Abstract
Monogonont rotifers are small, aquatic invertebrates capable of asexual and sexual reproduction. Sexual reproduction is required to produce diapausing eggs, which are able to survive adverse periods that typically occur every year. Their cyclically parthenogenetic life-cycle is believed to retain the advantages of recombination while minimizing the cost of sex. However, this life cycle is also thought to be unstable due to periodic loss of sexual reproduction by directional selection. Explaining the evolutionary dynamics of the monogonont rotifer life cycle is important for understanding how cyclical parthenogenesis is maintained, and for comparing monogononts with their close relatives, the bdelloid rotifers, which are ancient obligate asexuals. Our analysis clarifies that the cost of sex in monogononts is two-fold when compared to an obligate asexual lineage on an annual time-scale. However, when compared to an obligate sexual, cyclical parthenogens avoid the cost of sex in every parthenogenetic generation. In monogonont rotifers, where sexual reproduction is triggered by crowding, reproducible loss of sex has been reported in laboratory experiments. The mechanistic hypothesis is that some obligate asexual clones arise by spontaneous mutation, and they fail to respond to the sex triggering chemical signals produced by conspecifics. Hence, in these clones, asexual females never produce sexual daughters. Using a simple model, we show that as a result of this association of sex with dormancy, sex loss results in a huge short-term advantage, because sexual females only produce males or diapausing eggs, and do not contribute to current population growth. However, the requirement of sex for dormancy should result in a mid-term selection pressure to retain sex. It is this mid-term pressure that stabilizes cyclical parthenogenesis and allows it to persist. From this analysis, the periodic occurrence of obligate asexuals is predicted in monogonont rotifer populations, especially those with infrequent adverse periods.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
14.1 Introduction
Cyclical parthenogenesis is a life cycle combining asexual (parthenogenetic) and sexual reproduction. This life cycle is found in approximately 15,000 animal species (Hebert 1987) belonging to several taxa including aphids, cladocerans, and monogonont rotifers. It therefore has evolved independently several times. Cladocerans and rotifers are short-lived invertebrates commonly found in the zooplankton of ponds and lakes. In these habitats there is substantial temporal heterogeneity that is seasonal and/or unpredictable, conditions thought to favor cyclical parthenogenesis (Serra et al. 2003).
Rotifera is a phylum with a variety of life cycles regarding sex. The class Seisonidea reproduces by obligate sex, the class Bdelloidea reproduces by obligate parthenogenesis, and the class Monogononta – which is the taxon this chapter focuses on- reproduces by cyclical parthenogenesis (Wallace et al. 2006). Bdelloids are regarded as an “evolutionary scandal”, because they are ancient obligate asexuals and a diversified group (Maynard Smith 1986). How this long-term survival and relative evolutionary success was possible in the absence of sex requires explanation because it is counter to evolutionary theory and is the subject of a large ongoing research effort (e.g., Mark Welch et al. 2004a, b; see Chapter 13 in this book). The most parsimonious explanation is that bdelloids and monogononts evolved from a cyclically parthenogenetic ancestor, but bdelloids have lost sexual reproduction from their life cycle (Normark et al. 2003). Therefore, knowledge of the monogonont life cycle, genomic organization, and selection pressures on sexual reproduction is crucial to understand the evolutionary success of obligate asexuality in bdelloids.
Another feature of monogonont rotifers that makes them useful for examining the evolution of sex is the phenomenon of sex loss. A common observation is that Brachionus calyciflorus newly isolated from natural populations has a high frequency of sexual reproduction (Boraas 1983). However, after 20–30 generations in chemostat culture, sexual reproduction of the cyclically parthenogenetic life cycle is often eliminated (Fussmann et al. 2003). This ability to experimentally manipulate sex loss in monogonont rotifers makes them an excellent model for exploring the adaptive dynamics of this process.
Our objectives in this chapter are to examine the cost of sex in cyclically parthenogenetic life cycles, to investigate the mechanisms of sex loss in monogononts and the selective processes involved, and to explore the constraints on monogonont rotifers that limit the loss of sex.
14.2 The Monogonont Life Cycle
Monogonont rotifers are an evolutionarily successful group. There are approximately 1450 named species (Wallace et al. 2006). This number is likely a substantial underestimation, since molecular techniques are revealing the existence of many cryptic species (Gómez et al. 2002; Suatoni et al. 2006). Monogononts have colonized a diversity of aquatic and moist habitats; they are found in the plankton of fresh and brackish waters, in the soil, in mosses, and other habitats that are wet for more than a few days (Wallace et al. 2006). However, only a few species inhabit in the open sea, presumably because their vulnerability to predation. Monogonont rotifers, despite their small size (<2000 μm), are quite efficient grazers, with an important role in many aquatic food webs (e.g. Armengol et al. 2001).
