Abstract
Sex poses an immediate cost for monogonont rotifers, which combine exclusively asexual reproduction with periods of co-occurrence between asexual and sexual reproduction in their life cycle. Because sex is linked to dormancy, sexual daughters do not contribute to the current growth of a clonal genotype, as they only produce males or diapausing eggs. Therefore, the expectation under the all-else equal assumption (i.e., sexual and asexual females having equivalent life-history traits) is that female genotypes investing more in sexual daughters during the planktonic growing season will have slower rates of clonal growth. Here, we tested if these genotypes compensate for this greater investment in sex with higher fecundity and/or survival. We studied 45 genotypes (clones) of Brachionus plicatilis established from diapausing eggs isolated from sediments of ten brackish ponds in Spain. Using a life-table experiment, we estimated the investment in sexual reproduction of these genotypes and several life-history traits (lifespan, lifetime reproductive success, generation time and intrinsic growth rate). Results showed that there was a lack of correlation between sex-investment and any other life-history trait. Neither fecundity nor survival compensated the investment in sexual reproduction, so we conclude that the all-else-equal assumption holds and sex is costly in B. plicatilis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most interesting and enduring problems in evolutionary biology is the maintenance of sexual reproduction (Maynard-Smith, 1971, 1978; Williams, 1975; Bell, 1982; West et al., 1999; Keightley & Otto, 2006; Roze & Barton, 2006). It constitutes an evolutionary paradox because sex is predicted to be costly in evolutionary terms, but at the same time ubiquitous in nature (Maynard-Smith, 1971; Williams, 1975; Butlin, 2002; Simon et al., 2002; Lehtonen et al., 2012; Meirmans et al., 2012; Stelzer, 2015). Since sexual reproduction implies male production, all else being equal, asexual populations have a twofold fitness advantage over their sexual counterparts (Maynard-Smith, 1971, 1978; Williams, 1975; Bell, 1982). The reason is that while an asexual female invests their entire reproductive budget into clonal daughters, sexual females must spend half of their resources in sons, which cannot themselves make offspring. Therefore, in a two offspring per individual situation, the asexual lineage will increase exponentially while the sexual one will remain constant. This so-called “twofold cost of sex (or of males)” (Maynard-Smith, 1971, 1978) has been considered one of the main costs of sex (Lehtonen et al., 2012). Consequently, evolutionary biologists have sought advantages for sexual reproduction that could compensate the twofold cost and explain sex persistence (e.g., West et al., 1999; Burt, 2000; Otto & Lenormand, 2002; Rice, 2002; Hadany & Comeron, 2008; Otto, 2009; Lively & Morran, 2014). This body of theories suggests that sex reduces the accumulation of deleterious allele combinations, promotes the fixation of beneficial mutations, generates variation that facilitates adaptation, and improves evolvability. On the other hand, another approximation to address the maintenance of sexual reproduction has been to look for violations of the all-else-equal assumption (i.e., that sexual and asexual females have equivalent life-history traits; Jokela et al., 1997; Gibson et al., 2017). For instance, asexual females have been reported to have lower lifetime reproductive success or higher mortality rate than sexual females, implying a smaller cost than the one predicted by the all-else-equal assumption (e.g., Roth, 1974; Lamb & Willey, 1979; Wetherington et al., 1987; Corley & Moore, 1999; Kramer & Templeton, 2001; Neiman 2004; Kearney & Shine, 2005). However, the basis of these differences among reproductive types is poorly understood (Lehtonen et al., 2012), empirical studies suggesting as explanatory causes developmental constrains associated with asexual reproduction, divergent selective pressures on sexual and asexual linages resulting in different reproductive strategies, susceptibility to deleterious mutation accumulation and the inability to generate genetically rare or novel offspring of asexuals. It should be borne in mind that these explanatory mechanisms are dependent on the system studied.
Measuring directly the cost of sex is a challenge (Gibson et al., 2017), which is why most of the available empirical studies have indirectly addressed the question by testing the all-else-equal assumption (reviewed in Lehtonen et al., 2012; Meirmans et al., 2012; Stelzer, 2015); that is, testing whether life-history traits differ between sexual and asexual females. Therefore, among the different definitions of the cost of sex (Lehtonen et al., 2012), this will be the approach used in the present study.
Monogonont rotifers combine clonal reproduction with bouts of sexual reproduction in their life cycle, namely, cyclical parthenogenesis (Birky & Gilbert, 1971; Wallace & Smith, 2009). In most cyclically parthenogenetic rotifers, sexual reproduction is needed for diapausing-egg production, which is essential to survive unsuitable conditions in the water column, this production being a major component of fitness in these rotifers (Serra & King, 1999). Sexual reproduction requires the production of sexual (mictic) females that do not contribute to the immediate population growth, as they produce males or diapausing eggs. Under the all-else-equal assumption, monogononts are predicted to incur the twofold cost of sex in the sexual phase of their life cycle (Serra & Snell, 2009). This would occur as a result of the expectation that only half of the sexual females will produce diapausing eggs (Aparici et al., 1998, 2002). However, this expected cost would be diluted as an effect of the several generations of asexual reproduction; that is, when the complete lifecycle is considered (for a detailed discussion see Serra & Snell, 2009).
