in length. In humans, nematodes are of great medical importance and it has been estimated that a quarter of the world’s population suffer from a nematode infection of some sort. One of the most familiar diseases caused by nematodes is elephantiasis, caused by Wuchereria bancrofti. Ascaris lumbricoides is a major parasite of the intestine as is Enterobius vermicularis, which is probably familiar to many mothers as the ‘pin worm’ parasite of children. Dogs and cats are infected by several nematodes, among which are the microscopic Toxocara cati (in cats) and T. canis (in dogs). If the animals are not wormed the eggs voided in the faeces in parks and play areas, for example, can attach to fingers and, if ingested by humans, the juvenile nematode will hatch. The juvenile does not develop further, but not being in the proper host will wander around the body causing serious damage to organs. If the nematodes enter the cerebrospinal fluid and migrate to the brain, the victim can suffer brain damage and blindness. Deaths, usually of small children, have been reported. These are only a few examples of animal-parasitic nematodes; for further reading illustrating these pests and the horrific diseases they cause see Matthews (1998) and Lee (2001).
The subjects of this book, plant-parasitic nematodes, do not have such obvious and unpleasant effects. However, their economic and social impacts are no less severe, especially in developing countries where crop loss due to nematodes may be disastrous. All crop plants have one or more species of nematodes that feed on the roots as ectoparasites or invade the roots and feed internally as endoparasites. Some species are migratory endoparasitic, moving in and out of the plants and there are also species that feed on the aerial parts of plants (stems, leaves, buds and seeds). As well as the detrimental effects on the growth of the plants, causing stunting, early senescence and in severe cases total crop loss, the damage caused, especially to root crops such as carrots, can render the produce unmarketable and eliminate income. A major difficulty in controlling the plant-parasitic species is convincing farmers, growers and advisors that the crop problems are actually caused by these microscopic pests. With good reason, plant-parasitic nematodes have been called ‘The Invisible Enemy’.
Among the most economically important nematodes are those endoparasitic species that form complex feeding structures in the roots of their host plants. The most damaging are the root-knot (Meloidogyne spp.) and cyst (Heterodera and Globodera spp.) nematodes. The root-knot nematodes cause most damage worldwide (Moens et al. 2009). In general, species of Meloidogyne have broad host ranges, and are able to infect almost all species of flowering plants. The world-wide spread of root-knot and cyst nematodes and their enormous economic impact has formed the justification for much research; information on these two groups is extensive and this bias is, of necessity, reflected in the following sections. However, there are other groups that have a major agricultural impact, especially species of the genus Nacobbus (Manzanilla-López et al. 2002; Manzanilla-López 2010; Fig. 1.2) and the migratory endoparasitic root lesion nematodes of the genus Pratylenchus (Castillo and Vovlas 2007).
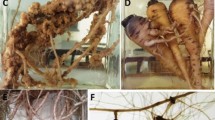
Galls of the false root-knot nematode, Nacobbus bolivianus, on potato roots. (Courtesy Rosa H Manzanilla-López, Rothamsted Research, UK; from Manzanilla-López 2010)
With the decline in use or banning of many chemicals because of adverse environmental impacts, it is imperative that new strategies for nematode control and management are developed and implemented. In this context, understanding plant-nematode interactions will be vital, and the ability to exploit genomics will not only indicate novel control targets, but also justify re-examination of some older suggestions for control based on interrupting certain phases of the life cycle.
2 Evolution of Plant Parasitism
The conserved morphology of nematodes and the absence of extensive fossil records make discussion of the evolution of parasitism in nematodes problematic (Poinar 2011). Several hypotheses about the origins of plant parasitism have been put forward by nematode taxonomists (for example, Maggenti 1971; Poinar 1983; Siddiqi 1983) with little agreement. However, more recent studies using molecular phylogenies based on the small subunit ribosomal RNA (SSU RNA) demonstrate that parasitism of plants by nematodes has arisen independently on at least three separate occasions (reviewed by Baldwin et al. 2004). The rRNA phylogenies also support convergent evolution of sedentary endoparasitism and feeding site establishment by root-knot and cyst nematodes, rather than the theory that the two groups shared a common ancestor.
The co-evolution of plants and plant-parasitic nematodes has resulted in remarkable synchrony of the host and parasite life cycles that enhances the chances of the nematode infection and, thus, survival and reproduction. This integration between host and nematode has progressed furthest in cyst nematodes and the dependency of some species on stimulation from host plants to cause hatch is one aspect of this integration (see Sect. 1.3).
Comparative genomics have been used to provide insights into the evolution of parasitism in the phylum Nematoda, especially the acquisition of novel genes associated with parasitic lifestyles (Rosso et al. 2009). Several genes have been identified in the transcriptomes of plant-parasitic nematodes that are most similar to microbial genes, and these may have been acquired by horizontal gene transfer (HGT) from microbes associated with ancestral nematodes. Jones et al. (2005) argued that acquisition of such genes via HGT has played a critical role in the evolution of plant parasitism. For example, several genes coding for enzymes such as cellulase, pectate lyase and chorismate mutase have been identified in cyst and root-knot nematodes with bacteria as the likely origin (Jones et al. 2005). Fungi are the probable origin for the gene coding for GHF45 cellulase, which is vital for the parasitic phase of the life cycle of Bursaphelenchus xylophilus (Kikuchi et al. 2004).
The use of RNAi enables loss-of-function phenotypes to be analysed and will provide information on the evolution of nematode parasitism. Rosso et al. (2009) consider that RNAi will facilitate the elucidation of the molecular determinants of parasitism. Information from this approach, together with functional and behavioural data, may provide pointers to new control targets centred on perturbing aspects of the parasitic life cycle such as hatching, host location and survival.