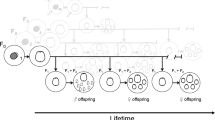
In monogonont populations, asexual (also called amictic) females produce asexual daughters by ameiotic (also called apomictic or amictic) parthenogenesis, so that the offspring is genetically identical to the mother in the absence of mutation (Fig. 14.1). This asexual reproduction can occur for an indefinite number of generations, causing clonal propagation. Episodically, in response to certain environmental cues such as population density and photoperiod (Gilbert 1963; Pourriot and Clément 1981), asexual females produce sexual daughters (also called mictic females). These sexual females produce meiotic eggs, which, if not fertilized, develop into haploid males (arrhenotoky). If these females are inseminated by a male, their fertilized eggs develop into cysts (diapausing or resting eggs) which undergo diapause. Diapausing eggs are resistant to adverse conditions such as drying and freezing, and can remain dormant in sediments for decades (Marcus et al. 1994; Kotani et al. 2001; Garcia-Roger et al. 2006). They are considered to be the main dispersal stage of monogononts. When diapausing eggs hatch, the asexual cycle is renewed as asexual females reproduce clonally until the next round of sexual reproduction. Some variation of this life cycle has been described in a few species, including eggs produced parthenogenetically that go into a short diapause (Gilbert 1995), females capable of producing both ameiotic and meiotic eggs (amphoteric females; e.g., King and Snell 1977), and sexual females hatched from diapausing eggs (Schröder et al. 2007). There are theoretical reasons to expect that half of sexual females will be male-producing, and half will be diapausing-egg producing. Fertilization rate of the sexual female eggs is controlled by the density of sexual females -which affects the density of males- and by the threshold age for fertilization. By developing the sex ratio theory for the monogonont life cycle, Aparici et al. (1998) showed that fertilization rate would evolve so that half of sexual females would not be inseminated before the threshold age, and so they will be male-producing, and half would be inseminated, and so they will produce diapausing eggs. There is some empirical evidence supporting these theoretical expectations (Aparici et al. 2002).
Monogonont rotifer life cycle. Asexual reproduction continues indefinitely, until environmental cues trigger the production of sexual females. If a young sexual female is inseminated and her haploid eggs fertilized, she produces diapausing eggs. If unfertilized, she produces haploid males. Processes are shown in italics
Most natural rotifer populations are temporary, inhabiting the water column of ponds and lakes for only a limited period of the year. Population growth typically begins when diapausing eggs in the sediment hatch. There is an initial phase of growth where the population is composed of exclusively asexual females. After a variable time period, sexual reproduction is triggered by environmental cues, and males and diapausing egg are produced, though asexual reproduction does not cease. The investment in sexual reproduction of a clone during a growing season can be described as a time series of the proportion of sexual daughters in the offspring of the asexual females (Serra et al. 2003). This time series has been called sexual reproduction pattern or mixis pattern. The pattern of sexual reproduction can be approximately described using two parameters: the timing of sexual reproduction initiation, and the sexual reproduction (mixis) ratio (i.e., the proportion of sexual daughters when sexual reproduction is initiated). In the genus Brachionus, where population density triggers sexual reproduction, the timing of sexual reproduction can be estimated by the threshold density for the sexual reproduction, a parameter that is easily quantified (Snell et al. 2006).
14.3 The Timing of Sex
Selection is expected to optimize sexual reproduction in rotifers from the trade-off in costs due to initiating sexual reproduction too early or too late (Snell 1987; Serra and Carmona 1993). If sex is early, population density would be low and diapausing egg production would be poor. The expected half-fertilization rate of sexual females might be unattainable because male-female encounters are unlikely. Moreover, investment in early sexual reproduction would waste the opportunity to achieve a larger population size by using up the available resources. Sex late in the season might also result in sex at low population size if growth rate has become negative due to environmental deterioration. This trade-off operating on the production of diapausing eggs has been well studied theoretically, and the optimal pattern of sexual reproduction has been related to different ecological scenarios (Serra et al. 2003). Either density-dependent growth or environmental uncertainty favor intermediate investment in sexual reproduction, while density-independent growth in predictable habitats favors a bang-bang pattern (early in the growing season all asexual reproduction, then all sexual reproduction).
