Abstract
Compared to their putative insectivore-like ancestors, extant primates show an enlarged brain relative to body weight, a larger neocortex and proportionally decreased olfactory bulbs. Besides hypotheses based on the comparative neuroanatomy of extant taxa, the only direct evidence documenting such long-term evolutionary history is provided by fossil endocasts. However, due to the unpredictable yet unavoidable impact of taphonomic processes, the reliability of data from the fossil record is complicated by the nature of the investigated structures themselves. Nonetheless, palaeoneurology has recently enlarged its traditional investigative toolkit by integrating descriptive morphology with advanced methods of high-resolution 3D imaging and computing. In addition to the development of digital restoration techniques, the introduction of analytical methods for investigating topographic differences in morphostructural organization and quantitatively characterizing intra- and interspecific variation patterns provides new possibilities for the study of the primate fossil record, especially for assessing brain evolutionary tracks.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Palaeoneurology
- Primate brain evolution
- Deformation-based models
- Semi-automatic sulci detection
- Computer-assisted reconstruction
4.1 A Review of Non-human Primate Palaeoneurology
4.1.1 Evidence from Primate Palaeoneurology
One of the most fascinating unsolved problems in palaeoneurology centres on unravelling the selective pressures that were responsible for both the increase in relative brain size and the cerebral reorganization in primates (Radinsky 1975). Investigating the evolutionary changes of the brain across various species is critical to characterize phylogenetic specializations and provide insights into the interaction between an organism and its environment (Barton and Harvey 2000; de Winter and Oxnard 2001). As stated by Le Gros Clark (1971: 227–228): “[Thus] the progressive elaboration and differentiation of the cortex in the evolving Primates have led to increasing powers of apprehending the nature of external stimuli, a greater capacity for a wider range of adjustments to any environmental change, and an enhancement of the neural mechanisms for effecting more delicately co-ordinated reactions”.
The relative importance and the timing of two critical processes in the evolution of the primate brain, i.e. cortical reorganization and size increase, has been largely questioned (Gonzales et al. 2015). Recent evidence that brain size and level of gyrification are controlled by different genes in extant catarrhine primates corroborates the often observed lack of correlation between these two structural parameters (Welker 1990) and suggests that encephalization and cerebral complexity could have evolved independently (Rogers et al. 2010). Despite the relative scarcity of direct fossil evidence, palaeontological studies provide valuable evidence, notably by revealing that cerebral complexity preceded enlarged brain size in particular primate lineages (Gonzales et al. 2015).
For a better understanding of primate brain evolution, it is then crucial to integrate and combine different approaches as well as various sources of data. However, this prerequisite represents a critical challenge since the data based either directly on the brain or indirectly on endocasts are by nature very different (Neubauer 2014). Our knowledge of the evolution of the primate brain primarily relies on the interpretation of palaeoneurological evidence and on comparative information from extant species, in which the brain and behaviour can be investigated directly (Armstrong and Falk 1982). Fossil endocasts are replicas of the internal table of the bony braincase and provide the only direct evidence of brain evolution. When the neurocranium is filled with sediment during fossilization, morphological information about the external brain surface may be preserved as a natural endocast, as remarkably illustrated by the South African primate fossil records (e.g. the hominin specimens Taung, Sts 60, SK 1585 or the cercopithecoid specimens MP3a, MP 36, STS 538, STS 564, STS 565; Brain 1981; Holloway et al. 2004). Endocasts thus constitute a proxy for investigating and quantifying variations in brain size, global brain shape and neocortical surface morphology (if the dura mater was thin enough in the living animal), including imprints of cerebral convolutions (i.e. gyri and sulci) (Holloway 1978; Holloway et al. 2004; Falk 2014; Neubauer 2014). The imprinted cerebral surface corresponds to much of the forebrain, especially the neocortex, which includes visual, auditory, somatosensory, motor as well as association areas (Radinsky 1975). However, the information the neocortical surface provides is limited to the external morphology, and little can be said about the evolution of subcortical elements.
In 1978, Holloway defined at least six levels of useful evidence to be gleaned from endocasts, depending on their completeness and the replicability of neocortical details: (1) the gross brain size, which corresponds to the volume of neural mass; (2) the areal determination, which is the surface of the endocast divided into major lobar regions; (3) the major sulcal and gyral identifications and blood vessel patterns; (4) the identification of secondary and tertiary sulcal and gyral convolutions and their functional correspondence; and (5), to some extent, the subcortical relationships of these parameters.
Two levels received particular interest in palaeoneurology. Firstly, brain size has been extensively explored in the primate fossil record, even used as a criterion to define taxa at some points (e.g. definition of the genus Homo by Leakey et al. 1964). Secondly, the pattern of cerebral fissures apparent on the endocasts can suggest the location of cortical functional divisions (Kaas 2006). Based on cortical maps determined by neurophysiologists, the coincidence between architectonic and functional entities has been used to interpret fossil endocast (Radinsky 1975; Falk 1981, 1982). Moreover, sulcal variation has been demonstrated to be a reasonable predictor of cytoarchitecture for primary and secondary regions, such as visual, somatosensory and motor areas (Fischl et al. 2008). However, it has been shown that the cerebral areas delimitated by sulci on the external cortical surface do not systematically coincide with functional areas (Amunts et al. 1999).
4.1.2 General Evolutionary Trends and Comparative Studies of Living Mammals
Increase in size and development of the fissuration pattern are two key processes in primate brain evolution. In 1971, Le Gros Clark pointed out that “undoubtedly the most distinctive trait of the Primates, wherein this order contrasts with all other mammalian orders in its evolutionary history, is the tendency towards the development of a brain which is large in proportion to the total body weight, and which is particularly characterized by a relatively extensive and often richly convoluted cerebral cortex” (Le Gros Clark 1971: 227). In his review of primate brain evolution based on living mammal and fossil endocasts, Radinsky (1975) added supplementary fundamental neuronal changes: “To summarize, evolutionary trends suggested by comparative studies of the brains of living insectivores and primates include: increase in size of brain relative to body weight, increase in amount of neocortex (beyond what one would expect from the increase in brain size); decrease in relative size of olfactory bulbs; increase in amount of visual cortex and size of lateral geniculate body (possibly accounted for by the overall increase in brain size); development of a central sulcus in anthropoids rather than the coronal sulcus seen in prosimians” (Radinsky 1975: 659).