3 Hatching
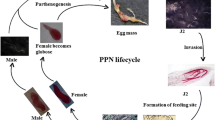
The embryo in each nematode egg develops through embryogenesis to a first-stage juvenile (J1), which, in some longidorids and trichodorids, hatches. However, in most species of plant-parasitic nematodes, the J1 moults within the egg to the second-stage juvenile (J2). It is this invasive J2 that hatches and then feeds on a host plant. There is little variation in the average size of nematode eggs, irrespective of the size of the adult, and the eggshell of plant-parasitic nematodes typically consists of three layers, an outer vitelline layer, a middle chitinous layer and an inner lipid layer. The lipid layer is the main permeability barrier of the eggshell and makes the egg resistant to chemicals, including non-fumigant nematicides. Physiological adaptations, such as different states of dormancy, are an essential component of the survival of nematodes in the absence of a host and are frequently associated with the unhatched juveniles (Perry 1989, 2002). In the majority of species of plant-parasitic nematodes, the juvenile hatches provided environmental conditions, including temperature and moisture content of the soil, are favourable. However, in some species, co-evolution of host and parasite has resulted in a sophisticated relationship whereby the nematode does not hatch unless stimulated by chemicals emanating from the host roots. These emanations have been termed root diffusates, root leachates or root exudates. Root diffusates is the preferred term of the present authors because ‘diffusate’ conveys the idea of volatile and non-volatile components diffusing through the soil and establishing a concentration gradient; thus, it is an especially apposite term in relation to hatching and attraction of nematodes.
The hatching process can be divided into three phases: changes in the eggshell permeability, metabolic activation of the juvenile, and eclosion (or hatch from the egg). The chronological order of the first two phases differs between genera. For example, in Meloidogyne spp., activation of the juvenile appears to occur first and causes eggshell changes; in others, such as Globodera spp., alteration of eggshell permeability characteristics appears a necessary pre-requisite for activation of the juvenile (Perry 2002). The agents for initiation of these responses vary between species and genera of nematodes but have been studied most extensively in species of root-knot and cyst nematodes. Hatching and survival attributes of these species are associated with the ‘packaging’ of eggs into ecological units (Perry and Moens 2011). Females of root-knot nematodes lay eggs into a gelatinous matrix, which comprises an irregular meshwork of glycoprotein material (Sharon and Spiegel 1993). The gelatinous matrix surrounds the eggs and retains them in a package termed an egg mass (Fig. 1.3). With cyst nematodes, the death of the mature females is followed by polyphenol oxidase tanning of the cuticle resulting in a hard, brown cyst. Egg masses and cysts can each contain several hundred eggs. Egg packaging units similar to cysts and egg masses are not found in animal-parasitic or free-living nematodes.
Hatching of Meloidogyne spp. is, in general, temperature dependent and hatching occurs when temperatures are favourable without the need for stimulus from root diffusates. However, there are exceptions and a proportion of the unhatched juveniles of M. hapla, M. triticoryzae and M. chitwoodi, for example, have been shown to be dependent on root diffusates for hatch, especially in later generations during a host growing season (Gaur et al. 2000; Perry and Wesemael 2008). Although a few other species from other groups (e.g. Rotylenchulus reniformis and Hypsoperine ottersoni) hatch in response to host root diffusates, this phenomenon is most common among the cyst nematodes but even in this group reliance on host stimulation for hatch varies. Globodera rostochiensis and G. pallida, have a very restricted host range and are almost completely dependent on host diffusates for hatch, whereas H. schachtii, for example, has a very wide host range (some 218 plant species, including many weeds) and hatches well in water (Perry 2002). Heterodera avenae has a large hatch in water but a relatively narrow host range; however, the hosts are very common (Turner and Rowe 2006). The dependence of G. rostochiensis and G. pallida on a plant-derived hatching stimulus is an obvious control target, with the aim of inducing hatch in the absence of a host plant and thus causing the nematodes to die of starvation. However, although much research effort has been expended in elucidating the chemicals, termed hatching factors, in root diffusates, there has been no successful control strategy using analogues of the hatching factors to induce hatch in the field.
Host root diffusates induce a cascade of inter-related changes leading to eclosion, and the sequence of events has been discussed in detail by Jones et al. (1998) and Perry (2002). Unhatched J2 of Globodera and Heterodera spp. are surrounded by perivitelline fluid, which contains trehalose. Trehalose generates an osmotic pressure that reduces the water content of the J2 and inhibits movement because the turgor pressure is insufficient to antagonise the longitudinal musculature. For hatching to occur, the pressure needs to be removed. In G. rostochiensis and some other species, this is achieved by a change in permeability of the inner lipoprotein membranes of the eggshell via HF binding or displacing internal Ca2+ (Clarke et al. 1978). In both G. rostochiensis and G. pallida, a 5 min exposure to host diffusate is sufficient to stimulate hatch (Perry and Beane 1982), suggesting the involvement of a receptor-ligand interaction between the HF and the eggshell lipoprotein membrane. The change in eggshell permeability enables trehalose to leave the egg, with a concomitant influx of water and subsequent rehydration of the J2 to a water content commensurate with movement. The eggshell of G. rostochiensis remains rigid during the hatching process and there is no evidence of enzyme involvement. Devine et al. (1996) demonstrated that the potato steroidal glycoalkaloids, α-solanine and α-chaconine, induce hatch of G. rostochiensis; glycoalkaloids are known to destabilise lipid membranes during which leakage of trehalose is possible. However, enzymes have been implicated in softening of the eggshell prior to eclosion in other species, including Xiphinema diversicaudatum, Aphelenchus avenae and M. incognita; in M. incognita lipase activity has been positively correlated with hatch (Perry et al. 1992). Rehydration of the J2 of G. rostochiensis is accompanied by increased metabolic activity due in part to removal of osmotic pressure and hydration and in part to direct stimulation of the J2 by root diffusate. Changes in gene expression of G. rostochiensis J2 appear to occur during or immediately after the hatching process (Jones et al. 1997), but more work is needed on the molecular aspects of the hatching response.