The timing of sex affects not only the number of diapausing eggs produced, but also the amount of genetic diversity retained in the diapausing egg bank (Williams 1975; King, 1980). During the period of clonal propagation, natural selection acts on all the expressed genetic variance, either additive or non-additive, since the genome is inherited without recombination. Clonal selection typically leads to an erosion of clonal diversity, as it has been observed in a few studies (Gómez and Carvalho 2000; Ortells et al. 2006). As a result, populations with longer periods of parthenogenetic growth tend to contain lower genetic diversity (Ortells et al. 2006). Nevertheless, clonal selection on quantitative traits may cause selection of phenotypically similar clones, but having hidden genetic variance, which could be expressed after sexual recombination (Lynch and Deng 1994). If sexual reproduction pattern has an effect on genetic diversity and fitness, then this effect would be relevant to shaping the optimal timing of sex. However, the consequences of genetic diversity for the optimal timing of sex have yet to be explored, probably due to the difficulty of determining the optimal amount of genetic variance within diapausing eggs.
14.4 The Cost of Sex in Cyclically Parthenogenetic Life Cycles
Conventional wisdom on the monogonont life cycle states that it has evolved as an adaptation for fast population growth via parthenogenesis, in order to exploit ephemeral resource abundance without losing the advantages of sex. Cyclical parthenogenesis can be seen as combining the best of sexual and asexual reproduction, particularly because theoretical studies support the idea that a little sex is enough to fully provide all of the benefits of recombination (Peck and Waxman 2000). The paradox of sex -why sexual reproduction is so prevalent in the living world- is based on the assumption that sex has to compensate for its large cost when compared to asexual reproduction. The cost of sex in dioecious organisms is usually assumed to be two-fold (Maynard Smith 1978) because half of the offspring are typically males. Additionally, other costs are implied, such as those of maintaining the meiotic cytological mechanisms and the costs of mating activity. It is difficult to find a sufficiently large advantage for sex to compensate for such high costs. However, at least a small advantage is likely associated with recombination. Whichever the advantages of recombination, a little sex would secure these advantages while minimizing the cost of sex (Fig. 14.2). As a consequence, rather than a paradox of sex, the paradox is more accurately viewed as why cyclical parthenogenesis is so rare.
Hypothetical relationship between the cost and benefits of sex and the amount (frequency) of sex. It is assumed that a little sex provides most of its benefits (Peck and Waxman 2000), and that obligate asexuals compensate for the two-fold cost of sex (1). Cyclical parthenogenesis (partially sexual) maximizes the difference between benefits and costs (2), and hence is optimal. Sigmoid curve for the benefits is assumed, so that a little sex is necessary and sufficient to compensate the cost of sex
How much is the cost of sex in monogonont rotifers? A convenient way to address this question is to compare obligate asexuals, obligate sexuals and cyclical parthenogens, assuming that both obligate asexuals and obligate sexuals can produce non-diapausing and diapausing eggs, while in cyclical parthenogens sex is necessary for dormancy. In order to neutralize the effect of dormancy on population growth, we will assume that the pattern of producing diapause stages is the same for all three life cycles.
As monogonont populations are usually seasonal, and need to be re-established yearly from diapausing eggs, the annual production of diapausing eggs can be assumed to determine the fitness of a rotifer clone, all the other things being equal (Serra and King 1999). Assuming that half of the sexual females are diapausing egg producers, the cost of sex is two-fold because two-fold more diapausing eggs could be produced if they could be produced asexually. Therefore, when compared to an obligatory asexual lineage, cyclical parthenogenetic rotifers incur the two-fold cost on an annual time-scale. However, when compared to an obligatory sexual lineage where the two-fold cost of sex is incurred every generation, cyclical parthenogens avoid this cost every parthenogenetic generation. There is no paradox in this different computation for the sex cost, as one is computed for every sexual generation (from diapausing egg to diapausing egg) of a cyclical parthenogen, and the other cost is the sex cost per generation, averaged over both asexual and sexual generations.
Another way to understand the cost of sex in monogononts is to view partial or complete sex loss as a way to minimize the cost of sex. Under this scenario, whenever selection favoring dormancy is relaxed (i.e. in chemostats), the loss of sex rapidly follows (Boraas 1983; Fussmann et al. 2003). If selection for recombination is also weak, this loss of sex would be evolutionarily stable. Consequently, if we can identify environments where sex is consistently lost, it will provide insight into the selective pressures maintaining sex in the cyclically parthenogenetic monogonont life cycle.
14.5 Mechanisms of Sex Loss in Monogononts
Obligate asexuality has been observed in some natural populations of cladocerans (Colbourne and Hebert 1996; see also Chapter 15). For monogonont rotifers, many limnological studies, which do not focus on sex loss, report rotifer populations where males have never been observed. However, this could be due to inappropriate sampling frequency (Snell 1989) or to the fact that rotifer males are dwarf and difficult to identify (Ricci and Melone 1998).
