Abstract
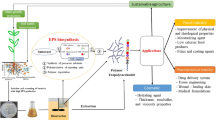
Inoculants with beneficial microorganisms comprise both selected strains and carriers that ensure a favorable microenvironment for cell survival and stability. Formulations of inoculants using synthetic polymers as carriers are common. However, only a few studies are available in the literature regarding the formulation of inoculants using natural biomolecules as carriers. Exopolysaccharides (EPS) are biomolecules produced by a vast array of microbial species, including symbiotic nitrogen-fixing bacteria, commonly known as rhizobia. EPS perform several functions, such as the protection against the deleterious effects of diverse environmental soil stresses. Two Rhizobium tropici strains and one Paraburkholderia strain were selected after semiquantitative analysis by scanning electron microscopy (SEM) of their EPS production in liquid YMA medium. Their EPS were characterized through a series of analytical techniques, aiming at their use in the formulation of plant inoculants. In addition, the effect of the carbon source on EPS yield was evaluated. Multi-stage fragmentation analysis showed the presence of xylose, glucose, galactose, galacturonic acid, and glucuronic acid in EPS chemical composition, which was confirmed by FT-IR spectra and 13C NMR spectroscopy. Thermal stability (thermogravimetric) was close to 270 °C and viscosity ranged from 120 to 1053.3 mPa.s. Surface morphology (SEM) was rough and irregular, with a cross-linked spongy matrix, which, together with the hydrophilic functional groups, confers water holding capacity. The present study showed that the three EPS have potential as microorganism carriers for formulation of microbial inoculants to be applied in plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological nitrogen fixation (BNF) is an essential process for nitrogen supply in the biosphere and is a successful biotechnology used in agriculture [1]. Rhizobia inoculants are commercially produced worldwide, generating a great impact on agricultural yield and sustainability. The annual market demand for plant growth promoting rhizobacteria is increasing globally, with an estimated grown rate of 14% in recent years and worldwide market of USD 1.88 billion in 2020 [2].
The production of rhizobial inoculants requires both selection of strains efficient in fixing N2 and the development of carriers that ensure a favorable microenvironment for cell survival and stability. Biopolymers used in inoculants should provide physical protection to the bacteria against the deleterious effects of the environment, supplying a sufficient number of viable cells to ensure the efficacy of the product [3].
Each year, studies reveal new strains that are efficient for several crops [4, 5], as well as specific formulations of inoculants using synthetic polymers [1, 3, 6,7,8]. However, only a few studies are available in the literature regarding the formulation of bacterial inoculants using natural biomolecules as carriers [9].
Exopolysaccharides (EPS), which are synthesized by a wide variety of bacteria, are a good example of these biomolecules [10]. EPS perform several functions, such as protection against the deleterious effects of temperature, desiccation, salinity, pH, antibiotics, and heavy metals. Furthermore, EPS provide resistance and defense to the host and aid in nutrient uptake, surface adhesion, and signaling and symbiotic infection processes for the development of root nodules [10,11,12,13].
Rhizobia are gram-negative α- and β-proteobacteria from the soil and are nitrogen fixers in symbiosis with legumes. These microorganisms have high EPS production, with extensive variation in structure and chemical composition [13]. In spite of the wide diversity of isolated rhizobia and their efficiency in nitrogen fixation in legumes, few studies have characterized the physicochemical properties of rhizobia exopolysaccharides with potential for biotechnological application [14]. Physicochemical characterization is a key step in understanding the behavior of biopolymers in order to use them in the formulation of rhizobia inoculants, as well as in other industrial applications.
Therefore, in this study, we investigated the physicochemical characteristics of EPS isolated from three rhizobia strains in order to verify their suitability as carriers and as bacterial cell protectors in bacterial inoculants.
Materials and methods
Materials and chemicals
In this study, all chemicals used in the preparation of the solutions were of analytical reagent grade from Sigma. Xanthan gum (CAS number: 11138–66-2) was purchased from Sigma-Aldrich (G1253-100 g). In most analyses, xanthan gum was used as reference for comparison with the EPS from rhizobia strains.
Bacterial strains and growth conditions
Initially, the three bacterial strains were chosen by their characteristic of producing significant amounts of EPS in 79 solid medium [15], also called YMA [16], at pH 6.8 (Figure S1). The strains selected were Paraburkholderia sp. (UFLA 04–269), isolated by using Macroptilium atropurpureum as a trap plant from Cerrado soils [17], and Rhizobium tropici UFLA 05–16, isolated from nodules of Crotalaria spectabilis growing in an As-contaminated gold mining site [18]. In addition, the Rhizobium tropici strain CIAT 899 T, isolated from South American acid soils [19], was also studied and used as a reference material in some analyses [20, 21]. All these strains belong to the collection of the Laboratory of Soil Biology, Microbiology and Biological Processes of the Department of Soil Science of UFLA–MG, where cultures are lyophilized and also stored at a temperature of − 80 °C. Liquid cultures of these strains were prepared for visualization by scanning electron microscopy (SEM). To standardize the inoculum concentration, the strains were inoculated separately in 79 liquid medium and incubated in an orbital shaker at 110 rpm and 28 °C for 72 h, up to optical density from 0.6 to 600 nm (OD600), equivalent to 1.5 × 109 CFU mL−1 by the McFarland scale. Subsequently, 20-µL aliquots were inoculated with applying the poly-l-lysine technique in a glass coverslip, and fixed to the SEM supports. Samples were then coated with a carbon layer for EDS analysis, and with gold of approximately 10-nm thickness for SEM analysis. Samples were observed in a SE microscope (LEO EVO 40) at the Laboratory of Electron Microscopy and Structural Analysis of UFLA; the accelerated voltage was operated at 15.0 kV. The EPS produced by the three strains were semiquantitatively analyzed regarding the amount of EPS produced.