Cyclically parthenogenetic organisms are useful for assessing the cost of sex assumption because they provide a system in which both modes of reproduction coexist in the same population (Green & Noakes, 1995; Simon et al., 2002; Wolinska & Lively, 2008; Stelzer, 2015). Moreover, in many cyclical parthenogens, recent transitions to obligate asexuality allow for comparison between them and their derived obligate parthenogenetic lineages (reviewed e.g., Lynch 1984; Decaestecker et al., 2009; Serra & Snell, 2009; Stelzer, 2011, 2015). However, in contrast to the substantial theoretical development, empirical studies on the cost of sex in cyclical parthenogenetic organisms are scarce. Several empirical studies have tested the all-else-equal assumption by comparing life-history traits of cyclical parthenogens against other reproductive modes. For instance, (1) cyclical parthenogenetic versus obligate asexual lineages in the rotifer Brachionus calyciflorus Pallas 1766 (Stelzer, 2011), in the aphid Sitobion avenae Fabricio 1975 (Helden & Dixon, 2002) and in the cladoceran Daphnia pulex Leydig 1860 (Scheiner & Yampolsky, 1998; Innes et al., 2000; Wolinska & Lively, 2008), and (2) the two previous modes of reproduction versus asexual lineages retaining the ability to produce males in D. pulex (Wolinska & Lively, 2008). These comparisons yielded mixed results. Some studies showed cyclical parthenogens and obligate asexuals having no differences in survival and fecundity (Scheiner & Yampolsky, 1998; Stelzer, 2011), whereas in others, cyclical parthenogens were reported either to have lower (Wolinska & Lively, 2008) or higher fecundity (Innes et al., 2000) than their asexual counterparts. Interestingly, some of these studies have been supplemented using pairwise competition experiments raising together both reproductive modes (Wolinska & Lively, 2008; Stelzer, 2011; Innes & Ginn, 2014). Their experimental outcomes varied. Thus, asexual lineages can invade and displace populations of cyclical parthenogens in B. calyciflorus, whereas the opposite was observed in D. pulex. These contrasting results suggest that the cost of sex might vary markedly among taxa (Meirmans et al., 2012). Therefore, estimating such a cost is a decisive starting point for addressing the paradox of sex in any species or group of species (Gibson et al., 2017).
Besides approaches based on the comparison of separate linages that have different reproductive modes, an alternative one is to compare individual cyclical parthenogenetic females (Corley & Moore, 1999). Cyclical parthenogenetic rotifers, offer a unique system for addressing the cost of sex using this approach because individual females retain the ability to invest into both sexual and asexual reproduction. When sex is induced in their parthenogenetic life cycle, asexual females parthenogenetically produce sexual daughters as some fraction of their offspring (i.e., the so-called sexual reproduction ratio or mixis ratio), so that both types of reproduction occur simultaneously (Gilbert, 1974; Schröder, 2005). This sexual reproduction ratio allows for quantifying the investment in sex (Serra & Snell, 2009; Carmona et al., 2009). The sexual reproduction ratio is extremely variable among clones representing different genotypes (e.g. Snell & Boyer, 1988; Carmona et al., 1994, 1995, 2009; Gilbert, 2002; Schröder & Gilbert, 2004; Gilbert & Schröder, 2007; Gilbert & Diéguez, 2010; Stelzer, 2017). The expectation under the all-else-equal assumption is that genotypes investing more in sexual daughters during the planktonic growing season will have slower rates of clonal growth. However, the assumption could not be fulfilled if these genotypes have a higher fecundity and/or survival, as reported in other cyclical parthenogens (e.g., Innes et al., 2000).
In this paper, we made experiments specifically designed to assess the all-else-equal assumption by analyzing life-history traits (LHTs) of individual female genotypes sharing the same reproductive mode (cyclical parthenogenesis), using the rotifer Brachionus plicatilis O. F. Müller 1786. Rotifers are commonly used as model organisms in population and evolutionary studies (e.g., Fussmann et al., 2007; Snell, 2014; Declerck & Papakostas, 2017; Stelzer, 2017). The studied species is known to have high among-clone variability in the investment in sex in natural populations (Aparici et al., 2001; Carmona et al., 2009; Campillo et al., 2011; Franch-Gras et al., 2017) so that the studied genotypes were expected to invest differently in sexual reproduction. We performed a dynamic life table experiment by following a cohort of B. plicatilis stem females (i.e., hatched from diapausing eggs) to test if genotypes investing higher in sexual daughters (i.e., male-producing and diapausing-egg-producing) could compensate this investment by having a higher fecundity, survival and/or generation time. We did so by analyzing if sexual investment was correlated with other LHTs estimated from life table analysis. The rationale is that if investment in sexual reproduction is not positively correlated with fecundity, survival and/or generation time, it will mean that there is no compensation and, therefore, sex will have a cost and the explanation of its maintenance would rely on compensatory benefits of sex.
Materials and methods
Rotifer isolation and species identification
To obtain the cohort of experimental females, diapausing eggs of B. plicatilis were isolated from the sediment of ten brackish ponds in Spain (Poza Sur, acronym: TOS; Hoya Turnera: HTU; Hoya Monte: HMT; Ontalafía: ONT; Hoya Yerba: HYB; Hoya Rasa: HYR; Saladar: SLD; Hondo Sur: HOS; Salobralejo: SAL and Turies: TUR). Pond selection was based on previous reports of diapausing egg banks of B. plicatilis in the sediments (Gómez et al., 2000; Ortells et al., 2000; Montero-Pau et al., 2011). Because rotifer diapausing eggs are the product of sexual reproduction, each hatchling (stem female) represents an individual female genotype. By collecting diapausing eggs from several ponds it was sought to get a cohort genetically diverse for the life table experiment. The sediments were collected in different sampling programs and, at the time of diapausing egg isolation, they had been stored in darkness at 4°C during a variable period of time: 4 years (HOS; SAL; TUR), 5 years (HTU; HMT; ONT; HYB; HYR; SLD) and 8 years (TOS).
Rotifer diapausing eggs potentially belonging to a mixture of species were isolated from the sediment samples using a sucrose flotation technique (Onbé, 1978; Gómez et al., 2000). Eggs were concentrated using a mesh of 30 µm and kept in Petri dishes with saline water prepared with commercial sea salt (Instant Ocean®; Aquarium Systems) at 15 g l−1 salinity, in darkness, and 4°C for approx. 16 h until their selection. Putative diapausing eggs of B. plicatilis were randomly picked and individually placed in a 96-multiwell plate (Nunc™) with 200 μl of 15 g l−1 saline water, where they were induced to hatch under constant illumination (150–170 μmol quanta m−2 s−1) at 25°C. The stem females hatched from these diapausing eggs constituted the initial cohort in the life-table experiment described below. Because B. plicatilis belongs to a cryptic species complex (Gómez et al., 2000), the species-level identification of the cohort females was carried out by PCR–RFLP analysis of cytochrome c oxidase subunit I (COI) fragments extracted by sampling the clonal lineage from each stem female, that lineage being initiated by parthenogenetic proliferation of one of the first daughters of the stem female. Once identification was performed, females that were not B. plicatilis (10 out of 55) were discarded from the study cohort.