Several lines of evidence highlight various evolutionary trends described by Le Gros Clark and Radinsky, which could be summarized as follows: (1) relative increase in brain size, (2) relative increase of the neocortical surface, (3) reorganization of the sensory system and (4) fissuration pattern reorganization. The four lines are briefly detailed here.
4.1.2.1 Brain Size
On average, compared to other mammalian orders, living primates have larger brains relative to body size. Based on Radinsky’s estimations (1975), relative brain size in living strepsirhines exceeds from 2.4 to 7.0 times what would be expected in a basal insectivores (sensu Stephan et al., 1991) of comparable weight. The interpretation of the increase over time in primate brain size and the earliest evidence of this evolutionary trend were largely debated in the literature (for a review, see Radinsky 1982). Because several features of brain morphology scale to absolute brain size (e.g. volume of the frontal cortex, degree of gyrification), variation in the primate brain size is critical for understanding brain evolution and evolutionary process (Zilles et al. 1988; Semendeferi et al. 2002).
4.1.2.2 Neocortical Surface
Since its emergence in a mammalian ancestor, the neocortex has expanded in both relative and absolute size, particularly in anthropoid primates, in which the neocortex comprises up to 80% of the brain mass (Rakic and Kornack 2001). Insectivore brains are relatively small and have little neocortex with few (if any) neocortical folds. Indeed, folds appeared on the primate brain with the evolution of larger brains containing more neocortex (Radinsky 1975). The causes and mechanisms of cortical folding are largely debated in terms of either selective pressure or developmental and structural processes (Van Essen 1997; Hilgetag and Barbas 2005; Toro 2012; Zilles et al. 2013; Bayly et al. 2014; Ronan and Fletcher 2015; Tallinen et al. 2016).
4.1.2.3 Sensory System
Visual areas represent about 50% of the entire primate neocortex, and the neocortical expansion is associated with variance in functionally specific parts of the visual system (Barton 1998). Comparative studies between neural structures in extant primates demonstrate a greatly modified visual system in comparison with their earliest relatives, with an increased number of visual cortical areas enlarged and specialized for detailed vision (Felleman and Van Essen 1991; Kaas 2006). Besides the development of the visual cortex, the other remarkable change in early primate neocortex was the reorganization of the motor and premotor cortex. In contrast with their mammalian relatives, both strepsirhines and haplorhines have a large and well-defined primary motor field (M1) (Kaas 2006).
4.1.2.4 Fissuration Pattern
As described by Radinsky (1975), a longitudinally oriented fissure (i.e. the coronal sulcus) separates the representation of the head from that of the forelimb in the primary motor and somatosensory cortex in strepsirhines and in most mammals with a comparable degree of neocortical folding. On the contrary, in anthropoids, no major fissure exists in that position, and the primary motor cortex is instead separated from the primary somatosensory cortex by a transverse fissure (i.e. the central sulcus) (Radinsky 1975).
4.1.3 Reconstructing the Primate Brain Evolution from Extant Diversity and Fossil Endocasts
4.1.3.1 Evidence from Extant Diversity
All stages experienced in the course of primate brain evolution, from the simple smooth hemisphere to a highly convoluted one, are suggested to be represented among living primate taxa (Hill 1972). Based on previous work by Le Gros Clark (1971), Hill (1972) proposed an evolutionary model that included living mammals as representatives of each evolutionary stage, from fundamental to a more complex pattern. Accordingly, the elephant shrew Macroscelides, now included in the order Afrotheria, represents the basal brain morphology.
The diurnal tree shrew Tupaia was regarded as an example of the early reduction of the olfactory lobe and the development of visual areas in the primate lineage (Hill 1972). However, it now seems that many of the primate-like features observed in the visual system of tree shrews are actually evolutionary convergences that arose independently from those observed in primates (Kaas 2002). Such parallel evolution appears to also have occurred during early primate evolution: for instance, late Palaeocene to middle Eocene microsyopids probably expanded their cerebral cortex and evolved an improved visual sense independently from euprimates (Silcox et al. 2010).
Changes in cortical regions are characterized by different reorganizational tempos. Comparison of the myelo- and cytoarchitecture of the granular frontal cortex in the strepsirhine Galago and in the anthropoid Macaca suggests that considerable changes occurred in this cortical region during primate evolution, including the addition of new areas in anthropoids (Preuss and Goldman-Rakic 1991a). In contrast, many, although possibly not all, of the parietal and temporal association areas present in Macaca appear to have evolved early in primate history, prior to the divergence of the lineages that led to strepsirhines and haplorhines (Preuss and Goldman-Rakic 1991b).
The neopallium expansion in both strepsirhines and haplorhines is accompanied by a complex cortical folding and by a definite pattern of sulci (e.g. deep Sylvian fissure, calcarine complex, superior temporal sulcus, rectus sulcus, intraparietal sulcus). In Cebidae, Cercopithecidae and Colobidae, the hemispheres are enlarged, and the neocortical surface is richly convoluted, with the occurrence of a well-marked central sulcus and of various fissures on the occipital lobe, including the lunate sulcus. The degree of neocortical fissuration is particularly high in hominoids, where the visual neocortex is shifted further back, around the occipital pole on the mesial aspect of the cortex (von Bonin and Bailey 1961).