The J2 of Globodera spp. uses its stylet to cut a regular series of perforations in the subpolar region of the eggshell, and the J2 hatches through the resulting slit. J2 of D. dipsaci use a similar approach, except that the stylet thrusts are more random and the J2 uses its head to force open the slit in the eggshell. In Pratylenchus penetrans and H. avenae, a single stylet thrust penetrates the eggshell and the head extends this into a tear.
Once hatched, nematodes are vulnerable to environmental extremes and have to locate a host to start feeding. For example, under optimal conditions for movement, J2 of G. rostochiensis must locate a host root and set up a feeding site within 6–11 days of hatching otherwise it will exhaust its energy reserves and die (Robinson et al. 1987). Hatching in response to host root diffusates has the advantage of ensuring that the nematodes hatch and leave the protection of the egg and cyst when host roots are close by; thus, synchrony of host availability and nematode hatch is advantageous for nematode survival.
4 Attraction to Plants
Around actively growing roots there exist several gradients of volatile and non-volatile compounds, including amino acids, ions, pH, temperature and CO2. It is evident that nematodes use their chemosensory sensilla, the amphids, to orientate towards the roots using at least some of these gradients. The ability to orientate towards stimuli from plant roots enhances the chances of host location and reduces the time without food (Perry 1997). Evaluating the reality of the attractiveness or otherwise of an individual compound is difficult. Information is usually based on in vitro behavioural studies, often using agar plate movement bioassays, which bear little if any resemblance to the situation in the soil; care must therefore be exercised in extrapolating from such assays to the field situation (Spence et al. 2009). It will be especially important in the future for nematologists to link with plant physiologists to determine the temporal and special attributes of putative attractants in the soil. However, some generalisations can be made and certain compounds are strongly implicated in orientating nematodes to the roots.
Perry (2005) separated gradients into three types: ‘long distance attractants’ that enable nematodes to move to the root area, ‘short distance attractants’ that enable the nematode to orientate to individual roots, and ‘local attractants’ that are used by endoparasitic nematodes to locate the preferred invasion site. There is clear experimental evidence that CO2 is a long distance attractant (Robinson and Perry 2006). With cyst nematodes, such as Globodera spp., it is apparent that the J2 responds to host root diffusate and the evidence is persuasive that diffusate contains chemicals that constitute short distance attractants (Perry 1997; Rolfe et al. 2000). Diffusates from the roots of the host plant, potato, increased the activity of the infective J2 of G. rostochiensis and also attracted them to the roots. As detailed in Sect. 1.3 potato root diffusate (PRD) is required to stimulate hatching of the majority of J2 of the potato cyst nematodes G. rostochiensis and G. pallida but work by Devine and Jones (2002) has shown that the chemicals in PRD responsible for hatching differ from those responsible for attracting the J2 to the root. Electrophysiological analysis of sensory responses (Perry 2001) demonstrated that spike activity of J2 of G. rostochiensis increased on exposure to PRD but not to root diffusate from the non-host sugar beet, thus indicating that responses to diffusates may be host specific. Pudasaini et al. (2007) found that the migration of P. penetrans towards a host depends on both the initial distance between the nematode and the host and the nature of the host. These authors considered that the attractiveness of the host to P. penetrans seems to be correlated with its efficiency as a host; the attractiveness of hosts also declines with age.
The orientation of J2 of cyst and root-knot nematodes to the preferred invasion site, the root tip, is well established but the active factors that constitute the ‘local attractants’ are unknown. The nematodes may orient to an electrical potential gradient at the elongation zone of the root tip but the relative importance of electrical and chemical attractants for root tip location has not been evaluated; in addition the elevated temperature at the zone of root elongation may influence nematode perception.
Blocking sensory perception so that the nematodes are unable to orientate to roots and thus exhaust their food reserves and die is an attractive control option but may be difficult to achieve. Exposure of J2 of M. javanica and G. rostochiensis to antibodies to amphidial secretions blocked the response to host root allelochemicals (Stewart et al. 1993; Perry and Maule 2004) but responses were not permanently blocked as, after a period of between 0.5 and 1.5 h, turnover of sensilla secretions presumably ‘unblocked’ the amphids. A similar effect occurred with the bionematicide, DiTera®, where disruption of sensory perception was reversible (Twomey et al. 2002).
5 Penetration and Feeding
As mentioned in Sect. 1.1, plant-parasitic nematodes exhibit several feeding strategies. However, for all species, feeding is dependent on the use of the hollow, needle-like mouth spear, or stylet, which is inserted into a cell in order to extract its contents. A framework of definitions for biological function is rarely exact; however, some general groupings can be made that reflect the different feeding strategies.
Sedentary e ndoparasitic nematodes enter host roots, set up a feeding site within the root tissue and feed internally. As with other aspects of plant-parasitic nematode biology, the focus on feeding by endoparasitic species now and in the recent past has been on cyst and root-knot nematodes. However, other nematodes, such as Nacobbus, set up a feeding site as a nutrient sink (Manzanilla-López et al. 2002) and may become important research subjects, especially as comparative genomics progresses.
The convergent evolution of cyst and root-knot nematodes has resulted in the same outcome, feeding on nematode induced nutrient sinks, but the method of achieving the end result and the feeding sites themselves show interesting differences. The preferred invasion site for both groups is behind the root tip in the zone of elongation. J2 of cyst nematodes, with their more robust stylets, cut cortical cell walls and migrate through cells until they reach the differentiating vascular cylinder. This intracellular migration causes considerable damage to host tissue, resulting in necrosis from the invasion point to the feeding site. By contrast, root-knot nematodes migrate intercellularly. After invasion, J2 of root-knot nematodes move towards the root tip until they reach the root apex where they turn around, avoiding the barrier of the endodermis, and migrate back up the root until they reach a site near the vascular cylinder.