Culture media
In experiments, rotifers grew in the medium where the microalgae Tetraselmis suecica (Kylin) Butcher 1959, used as food, had been previously cultured. Microalgae were grown in modified f/2 medium (Guillard & Ryther, 1962), 15 g l−1 salinity, at 20°C and under constant illumination (150–170 μmol quanta m−2 s−1) using a semi-continuous culture system (dilution rate: 0.65 day−1). Microalgae density was estimated by 750-nm wavelength light extinction using a calibration curve of absorption versus density.
Experimental set-up
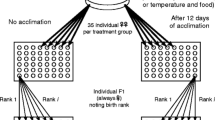
A dynamic life-table experiment was performed following a cohort of 45 B. plicatilis stem females (F0 generation, see Fig. 1), each one representing a different genotype (1-6 genotypes per pond). The common garden experiment started by individually transferring each stem female (< 12 h old) to a Petri dish with 15 ml of culture medium (0.07 females ml−1) containing a concentration of algae equivalent to 8 mg C l−1 (approx. 42.000 cells ml−1), well above the incipient-limiting level (Ciros-Pérez et al., 2001). Experimental cultures containing each stem female were randomly placed inside wet chambers, over an orbital shaker (approx. 28 rpm), at 20°C and in darkness. The cultures were monitored every 24 h, checking for stem female survival, counting and transferring daughters (F1 generation, Fig. 1), and renewing 15% of the medium. Since the density of rotifers (stem female + daughters) in the cultures was continuously low (< 0.67 females ml−1) and the culture medium was partially daily renewed, food concentration in the cultures was not limiting during the experiment. Sexual reproduction in Brachionus spp. is triggered in a quorum-sensing process by a pheromone called the mixis-inducing protein (MIP) that the females secrete into the medium (Snell et al., 2006). Therefore, the partial renewal of the culture medium allows MIP to accumulate in the cultures, so that induction of sexual reproduction of stem females can take place. Daughters (F1) were individually transferred to wells of a 96-multiwell plate (Nunc™) until they produced their offspring (F2 generation). Each F1 female was classified as asexual if her offspring were females or sexual if her offspring were males. The life-table experiment finished when all the cohort females died.
Scheme of the experimental set-up. Diapausing eggs isolated from the sediments of Spanish brackish ponds were incubated under standard hatching conditions. A total of 45 stem females hatched from these eggs constituted the initial cohort (F0 generation) for the life-table experiment. Stem females grew in Petri dishes under common garden conditions producing a number of daughters (F1 generation) by asexual proliferation throughout her life (i.e. horizontal segment). Every day survival of each stem female was monitored and all daughters were transferred (see downward arrows) to new Petri dishes, allowed to proliferate (horizontal arrows), and classified as sexual or asexual depending on whether their offspring (F2 generation) were males or females, respectively. The experiment finished when the last stem female died. See details in the text
Data records from the life-table experiment were used to calculate individually for each stem female the following life history traits (LHTs): lifespan, lifetime reproductive success, generation time (i.e., weighted mean age at birth of the offspring), intrinsic growth rate, and investment in sexual reproduction (i.e., proportion of sexual daughters produced in the stem female total offspring). Additionally, age-specific investment in sexual reproduction was calculated from the daily production of asexual and sexual daughters. Two different measures of lifetime reproductive success (LRS) were computed: (1) for the total offspring (hereafter, LRStotal), and (2) only for asexual daughters (hereafter, LRSasex). Generation time was calculated as
where x = age of the stem female at the observation event and fx= number of offspring produced from x to x + 1. Three different measures of T were computed: (1) for the total offspring (hereafter, Ttotal), (2) for asexual daughters (hereafter, Tasex), and (3) for sexual daughters (hereafter, Tsex). When options (2) or (3) are used in the reported results, it is explicitly indicated. Intrinsic growth rate (r) was calculated from the fecundity schedule of each stem female by solving Euler–Lotka’s equation using Microsoft Excel Solver tool
where nx is the offspring produced from the age x to the age x + 1 (Birch, 1948). Some words of caution are needed to interpret our estimation of this r as the intrinsic population growth rate. First, a necessary assumption for this interpretation is that the cohort (here, a single female) and the offspring are both samples of the same ideal population. Second, as we were interested in the short-term cost of sex, asexual and sexual daughters were considered to be equivalent, so that our estimation was at best the potential intrinsic growth rate (i.e., the rate of increase that a population/genotype would have if no investment in diapausing eggs would occur; see Montero-Pau et al., 2014). Third, the physiological conditions of a stem female are expected to be different of that of her offspring, at least due to the fact that the former came from a diapausing egg. Nevertheless, our estimation of r is expected to be a composite metric correlated to fitness.
To avoid biases due to the correlation between individual lifespan and probability of accidental lost during experimental manipulation, females that were accidentally lost (a total of 6 stem females on days sixth and seventh) were included in the analyses as censored data using the mean substitution method (e.g., Little & Rubin, 2002; Enders, 2010). This is, it was assumed that, after being lost, the female would have had the same demographic parameters than the average for the rest of the stem females of the cohort.
