Abstract
Atypical antipsychotics have an important role in the acute and maintenance treatment of bipolar disorder. While robust evidence supports the efficacy of these agents in the treatment of mania and in the prevention of manic relapse, few atypical antipsychotics have shown efficacy in the treatment or prevention of depressive episodes. These agents pose a lower risk of extrapyramidal side effects compared to typical neuroleptics, but carry a significant liability for weight gain and other metabolic side effects such as hyperglycemia and hyperlipidemia. More comparative effectiveness studies are needed to assess the optimal treatment regimens, including the relative benefits and risks of antipsychotics versus mood stabilizers. The exploration of the molecular mechanisms of antipsychotics has helped to shed further light on the underlying neurobiology of bipolar disorder, since these compounds target systems thought to be key to the pathophysiology of bipolar disorder. In addition to modulating monoaminergic neurotransmission, atypical antipsychotics appear to share properties with mood-stabilizing agents known to alter intracellular signal transduction leading to changes in neuronal activity and gene expression. Atypical antipsychotic drugs have been shown to exhibit neuroprotective properties that are mediated by upregulation of trophic and cellular resilience factors. Building on our understanding of existing therapeutics, especially as it relates to underlying disease pathology, newer “plasticity enhancing” strategies hold promise for future treatments of bipolar disorder.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bipolar disorder
- Atypical antipsychotics
- Monoamines
- Signal transduction
- Cellular resilience
- Neuroplasticity
1 Introduction
Bipolar disorder is a common, chronic, recurrent mental illness that affects the lives and functioning of millions of individuals worldwide. High rates of relapse, chronicity, lingering residual symptoms, sub-syndromes, cognitive and functional impairments, psychosocial disability, and diminished well-being are unfortunately common occurrences in bipolar disorder (Goodwin and Jamison 2007; Levy and Manove 2011). Recent studies have shown the efficacy of several atypical antipsychotics, both as monotherapy and in combination with mood stabilizers such as lithium and valproate, for the treatment of acute mania, manic relapse prevention, and acute bipolar depression (Calabrese et al. 2005a, b; Surja et al. 2006; Thase et al. 2006; Tohen and Vieta 2009).
In this review, we discuss contemporary research with antipsychotics in bipolar disorder including clinical treatment, neuroimaging, genetic association studies, and molecular and preclinical pharmacological studies. This research has elucidated some of the cellular and molecular effects of antipsychotics that include their antidopaminergic effects, as well as other mechanisms of action. We conclude by highlighting key achievements, shortcomings and unmet medical needs, as well as emerging new targets and next steps forward. An understanding of the neurobiology of this complicated and multifactorial disease is still developing, yet critical for the future development of targeted therapies.
2 Clinical Studies
2.1 Introduction to Clinical Studies
Antipsychotic medications have a long history in the treatment of bipolar disorder, especially in the management of acute mania and depressive episodes with psychotic features (Gentile 2007). Conventional antipsychotics, such as chlorpromazine and haloperidol, have been reported to be effective in up to 70 % of patients with acute mania, particularly in those with psychomotor agitation (Cousins and Young 2007; Tohen and Vieta 2009; Vestergaard 1992). Additionally, retrospective reports suggest that conventional agents are effective in the maintenance treatment of bipolar disorder (Cousins and Young 2007). While efficacious, use of these drugs has been limited by their neurological side effects. These untoward effects may be of particular concern in bipolar disorder, as patients with affective illness appear to be especially vulnerable to acute extrapyramidal side effects and tardive dyskinesia (Gao et al. 2008). Further, several reports suggest that conventional antipsychotics have depressogenic potential (Kusumakar 2002).
The introduction of atypical antipsychotics held the promise of fewer neurological side effects and the potential for greater utility in bipolar disorder. Over the past decade nearly all of the drugs within this class have been systematically studied, either as monotherapy or adjunctive therapy to a mood stabilizer, both in acute mania as well as in maintenance therapy (Tables 1 and 3). As a result, most of these agents have been approved for these indications by health authorities around the world. More recently, some of these agents have been studied in bipolar depression (Tables 1 and 3). Current treatment guidelines include atypical antipsychotics as first-line treatment in bipolar disorder (APA 2002; Beckford-Ball 2006; Giese 2009; Toprac et al. 2006).
Despite the widespread use of atypical antipsychotics in bipolar disorder, there are limited data on the relative efficacy of (1) the various agents within the class; (2) atypical versus conventional antipsychotics; and (3) atypical antipsychotics compared to mood stabilizers, such as lithium or valproate. Likewise, there are little data on the comparative safety of atypical antipsychotics in this patient population. This section provides a review of the randomized placebo-controlled trials of atypical antipsychotics in bipolar disorder and recent meta-analyses of these agents in acute mania.
2.2 Clinical Studies in Acute Mania
Table 1 provides a summary of randomized placebo-controlled trials of atypical antipsychotics in acute mania, when used either as monotherapy or adjunctive therapy to mood stabilizers. Aripiprazole, asenapine, olanzapine, quetiapine, and risperidone have demonstrated efficacy and got health authority approval in the United States (US) and Europe (EU) for use in acutely manic adults with and without psychosis, as either mono- or adjunctive therapy. All adjunctive trials included subjects who had inadequate response to current treatment with a mood stabilizer, although the study of quetiapine also included subjects who may not have received an adequate course of treatment with lithium or valproate prior to randomization (Sachs et al. 2004). Ziprasidone has demonstrated efficacy and got approved US and EU indications as a monotherapy for acute mania (Bowden et al. 2010). Most pivotal trials of atypical antipsychotics in acute mania included subjects with mixed episodes (with the exception of quetiapine) (Bowden et al. 2005). However, only aripiprazole, olanzapine, and risperidone have been studied in subjects with rapid-cycling bipolar disorder (Zupancic 2011). Aripiprazole, olanzapine, quetiapine, and risperidone have demonstrated efficacy and safety in children and adolescents and are indicated for the treatment of acute mania in pediatric bipolar disorder (Chang 2008; Fraguas et al. 2010; Scheffer et al. 2011).
Few studies exist that directly compare the efficacy of the various atypical antipsychotics in acute mania. Perlis and coworkers conducted a meta-analysis of randomized placebo-controlled, mono- and adjunctive therapy trials of atypical antipsychotics published through 2004 (Perlis et al. 2006). Little difference between agents was observed in efficacy scores versus placebo or in differential response rates among the individual atypical antipsychotics whether used alone or adjunctive to a mood stabilizer. The three monotherapy studies that included active comparators suggest that atypical antipsychotics had similar efficacy scores compared to lithium and haloperidol, a typical antipsychotic (Yildiz et al. 2011). As expected, the meta-analysis confirmed that adjunctive therapy with an atypical antipsychotic provides additional benefit over monotherapy with a mood stabilizer. Another meta-analysis suggests that antipsychotics are more effective than anticonvulsants when used as monotherapy (Cipriani et al. 2011). However, the relevant question of whether the combination of atypical antipsychotic and mood stabilizer is clinically superior to atypical antipsychotic alone remains unanswered. Nonetheless there appears to be a clear role for atypical antipsychotics in the treatment of acute mania, with a large body of data giving clinicians evidence for use as monotherapy or in combination with a mood stabilizer, in patients with pure and mixed mania, and in those with and without psychosis.
2.3 Clinical Studies in Acute Agitation
Intramuscular (IM) formulations of both aripiprazole and olanzapine are approved for the acute treatment of agitation associated with manic or mixed episodes of bipolar I disorder. In a placebo-controlled study of 291 agitated inpatients with manic or mixed episodes, both 9.75 and 15 mg doses of IM aripiprazole were superior to placebo in reducing acute agitation 2 h post-dose, as measured by the PANSS Excited Component (PEC). Likewise, 10 mg of IM olanzapine was superior to placebo in reducing the PEC 2 h post-dose in a study of 201 acutely agitated inpatients with a manic or mixed episode (Wagstaff et al. 2005). The efficacy for the two appears to be similar (Kinon et al. 2008).