4.1.3.2 Evidence from Fossil Endocasts
Despite the paucity of available hard evidence, Radinsky pointed out that several of the known fossil specimens provide relevant information for understanding primate brain evolution, notably the tempo of the emergence of the extant primate condition, and published most of the earliest critical descriptions (Radinsky 1967, 1970, 1973, 1974). The fossil record suggests that the expansion of the visual cortex and the reduction in size of the olfactory bulbs that distinguish strepsirhines from basal insectivores had already appeared at the beginning of the second major primate evolutionary radiation, 55 million years ago. Indeed, from early Eocene deposits of North America, the euprimate Tetonius homunculus shows a remarkably advanced brain compared to the condition typical of basal insectivores, with enlarged occipital and temporal lobes and reduced olfactory bulbs. However, brain morphology remains less derived than later primates because of relatively small frontal lobes (Radinsky 1967, 1974). The features observed on the Tetonius’ endocast surface corroborate the results of the cytoarchitectonic studies published by Preuss and Goldman-Rakic (1991a, b). In addition, with the unique exception of a visible Sylvian fissure, in this fossil representative, the neocortical surface is featureless (Radinsky 1967, 1970), which is a typical feature of euprimates (Orliac et al. 2014), although also missing in another early euprimate, Smilodectes gracilis, a middle Eocene basal adapoid (Gazin 1965).
In early euprimates such as Tetonius, but also in Smilodectes gracilis, the presence of enlarged occipital and temporal cortical areas suggests that the expansion of these regions involved in optic and auditory functions, respectively, may have been one among the critical adaptations responsible for the early Eocene primate radiation (Radinsky 1967, 1970, 1974).
According to Radinsky, the anthropoid record suggests that, 25–30 million years ago, the visual cortex and the olfactory bulb relative size had reached the condition observed in extant taxa. The transversely oriented central sulcus, distinguishing modern anthropoids from most strepsirhines, was also present in at least some among the earliest anthropoids, such as Aegyptopithecus from the Oligocene deposits of Fayum, Egypt (Radinsky 1973, 1974).
4.2 Extracting, Reconstructing and Characterizing Fossil Endocasts
4.2.1 Extracting and Reconstructing Fossil Endocasts
When the neurocranium is filled with sediment during the fossilization process, information about brain morphology and organization may be recorded as a natural endocast. For fossil specimens not preserving a natural endocast, it is possible to generate an imprint of the endocranial surface by filling the braincase with casting material (Holloway et al. 2004). However, thanks to recent developments in imaging techniques, virtual endocasts may now be generated from computed (micro)tomography (CT and μCT) data (Zollikofer and Ponce de León 2005; Weber and Bookstein 2011; Neubauer 2014).
Dealing with the fossil record nearly invariably implies facing the problem of fragmentation and distortion of the remains. These factors can be partially balanced by using advanced virtual imaging techniques (Zollikofer et al. 1998; Zollikofer 2002; Gunz et al. 2009; Weber and Bookstein 2011). To illustrate the valuable contribution provided by virtual imaging to primate palaeoneurology, here we present three selected cases of fossil cercopithecoids from Plio-Pleistocene South African deposits: (1) the female individual MP 224 from the site of Makapansgat, attributed to Parapapio broomi by Freedman (1976); (2) the specimen STS 564 that corresponds to the holotype of Parapapio broomi, from Sterkfontein Member 4 (Freedman 1957); and (3) the female Parapapio antiquus TP 8 from Taung (formerly Tvl. 639), originally described by Freedman (1957, 1961). The specimens are currently housed at the University of the Witwatersrand, Johannesburg, and at the Ditsong National Museum of Natural History, Pretoria, and have been detailed by microtomography at the South African Nuclear Corporation (Necsa) of Pelindaba and at the Palaeosciences Centre of the University of the Witwatersrand, Johannesburg (spatial resolution ranging from 0.072 to 0.082 mm).
In principle, sediments can be removed digitally, and the endocranial cavity can be virtually separated from the braincase using semi-automatic threshold-based segmentation (Spoor et al. 1993; see also the region-based segmentation approach that relies on topographic concepts and which is known as the “watershed transform” in Meyer and Beucher 1990; Roerdink and Meijster 2001) (Fig. 4.1). Lately, additional methods were developed for the automatic extraction of endocasts. Among the most effective, the Endex software (Subsol et al. 2010; http://liris.cnrs.fr/gilles.gesquiere/wiki/doku.php?id=endex) is particularly suitable for specimens preserving an empty endocranial cavity. A mesh represented by a sphere is positioned inside the endocranial space and deformed along the three orthogonal directions (Fig. 4.2). Through this deformation process, the mesh moulds the inner cranial surface and produces a virtual endocast. Both regularity and constrained smoothing contribute to deform the mesh within the limits imposed by the braincase (e.g. the virtual endocast could not spread out of natural openings such as the foramen magnum).
The type of damage most commonly observed on fossil specimens results from post-depositional taphonomic dynamics and can be considered within three main categories: (1) missing parts (e.g. MP 224, STS 564), (2) fragment assembling (e.g. TP 8) and (3) plastic deformation (not specifically illustrated here, but see comments below).
4.2.1.1 Missing Parts
The fossil specimen may lack some endocranial areas, as in MP 224, where part of the right temporal region is missing, and in STS 564, lacking a significant portion of the left hemisphere (Fig. 4.3). When topographically limited, the missing parts can be estimated and reconstructed based on the remaining bony parts by using classical landmark-based geometric morphometric tools and algorithmic approaches (i.e. statistical and geometric reconstructions; Gunz et al. 2004, 2009). These methods rely on the fact that information captured by shape coordinates is typically redundant, especially when the measurement points are closely spaced (Gunz et al. 2009). The non-preserved regions can now be digitally and automatically closed by digitizing a curve network around the margin of the missing area and by creating a NURBS (non-uniform rational basis spline) surface that matches the points along the curves through, for instance, the Rhino v5.0 software (R. McNeel & Associates) (Benazzi et al. 2011). Here, the result of the application of the automatic closure process to the MP 224 Parapapio specimen from Makapansgat is illustrated in Fig. 4.3. When the missing regions affect a larger endocranial area (e.g. part of the cranium is not preserved), because of the bilateral symmetry of the brain, bony portions that are preserved on one side can be mirrored in order to reconstruct the missing side (Gunz et al. 2009). Here, the right hemisphere of the specimen STS 564 was mirrored to reconstruct the left counterpart (Fig. 4.3).
