Abstract
Flavodiiron proteins (FDPs) constitute a large family of enzymes, widespread among the two prokaryotic domains, Bacteria and Archaea, including anaerobes, facultative anaerobes and oxygenic phototrophs. Recently, homologues have also been found in Eukarya, namely algae, mosses and anaerobic protozoa. The distinctive feature of this protein family is a common core built by the fusion of a metallo-β-lactamase-like and flavodoxin-like domains harbouring, respectively, a diiron center and a flavin mononucleotide. These enzymes are endowed with oxygen reductase and/or nitric oxide reductase activities, playing an important role in resistance towards oxidative and/or nitrosative stresses. Cyanobacteria are a particularly interesting example of organisms containing multiple and several unique types of FDPs, whose function appears to be mainly related to protection against oxidative stress conditions, reducing dioxygen to water, and therefore avoiding the formation of reactive oxygen species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nitrosative Stress
- Electron Transfer Chain
- Flavin Mononucleotide
- Diiron Centre
- Oxygenic Photosynthetic Organism
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The flavodiiron proteins (FDP) constitute a large family of enzymes that reduce nitric oxide (nitrogen monoxide) to nitrous oxide (N2O) or oxygen to water (for recent reviews see Saraiva et al. (2004); Vicente et al. (2008a–d)). These enzymes were given this name because they contain a flavin mononucleotide (FMN) and a diiron centre as the common prosthetic groups. The first reported example was rubredoxin:oxygen oxidoreductase, ROO, from the sulphate reducing bacterium Desulfovibrio (D.) gigas. This enzyme was shown to be the terminal oxidase of a three-component electron transfer chain coupling NADH oxidation (by an NADH:rubredoxin oxidoreductase, NRO) to oxygen reduction (Chen et al. 1993a, b). Electron transfer from NRO to ROO is mediated by a small iron-protein, rubredoxin (Rd), which contains a FeCys4 centre (Chen et al. 1993b; Gomes et al. 1997). This process, which occurs in the cytoplasm, was proposed to confer oxygen tolerance to this anaerobic bacterium, simultaneously enabling NAD+ regeneration (Fareleira et al. 2003). The first structure of an enzyme of the flavodiiron family was also that obtained for the D. gigas ROO, that allowed to clearly identify the catalytic centre, built by two iron ions ligated by the side chains of histidines and aspartates/glutamates (H79-X-E81-X-D83-X62-H146-X18-D165-X60-H226, numbering of D. gigas ROO), and to envisage the intramolecular electron transfer pathways. Whereas the initial studies suggested a general role in oxygen stress alleviation in anaerobes, evidence for a role in nitric oxide detoxification broadened the interest on this protein family. In fact, nitric oxide is a well recognized toxic molecule, in concentrations above the nanomolar range, being either a weapon of the immune system or an intermediate of microbial denitrification; thus, organisms need to have enzymes able to deal with NO or its derived and toxic species. Until the discovery of this function of the FDPs, NO detoxification was thought to be mainly restricted to the flavohemoglobin family (for a recent review see Lewis et al. (2008)), while the heme-iron membrane-bound NO reductases are mainly considered as enzymes of the denitrification pathway.
Since those early findings, a wealth of data has been accumulated in this field for different organisms. The main features of this challenging enzyme family will be discussed, with a particular focus on what is so far known on flavodiiron enzymes from cyanobacteria.
2 FDPs: A Family of Modular Enzymes
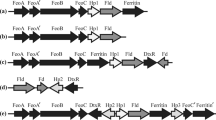
The analysis of the primary structure of the large number of FDPs encoded in microbial and eukaryotic genomes revealed a common flavodiiron core with ca 400 amino acids, composed by a ~250-amino acids metallo-β-lactamase domain and a ~150-amino acids flavodoxin domain, and the presence of extra structural domains fused at the C-terminus of the flavodiiron core. This latter observation led us to establish four subfamilies (Classes A–D), according to the type of structural domains present in each protein (Fig. 22.1a–d); the domain arrangement is intimately associated with the type of electron transfer chains involved in each case (e.g., Saraiva et al. 2004; Vicente et al. 2008b)
Flavodiiron proteins are modular enzymes. Scheme depicting the modular nature of flavodiiron proteins, which in several organisms display extra C-terminal structural domains and were classified accordingly. a—Class A FDPs are the structural prototype of this protein family, being composed of an N-terminal β lactamase-like domain (light blue box) and a C-terminal flavodoxin-like domain (light yellow box). b—Class B FDPs, found thus far only in enterobacteria, bear an extra C-terminal rubredoxin domain (dark red box), harbouring a FeCys4 center. c—Class C FDPs, from cyanobacteria and some eukaryotic oxygenic phototrophs, having a NAD(P)H:flavin oxidoreductase domain (light green box) fused at the C-terminus of the flavodiiron core, that may bind one FMN or one FAD moiety. d—Class D FDPs are, so far, only found in the genomes of Trichomonas vaginalis and in a few Clostridia species. These FDPs appear to result from a fusion between a Class B FDP and its reductase partner, of the rubredoxin reductase family (light orange box)
The prototype enzymes, which constitute the bulk of identified FDPs, are those that have only the core domains, i.e., the β-lactamase and the flavodoxin-like domains (Class A, Fig. 22.1a). In several organisms these enzymes receive electrons from rubredoxins, acting as the terminal oxidase of a three components chain that involves also an NADH:rubredoxin oxidoreductase. However, in most organisms the immediate electron donor is not known and, as far as it can be predicted from genomic data, it is not a rubredoxin, since genes coding for this type of proteins are absent. Distinct types of electron donors have been reported, namely the F420H2 co-factor in methanogenic Archaea (Seedorf et al. 2007), and the pyruvate:ferredoxin oxidoreductase system for the Trichomonas vaginalis hydrogenosomal FDP (Smutna et al. 2009).
The second type of enzyme to be described was the so-called flavorubredoxin, FlRd, since it contains at the C-terminus a third structural domain, similar to rubredoxins. The ca 50 amino acids rubredoxin domain is fused to the flavodiiron core by an apparently unstructured linker of about 20 amino acids. So far, this type of enzymes (Class B, Fig. 22.1b) is restricted to enterobacteria, such as Escherichia and Salmonella species. FlRd has been shown to accept electrons directly from an NADH:FlRd oxidoreductase (from the family of NADH:rubredoxin oxidoreductases), establishing a two component electron transfer chain (Gomes et al. 2000, 2002; Vicente et al. 2007). The crystallographic structure of these enzymes remains to be determined, but structural studies by small angle X-ray scattering proposed that the linker between the Rd domain and the flavodiiron core is highly flexible, and thus the Rd domain protrudes into the solvent extending from the tetrameric quaternary structure, resembling an independently-behaving “quasi” free rubredoxin (Petoukhov et al. 2008).
Class C FDPs (Fig. 22.1c) were firstly identified in cyanobacteria (further discussed below)—these enzymes also bear a third domain at the C-terminus, homologous to NAD(P)H flavin reductases (Wasserfallen et al. 1998; Saraiva et al. 2004; Vicente et al. 2002, 2008a, b). Therefore, these FDPs have the interesting feature of condensing in a single polypeptide chain the whole electron transfer chain that couples NAD(P)H oxidation to oxygen and/or NO reduction.