Cell wall-degrading enzymes from the subventral glands are secreted through the stylets of both cyst and root-knot nematodes to facilitate migration by weakening or breaking down cell walls. Among the enzymes identified in both nematode groups are cellulases and pectate lyases, and in root-knot nematodes xylanase and polygalacturonase (Davis et al. 2000; Gheysen and Jones 2006). Before the discovery of these enzymes in nematodes, they had been reported only from plants and pathogenic bacteria and fungi. They are not present in non-parasitic nematodes or other invertebrates and it is likely that they were acquired by horizontal gene transfer from bacteria to plant-parasitic nematodes (see Sect. 1.2).
Detailed information of the induction and maintenance of the feeding sites for cyst and root-knot nematodes are given in Chaps. 4 and 5 respectively, this volume. Briefly, a cyst nematode selects a cell, becomes sessile, and a multinucleate feeding site, termed a syncytium, is formed by gradual incorporation of hundreds of adjacent cells as the intervening cell walls disintegrate. Root-knot nematodes become sessile and induce the formation of several binucleate cells followed by mitosis that does not cause cell division. These nuclei further divide and several large multinucleate giant cells result and cells surrounding them also enlarge to form a gall or root-knot. In both cyst and root-knot nematodes, secretions from the dorsal and ventral pharyngeal glands play a central role in the induction and maintenance of the feeding sites.
Only the J2 and adult males of root-knot and cyst nematodes are migratory, whereas in Nacobbus, for example, all juvenile stages, the male and the immature vermiform female are migratory, only the mature female being sedentary. All mobile stages of m igratory endoparasit ic nematodes, such as Pratylenchus and Radopholus, invade plant hosts but do not become sessile and have no fixed feeding site within the plant, moving around and feeding off numerous cells and causing considerable damage to plant tissue.
Semi-endoparasitic nematodes, such as Rotylenchulus and Tylenchulus, become sessile after penetrating the root but only the anterior part of the nematode penetrates and remains embedded in the root tissue, the rest of the body remains outside the root in the soil. Some genera, such as Hoplolaimus or Helicotylenchus, may be either semi-endoparasitic or migratory ecto-endoparasitic, depending on the host. Ectoparasitic nematodes remain outside the roots to feed externally, puncturing individual cells with their stylet, extracting food and then withdrawing the stylet to move on to another cell to repeat the process. Some ectoparasitic species, e.g. Cacopaurus, become permanently attached to the root by the deeply embedded stylet.
6 Moulting
Once feeding commences the nematode can continue development. The majority of nematodes have four juvenile stages before development to the adult; thus, there are four moults where the old cuticle is replaced by a new cuticle. Exceptions include certain species of Xiphinema, which have only three moults and three juvenile stages. During the moulting process the cuticle is either shed completely or, as in Meloidogyne, is partially absorbed. Unlike insects, the nematodes increase in size between moults and not during the moulting process.
Moulting may be a putative control target. Soriano et al. (2004) examined the effects of the ecdysteroid 20-hydroxyecdysone (20E), a major moulting hormone of insects, on M. javanica. Exogenous application of 20E resulted in immobility and death of J2, and invasion was partially inhibited and development was halted in spinach with induced high levels of endogenous 20E; however, of the few J2 that invaded, no abnormal moulting was observed. The biosynthesis of ecdysteroids by any nematode has yet to be demonstrated, and specific efforts to detect 20E and its precursor, ecdysone, in M. arenaria and M. incognita were unsuccessful (Chitwood et al. 1987). The complete sequences of the genomes of M. hapla (Opperman et al. 2008) and M. incognita (Abad et al. 2008) will provide information about the genes involved in moulting in Meloidogyne, and this, together with data from genome projects on other species of nematodes, may provide the basis for a realistic assessment of inhibiting or disrupting moulting as a control strategy.
7 Reproduction
Nematodes exhibit a variety of sexual and asexual reproductive methods (reviewed by Evans 1998) and, of the plant-parasitic nematodes, most information is available on Meloidogyne (reviewed by Chitwood and Perry 2009). Sexual reproduction (amphimixis) can occur in species where there are two separate sexes, where the haploid (n) male (spermatocytes) and female (oocytes) gametes fuse to form the zygote and restore the diploid (2n) complement of chromosomes. In A. tritici and D. dipsaci, for example, female nematodes are the homogametic sex (genetically XX) and males are heterogametic (sometimes XO, usually XY), and the sex ratio is determined genetically. In genera such as Globodera, Heterodera and Meloidogyne sex chromosomes are absent and the sex ratio may be environmentally influenced. In many genera, including Heterodera, Pratylenchus and Radopholus, the female can be fertilised by several males, thus enhancing the genetic diversity of the offspring.
In species of nematodes that reproduce asexually, males are absent or occur only rarely. There are two main types of parthenogenesis, meiotic parthenogenesis (automixis) and mitotic parthenogenesis (apomixis). In meiotic parthenogenesis, there is a first meiotic division in the oocytes, although there are variations between species. This meiotic division allows some genetic reorganisation, even though the diploid chromosome number is restored by self-fertilization. Meloidogyne hapla race A exhibits facultative meiotic parthenogenesis, oogenesis and spermatogenesis proceeding as in amphimictic species to yield one haploid nucleus and two polar bodies per oocyte. Although parthogenesis predominates, amphimixis can occur if sperm are present. However, if the sperm are not present, the egg pronucleus recombines with the second (haploid) polar body to restore the diploid state. In monosexual populations of Aphelenchus avenae, females produce only female progeny and reproduction is by obligate meiotic parthenogenesis. Meiosis produces only one polar body, with the egg nucleus having the 2n chromosome number that then develops into the zygote.