Data analysis
To test the all-else-equal assumption, we analysed the relationships between investment in sexual reproduction versus each LHTs (lifespan, LRS T and r) by calculating Pearson’s correlation coefficients using statistical package SPSS v.19.0 (SPSS Inc., Chicago, USA). Additionally, other pairwise relationships between LHTs were also explored. Potential effects due to population differences and storage time of diapausing eggs on LHTs were discarded (P > 0.05 in all cases) prior to further analyses by means of generalized linear models (GLMs) assuming different error distributions and link functions depending on each LHT (i.e., Poisson distribution of errors and log as link function for LRS and reproductive period; Gaussian distribution of errors and identity link function for lifespan, T and investment in sexual reproduction). Finally, to analyse the relationship between age-specific investment in sexual reproduction and female age, we used another GLM on number of offspring (F1) produced by the stem females as the response variable, and assumed a Poisson distribution of errors with log as link function. Female age, offspring type (sexual or asexual), and their interaction were entered as fixed-effect factors. All GLM analyses were implemented using the glm function from the “stats” package in R statistical software v.3.1.1 (R Development Core Team, 2014).
Results
Table 1 shows average values for LHTs obtained for the B. plicatilis cohort. The investment in sexual reproduction averaged ca. 9% in the cohort (range 0–33%), while 62% of the stem females had sexual daughters. For this fraction of females, sexual daughters were much less abundant (3.3 ± 0.4 females) than asexual ones (20.5 ± 0.9 females).
Figure 2 shows pairwise relationships of LHTs. Regarding the assessment of the all-else-equal assumption, investment in sexual reproduction (ranging from 0 to 0.333) was not significantly correlated with any other LHT accounting for the total F1 offspring (Fig. 2a–c), which indicates that the investment in sexual reproduction did not affect lifespan, LRStotal or T of the stem females. Accordingly, there was also a lack of correlation between r and the investment in sex (Pearson’s r = − 0.09, P = 0.55; data not plotted), r ranging between 0.696 and 0.921 in stem females without investment in sexual reproduction and between 0.676 and 0.883 in those with an investment greater than 0.150. However, investment in sexual reproduction was negatively correlated with LRSasex (Pearson’s r = − 0.68, P < 0.001; Fig. 2a), evidencing a trade-off. A weak but significant positive relationship exists between the lifespan and the Ttotal (Pearson’s r = 0.31, P < 0.05; Fig. 2f); no significant correlation was found between lifespan and Tasex (Pearson’s r = 0.26, P = 0.08, data not plotted) or Tsex (Pearson’s r = 0.20, P = 0.32, data not plotted). Figure 3 shows that a post-reproductive period occurred in almost all the females, which is consistent with the lack of a significant correlation between lifespan and both LRStotal and LRSasex (Fig. 2e).
Display of age-specific survival and lifetime fecundity of 45 genetically diverse stem females of the rotifer B. plicatilis from ten Spanish ponds. Each horizontal bar represents the longevity of a single female and the fecundity is coded in a grayscale. a production of asexual daughters and b production of sexual daughters. Lifespans are ranked from the shortest in the top to the longest in the bottom (according to Carey et al., 1998)
According to GLM analysis (Table 2), there was a significant effect of both the age of the stem female (P < 0.001) and the F1 offspring type (sexual or asexual) (P < 0.001) on the number of F1 offspring produced, as well as a significant interaction effect (P = 0.058) of the age and offspring type (P = 0.008), which is also suggested by the visual comparison of the two graphs displaying age-specific individual female fecundity for asexual and sexual offspring (Fig. 3a, b), where fecundity patterns clearly differ depending on F1 offspring type. Sexual daughters were produced earlier by the stem females (generation time: 4.08 ± 0.04 days) than asexual daughters (4.57 ± 0.09 days) and during a shorter time (4 days vs. 6 days of reproductive period). We tested for an effect of the population from which the experimental clones were isolated on LHTs, and the results confirmed that no significant effect was affecting the data.
Discussion
It appears that the cost of sex holds true when comparing LHTs between cyclical parthenogenetic females of B. plicatilis having different investment in sexual offspring. The lack of correlation between the investment in sexual reproduction and any other studied LHTs implies that a higher investment in sex is not compensated with a higher lifespan, lifetime reproductive success and/or generation time. Therefore, our experimental data support the hypothesis that sex is costly in these rotifers. A caveat to be considered is if these results may be due to inadequate statistical power. However, this is unlikely because the correlations that we found between investment in sexual offspring and the studied LHTs, although not significant, were negative (i.e., the opposite of what is expected if there was compensation).
The studied LHTs (i.e., lifespan, lifetime reproductive success, generation time and intrinsic growth rate) were similar irrespectively of the investment in sexual offspring, supporting the assumption of equal life-history patterns. These findings are in agreement with a previous life-table study by Stelzer (2011) comparing cyclical and obligate parthenogenetic females derived from the same maternal genotype in the rotifer B. calyciflorus, in which the all-else-equal assumption regarding the population growth rate was fulfilled. This finding is in agreement with previous studies in other systems (e.g., Jokela et al., 1997; Crummett & Wayne, 2009; Schlupp et al., 2010; Gibson et al., 2017), including other cyclical parthenogens (Scheiner & Yampolsky, 1998; Wolinska & Lively, 2008). However, the equivalence in LHTs between sexual and asexual females is not the general rule (reviewed in Lehtonen et al., 2012; Meirmans et al., 2012; Stelzer, 2015). This variability in outcomes among studies indicates that the realized cost of sex could be dependent on certain LHTs of a species and that the maintenance of sex might be more species-specific than previously assumed (West et al., 1999; Meirmans & Strand, 2010; Meirmans et al., 2012).
The absence of compensation between investment in sex and the studied LHTs indirectly evidences the cost of sex in B. plicatilis and calls for alternative compensatory factors to explain the maintenance of sex in this cyclical parthenogenetic rotifer. It can be proposed that, as in other facultative sexual organisms, rotifer females by investing little in sexual offspring could reduce the cost of sex while gaining its full advantages (Green & Noakes, 1995; Peck & Waxman, 2000; D’Souza & Michiels, 2010; Stelzer & Lehtonen, 2016). However, this is not the case in heterogeneous and novel environments where studies using rotifers have found that a high investment in sex evolves (Becks & Agrawal, 2010, 2012). As expected, the investment in sexual reproduction was variable among stem females, which raises the question of why they incur in different cost of sex. Despite unambiguous evidence for genetic differences among females cannot be claimed due to the lack of replicates from each clone, our common garden experiment minimizes environmental variation, so that a genetic basis for their different investment in sex is plausible. It is possible that these females have diverse strategies regarding the investment in diapausing eggs that would be maintained by the link between sex and diapause, due to the important selection pressure exerted by the need to survive unsuitable periods through diapause (e.g., Tarazona et al., 2017). The association between sex and diapause reduces the cost of sex by providing the correlated advantage of dormancy (Serra & Snell, 2009; Stelzer & Lehtonen, 2016). Further, since these propagules are the result of sexual recombination, a relationship between genetic variation and investment in sex is expected, and this genetic variation would be advantageous over the long term to cope with environmental novelties (Becks & Agrawal, 2012; Lehtonen et al., 2012; Stelzer & Lehtonen, 2016).