The fourth class of FDPs (Class D, Fig. 22.1d) is composed by enzymes that appear to result from the fusion of a Class B FDP with the respective NADH:FlRd oxidoreductase, yielding a condensed four-domain polypeptide that is likely to directly accomplish O2 or NO reduction at the expense of NAD(P)H oxidation. So far, the only examples were found in the genomes of the anaerobic protozoan Trichomonas vaginalis (Carlton et al. 2007) and of the anaerobic bacterial pathogen Clostridium perfringens (Shimizu et al. 2002).
3 Amino Acid Sequences Analyses and FDPs Distribution
A BLAST search on the sequence databases using several queries (such as the sequences of the enzymes from cyanobacteria and from anaerobic bacteria) retrieved close to 500 sequences of flavodiiron proteins, which were aligned using ClustalX (Larkin et al. 2007). A dendrogram was constructed, based only on the common FDP core (discarding the extra C-terminal domains and several types of signal peptides at the N-terminus), using the neighbour joining method implemented in ClustalX (Fig. 22.2). FDPs, which were originally thought to be present only in prokaryotes, are also found in uni- and multicellular Eukarya, including oxygenic photosynthetic organisms (Table 22.1) and anaerobic protozoa (Trichomonas, Giardia and Entamoeba species). As previously proposed, there are evidences for multiple gene transfer events, but it is also possible to distinguish groups corresponding to particular phylogenies (Andersson et al. 2003, 2006; Vicente et al. 2008b). A striking example is the observation that sequences retrieved for the cyanobacteria and for all the other oxygenic photosynthetic organisms so far known to contain genes encoding FDPs (algae, mosses, lycophytes or even the higher plant Picea sitchensis, see Table 22.1) form a distinct clade from the other classes of FDPs. The sequences of 82 putative FDPs of cyanobacteria were aligned separately, together with those from eukaryotic oxygenic phototrophs (Table 22.1) and with a few other prototypic FDPs, which served as a reference; a subset of this alignment is presented in Fig. 22.3, and the resulting dendrogram in Fig. 22.2b. Genes coding for FDPs are present in all complete genomes of cyanobacteria, and each cyanobacterium sequenced has always at least two FDP encoding genes; cyanobacteria are also among the organisms that have a higher number of homologues in a single genome (up to six homologues, as in Anabaena sp. PCC7120 and Anabaena variabilis ATCC 29413, see Table 22.1). As already mentioned, all cyanobacterial FDPs and those from oxygenic phototrophs are of the C-type, i.e., have the extra C-terminal flavin-reductase domain; the only exception is the FDP from Picea sitchensis, which, in spite of having high amino acid identity/similarity with the homologues of the remaining photosynthetic organisms, lacks the flavin reductase domain. The fact that the FDP sequences of the eukaryotic oxygenic photosynthetic organisms are closer to the cyanobacterial ones than to those from other eukaryotes (see Fig. 22.2a, b), indicates that eukaryotes acquired these genes more than once, i.e., their origin is not monophyletic. It had already been proposed that the anaerobic protozoa had acquired FDPs from multiple lateral gene transfers from anaerobic prokaryotes (Andersson et al. 2003, 2006). Although a similar in-depth analysis is out of the context of this review, at least two arguments suggest a different scenario for the origin of the FDPs in oxygenic eukaryotes: (1) a close inspection of the sub-tree obtained only for the oxygenic photosynthetic organisms (Fig. 22.2b) strongly suggests that the higher organisms obtained FDPs from an ancient, possibly non extant, cyanobacterium; (2) several putative FDPs of algae, mosses, lycophytes and of the tree Picea sitchensis, are nuclear encoded, and the deduced amino acid sequences reveal clear signal peptides at the N-terminus (peptides rich in aliphatic residues, as well as in basic residues, namely arginines (Gould et al. 2008)) which suggest that the FDPs will be present in cellular organelles. Quite interestingly, the FDPs of Paulinella chromatophora (an organism considered to have acquired its plastid from a recent endosymbiotic event that may have occurred only a few millions years ago (Bodyl et al. 2007; Yoon et al. 2009)), lack those signal peptides and, accordingly, are encoded in the plastidic genome. In a speculative way, it appears that migration of the FDP genes from the plastidic chromosome to the nucleus did not yet occur in Paulinella chromatophora, contrary to what already happened quite longer ago in the other photosynthetic organisms. On this basis, as well as taking into account the proposed function for FDPs in cyanobacteria (see Sect. 22.7), it is tempting to suggest localization in the chloroplasts for the FDPs of the other eukaryotic phototrophs, but experimental work will be needed to prove this hypothesis. This situation is reminiscent of that for at least one of the FDPs of the protozoan Trichomonas vaginalis, which possesses a signal peptide targeting the enzyme to the hydrogenosome (Smutna et al. 2009).
a Dendrogram of selected flavodiiron proteins. A few sequences are highlighted: D. gigas (Dg), Moorella thermoacetica (Mth), E. coli (Ec), Methanothermobacter marburgensis (Mm), Giardia intestinalis (Gi), Trichomonas vaginalis (Tv1, Tv2), and several Entamoeba species (Ent). b Dendrogram of the flavodiiron proteins from cyanobacteria and oxygenic photosynthetic eukaryotes. The numbers correspond to the several Types of FDPs. Colors according to each type: green—1; red—2; dark blue—3; light blue—4; magenta—5; light orange—6; gray—7; black—8; light green—9; pink—10; ocean blue—11; dark orange—12. The dendrogram was obtained using ClustalX (Larkin et al. 2007), and Dendroscope, version 2.4 (Huson et al. 2007)
Amino acid sequence alignment of the FDPs from Class C—Types 1–12. The alignment was performed using one example from each type, and the sequence of M. thermoacetica FDP as a reference (Mth). Syn 1 and Syn2: Sll0550 and Sll1521, respectively from Synechocystis sp. PCC6803; Ana: All0177 from Anabaena sp. PCC7120; Pma1: P9215_00501 from Prochlorococcus (P.) marinus MIT 9215; Pma2: P9515_00491 from P. marinus MIT 9515; Pma3: PMN2A_1375 from P. marinus NATL2A; Sno: SynRCC307_2387 from Synechococcus sp: RCC307; Ter: Tery_0770 from Trichodesmium erythraeum IMS101; Mpu: EEH_58658 from Micromonas pusilla CCMP1545; Olu: XP_001416100 from Ostreococcus lucimarinus CCE9901; Cre: XP_001692916 from Chlamydomonas reinhardtii; Ota: CAL52487 from Ostreococcus tauri. Several N-terminal extensions were deleted. The residues implicated in the binding of the diiron site in Moorella thermoacetica FDP are marked with an asterisk (*). Black shadows correspond to strictly conserved residues; Gray shadows correspond to the putative diiron site ligands. Too large non-conserved segments from the sequences designated by Mpu and Ota were removed for clarity and are represented by -//-
As recently noted by E.M. Aro and co-workers (Zhang et al. 2009), the FDP sequences of the cyanobacteria form two distinct clusters (Fig. 22.2b), which those authors named A and B. However, due to the above mentioned classification of the FDPs according to the respective modular arrangement, we prefer a different nomenclature for this division. Indeed, a detailed analysis of the cyanobacterial sequences (c.f. Fig. 22.3) reveals another remarkable characteristic, as compared to the remaining FDP-encoding organisms: while about half of the sequences have conserved residues matching the “canonical” ones known to be involved in iron coordination (H81-X-E83-X-D85H86-X62-H148-X18-D167-X60-H228, Morella thermoacetica FDP numbering), which correspond to what we now propose to designate as Class C, Type 1 FDPs (Cluster B in Fig. 8 of Zhang et al. (2009)), a significant variation in these residues is present in the sequences forming the other cluster (Cluster A in Fig. 8 of that reference): up to eleven possible different ligand substitution combinations could be detected (Types 1–12, see Table 22.2, Figs. 22.2b and 22.3), with the corresponding sequences scattered along that second cluster, in between the second major group, the Type 2 enzymes. It is striking that the amino acid changes involve, in general, substitutions by neutral or positively charged residues (arginines, lysines and asparagines), or even aliphatic residues, such as isoleucines or alanines. It should also be noted that almost all cyanobacterial and photosynthetic eukaryotic organisms contain at least two FDP genes, one coding for a “canonical”, Type 1 enzyme, and a second, encoding one of the multiple types identified (Tables 22.1, 22.2 and Fig. 22.3). Some sub-types appear to be more common than others (c.f. Table 22.2), and Types 9–12 are, so far, restricted to eukaryotes.