Mitotic parthenogenesis, which occurs in several species of Meloidogyne, is the most common method of asexual reproduction and is always obligate; the only division is mitosis and the oocytes retain the diploid chromosome number. This would seem to prevent any genetic reorganisation, except for that resulting from mutations. Frequently, mitotic parthenogenesis is associated with polyploidy, which may increase the likelihood of mutation. Several of the most widespread and economically important species of Meloidogyne are obligate mitotic parthenogens. Populations of the same Meloidogyne species may differ in mode of reproduction; for example, 29 of 32 populations of M. hapla reproduced by facultative meiotic parthenogenesis, the others by mitotic parthenogenesis (Triantaphyllou 1966).
In hermaphrodites, both egg and sperm are produced in the same individual. Usually the sperm is produced first and is stored in the spermatheca, then the gonad produces oocytes, which are fertilized by the sperm until the sperm supply is exhausted. Hermaphroditism is a common method of reproduction amongst free-living nematodes but is relatively rare in plant-parasitic nematodes, being found in some members of the Criconematoidea and in Radopholus similis and species of Paratrichodorus.
8 Survival
For obligate parasitic species there are situations where persistence of a population requires survival of the infective stages outside the host. This may occur when the host is not available, requiring the nematode to survive in the absence of food, or environmental conditions exist that are not commensurate with life cycle progression, such as temperature extremes, osmotic stress and dehydration. Of the environmental extremes, desiccation survival has attracted most interest, partly because the ability to survive dry is implicit in the distribution of infective stages by wind and in dry plant material, such as seeds. Interest has also been generated by the spectacular abilities of some nematode to survive desiccation for periods considerably in excess of the normal life cycle. For example, D. dipsaci fourth-stage juveniles (J4) have been recorded as surviving for more than 20 years in the dry state, yet the whole life cycle of the nematode in favourable environmental conditions only takes about 4 weeks from egg to egg-laying adult; thus, the ability to survive in the dry state (anhydrobiosis) can prolong life by a factor exceeding 240 times!
Although some of the most astonishing survival attributes are found in species of nematodes, such as A. tritici, and D. dipsaci, that live within aerial parts of plants, the infective J2 of root-knot and, especially, cyst nematodes also survive for long periods as unhatched individuals inside the egg mass or cyst (Perry 1999). Morphological features such as the cyst, gelatinous matrix and eggshell all protect the J2 and ensure a slow rate of drying to ensure effective entry into anhydrobiosis. There is no one stage that can be termed the ‘nematode survival stage’. Where species have the ability to suspend development and withstand environmental extremes, the stage(s) involved often differ. Similarly, there are variations in the behavioural and morphological adaptations that ensure desiccation survival, but all have the role of reducing the rate at which water is lost from the nematode as it experiences drying conditions (Perry and Moens 2011).
Galls induced by A. tritici in the tissues of host plants contain tightly packed aggregates of J2, each of which remains uncoiled when dry, yet the galls induced by A. amsinckia contain hundreds of desiccated adults and juveniles of all stages, many of which are coiled. Coiling and clumping are two behavioural responses that effectively reduce the surface area exposed to drying conditions (Perry 1999; Moens and Perry 2009). Reproduction of Aphelenchoides besseyi stops as rice grains ripen and adults coil in clumps beneath the hulls of grains, where the nematodes can remain viable for 2–3 years in dry grains. As mentioned above, in D. dipsaci development stops at the J4 stage and hundreds of desiccated individuals coil and clump in masses termed ‘eelworm wool’, often associated with infected narcissus bulbs (Fig. 1.4) or inside bean pods. The death of the peripheral J4 apparently provides a protective coat that aids survival of the enclosed nematodes by slowing their rate of drying, in a manner that Ellenby (1969) termed the ‘eggshell effect’. Survival of J4 of D. dipsaci and J2 of Anguina spp. is also associated with an intrinsic property of the cuticle, involving an outer lipid layer (Preston and Bird 1987; Wharton et al. 1988), to control the rate of water loss. The cuticle dries more rapidly than other layers and slows down the rate of water loss of internal, and perhaps more vital, structures. As dry individuals in galls, as ‘eelworm wool’, in plant tissue, or in cysts the nematodes can survive for many years and withstand other adverse conditions, such as extremes of temperature; they are also more resistant to non-fumigant nematicides and can be dispersed effectively by wind.
Narcissus bulb with accumulation of Ditylenchus dipsaci fourth-stage juveniles (J4) as dry ‘eelworm wool’, and an inset showing a transmission electron microscope image of the eelworm wool with coiled, clumped, desiccated J4. (Photos courtesy of Roland N Perry, Rothamsted Research, UK; from Moens and Perry 2009)
Why is the rate of water loss important for survival even though the nematodes eventually lose all their body water? A slow rate of water loss appears to allow orderly packing and stabilization of structures to maintain functional integrity during desiccation. At water contents below about 20%, there is no free water in the cells. This 20%, usually referred to as ‘bound water’, is involved in the structural integrity of macromolecules and macromolecular structures, such as membranes. In desiccated, anhydrobiotic nematodes it is probable that the bound water has been lost and research has centred on molecules that might replace bound water and preserve structural integrity. The control of water loss enables biochemical changes to take place, including replacement of bound water, thus ensuring long-term survival of true anhydrobiotes (Barrett 1991; Perry 1999).
Ditylenchus dipsaci J4 and A. tritici J2 sequester trehalose, which has frequently been suggested as a desiccation protectant because of its role in preserving membrane stability, preventing protein denaturation and acting as a free-radical scavenging agent to reduce random chemical damage (Barrett 2011). However, there are contradictory reports about the importance of trehalose (Burnell and Tunnacliffe 2011). For example, all stages of the mycophagous D. myceliophagus survive desiccation poorly, even at high humidities (Perry 1977). The survival of D. myceliophagus was unrelated to their trehalose content, and elevated levels of trehalose, generated by providing the nematodes with different food sources, did not enhance anhydrobiotic survival of this species (Womersley et al. 1998). Synthesizing trehalose during dehydration may indicate preliminary preparation for a period in the dry state, but it does not necessarily mean that survival during subsequent severe desiccation is assured. Following trehalose synthesis, it appears that other, at present unknown, adaptations are required at the cellular and subcellular levels for nematode survival, and rate of drying still has to be controlled (Higa and Womersley 1993).