Virtual rendering of the South African fossil cercopithecoid specimens MP 224 (a Parapapio broomi from Makapansgat), STS 564 (b Parapapio broomi from Sterkfontein), and TP 8 (c from Parapapio antiquus from Taung) (top row) and digital extraction and reconstruction of the corresponding endocasts (middle and bottom rows) (Beaudet 2015; Beaudet et al. 2016). Surfaces in red and green represent the original and the corrected volumes, respectively. The missing temporal area in MP 224 has been digitally filled. The left hemisphere in STS 564 has been rendered by mirroring the right counterpart. The fissure running through the TP 8 right hemisphere has been corrected by digitally reassembling the two halves. Images not to scale
4.2.1.2 Fragment Assembling
Virtual reconstructions have also focused on assembling fragments. In some cases, pieces of skulls physically restored do not perfectly articulate, and this impacts on, and biases, the endocast reconstruction. However, once segmented out of volumetric images, virtual bones can be readily reassembled manually on the computer. Natural endocasts affected by breakages and separated into several fragments, such as in the case of the Parapapio antiquus endocast TP 8 from Taung divided in two halves because of an anteroposterior fracture, can be corrected by virtually reassembling the two parts (Fig. 4.3).
4.2.1.3 Crushing and Deformation
Plastic distortions affecting the bony remains or the natural endocasts are difficult to identify and assess, especially when the morphological inter- and intra-individual variability is unknown, which is nearly always the case for extinct species. The operation of reconstructing an ante-mortem shape from a deformed specimen is called “retrodeformation” (Tallman et al. 2014). Assuming a low degree of developmentally related normal deviation from symmetry, one can correct it by a symmetrization process (e.g. reflection and averaging method in Gunz et al. 2009 or algorithmic symmetrization in Tallman et al. 2014). As stated by Gunz et al. (2009), any reconstruction is based on assumptions about functional constraints, integration, symmetry as well as sex variation, species affinity and taphonomic distortion, regardless of whether it relies on plaster material handling or computer processing.
4.2.2 Characterizing Fossil Endocasts
In 1978, Holloway noted that “Most of the research done in primate (and other animal) paleoneurology is necessarily of a qualitative nature which can, and often does, incorporate each of the first five levels of description mentioned above-that is, whether gyrus x or sulcus y can be seen, whether the frontal lobe is relatively small or large (or smaller or larger than z), whether the primary visual striate cortex is expanded or not, or whether or not the olfactory bulbs are reduced” (Holloway 1978: 187). However, palaeoneurology has recently enlarged its traditional investigative toolkit by integrating methods of high-resolution imaging, 3D modelling and statistical analyses granting a higher degree of reliability of the quantitative and qualitative estimates. In addition to basic descriptions and traditional metrical analyses, recent incorporation and validation of computer-based techniques for reconstructing and comparing endocranial casts have substantially improved the quality of data delivered by endocasts (Zollikofer et al. 1998; Zollikofer 2002; Gunz et al. 2009; Weber and Bookstein 2011). Accordingly, the advanced methods in virtual palaeoanthropology contribute to the characterization of the organization and morphology of lobes and to the identification of gyral and sulcal pattern, corresponding, respectively, to levels (ii) and (iii) in Holloway’s description (Holloway 1978).
4.2.2.1 Deformation-Based Models: Interspecific Variation
Digital data make quantitative analysis of overall endocranial shape possible, notably through the landmark-/semilandmark-based geometric morphometrics (Bruner et al. 2009; Bruner 2004; Neubauer et al. 2009, 2010; Gunz 2015), or via the registration of surfaces from the correspondence of anatomical landmarks (Specht et al. 2007). However, even if efficient in compartmentalizing the endocranial cavity, the use of methodological toolkits based on landmarks and semilandmarks positioned on surfaces or extracted from curves captures little information about the brain itself and its subdivisions (Neubauer 2014). One potential alternative is to combine a detailed analysis of the sulcal pattern together with the characterization of the overall endocranial shape via the deformation-based models (Durrleman et al. 2012a, b; Beaudet et al. 2016; Dumoncel et al. 2014; Beaudet 2015).
The deformation-based model has been demonstrated to be a relevant tool for the registration of morphoarchitectural variations in primate endocranial ontogenetic trajectories, depicting both global and local changes (Durrleman et al. 2012a), and also valuable for taxonomic and evolutionary studies (Dumoncel et al. 2014; Beaudet 2015; Beaudet et al. 2016). Through the deformation computation process, both global and local changes could be rendered, compared and discussed. This mathematical approach relies on the construction of group-average surface models (i.e. global mean shape) and their deformation to the investigated surfaces (i.e. specimens). Contrary to landmark-based geometric morphometric analyses, comparison between surfaces does not assume a point-to-point correspondence between samples (Glaunès and Joshi 2006; Durrleman et al. 2012a, b; Dumoncel et al. 2014). The magnitude of displacements from the reference to specimens is rendered by colour maps from, for instance, dark blue (lowest values) to red (highest values) onto the surfaces. Figure 4.4 illustrates the displacements from a global mean shape to the cercopithecoid fossil specimens MP 224, STS 564 and TP 8 (see also Beaudet et al. 2016). When compared to the mean shape, MP 224 is antero-posteriorly elongated and the temporal lobes are convergent, while in STS 564 the parietal area is elevated and the volume of the temporal lobes is reduced. The large vectors recorded inferiorly for STS 564 and TP 8 may indicate taphonomic distortion and/or approximation in the segmentation process. It is noteworthy that the reconstructed temporal area in MP 224 does not affect the overall deformation results.
