Abstract
Background: Germinal matrix hemorrhage (GMH) is a neurological disorder associated with very low birth weight premature infants. This event can lead to post-hemorrhagic hydrocephalus, cerebral palsy, and mental retardation. This study developed a novel animal model for pre-clinical investigations.
Methods: Neonatal rats underwent infusion of clostridial collagenase into the right germinal matrix (anterior caudate) region using stereotaxic techniques. Developmental milestones were evaluated over 10 days, cognitive function at 3 weeks, and sensorimotor function at 4 weeks after collagenase infusion. This was accomplished by anthropometric quantifications of cranial, cerebral, cardiac, and splenic growths.
Results: Collagenase infusion led to delays in neonatal developmental milestones, followed by cognitive and sensorimotor dysfunctions in the juvenile animals. Cranial growth was accelerated during the first week after injury, and this was followed by significant brain atrophy, splenomegaly, and cardiac hypertrophy 3 weeks later.
Conclusion: This study characterized the developmental delays, mental retardation, and cerebral palsy features resembling the long-term clinical course after germinal matrix hemorrhage in premature infants. Pre-clinical testing of therapeutics in this experimental model could lead to improved patient outcomes while expanding upon the pathophysiological understanding of this disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
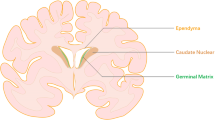
Germinal matrix hemorrhage (GMH) is the rupture of immature blood vessels within the subventricular (anterior caudate) progenitor cell region of neonatal brains [1] during the first 7 days of life [2]. GMH occurs in 20–25% of very low birth weight (VLBW ≤ 1,500 g) premature infants [3–5] and affects 3.5/1,000 births in the United States each year [6]. This is an important clinical problem, since the consequences are hydrocephalus (post-hemorrhagic ventricular dilation), cognitive and motor developmental delay, cerebral palsy, and mental retardation [4, 7]. However, available animal models to study the pathophysiological basis of these outcomes are lacking [8].
An important research priority is the development and validation of experimental models of brain hemorrhage for translational studies of human conditions [9]. Elevated MMP-2 and MMP-9 are associated with GMH induction in humans [10, 11]. Stereotaxic collagenase infusion is one of the most commonly used methods in adult experimental intracerebral hemorrhage (ICH) studies [12, 13] and functions as an MMP to lyse the extracellular-matrix around blood vessels to cause vascular rupture [13, 14]. This approach enables investigations of neurological and brain injury outcomes [12–19].
In this study, we hypothesized that unilateral germinal-matrix collagenase infusion in neonatal rats would model features similar to clinical GMH [4, 7]. With this approach, applications of therapeutic strategies can be tested to improve outcomes and to gain a better pathophysiological understanding of this disease [9].
Methods and Materials
Animal Groups and General Procedures
This study was in accordance with the National Institutes of Health guidelines for the treatment of animals and was approved by the Institutional Animal Care and Use Committee at Loma Linda University. Timed pregnant Sprague-Dawley rats were housed with food and water available ad libitum. Postnatal day 7 (P7) pups were blindly assigned to the following (n = 8/group): sham (naive), needle (control), and collagenase infusion. All groups were evenly divided within each litter.
Experimental Model of GMH
Using an aseptic technique, rat pups were gently anesthetized with 3% isoflurane (in mixed air and oxygen) while placed prone on a stereotaxic frame. Betadine sterilized the surgical scalp area, which was incised in the longitudinal plane to expose the skull and reveal the bregma. The following stereotactic coordinates were determined: 1 mm (anterior), 1.5 mm (lateral) and 3.5 mm (ventral) from bregma. A bore hole (1 mm) was drilled, into which a 27-gauge needle was inserted at a rate of 1 mm/min. A microinfusion pump (Harvard Apparatus, Holliston, MA) infused 0.3 units of clostridial collagenase VII-S (Sigma, St Louis, MO) through a Hamilton syringe. The needle remained in place for an additional 10 min after injection to prevent “back-leakage.” After needle removal, the burr hole was sealed with bone wax, the incision sutured closed, and the animals were allowed to recover. The entire surgery took an average of 20 min. Upon recovering from anesthesia, the animals were returned to their dams. Needle controls consisted of needle insertion alone without collagenase infusion, while naïve animals did not receive any surgery.