Effect of the carbon source on EPS production
To evaluate the effect of the carbon source on EPS production, liquid medium 79 was used with its original composition, i.e., with mannitol, and modified by replacing mannitol with glucose, sucrose, or glycerol at the same concentration. Each strain was inoculated into an Erlenmeyer flask (125.0 mL) containing 45.0 mL of the original or modified liquid medium 79 (pH 6.8). These cultures were incubated on an orbital shaker at 110 rpm and 28 °C for 72 h, until reaching an optical density of 0.6 at 600 nm (OD600). Erlenmeyer flasks were capped with sterilized cotton plugs. Aliquots corresponding to 10% (v/v) of the cultures were transferred to Erlenmeyer flasks (250 mL) with 200 mL of liquid medium 79 on a rotary shaker. They were shaken for 360 h at 150 rpm and 28 °C for analyses of EPS production. Each strain/carbon source treatment was tested in triplicate.
EPS extraction
After incubation, bacterial cells were removed from the culture medium by centrifugation at 10,000 g for 10 min at 4 °C, and cold 96% ethanol (4 °C) was added to the cell-free supernatant mixture at a ratio of 3:1 (v/v) [22]. At this stage, the formation of a supernatant gel and a precipitate was immediately observed. The mixture was cooled to 4 °C for 24 h. Ethanol was evaporated in a drying oven at 60 °C. The precipitation solvent enabled partial purification of the polymer by eliminating the soluble components of the culture medium [23]. Then, the dialysis of the raw EPS using Sigma-Aldrich dialysis membrane (dialysis tubing cellulose membrane weight cut-off = 14,000) was performed. Two dialyses were performed, totaling 48 h of dialysis, with replacement of deionized water twice a day. The precipitated product was dried to a constant weight using a Labconco FreeZone 2.5 lyophilizer to verify the amount of EPS obtained (gram of EPS per liter of culture medium).
Characterization
All analyses of EPS chemical characterization were performed in cultures of strains grown in liquid medium 79 with mannitol as a carbon source.
Determination of protein content in the EPS
The soluble protein content in the samples was determined by the method described by Bradford [24]. Quantitative determination was performed with the Coomassie Brilliant Blue G-250 dye, which in the protein-bound form has an absorption peak at 595 nm determined by spectrophotometry. The concentration of soluble proteins in the samples was obtained from their absorbance using a calibration curve prepared with known concentrations of bovine serum albumin (BSA) as a standard. Each sample was analyzed in triplicate. Data were expressed as milligrams of soluble protein per milliliter of sample extract.
Structural characterization of EPS
The EPS was characterized by mass spectrometry (FIA-ESI-IT-MSn), Fourier transform-infrared (FT-IR) spectroscopy, solid-state nuclear magnetic resonance analysis of the nuclide 13C, and scanning electron microscopy (SEM). A clean-up step was performed to remove contaminants for the FIA-ESI-IT-MSn. The sample was purified by solid-phase extraction (SPE) using Phenomenex Strata C18 cartridges (500 mg, stationary phase) that were previously activated with MeOH (5 mL) and equilibrated with MeOH:H2O (5 mL, 1:1, v/v). The purified extracts were eluted from cartridges using MeOH:H2O (5 mL, 1:1, v/v), filtered through a 0.2- µm PTFE filter, and dried. Then, the extracts were diluted to 10 µg/mL using the HPLC solvent. Aliquots of 20 µL were injected directly into the FIA-ESI-IT-MSn.
For sample preparation, we used the KBr pellet method using a manual press. Initially, we macerated a portion of pure analytical KBr salt in grain to obtain a powder. We then dilute our EPS powder samples in the mortar into the powdered KBr salt in a 1:1 ratio (5 mg EPS + 5 mg KBr). After dilution, the mixture was macerated until a powder was obtained. Then the EPS + KBr sample was inserted into the pastillator and then manually pressed to obtain the shares. For analysis, we used pure KBr tablets (standard) and EPS + KBr tablets.
Determination of the monosaccharide composition by mass spectrometry (FIA-ESI-IT-MS.n)
The monosaccharide composition of the EPS was determined by direct infusion of the samples in a mass spectrometer with an ion trap analyzer, LTQ XL™, equipped with negative electrospray ionization (ESI) (Thermo, San Jose, CA, USA). The experimental conditions were flow of 1 mL min−1, 280 °C capillary temperature, − 90 V capillary voltage, 5.00 kV spray voltage, and − 100-V tube lens. Multi-stage fragmentation (ESI-MSn) was performed using the collision-induced dissociation method (CID), at a range of m/z 100–1000, and using helium as a collision gas. The first stage was a complete mass spectrum to obtain ion data at this range of m/z. The second scan event was an MSn experiment performed using a data-dependent scan performed on deprotonated molecules from the compounds at collision energy of 20–30% and activation time of 25–35 ms. In the negative mode, deprotonated neutral carbohydrates produce C- and Z-type ion fragments, when the acidic sugars undergo B- and Y-type fragmentation.
The ESI–MS/MS analysis of the ions found in the MS spectrum allows the sequence to be assessed by studying the fragmentation ions. The product ions were subjected to further fragmentation, under the same conditions, until no fragments were observed.
Fourier transform-infrared spectroscopy analysis (FT-IR)
The functional groups were analyzed by vibrational spectroscopy in the infrared region with Fourier transform (FT-IR), using the IRAffinity equipment (SHIMADZU), with a spectral range of 4000–400 cm−1, 64 scans, and a resolution of 4 cm−1, using the method of pasting with KBr.