2.4 Clinical Studies in Acute Treatment of Bipolar Depression
Table 2 summarizes the placebo-controlled, randomized monotherapy and adjunctive therapy studies of atypical antipsychotics in the acute treatment of bipolar depression. Quetiapine is the only atypical antipsychotic indicated as a monotherapy for treatment of acute bipolar depression, with efficacy demonstrated in a population of bipolar I and II subjects (Thase et al. 2006). Little is known whether other atypical antipsychotics would be effective as monotherapy in bipolar depression, though two studies of aripiprazole in this population were negative. The combination of olanzapine and fluoxetine is also approved for the acute bipolar I depression. While olanzapine monotherapy did show superiority over placebo, the magnitude of antidepressant effect was greatest with the olanzapine–fluoxetine combination (Tohen et al. 2003). Additionally, an open-label follow-up study of olanzapine–fluoxetine combination showed little risk of inducing mania (Corya et al. 2006).
2.5 Clinical Studies in Maintenance Treatment of Bipolar Disorder
Table 3 summarizes the randomized placebo-controlled trials of atypical antipsychotics as maintenance therapy in bipolar I disorder. Aripiprazole and long-acting risperidone have shown efficacy in the maintenance treatment of bipolar I disorder as mono- and adjunctive therapy (Popovic et al. 2010; Rybakowski 2005; Smith et al. 2007). Olanzapine has proven efficacy as a monotherapy in preventing mood episodes (Weisler et al. 2010), whereas quetiapine and ziprasidone have only been shown to prevent such relapses when given in combination with a mood stabilizer (Vieta et al. 2011).
Although the study designs differed among the pivotal trials for the various atypical antipsychotics, all trials employed a randomized discontinuation after a period of stabilization. While studies of all of the agents included subjects stabilized from an acute manic or mixed state, maintenance studies of quetiapine also included subjects stabilized from acute depressive episodes. The adjunctive study of long-acting injectable risperidone also enrolled euthymic patients and patients stabilized from hypomanic and depressed states.
Although data from most maintenance trials of atypical antipsychotics show greater differences between the active treatment and placebo groups in the number of manic relapses compared to the number of depressive relapses, only the long-term study of quetiapine showed a treatment effect of delaying both manic and depressive relapses (Vieta et al. 2008a, b; Weisler et al. 2010). However, it is important to note that these maintenance trials were not designed to conclude that atypical antipsychotics are more effective in preventing mania than depression. In general, these trials were powered to detect a drug–placebo difference only in the total number of relapses. Moreover, most subjects entered the maintenance phase after an acute manic or mixed episode, thereby biasing the outcome towards more manic episodes.
Little data exist on the comparative long-term efficacy of atypical antipsychotic monotherapy versus mood stabilizers in preventing relapses. Olanzapine, however, has been compared to both lithium and valproate in two separate randomized controlled trials (Suppes et al. 2005a, b; Tohen et al. 2005). Both of these studies showed similar rates of overall relapses in subjects receiving olanzapine and each of the mood stabilizers; however, olanzapine appeared to be more effective than lithium in preventing manic relapses (13.8 % vs. 23.4 %, p = 0.002) (Tohen et al. 2005).
2.6 Tolerability and Safety in Clinical Studies
Table 4 summarizes the safety profiles of the various atypical antipsychotics in patients with bipolar disorder. While this newer generation of agents has a lower risk of extrapyramidal side effects, they carry a significant risk of weight gain and other metabolic side effects, such as hyperglycemia and hyperlipidemia. Weight gain may be particularly problematic in the bipolar patient population, especially if it is additive to the weight gain caused by mood stabilizers. Certain agents, such as olanzapine, have an increased potential for weight gain and hyperlipidemia, and these effects may be more pronounced in adolescents compared to adults (olanzapine prescribing information). Additionally, the sedation associated with some atypical antipsychotics may be especially bothersome to patients who are students or are employed.
2.7 Summary of Clinical Data
A relatively large body of evidence now exists to support the efficacy of atypical antipsychotics in various stages of bipolar disorder. As a class, these drugs appear to be effective in the treatment of acute mania. Indeed, several treatment guidelines have recommended atypical antipsychotic monotherapy as a first-line treatment for acute mania (APA 2002; Suppes et al. 2005a, b), and several studies suggest that the combination of an atypical antipsychotic plus a mood stabilizer is more effective than a mood stabilizer alone, especially in patients inadequately responsive to mood stabilizers. Yet, it is not clear if an antipsychotic adjunctive to a mood stabilizer is superior to an antipsychotic alone. Intramuscular formulations of certain atypical antipsychotics are effective in the treatment of acute agitation associated with mania (Kinon et al. 2008; Tran-Johnson et al. 2007). Quetiapine is the only antipsychotic monotherapy to demonstrate efficacy in acute bipolar depression, whereas olanzapine in combination with fluoxetine appears to be effective in bipolar depression with little risk of inducing mania (Benyamina and Samalin 2012). Either of these treatments may have utility in acutely depressed patients requiring rapid treatment response. Most of the atypical antipsychotics have demonstrated the ability to prevent relapse of a mood episode after stabilization from a manic or mixed state, with clear benefit in preventing manic relapses.
The decision to use an atypical antipsychotic in a patient with bipolar disorder, as well as the selection of a particular agent, will depend on factors other than efficacy alone, such as safety, tolerability, compliance, and cost (Perlis et al. 2006). The atypical antipsychotics carry the risk of weight gain and metabolic abnormalities, sedation, and other side effects, some of which may be compounded when used in combination with mood stabilizers. Long-acting injectable risperidone may be a particularly valuable treatment option for bipolar patients with a history of poor medication compliance (Walburn et al. 2001). While pharmacoeconomic factors may also play a role in treatment selection, there are now several generic atypical antipsychotics available.
Further comparative effectiveness trials of atypical antipsychotics are still needed to best evaluate the full utility and value of these agents in clinical practice. Specifically, more data are needed to determine the relative acute efficacy of atypical antipsychotic versus mood stabilizer monotherapy, as well as atypical antipsychotic monotherapy versus atypical antipsychotic plus a mood stabilizer. Moreover, far fewer agents have demonstrated efficacy for preventing depressive relapse and additional research is needed to establish effective treatments for this phase of the illness.
Atypical antipsychotics have broadened the pharmacological armamentarium for the treatment of bipolar disorder. Furthermore, the exploration of the molecular mechanisms of antipsychotics has helped to shed greater light on the underlying neurobiology of the condition. The subsequent section of this chapter reviews the putative pharmacological mechanisms of antipsychotics, including their effect on monoaminergic neurotransmitters and signaling cascades implicated in the control of neuroplasticity, and cellular resilience.
3 Mechanisms of Antipsychotics in the Treatment of Bipolar Disorder
3.1 Introduction to Mechanisms of Action
As described above, many antipsychotics have been shown to have an antimanic effect, and some of the atypical antipsychotics have also been shown to have antidepressant effects as monotherapy (e.g., quetiapine) or as an adjunct to an antidepressant (e.g., aripiprazole and olanzapine). The primary mechanism of action of antipsychotics is via blockade of dopamine D2 receptors. Atypical antipsychotics also block serotonin 5-HT2 receptors and may restore the dampened firing rate of norepinephrine (NE) neurons produced by selective 5-HT reuptake inhibitors, thereby enhancing their antidepressant activity (Blier and Blondeau 2011). In addition, antipsychotics have differing activity on α-adrenoceptors, muscarinic receptors, and histamine receptors. Beyond their effects on neurotransmission, atypical antipsychotics alter intracellular signal transduction and exhibit neuroprotective properties that are mediated by upregulation of trophic and cellular resilience factors.