Comparative maps of morphological deformations from the global mean shape (a) to the South African fossil cercopithecoid specimens MP 224 (b Parapapio broomi from Makapansgat), STS 564 (c Parapapio broomi from Sterkfontein) and TP 8 (d Parapapio antiquus from Taung) (Modified from Beaudet et al. 2016). Cumulative displacement variations (in mm) are rendered by a pseudo-colour scale ranging from dark blue (lowest values) to red (highest values). Vectors represent the magnitude and the orientation of the deformations from the mean shape. Images not to scale
4.2.2.2 Deformation-Based Models: Intraspecific Variation
For an accurate evaluation of interspecific variation, intraspecific variability should be considered and quantified. Intraspecific studies could help one to elucidate whether different regions of the brain are more or less prone to evolve in a coordinated or independent way (Gómez-Robles et al. 2014). In the absence of large fossil assemblages, living populations have to be considered as reliable proxies for the assessment of reference variation patterns. Previous landmark-based studies attempted to characterize the primate endocranial and brain morphological variation (e.g. Gómez-Robles et al. 2013). The variability of primate brains, including those of humans, has been also explored and mapped using coarse spatial matching (e.g. Gilissen 2001; Zilles et al. 2001). In this respect, deformation-based models are particularly suitable (Durrleman 2010). Intra-group variability may be explored by performing a principal component analysis using the deformation momenta from the mean shape to specimens (Durrleman 2010). Deformations along the axis illustrate how the mean shape varies within the population from the mean to the standard deviation (σ) (i.e. the square root of variance, the corresponding eigenvalue). The extent of morphological variation revealed by a sample of eight endocasts representing five extant Papio taxa (anubis n = 2, cynocephalus n = 1, cynocephalus kindae n = 2, hamadryas n = 1 and ursinus n = 2) is shown in Fig. 4.5. In this case, a mean shape was computed from the Papio sample and deformed along the two axes of the principal component analysis. Along both PC1 and PC2, the observed variation mainly affects the temporal lobes and the superior part of the parietal area and, in some extent, the frontal pole, indicating that these regions are relatively variable within this taxon.
Morphological variability of the taxon mean shape (in grey) computed for the extant Papio endocasts along the first (PC1) and second (PC2) axes of the principal component (Beaudet 2015). Deformations illustrate how the mean shape varies within the sample from the mean to the standard deviation (+/−σ). Cumulative displacement variations (in mm) are rendered by a pseudo-colour scale ranging from dark blue (lowest values) to red (highest values). Images not to scale
4.2.2.3 Semi-automatic Sulci Detection
Because of variable degrees of preservation, individual variation and ambiguous homology, identification of cortical convolutions from endocast may be problematic (Conolly 1950; Holloway et al. 2004; Falk 2009). However, sulcal patterns reproduced on endocast provide important information for studying the evolution of the primate cerebral cortex, and significant effort should be performed in order to extract as much details from any available fossil specimen (Falk 2014). The sulcal pattern is usually identified and described by visual inspection of the endocranial surface (e.g. Holloway et al. 2004; Falk 2014). Based on previous similar studies (Subsol 1995, 1998), an automatic method for the detection of the neocortical reliefs based on the algorithm introduced by Yoshizawa and co-workers (2007, 2008) in 3D meshes of topographical variation (i.e. crest lines) has been recently developed (Beaudet 2015; Beaudet et al. 2016). Following the automatic detection process, manual corrections can be performed by removing the non-anatomical features using references from extant primates (e.g. Primate Brain Bank; Netherlands Institute for Neuroscience; the Netherlands, www.primatebrainbank.org; Comparative Mammalian Brain Collections; the University of Wisconsin; Michigan State University; and the National Museum of Health and Medicine, http://brainmuseum.org/).
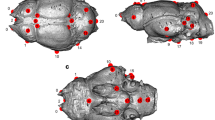
Results of the automatic detection and manual correction computed on the three fossil specimens used as examples in this contribution are illustrated in Fig. 4.6, where they have been compared to the condition of a living baboon individual (Papio hamadryas) derived from the online database Primate Brain Bank (see also Beaudet et al. 2016). In STS 564, only the right hemisphere is represented. The three specimens preserve the arcuate (arc) and principal (p) sulci, as well as part of the Sylvian fissure(s). Imprints of the lateral calcarine (lc) and inferior occipital sulcus (oci) are visible in STS 564, while the central (c), intraparietal (ip) and temporal superior (ts) sulci are detectable in MP 224 and STS 564. These results convincingly demonstrate that, in the case of the fossil record, the variable degree of preservation of the investigated specimens, the quality of the imaging system and the efficiency of the semi-automatic detection all influence the degree of reliability of the sulcal pattern assessment based on fossil endocasts (Fig. 4.6). Moreover, the correspondence of endocranial impressions to cerebral sulci and gyri has been largely questioned in primate palaeoneurology (e.g. Le Gros Clark et al. 1936), and further studies are needed to assess the reliability of cerebral cranial imprints for inferring fossil neuroanatomical evidence (e.g. Kobayashi et al. 2014).
Superior view of the brain of a specimen of the extant Papio hamadryas (a image courtesy of the Primate Brain Bank, Netherlands Institute for Neuroscience, the Netherlands) compared with virtual reconstructions of the endocasts extracted from the South African fossil cercopithecoid specimens MP 224 (b Parapapio broomi from Makapansgat), STS 564 (c Parapapio broomi from Sterkfontein) and TP 8 (d Parapapio antiquus from Taung), also rendered with their sulcal impressions (in upper and lateral views) (Modified from Beaudet et al. 2016). Because in TP 8 the left hemisphere was reconstructed as mirrored surface of the right counterpart, only the right side is annotated. Arc arcuate sulcus, ip intraparietal sulcus, lc lateral calcarine sulcus, oci inferior occipital sulcus, p principal sulcus, s Sylvian (lateral) fissure, sca subcentral anterior sulcus, scp subcentral posterior sulcus, ts superior temporal sulcus. Only the sulci mentioned in the text are labelled here. Images not to scale
4.3 Conclusions and Perspectives
Although the primate fossil record had steadily increased, subtle aspects concerning the tempo and mode of some of the most critical changes occurred during brain evolution – i.e. increase in brain size and expansion of the neocortical surface and reorganization of the sensory system and of the fissuration pattern – remain to be elucidated. Endocasts, the only direct evidence of extinct neuroanatomical conditions, preserve two fundamental bodies of information: the gross cerebral morphology and the sulcal pattern (Holloway 1978). Recent advances in the field of palaeoneurological research convincingly demonstrate that, despite some unavoidable limitations intimately related to fossilization dynamics, such information can be virtually extracted through automatic segmentation methods (e.g. Endex software, watershed transform) and confidently reconstructed in cases of missing data, fragment assembling or plastic deformation via mirroring processes or automatic closure applications. Combining the quantitative description of the overall endocranial conformation and the sulcal pattern through newly developed analytical approaches offers the unique opportunity to track some of the finest cortical changes that occurred in primate neuroanatomy evolution. Also, because the deformation-based models do not assume a point-to-point correspondence between samples, as it is the case of classical landmark-based geometric morphometrics, and concern the overall endocranial surface, this methodology represents a powerful tool for the comparative assessment of most neuroanatomical features, especially given the potential modular organization of the brain and the associated mosaic evolutionary patterns described for some taxa (Preuss and Goldman-Rakic 1991a, b; Gómez-Robles et al. 2014). Since sulcal variation has been accepted as a reasonable predictor of cytoarchitecture for primary and secondary regions such as the visual, somatosensory and motor areas (Fischl et al. 2008), an accurate description of the neocortical sulci topographic distribution and arrangement is necessary for detecting the functional determinants of cerebral changes and for challenging ongoing evolutionary hypotheses. In this perspective, a further investigative step will be represented by the development of analytical protocols granting the automatic sulci recognition and labelling in fossil endocasts, as already occurs for human brains (e.g. BrainVISA, http://brainvisa.info/index_f.html).