These multiple putative ligand substitutions will be further discussed in the next section, regarding the possible involvement of these amino acids as ligands for the iron ions. At present, no rationale can be proposed for this diversity and its apparent occurrence only in oxygenic phototrophs, either from the Bacteria or from the Eukarya domains.
The genomic organization of the genes encoding flavodiiron proteins is, in general, very diverse. In enterobacteria, the gene coding for the FDP forms a dicistronic unit with that coding its NADH oxidoreductase; in D. gigas, roo forms also a dicistronic unit with the gene encoding rubredoxin, the immediate electron donor to ROO. However, in the majority of the cases, the FDP-encoding genes have in their vicinity genes coding for proteins whose functions are unrelated to oxidative or nitrosative stress responses. We analysed the genome regions surrounding the 82 FDP-encoding genes retrieved from the Cyanobase website (http://genome.kazusa.or.jp/cyanobase/). From the 35 genomes scrutinized, 20 have genes coding for FDPs adjacently transcribed (Fig. 22.4a), where one of the genes corresponds always to a type 1 FDP, and the other to types 2–7. The flanking regions surrounding these contiguous FDP-encoding genes are variable. However, we found some examples of a common organization, as depicted in Fig. 22.4a. A particularly interesting case is found in a few Prochlorococcus marinus strains, where a rubrerythrin-encoding gene (a protein involved in oxidative stress response, as an H2O2 reductase (Kurtz 2006)) is adjacently upstream to one of the FDP coding genes and transcribed in the same direction.
Genomic regions surrounding cyanobacterial FDP encoding genes. The genome regions around each cyanobacterial FDP encoding gene were analyzed in the Cyanobase website (http://genome.kazusa.or.jp/cyanobase). a Types of genome loci that were found in at least two genomes, and the corresponding Type of Class C FDPs encoded. b In four genomes (including that of Synechocystis sp. PCC6803), genes encoding FDPs were found to be interspaced by a conserved gene encoding a hypothetical protein containing a transmembrane domain; these encoded FDPs are in every case a Type 1 and a Type 2
In four genomes (including that of Synechocystis sp. PCC6803), two of the genes coding for FDPs are almost contiguous (always encoding a type 1 and a type 2 FDP enzymes), displaying only one gene (encoding a hypothetical protein) in between them, and the three genes are transcribed in the same direction (sll0217-sll0219 in Synechocystis sp. PCC6803, Fig. 22.4b). That same gene is conserved among the genomes displaying this organization, which suggests that transfer events may have included the set of FDP genes interspaced by this gene. Another interesting observation is that a secondary structure prediction (using the PSI-PRED server (Jones 1999, p. 839)) suggests the presence of transmembrane helices in that putative gene product. It should be recalled that the Synechocystis sp. PCC6803 sll0217 and sll0219 gene products (the type 1 and 2 FDPs in this kind of genomic organization) are found, at least partially, in the bacterial membranes, despite lacking any predictable transmembrane helices. Whether that hypothetical transmembranar protein contributes to this localization is an interesting question that requires experimental data to be answered.
4 Three Dimensional Structure of Flavodiiron Enzymes
The first step in the elucidation of the structural features of FDPs consisted in the resolution of the crystallographic structure of D. gigas FDP, rubredoxin:oxygen oxidoreductase (Frazão et al. 2000). This three dimensional structure confirmed the homodimeric quaternary arrangement determined in solution, with each ~43 kDa monomer being built by two structural domains: a diiron-containing domain, structurally analogous to zinc β-lactamases (despite the poor amino acids sequence similarity), followed by a short-chain flavodoxin-like domain, harbouring an FMN. In each monomer, the redox active cofactors are placed ca. 30 Å apart, precluding biologically active electron transfer between each other. However, the quaternary structure is such that, due to the “head-to-tail” arrangement of each monomer within the dimer, the diiron site of one monomer is almost in Van der Waals contact with the FMN from the other monomer, ensuring a fast electron transfer between the two redox centres (c.f. Fig. 22.5). The iron ions from the diiron centre are bound by histidines and glutamates/aspartates, and are bridged by a µ-hidroxo(oxo) species and one aspartate residue (Fig. 22.5c). Several FDP´s structures are now available (Silaghi-Dumitrescu et al. 2005; Seedorf et al. 2007; Di Matteo et al. 2008), revealing a conservation of the dimeric “head-to-tail” arrangement (even when the quaternary structure is a tetramer), as well as of the diiron ligand sphere.