3.2 Role of Dopamine
The role of dopamine in bipolar disorder has been reviewed extensively (Berk et al. 2007; Cousins et al. 2009; Goodwin 2007; Kapur and Mann 1992). In summary, dopaminergic pathways have been implicated in the core symptoms of bipolar disorders. Historically, dopaminergic models of bipolar disorder were simplistic, dichotomous models suggesting mania as a hyperdopaminergic state and depression as a hypodopaminergic state. However, such a model fails to explain the complexity of the illness and various symptoms or comorbid conditions (e.g., psychotic symptoms during bipolar depression episodes or attentional deficits during manic episodes). Nonetheless, studies of regional dopaminergic pathways and cell signaling pathways have provided some insight into the pathophysiology of bipolar disorder, as these pathways subserve many of the physical and psychological processes known to be altered in this condition.
Bipolar disorder is a highly heritable illness. Genes encoding the dopamine transporter (DAT), SLC6A3, and rs27072 (Kato 2007; Pinsonneault et al. 2011) have been implicated bipolar disorder, although not consistently (Sklar et al. 2011). Brain imaging studies have not consistently shown any direct evidence of increased or reduced dopaminergic activity, dopamine transporter (DAT) activity, or striatal D2 dopamine receptor binding in bipolar disorder (Pearlson et al. 1995). Interpretation of neuroimaging findings of the dopaminergic system in bipolar illness is hampered by the fact that subjects were assessed during different phases of illness, bipolar depression, mania or during a euthymic state, as well as while medicated and drug-free in the few studies conducted (Nikolaus et al. 2009).
Dopaminergic psychostimulants, such as amphetamine and methylphenidate, can lead to euphoria or can mimic hypomania. High doses, particularly when taken repeatedly, can cause a number of symptoms including alterations in drive, motivation, impulsivity, and sleep, as well as a full manic episode. Interestingly, these symptoms can be attenuated with treatment with antipsychotics, lithium, or valproate (Berk et al. 2007; Kapur and Mann 1992). Lithium and valproate at therapeutically relevant concentrations have been shown to modulate dopaminergic activity by attenuating the downstream pathways activated by dopamine receptors as shown by studies using forskolin-raised cAMP concentrations which were inhibited by lithium, valproate, and carbamazepine, both in vitro and in vivo (Montezinho et al. 2006). Data from pharmacological interventions support the role of dopamine in bipolar disorder, with most of the antipsychotics having shown antimanic activity (reviewed above).
3.3 Role of Serotonin and Norepinephrine
As discussed above, a few atypical antipsychotics have been shown to improve depressive symptoms. In addition to their affinity to D2 receptors, atypical antipsychotics are also characterized by their affinity for 5-HT2A and 5-HT2C receptors and effects on norepinephrine (NE) transmission. Potent α2-adrenergic antagonist activity has been reported for aripiprazole, quetiapine, risperidone, and paliperidone. Blier and others have proposed that blockade of these autoreceptors on the cell body of NE neurons and their terminals can lead to enhanced NE neurotransmission. This effect may be seen especially with norquetiapine, an active metabolite of quetiapine, potentially conferring antidepressant effects while the D2 blockade may prevent a switch to mania (Blier and Blondeau 2011; Jensen et al. 2008; McIntyre et al. 2009; Prieto et al. 2010).
3.4 Signal Transduction Pathways
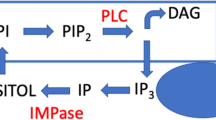
Activation or blockade of dopaminergic receptors results in changes in intracellular signal transduction leading to alterations in neuronal activity and gene expression. Protein kinase C (PKC) has generated considerable interest as a common target for mood stabilization. Lithium has been shown to act directly on pathways involving phospholipase2 (PLA2), and lithium and valproate on PKC pathways (Fig. 1) (Zarate and Manji 2009). Based upon this hypothesis that PKC is a common target, a number of studies have demonstrated the potential involvement of PKC in the pathophysiology and treatment of bipolar disorder (reviewed in Zarate and Manji 2009). Most critically a number of small proof of concept studies with tamoxifen, a PKC inhibitor, demonstrated a fast onset of efficacy in acute mania (Amrollahi et al. 2011; Yildiz et al. 2008; Zarate et al. 2007), thus corroborating the PKC hypothesis of mania. Although tamoxifen also has antiestrogenic effects, studies with other antiestrogen compounds such as medroxyprogesterone or clomiphene did not attenuate the amphetamine-induced behavioral changes in an animal model of mania that are attenuated by tamoxifen, suggesting that the antiestrogenic effect of tamoxifen is unlikely to contribute to its antimanic property (Pereira et al. 2011). Chronic administration of clozapine and haloperidol has previously been shown to reduce PKC activity in discrete brain areas, e.g., the hippocampus (Dwivedi and Pandey 1999), which may be relevant for the mechanism of antipsychotic drugs and may in part play a role in the antimanic activity of antipsychotics mediated through PKC inhibition (Zarate and Manji 2009). A recent meta-analysis of the efficacy of antimanic agents supports a larger effect size of tamoxifen compared to antipsychotics (Yildiz et al. 2011). These data add support to the relevance of PKC as a target in bipolar disorder and warrants further studies (DiazGranados and Zarate 2008; Zarate and Manji 2009).
Protein kinase C (PKC) in the pathophysiology and treatment of manic behavior. DA dopamine; GAP-43 growth-associated protein of 43 kDa; NA noradrenaline (norepinephrine); ↑ indicates increased. Figure adapted from Zarate and Manji (2009)
3.4.1 Akt/GSK-3 and Wnt Pathway
Akt is a protein kinase (also known as protein kinase B) that is involved in multiple cellular functions including metabolism, cell stress, cell-cycle regulation, and apoptosis. Akt has a basic role in regulating neuronal cell size and survival (Freyberg et al. 2010). Regulation of Akt by phosphorylated phosphatidylinositol has been associated with the action of insulin, insulin-related peptides (e.g., insulin-like growth factor), and neurotrophins (e.g., nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin (NT)-3) that exert their biological function by stimulating receptor tyrosine kinase (Beaulieu and Gainetdinov 2011). Activation of Akt via phosphorylation by the intracellular kinases PDK1 (3-phosphoinoitide-dependent protein kinase 1) and rictor-mTORC2 (mammalian target of rapamycin complex 2) leads to phosphorylation of other molecules including GSK-3, which plays a significant role in glucose metabolism and in differentiation and development, intracellular trafficking, modulation of synaptic plasticity, apoptosis, and regulation of gene transcription.
In addition to the Akt/GSK-3 pathway, signaling through the Wingless (Wnt) pathway is also of relevance. Wnt proteins are important mediators of cell–cell communication and are involved in diverse cellular processes, including the development of the CNS and play a crucial role in modulation of synaptic plasticity and in neurogenesis and in maintaining and protecting neuronal connections throughout the entire life span (Inestrosa and Arenas 2010). Activated GSK3 promotes β-catenin (a downstream mediator on Wnt signaling) and inhibits protein synthesis. DISC1, one of the most consistently reported risk mutations in schizophrenia and a risk factor for mood disorders (Lipina et al. 2011), regulates adult progenitor cells through GSK-3–β-catenin signaling. Mice lacking DISC1 in the dentate gyrus exhibited schizophrenia-like and depression-like behavior that could be normalized by treatment with a GSK-3 inhibitor (Lipina et al. 2011).
Dopamine-mediated decreases in Akt activity are considered to be mediated by postsynaptic dopamine D2 receptors. Activation of dopamine D2 receptor by dopamine or amphetamines leads to recruitment of beta-arrestin 2 and Akt along with the phosphatase PP2A, which dephosphorylates and consequently inactivates Akt, leading to an increase in GSK-3 activity. GSK-3 is constitutively active in resting cells, requiring phosphorylation by kinases such as Akt to inactivate it (Beaulieu et al. 2009). Disruption of Akt’s regulation of GSK-3 activity in the brain may also play a role in dysregulation of brain function in schizophrenia and mood disorders (Freyberg et al. 2010).