Loss of sex has been demonstrated in experimental populations of monogonont rotifers. Boraas (1983) found that newly established cultures of Brachionus calyciflorus collected from the field produced 40% mictic (sexual) females when induced. After 2–3 months in a chemostat, that percentage was reduced to 0 in similarly inducing environments. He argued that the loss of sex was permanent, due to selection against sexual reproduction. This work has been repeated by Bennet and Boraas (1989) and more recently by Fussmann et al. (2003). Bennet and Boraas (1989) founded their cultures with a single female, and hence genetic variation causing sex loss had to arise during experimental culture. In other experiments not initiated with one female, genetic variation for investment in sex might be present in the founder populations. Nevertheless, the observed evolutionary dynamics did result in selection for sex loss. It could be argued that the loss of sex in these chemostat cultures is due to the evolution of an increased density threshold for sexual reproduction, and thus is reversible. Loss of sex has been found to be stable in strains used in different laboratories for years (Stelzer 2008). On the other hand, absence of sex has been observed at densities much higher than those observed in natural rotifer populations, making loss of sex evolutionarily permanent.
These experiments show that rotifers are good models for investigating sex loss because the level of sex can be manipulated experimentally (Snell and Boyer 1988; Stelzer and Snell 2003; Snell et al. 2006). Also, experimental selection can reproducibly produce obligate parthenogens in laboratory chemostats in a few months (20–30 generations). It furthermore shows that in rotifers evolutionary and ecological time scales overlap (Fussmann et al. 2003; Yoshida et al. 2003).
In some cladocerans, such as Daphnia pulex, obligate asexuality is caused by a meiosis suppressor gene, which is expressed only in females (Innes and Hebert 1988; Crease et al. 1989). This gene spreads because males are produced by obligate asexual clones, and these males copulate with females belonging to cyclically parthenogenetic clones. Interestingly, while this “contagious” process is going on, asexuals are capturing genetic variance from their sexual conspecifics (Simon et al. 2003). Since in monogonont rotifers meiosis only occurs in females -males are haploids-, a sex-dependent meiosis suppressor gene is not possible, and obligate asexuality cannot spread as in cladocerans.
Brachionid rotifers obtain information about their current population density by sensing one or more chemicals produced by the rotifers themselves in a process analogous to quorum sensing in bacteria (Miller and Bassler 2001; Kubanek and Snell 2008). These compounds accumulate in the medium as population density increases to a threshold that triggers the switch from asexual to sexual reproduction; the threshold is about 70 rotifers per liter in B. plicatilis (Snell et al. 2006). There is extensive variation among clones from the same population in sexual response as well as among geographical isolates (Gilbert 2003; Stelzer and Snell 2006). Our current model of sex loss envisions mutations appearing somewhere in the mixis signal transduction pathway. This pathway probably includes genes involved in mixis signal production, signal reception, G-protein signaling, intracellular messengers, and transcription factors. None of these genes and proteins has been characterized from rotifers, but such pathways are known to be involved in the chemosensory mating behavior of Drosophila (Bray and Amrein 2003). Stelzer (2008) performed cross-induction experiments using both cyclically parthenogenetic strains and obligate asexual strains of B. calyciflorus. He found that conditioned medium from asexual strains was able to elicit sex in cyclically parthenogenetic strains, but not reciprocally in obligately asexual strains. This finding supports the hypothesis that asexual strains produce the mixis signal but do not respond to it.
Loss of sex by becoming unresponsive to the mixis signal has the added advantage of truncating the sex response at the first step, so that no energy is wasted on a failed sexual attempt. A single gene mutation could eliminate the ability of the mixis signal to bind to its receptor (Snell et al. 2006). It is known that some Brachionus species have delayed responsiveness to the mixis signal for several generations after diapause egg hatching (Gilbert 2002). A reversible physiological mechanism must exist capable of operating for several generations to block responsiveness to mixis signal. It is therefore easy to imagine a mutant capable of blocking mixis responsiveness indefinitely. There also is species-specificity in the response, so that signals from closely related B. calyciflorus species are sufficiently different so that there is little cross-reactivity (Gilbert 2003). It may be easy for rotifers to evolve a new reaction norm to mixis signals through changes in the receptor. However, Stelzer and Snell (2006) found that crowding by several putative species from the B. plicatilis species complex were capable of cross-inducing mixis. More work is needed on the selection pressures shaping the species-specificity of mixis signals among closely related species to understand when to expect cross-induction and when to expect differentiation.