Structural models of cyanobacterial flavodiiron proteins. a Structures of β-lactamase domains of a Class A FDP (Moorella thermoacetica FDP, PDB code 1YCG, in blue), and of a Class C Type 3 FDP (Anabaena sp. PCC7120 all0177, PDB code 3HNN, in green). The structures were aligned in Pymol (DeLano 2002) with an RMS of 1.364 Å for 185 aligned residues. b Models of the flavodiiron core of cyanobacterial FDPs (the C-terminal flavin reductase domains were removed from the modelled sequences). Two types of Synechocystis sp. PCC6803 FDPs were modelled using deposited FDPs structures as templates. sll550 (Type 1 FDP) was modelled using 1E5D, 1VME and 2OHH as templates (model in blue); sll1521 (Type 2 FDP) represented in red and orange, modelled using 2OHH as template. Each monomer is displayed either in cartoon or ribbon representation. c Superimposed modelled diiron site of sll550 (in blue) and of Moorella thermoacetica FDP (in red); iron ions as orange spheres. d Superimposition of the modelled diiron sites of sll1521 (Type 2), using different modelling strategies: blue, ligand substitutions modelled with structures of “canonical” Class A FDPs structures; yellow, the same “substituting” residues modelled with the structure of Anabaena sp. PCC7120 Type 3 FDP lactamase domain (PDB code 3HNN)
As this revision was being written, the structure of a truncated form of Anabaena sp. PCC7120 FDP encoded by all0177 (a Type 3 FDP), consisting solely of its lactamase-like domain was deposited in the Protein Data Bank (code 3HNN). The amino acids that act as iron ligands in “canonical”, Type 1, FDPs, are herein replaced in several positions (Table 22.2); furthermore, the structure model does not contain any metal ion. Whereas the overall fold of this truncated domain resembles the correspondent ones in the other FDPs (Fig. 22.5a), the structure of the diiron site pocket is markedly different (Fig. 22.5d). In this structure, the side chain of a lysine (K174, all0177 numbering, equivalent to K197 in Fig. 22.5d—see sequence alignment in Fig. 22.3), which replaces the bridging aspartate in Type 1 FDPs, protrudes into the space occupied by the iron ions in the other FDPs. Moreover, the number of basic residues, such as asparagine and arginine (that replace the “canonical” carboxylate ligands) crowding the pocket may be the reason why a phosphate ion is stabilized inside the cavity. Since this structure was obtained for a truncated domain, one has to take into account a possible destabilization of the overall structure, in spite of the apparently retention of the lactamase fold (as described above and shown in Fig. 22.5a). This raises the question whether iron is missing because of the nature of the “alternative ligands”, or due to any technical issue related with the protein production, purification or crystallization processes, which may trigger a rearrangement of the binding pocket. It should be noted that in several of the other types of enzymes (Types 2–8) the residue that replaces the bridging aspartate is preceded by another aspartate or by a glutamate, which may fulfil the same role, as will be discussed below. However, from the amino acid sequences comparison (see data on Table 22.2 and Fig. 22.3), it is clear that while some amino acid substitutions are conservative, in the sense that the amino acid substitute may bind to a metal ion through its side chain (even if representing less frequent modes of biological metal coordination), other amino acids may bind only through the backbone carbonyls, as the respective side chains are aliphatic (for example, the cases of isoleucine and alanine). Also, in many cases it is not possible to find in the sequences possible sensible amino acid substitutes, being the bridging aspartate a possible exception to this observation. These multiple amino acid substitutions raise the question whether some of those “unusual” FDPs are indeed metalloenzymes, which may be clarified only by appropriate experimental data. Of course, the possible absence of the iron ions will have unpredictable influences on the protein function.
Physiological studies on cyanobacterial FDPs have thus far focused on Synechocystis sp. PCC6803 enzymes (Vicente et al. 2002; Helman et al. 2003; Hackenberg et al. 2009; Zhang et al. 2009). This organism’s genome codes for four FDP homologues, two of which fall into Type 1 and the other two into Type 2 subgroups. Since there appears to be no difference in terms of physiological roles between the two types of FDPs (see Sect. 22.7), we attempted to predict the structures of one of each type using deposited PDB files as templates. Since Class C FDPs have the extra C-terminal flavin reductase domain, the sequences used for modelling the structure were previously truncated, in order to model only the flavodiiron core. The protein coded by gene sll0550 (a Type 1 enzyme) was modelled by combining the generated optimal structural alignments between sequence segments and several FDP structures (D. gigas ROO (1E5D), Thermotoga maritima FDP (1VME) and Methanothermobacter marburgensis F420H2 oxidase (2OHH)). The resulting model is thus an assembly of the best-fit models for different parts of the sll0550 sequence. The predicted structure (Fig. 22.5b) is quite similar to those of the “canonical” FDPs. Looking in detail at the binuclear site (Fig. 22.5c, red), it is observed that the conserved diiron ligands are modelled in the same positions and geometries as those of Moorella thermoacetica FDP (Fig. 22.5c, blue).
The Type 2 FDP, encoded by sll1521, was modelled by two different strategies. First, it was modelled using as template the structure of Methanothermobacter marburgensis F420H2 oxidase (2OHH). By this approach, it was possible to obtain a model where the residues substituting the “canonical” ligands display a geometry compatible with an iron coordination equivalent to Type 1 FDPs (Fig. 22.5d). Moreover, this modelling strategy resulted for sll1521 in a structure where the iron ions are present and the aspartate preceding the substituting lysine is placed in the same three dimensional position as the “canonical” aspartate. This model for sll1521 is thus compatible with a protein where the alternative amino acids indeed bind the iron ions. However, when we modelled sll1521 using the structure of the lactamase-like domain of Anabaena sp. PCC7120 Type 3 FDP (PDB code 3HNN) as a template, the structure of the diiron binding pocket changed dramatically, as expected (Fig. 22.5d). As already noted for the Anabaena truncated enzyme, the side chain of the lysine that substitutes in the sequence of all177 the bridging aspartate protrudes into the space occupied by iron in Type 1 FDPs. Moreover, the equivalent basic residues that substitute the Type 1 ligands are placed in the same position as those in the al177 structure. Therefore, the fact that both models are plausible leaves an open question, whether the metal ions that constitute the diiron site are present or not in the native sll1521 enzyme, and which would be the effect of their presence or absence on the protein’s function. As above mentioned, this question may be answered only with further structural and functional studies of the different types of FDPs.
5 Biochemical Properties
The FDPs thus far characterized are homodimers or homotetramers (a dimer of “head-to-tail” homodimers) in solution. Each monomer has ~45 kDa (Class A), ~54 kDa (Class B), and ~63 kDa (Class C) (no Class D FDP has been characterized), and contains two iron ions and one FMN per flavodiiron core, plus one iron per rubredoxin domain in Class B FDPs, and one flavin per flavin reductase domain in Class C FDPs. The latter extra domain is promiscuous in terms of flavin, being capable of harbouring FMN or FAD (Vicente et al. 2002). Due to the very low molar absortivity of the diiron centres, the electronic spectra of these enzymes are dominated by the flavin absorption, with maxima at ~460 and ~380 nm. In Class B enzymes, due to the presence of the FeCys4 centre the band at ~460 nm shifts to ~470 nm and an extra band appears at ~570 nm. Most Class A flavodiiron proteins thus far characterized displayed visible spectra which were broad and smooth in the band centred at ~460 nm. However, the spectra of a cyanobacterial FDP (Synechocystis sp. PCC6803 sll0550 (Vicente et al. 2002)) and of a methanogenic FDP (Wasserfallen et al. 1995) display this band with two shoulders. It has been noted that the FDPs with this kind of spectrum, lack a conserved tryptophan residue in the flavodoxin-like domain (Trp347 in D. gigas ROO numbering) which is coplanar with the FMN isoalloxazine ring. It was thus proposed that the presence/absence of this Trp residue was responsible for the heterogeneity in the spectral shape of the flavin moiety (Saraiva et al. 2004).
The diiron centre was first assessed by the structure of the D. gigas enzyme (Frazão et al. 2000). Later, it was studied in detail by EPR spectroscopy for the E. coli enzyme (Vicente and Teixeira 2005), and more recently for the Giardia and Thrichomonas enzymes (Vicente et al. 2009; Smutna et al. 2009). As characteristic of this type of iron centre, it can exist in three different oxidation states: diferric (Fe(III) Fe(III)), mixed valence (Fe(III) Fe(II)) and diferrous (Fe(II) Fe(II)). The diferric state is EPR silent, since the two iron ions (high-spin, S = 5/2) are antiferromagnetically coupled, yielding a total spin S = 0; for the E. coli enzyme a resonance at g ~ 11 for the diferrous state was detected, suggesting a S = 4 spin ground state. The mixed valence state has a total spin S = 1/2 and is EPR active, yielding a typical set of resonances below g = 2.0. The diiron centre of Moorella thermoacetica FDP was characterized by Mössbauer spectroscopy, confirming that the iron ions in the binuclear site are magnetically coupled (Silaghi-Dumitrescu et al. 2003).
