Abstract
Long-term field research on wild animals is essential for understanding life history and social systems of long-lived organisms like primates. Gibbons (family Hylobatidae) live surprisingly slow lives, given their relatively small body mass. Following an approximately 7-year-long juvenile period, one of the longest among all primates, Khao Yai white-handed gibbon females begin reproducing at an average age of 10.5±1.2 years. This is much later than in monkeys of at least the same body mass and, remarkably, at about the same age as in mountain gorillas. Our long-term research also revealed remarkable social flexibility analogous to that seen in other apes. At Khao Yai, white-handed gibbons form pairs or small two-male/one-female reproductive units, although individuals may temporarily also live in single-male/multi-female groups, and here we report a novel, semi-solitary life stage of two older males for the first time. Mating patterns also turned out to be flexible, with males and females mating polygamously, including extra-pair copulations and regular polyandrous mating of females living in multi-male groups. We have also found that in accordance with this variability in male–female socio-sexual bonds, female gibbons at Khao Yai show cyclical sexual swellings that advertise the probability of ovulation without allowing males to exactly pinpoint the day of ovulation. After decades of research, we have come to recognize more clearly the importance of the gibbon community and feel confident that we understand the basic social and mating systems of the Khao Yai white-handed gibbon population, but we also continue to discover new details of the evolutionary forces that shape gibbons complex social life.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
A unifying theme of early and current primate field studies is their “individual-centric” approach, which means that particular individuals and their lives become the focus of a researcher’s attention and systematic data collections (e.g., Goodall 1986). Working with well-known individuals is a unique strength of long-term field studies and one that continuously draws students, volunteers, and periodically the media to our field. Hearing of the adventures of primate characters and following the fate of individuals through time often seems just as fascinating as vividly telling their stories and presenting data from the field (e.g., Perry and Manson 2008), which now sometimes even happens in “near-real-time” in the new format of primate field blogs. Beyond scientific curiosity and theoretically well-grounded questions, many primatologists, students, and professionals alike, feed off direct contact with well known, habituated individuals as their source of energy to write grant proposals, and involvement in the lives of their study subjects can bring researchers back to a field site year after year. Dedication and developing relationships with primate subjects and human communities living closest to them are emotional and intellectual reservoirs field workers use until a long-term study emerges, which is a necessary step to document life-history strategies of long-lived mammals.
2 History of the Khao Yai White-Handed Gibbon Study Site
Research on white-handed gibbons (Hylobates lar >) of Khao Yai National Park (KY), Thailand began in 1977. Like other primate field projects, ours began small, but it gradually grew to become the longest ongoing gibbon study, and we have accumulated demography data on 14 habituated groups (Fig. 11.1). Like others, we believe that longitudinal research, although slow and difficult to maintain, is essential as it is often the only way to generate life history data, to decode strategies underlying complex behaviors in wild populations, like those that involve reciprocity, cooperation, conflict resolution, and to understand primate social dynamics more broadly (Wells 1991; Boesch and Boesch-Achermann 2000; Strier et al. 2002; Watts 2002). The complex social dynamics of KY white-handed gibbons would have been difficult to detect in a short-term study (see below), even if it covered several years. Lack of long-term documentation of gibbon demography, life-history strategies, and social dynamics until recently is the reason why the subtleties and complexity of their social organization remained unnoticed for a long time.
Over the years, many individuals have contributed to the ongoing demographic data collection (for a complete list, see Brockelman et al. 1998). Key people at the beginning were Treesucon (1984), Raemaekers and Raemaekers (1985) and W.Y. Brockelman, of whom only Brockelman continues to do research at the site. By the end of 1989, Reichard (1991) became involved, and in the mid 1990s, C. Barelli joined the research effort; since then, they have coordinated systematic recording of demography data. Quantitative data presented here were collected by C. Barelli and U.H. Reichard between 1989 and 2010.
Today, KY is a patch of 2,168 km2 (Smitinand 1977) of forest surrounded by agricultural land on all sides except one. The park was established in 1962 and, in 2005, was included in Thailand’s 6,199 km2 large Dong Phayayen – Khao Yai Forest Complex (DPKY) as part of a new World Heritage site (UNESCO 2005) because it is a biodiversity hotspot in Asia (Lynam et al. 2006).