References
Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HBM, Zilles K (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412:319–341
Armstrong E, Falk D (1982) Primate brain evolution: methods and concepts. Plenum Press, New York
Barton RA (1998) Visual specialization and brain evolution in primates. Proc Biol Sci 265:1933–1937
Barton RA, Harvey PH (2000) Mosaic evolution of brain structure in mammals. Nature 405:1055–1058
Bayly P, Taber L, Kroenke C (2014) Mechanical forces in cerebral cortical folding: a review of measurements and models. J Mech Behav Biomed Mater 29:568–581
Beaudet A (2015) Caractérisation des Structures Crânio-Dentaires Internes des Cercopithécoïdes et Etude Diachronique de leurs Variations Morphologiques dans la Séquence Plio-Pléistocène Sud-Africaine. PhD dissertation, Université de Toulouse
Beaudet A, Dumoncel J, de Beer F, Duployer B, Durrleman S, Gilissen E, Hoffman J, Tenailleau C, Thackeray JF, Braga J (2016) Morphoarchitectural variation in South African fossil cercopithecoid endocasts. J Hum Evol 101:65–78
Benazzi S, Bookstein FL, Strait DS, Weber GW (2011) A new OH5 reconstruction with an assessment of its uncertainty. J Hum Evol 61:75–88
Brain CK (1981) The hunters of the hunted? An introduction to African cave Taphonomy. University of Chicago Press, Chicago
Bruner E (2004) Geometric morphometrics and paleoneurology: brain shape evolution in the genus Homo. J Hum Evol 47:279–303
Bruner E, Mantini S, Ripani M (2009) Landmark-based analysis of the morphological relationship between endocranial shape and traces of the middle meningeal vessels. Anat Rec 292:518–527
Connolly CJ (1950) External morphology of the primate brain. C.C. Thomas, Springfield
de Winter W, Oxnard CE (2001) Evolutionary radiations and convergences in the structural organization of the mammalian brain. Nature 409:710–714
Dumoncel J, Durrleman S, Braga J, Jessel J-P, Subsol G (2014) Landmark-free 3D method for comparison of fossil hominins and hominids based on endocranium and EDJ shapes. Am J Phys Anthropol 153(suppl 56):110 (abstract)
Durrleman S (2010) Statistical models of currents for measuring the variability of anatomical curves, surfaces and their evolution. PhD dissertation, Université Nice-Sophia Antipolis
Durrleman S, Pennec X, Trouvé A, Ayache N, Braga J (2012a) Comparison of the endocranial ontogenies between chimpanzees and bonobos via temporal regression and spatiotemporal registration. J Hum Evol 62:74–88
Durrleman S, Prastawa M, Korenberg JR, Joshi S, Trouvé A, Gerig G (2012b) Topology preserving atlas construction from shape data without correspondence using sparse parameters. In: Ayache N, Delingette H, Golland P, Mori K (eds) MICCAI 2012, Part III. LNCS, vol 7512. Springer, Heidelberg, pp 223–230
Falk D (1981) Sulcal patterns of fossil Theropithecus baboons: phylogenetic and functional implications. Int J Primatol 2:57–69
Falk D (1982) Mapping fossil endocasts. In: Armstrong E, Falk D (eds) Primate brain evolution: methods and concepts. Plenum Publishing Company, New York, pp 217–226
Falk D (2009) The natural endocast of Taung (Australopithecus africanus): insights from the unpublished papers of Raymond Arthur Dart. Am J Phys Anthropol 49:49–65
Falk D (2014) Interpreting sulci on hominin endocasts: old hypotheses and new findings. Front Hum Neurosci 8:134
Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1:1–47
Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BT, Mohlberg H, Amunts K, Zilles K (2008) Cortical folding patterns and predicting cytoarchitecture. Cereb Cortex 18:1973–1980
Freedman L (1957) The fossil Cercopithecoidea of South Africa. Ann Transv Mus 23:121–262
Freedman L (1961) New cercopithecoid fossils, including a new species, from Taung, Cape Province, South Africa. Ann S Afr Mus 46:1–14
Freedman L (1976) South African fossil Cercopithecoidea: a re-assessment including a description of new material from Makapansgat, Sterkfontein and Taung. J Hum Evol 5:297–315
Gazin CL (1965) An endocranial cast of the Bridger middle Eocene primate Smilodectes gracilis. Smithson Misc Coll 149:1–14
Gilissen E (2001) Structural symmetries and asymmetries in human and chimpanzee brains. In: Falk D, Gibson KR (eds) Evolutionary anatomy of the primate cerebral cortex. Cambridge University Press, Cambridge, pp 187–215
Glaunès JA, Joshi S (2006) Template estimation from unlabeled point set data and surfaces for computational anatomy. In: Pennec X, Joshi S (eds) Proceedings of the international workshop on the Mathematical Foundations of Computational Anatomy. Copenhagen, pp 29–39
Gómez-Robles A, Hopkins D, Sherwood C (2013) Increased morphological asymmetry, evolvability and plasticity in human brain evolution. Proc R Soc B Biol Sci 280:20130575
Gómez-Robles A, Hopkins D, Sherwood CC (2014) Modular structure facilitates mosaic evolution of the brain in chimpanzees and humans. Nat Comm 5:4469
Gonzales LA, Benefit BR, McCrossin ML, Spoor F (2015) Cerebral complexity preceded enlarged brain size and reduced olfactory bulbs in old world monkeys. Nat Comm 6:7580
Gunz P (2015) Computed tools for paleoneurology. In: Bruner E (ed) Human paleoneurology. Springer, Zurich, pp 39–55