NMR 13C analysis
Solid-state 13C NMR spectra were acquired using a Bruker Avance 400 spectrometer, equipped with a Bruker 4-mm magic angle sample spinning (MAS) probe. The spectra were acquired with a cross polarization sequence, with π/2 pulse lengths of 4 µs for 13C (100.5 MHz), a 2-s recycle delay, a 10-kHz spinning frequency, and 2000 scans. The spectra were filtered by an exponential function (line broadening equal to 20 Hz).
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS)
The microstructure and elemental composition of the lyophilized EPS were observed using a scanning electron microscope from Carl Zeiss, model Leo Evo 40, at an accelerating voltage of 20 keV. The EPS samples were mounted on a metal stub and coated with a carbon layer for EDS analysis, and with gold of approximately 10-nm thickness for SEM analysis.
Thermal analyses
The thermal decomposition behavior of the compounds investigated was assessed using thermogravimetric analysis (TGA) and differential thermal analysis (DTA).
Thermogravimetry (TG)
The thermal behavior of the samples was analyzed by thermogravimetry using a SHIMADZU thermomechanical analyzer, model DTG-60AH. Approximately 5.0 mg of each sample was heated at a rate of 10 °C min−1, from 30 to 800 °C, under a flow of 50 mL min−1 of N2.
Differential scanning calorimetry (DSC)
Samples were analyzed by differential scanning calorimetry using a Shimadzu calorimeter, model DSC-60 A, with an auto-cooling system. Samples of 5 mg at an initial temperature of 30 °C were cooled at a rate of − 10 °C min−1 to − 30 °C (remaining for 2 min), and heated at a rate of 10 °C min−1 to 550 °C in an N2 atmosphere (flow of 30 mL min−1).
Viscosity
For viscosity analysis, 5 mg of EPS was dissolved in 0.5% (m/v) distilled water. Viscosity was determined using a Brookfield viscometer (Model RVDV-II +) equipped with spindle-RV1 at a temperature of 18 °C, subjected to a speed of 10 rpm and to a torque ranging from 11.7 to 22.3%. Viscosity values (mPa) were obtained by readings of the equipment in triplicate.
Water holding capacity (WHC)
EPS samples were characterized by their water holding capacity (WHC) by dissolving 0.01 g of powdered EPS in 1.5 mL of distilled water and maintained it at 100 °C for 10 min, according to the methodology previously described [25]. The supernatant was then discarded and the product was placed in a laboratory oven at 60 °C. The test tube was preweighed and the total weight was measured. WHC was calculated using the equation:
Statistical analysis
Data were expressed as mean (± standard error) and analyzed statistically using the one-way ANOVA procedure of the R software (version 3.3.1). Means were clustered by the Scott-Knott test at 95% significance. Prior to the analysis of variance and the F test, normality and homoscedasticity tests were performed on the data of each evaluation. When necessary, data were first transformed by the formula (Y + 0.5)0,5.
Results and discussion
Carbon source and EPS production
The effect of four carbon sources on EPS production was studied in three rhizobia strains, Paraburkholderia sp. UFLA 04–269 and Rhizobium tropici UFLA 05–16 and CIAT 899 T. The variation in the carbon source resulted in different amounts of EPS produced by the three strains, as revealed by the F test (p < 0.05) (Fig. 1).
EPS production of Paraburkholderia sp. UFLA 04–269 and Rhizobium tropici UFLA 05–16 and CIAT 899 T in four carbon sources [glucose (Glu), sucrose (Suc), mannitol (Man), and glycerol (Gly)] at 150 rpm and 28 °C for 15 days. Lowercase letters compare the means among carbon sources in the same strain, and uppercase letters compare means among strains in the same carbon source, according to the Scott-Knott test at 5% probability
The strain UFLA 05–16 (Rhizobium tropici) produced 3.11 ± 0.64 g L−1 of EPS using sucrose as a carbon source, not statistically different from the strain CIAT 899 T (Rhizobium tropici) at 3.39 ± 0.61 in mannitol (Fig. 1). EPS production for the strain UFLA 04–269 (Paraburkholderia sp.) showed no statistical difference among the carbon sources (Fig. 1). The three strains produced the same amount of EPS with glucose and glycerol.
These results corroborate recent studies involving Rhizobium tropici, which show maximum EPS production of 3.48 g L−1 in PSYL liquid medium (30.0 g of sucrose per liter, 6.8 pH, 150 rpm, 28 °C, 6 days) and 2.52 ± 0.45 in liquid PSYL medium (140 rpm, 29 °C, 6 days) [22, 25]. Another study with Rhizobium tropici CIAT 899 T (SEMIA 4077) reported a maximum EPS production of 4.08 g L−1 at C/N = 20 under optimized conditions [21]. However, other studies showed that EPS production by Rhizobium tropici reached 7.67 ± 0.11 g L−1 (mutant 4077::Z04) in PSYL medium (registration PI0304053-4) with 3% sucrose as a carbon source, when incubated for 144 h at 140 rpm and 29 °C [14]. The difference in the composition between PSYL and 79 media may explain the differences in the results for CIAT 899 T.
Studies have revealed that the amount of EPS and their properties depend on the microorganism, on the bacterial culture conditions, and on the composition of the media [10, 26, 27]. Our study supports these findings since it showed variation in EPS production in relation to the microorganisms and the carbon sources evaluated.
These results are significant, especially for glycerol, since this is a by-product of the biodiesel production chain and a cheap carbon source for EPS production. Therefore, rather than an environmental liability, glycerol becomes a biologically managed material.