Late embryogenesis abundant (LEA) proteins have been associated with survival in some nematodes, including C. elegans (Gal et al. 2004; Burnell and Tunnacliffe 2011), and LEA proteins may protect cellular components against the effects of desiccation (Goyal et al. 2005). Homologues of LEA genes have been identified in B. xylophilus (Kikuchi et al. 2007).
Aspects of the biochemical adaptations and the genes that are switched on during the induction of anhydrobiosis are likely to become evident when progress is made with comparative genomics and transcriptome analyses. In addition, the molecular information will also progress our understanding of the extent of the ‘dauer’ phenomenon in plant-parasitic nematodes. The dauer stage of the free-living nematode, Caenorhabditis elegans, represents a developmental arrest (Riddle and Albert 1997). Dauer larvae are enclosed by a dauer-specific cuticle and exhibit several characteristics associated with survival of adverse conditions, including reduced metabolism, elevated levels of several heat shock proteins and an enhanced resistance to desiccation (Kenyon 1997). Bird and Bird (1991) suggested that the survival forms of Anguina, may be regarded as dauers. In Ditylenchus dipsaci, the J4 that accumulate in response to adverse conditions are larger, have more lipid reserves and show a propensity to aggregate compared with J4 in a population feeding and developing under ideal conditions (Perry, unpublished), all properties that reflect the dauer state. In some species of the genus Bursaphelenchus a dauer form (J4) is present as a specialised survival and dispersal stage of the life cycle. Bursaphelenchus xylophilus is a migratory endoparasitic nematode that has a complex life cycle involving beetles of the genus Monochamus as the vector (Mota et al. 2008). Bursaphelenchus xylophilus has a dauer stage, which uses the insect for transport to susceptible hosts where the nematodes enter the shoots of trees through the feeding wounds caused by the vector. In an analysis of more than 13,000 ESTs from B. xylophilus, Kikuchi et al. (2007) looked for homologues of 37 genes involved in dauer entry and maintenance in C. elegans. They identified 31 homologues of 18 C. elegans genes, including nine homologues for daf (dauer formation) genes.
Meloidogyne hapla carries 14 orthologues of C. elegans daf genes as well as three further matches that are weak (Abad et al. 2008; Abad and Opperman 2009) but it does not carry the daf-28 orthologue, which is key in the signal transduction pathway. Abad and Opperman (2009) conclude that basic development mechanisms are conserved, although signalling is not. Thus, there may be marked differences between free-living and parasitic nematodes in developmental response to adverse changes in the environment.
These studies provide initial evidence that the dauer phenomenon may be more widespread than currently recognised. Certainly, the indications in some species of plant-parasitic nematodes of an alternative developmental stage similar to a dauer larva are convincing (see Sect. 1.8 above). However, there are difficulties in relating information on dauer formation in C. elegans to parasitic nematodes. Comparison of expression profiles of dauer genes in C. elegans and in survival stages of parasitic nematodes (Elling et al. 2007) reveals marked differences in expression patterns between C. elegans and other nematodes; as yet, there is insufficient information to be able to link individual daf genes to specific survival traits.
9 Conclusions
There are many putative control targets in the life cycle of plant-parasitic nematodes. Comparative genomics will undoubtedly increase our knowledge of the parasitic life style and the vulnerable phases. However, the challenge will be to translate this information to viable, environmentally benign control or management options. This chapter has focused on the nematode side of the plant-nematode interaction and has highlighted some putative targets. We have not discussed plant resistance or genetic engineering to interfere with nematode invasion and development. This research area of developing plant resistance to nematodes is undoubtedly important, but it is possible that future management options may be based on perturbing several different aspects of the nematode’s life cycle to achieve a desired level of control.