Gunz P, Mitteroecker P, Bookstein, FL, Weber GW (2004) Computer-aided reconstruction of incomplete human crania using statistical and geometrical estimation methods. Enter the past: computer applications and quantitative methods in archaeology. BAR International Series, Oxford, pp 96–98
Gunz P, Mitteroecker P, Neubauer S, Weber GW, Bookstein FL (2009) Principles for the virtual reconstruction of hominin crania. J Hum Evol 57:48–62
Hilgetag CC, Barbas H (2005) Developmental mechanics of the primate cerebral cortex. Anat Embryol 210:411–417
Hill WCO (1972) Evolutionary biology of the primates. Academic, London
Holloway RL (1978) The relevance of endocasts for studying primate brain evolution. In: Noback CR (ed) Sensory systems of primates. Plenum Press, New York, pp 181–200
Holloway RL, Broadfield DC, Yuan MS (2004) The human fossil record: brain endocasts – the paleoneurological evidence. Wiley-Liss, New York
Kaas JH (2002) Convergences in the modular and areal organization of the forebrain of mammals: implications for the reconstruction of forebrain evolution. Brain Behav Evol 59:262–272
Kaas JH (2006) Evolution of the neocortex. Curr Biol 16:910–914
Kobayashi Y, Matsui T, Haizuka Y, Ogihara N, Hirai N, Matsumura G (2014) Cerebral sulci and gyri observed on macaque endocasts. In: Akazawa T, Ogihara N, Tanabe HC, Terashima H (eds) Dynamics of learning in Neanderthals and modern humans, vol 2. Springer, Japan, pp 131–137
Le Gros Clark WE (1971) The antecedents of man: an introduction to the evolution of the primates. Edinburgh University Press, Edinburgh
Le Gros Clark WE, Cooper DM, Zuckerman S (1936) The endocranial cast of the chimpanzee. J Roy Anthropol Inst 66:249–268
Leakey LSB, Tobias PV, Napier JR (1964) A new species of genus Homo from Olduvai Gorge. Nature 202:7–9
Meyer F, Beucher S (1990) Morphological segmentation. J Vis Commun Image Represent 1:21–46
Neubauer S (2014) Endocasts: possibilities and limitations for the interpretation of human brain evolution. Brain Behav Evol 84:117–134
Neubauer S, Gunz P, Hublin J-J (2009) The pattern of endocranial ontogenetic shape changes in humans. J Anat 215:240–255
Neubauer S, Gunz P, Hublin J-J (2010) Endocranial shape changes during growth in chimpanzees and humans: a morphometric analysis of unique and shared aspects. J Hum Evol 59:555–566
Orliac MJ, Ladevèze S, Gingerich PD, Lebrun R, Smith T (2014) Endocranial morphology of Palaeocene Plesiadapis tricuspidens and evolution of the early primate brain. Proc R Soc B 281:20132792
Preuss TM, Goldman-Rakic PS (1991a) Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol 310:429–474
Preuss TM, Goldman-Rakic PS (1991b) Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared to the anthropoid primate Macaca. J Comp Neurol 310:475–506
Radinsky L (1967) The oldest primate endocast. Am J Phys Anthropol 27:385–388
Radinsky L (1970) The fossil evidence of prosimian brain evolution. In: Noback CR, Montagna W (eds) Primate brain. Appleton-Century-Croft, New York, pp 209–224
Radinsky L (1973) Aegyptopithecus endocasts: oldest record of a pongid brain. Am J Phys Anthropol 39:239–247
Radinsky L (1974) The fossil evidence of anthropoid brain evolution. Am J Phys Anthropol 41:15–28
Radinsky L (1975) Primate brain evolution. Am Sci 63:656–663
Radinsky L (1982) Some cautionary notes on making inferences about relative brain size. In: Armstrong E, Falk D (eds) Primate brain evolution: methods and concepts. Plenum, New York, pp 29–37
Rakic P, Kornack DR (2001) Neocortical expansion and elaboration during primate evolution: a view from neuroembryology. In: Falk D, Gibson KR (eds) Evolutionary anatomy of the primate cerebral cortex. Cambridge University Press, Cambridge, pp 30–56
Roerdink JBTM, Meijster A (2001) The watershed transform: definitions, algorithms and parallelization strategies. Fund Inform 41:187–228
Rogers J, Kochunov P, Zilles K, Shelledy W, Lancaster J, Thompson P, Duggirala R, Blangero J, Fox PT, Glahn DC (2010) On the genetic architecture of cortical folding and brain volume in primates. NeuroImage 53:1103–1108
Ronan L, Fletcher PC (2015) From genes to folds: a review of cortical gyrification theory. Brain Struct Funct 220:2475–2483
Semendeferi K, Lu A, Schenker N, Damasio H (2002) Humans and great apes share a large frontal cortex. Nat Neurosci 5:272–276
Silcox MT, Benham AE, Bloch JI (2010) Endocasts of Microsyops (Microsyopidae, primates) and the evolution of the brain in primitive primates. J Hum Evol 58:505–521
Specht M, Lebrun R, Zollikofer CPE (2007) Visualizing shape transformation between chimpanzee and human braincases. Vis Comput 23:743–751
Spoor F, Zonneveld F, Macho GA (1993) Linear measurements of cortical bone and dental enamel by computed tomography: applications and problems. Am J Phys Anthropol 91:469–484
Stephan H, Baron G, Frahm HD (1991) Comparative brain research in mammals volume 1. Insectivora. Springer, New York
Subsol G (1995) Construction Automatique d’Atlas Anatomiques Morphométriques à Partir d’Images Médicales Tridimensionnelles. PhD dissertation, Ecole Centrale de Paris
Subsol G (1998) Crest lines for curve based warping. In: Toga A (ed) Brain warping. Academic Press, San Diego, pp 241–262
Subsol G, Gesquière G, Braga J, Thackeray F (2010) 3D automatic methods to segment “virtual” endocasts: state of the art and future directions. Am J Phys Anthropol 141(suppl 50):226–227