Determination of protein content in EPS
No protein was observed in the EPS using the Bradford method [24], except for the strain UFLA 05–16, which had a protein content of 0.19% (w/v). Results confirm that the solvent used in EPS isolation is effective in primary purification of these macromolecules of the soluble components of the culture medium, as presented by other studies using the same methods of extraction and analysis [22, 23].
Structural characterization
FIA-ESI-IT-MS n spectrum analysis
All the EPS isolated from our strains are heteropolysaccharides. The ESI-MSn analysis revealed the presence of glucose, galactose, xylose, and sugar acids (glucuronic acid and galacturonic acid) in the chemical composition of both the EPS produced by rhizobia and in xanthan gum. In the current study, the compounds were unambiguously identified based on their MS fragment behaviors (Table 1) and by comparisons with the reference standards.
Studies show that the monosaccharide composition of Rhizobium tropici CIAT 899 T (ATCC 49,672) determined by GC/MS differed according to the carbon source used in the growth process [21]. This may explain the absence of xylose production in other studies with CIAT 899 T and the presence of rhamnose, as reported by [21].
Glucose and galactose are commonly found in EPS from other rhizobia strains, including R. tropici CIAT 899 T, analyzed by other methods, such as RP-HPLC [20, 25] and HPAE/PAD [28].
FT-IR spectrum analysis
Figure 2a shows the FT-IR spectrum of xanthan gum (EPS standard), which had major absorption bands at 3700–3000 cm−1, 2970–2850 cm−1, 1730 cm−1, 1610 cm−1, 1425 cm−1, and 1048 cm−1 due to the presence of the following functional groups: ν(OH), ν(CH) of the groups CH2 and CH3, ν(CO), ν(C = C) of the glycosidic ring conjugated with δ(OH), δ(CH), and ν(COO−)ass, respectively, as was also shown in other papers [14, 29].
As expected, and according to FIA-ESI-IT-MSn analyses (see Table 1), FT-IR spectrum analysis of the EPS produced (Fig. 2b–d) showed vibrational modes similar to those of the xanthan gum spectrum, thus confirming their polysaccharide nature. The strong hydroxyl stretching vibration (3700–3000 cm−1) may explain the high water solubility of these materials and their extraction procedure. Absorption bands in the 2970 to 2850 cm−1 region, due to C-H stretching, can reveal the presence of sugars in the EPS composition. The vibrational mode at 1730 cm−1, assigned to ν(CO), indicates the presence of esters or of their respective carboxylic acids, like glucuronic or galacturonic acids. The other vibrational modes identified in the xanthan gum spectrum were also present in the EPS samples and have received identical assignments.
Although there was spectral identification among all the polysaccharides studied, the greatest similarities were observed between the xanthan gum and the UFLA 05–16 EPS samples (Fig. 2a–d), as well as between the UFLA 04–269 and CIAT 899 T EPS samples (Fig. 2b–c). These differences are more easily observed in the fingerprint region (below 1500 cm−1), which contains more specific signals for each polysaccharide [30]. Although several biopolymers obtained from rhizobia have already been reported in the literature [13, 14], such as for the strain Rhizobium tropici CIAT 899 T, the EPS produced by this strain deserves further consideration [14, 31]. According to [32], the EPS structure excreted by the CIAT 899 T variety is formed by repeating units of an octasaccharide mainly composed of D-glucose and D-galactose at a 6:2 ratio, which is in agreement with the infrared spectra observed for this biopolymer (Fig. 2b). Thus, in full agreement with the FIA-ESI-IT-MSn results, the FT-IR spectra confirm the heteropolysaccharide composition of the obtained EPS from strains of Paraburkholderia sp. and Rhizobium tropici.
NMR 13 C analysis
NMR spectroscopy is an excellent tool for determining the structure and the nature of the materials. In this case, 13C NMR analysis allowed the determination of the main components of extracellular polysaccharide. Based on chemical shifts, all the 13C NMR observed spectra (Fig. 3) can be divided into different regions: δC resonances at around 18 ppm, corresponding to the methyl groups; δC signals in the region of 50 to 110 ppm, related to CHOH and CH2OH carbons from the carbohydrate residues; and δC values over 175 ppm, corresponding to the resonances of the carbonyl acid residues (acetate, glucuronic, and galacturonic acid moieties). The intensities of the carbonyl signals at 175 ppm decline in the following order: UFLA 04–269 > UFLA 05–16 = CIAT 899 T. The methyl signal of acetate at 21 ppm is observed only in the spectra of the UFLA-04–269 EPS. The methyl signal at 26 ppm is observed in the spectra of CIAT 899 T, UFLA 05–16, and UFLA 04–269 EPS. The methylene signal of succinate at 32 ppm is observed in the spectra of UFLA 04–269 EPS. The solid-state 13C NMR spectra of the EPS samples exhibited an additional signal in the anomeric regions at 106 ppm (CIAT 899 T), 105 ppm (UFLA 05–16), and 103 ppm (UFLA 04–269), which was attributed to the β-anomeric carbon of the glucopyranosyl unit. These data are in agreement with the results reported for xanthan gum, where the 13C signals for anomeric carbons of the glucose are at 106 ppm. Therefore, through the observation by mass spectrometry technique, it was possible to identify different sugars and glucuronic acid produced by samples. These sugar units confirm that samples produce the polysaccharides identified in the other techniques.