Most of the referred investigations into the all-else-equal assumption have focused on comparisons between lineages within a species having different reproductive modes (Kondrashov, 1993; Hurst & Peck, 1996; Neiman et al., 2010). However, these comparisons have led to some criticisms. For instance, confounding effects may appear due to differences in origin, genetic background, environmental and evolutionary histories between the asexual and sexual females object of comparison, even if they belong to the same population (Jokela et al., 1997; Corley & Moore, 1999; Kramer & Templeton, 2001; Simon et al., 2003; Allen & Lynch, 2008; Stelzer, 2011). In our model of study, we adopted a different approach, instead of comparing cyclical parthenogenetic lineages versus asexual ones, we directly compared LHTs of individual rotifer females with capacity to produce both sexual and asexual daughters using a common benign environment (Stelzer, 2015; Ram & Hadany, 2016). This approach facilitates to interpret the comparison among a variable investment in sex by otherwise equivalent females.
According to our findings, lifetime reproductive success was equal irrespective of the investment in sex done by an individual rotifer female. Therefore, it necessarily follows that the greater the investment in sex the greater the decrease in asexual daughter production, as evidenced by the negative correlation we found between the investment in sex and the lifetime reproductive success, when it was calculated accounting only for asexual offspring. This trade-off is straightforward, as the asexual and sexual success of a genotype are not independent, but traded-off against each other in the life history of cyclically parthenogenetic rotifers, which has been well studied theoretically (Snell, 1987; Serra & Carmona, 1993; Serra & King, 1999; Stelzer, 2005). Therefore, the costs of sex cause a trade-off between investment into current population growth by asexual (clonal) proliferation and long-term population persistence throughout the sexually-produced diapausing eggs. This trade-off may affect the optimal patterns of sexual reproduction, which have been correlated with habitat features (Carmona et al., 1995, 2009; Serra et al., 2004; Campillo et al., 2011; Franch-Gras et al., 2017). Few studies have addressed the all-else-equal assumption in natural settings (e.g., Jokela et al., 1997; Gibson et al., 2016, 2017). In this regard, the finding that the assumption holds in our laboratory experiment is consistent with the observed decline in frequency of genotypes investing more in sex during the growing season in natural populations of B. plicatilis (Carmona et al., 2009).
Initiation of sex in the genus Brachionus is induced by population density through a quorum-sensing factor, the so-called mixis inducing protein, MIP (Stelzer & Snell, 2003; Snell et al., 2006; Kubanek & Snell, 2008), but this response may depend on several endogenous factors (Hagiwara et al., 2005; Gilbert, 2007; Gilbert & Schröder, 2007). An endogenous factor affecting investment in sex that has been extensively studied is female age. Our finding of a higher investment in sex at earlier ages is a quite general pattern found in previous studies in B. plicatilis (Lubzens & Minkoff, 1988; Carmona et al., 1994), B. calyciflorus (Pourriot & Rougier, 1976, 1977; Rougier & Pourriot, 1977; Rougier et al., 1977) and B. rubens Ehrenberg 1838 (Pourriot & Rougier, 1976; Pourriot et al., 1986; Fussmann et al., 2007). Instead, other studies showed a pattern with production of sexual daughters being less likely as mothers aged (Gilbert & Schröder, 2007) or even no effect of age (Pourriot & Rougier, 1978; Gilbert & Schröder, 2007). These differing patterns have been attributed to genetic differences between clones and species, causing age-related differences in MIP production, in MIP responsiveness or in both (Gilbert & Schröder, 2007). The effect of female age on sex investment varied among the genotypes studied here. According to theoretical predictions the timing of production of sexual daughters affects the cost of sex in rotifers, so that, for a given lifetime sexual reproduction ratio, producing the sexual offspring late in life minimizes the cost of sex if compared with producing it early (Stelzer, 2011). Therefore, there will be clear consequences for the competitiveness during the planktonic growing season of clonal genotypes having different lifetime patterns of sexual offspring production, which deserves further investigation to fully understand the role of life history variation in the maintenance of sex in cyclical parthenogenetic rotifers.
References
Allen, D. E. & M. Lynch, 2008. Both costs and benefits of sex correlate with relative frequency of asexual reproduction in cyclically parthenogenic Daphnia pulicaria populations. Genetics 179: 1497–1502.
Aparici, E., M. J. Carmona & M. Serra, 1998. Sex allocation in haplodiploid cyclical parthenogens with density-dependent proportion of males. The American Naturalist 152: 652–657.
Aparici, E., M. J. Carmona & M. Serra, 2001. Variability for mixis initiation in Brachionus plicatilis. Hydrobiologia 446(447): 45–50.
Aparici, E., M. J. Carmona & M. Serra, 2002. Evidence for an even sex allocation in haplodiploid cyclical parthenogens. Journal of Evolutionary Biology 15(1): 65–73.
Becks, L. & A. F. Agrawal, 2010. Higher rates of sex evolve in spatially heterogeneous environments. Nature 468: 89–92.
Becks, L. & A. F. Agrawal, 2012. The evolution of sex is favoured during adaptation to new environments. PLoS Biology 10: e1001317.
Bell, G., 1982. The masterpiece of nature: the evolution and genetics of sexuality. Croom Helm, London.