The spectroscopic signatures from the several redox cofactors of FDPs were used to study their redox properties, combining Visible and EPR spectroscopies with potentiometric titrations. The flavodoxin domain-bound FMN cofactor of several FDPs displayed reduction potentials in the −224 to +25 mV range for the FMNoxàFMNsq transition and −117 to +25 mV range for the FMNsqàFMNred transition (Gomes et al. 1997; Silaghi-Dumitrescu et al. 2003; Vicente and Teixeira 2005; Vicente et al. 2009; Smutna et al. 2009). The majority of the FDPs studied have reduction potentials that are enough separated to allow transient stabilization of the one electron-reduced semiquinone (Sq) state, which so far is always of the red, anionic type. However, recent studies on protozoan FDPs reported very close reduction potentials for the two redox transitions of the flavin, suggesting that the semiquinone state is not stabilized in these enzymes. Notably, those enzymes are only able to efficiently reduce oxygen, whereas the other FDPs, which stabilize the semiquinone flavin state, are either only NO (Vicente and Teixeira 2005) or ambivalent NO/O2 reductases (Silaghi-Dumitrescu et al. 2003; Rodrigues et al. 2006). Until now, it has not been possible to propose a rationale for this difference in redox behaviour between O2-reducing and NO/O2-reducing FDPs, mainly because, at the available structural resolutions, the flavin-binding pockets are essentially identical among the several FDPs. Concerning the binuclear iron active site, the reduction potentials have been determined only for the E. coli flavorubredoxin (Vicente and Teixeira 2005) and for the protozoan FDPs (of Trichomonas vaginalis and Giardia intestinalis) (Di Matteo et al. 2008; Smutna et al. 2009), coupling redox potentiometry to EPR spectroscopy. The diiron sites of the protozoan enzymes have much higher reduction potentials than those of the E. coli FDP. However, whereas the reduction potentials in all three cases are compatible with oxygen or nitric oxide reduction, it is noteworthy that the two protozoan enzymes, that display only O2 reductase activity, have reduction potentials markedly different from those of the NO-reducing E. coli enzyme. As mentioned for the flavin cofactor, the available structures do not allow yet understanding the differences in redox properties of the diiron centers.
One interesting aspect of FDPs redox properties is the observation, for the E. coli FlRd, that the reduction potentials of the iron center are altered in the presence of the reductase partner, which strongly suggests the formation of an electron transfer complex between the two enzymes (Vicente and Teixeira 2005).
6 Enzymatic Studies
Enzymatic studies to assess the putative role of FDPs as oxygen or nitric oxide reductases have been performed in Clark-type electrodes specific for each molecule. In typical experiments, in vitro electron transfer chains were assembled to mediate, under non rate-limiting conditions, electron delivery from NAD(P)H to FDP and determine its reductase activity towards each substrate. Fast kinetics methods, namely stopped-flow coupled to Visible spectroscopy was also used to further confirm the steady-state activity of the Giardia enzyme (Di Matteo et al. 2008).
The first enzymatic studies on a member of this protein family, performed for D. gigas ROO, led to the proposal that FDPs would be involved in the detoxification of dioxygen, which is both an inhibitor of many enzymes from anaerobes and a source of deleterious reactive oxygen species. Following the initial characterization of the E. coli enzyme that was shown to be capable of binding nitric oxide (Gomes et al. 2000), Gardner and co-workers proposed that E. coli FlRd was involved in NO detoxification under anaerobic conditions (Gardner et al. 2002). This proposal led to the in vitro demonstration of nitric oxide reduction by FlRd, coupled to NADH oxidation by its physiological partner, the NADH:FlRd oxidoreductase, with a turnover of 15–20 s− 1 (Gomes et al. 2002). Since then, nitric oxide reductase activities of the same order of magnitude have been determined for several other FDPs (Gomes et al. 2002; Silaghi-Dumitrescu et al. 2003, 2005; Rodrigues et al. 2006). However, the data so far available indicate that the FDPs may have an ambivalent activity, i.e., some are able to reduce mainly NO, others mainly, or even exclusively, O2 (Di Matteo et al. 2008; Smutna et al. 2009), and there are few cases for which comparable activities with both substrates were measured (Silaghi-Dumitrescu et al. 2003, 2005; Rodrigues et al. 2006; Hillmann et al. 2009). Concerning Synechocystis sp. PCC6803, preliminary data obtained for Sll0550 revealed that the enzyme has a relatively low oxygen reductase activity, which could have been hampered by the sub-stoichiometric flavin load (Vicente et al. 2002). It is important to emphasize that the data available also suggest a sluggish reaction of the reduced enzymes with hydrogen peroxide, H2O2, and with several reactive nitrogen species (Di Matteo et al. 2008; Smutna et al. 2009 and our own unpublished data), i.e., the enzymes appear to favour NO and O2 as substrates. In this respect, it should be mentioned that the substrate binding cavity present in zinc β-lactamases is occluded in FDPS (e.g., Frazão et al. 2000). The dual oxygen/nitric oxide reducing activity raises the question whether both activities are physiologically relevant and what dictates the preference for each substrate. The evidences for the physiological roles of FDPs will be further discussed in the next section.
7 Physiological Roles of FDPs: FDPS as O2 and/or NO Reductases
The work by Gardner and co-workers first showed that FlRd protects E. coli against nitric oxide; deletion of the flrd gene highly compromised the growth viability of E. coli upon exposure to NO, under anaerobic conditions (Gardner et al. 2002). Furthermore, it was shown that flrd transcription is regulated by NO via NorR (Gardner et al. 2003), a transcriptional regulator that in enterobacteria is located immediately upstream, and divergently transcribed of the dicistronic unit that encodes both FlRd and its NADH oxidoreductase (da Costa et al. 2003). Saraiva and co-workers (Justino et al. 2005a) further showed that the binding of the NorR trimer to three sites of the flavorubredoxin gene promoter is required for nitric oxide-dependent induction of flrd. Several transcriptomic studies confirmed that flrd transcription is indeed up-regulated by NO (Mukhopadhyay et al. 2004; Flatley et al. 2005; Justino et al. 2005b; Pullan et al. 2006), including in E. coli cells grown under anaerobic conditions (Justino et al. 2005b). On the contrary, transcriptional studies performed for E. coli under several oxidative stress conditions did not reveal a significant alteration on the expression of the FlRd-encoding gene. These in vitro studies established a role for flavorubredoxin (a Class B FDP) in E. coli, as an enzyme responsive to the deleterious action of nitric oxide, namely under anaerobic conditions. Similar evidences were later on obtained for a few other bacteria, such as D. gigas (Rodrigues et al. 2006). But, in apparent agreement with the fact that the D. gigas enzyme has comparable NO and O2 reducing activities, ROO seems to be important also under oxidative stress conditions (Rodrigues et al. 2006). A similar situation was recently reported for Clostridium acetobutylicum, in which the flavodiiron proteins were shown to be up-regulated upon exposure of C. acetobutylicum to O2 and important to protect this anaerobic bacterium against oxygen (Hillmann et al. 2009).
For cyanobacteria, there is no data regarding the role of FDPs in response to nitrosative stress conditions; however, as will be described below, several experimental data clearly suggest a function for their FDPs in oxidative stress protection.