There is increasing evidence that Akt and GSK-3-related intracellular signaling may at least partially be responsible for the ability of antipsychotic medications to treat symptoms of psychosis and may be a common mechanism for antipsychotics, mood stabilizers, and antidepressants. Some atypical antipsychotics (e.g., clozapine, risperidone, olanzapine, aripiprazole) and typical antipsychotics (e.g., haloperidol) have been shown to either activate Akt or mimic Akt activity by increasing the phosphorylation of its substrates GSK-3 (Roh et al. 2007). However, differences between haloperidol and atypical antipsychotics have emerged in the kinetics of Akt/GSK-3 phosphorylation, the levels of proteins expressed following drug exposure, and the pathway that is preferentially activated (i.e., Akt vs. Wnt pathway signaling) (Roh et al. 2007). Treatment with either haloperidol or clozapine led to phosphorylation of GSK-3α and GSK-3β in rat frontal cortex (Roh et al. 2007). However, whereas levels of phosphorylated Akt1 returned to baseline within 1 hour following acute haloperidol exposure, Akt remained phosphorylated after a similar acute clozapine treatment. The prolonged duration of the effect of clozapine on Akt relative to a typical antipsychotic such as haloperidol may account for its greater effect on downstream molecules in the Wnt pathway (Sutton and Rushlow 2011). In addition, GSK-3 activity is regulated by 5-HT neurotransmission, through the activation of 5-HT2A receptors. It is therefore possible that atypical antipsychotics, which are antagonists of dopamine D2 receptors and serotonin 5-HT2A receptors, might inhibit GSK-3 activity by acting on dopamine and 5-HT receptor functions (Beaulieu et al. 2007).
Lithium exerts some of its biochemical and behavioral effects by interfering with a β-arrestin signaling complex involved in the regulation of Akt and GSK-3. Lithium’s effects on circadian rhythms and mood stabilization have been suggested to be mediated through direct inhibition of GSK-3 at therapeutically relevant concentrations (Gould et al. 2006). Valproate and lamotrigine (but not carbamazepine) have also been shown to indirectly inhibit GSK-3, and this mechanism has been demonstrated by various techniques to result in mood stabilizer-like behavior in rodent models (Gould et al. 2006, O’Brien and Klein 2007). The action of SSRIs and other 5-HT-related antidepressants on GSK-3 as well as the apparent antidepressant-like action of GSK-3 inhibitors in behavioral tests are strongly suggestive of an involvement of GSK-3 in the effects of antidepressants. In summary, in both animal models and the clinical population, GSK-3 manipulation appears to have antidepressant, antipsychotic, and antimanic effects.
Downstream targets of Akt and/or GSK-3 in the action of psychotropic drugs need to be identified and investigated. Whether the role of the Akt/GSK-3/Wnt signaling cascade in mediating behavioral outcomes and actions of psychotropic drugs is confined to certain brain areas is of interest to explore. Interestingly the mGlu2/3 agonist, LY379268, which has demonstrated preliminarily clinical antipsychotic efficacy, has also been shown to target Akt and Wnt signaling (Sutton and Rushlow 2011). It is tempting to speculate that this pathway may play a pivotal role in the therapeutic action of antipsychotics.
3.4.2 Neurotrophic Signaling Cascades
Neurotrophins (NTs), a family of peptide growth factors for nerve and glial cells, influence cell cycle, growth, differentiation and survival of neurons and thereby regulate synaptic plasticity in the adult brain. Members of the NT family include NGF, BDNF, NT-3, NT-4, NT-5, and NT-6. BDNF and other neurotrophic factors are necessary for the survival and function of neurons; thus, sustained reductions of these factors could affect neuronal viability.
Endogenous neurotrophic factors have traditionally been viewed as increasing cell survival by providing necessary trophic support. However, it is now clear that their survival-promoting effects are mediated largely by inhibiting cell death (apoptosis) cascades (Lee et al. 2001). Increasing evidence suggests that neurotrophic factors inhibit cell death cascades by activating the extracellular-regulated kinase (ERK) signaling pathway [cyclic adenosine monophosphate (cAMP) response element binding (CREB) is directly phosphorylated and activated by phospho-ERK1/2], the phospholipase C (PLC) cascade, and the phosphoinositide 3-kinase (PI3K)/Akt pathway.
Atypical antipsychotic drugs have been shown to exhibit neuroprotective properties that are mediated by upregulation of trophic factors (Lieberman et al. 2008). Quetiapine reverses the stress-induced decrease in hippocampal BDNF (Fumagalli et al. 2004) and prolongs neuronal survival similar to that shown with antidepressants. Quetiapine and olanzapine have been shown to elicit trophic effects in cultured neuronal cells by activation of Akt and ERK, which could indicate mitogenic and neuroprotective effects (Di Benedetto et al. 2011). Although antidepressants and antipsychotics both increase neurogenesis, the effect of antidepressants is restricted mainly to the subgranular zone (SGZ) of the dentate gyrus, with no impact on subventricular zone (SVZ) of the lateral ventricles in the forebrain (Samuels and Hen 2011). However, antipsychotic drugs have been reported to promote neurogenesis in both the SGZ and SVZ (Newton and Duman 2007). Additionally, haloperidol, a typical antipsychotic drug and potent D2 receptor antagonist, significantly increases cell proliferation in the SVZ, resulting in new neurons in the olfactory bulb and non-neuronal cells in the striatum (Deutch et al. 1995). The striatal cell proliferation would explain the caudate enlargement (Newton and Duman 2007) that has been reported with long-term administration of typical antipsychotic drugs but not atypical agents. Despite the robust SVZ neurogenesis, antipsychotic drugs with high D2 receptor specificity do not seem to increase hippocampal proliferation. In contrast, atypical antipsychotic drugs that exhibit affinity for serotonin receptors increase SGZ neurogenesis in addition to non-neuronal proliferation in the frontal cortex (Newton and Duman 2007). Whether atypicals are still able to induce SVZ proliferation is somewhat controversial as some studies report this effect (Green et al. 2006) while others do not (Councill et al. 2006).
The overlap that is seen in proliferation profiles of SSRI antidepressants and atypical antipsychotic drugs, particularly with 5-HT2 inhibitory activity, is striking (Nasrallah et al. 2010) but still only correlative, with the precise underlying mechanism remaining unclear. Studies with specific serotonin agonists and antagonists have shed light on some of these mechanisms. The role of D2 receptors in haloperidol-induced proliferation was addressed by generating D2 receptor null mice that do not exhibit an increase in neural stem cells with haloperidol administration (Newton and Duman 2007). These data also suggest that dopamine signaling via activation of D2 receptors has an anti-proliferative effect, which is overcome by the antagonistic effect of haloperidol (Newton and Duman 2007). However, there is still significant controversy regarding the contribution of D2 receptors, as D3 receptor activation was earlier shown to stimulate adult SVZ (Merlo et al. 2011) and substantia nigra (Collo et al. 2008) neurogenesis.
3.4.3 The Bcl-2 Family of Proteins
The B-cell lymphoma protein (Bcl-2) family includes pro- (such as Bax and Bad) and anti-apoptotic (such as Bcl-2 and Bcl-xL) proteins (Youle and Strasser 2008). Several preclinical studies have shown that atypical antipsychotics, including olanzapine, risperidone, and quetiapine upregulate levels of Bcl-2 or Bcl-xL in the brain after chronic administration (Hammonds and Shim 2009; He et al. 2004, 2006; Keilhoff et al. 2010; Luo et al. 2004). The mechanism through which atypical antipsychotics upregulate Bcl-2 is still largely unknown. However, Bcl-2 effects of atypical antipsychotics illustrate that atypical antipsychotics and mood stabilizers can produce similar intracellular actions, and this might explain in part the efficacy of atypical antipsychotics in the treatment of mood episodes and in the prevention of recurrence.