Birch, L. C., 1948. The intrinsic rate of natural increase of an insect population. The Journal of Animal Ecology 17: 15–26.
Birky, C. W. & J. J. Gilbert, 1971. Parthenogenesis in rotifers: the control of sexual and asexual reproduction. American Zoologist 11: 245–266.
Burt, A., 2000. Perspective: sex, recombination, and the efficacy of selection - Was Weismann right? Evolution 54: 337–351.
Butlin, R., 2002. The costs and benefits of sex: new insights from old asexual lineages. Nature Reviews Genetics 3: 311–316.
Campillo, S., E. M. García-Roger, M. J. Carmona & M. Serra, 2011. Local adaptation in rotifer populations. Evolutionary Ecology 25: 933–947.
Carey, J. R., P. Liedo, H. G. Müller, J. L. Wang & J. W. Vaupel, 1998. A simple graphical technique for displaying individual fertility data and cohort survival: case study of 1000 Mediterranean fruit fly females. Functional Ecology 12: 359–363.
Carmona, M. J., M. Serra & M. R. Miracle, 1994. Effect of population density and genotype on life-history traits in the rotifer Brachionus plicatilis O.F. Müller. Journal of Experimental Marine Biology and Ecology 182: 223–235.
Carmona, M. J., A. Gómez & M. Serra, 1995. Mictic patterns of the rotifer Brachionus plicatilis Müller in small ponds. Hydrobiologia 313(314): 365–371.
Carmona, M. J., N. Dimas-Flores, E. M. García-Roger & M. Serra, 2009. Selection of low investment in sex in a cyclically parthenogenetic rotifer. Journal of Evolutionary Biology 22: 1975–1983.
Ciros-Pérez, J., M. J. Carmona & M. Serra, 2001. Resource competition between sympatric sibling rotifer species. Limnology & Oceanography 46: 1511–1523.
Corley, L. S. & A. J. Moore, 1999. Fitness of alternative modes of reproduction: developmental constraints and the evolutionary maintenance of sex. Proceedings of the Royal Society of London B: Biological Sciences 266: 471–476.
Crummett, L. T. & M. L. Wayne, 2009. Comparing fecundity in parthenogenetic versus sexual populations of the freshwater snail Campeloma limum: is there a two-fold cost of sex? Invertebrate Biology 12: 1–8.
Decaestecker, E., L. De Meester & J. Mergeay, 2009. Cyclical parthenogenesis in Daphnia: sexual versus asexual reproduction. In Schön, I., K. Martens & P. Dijk (eds), Lost Sex. Springer, Netherlands: 295–316.
Declerck, S. A. & S. Papakostas, 2017. Monogonont rotifers as model systems for the study of micro-evolutionary adaptation and its eco-evolutionary implications. Hydrobiologia 796(1): 131–144.
D’Souza, T. G. & N. K. Michiels, 2010. The costs and benefits of occasional sex: theoretical predictions and a case study. Journal of Heredity 101: S34–S41.
Enders, C. K., 2010. Applied Missing Data Analysis. The Guilford Press, New York.
Franch-Gras, L., E. M. García-Roger, B. Franch, M. J. Carmona & M. Serra, 2017. Quantifying unpredictability: a multiple-model approach based on satellite imagery data from Mediterranean ponds. PLoS ONE 12: e0187958.
Fussmann, G. F., G. Kramer & M. Labib, 2007. Incomplete induction of mixis in Brachionus calyciflorus: patterns of reproduction at the individual level. Hydrobiologia 593: 111–119.
Gibson, A. K., J. Y. Xu & C. M. Lively, 2016. Within-population covariation between sexual reproduction and susceptibility to local parasites. Evolution 70: 2049–2060.
Gibson, A. K., L. F. Delph & C. M. Lively, 2017. The two-fold cost of sex: experimental evidence from a natural system. Evolution Letters 1: 6–15.
Gilbert, J. J., 1974. Dormancy in rotifers. Transactions of the American Microscopical Society 93: 490–513.
Gilbert, J. J., 2002. Endogenous regulation of environmentally induced sexuality in a rotifer: a multigenerational parental effect induced by fertilization. Freshwater Biology 47: 1633–1641.
Gilbert, J. J., 2007. Induction of mictic females in the rotifer Brachionus: oocytes of amictic females respond individually to population-density signal only during oogenesis shortly before oviposition. Freshwater Biology 52: 1417–1426.
Gilbert, J. J. & M. C. Diéguez, 2010. Low crowding threshold for induction of sexual reproduction and diapause in a Patagonian rotifer. Freshwater Biology 55: 1705–1718.
Gilbert, J. J. & T. Schröder, 2007. Intraclonal variation in propensity for mixis in several rotifers: variation among females and with maternal age. Hydrobiologia 593: 121–128.
Gómez, A., G. R. Carvalho & D. H. Lunt, 2000. Phylogeography and regional endemism of a passively dispersing zooplankter: mitochondrial DNA variation in rotifer resting egg banks. Proceedings of the Royal Society of London Series 267: 2189–2197.
Green, R. F. & D. L. Noakes, 1995. Is a little bit of sex as good as a lot? Journal of Theoretical Biology 174: 87–96.
Guillard, R. R. L. & J. H. Ryther, 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve). Canadian Journal of Microbiology 8: 229–239.
Hadany, L. & J. M. Comeron, 2008. Why are sex and recombination so common? Annals of the New York Academy of Sciences 1133: 26–43.
Hagiwara, A., Y. Kadota & A. Hino, 2005. Maternal effect by stem females in Brachionus plicatilis: effect of starvation on mixis induction in offspring. Hydrobiologia 546: 275–279.
Helden, A. J. & A. F. G. Dixon, 2002. Life-cycle variation in the aphid Sitobion avenae: costs and benefits of male production. Ecological Entomology 27: 692–701.
Hurst, L. D. & J. R. Peck, 1996. Recent advances in understanding of the evolution and maintenance of sex. Trends in Ecology & Evolution 11: 46–52.