Tallinen T, Chung JY, Rousseau F, Girard N, Lefèvre J, Mahadevan L (2016) On the growth and form of cortical convolutions. Nat Phys. doi:10.1038/nphys3632
Tallman M, Amenta N, Delson E, Frost SR, Ghosh D, Klukkert ZS, Morrow A, Sawyer GJ (2014) Evaluation of a new method of fossil retrodeformation by algorithmic symmetrization: crania of papionins (primates, Cercopithecidae) as a test case. PLoS One 9(7):e100833
Toro R (2012) On the possible shapes of the brain. Evol Biol 39:600–612
Van Essen DC (1997) A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385:313–318
von Bonin G, Bailey P (1961) Pattern of the cerebral isocortex. Primatologia II/2. Karger, New York
Weber GW, Bookstein FL (2011) Virtual anthropology: a guide to a new interdisciplinary field. Springer, London
Welker W (1990) Why does cerebral cortex fissure and fold? A review of determinants of gyri and sulci. In: Jones EG, Peters A (eds) Cerebral cortex. Vol 8b. Comparative structure and evolution of cerebral cortex, Part II. Plenum Press, New York, pp 3–136
Yoshizawa S, Belyaev A, Yokota H, Seidel HP (2007) Fast and faithful geometric algorithm for detecting crest lines on meshes. Proceedings of the 15th Pacific conference on computer graphics and applications, pp 231–237
Yoshizawa S, Belyaev A, Yokota H, Seidel HP (2008) Fast, robust, and faithful methods for detecting crest lines on meshes. Comput Aided Geom D 25:545–560
Zilles K, Armstrong E, Schleicher A, Kretschmann HJ (1988) The human pattern of gyrification in the cerebral cortex. Anat Embryol 179:173–179
Zilles K, Kawashima R, Dabringhaus A, Fukuda H, Schormann T (2001) Hemispheric shape of European and Japanese brains: H 3-D MRI analysis of intersubject variability, ethnical, and gender differences. NeuroImage 13:262–271
Zilles K, Palomero-Gallagher N, Amunts K (2013) Development of cortical folding during evolution and ontogeny. Trends Neurosci 36:275–284
Zollikofer CPE (2002) A computational approach to paleoanthropology. Evol Anthropol 11:64–67
Zollikofer CPE, Ponce de León MS (2005) Virtual reconstruction: a primer in computer-assisted paleontology and biomedicine. Wiley Interscience, Hoboken
Zollikofer CPE, Ponce de León MS, Martin RD (1998) Computer-assisted paleoanthropology. Evol Anthropol 6:41–54
Acknowledgements
We are grateful to the editors E. Bruner, N. Ogihara and H. Tanabe for their kind invitation to contribute this volume. We are indebted to J. Cuisin (Paris), G. Fleury (Toulouse), S. Potze (Pretoria), W. Wendelen (Tervuren) and B. Zipfel (Johannesburg) for having granted access to fossil and comparative materials under their care from the Ditsong National Museum of Natural History (Pretoria), the Musée d’Histoire naturelle in Toulouse, the Musée national d’Histoire naturelle (Paris), the Royal Museum for Central Africa (Tervuren) and the University of the Witwatersrand (Johannesburg). We also thank K. Carlson and T. Jashashvili (Johannesburg), G. Clément and M. Garcia-Sanz (Paris), B. Duployer and C. Tenailleau (Toulouse), L. Bam, F. de Beer and J. Hoffman (Pretoria) for microtomographic acquisitions performed at the Accès Scientifique à la Tomographie à Rayons-X (AST-RX) imagery platform set at the Musée national d’Histoire naturelle (Paris), at the French Research Federation FERMaT (Toulouse), at the Palaeosciences Centre of the University of the Witwatersrand (Johannesburg) and at the South African Nuclear Corporation (Pelindaba). For scientific contribution and/or discussion and comments to the results summarized in this study, we are especially grateful to J. Braga (Toulouse), L. Bruxelles (Toulouse), M. Cazenave (Pretoria), E. Delson (New York), J. Dumoncel (Toulouse), S. Durrleman (Paris), D. Ginibriere (Toulouse), J. Heaton (Birmingham), R. Holgate (Pretoria), N. Jablonski (University Park), J.P. Jessel (Toulouse), O. Kullmer (Frankfurt), R. Macchiarelli (Poitiers & Paris), M. Nakatsukasa (Kyoto), L. Pan (Toulouse), G. Subsol (Montpellier), D. Stratford (Johannesburg), J.F. Thackeray (Johannesburg) and C. Zanolli (Toulouse). The French research federation FERMaT (FR3089), the National Research Foundation (NRF) and Department of Science and Technology (DST) of South Africa are acknowledged for providing micro-X-ray tomography laboratory facilities. This work was granted access to the HPC resources of CALMIP supercomputing centre under the allocation 2015-[P1440] attributed to the laboratory AMIS (Toulouse). Research is supported by the Centre of Research and Higher Education (PRES) of Toulouse, the Midi-Pyrénées Region and the French Ministry of Foreign Affairs.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Japan KK
About this chapter
Cite this chapter
Beaudet, A., Gilissen, E. (2018). Fossil Primate Endocasts: Perspectives from Advanced Imaging Techniques. In: Bruner, E., Ogihara, N., Tanabe, H. (eds) Digital Endocasts. Replacement of Neanderthals by Modern Humans Series. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56582-6_4
Download citation
DOI: https://doi.org/10.1007/978-4-431-56582-6_4
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56580-2
Online ISBN: 978-4-431-56582-6
eBook Packages: Social SciencesSocial Sciences (R0)