A survey of DNA microarray data so far reported for Synechocystis sp. showed that the genes encoding FDPs have their expression modified under certain conditions. The expression of the already mentioned gene cluster sll0217-0219 was found to be induced during Synechocystis acclimation from low- to high-light intensity (Hihara et al. 2001). The sll0217 gene is also among the 36 genes encoding potential FMN-containing proteins that were induced by UV-B light (Huang et al. 2002). The induction of the FDP encoding genes observed under high energy supply is possibly related to the higher generation of reactive oxygen species under these conditions; in fact, other genes encoding ROS scavenging enzymes, such as superoxide dismutase and glutathione peroxidase, were also up-regulated under those conditions (Huang et al. 2002). Chauvat and co-workers (Houot et al. 2007) reported that the transcription of the sll1521, sll0217 and sll0219 genes, encoding FDPs, increases in Synechocystis cells exposed to 3 mM hydrogen peroxide, or grown in the presence of excess of metals such as cadmium, iron and zinc. Nevertheless, the observed variations were low (2-4-fold) and contradictory data has been reported (Hihara et al. 2003; Kobayashi et al. 2004; Li et al. 2004). In summary, the DNA array data is still too scarce and somehow ambiguous. More specific and clarifying studies were performed also for Synechocystis sp. PCC6803, mainly based on the analysis of the behaviour of several single and multiple deletion mutants of its FDP genes under different growth conditions, namely illumination and carbon dioxide fluxes, and combinations of both these factors (Helman et al. 2003; Hackenberg et al. 2009; Zhang et al. 2009). It was concluded that sll1521 and sll0550 (enzymes of Types 2 and 1, respectively) were essential for the Mehler reaction, i.e., for electron transport from photosystem I to oxygen, without formation of reactive oxygen species. In the same report it was proposed that the two enzymes could assemble as a heterodimer, and that sll0550 could be sufficient to sustain photoreduction of dioxygen. More recently was studied in further detail the possible role of the other two FDPs, sll0217 and sll0219 (Zhang et al. 2009). The main conclusion was that both enzymes were important to protect photosystem II against oxidative stress, caused by conditions of high illumination intensity and/or low carbon dioxide fluxes. Interestingly, it was further shown that those FDPs are membrane associated, and that the expression of sll0217 was enhanced in the single deletion mutants of sll1521 or sll0550, i.e., it appears that sll0217 may substitute also for either of those two enzymes.
These proposals are in line with an oxygen reductase activity of the cyanobacterial enzymes, as first shown for the gene product of sll0550 (Vicente et al. 2002): the depletion of dioxygen by a NAD(P)H:oxygen oxidoreductase activity of the FDPs is fully consistent with their proposed roles in acting against oxidative stressing conditions, avoiding the formation of reactive oxygen species through the direct reduction of oxygen to water and enabling the elimination of excess reducing equivalents in the photosynthetic electron transfer chain. Nevertheless, these activities remain to be directly proven in vitro for the other three Synechocystis FDPs. This is particularly relevant, due to the fact that there is no apparent correlation between the proposed functions for the Synechocystis enzymes and their types, i.e., the presence or absence of the “canonical” ligands: sll1521 and sll0219 are enzymes of Type 2, lacking some of those ligands, while sll0550 and sll0217 are enzymes of the “canonical” Type 1.
8 Concluding Remarks
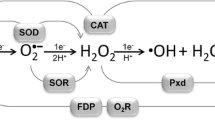
In summary, the flavodiiron proteins are a family of modular enzymes having nitric oxide and/or oxygen reductase activities. Both activities are supported either by in vitro experiments or by in vivo approaches, namely through transcriptional and phenotypic analysis of wild type and deletion strains. The abundance of homologues in cyanobacteria is particularly striking and may be related to the fact that oxygenic phototrophs are particularly prone to oxidative stress, due in special to the endogenous production of dioxygen and to the delicate balance between photonic energy supply and carbon dioxide availability. As flavodiiron enzymes appear to have evolved quite early, eventually before the split between Bacteria and Archaea, they may have been already present when oxygenic photosynthesis started, ca 3–3.5 billion years ago, as one of the first mechanisms to respond to the “oxygen paradox”, i.e., to avoid its intracellular toxicity. A similar scenario has been proposed for another type of diiron proteins, the rubrerythrins (Gomes et al. 2001), which more recently were proposed to act as hydrogen peroxide reductases, forming water directly through reduction of H2O2 (e.g., Kurtz 2006) and which are also quite spread among cyanobacteria (our unpublished observation). A schematic representation of the diverse mechanisms involved in oxidative stress protection in cyanobacteria is presented in Fig. 22.6, where it becomes clear the importance of having enzymes, such as FDPs, that avoid the production of reactive oxygen species, like the superoxide anion (detoxified by superoxide dismutases), or hydrogen peroxide (scavenged by catalases, peroxidases, rubrerythrins or ascorbate peroxidases). It has still to be shown if in cyanobacteria FDPs play also a role in nitrosative stress defence. But whatever the actual function of the several homologues of FDPs will be, their multiplicity suggests a crucial role in cyanobacteria.
Scheme illustrating oxidative stress and response mechanisms in cyanobacteria. Zoom-in into the thylakoid membranes in cyanobacteria and the cytoplasmatic space in between, where formation of reactive oxygen species and their breakdown by specialized enzymes are likely to take place. Oxygen is produced by water oxidation at the oxygen-evolving complex in Photosystem II (PS II, in dark green). Excess electrons in Photosystem I (PS I, in light green) generate superoxide anion from reaction with molecular oxygen; superoxide can self-dismutate to yield oxygen and hydrogen peroxide. Excess oxygen can be fully reduced to water by flavodiiron proteins (FDP, in orange), soluble and possibly also membrane-associated. Besides its self-dismutation, superoxide anion can be efficiently scavenged by superoxide dismutase (SOD, in light blue), both soluble and membrane-bound, whose products are O2 and H2O2. Hydrogen peroxide can be removed by at least three different enzymatic systems detected in the genomes of cyanobacteria: both ascorbate peroxidase (APX, in yellow, soluble and membrane-bound forms) and rubrerythrin (Rbr, in red) can reduce hydrogen peroxide to water; catalase (Kat, in darker blue) generates oxygen and water
Several challenging questions remain to be answered: one relates to the evolution of FDPs and their function in higher eukaryotes; the second, the unusual diversity of putative “ligands” to the diiron site—do all types of FDPs here described contain indeed a metal site? If yes, they will have quite unprecedented ligand combinations; if no, which will then be the function of the non-metal containing enzymes? Answers to these questions will bring new insights into the structure and function of diiron proteins and into the field of oxidative stress, and will further enlarge the knowledge on the physiology of oxygenic phototrophs.