These Bcl-2 family proteins are known to play essential roles in apoptosis. They also play non-apoptotic regulatory roles in mitochondrial function, endoplasmic reticulum stress, calcium homeostasis, neurite growth, axonal regeneration, AMPA receptor trafficking and synaptic plasticity (Chen et al. 2010; Hunsberger et al. 2009; Jiao and Li 2011; Jonas 2006, 2009; Kim et al. 2008; Li and Jope 2010; Youle and Strasser 2008). Chen et al. (1999) found that upregulation of Bcl-2 in the brain is a common effect of two structurally distinct mood stabilizers, lithium and valproate. They also determined that the upregulation is at least in part through activation of the ERK MAP kinase (Creson et al. 2009; Einat et al. 2003; Yuan et al. 2001). The ERK activation led to activation of CREB, a transcription factor, and upregulation of bcl-2 gene transcription (Creson et al. 2009; Einat et al. 2003; Yuan et al. 2001). The follow-up studies from their group and others show that mood stabilizers also enhance cellular function of Bcl-2, including neuronal protection against death-inducing insults, neurite and axonal growth, calcium signaling, and mitochondrial function (Chen et al. 2010; Hunsberger et al. 2009; Machado-Vieira et al. 2011). The same group also demonstrated that lithium and valproate upregulated another Bcl-2-related protein, BAG1, and modulated a BAG1 unique function, glucocorticiod receptor translocation to nuclei (Zhou et al. 2005). Their follow-up behavioral studies show that Bcl-2 proteins including Bcl-2, BAG1, BI-1, and tBid are sufficient to modulate behavioral outcomes in a wide range of rodent models of mood disorders (Chen et al. 2010; Einat et al. 2005; Hunsberger et al. 2011; Lien et al. 2008; Maeng et al. 2008; Malkesman et al. 2011). The paradigms include the forced swim test, tail suspension test, learned helplessness paradigm, anhedonia-like deficits induced by serotonin and catecholamine depletion, amphetamine-induced hyperlocomotion, and amphetamine-induced behavioral sensitization. Human postmortem brain studies reveal lower levels of Bcl-2 (Kim et al. 2010), higher levels of Bax and Bad (Kim et al. 2010), and lower levels of ERK pathway components in cortical tissues from bipolar patients (P. Yuan et al. 2010). These coherent data indicate the possible role of Bcl-2 dysfunction in bipolar disorder. Some studies also revealed lower levels of Bcl-2 (Jarskog et al. 2000); a higher ratio of Bax vs. Bcl-2 (Jarskog et al. 2004), and lower levels of ERK/MAP kinase pathway components in the postmortem brain tissues from schizophrenia patients (Yuan et al. 2010). These data suggest that Bcl-2 dysfunction is a common intracellular arbiter of both illnesses, though the Bcl-2 dysfunction might reside in different neuronal circuitries and/or different cell types in these two different illnesses.
3.5 Summary of Preclinical Studies and Future Directions
A considerable body of evidence supports abnormalities in the regulation of cellular plasticity cascades as an integral part of the neurobiology underlying bipolar disorder. Many of these pathways play critical roles in immediate synaptic plasticity, and in long-term cell growth/atrophy and cell survival/cell death. Indeed, the atrophic changes observed in multiple cell types (neurons and glia), as well as the reversibility of the changes with treatment, support a role for intracellular plasticity cascades. It is likely that the major defect is in the ability to regulate neuroplastic adaptations to perturbations (both physiological and pathophysiological) and the inability to handle “normal loads” (neurochemical, hormonal, stress-induced, pharmacologically induced, etc.) without failing or invoking compensatory adaptations that overshoot and predispose to oscillations.
Antipsychotics appear to affect a number of these targets, underlying their utility in mood disorders. Newer “plasticity enhancing” strategies that may have utility in the treatment of mood disorders include inhibitors of glutamate release, N-methyl-d-aspartate antagonists, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid potentiators, and PKC inhibitors. Indeed, such potential next-generation drugs, in addition to treating the core symptoms of bipolar disorder, might be able to target other important aspects of the illness. These aspects include enhancing cognition independent of any improvement in mood symptoms, and preventing or reversing epigenetic factors that may have long-term negative impacts on the course of the illness. The development of novel therapeutics holds much promise for the long-term treatment of severe mood disorders and for improving the lives of the many who suffer from them.
4 Conclusions
Atypical antipsychotics have an important role in the acute and maintenance treatment of bipolar disorder. While a large body of evidence supports the efficacy of these agents in the various stages of bipolar disorder, more comparative effectiveness studies are needed to assess the optimal treatment regimens, including the relative benefits and risks of antipsychotics versus mood stabilizers. The growing understanding of the underlying neurobiology of bipolar disorder, along with the ability of atypical antipsychotics to target key pathophysiological pathways of this condition, suggests that these medications exert their therapeutic effect through a variety of mechanisms. Further, the development of novel “plasticity-enhancing” therapeutics brings hope for the future treatment of patients with bipolar disorder.
References
Amrollahi Z, Rezaei F, Salehi B, Modabbernia AH, Maroufi A, Esfandiari GR et al (2011) Double-blind, randomized, placebo-controlled 6-week study on the efficacy and safety of the tamoxifen adjunctive to lithium in acute bipolar mania. J Affect Disord 129(1–3):327–331
APA (2002) Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 159(4 Suppl):1–50
Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63(1):182–217
Beaulieu JM, Gainetdinov RR, Caron MG (2007) The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci 28(4):166–172
Beaulieu JM, Gainetdinov RR, Caron MG (2009) Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol 49:327–347
Beckford-Ball J (2006) An overview of the new NICE guidelines on bipolar disorder. Nurs Times 102(34):23–24
Benyamina A, Samalin L (2012) Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract 16(1):2–7
Berk M, Dodd S, Kauer-Sant’anna M, Malhi GS, Bourin M, Kapczinski F et al (2007) Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand Suppl 434:41–49
Berwaerts J, Lane R, Nuamah IF, Lim P, Remmerie B, Hough DW (2011) Paliperidone extended-release as adjunctive therapy to lithium or valproate in the treatment of acute mania: a randomized, placebo-controlled study. J Affect Disord 129(1–3):252–260
Blier P, Blondeau C (2011) Neurobiological bases and clinical aspects of the use of aripiprazole in treatment-resistant major depressive disorder. J Affect Disord 128(Suppl 1):S3–10
Bowden CL, Grunze H, Mullen J, Brecher M, Paulsson B, Jones M et al (2005) A randomized, double-blind, placebo-controlled efficacy and safety study of quetiapine or lithium as monotherapy for mania in bipolar disorder. J Clin Psychiatry 66(1):111–121
Bowden CL, Vieta E, Ice KS, Schwartz JH, Wang PP, Versavel M (2010) Ziprasidone plus a mood stabilizer in subjects with bipolar I disorder: a 6-month, randomized, placebo-controlled, double-blind trial. J Clin Psychiatry 71(2):130–137
Calabrese JR, Elhaj O, Gajwani P, Gao K (2005a) Clinical highlights in bipolar depression: focus on atypical antipsychotics. J Clin Psychiatry 66(Suppl 5):26–33
Calabrese JR, Keck PE Jr, Macfadden W, Minkwitz M, Ketter TA, Weisler RH et al (2005b) A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 162(7):1351–1360