Developmental Milestones
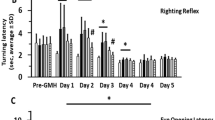
Animals were assessed over 10 days after collagenase infusion. For the righting reflex, time needed for the rat pups to completely roll over onto all four limbs after being placed on their backs was measured [20]. For negative geotaxis, the time needed for complete rotation (180°) after being placed head down on a slope (20° angle), was recorded [20]. The maximum allotted time was 60 s/trial (two trials/day).
Cognitive Measures
Higher order brain function was assessed during the third week after collagenase infusion. The T-Maze assessed short-term (working) memory [21]. Rats were placed into the stem (40 cm × 10 cm) of a maze and allowed to explore until one arm (46 cm × 10 cm) was chosen. From the sequence of ten trials, of left and right arm choices, the rate of spontaneous alternation (0% = none and 100% = complete, alternations/trial) was calculated, as routinely performed [22, 23]. The Morris water maze assessed spatial learning and memory on four daily blocks, as described previously in detail [16, 17]. The apparatus consisted of a metal pool (110 cm diameter), filled to within 15 cm of the upper edge, with a platform (11 cm diameter) for the animal to escape onto, that changed location for each block (maximum = 60 s/trial), and was digitally analyzed by Noldus Ethovision tracking software. Cued trials measured place learning with the escape platform visible above water. Spatial trials measured spatial learning with the platform submerged, and probe trials measured spatial memory once the platform had been removed. For the locomotor activity, in an open field, the path length in open-topped plastic boxes (49 cm-long, 35.5 cm-wide, 44.5 cm-tall) was digitally recorded for 30 min and analyzed by Noldus Ethovision tracking software [17].
Sensorimotor Outcome
At 4 weeks after collagenase infusion, animals were tested for functional ability. Neurodeficit was quantified using a summation of scores (maximum = 12), given for (1) postural reflex, (2) proprioceptive limb placing, (3) back pressure towards the edge, (4) lateral pressure towards the edge, (5) forelimb placement, and (6) lateral limb placement (2 = severe, 1 = moderate, 0 = none), as routinely performed [22]. For the rotarod, striatal ability was assessed using an apparatus consisting of a horizontal, accelerated (2 rpm/5 s), rotating cylinder (7 cm diameter × 9.5 cm wide), requiring continuous walking to avoid falling recorded by photobeam circuit (Columbus Instruments) [16, 17]. For foot fault, the number of complete limb missteps through the openings, was counted over 2 min while exploring over an elevated wire (3 mm) grid (20 cm × 40 cm) floor [23].
Assessment of Growth
Over 28 days after collagenase infusion, the head (width and height) and rump-to-crown (length) measurements were performed using a Boley Gauge (Franklin Dental Supply, Bellmore, NY), as previously described [24]. Head width was measured anterior to the side of the ears, head height from posterior to the adjacent mandible, and rump-to-crown was the greatest cranial (caudal) to tail (rostral) extension. At the completion of experiments, the brains were removed, and hemispheres separated by a midline incision (loss of brain weight has been used as the primary variable to estimate brain damage in juvenile animals after neonatal brain injury [25]). For organ weights, the spleen and heart were separated from surrounding tissue and vessels. The quantification was performed using an analytical microbalance (model AE 100; Mettler Instrument Co., Columbus, OH) capable of 1.0 μg precision.
Statistical Analysis
Significance was considered at P < 0.05. Data were analyzed using analysis of variance (ANOVA), with repeated measures (RM-ANOVA) for long-term neurobehavior. Significant interactions were explored with conservative Scheffe post hoc and Mann-Whitney rank sum tests when appropriate.