SEM and EDS analysis
The study of the surface micromorphology of polysaccharides using scanning electron microscopy (SEM) assists in understanding EPS physical properties [33]. The micrographs show rough and irregular surfaces for the three EPS with an approximation of 80 µm (Fig. 4a, d, g). The morphology of xanthan gum, different from the EPS, has compact and smoother agglomerates (Fig. 4j). At 8 µm, the micromorphology of the three EPS revealed a cross-linked spongy matrix encrusted with canals (Fig. 4b, e, h), in contrast with the dense structure of xanthan gum (Fig. 4k). These results are in agreement with previous studies that describe the porous and cross-linked structure of microbial EPS [34, 35]. The presence of canals in the meshes of the three EPS allows accommodation of nitrogen-fixing bacteria in a microenvironment favorable to cell protection. According to [36], structures containing canals have functional groups on the walls and are responsible for increasing the contact surface of the water with the polysaccharide. There is not yet any information in the literature on mesh size of EPS in rhizobia.
SEM and EDX showing the microstructure and elemental composition of lyophilized exopolysaccharides. Images (a) and (b) correspond to SEM and (c) to EDX of the EPS of CIAT 899.T. Images (d) and (e) correspond to SEM and (f) to EDX of the EPS of UFLA 04–269. Images (g) and (h) correspond to SEM and (i) to EDX of the EPS of UFLA 05–16
Energy-dispersive X-ray spectroscopy (EDX) recorded the presence of carbon (C) and oxygen (O) associated with the monosaccharides of the polysaccharides. Signals of phosphorus (P) and potassium (K) in the three EPS were recorded, as well as of other minerals, such as calcium (Ca), magnesium (Mg), sodium (Na), and chlorine (Cl), which may be associated with the salts used in the culture medium. Studies using the same technique of analysis show the presence of calcium and chlorine, as well as carbon and oxygen in the EPS of Azotobacter chroococcum [37].
Thermal analyses
The TG and DSC curves of thermal degradation of EPS samples in a nitrogen atmosphere are shown in Figs. 5a and b, respectively. The TG curves showed three weight loss steps for all EPS samples. The first weight loss, below 150 °C, occurs with weight loss of 5 to 10%. This is an endothermic peak and it is related to the weight loss of absorbed and structural water. The second weight loss begins at 150 °C and goes up to 320 °C and can be attributed to depolymerization and to initiation of biomolecule (exopolysaccharide) pyrolysis, with a slow process followed by a fast process and weight loss of 57.0%. From the results of DSC, this event is characterized as heat release (exothermic) [38, 39]. Finally, the mass is carbonized at approximately 400 °C in the third stage, and weight continues to decrease gradually as the temperature increases; at 600 °C, the amount of fixed carbon is between 20 and 40%, as a result of carbonization of the material. The three EPS had thermostability of approximately 270 °C, surpassing other groups of EPS that have already been studied [35]. From the weight loss percentage in the TGA curve, it is clear that the thermal stability of the EPS of UFLA 05–16 is greater than that of CIAT 899 T and UFLA 04–269. These results indicate that the higher thermal stability observed in this study may come from stronger intra- and inter-molecular hydrogen bonding interactions, resulting in a stronger polysaccharide chain [40].
Thermal analyses of EPS produced by Rhizobium tropici CIAT 899 T (—), Paraburkholderia sp. UFLA 04–269 (‑‐‐), and Rhizobium tropici UFLA 05–16 (···): a TGA, heating rate equal to 10 °C min−1 and nitrogen gas atmosphere under flow of 50 mL min−1; and b DSC, heating rate equal to 10 °C min−1 and nitrogen gas atmosphere under flow of 30 mL min.−1
Not all assignments are in agreement. [38] attributed the volatilization of residual ethanol from EPS extraction, as well as impurities, to the weight loss observed in the first stage. Considering that they are amorphous biopolymers and that the increase in temperature allows greater movement of molecules and formation of a crystalline state, [41] attributed the greater weight loss in the second stage to these movements and to interactions of the side chains and main chain. Thus, the exothermic peaks of the DSC thermogram between 142 and 243 °C would confirm the transition from an amorphous solid to a crystalline solid state (exothermic). No studies on the thermal analyses of rhizobia EPS have been reported in the literature. Therefore, the results obtained from the strains Paraburkholderia sp. UFLA 04–269, Rhizobium tropici UFLA 05–16, and Rhizobium tropici CIAT 899 T had to be compared with those of other bacterial genera.
Viscosity
Viscosity measures the contribution of a material to the flow resistance of a solution or to dispersion [42]. The viscosity of the EPS of CIAT 899 T (1053.3 ± 37.05), subjected to a speed of 10 rpm and a torque of 13.2%, was higher than that of the EPS of UFLA 05–16 (872.0 ± 4.62 mPa) and of the EPS of UFLA 04–269 (120.0 ± 7.00 mPa), subjected to the same speed (10 rpm) and torque of 11.7% and 12.6%, respectively. However, the xanthan gum had the highest viscosity value (1776.0 ± 13.86 mPa), at a speed of 10 rpm and a torque of 22.3% (Fig. 6). Studies with the EPS of Rhizobium tropici (SEMIA 4077) at a concentration of 0.5% (m/v) reported viscosity of 957.7 mPa at a shear rate of 5.01 s−1, decreasing to 82.7 mPa with an increase in the shear rate for 100 s−1, exhibiting non-Newtonian and pseudoplastic behavior [14]. Other studies describe viscosity change from 23.4 to 21.8 mPa of a concentration of 0.25% (m/v) EPS of Rhizobium sp. with an increase in the shear rate from 75 to 82.5 s−1 at 25 °C [43]. The low viscosity of the EPS of UFLA 04–269, despite having monosaccharide composition similar to that of the other EPS, may be related to chain length, lateral branches, and molar mass, since Rhizobium can synthesize EPS with two molar masses [44].