14.6 Selection for Sex Loss
The rarity of cyclical parthenogenesis in nature could be due to the possible instability of such a life cycle (Simon et al. 2002). Following this idea, cyclical parthenogenesis is inherently unstable because the transition to obligate asexuality does not imply the acquisition of a new function, but only the loss of the sexual function. The rationale behind this argument can be better understood if the evolutionary dynamics of obligate sexual and obligate asexual counterparts is analyzed in a hypothetical species. If passing from obligate sexuality to obligate asexuality is difficult, but not impossible, asexuality could invade some populations due to its short-term advantage (i.e., the two-fold cost of sex). However, other populations would remain obligately sexual. These obligate sexual populations would have higher survival chances than obligate asexual populations, due to the higher capability of the former to adapt to environmental change and/or to purge deleterious mutations. As a result, most of the observed species will be sexual. In some sense, the unlikely transition from sex to asexuality makes similar the time scales of mutation and selection, both being long-term. This makes selection an efficient factor in maintaining sex. However, in cyclical parthenogens, the transition to asexuality would be much easier than in obligate sexuals. Therefore, they would become obligate asexuals due to the cost of sex, and consequently become more prone to extinction (but see the discussion below on the bdelloid case).
In order to clarify this issue, it is useful to analyze sex costs within an annual growing season of a cyclical parthenogen as compared to an obligate asexual when the latter is unable to produce diapausing eggs. This comparison is interesting because we imagine that this type of obligate asexual reproduction often arises in natural rotifer populations through selection for sex loss as described above. We will call this cost the within-growing-season cost of sex in cyclical parthenogens. We assume that when obligate asexuals appear, they produce only asexual daughters, with no recruitment from diapausing eggs. Under our assumptions, the within-growing-season cost of sex is the proportion of sexual daughters in the offspring of the asexual females belonging to the cyclical parthenogenetic lineage. Sexual daughters of cyclically parthenogenetic rotifers do not contribute to the current population growth, as they will only produce either males or diapausing eggs. By contrast, all of the daughters of the obligate asexual clone contribute to the current growth. Fig. 14.3 shows the dynamics for a simulated population composed of cyclical parthenogens and obligate asexuals, and for a simultated population of exclusively cyclical parthenogens. Notice that within-growing-season costs of sex in monogononts as stated here are due to the association between sex and dormancy. However, when a period of adverse conditions occurs, the production of resistant, diapausing eggs is required and it imposes a short-term (annual) selection pressure for the maintenance of sex, similar to what occurs in other cyclical parthenogens (Simon et al. 2002). Therefore, in an annual cycle of a seasonal population, we expect to observe fluctuating selection for sex. Early in the growing season, before sex is induced in cyclical parthenogens, both the cyclical parthenogens and the newly mutated obligate asexual lineages would have no cost of sex. After sex induction, cyclical parthenogens would be selected against due to its within-growing-season cost. In contrast, only dormant stages survive adverse environments, so obligate asexuals would have zero fitness when the water column becomes uninhabitable. Nevertheless, cyclical parthenogens might enjoy short-term benefits associated with recombination, which would compensate at least in part for the within-growing season cost of sex. For instance, genetic recombination might cause the production of some F1 clones with low mutational load, or decreased interclonal competition due to their diversity, as found in experimental populations of cladocerans (Tagg et al. 2005).