References
Abad P, Opperman CH (2009) The complete sequence of the genomes of Meloidogyne incognita and Meloidogyne hapla. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. CABI Publishing, Wallingford
Abad P, Gouzy J, Aury J-M et al (2008) Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 8:909–915
Baldwin JG, Nadler SA, Adams BJ (2004) Evolution of plant parasitism among nematodes. Ann Rev Phytopathol 42:83–105
Barrett J (1991) Anhydrobiotic nematodes. Agric Zool Rev 4:161–176
Barrett J (2011) Biochemistry of survival. In: Perry RN, Wharton DA (eds) Molecular and physiological basis of nematode survival. CABI Publishing, Wallingford
Bird AF, Bird J (1991) The structure of nematodes, 2nd edn. Academic Press, New York
Burnell AM, Tunnacliffe A (2011) Gene induction and desiccation stress in nematodes. In: Perry RN, Wharton DA (eds) Molecular and physiological basis of nematode survival. CABI Publishing, Wallingford
Castillo P, Vovlas N (2007) Pratylenchus (Nematoda; Pratylenchidae): diagnosis, biology, pathogenicity and management. In: Hunt DJ, Perry RN (eds) Nematology monographs and perspectives, vol 6. Brill, Leiden
Chitwood DJ, Perry RN (2009) Reproduction, physiology and biochemistry. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. CABI Publishing, Wallingford
Chitwood DJ, McClure MA, Feldlaufer MF, Lusby WR, Oliver JE (1987) Sterol composition and ecdysteroid content of eggs of the root-knot nematodes, Meloidogyne incognita and M. arenaria. J Nematol 19:352–360
Clarke AJ, Perry RN, Hennessy J (1978) Osmotic stress and the hatching of Globodera rostochiensis. Nematology 24:384–392
Cobb NA (1914) Antarctic marine free-living nematodes of the Shackleton Expedition. Contrib Sci Nematol 1:1–33
Davis EL, Hussey RS, Baum TJ, Bakker J, Schots A (2000) Nematode parasitism genes. Ann Rev Phytopath 38:365–396
Devine KJ, Jones PW (2002) Investigations into the chemoattraction of the potato cyst nematodes Globodera rostochiensis and G. pallida towards fractionated potato root leachate. Nematology 5:65–75
Devine KJ, Byrne J, Maher N, Jones PW (1996) Resolution of natural hatching factors for the golden potato cyst nematode, Globodera rostochiensis. Ann Appl Biol 129:323–334
Ehlers R-U (2001) Mass production of entomopathogenic nematodes for plant protection. Appl Microbiol Biotechnol 56:623–633
Ellenby C (1969) Dormancy and survival in nematodes. Symp Soc Exp Biol 23:83–97
Elling AE, Mitreva M, Recknor J, Gai X, Martin J et al (2007) Divergent evolution of arrested development in the dauer stage of Caenorhabditis elegans and the infective stage of Heterodera glycines. Genome Biol 8:R211. doi:10.1186/gb-2007-8-10-r211
Evans AAF (1998) Reproductive mechanisms. In: Perry RN, Wright DJ (eds) The physiology and biochemistry of free-living and plant-parasitic nematodes. CAB Publishing, Wallingford
Gal TZ, Glazer I, Koltai H (2004) An LEA group 3 family member is involved on survival of C. elegans during exposure to stress. FEBS Lett 577:21–26
Gaugler R (2002) Entomopathogenic nematology. CABI Publishing, Wallingford
Gaugler R, Han R (2002) Production technology. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, Wallingford
Gaur HS, Beane J, Perry RN (2000) The influence of root diffusate, host age and water regimes on hatching of the root-knot nematode, Meloidogyne triticoryzae. Nematology 2:191–199
Gheysen L, Jones JT (2006) Molecular aspects of plant-nematode interactions. In: Perry RN, Moens M (eds) Plant nematology. CABI Publishing, Wallingford
Goyal K, Walton LJ, Tunnacliffe A (2005) LEA proteins prevent protein aggregation due to water stress. Biochem J 388:151–157
Higa LM, Womersley CZ (1993) New insights into the anhydrobiotic phenomenon: the effects of trehalose content and differential rates of evaporative water loss on the survival of Aphelenchus avenae. J Exp Zool 267:120–129
Jones JT, Furlanetto C, Kikuchi T (2005) Horizontal gene transfer from bacteria and fungi as a driving force in the evolution of plant parasitism in nematodes. Nematology 7:641–646
Jones JT, Robertson L, Perry RN, Robertson WM (1997) Changes in gene expression during stimulation and hatching of the potato cyst nematode Globodera rostochiensis. Parasitology 114:309–315
Jones PW, Tylka GL, Perry RN (1998) Hatching. In: Perry RN, Wright DJ (eds) The physiology and biochemistry of free-living and plant-parasitic nematodes. CABI Publishing, Wallingford
Kenyon C (1997) Environmental factors and gene activities that influence life span. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds) C. elegans II. Cold Spring Harbor Press, Cold Spring Harbor
Kikuchi T, Jones JT, Aikawa T, Kosaka H, Ogura N (2004) A family of glycosyl hydrolase family 45 cellulases from the pine wood nematode Bursaphelenchus xylophilus. FEBS Lett 572:201–205
Kikuchi T, Aikawa T, Kosaka H, Pritchard L, Ogura N, Jones JT (2007) Expressed sequence tag (EST) analysis of the pine wood nematodes Bursaphenelchus xylophilus and B. mucronatus. Mol Biochem Parasitol 155:9–17
Lee DL (2001) The biology of nematodes. Taylor and Francis, London
Maggenti AR (1971) Nemic relationships and the origins of plant parasitic nematodes. In: Zuckerman BF, Mai WM, Rohde RA (eds) Plant parasitic nematodes, vol 1. Academic Press, New York
Manzanilla-López R (2010) Speciation within Nacobbus: consilience or controversy? Nematology 12:321–334
Manzanilla-López R, Costilla MA, Doucet M, Franco J, Inserra RN, Lehman PS, Cid del Prado-Vera I, Souza RM, Evans K (2002) The genus Nacobbus Thorne and Allen, 1944 (Nematoda: Pratylenchidae): systematics, distribution, biology and management. Nematropica 32:149–227
Matthews B (1998) An introduction to parasitology. Cambridge University Press, Cambridge
Moens M, Perry RN (2009) Migratory plant endoparasitic nematodes: a group rich in contrasts and diversity. Ann Rev Phytopathol 47:313–332
Moens M, Perry RN, Starr JL (2009) Meloidogyne species—a diverse group of novel and important plant parasites. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. CABI Publishing, Wallingford