Water holding capacity (WHC)
The WHC of the EPS of CIAT 899 T (65.6% ± 1.7%), UFLA 04–269 (69.6% ± 1.9%), and UFLA 05–16 (67.1% ± 3.0%) is similar to that of xanthan gum (66.9% ± 3.6%) and guar gum (67.4% ± 2.9%), and higher than that of carboxymethylcellulose (CMC, 57.4% ± 2.0%), polyvinylpyrrolidone (PVP, 46.9% ± 0.5%), and alginate (43.2% ± 1.2%) (Fig. 7). [45] reported that the WHC of the EPS of Leuconostoc lactis KC117496 is 117% ± 7.5%. [46], working with two EPS of Agrobacterium HX1126, reported WHC of 22.4% and 36.2%. The structure of the three EPS, which contains canals, revealed in the SEM images, and the chemical composition characterized by a high number of hydrophilic groups, such as carboxylates and hydroxyls in the chain, can also be considered determinants in WHC [37, 47, 48]. For some studies, this property is also attributed to hydrogen bonds present in large number in the structure of the EPS polymer chain [48]. A good WHC and a rough structure with canals are physical properties that make it feasible for EPS to be applied as carriers in the formulation of bacterial inoculants [35]. They ensure protection against deleterious environmental effects and ensure the good hydration capacity of bacterial cells. WHC analyses for rhizobia EPS have not yet been reported in the literature.
Conclusions
This study described three heteropolysaccharides produced by isolated strains of bacteria, Paraburkholderia sp. UFLA 04–269 and Rhizobium tropici UFLA 05–16 and CIAT 899 T. The strains CIAT 899 T, with mannitol and sucrose, and UFLA 05–16, with sucrose, had the highest EPS production, confirming that production depends on the strain and the carbon source used. All strains also showed high production in glycerol. Glycerol is a viable and inexpensive carbon alternative for EPS production through the strains evaluated. The EPS produced by strains in this study are heteropolysaccharides composed of glucose, galactose, and xylose and sugar acids (glucuronic acid and galacturonic acid). They have hydrophilic chemical groups (carboxyls, hydroxyls, and carbonyls) characteristic of acids and esters, capable of storing nutrients that can be used by the bacteria. EPS has high thermal stability and viscosity, which ensure its industrial application. The high cross-linked and three-dimensional porosity of the meshes, together with the hydrophilic functional groups, confers WHC of more than 65% for the three EPS. Therefore, the three EPS studied have properties that make them excellent carriers of microorganisms in the formulation of microbial inoculants. Indeed, polymeric formulations of liquid inoculants with these EPS were shown to increase the survival and symbiotic efficiency of elite Bradyrhizobium strains with soybeans and cowpea [49].
Data availability
Available upon reasonable request.
Code availability
Not applicable.
References
Bashan Y, de-Bashan LE, Prabhu SR, Hernandez J-P (2014) Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant Soil 378(1–2):1–33. https://doi.org/10.1007/s11104-013-1956-x
Keswani C, Prakash O, Bharti N et al (2019) Re-addressing the biosafety issues of plant growth promoting rhizobacteria. Sci Total Environ 690:841–852. https://doi.org/10.1016/j.scitotenv.2019.07.046
Ruíz-Valdiviezo VM, Canseco LMCV, Suárez LAC, Gutiérrez-Miceli FA, Dendooven L, Rincón-Rosales R (2015) Symbiotic potential and survival of native rhizobia kept on different carriers. Braz J Microbiol 46(3):735–742. https://doi.org/10.1590/S1517-838246320140541
Farias TP, Trochmann A, Soares BL, Moreira FMS (2016) Rhizobia inoculation and liming increase cowpea productivity in Maranhão State. Acta Sci Agron 38(3):387. https://doi.org/10.4025/actasciagron.v38i3.28630
de Oliveira-Longatti SM, Marra LM, de Carvalho TS, de Moreira FM, S, (2020) The culture medium volume and the inoculation method should be considered in semi-quantitative screening of calcium phosphate solubilization by bacteria. Acta Sci Agron 42:e44332. https://doi.org/10.4025/actasciagron.v42i1.44332
Lee S-K, Lur H-S, Lo K-J et al (2016) Evaluation of the effects of different liquid inoculant formulations on the survival and plant-growth-promoting efficiency of Rhodopseudomonas palustris strain PS3. Appl Microbiol Biotechnol 100(18):7977–7987. https://doi.org/10.1007/s00253-016-7582-9
Rivera D, Obando M, Barbosa H, Rojas Tapias D, Bonilla Buitrago R (2014) Evaluation of polymers for the liquid rhizobial formulation and their influence in the Rhizobium-Cowpea interaction. Univ Sci 19(3):265–275. https://doi.org/10.11144/Javeriana.SC19-3.eplr
Schoebitz M, López MD, Roldán A (2013) Bioencapsulation of microbial inoculants for better soil–plant fertilization. A review Agron Sustain Dev 33(4):751–765. https://doi.org/10.1007/s13593-013-0142-0
Marcelino PRF, Milani KML, Mali S, dos Santos OJAP, de Oliveira ALM (2016) Formulations of polymeric biodegradable low-cost foam by melt extrusion to deliver plant growth-promoting bacteria in agricultural systems. Appl Microbiol Biotechnol 100(16):7323–7338. https://doi.org/10.1007/s00253-016-7566-9
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633. https://doi.org/10.1038/nrmicro2415
Cieśla J, Kopycińska M, Łukowska M, Bieganowski A, Janczarek M (2016) Surface properties of wild-type Rhizobium leguminosarum bv. trifolii strain 24.2 and its derivatives with different extracellular polysaccharide content Martinez-Abarca F, ed. PLOS ONE 11(10):e0165080. https://doi.org/10.1371/journal.pone.0165080
Kawaharada Y, Kelly S, Nielsen MW et al (2015) Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523(7560):308–312. https://doi.org/10.1038/nature14611
Bomfeti CA, Florentino LA, Guimarães AP, Cardoso PG, Guerreiro MC, de Moreira FMS (2011) Exopolysaccharides produced by the symbiotic nitrogen-fixing bacteria of leguminosae. Rev Bras Ciênc Solo 35(3):657–671. https://doi.org/10.1590/S0100-06832011000300001
Castellane TCL, Persona MR, Campanharo JC, de Macedo Lemos EG (2015) Production of exopolysaccharide from rhizobia with potential biotechnological and bioremediation applications. Int J Biol Macromol 74:515–522. https://doi.org/10.1016/j.ijbiomac.2015.01.007
Fred EB, Waksman SA (1928) Laboratory manual of general microbiology with special reference to the microorganisms of the soil. McGraw-Hill Book Co., Published online, p 145
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. Vol 15. 15th ed. International Biological Programme.