Hypothetical dynamics of monogonont rotifer populations. Upper panel: Population composed of cyclical parthenogens (initial frequency: 99.99%) and an obligatorily asexual invader (initial frequency: 0.01%). Lower panel: Monomorphic cyclically parthenogenetic population. Curves were produced by simulating the following model (modified from Serra and King 1999): \({\rm{dA/dt = b}}\left( {\rm{N}} \right)\left( {1 - {\rm{m}}\left( {\rm{N}} \right)} \right){\rm{A}} - {\rm{q A,}}\) \({\rm{dM/dt = b}}\left( {\rm{N}} \right){\rm{ m}}\left( {\rm{N}} \right)\,\,{\rm{A}} - {\rm{q M,}}\) \({\rm{dO/dt = b}}\left( {\rm{N}} \right) - {\rm{q O,}}\)where A, M and O are respectively the densities of cyclical parthenogenetic asexual females, cyclical parthenogenetic sexual females, and obligatorily asexual females, q is the mortality rate (assumed to be 0.4 d–1), N is the total density (i.e., A + M + O; initial N = 1 L–1), b(N) is the birth rate, and m(N) is the proportion of asexual females, both assumed to be density-dependent. b was assumed to be bmax – (bmax – q)(N/K), where bmax is the maximum birth rate (0.5 d–1) and K is the carrying capacity (200 L–1). m(N) was assumed to be 0 if N < 70 L−1) and 0.05 otherwise. Parameter values are conservative ones obtained from the literature (see Snell et al. 1998)
The pattern of selection observed in chemostats (Boraas 1983; Fussmann et al. 2003) may be duplicated in nature. Cyclical parthenogens probably dominate in seasonal environments (e.g. temperate lakes) where dormancy is required to survive the winter. However, obligate asexuals could be favored in regions where diapausing egg production is not necessary (tropics, permanent habitats). Unnoticed coexistence of cyclical parthenogens and obligate asexuals might be common in natural rotifer populations. More than 30% of aphid species are polymorphic mixes of cyclical parthenogens and obligate asexuals (Moran 1992). Rotifer populations commonly number in the billions of individuals, so most monogonont populations probably rapidly generate new obligate asexual mutants soon after diapausing egg hatching. Therefore, even in non-permanent populations, the frequency of clones with no or low investment in sex is expected to increase during the growing season due to clonal selection. The longer the period, in which cyclical parthenogens engage in sex, the higher the probability of observing asexual clones. Specific ecological conditions promoting long periods of sexual reproduction in cyclical parthenogens are (1) density-dependent population growth, and (2) a large variance in the length of the rotifer growing season (Serra and King 1999).
14.7 Dormancy and Sex
According to the fluctuating selection scheme for sex loss outlined above, the linkage between dormancy and sex promotes the maintenance of sex through short-term selection favoring dormancy (Simon et al. 2002). This linkage reduces the disadvantage of sex (the two-fold cost) by providing a correlated advantage (dormancy). The linkage does not need to be absolute, it only needs to be unlikely to be broken. Similarly to the obligate sexual-asexual dynamics described above, the long time scale for the dissociation between sex and dormancy could be equivalent to the time scale for the long-term advantages of recombination.
This argument prompts a crucial question: Why are dormancy and sex linked? The association could have arisen by chance, as a fortunate constraint allowing the evolution of a life cycle of cyclical parthenogenesis. More likely, it seems that when two types of reproduction are available (sexual and asexual), sexual reproduction tends to be associated with dispersal in time or space. This applies, for instance, to vegetative reproduction and seed production in plants. Williams (1975) stressed that natural selection would favor a correlation between parent-offspring genetic similarity and parent-offspring environmental similarity, as a cause to expect association between sex and dormancy in his rotifer-aphid model. In fact, the association between dormancy and sex makes it difficult to ignore a role for ecology in the evolution of sex.
Bdelloid rotifers have managed to uncouple dormancy and sex. Instead of producing diapausing eggs through fertilization like monogononts, bdelloids undergo anhydrobiosis where adults desiccate and can be revived after 20 or more years of dormancy (Caprioli and Ricci 2001; see also Chapter 13). An individual bdelloid rotifer can become anhydrobiotic in minutes without reproduction. Likewise, revival from an anhydrobiotic state is rapid and independent of reproduction. Uncoupling dormancy and sex has eliminated the correlated advantage of sex and it has been lost in bdelloids. In fact, bdelloid rotifers have done without sex for millions of years (Mark Welch and Meselson 2000, 2001) and they are arguably the most successful animal group of ancient obligate asexuals. The bdelloid case illustrates the delicate balance of selective forces maintaining sex in populations of cyclical parthenogens. Loss of a short-term correlated advantage like dormancy can lead to sex loss which can be stable over evolutionary time. The challenge is to explain the adaptations in the bdelloid genome that have allowed this group to continue to evolve at rates sufficient to avoid extinction in the absence of recombination.