References
Andersson JO, Sjogren AM, Davis LA, Embley TM and Roger AJ (2003) Phylogenetic analyses of diplomonad genes reveal frequent lateral gene transfers affecting eukaryotes. Curr Biol 13 (2): 94–104
Andersson JO, Hirt RP, Foster PG and Roger AJ (2006) Evolution of four gene families with patchy phylogenetic distributions: influx of genes into protist genomes. BMC Evol Biol 6: 27
Bodyl A, Mackiewicz P and Stiller JW (2007) The intracellular cyanobacteria of Paulinella chromatophora: endosymbionts or organelles? Trends Microbiol 15 (7): 295–296
Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UC, Besteiro S, Sicheritz-Ponten T, Noel CJ, Dacks JB, Foster PG, Simillion C, Van de Peer Y, Miranda-Saavedra D, Barton GJ, Westrop GD, Muller S, Dessi D, Fiori PL, Ren Q, Paulsen I, Zhang H, Bastida-Corcuera FD, Simoes-Barbosa A, Brown MT, Hayes RD, Mukherjee M, Okumura CY, Schneider R, Smith AJ, Vanacova S, Villalvazo M, Haas BJ, Pertea M, Feldblyum TV, Utterback TR, Shu CL, Osoegawa K, de Jong PJ, Hrdy I, Horvathova L, Zubacova Z, Dolezal P, Malik SB, Logsdon JM, Jr., Henze K, Gupta A, Wang CC, Dunne RL, Upcroft JA, Upcroft P, White O, Salzberg SL, Tang P, Chiu CH, Lee YS, Embley TM, Coombs GH, Mottram JC, Tachezy J, Fraser-Liggett CM and Johnson PJ (2007) Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315 (5809): 207–212
Chen L, Liu MY, Legall J, Fareleira P, Santos H and Xavier AV (1993a) Purification and characterization of an NADH-rubredoxin oxidoreductase involved in the utilization of oxygen by Desulfovibrio gigas. Eur J Biochem 216 (2): 443–448
Chen L, Liu MY, LeGall J, Fareleira P, Santos H and Xavier AV (1993b) Rubredoxin oxidase, a new flavo-hemo-protein, is the site of oxygen reduction to water by the “strict anaerobe” Desulfovibrio gigas. Biochem Biophys Res Commun 193 (1): 100–105
da Costa PN, Teixeira M and Saraiva LM (2003) Regulation of the flavorubredoxin nitric oxide reductase gene in Escherichia coli: nitrate repression, nitrite induction, and possible post-transcription control. FEMS Microbiol Lett 218 (2): 385–393
DeLano WL (2002) The PyMOL User’s Manual DeLano Scientific. Palo Alto, CA, USA
Di Matteo A, Scandurra FM, Testa F, Forte E, Sarti P, Brunori M and Giuffre A (2008) The O2-scavenging flavodiiron protein in the human parasite Giardia intestinalis. J Biol Chem 283 (7): 4061–4068
Fareleira P, Santos BS, Antonio C, Moradas-Ferreira P, LeGall J, Xavier AV and Santos H (2003) Response of a strict anaerobe to oxygen: survival strategies in Desulfovibrio gigas. Microbiology 149 (Pt 6): 1513–1522
Flatley J, Barrett J, Pullan ST, Hughes MN, Green J and Poole RK (2005) Transcriptional responses of Escherichia coli to S-nitrosoglutathione under defined chemostat conditions reveal major changes in methionine biosynthesis. J Biol Chem 280 (11): 10065–10072
Frazão C, Silva G, Gomes CM, Matias P, Coelho R, Sieker L, Macedo S, Liu MY, Oliveira S, Teixeira M, Xavier AV, Rodrigues-Pousada C, Carrondo MA and Le Gall J (2000) Structure of a dioxygen reduction enzyme from Desulfovibrio gigas. Nat Struct Biol 7 (11): 1041–1045
Gardner AM, Helmick RA and Gardner PR (2002) Flavorubredoxin, an inducible catalyst for nitric oxide reduction and detoxification in Escherichia coli. J Biol Chem 277 (10): 8172–8177
Gardner AM, Gessner CR and Gardner PR (2003) Regulation of the nitric oxide reduction operon (norRVW) in Escherichia coli. Role of NorR and sigma54 in the nitric oxide stress response. J Biol Chem 278 (12): 10081–10086
Gomes CM, Silva G, Oliveira S, LeGall J, Liu MY, Xavier AV, Rodrigues-Pousada C and Teixeira M (1997) Studies on the redox centers of the terminal oxidase from Desulfovibrio gigas and evidence for its interaction with rubredoxin. J Biol Chem 272 (36): 22502–22508
Gomes CM, Vicente JB, Wasserfallen A and Teixeira M (2000) Spectroscopic studies and characterization of a novel electron-transfer chain from Escherichia coli involving a flavorubredoxin and its flavoprotein reductase partner. Biochemistry 39 (51): 16230–16237
Gomes CM, Le Gall J, Xavier AV and Teixeira M (2001) Could a diiron-containing four-helix-bundle protein have been a primitive oxygen reductase? Chembiochem 2 (7–8): 583–587
Gomes CM, Giuffre A, Forte E, Vicente JB, Saraiva LM, Brunori M and Teixeira M (2002) A novel type of nitric-oxide reductase. Escherichia coli flavorubredoxin. J Biol Chem 277 (28): 25273–25276
Gould SB, Waller RF and McFadden GI (2008) Plastid evolution. Annu Rev Plant Biol 59 491–517
Hackenberg C, Engelhardt A, Matthijs HC, Wittink F, Bauwe H, Kaplan A and Hagemann M (2009) Photorespiratory 2-phosphoglycolate metabolism and photoreduction of O2 cooperate in high-light acclimation of Synechocystis sp. strain PCC6803. Planta 230 (4): 625–637
Helman Y, Tchernov D, Reinhold L, Shibata M, Ogawa T, Schwarz R, Ohad I and Kaplan A (2003) Genes encoding a-type flavoproteins are essential for photoreduction of O(2) in cyanobacteria. Curr Biol 13 (3): 230–235
Hihara Y, Kamei A, Kanehisa M, Kaplan A and Ikeuchi M (2001) DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13 (4): 793–806
Hihara Y, Sonoike K, Kanehisa M and Ikeuchi M (2003) DNA microarray analysis of redox-responsive genes in the genome of the cyanobacterium Synechocystis sp. strain PCC6803. J Bacteriol 185 (5): 1719–1725
Hillmann F, Riebe O, Fischer RJ, Mot A, Caranto JD, Kurtz DM, Jr. and Bahl H (2009) Reductive dioxygen scavenging by flavo-diiron proteins of Clostridium acetobutylicum. FEBS Lett 583 (1): 241–245
Houot L, Floutier M, Marteyn B, Michaut M, Picciocchi A, Legrain P, Aude JC, Cassier-Chauvat C and Chauvat F (2007) Cadmium triggers an integrated reprogramming of the metabolism of Synechocystis PCC6803, under the control of the Slr1738 regulator. BMC Genomics 8: 350
Huang L, McCluskey MP, Ni H and LaRossa RA (2002) Global gene expression profiles of the cyanobacterium Synechocystis sp. strain PCC6803 in response to irradiation with UV-B and white light. J Bacteriol 184 (24): 6845–6858
Huson DH, Richter CD, Rausch C, Dezulian T, Franz M and Rupp R (2007) Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinformatics 8: 460
Jones DT (1999) Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol 292 (2): 195–202
Justino MC, Goncalves VM and Saraiva LM (2005a) Binding of NorR to three DNA sites is essential for promoter activation of the flavorubredoxin gene, the nitric oxide reductase of Escherichia coli. Biochem Biophys Res Commun 328 (2): 540–544