Araújo KS, Carvalho F de, Moreira FM de S (2017) Bukholderia strains promote Mimosa spp. growth but not Macroptilium atropurpureum. Rev Ciênc Agronômica 48. https://doi.org/10.5935/1806-6690.20170005
de Rangel WM, de Oliveira Longatti SM, Ferreira PAA et al (2017) Leguminosae native nodulating bacteria from a gold mine As-contaminated soil: multi-resistance to trace elements, and possible role in plant growth and mineral nutrition. Int J Phytoremediation 19(10):925–936. https://doi.org/10.1080/15226514.2017.1303812
Graham PH, Draeger KJ, Ferrey ML et al (1994) Acid pH tolerance in strains of Rhizobium and Bradyrhizobium, and initial studies on the basis for acid tolerance of Rhizobium tropici UMR1899. Can J Microbiol 40(3):198–207. https://doi.org/10.1139/m94-033
Castellane TCL, Otoboni AMMB, de Lemos EGM (2015) Characterization of exopolysaccharides produced by rhizobia species. Rev Bras Ciênc Solo 39(6):1566–1575. https://doi.org/10.1590/01000683rbcs20150084
Staudt AK, Wolfe LG, Shrout JD (2012) Variations in exopolysaccharide production by Rhizobium tropici. Arch Microbiol 194(3):197–206. https://doi.org/10.1007/s00203-011-0742-5
Castellane TCL, Lemos MVF, de Lemos EGM (2014) Evaluation of the biotechnological potential of Rhizobium tropici strains for exopolysaccharide production. Carbohydr Polym 111:191–197. https://doi.org/10.1016/j.carbpol.2014.04.066
Castellane TCL, de Lemos EGM (2007) Composição de exopolissacarídeos produzidos por estirpes de rizóbios cultivados em diferentes fontes de carbono. Pesqui Agropecuária Bras 42(10):1503–1506. https://doi.org/10.1590/S0100-204X2007001000019
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Moretto C, Castellane TCL, Lopes EM, Omori WP, Sacco LP, de Lemos EGM (2015) Chemical and rheological properties of exopolysaccharides produced by four isolates of rhizobia. Int J Biol Macromol 81:291–298. https://doi.org/10.1016/j.ijbiomac.2015.07.056
Donot F, Fontana A, Baccou JC, Schorr-Galindo S (2012) Microbial exopolysaccharides: main examples of synthesis, excretion, genetics and extraction. Carbohydr Polym 87(2):951–962. https://doi.org/10.1016/j.carbpol.2011.08.083
Polak-Berecka M, Choma A, Waśko A, Górska S, Gamian A, Cybulska J (2015) Physicochemical characterization of exopolysaccharides produced by Lactobacillus rhamnosus on various carbon sources. Carbohydr Polym 117:501–509. https://doi.org/10.1016/j.carbpol.2014.10.006checardoi
Monteiro NK, Aranda-Selverio G, Exposti DTD et al (2012) Caracterização química dos géis produzidos pelas bactérias diazotróficas Rhizobium tropici e Mesorhizobium sp. Quím Nova 35(4):705–708. https://doi.org/10.1590/S0100-40422012000400009
Faria S, de Oliveira Petkowicz CL, de Morais SAL et al (2011) Characterization of xanthan gum produced from sugar cane broth. Carbohydr Polym 86(2):469–476. https://doi.org/10.1016/j.carbpol.2011.04.063
Kanamarlapudi SLRK, Muddada S (2017) Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. BioMed Res Int 2017:1–11. https://doi.org/10.1155/2017/4201809
Pereira PAA, Oliver A, Bliss FA, Crowe L, Crowe J (2002) Preservation of rhizobia by lyophilization with trehalose. Pesqui Agropecuária Bras 37(6):831–839. https://doi.org/10.1590/S0100-204X2002000600012
Gil-Serrano A, del Junco AS, Tejero-Mateo P, Megias M, Caviedes MA (1990) Structure of the extracellular polysaccharide secreted by Rhizobium leguminosarum var. phaseoli CIAT 899. Carbohydr Res. 204:103–107. https://doi.org/10.1016/0008-6215(90)84025-P
Miao M, Ma Y, Huang C, Jiang B, Cui SW, Zhang T (2015) Physicochemical properties of a water soluble extracellular homopolysaccharide from Lactobacillus reuteri SK24.003. Carbohydr Polym 131:377–383. https://doi.org/10.1016/j.carbpol.2015.05.066
Dertli E, Toker OS, Durak MZ et al (2016) Development of a fermented ice-cream as influenced by in situ exopolysaccharide production: rheological, molecular, microstructural and sensory characterization. Carbohydr Polym 136:427–440. https://doi.org/10.1016/j.carbpol.2015.08.047
Han Y, Liu E, Liu L et al (2015) Rheological, emulsifying and thermostability properties of two exopolysaccharides produced by Bacillus amyloliquefaciens LPL061. Carbohydr Polym 115:230–237. https://doi.org/10.1016/j.carbpol.2014.08.044
Kaith BS, Sharma R, Kalia S (2015) Guar gum based biodegradable, antibacterial and electrically conductive hydrogels. Int J Biol Macromol 75:266–275. https://doi.org/10.1016/j.ijbiomac.2015.01.046
Rasulov B, Rozi P, Pattaeva M, Yili A, Aisa H (2016) Exopolysaccharide-based bioflocculant matrix of Azotobacter chroococcum XU1 for synthesis of AgCl nanoparticles and its application as a novel biocidal nanobiomaterial. Materials 9(7):528. https://doi.org/10.3390/ma9070528