Results
Collagenase infusion delayed the developmental acquisition of eye opening, negative geotropism and righting reflex by 2–3 days (Fig. 1a–c, P < 0.05). Three weeks after GMH, significant deficits were discovered in spatial learning and memory (Fig. 2a, b, P < 0.05), T-maze (working) memory (Fig. 2c, P < 0.05), and hyperactivity, in the open field (decreased corner time and increased center crossings, Fig. 2d, e, P < 0.05). Juvenile animals had significant sensorimotor dysfunction, as revealed by the neurodeficit score, accelerating rotarod and foot fault (Fig. 3a–c, P < 0.05). These dysfunctions were associated with increased cranial size at 7 days (Fig. 4a, P < 0.05), and dysfunctional growth of the body, brain, heart, and spleen (Fig. 4b–e, P < 0.05) 3 weeks later.
Cognitive dysfunction: Higher order function was measured at the 3rd week after collagenase infusion. (a) Cued and spatial learning water maze, (b) probe (spatial memory) water maze, (c) T-maze, (d) open field (percent time in corner), and (e) open field (center crossing frequency). Values expressed as mean ± 95th C.I. (probe quadrant) or mean ± SEM (all others), n = 8 (per group), *P < 0.05 compared with controls (sham and needle trauma) and ‡P < 0.05 compared with block 1 (spatial learning water maze)
Discussion
Germinal matrix hemorrhage (GMH) is an important problem affecting approximately 12,000 births in the United States each year [6]. The clinical consequences of GMH are developmental delay, cerebral palsy, and mental retardation [4, 7]. In this study collagenase was infused into the germinal matrix of neonatal rats as an approach to model these features, since animal models to study the basis of these outcomes are lacking [8].
This neonatal rat model of GMH resembles the neurological consequences seen in the pediatric population after hemorrhagic brain injury. Collagenase infusion led to developmental delays in the neonates that were followed by cognitive and sensorimotor dysfunction in the juvenile developmental stage. The cranium was enlarged compared to somatic growth during the first week, with significant brain atrophy 3 weeks later. This presentation is likely a reflection of hydrocephalic cerebrospinal fluid build-up, leading to cranial expansion and compression of the brain tissue into an atrophic developmental growth pattern. Splenomegaly and cardiac hypertrophy presented at 1 month after injury, and this could either be a reflection of the disproportionate somatic growth or of prolonged peripheral hemostatic or inflammatory consequences of the brain bleed.
In summary, we have characterized a highly reliable and easily reproducible experimental model of germinal matrix hemorrhage using neonatal rats. This provides the basis for studying the clinical and pathophysiological features of this disease, and establishes a foundation for performing further preclinical therapeutic investigations.
Acknowledgement This study is partially supported by NIH NS053407 to J.H. Zhang and NS060936 to J. Tang.
References
Ballabh P (2010) Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res 67:1–8. doi:10.1203/PDR.0b013e3181c1b176
Kadri H, Mawla AA, Kazah J (2006) The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst 22:1086–1090. doi:10.1007/s00381-006-0050-6
Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, Simon NP, Wilson DC, Broyles S, Bauer CR, Delaney-Black V, Yolton KA, Fleisher BE, Papile LA, Kaplan MD (2000) Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics 105:1216–1226
Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ (2002) Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed 87:F37–F41
Vermont-Oxford (1990) The Vermont-Oxford Trials Network: very low birth weight outcomes for 1990. Investigators of the Vermont-Oxford Trials Network Database Project. Pediatrics 91:540–545
Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B (2010) Annual summary of vital statistics: 2007. Pediatrics 125:4–15. doi:10.1542/peds.2009-2416 [pii]