Calabrese J, Stet L, Kotari H (2010) Asenapine as adjunctive treatment for bipolar mania: results of a placebo-controlled 12-week study and 40-week extension. Eur Psychiatry (Supplement 1): 1447
Chang KD (2008) The use of atypical antipsychotics in pediatric bipolar disorder. J Clin Psychiatry 69(Suppl 4):4–8
Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH et al (1999) The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem 72(2):879–882
Chen G, Henter ID, Manji HK (2010) Translational research in bipolar disorder: emerging insights from genetically based models. Mol Psychiatry 15(9):883–895
Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S et al (2011) Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet 378:1306–15
Collo G, Zanetti S, Missale C, Spano P (2008) Dopamine D3 receptor-preferring agonists increase dendrite arborization of mesencephalic dopaminergic neurons via extracellular signal-regulated kinase phosphorylation. Eur J Neurosci 28(7):1231–1240
Corya SA, Perlis RH, Keck PE Jr, Lin DY, Case MG, Williamson DJ et al (2006) A 24-week open-label extension study of olanzapine-fluoxetine combination and olanzapine monotherapy in the treatment of bipolar depression. J Clin Psychiatry 67(5):798–806
Councill JH, Tucker ES, Haskell GT, Maynard TM, Meechan DW, Hamer RM et al (2006) Limited influence of olanzapine on adult forebrain neural precursors in vitro. Neuroscience 140(1):111–122
Cousins DA, Young AH (2007) The armamentarium of treatments for bipolar disorder: a review of the literature. Int J Neuropsychopharmacol 10(3):411–431
Cousins DA, Butts K, Young AH (2009) The role of dopamine in bipolar disorder. Bipolar Disord 11(8):787–806
Creson TK, Yuan P, Manji HK, Chen G (2009) Evidence for involvement of ERK, PI3K, and RSK in induction of Bcl-2 by valproate. J Mol Neurosci 37(2):123–134
Cruz N, Sanchez-Moreno J, Torres F, Goikolea JM, Valenti M, Vieta E (2009) Efficacy of modern antipsychotics in placebo-controlled trials in bipolar depression: a meta-analysis. Int J Neuropsychopharmacol 13(1):5–14
Deutch AY, Ongur D, Duman RS (1995) Antipsychotic drugs induce Fos protein in the thalamic paraventricular nucleus: a novel locus of antipsychotic drug action. Neuroscience 66(2):337–346
Di Benedetto B, Kuhn R, Nothdurfter C, Rein T, Wurst W, Rupprecht R (2011) N-desalkylquetiapine activates ERK1/2 to induce GDNF release in C6 glioma cells: A putative cellular mechanism for quetiapine as antidepressant. Neuropharmacology 62(1):209–16
DiazGranados N, Zarate CA Jr (2008) A review of the preclinical and clinical evidence for protein kinase C as a target for drug development for bipolar disorder. Curr Psychiatry Rep 10(6):510–519
Dwivedi Y, Pandey GN (1999) Effects of treatment with haloperidol, chlorpromazine, and clozapine on protein kinase C (PKC) and phosphoinositide-specific phospholipase C (PI-PLC) activity and on mRNA and protein expression of PKC and PLC isozymes in rat brain. J Pharmacol Exp Ther 291(2):688–704
Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L et al (2003) The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci 23(19):7311–7316
Einat H, Yuan P, Manji HK (2005) Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: further support for the involvement of mitochondrial function in anxiety disorders. Behav Brain Res 165(2):172–180
Fraguas D, Correll CU, Merchan-Naranjo J, Rapado-Castro M, Parellada M, Moreno C et al (2010) Efficacy and safety of second-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: comprehensive review of prospective head-to-head and placebo-controlled comparisons. Eur Neuropsychopharmacol 21(8):621–645
Freyberg Z, Ferrando SJ, Javitch JA (2010) Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry 167(4):388–396
Fumagalli F, Molteni R, Bedogni F, Gennarelli M, Perez J, Racagni G et al (2004) Quetiapine regulates FGF-2 and BDNF expression in the hippocampus of animals treated with MK-801. Neuroreport 15(13):2109–2112
Gao K, Kemp DE, Ganocy SJ, Gajwani P, Xia G, Calabrese JR (2008) Antipsychotic-induced extrapyramidal side effects in bipolar disorder and schizophrenia: a systematic review. J Clin Psychopharmacol 28(2):203–209
Gentile S (2007) Atypical antipsychotics for the treatment of bipolar disorder: more shadows than lights. CNS Drugs 21(5):367–387
Giese AA (2009) Closing the gap between guidelines for bipolar disorder treatment and clinical practice. Am J Psychiatry 166(11):1205–1206
Goodwin FK (2007) Manic-depressive illness: bipolar disorders and recurrent depression, 2nd edn. Oxford University Press, Oxford
Goodwin FK, Jamison KR (2007) Manic-depressive illness: bipolar disorders and recurrent depression, 2nd edn. Oxford University Press, Oxford
Gould TD, Picchini AM, Einat H, Manji HK (2006) Targeting glycogen synthase kinase-3 in the CNS: implications for the development of new treatments for mood disorders. Curr Drug Targets 7(11):1399–1409, Review. PubMed PMID: 17100580
Green W, Patil P, Marsden CA, Bennett GW, Wigmore PM (2006) Treatment with olanzapine increases cell proliferation in the subventricular zone and prefrontal cortex. Brain Res 1070(1):242–245
Hammonds MD, Shim SS (2009) Effects of 4-week treatment with lithium and olanzapine on levels of brain-derived neurotrophic factor, B-cell CLL/lymphoma 2 and phosphorylated cyclic adenosine monophosphate response element-binding protein in the sub-regions of the hippocampus. Basic Clin Pharmacol Toxicol 105(2):113–119
He J, Xu H, Yang Y, Zhang X, Li XM (2004) Neuroprotective effects of olanzapine on methamphetamine-induced neurotoxicity are associated with an inhibition of hyperthermia and prevention of Bcl-2 decrease in rats. Brain Res 1018(2):186–192
He J, Xu H, Yang Y, Rajakumar D, Li X, Li XM (2006) The effects of chronic administration of quetiapine on the phencyclidine-induced reference memory impairment and decrease of Bcl-XL/Bax ratio in the posterior cingulate cortex in rats. Behav Brain Res 168(2):236–242
Hirschfeld RM, Keck PE Jr, Kramer M, Karcher K, Canuso C, Eerdekens M et al (2004) Rapid antimanic effect of risperidone monotherapy: a 3-week multicenter, double-blind, placebo-controlled trial. Am J Psychiatry 161(6):1057–1065
Hunsberger J, Austin DR, Henter ID, Chen G (2009) The neurotrophic and neuroprotective effects of psychotropic agents. Dialogues Clin Neurosci 11(3):333–348
Hunsberger JG, Machado-Vieira R, Austin DR, Zarate C, Chuang DM, Chen G et al (2011) Bax inhibitor 1, a modulator of calcium homeostasis, confers affective resilience. Brain Res 1403:19–27
Inestrosa NC, Arenas E (2010) Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci 11(2):77–86
Jarskog LF, Gilmore JH, Selinger ES, Lieberman JA (2000) Cortical bcl-2 protein expression and apoptotic regulation in schizophrenia. Biol Psychiatry 48(7):641–650
Jarskog LF, Selinger ES, Lieberman JA, Gilmore JH (2004) Apoptotic proteins in the temporal cortex in schizophrenia: high Bax/Bcl-2 ratio without caspase-3 activation. Am J Psychiatry 161(1):109–115
Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL (2008) N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacology 33(10):2303–2312
Jiao S, Li Z (2011) Nonapoptotic function of BAD and BAX in long-term depression of synaptic transmission. Neuron 70(4):758–772
Jonas E (2006) BCL-xL regulates synaptic plasticity. Mol Interv 6(4):208–222
Jonas EA (2009) Molecular participants in mitochondrial cell death channel formation during neuronal ischemia. Exp Neurol 218(2):203–212
Kapur S, Mann JJ (1992) Role of the dopaminergic system in depression. Biol Psychiatry 32(1):1–17
Kato T (2007) Molecular genetics of bipolar disorder and depression. Psychiatry Clin Neurosci 61(1):3–19
Keck PE Jr, Versiani M, Potkin S, West SA, Giller E, Ice K (2003) Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry 160(4):741–748