References
Aparici E, Carmona MJ, Serra M (1998) Sex allocation in haplodiploid cyclical parthenogens with density-dependent proportion of males. Am Nat 152: 652–657
Aparici E, Carmona MJ, Serra M (2002) Evidence for an even sex allocation in haplodiploid cyclical parthenogens. J Evol Biol 15:65–73
Armengol X, Boronat L, Camacho A, Wurtsbaugh W (2001) Grazing by a dominant rotifer Conochilus unicornis Rousselet in a mountain lake: In situ measurements with synthetic microspheres. Hydrobiologia 446/447: 107–114
Bennet WN, Boraas ME (1989) A demographic profile of the fastest growing metazoan – a strain of Brachionus calyciflorus (Rotifera). Oikos 55: 365–369
Boraas ME (1983) Population dynamics of food-limited rotifers in two-stage chemostat culture. Limnol Oceanogr 28: 546–563
Bray S, Amrein H (2003) A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron 39: 1019–1029
Caprioli M, Ricci C (2001) Recipies for successful aanhydrobiosis in bdelloid rotifers. Hydrobiologia 446/447: 13–17
Colbourne JK, Hebert PDN (1996) The systematics of North American Daphnia (Crustacea: Anomopoda): a molecular phylogenetic approach. Philos Trans R Soc Lond 351: 349–360
Crease TJ, Stanton DJ, Hebert PDN (1989) Polyphyletic origins of asexuality in Daphnia pulex II. Mitochondrial-DNA variation. Evolution 43: 1016–1026
Fussmann GF, Ellner SP, Hairston NG Jr (2003) Evolution as a critical component of plankton dynamics. Proc R Soc Lond B 270: 1015–1022
Garcia-Roger EM, Carmona MJ, Serra M (2006) Patterns in rotifer diapausing egg banks: density and viability. J Exp Mar Biol Ecol 336: 198–210
Gilbert JJ (1963) Mictic female production in the rotifer Brachionus calyciflorus. J Exp Zool 153: 113–124
Gilbert JJ (1995) Structure, development and induction of a new diapause stage in rotifers. Freshw Biol 34: 263–270
Gilbert JJ (2002) Endogenous regulation of environmentally induced sexuality in a rotifer: a multigenerational parental effect induced by fertilisation. Freshw Biol 47: 1633–1641
Gilbert JJ (2003) Specificity of crowding response that induces sexuality in the rotifer Brachionus. Limnol Oceanogr 48: 1297–1303
Gómez A, Carvalho GR (2000) Sex, parthenogenesis and genetic structure of rotifers: microsatellite analysis of contemporary and resting egg bank populations. Mol Ecol 9: 203–214
Gómez A, Serra M, Carvalho GR, Lundt DH (2002) Speciation in ancient cryptic species complexes: evidence from the molecular phylogeny of Brachionus plicatilis (Rotifera). Evolution 56: 1431–1445
Hebert PDN (1987) Genotypic characteristics of cyclic parthenogens and their obligately asexual derivatives. In: Stearns SC (ed) The evolution of sex and its consequences. Birkhauser Verlag, Basel and Boston, pp. 175–195
Innes DJ, Hebert PDN (1988) The origin and genetic basis of obligate parthenogenesis in Daphnia pulex. Evolution 42: 1024–1035
King CE (1980) The genetic structure of zooplankton populations. In: Kerfoot WC (ed). Evolution and ecology of zooplankton communities. University Press of New England, Hanover, pp. 315–329
King CE, Snell TW (1977) Genetic basis of amphoteric reproduction in rotifers. Heredity 39: 361–364
Kotani T, Ozaki M, Matsuoka K, Snell TW, Hagiwara A (2001) Reproductive isolation among geographically and temporally isolated marine Brachionus strains. Hydrobiologia 446/447: 283–290
Kubanek J, Snell TW (2008) Quorum sensing in rotifers. In: Winans SC, Bassler BL (eds). Chemical communication among bacteria. ASM Press, Washington DC, pp. 453–461
Lynch M, Deng HW (1994) Genetic slippage in response to sex. Am Nat 144: 242–261
Marcus NH, Lutz R, Burnett W, Cable P (1994) Age, viability, and vertical distribution of zooplankton resting eggs from an anoxic basin: evidence of an egg bank. Limnol Oceanogr 39: 154–158
Mark Welch DB, Cummings MP, Hillis DM, Meselson M (2004a) When do gene copies in the asexual class Bdelloidea diverge? Proc Natl Acad Sci USA 101: 1622–1625
Mark Welch DB, Meselson M (2000) Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288: 1211–1215
Mark Welch DB, Meselson M (2001) Rates of nucleotide substitution in sexual and ancient asexual rotifers. Proc Natl Acad Sci USA 98: 6720–6724
Mark Welch, JL, Mark Welch DB, Meselson M (2004b) Cytogenetic evidence for asexual evolution of bdelloid rotifers. Proc Natl Acad Sci USA 101: 1618–1621
Maynard Smith J (1978) The evolution of sex. Cambridge University Press, Cambridge, UK
Maynard Smith J (1986) Contemplating life without sex. Nature 324: 300–301
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55: 165–199
Moran NA (1992) The evolution of aphid life cycles. Annu Rev Entomol 27: 321–348
Normark BB, Judson OP, Moran NA (2003) Genomic signatures of ancient asexual lineages. Biol J Linnean Soc 79: 69–84
Ortells R, Gomez A, Serra M (2006) Effects of duration of the planktonic phase on rotifer genetic diversity. Arch Hydrobiol 167: 203–216
Peck J, Waxman D (2000) What’s wrong with a little sex? J Evol Biol 13: 63–69
Pourriot R, Clément P (1981) Action de facteurs externes sur la reproduction et le cycle reproducteur des Rotifers. Acta Oecologica Generale 2: 135–151
Ricci C, Melone G (1998) Dwarf males in monogonont rotifers. Aquatic Ecol 32: 361–365
Schröder T, Howard S, Arroyo ML, Walsh EJ (2007) Sexual reproduction and diapause of Hexarthra sp (Rotifera) in short-lived ponds in the Chihuahuan Desert. Freshw Biol 52: 1033–1042
Serra M, Carmona MJ (1993) Mixis strategies and resting egg production of rotifers living in temporally-varying habitats. Hydrobiologia 255–256: 117–126
Serra M, King CE (1999) Optimal rates of bisexual reproduction in cyclical parthenogens with density-dependent growth. J Evol Biol 12: 263–271.