Justino MC, Vicente JB, Teixeira M and Saraiva LM (2005b) New genes implicated in the protection of anaerobically grown Escherichia coli against nitric oxide. J Biol Chem 280 (4): 2636–2643
Kobayashi M, Ishizuka T, Katayama M, Kanehisa M, Bhattacharyya-Pakrasi M, Pakrasi HB and Ikeuchi M (2004) Response to oxidative stress involves a novel peroxiredoxin gene in the unicellular cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol 45 (3): 290–299
Kurtz DM Jr. (2006) Avoiding high-valent iron intermediates: superoxide reductase and rubrerythrin. J Inorg Biochem 100 (4): 679–693
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ and Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23 (21): 2947–2948
Lewis ME, Corker HA, Gollan B and Poole RK (2008) A survey of methods for the purification of microbial flavohemoglobins. Methods Enzymol 436: 169–186
Li H, Singh AK, McIntyre LM and Sherman LA (2004) Differential gene expression in response to hydrogen peroxide and the putative PerR regulon of Synechocystis sp. strain PCC6803. J Bacteriol 186 (11): 3331–3345
Mukhopadhyay P, Zheng M, Bedzyk LA, LaRossa RA and Storz G (2004) Prominent roles of the NorR and Fur regulators in the Escherichia coli transcriptional response to reactive nitrogen species. Proc Natl Acad Sci U S A 101 (3): 745–750
Petoukhov MV, Vicente JB, Crowley PB, Carrondo MA, Teixeira M and Svergun DI (2008) Quaternary structure of flavorubredoxin as revealed by synchrotron radiation small-angle X-ray scattering. Structure 16 (9): 1428–1436
Pullan ST, Gidley MA, Jones RA, Barrett J, Stevanin TM, Read RC, Green J and Poole RK (2006) Nitric Oxide in Chemostat-Cultured Escherichia coli is Sensed by Fnr and Other Global Regulators; Unaltered Methionine Biosynthesis Indicates Lack of S-Nitrosation. J Bacteriol 189 (5): 1845–55
Rodrigues R, Vicente JB, Felix R, Oliveira S, Teixeira M and Rodrigues-Pousada C (2006) Desulfovibrio gigas flavodiiron protein affords protection against nitrosative stress in vivo. J Bacteriol 188 (8): 2745–2751
Saraiva LM, Vicente JB and Teixeira M (2004) The role of the flavodiiron proteins in microbial nitric oxide detoxification. Adv Microb Physiol 49: 77–129
Seedorf H, Hagemeier CH, Shima S, Thauer RK, Warkentin E and Ermler U (2007) Structure of coenzyme F420H2 oxidase (FprA), a di-iron flavoprotein from methanogenic Archaea catalyzing the reduction of O2 to H2O. FEBS J 274 (6): 1588–1599
Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Shiba T, Ogasawara N, Hattori M, Kuhara S and Hayashi H (2002) Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci U S A 99 (2): 996–1001
Silaghi-Dumitrescu R, Coulter ED, Das A, Ljungdahl LG, Jameson GN, Huynh BH and Kurtz DM Jr. (2003) A flavodiiron protein and high molecular weight rubredoxin from Moorella thermoacetica with nitric oxide reductase activity. Biochemistry 42 (10): 2806–2815
Silaghi-Dumitrescu R, Kurtz DM Jr., Ljungdahl LG and Lanzilotta WN (2005) X-ray crystal structures of Moorella thermoacetica FprA. Novel diiron site structure and mechanistic insights into a scavenging nitric oxide reductase. Biochemistry 44 (17): 6492–6501
Smutna T, Goncalves VL, Saraiva LM, Tachezy J, Teixeira M and Hrdy I (2009) Flavodiiron protein from Trichomonas vaginalis hydrogenosomes: the terminal oxygen reductase. Eukaryot Cell 8 (1): 47–55
Vicente JB and Teixeira M (2005) Redox and spectroscopic properties of the Escherichia coli nitric oxide-detoxifying system involving flavorubredoxin and its NADH-oxidizing redox partner. J Biol Chem 280 (41): 34599–34608
Vicente JB, Gomes CM, Wasserfallen A and Teixeira M (2002) Module fusion in an A-type flavoprotein from the cyanobacterium Synechocystis condenses a multiple-component pathway in a single polypeptide chain. Biochem Biophys Res Commun 294 (1): 82–87
Vicente JB, Scandurra FM, Rodrigues JV, Brunori M, Sarti P, Teixeira M and Giuffre A (2007) Kinetics of electron transfer from NADH to the Escherichia coli nitric oxide reductase flavorubredoxin. FEBS J 274 (3): 677–686
Vicente JB, Carrondo MA, Teixeira M and Frazao C (2008a) Structural studies on flavodiiron proteins. Methods Enzymol 437B: 3–19
Vicente JB, Carrondo MA, Teixeira M and Frazão C (2008b) Flavodiiron proteins: nitric oxide and/or oxygen reductases. Handbook of Metalloproteins, Ed. Albrecht Messerschmidt, John Wiley & Sons, Vol. 4: 1–19
Vicente JB, Justino MC, Goncalves VL, Saraiva LM and Teixeira M (2008c) Biochemical, spectroscopic, and thermodynamic properties of flavodiiron proteins. Methods Enzymol 437B: 21–45
Vicente JB, Scandurra FM, Forte E, Brunori M, Sarti P, Teixeira M and Giuffre A (2008d) Kinetic characterization of the Escherichia coli nitric oxide reductase flavorubredoxin. Methods Enzymol 437B: 47–62
Vicente JB, Testa F, Mastronicola D, Forte E, Sarti P, Teixeira M and Giuffre A (2009) Redox properties of the oxygen-detoxifying flavodiiron protein from the human parasite Giardia intestinalis. Arch Biochem Biophys 488 (1): 9–13
Wasserfallen A, Huber K and Leisinger T (1995) Purification and structural characterization of a flavoprotein induced by iron limitation in Methanobacterium thermoautotrophicum Marburg. J Bacteriol 177 (9): 2436–2441
Wasserfallen A, Ragettli S, Jouanneau Y and Leisinger T (1998) A family of flavoproteins in the domains Archaea and Bacteria. Eur J Biochem 254 (2): 325–332
Yoon HS, Nakayama T, Reyes-Prieto A, Andersen RA, Boo SM, Ishida K and Bhattacharya D (2009) A single origin of the photosynthetic organelle in different Paulinella lineages. BMC Evol Biol 9: 98
Zhang P, Allahverdiyeva Y, Eisenhut M and Aro EM (2009) Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC6803. PLoS One 4 (4): e5331
Acknowledgments
Work by the authors discussed in this review was supported by projects from the Fundação para a Ciência e Tecnologia (to MT and LST). VLG and JVB are recipients of grants SFRH/BD/29428/2006 and SFRH/BPD/26895/2006. We would like to thank our collaborators in this field whose names appear in the appropriate references, and Dr. Bruno Victor (ITQB) for performing the homology model of the Synechocystis FDP.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Gonçalves, V.L., Vicente, J.B., Saraiva, L.M., Teixeira, M. (2011). Flavodiiron Proteins and Their Role in Cyanobacteria. In: Peschek, G., Obinger, C., Renger, G. (eds) Bioenergetic Processes of Cyanobacteria. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0388-9_22
Download citation
DOI: https://doi.org/10.1007/978-94-007-0388-9_22
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0352-0
Online ISBN: 978-94-007-0388-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)