Mota M, Vieira PR (eds) (2008) Pine wilt disease: a worldwide threat to forest ecosystems. Springer, Berlin
Opperman CH, Bird D McK, Williamson VM et al (2008) Sequence and genetic map of Meloidogyne hapla: a compact nematode genome for plant parasitism. Proc Nat Acad Sci USA 105:14802–14807
Perry RN (1977) Desiccation survival of larval and adult stages of the plant parasitic nematodes, Ditylenchus dipsaci and D. myceliophagus. Parasitology 74:139–148
Perry RN (1989) Dormancy and hatching of nematode eggs. Parasitol Today 5:377–383
Perry RN (1997) Plant signals in nematode hatching and attraction. In: Grundler F, Ohl S, Fenoll C (eds) Cellular and molecular aspects of plant-nematode interactions. Kluwer Academic Publishers
Perry RN (1999) Desiccation survival of parasitic nematodes. Parasitology 119:S19–S30 (published Jan, 2001)
Perry RN (2001) Analysis of the sensory responses of parasitic nematodes using electrophysiology. Int J Parasitol 31:908–917
Perry RN (2002) Hatching. In: Lee DL (ed) The biology of nematodes. Taylor and Francis, London
Perry RN (2005) An evaluation of types of attractants enabling plant-parasitic nematodes to locate plant roots. Russ J Nematol 13:83–88
Perry RN, Beane J (1982) The effect of brief exposures to potato root diffusate on the hatching of Globodera rostochiensis. Rev Nématol 5:221–224
Perry RN, Maule AG (2004) Physiological and biochemical basis of nematode behaviour. In: Gaugler R, Bilgrami A (eds) Nematode behaviour. CAB International, Wallingford
Perry RN, Wesemael WML (2008) Host plant effects on hatching of root-knot nematodes. Russ J Nematol 16:1–5
Perry RN, Moens M (2011) Survival of parasitic nematodes outside the host. In: Perry RN, Wharton DA (eds) Molecular and physiological basis of nematode survival. CABI Publishing, Wallingford
Perry RN, Knox DP, Beane J (1992) Enzymes released during hatching of Globodera rostochiensis and Meloidogyne incognita. Fundam Appl Nematol 15:283–288
Poinar GO (1983) The natural history of nematodes. Prentice Hall, New Jersey
Poinar GO (2011) The evolutionary history of nematodes. In: Hunt DJ, Perry RN (eds). Nematology Monographs and Perspectives, vol 9. Brill, Leiden
Preston CM, Bird AF (1987) Physiological and morphological changes associated with recovery from anabiosis in the dauer larva of the nematode Anguina agrostis. Parasitology 95:125–133
Pudasaini MP, Viaene N, Moens M (2007) The influence of host and temperature on the vertical migration of Pratylenchus penetrans. Nematology 9:437–447
Rahm G (1937) Grenzen des Lebens. Studien in Heissen Quellen. Forsch Fortschr 13:318–383
Riddle DL, Albert PS (1997) Genetic and environmental regulation of dauer larva development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds) C. elegans II. Cold Spring Harbor Press, Cold Spring Harbor, USA
Robinson AF, Perry RN (2006) Behaviour and Sensory Perception. In: Perry RN, Moens, M (eds) Plant nematology. CABI International, Wallingford
Robinson MP, Atkinson HJ, Perry RN (1987) The influence of soil moisture and storage time on the motility, infectivity and lipid utilization of second stage juveniles of the potato cyst nematodes Globodera rostochiensis and G. pallida. Rev Nématol 10:343–348
Rolfe RN, Barrett J, Perry RN (2000) Analysis of chemosensory responses of second stage juveniles of Globodera rostochiensis using electrophysiological techniques. Nematology 2:523–533
Rosso MN, Jones JT, Abad P (2009) RNAi and functional genomics in plant parasitic nematodes: tools and discoveries in the post-genomic era. Ann Rev Phytopathol 47:207–232
Sharon E, Spiegel Y (1993) Glycoprotein characterization of the gelatinous matrix in the root-knot nematode Meloidogyne javanica. J Nematol 25:585–589
Siddiqi MR (1983) Evolution of plant parasitism in nematodes. In: Stone AR, Platt HM, Khalil LF (eds) Concepts in nematode systematic. Academic Press, London
Soriano IR, Riley IT, Potter MJ, Bowers WS (2004) Ohytoecdysteroids: a novel defense against plant-parasitic nematodes. J Chem Ecol 30:1885–1899
Spence KO, Lewis EE, Perry RN (2009) Host-finding and invasion by entomopathogenic and plant-parasitic nematodes: evaluating the ability of laboratory bioassays to predict field results. J Nematol 40:93–98
Stewart GR, Perry RN, Wright DJ (1993) Studies on the amphid specific glycoprotein gp32 in different life cycle stages of Meloidogyne species. Parasitology 107:573–578
Szewczyk NJ, Lamb W (2005) Surviving atmospheric spacecraft breakup. Wilderness Environ Med 16:27–32
Triantaphyllou AC (1966) Polyploidy and reproductive patterns in the root-knot nematode Meloidogyne hapla. J Morphol 118:403–413
Turner SJ, Rowe JA (2006) Cyst nematodes. In: Perry RN, Moens M (eds) Plant nematology. CABI Publishing, Wallingford
Twomey U, Rolfe RN, Warrior P, Perry RN (2002) Effects of the biological nematicide, DiTera®, on movement and sensory responses of second stage juveniles of Globodera rostochiensis, and stylet activity of G. rostochiensis and fourth stage juveniles of Ditylenchus dipsaci. Nematology 4:909–915
Wharton DA, Preston CM, Barrett J, Perry RN (1988) Changes in cuticular permeability associated with recovery from anhydrobiosis in the plant parasitic nematode, Ditylenchus dipsaci. Parasitology 97:317–330
Wilson M, Khakouli-Duarte T (eds) (2009) Nematodes as environmental indicators. CABI Publishing, Wallingford
Womersley CZ, Wharton DA, Higa, LM (1998) Survival biology. In: Perry RN, Wright DJ (eds) The physiology and biochemistry of free-living and plant-parasitic nematodes. CABI Publishing, Wallingford
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Perry, R.N., Moens, M. (2011). Introduction to Plant-Parasitic Nematodes; Modes of Parasitism. In: Jones, J., Gheysen, G., Fenoll, C. (eds) Genomics and Molecular Genetics of Plant-Nematode Interactions. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0434-3_1
Download citation
DOI: https://doi.org/10.1007/978-94-007-0434-3_1
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0433-6
Online ISBN: 978-94-007-0434-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)