Innes, D. J. & M. Ginn, 2014. A population of sexual Daphnia pulex resists invasion by asexual clones. Proceedings of the Royal Society of London B: Biological Sciences 281: 20140564.
Innes, D. J., C. J. Fox & G. L. Winsor, 2000. Avoiding the cost of males in obligately asexual Daphnia pulex (Leydig). Proceedings of the Royal Society B: Biological Sciences 267: 991–997.
Jokela, J., C. M. Lively, M. F. Dybdahl & J. A. Fox, 1997. Evidence for a cost of sex in the freshwater snail Potamopyrgus antipodarum. Ecology 78: 452–460.
Kearney, M. & R. Shine, 2005. Lower fecundity in parthenogenetic geckos than sexual relatives in the Australian arid zone. Journal of evolutionary biology 18: 609–618.
Keightley, P. D. & S. P. Otto, 2006. Interference among deleterious mutations favours sex and recombination in finite populations. Nature 443: 89–92.
Kondrashov, A. S., 1993. Classification of hypotheses on the advantage of amphimixis. Journal of Heredity 84: 372–387.
Kramer, M. G. & A. R. Templeton, 2001. Life-history changes that accompany the transition from sexual to parthenogenetic reproduction in Drosophila mercatorum. Evolution 55: 748–761.
Kubanek, J. & T. W. Snell, 2008. Quorum sensing in rotifers. In Winans, S. C. & B. L. Bassler (eds), Chemical Communication Among Bacteria. American Society of Microbiology Press, Washington: 453–461.
Lamb, R. Y. & R. B. Willey, 1979. Are parthenogenetic and related bisexual insects equal in fertility? Evolution 33: 774–775.
Lehtonen, J., M. D. Jennions & H. Kokko, 2012. The many costs of sex. Trends in Ecology & Evolution 27: 172–178.
Little, R. J. A. & D. B. Rubin, 2002. Statistical Analysis with Missing Data. Wiley, Hoboken.
Lively, C. M. & L. T. Morran, 2014. The ecology of sexual reproduction. Journal of Evolutionary Biology 27: 1292–1303.
Lubzens, E. & G. Minkoff, 1988. Influence of the age of algae fed to rotifer (Brachionus plicatilis O.F. Müller) on the expression of mixis in their progenies. Oecologia 75: 430–435.
Lynch, M., 1984. The genetic structure of a cyclical parthenogen. Evolution 38: 186–203.
Maynard-Smith, J., 1971. What use is sex? Journal of Theoretical Biology 30: 319–335.
Maynard-Smith, J., 1978. The Evolution of Sex. Cambridge University Press, Oxford.
Meirmans, S. & R. Strand, 2010. Why are there so many theories for sex, and what do we do with them? Journal of Heredity 101: S3–S12.
Meirmans, S., P. G. Meirmans & L. R. Kirkendall, 2012. The costs of sex: facing real-world complexities. The Quarterly Review of Biology 87: 19–40.
Montero-Pau, J., E. Ramos-Rodríguez, M. Serra & A. Gómez, 2011. Long-term coexistence of rotifer cryptic species. PLoS ONE 6: E21530.
Montero-Pau, J., C. Gabaldón, M. J. Carmona & M. Serra, 2014. Measuring the potential for growth in populations investing in diapause. Ecological Modelling 272: 76–83.
Neiman, M., 2004. Physiological dependence on copulation in parthenogenetic females can reduce the cost of sex. Animal Behaviour 67: 811–822.
Neiman, M., G. Hehman, J. T. Miller, J. M. Logsdon & D. R. Taylor, 2010. Accelerated mutation accumulation in asexual lineages of a freshwater snail. Molecular Biology and Evolution 27: 954–963.
Onbé, T., 1978. Sugar flotation method for sorting the resting eggs of marine cladocerans and copepods from sea-bottom sediment. Bulletin of the Japanese Society of Scientific Fisheries 44: 1411.
Ortells, R., T. W. Snell, A. Gómez & M. Serra, 2000. Patterns of genetic differentiation in resting egg banks of a rotifer species complex in Spain. Archiv für Hydrobiologie 149: 529–551.
Otto, S. P., 2009. The evolutionary enigma of sex. The American Naturalist 174: S1–S14.
Otto, S. P. & T. Lenormand, 2002. Evolution of sex: resolving the paradox of sex and recombination. Nature Reviews Genetics 3: 252–261.
Peck, J. R. & D. Waxman, 2000. What’s wrong with a little sex? Journal of Evolutionary Biology 13: 63–69.
Pourriot, R. & C. Rougier, 1976. Influence de l’âge des parents sur la production de femelles mictiques chez Brachionus calyciflorus (Pallas) et B. rubens Ehr. (Rotifère). Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences Série D 283: 1497–1500.
Pourriot, R. & C. Rougier, 1977. Effets de la densité de population et du groupement sur la reproduction de Brachionus calyciflorus (Pallas) [Rotifère]. Annales de Limnologie-International Journal of Limnology 13: 101–113.
Pourriot, R. & C. Rougier, 1978. Influences conjuguées du groupement et de la qualité de la nourriture sur la reproduction de Brachionus plicatilis O.F. Müller (Rotifère). Netherlands Journal of Zoology 29: 242–2264.
Pourriot, R., C. Rougier & D. Benest, 1986. Qualité de la nourriture et contrôle de la mixis chez le rotifère Brachionus rubens, Ehr. Bulletin de la Société zoologique de France 111: 105–111.
R Core Team, 2014. A language and environment for statistical computing, V.3.1.1. R Foundation for Statistical Computing, Vienna, Austria.
Ram, Y. & L. Hadany, 2016. Condition-dependent sex: who does it, when and why? Philosophical Transactions of the Royal Society of London. Series B, Biological sciences 371: 20150539.
Rice, W. R., 2002. Evolution of sex: experimental tests of the adaptive significance of sexual recombination. Nature Reviews Genetics 3: 241–251.
Roth, L. M., 1974. Reproductive potential of bisexual Pycnoscelus indicus and clones of its parthenogenetic relative. Pycnoscelus surinamensis. Annals of the Entomological Society of America 67: 215–223.