Serra M, Snell TW, King CE (2003) The timing of sex in cyclically parthenogenetic rotifers. In: Moya A, Font E (eds) Evolution: from molecules to ecosystems. Oxford University Press, New York, pp. 135–146.
Simon JC, Delmotte F, Rispe C, Crease T (2003) Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biol J Linn Soc 79: 151–163
Simon JC, Rispe C, Sunnucks P (2002) Ecology and evolution of sex in aphids. Trends Ecol Evol 17: 34–39
Snell TW (1987) Sex, population dynamics and resting egg production in rotifers. Hydrobiologia 144: 105–111
Snell TW (1989) Systematics, reproductive isolation and species boundaries in monogonont rotifers. Hydrobiologia 186/187: 299–310
Snell TW, Boyer EM (1988) Thresholds for mictic female production in the rotifer Brachionus plicatilis (Muller). J Exp Mar Biol Ecol 124: 73–85
Snell TW, Kubanek J, Carter J, Payne AB, Kim J, Hicks MK, Stelzer CP (2006) A protein signal triggers sexual reproduction in Brachionus plicatilis (Rotifera). Mar Biol 149: 763–773
Snell TW, Serra M, Carmona MJ (1998) Toxicity and sexual reproduction in rotifers: reduced resting egg production and heterozygosity loss. In: Forbes VE (ed) Genetics and Ecotoxicology. Taylor and Francis, London, pp. 169–185
Stelzer CP (2008) Obligate asexuality in a rotifer and the role of sexual signals. J Evol Biol 21: 287–293
Stelzer CP, Snell TW (2003) Induction of sexual reproduction in Brachionus plicatilis (Monogononta, Rotifera) by low density-dependent chemical cue. Limnol Oceanogr 48: 939–943
Stelzer CP, Snell TW (2006) Specificity of the crowding response in the Brachionus plicatilis species complex. Limnol Oceanogr 51: 125–130
Suatoni E, Vicario S, Rice S, Snell TW, Caccone A (2006) An analysis of species boundaries and biogeographic patterns in a cryptic species complex: the rotifer Brachionus plicatilis. Mol Phyl Evol 41: 86–98
Tagg N, Doncaster CP, Innes DJ (2005) Outcomes of reciprocal invasions between genetically diverse and genetically uniform populations of Daphnia obtusa (Kurz). Oecologia 143: 527–536
Wallace RL, Snell TW, Ricci C, Nogrady T (2006) Rotifera 1: Biology, Ecology and Systematics. Backhuys Publishers, Leiden
Williams GC (1975) Sex and Evolution. Princeton University Press, Princeton
Yoshida T, Jones LE, Ellner S, Fussmann GF, Hairston NG Jr (2003) Rapid evolution drives ecological dynamics in a predator-prey system. Nature 424: 303–306
Acknowledgements
We thank Maria José Carmona for valuable comments that improved this paper. Two anonymous reviewers made useful comments that we incorporated into this chapter. This work was supported by the National Science Foundation grants BE/GenEn MCB-0412674 to TWS and by the Spanish Ministry of Education and Science grants CLG2006-27069-E/BOS and BOS2003-0075 to MS.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Serra, M., Snell, T.W. (2009). Sex Loss in Monogonont Rotifers. In: Schön, I., Martens, K., Dijk, P. (eds) Lost Sex. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-2770-2_14
Download citation
DOI: https://doi.org/10.1007/978-90-481-2770-2_14
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-2769-6
Online ISBN: 978-90-481-2770-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)