Ballesteros LF, Cerqueira MA, Teixeira JA, Mussatto SI (2015) Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr Polym 127:347–354. https://doi.org/10.1016/j.carbpol.2015.03.047
Chen T, Wang Y, Li J et al (2016) Phthaloyl modification of a polysaccharide from Lachnum YM262 and immunomodulatory activity. Process Biochem 51(10):1599–1609. https://doi.org/10.1016/j.procbio.2016.08.004
Wang K, Li W, Rui X et al (2015) Chemical modification, characterization and bioactivity of a released exopolysaccharide (r-EPS1) from Lactobacillus plantarum 70810. Glycoconj J 32(1–2):17–27. https://doi.org/10.1007/s10719-014-9567-1
Mishra A, Kavita K, Jha B (2011) Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr Polym 83(2):852–857. https://doi.org/10.1016/j.carbpol.2010.08.067
Prasanna PHP, Bell A, Grandison AS, Charalampopoulos D (2012) Emulsifying, rheological and physicochemical properties of exopolysaccharide produced by Bifidobacterium longum subsp. infantis CCUG 52486 and Bifidobacterium infantis NCIMB 702205. Carbohydr Polym. 90(1):533–540. https://doi.org/10.1016/j.carbpol.2012.05.075
Priyanka P, Arun AB, Ashwini P, Rekha PD (2015) Versatile properties of an exopolysaccharide R-PS18 produced by Rhizobium sp. PRIM-18. Carbohydr Polym 126:215–221. https://doi.org/10.1016/j.carbpol.2015.03.017
Janczarek M (2011) Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia. Int J Mol Sci 12(12):7898–7933. https://doi.org/10.3390/ijms12117898
Saravanan C, Shetty PKH (2016) Isolation and characterization of exopolysaccharide from Leuconostoc lactis KC117496 isolated from idli batter. Int J Biol Macromol 90:100–106. https://doi.org/10.1016/j.ijbiomac.2015.02.007
Liu Y, Gu Q, Ofosu FK, Yu X (2016) Production, structural characterization and gel forming property of a new exopolysaccharide produced by Agrobacterium HX1126 using glycerol or d-mannitol as substrate. Carbohydr Polym 136:917–922. https://doi.org/10.1016/j.carbpol.2015.09.107
Mudgil D, Barak S, Khatkar BS (2014) Guar gum: processing, properties and food applications – a Review. J Food Sci Technol 51(3):409–418. https://doi.org/10.1007/s13197-011-0522-x
Ahmed Z, Wang Y, Anjum N, Ahmad A, Khan ST (2013) Characterization of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir – Part II. Food Hydrocolloids 30(1):343–350. https://doi.org/10.1016/j.foodhyd.06.009
Farias TP, Soares BL, D´eca CSB, Moreira FMSM (2022) Polymeric formulations of liquid inoculants with rhizobia exopolysaccharides increase the survival and symbiotic efficiency of elite Bradyrhizobium strains. Arch Microbiol 204:177–186. https://doi.org/10.1007/s00203-022-02779-z
Acknowledgements
We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (CAPES/PROEX AUXPE 593/2018), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process: 304527/2016-5; process: 431504/2016-4), and the Fundação de Amparo à Pesquisa do estado de Minas Gerais (Fapemig) (CAG-RED-00330-16) for financial support and for granting scholarships. This research is associated with the Brazilian National Institute of Science and Technology (Soil Biodiversity/INCT-CNPq). We also thank the Program for the Qualification of Public Servants of IFMA (PROQUALIS) for granting a PhD scholarship to the first author.
Funding
Financial support and for granting scholarships by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PROEX AUXPE 593/2018), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process: 304527/2016–5; process: 431504/2016–4), and the Fundação de Amparo e Pesquisa de Minas Gerais (Fapemig) (CAG- RED-00330–16).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
We warrant that all of the authors have contributed substantially to the manuscript and approved the final submission.
Consent for publication
We warrant that all of the authors have contributed substantially to the manuscript and approved the final submission to BJM.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Luiz Henrique Rosa
Highlights.
• Multi-technique-analyzed/characterized rhizobia EPS.
• Attractive EPS production from three rhizobia strains.
• Presence of xylose, glucose, and galacturonic acid determined.
• Interesting WHC and thermal stability close to 270 °C.
• Potential for use as biodegradable inoculant carriers.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Palhares Farias, T., de Melo Castro, E., Marucci Pereira Tangerina, M. et al. Rhizobia exopolysaccharides: promising biopolymers for use in the formulation of plant inoculants. Braz J Microbiol 53, 1843–1856 (2022). https://doi.org/10.1007/s42770-022-00824-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00824-z