Rougier, C. L. & R. Pourriot, 1977. Aging and control of the reproduction in Brachionus calyciflorus (Pallas) (Rotatoria). Experimental Gerontology 12: 137–151.
Rougier, C., R. Pourriot & P. Clément, 1977. Determination of mixis in Brachionus. Archiv für Hydrobiologie Beiheft Ergebnisse der Limnologie 8: 163–166.
Roze, D. & N. H. Barton, 2006. The Hill-Robertson effect and the evolution of recombination. Genetics 173: 1793–1811.
Scheiner, S. M. & L. E. V. Yampolsky, 1998. The evolution of Daphnia pulex in a temporally varying environment. Genetical Research 72: 25–37.
Schlupp, I., A. Taebel-Hellwig & M. Tobler, 2010. Equal fecundity in asexual and sexual mollies (Poecilia). Environmental Biology of Fishes 88: 201–206.
Schröder, T., 2005. Diapause in monogonont rotifers. Hydrobiologia 546: 291–306.
Schröder, T. & J. J. Gilbert, 2004. Transgenerational plasticity for sexual reproduction and diapause in the life cycle of monogonont rotifers: intraclonal, intraspecific and interspecific variation in the response to crowding. Functional Ecology 18: 458–466.
Serra, M. & M. J. Carmona, 1993. Mixis strategies and resting egg production of rotifers living in temporally-varying habitats. Hydrobiologia 255(256): 117–126.
Serra, M. & C. E. King, 1999. Optimal rates of bisexual reproduction in cyclical parthenogens with density-dependent growth. Journal of Evolutionary Biology 12: 263–271.
Serra, M. & T. W. Snell, 2009. Sex Loss in Monogonont Rotifers. In Schön, I., K. Martens & P. Dijk (eds), Lost Sex. Springer, Netherlands: 281–294.
Serra, M., T. W. Snell & C. E. King, 2004. The timing of sex in cyclically parthenogenetic rotifers. In Moya, A. & E. Font (eds), Evolution: From Molecules to Ecosystems. Oxford University Press, Oxford: 135–146.
Simon, J. C., C. Rispe & P. Sunnucks, 2002. Ecology and evolution of sex in aphids. Trends in Ecology & Evolution 17: 34–39.
Simon, J. C., F. Delmotte, C. Rispe & T. Crease, 2003. Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biological Journal of the Linnean Society 79: 151–163.
Snell, T. W., 1987. Sex, population dynamics and resting egg production in rotifers. Hydrobiologia 144: 105–111.
Snell, T. W., 2014. Rotifers as models for the biology of aging. International Review of Hydrobiology 99(1–2): 84–95.
Snell, T. W. & E. M. Boyer, 1988. Thresholds for mictic female production in the rotifer Brachionus plicatilis (Müller). Journal of Experimental Marine Biology and Ecology 124: 73–85.
Snell, T. W., C. William, B. P. Audra, K. Jerry, K. H. Melissa & C. P. Stelzer, 2006. A protein signal triggers sexual reproduction in Brachionus plicatilis (Rotifera). Marine Biology 149: 1432–1793.
SPSS. V.19.0, Inc., Chicago, USA.
Stelzer, C. P., 2005. Evolution of rotifer life histories. Hydrobiologia 546: 335–346.
Stelzer, C. P., 2011. The cost of sex and competition between cyclical and obligate parthenogenetic rotifers. The American Naturalist 177: E43–E53.
Stelzer, C. P., 2015. Does the avoidance of sexual costs increase fitness in asexual invaders? Proceedings of the National Academy of Sciences 112: 8851–8858.
Stelzer, C. P., 2017. Life history variation in monogonont rotifers. In Hagiwara, A. & T. Yoshinaga (eds), Rotifers. Fisheries Science Series Springer, Singapore: 89–109.
Stelzer, C. P. & J. Lehtonen, 2016. Diapause and maintenance of facultative sexual reproductive strategies. Philosophical Transactions of the Royal Society B: Biological Sciences 371: 20150536.
Stelzer, C. P. & T. W. Snell, 2003. Induction of sexual reproduction in Brachionus plicatilis (Monogononta, Rotifera) by a density-dependent chemical cue. Limnology and Oceanography 48: 939–943.
Tarazona, E., E. M. García-Roger & M. J. Carmona, 2017. Experimental evolution of bet hedging in rotifer diapause traits as a response to environmental unpredictability. Oikos 126: 1162–1172.
Wallace, R. L. & H. Smith, 2009. Rotifera. In Likens, G. E. (ed.), Encyclopedia of Inlands Waters. Elsevier, Oxford: 698–703.
West, S. A., C. M. Lively & A. F. Read, 1999. A pluralist approach to sex and recombination. Journal of Evolutionary Biology 12: 1003–1012.
Wetherington, J. D., K. E. Kotora & R. C. Vrijenhoek, 1987. A test of the spontaneous heterosis hypothesis for unisexual vertebrates. Evolution 41: 721–731.
Williams, G. C., 1975. Sex and Evolution. Pinceton University Press, New Jersey.
Wolinska, J. & C. M. Lively, 2008. The cost of males in Daphnia pulex. Oikos 117: 1637–1646.
Acknowledgements
This study was supported by the Spanish Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica from the Spanish Ministry of Economy and Competitiveness, Grants CGL2006-07267 and CGL2015-65422-P (co-financed by FEDER funds, European Union). N. Dimas-Flores was supported by a fellowship (171031) from the Consejo Nacional de Ciencia y Tecnología of Mexico.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Steven A. J. Declerck, Diego Fontaneto, Rick Hochberg & Terry W. Snell / Crossing Disciplinary Borders in Rotifer Research
Rights and permissions
About this article
Cite this article
Dimas-Flores, N., Serra, M., García-Roger, E.M. et al. Evidencing the cost of sexual reproduction in the rotifer Brachionus plicatilis. Hydrobiologia 844, 243–255 (2019). https://doi.org/10.1007/s10750-019-3906-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-3906-y