Keck PE Jr, Calabrese JR, McIntyre RS, McQuade RD, Carson WH, Eudicone JM et al (2007) Aripiprazole monotherapy for maintenance therapy in bipolar I disorder: a 100-week, double-blind study versus placebo. J Clin Psychiatry 68(10):1480–1491
Keilhoff G, Grecksch G, Bernstein HG, Roskoden T, Becker A (2010) Risperidone and haloperidol promote survival of stem cells in the rat hippocampus. Eur Arch Psychiatry Clin Neurosci 260(2):151–162
Khanna S, Vieta E, Lyons B, Grossman F, Eerdekens M, Kramer M (2005) Risperidone in the treatment of acute mania: double-blind, placebo-controlled study. Br J Psychiatry 187:229–234
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7(12):1013–1030
Kim HW, Rapoport SI, Rao JS (2010) Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis 37(3):596–603
Kinon BJ, Stauffer VL, Kollack-Walker S, Chen L, Sniadecki J (2008) Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. J Clin Psychopharmacol 28(6):601–607
Kusumakar V (2002) Antidepressants and antipsychotics in the long-term treatment of bipolar disorder. J Clin Psychiatry 63(Suppl 10):23–28
Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294(5548):1945–1948
Levy B, Manove E (2011) Functional outcome in bipolar disorder: the big picture. Depress Res Treat 2012:949248
Li X, Jope RS (2010) Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology 35(11):2143–2154
Lien R, Flaisher-Grinberg S, Cleary C, Hejny M, Einat H (2008) Behavioral effects of Bcl-2 deficiency: implications for affective disorders. Pharmacol Rep 60(4):490–498
Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, Aprille JR, Dwyer DS, Li XM, Mahadik SP, Duman RS, Porter JH, Modica-Napolitano JS, Newton SS, Csernansky JG (2008) Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev 60(3):358–403, Review. Erratum in: Pharmacol Rev. 2008 Dec;60(4):582. PubMed PMID: 18922967
Lipina TV, Kaidanovich-Beilin O, Patel S, Wang M, Clapcote SJ, Liu F et al (2011) Genetic and pharmacological evidence for schizophrenia-related Disc1 interaction with GSK-3. Synapse 65(3):234–248
Luo C, Xu H, Li XM (2004) Post-stress changes in BDNF and Bcl-2 immunoreactivities in hippocampal neurons: effect of chronic administration of olanzapine. Brain Res 1025(1–2):194–202
Macfadden W, Alphs L, Haskins JT, Turner N, Turkoz I, Bossie C et al (2009) A randomized, double-blind, placebo-controlled study of maintenance treatment with adjunctive risperidone long-acting therapy in patients with bipolar I disorder who relapse frequently. Bipolar Disord 11(8):827–839
Machado-Vieira R, Pivovarova NB, Stanika RI, Yuan P, Wang Y, Zhou R et al (2011) The Bcl-2 gene polymorphism rs956572AA increases inositol 1,4,5-trisphosphate receptor-mediated endoplasmic reticulum calcium release in subjects with bipolar disorder. Biol Psychiatry 69(4):344–352
Maeng S, Hunsberger JG, Pearson B, Yuan P, Wang Y, Wei Y et al (2008) BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc Natl Acad Sci U S A 105(25):8766–8771
Malkesman O, Austin DR, Tragon T, Henter ID, Reed JC, Pellecchia M, et al (2011) Targeting the BH3-interacting domain death agonist to develop mechanistically unique antidepressants. Mol Psychiatry
Marcus R, Khan A, Rollin L, Morris B, Timko K, Carson W et al (2011) Efficacy of aripiprazole adjunctive to lithium or valproate in the long-term treatment of patients with bipolar I disorder with an inadequate response to lithium or valproate monotherapy: a multicenter, double-blind, randomized study. Bipolar Disord 13(2):133–144
McIntyre RS, Brecher M, Paulsson B, Huizar K, Mullen J (2005) Quetiapine or haloperidol as monotherapy for bipolar mania–a 12-week, double-blind, randomised, parallel-group, placebo-controlled trial. Eur Neuropsychopharmacol 15(5):573–585
McIntyre RS, Cohen M, Zhao J, Alphs L, Macek TA, Panagides J (2009) Asenapine versus olanzapine in acute mania: a double-blind extension study. Bipolar Disord 11(8):815–826
McIntyre RS, Cohen M, Zhao J, Alphs L, Macek TA, Panagides J (2010) Asenapine in the treatment of acute mania in bipolar I disorder: a randomized, double-blind, placebo-controlled trial. J Affect Disord 122(1–2):27–38
McQuade R, Marcus R, Sanchez R (2003) Aripiprazole vs placebo in acute mania: safety and tolerability pooled analysis. Paper presented at the 5th International Conference on Bipolar Disorder, Pittsburgh, PA
Merlo S, Canonico PL, Sortino MA (2011) Distinct effects of pramipexole on the proliferation of adult mouse sub-ventricular zone-derived cells and the appearance of a neuronal phenotype. Neuropharmacology 60(6):892–900
Montezinho LP, Castro MM, Duarte CB, Penschuck S, Geraldes CF, Mork A (2006) The interaction between dopamine D2-like and beta-adrenergic receptors in the prefrontal cortex is altered by mood-stabilizing agents. J Neurochem 96(5):1336–1348
Mullen J, Devine N, Sweitzer D (2003) Quetiapine adjunctive therapy for acute mania associated with bipolar disorder (SIAM) Paper presented at the 156th Annual Meeting of the American Psychiatric Association, San Francisco, CA
Nasrallah HA, Hopkins T, Pixley SK (2010) Differential effects of antipsychotic and antidepressant drugs on neurogenic regions in rats. Brain Res 1354:23–29
Newton SS, Duman RS (2007) Neurogenic actions of atypical antipsychotic drugs and therapeutic implications. CNS Drugs 21(9):715–725
Nikolaus S, Antke C, Muller HW (2009) In vivo imaging of synaptic function in the central nervous system: II Mental and affective disorders. Behav Brain Res 204(1):32–66
O'Brien WT, Klein PS (2007) Regulation of glycogen synthase kinase-3 in patients with affective disorders. Biol Psychiatry 61(2):139–141
Pearlson GD, Wong DF, Tune LE, Ross CA, Chase GA, Links JM et al (1995) In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Arch Gen Psychiatry 52(6):471–477
Pereira M, Martynhak BJ, Baretta IP, Correia D, Siba IP, Andreatini R (2011) Antimanic-like effect of tamoxifen is not reproduced by acute or chronic administration of medroxyprogesterone or clomiphene. Neurosci Lett 500(2):95–98
Perlis RH, Welge JA, Vornik LA, Hirschfeld RM, Keck PE Jr (2006) Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry 67(4):509–516
Pinsonneault JK, Han DD, Burdick KE, Kataki M, Bertolino A, Malhotra AK et al (2011) Dopamine transporter gene variant affecting expression in human brain is associated with bipolar disorder. Neuropsychopharmacology 36(8):1644–1655
Popovic D, Reinares M, Amann B, Salamero M, Vieta E (2010) Number needed to treat analyses of drugs used for maintenance treatment of bipolar disorder. Psychopharmacology (Berl) 213(4):657–667
Potkin SG, Keck PE Jr, Segal S, Ice K, English P (2005) Ziprasidone in acute bipolar mania: a 21-day randomized, double-blind, placebo-controlled replication trial. J Clin Psychopharmacol 25(4):301–310
Prieto E, Mico JA, Meana JJ, Majadas S (2010) Neurobiological bases of quetiapine antidepresant effect in the bipolar disorder. Actas Esp Psiquiatr 38(1):22–32
Quiroz JA, Yatham LN, Palumbo JM, Karcher K, Kushner S, Kusumakar V (2010) Risperidone long-acting injectable monotherapy in the maintenance treatment of bipolar I disorder. Biol Psychiatry 68(2):156–162
Roh MS, Seo MS, Kim Y, Kim SH, Jeon WJ, Ahn YM et al (2007) Haloperidol and clozapine differentially regulate signals upstream of glycogen synthase kinase 3 in the rat frontal cortex. Exp Mol Med 39(3):353–360
Rybakowski J (2005) Maintenance treatment of bipolar disorders. Neuro Endocrinol Lett 26(Suppl 1):49–65
Sachs GS, Grossman F, Ghaemi SN, Okamoto A, Bowden CL (2002) Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry 159(7):1146–1154
Sachs G, Chengappa KN, Suppes T, Mullen JA, Brecher M, Devine NA et al (2004) Quetiapine with lithium or divalproex for the treatment of bipolar mania: a randomized, double-blind, placebo-controlled study. Bipolar Disord 6(3):213–223
Sachs G, Sanchez R, Marcus R, Stock E, McQuade R, Carson W et al (2006) Aripiprazole in the treatment of acute manic or mixed episodes in patients with bipolar I disorder: a 3-week placebo-controlled study. J Psychopharmacol 20(4):536–546