Ballabh P, Braun A, Nedergaard M (2004) The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16:1–13. doi:10.1016/j.nbd.2003.12.016S0969996103002833 [pii]
Balasubramaniam J, Del Bigio MR (2006) Animal models of germinal matrix hemorrhage. J Child Neurol 21:365–371
NINDS ICH Workshop Participants (2005) Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke 36:e23–e41. doi:01.STR.0000155685.77775.4c [pii] 10.1161/01.STR.0000155685.77775.4c
Cockle JV, Gopichandran N, Walker JJ, Levene MI, Orsi NM (2007) Matrix metalloproteinases and their tissue inhibitors in preterm perinatal complications. Reprod Sci 14:629–645. doi:14/7/629 [pii]10.1177/1933719107304563
Schulz CG, Sawicki G, Lemke RP, Roeten BM, Schulz R, Cheung PY (2004) MMP-2 and MMP-9 and their tissue inhibitors in the plasma of preterm and term neonates. Pediatr Res 55:794–801. doi:10.1203/01.PDR.0000120683.68630.FB01.PDR.0000120683.68630.FB [pii]
MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, Colbourne F (2008) Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab 28:516–525. doi:9600548 [pii]10.1038/sj.jcbfm.9600548
Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M (1990) Collagenase-induced intracerebral hemorrhage in rats. Stroke 21:801–807
Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT (1994) Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg 81:93–102
Foerch C, Arai K, Jin G, Park KP, Pallast S, van Leyen K, Lo EH (2008) Experimental model of warfarin-associated intracerebral hemorrhage. Stroke 39:3397–3404. doi:STROKEAHA.108.517482 [pii]10.1161/STROKEAHA.108.517482
Lekic T, Hartman R, Rojas H, Manaenko A, Chen W, Ayer R, Tang J, Zhang JH (2010) Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J Neurotrauma 27:627–637. doi:10.1089/neu.2009.1163
Hartman R, Lekic T, Rojas H, Tang J, Zhang JH (2009) Assessing functional outcomes following intracerebral hemorrhage in rats. Brain Res 1280:148–157. doi:S0006-8993(09)00957-3 [pii]10.1016/j.brainres.2009.05.038
Andaluz N, Zuccarello M, Wagner KR (2002) Experimental animal models of intracerebral hemorrhage. Neurosurg Clin N Am 13:385–393
Thiex R, Mayfrank L, Rohde V, Gilsbach JM, Tsirka SA (2004) The role of endogenous versus exogenous tPA on edema formation in murine ICH. Exp Neurol 189:25–32. doi:10.1016/j.expneurol.2004.05.021S0014488604001840 [pii]
Thullier F, Lalonde R, Cousin X, Lestienne F (1997) Neurobehavioral evaluation of lurcher mutant mice during ontogeny. Brain Res Dev Brain Res 100:22–28. doi:S0165380697000102 [pii]
Hughes RN (2004) The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev 28:497–505. doi:S0149-7634(04)00073-9 [pii]10.1016/j.neubiorev.2004.06.006
Fathali N, Ostrowski RP, Lekic T, Jadhav V, Tong W, Tang J, Zhang JH (2010) Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med 38:572–578. doi:10.1097/CCM.0b013e3181cb1158
Zhou Y, Fathali N, Lekic T, Tang J, Zhang JH (2009) Glibenclamide improves neurological function in neonatal hypoxia-ischemia in rats. Brain Res 1270:131–139. doi:S0006-8993(09)00520-4 [pii]10.1016/j.brainres.2009.03.010
Saad AY (1990) Postnatal effects of nicotine on incisor development of albino mouse. J Oral Pathol Med 19:426–429
Andine P, Thordstein M, Kjellmer I, Nordborg C, Thiringer K, Wennberg E, Hagberg H (1990) Evaluation of brain damage in a rat model of neonatal hypoxic-ischemia. J Neurosci Methods 35:253–260
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag/Wien
About this chapter
Cite this chapter
Lekic, T., Manaenko, A., Rolland, W., Tang, J., Zhang, J.H. (2011). A Novel Preclinical Model of Germinal Matrix Hemorrhage Using Neonatal Rats. In: Zhang, J., Colohan, A. (eds) Intracerebral Hemorrhage Research. Acta Neurochirurgica Supplementum, vol 111. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0693-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0693-8_10
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0692-1
Online ISBN: 978-3-7091-0693-8
eBook Packages: MedicineMedicine (R0)