Samuels BA, Hen R (2011) Neurogenesis and affective disorders. Eur J Neurosci 33(6):1152–1159
Scheffer RE, Tripathi A, Kirkpatrick FG, Schultz T (2011) Guidelines for treatment-resistant mania in children with bipolar disorder. J Psychiatr Pract 17(3):186–193
Sklar P, Ripke S, Scott LJ, Andreassen OA, Cichon S, Craddock N et al (2011) Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet 43(10):977–83
Smith LA, Cornelius V, Warnock A, Bell A, Young AH (2007) Effectiveness of mood stabilizers and antipsychotics in the maintenance phase of bipolar disorder: a systematic review of randomized controlled trials. Bipolar Disord 9(4):394–412
Smulevich AB, Khanna S, Eerdekens M, Karcher K, Kramer M, Grossman F (2005) Acute and continuation risperidone monotherapy in bipolar mania: a 3-week placebo-controlled trial followed by a 9-week double-blind trial of risperidone and haloperidol. Eur Neuropsychopharmacol 15(1):75–84
Suppes T, Brown E, Schuh LM, Baker RW, Tohen M (2005a) Rapid versus non-rapid cycling as a predictor of response to olanzapine and divalproex sodium for bipolar mania and maintenance of remission: post hoc analyses of 47-week data. J Affect Disord 89(1–3):69–77
Suppes T, Dennehy EB, Hirschfeld RM, Altshuler LL, Bowden CL, Calabrese JR et al (2005b) The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry 66(7):870–886
Suppes T, Vieta E, Liu S, Brecher M, Paulsson B (2009) Maintenance treatment for patients with bipolar I disorder: results from a north american study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry 166(4):476–488
Surja AA, Tamas RL, El-Mallakh RS (2006) Antipsychotic medications in the treatment of bipolar disorder. Curr Drug Targets 7(9):1217–1224
Sutton LP, Rushlow WJ (2011) Regulation of Akt and Wnt signaling by the group II metabotropic glutamate receptor antagonist LY341495 and agonist LY379268. J Neurochem 117(6):973–983
Thase ME, Macfadden W, Weisler RH, Chang W, Paulsson B, Khan A et al (2006) Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study). J Clin Psychopharmacol 26(6):600–609
Thase ME, Jonas A, Khan A, Bowden CL, Wu X, McQuade RD et al (2008) Aripiprazole monotherapy in nonpsychotic bipolar I depression: results of 2 randomized, placebo-controlled studies. J Clin Psychopharmacol 28(1):13–20
Tohen M, Vieta E (2009) Antipsychotic agents in the treatment of bipolar mania. Bipolar Disord 11(Suppl 2):45–54
Tohen M, Sanger TM, McElroy SL, Tollefson GD, Chengappa KN, Daniel DG et al (1999) Olanzapine versus placebo in the treatment of acute mania Olanzapine HGEH Study Group. Am J Psychiatry 156(5):702–709
Tohen M, Jacobs TG, Grundy SL, McElroy SL, Banov MC, Janicak PG et al (2000) Efficacy of olanzapine in acute bipolar mania: a double-blind, placebo-controlled study The Olanzipine HGGW Study Group. Arch Gen Psychiatry 57(9):841–849
Tohen M, Chengappa KN, Suppes T, Zarate CA Jr, Calabrese JR, Bowden CL et al (2002) Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 59(1):62–69
Tohen M, Vieta E, Calabrese J, Ketter TA, Sachs G, Bowden C et al (2003) Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 60(11):1079–1088
Tohen M, Greil W, Calabrese JR, Sachs GS, Yatham LN, Oerlinghausen BM et al (2005) Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry 162(7):1281–1290
Tohen M, Calabrese JR, Sachs GS, Banov MD, Detke HC, Risser R et al (2006) Randomized, placebo-controlled trial of olanzapine as maintenance therapy in patients with bipolar I disorder responding to acute treatment with olanzapine. Am J Psychiatry 163(2):247–256
Toprac MG, Dennehy EB, Carmody TJ, Crismon ML, Miller AL, Trivedi MH et al (2006) Implementation of the texas medication algorithm project patient and family education program. J Clin Psychiatry 67(9):1362–1372
Tran-Johnson TK, Sack DA, Marcus RN, Auby P, McQuade RD, Oren DA (2007) Efficacy and safety of intramuscular aripiprazole in patients with acute agitation: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 68(1):111–119
Vestergaard P (1992) Treatment and prevention of mania: a Scandinavian perspective. Neuropsychopharmacology 7(4):249–259
Vieta E, Suppes T, Eggens I, Persson I, Paulsson B, Brecher M (2008a) Efficacy and safety of quetiapine in combination with lithium or divalproex for maintenance of patients with bipolar I disorder (international trial 126). J Affect Disord 109(3):251–263
Vieta E, T’Joen C, McQuade RD, Carson WH Jr, Marcus RN, Sanchez R et al (2008b) Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially nonresponsive to valproate/lithium monotherapy: a placebo-controlled study. Am J Psychiatry 165(10):1316–1325
Vieta E, Nuamah IF, Lim P, Yuen EC, Palumbo JM, Hough DW et al (2010) A randomized, placebo- and active-controlled study of paliperidone extended release for the treatment of acute manic and mixed episodes of bipolar I disorder. Bipolar Disord 12(3):230–243
Vieta E, Gunther O, Locklear J, Ekman M, Miltenburger C, Chatterton ML et al (2011) Effectiveness of psychotropic medications in the maintenance phase of bipolar disorder: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol 14(8):1029–1049
Wagstaff AJ, Easton J, Scott LJ (2005) Intramuscular olanzapine: a review of its use in the management of acute agitation. CNS Drugs 19(2):147–164
Walburn J, Gray R, Gournay K, Quraishi S, David AS (2001) Systematic review of patient and nurse attitudes to depot antipsychotic medication. Br J Psychiatry 179:300–307
Weisler R, Dunn J, English P (2003) Ziprasidone in adjunctive treatment of acute bipolar mania: a randomized, placebo-controlled trial. Paper presented at the 16th congress of the European College of Neuro-psychopharmacology, Prague, Czech Republic
Weisler RH, Nolen WA, Neijber A, Hellqvist A, Paulsson B (2010) Continuation of quetiapine versus switching to placebo or lithium for maintenance treatment of bipolar I disorder (Trial 144: a randomized controlled study). J Clin Psychiatry 72(11):1452–1464
Yatham LN, Grossman F, Augustyns I, Vieta E, Ravindran A (2003) Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double-blind, randomised controlled trial. Br J Psychiatry 182:141–147
Yildiz A, Guleryuz S, Ankerst DP, Ongur D, Renshaw PF (2008) Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch Gen Psychiatry 65(3):255–263
Yildiz A, Vieta E, Leucht S, Baldessarini RJ (2011) Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology 36(2):375–389
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9(1):47–59
Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G (2001) The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem 276(34):31674–31683
Yuan P, Zhou R, Wang Y, Li X, Li J, Chen G et al (2010) Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J Affect Disord 124(1–2):164–169
Zarate CA, Manji HK (2009) Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs 23(7):569–582
Zarate CA Jr, Singh JB, Carlson PJ, Quiroz J, Jolkovsky L, Luckenbaugh DA et al (2007) Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord 9(6):561–570
Zhou R, Gray NA, Yuan P, Li X, Chen J, Chen G et al (2005) The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J Neurosci 25(18):4493–4502
Zupancic ML (2011) Role of atypical antipsychotics in rapid cycling bipolar disorder: a review of the literature. Ann Clin Psychiatry 23(2):141–149
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Singh, J., Chen, G., Canuso, C.M. (2012). Antipsychotics in the Treatment of Bipolar Disorder. In: Gross, G., Geyer, M. (eds) Current Antipsychotics. Handbook of Experimental Pharmacology, vol 212. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-25761-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-642-25761-2_8
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-25760-5
Online ISBN: 978-3-642-25761-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)