Located at latitudes 14°05′–14°15′ N and longitudes 101°05′–101°50′ E, KY is part of the Phanom Dongrak mountain range that runs north to south from the Thai–Laotian border before bending eastwards and eventually forming the Thai–Cambodian border in the region of Pang Sida and Ta Phraya National Parks. Elevation at KY ranges from ~250 to 1,351 m a.s.l., and the terrain is rugged. The climate is seasonally wet following the Asian southwest monsoon cycle (Singhrattna et al. 2005), with annual precipitation averaging 2,477 mm/year (range 2,038–3,111) (Tangtam 1992; Boonpragob et al. 1998; Kitamura et al. 2004, 2005, 2008; Bartlett 2009a; Gale et al. 2009). The wet months are March–October.
KY can be broadly classified as a tropical seasonal forest (Smitinand 1989; Kitamura et al. 2005, 2008) or moist evergreen forest (Round and Gale 2008), because this vegetation type occupies 64% of the park’s land area found between 400 and 1,000 m elevation. Several gibbon study groups have established home ranges that partially include old secondary growth (i.e., groups A, H, and D). The gibbons have continuously and increasingly used these areas for travel and foraging since observations began. The park also includes areas of grassland where villagers living around the present day headquarters had cleared fields prior to the establishment of KY as a National Park; these are now maintained by annual burning and mowing.
2.1 Threats to Khao Yai Wildlife
Field sites vary greatly in the degree and form of threats they receive from humans. Due to its large size, systematic law enforcement is a constant challenge to park management at KY (Albers and Grinspoon 1997). Small-scale encroachment and hunting occur, although gibbons are not specifically targeted by poachers and, compared to other protected areas in Thailand, these pressures are low at KY (Lynam et al. 2006; Brodie et al. 2009). In our experience, the biggest threat to wildlife comes from selective, non-timber harvesting of Mai hom trees, Aquilaria crassna (Family Thymelaeaceae), by villagers and organized poacher groups. Mai hom trees produce agarwood, also known as aloewood or eaglewood, used by the perfume industry. The tree family occurs naturally in primary evergreen and semi-evergreen forests from ~600 to 1,400 m a.s.l. in many Southeast Asian countries and the commercially valuable resin is traditionally harvested by local people (Jensen and Meilby 2010).
At KY entire trees are sometimes felled, but more commonly mature trees are injured repeatedly to stimulate resin production (Zhang et al. 2008). Several months after a tree has been damaged, poachers return to chisel resin-soaked woodchips off until a tree eventually falls (Zhang et al. 2008). Large-scale harvest of Mai hom is obviously destructive because it involves bringing heavy machinery into the forest to fell and transport stems. But also small-scale poaching, i.e., poachers targeting specific trees and removing large quantities of woodchips, negatively affects wildlife because poachers stay in the forest for more days than they can carry food, and when their provisions are exhausted they hunt for food. Poachers often carry firearms, which makes encounters with them dangerous to park rangers, researchers, and our Thai field assistants alike. Poaching of Aquilaria trees also directly, although marginally affects gibbons, who feed on the tiny sprout of Aquillaria seeds during the trees’ short fruiting period after biting off the thick husk with their long, sharp canines. Selective harvest of agarwood is not unique to KY; it also occurs at other protected sites in Thailand (Grassman et al. 2005). The market value of agarwood varies according to quality, and agarwood from KY consistently yields high market prices, which makes effective control of Mai hom harvest and trade difficult.
3 Highlights of Long-Term Gibbon Research
Identifying results and benefits of long-term research on KY white-handed gibbons is straightforward and well documented through numerous publications that span a wide variety of topics ranging from vocal communication (Raemaekers et al. 1984; Raemaekers and Raemaekers 1985) to ecology (Bartlett 2009a, b; Brockelman 2009), social behavior (Reichard 1995, 1998, 2003; Reichard and Sommer 1997; Brockelman et al. 1998; Sommer and Reichard 2000; Barelli et al. 2008a), reproduction (Barelli et al. 2007, 2008b; Barelli and Heistermann 2009), life history (Reichard and Barelli 2008), and cognition (Asensio et al. 2011).
In the following, we highlight advances in three areas of research on white-handed gibbons with which we have been particularly involved: (1) social organization, (2) reproductive strategies, and (3) life histories. Research on all of these topics substantially advances our knowledge about gibbons and helps shift understanding of gibbon social organization from a simplistic focus on monogamy to a more complex community model.
3.1 Flexible Social Organization>
Our long-term research revealed a formerly unrecognized extent of social flexibility in white-handed gibbons. Although anecdotal reports of gibbon groups with more than one adult of one sex existed for some time (summarized in Fuentes (2000) and Reichard (2003)), systematic data allowing quantification of the frequency of social units not consisting of pairs became first available at KY (Barelli et al. 2007, 2008b; Reichard and Barelli 2008; Reichard 2009).
An important insight from our long-term observations is that white-handed gibbons are not per se committed to pair-living or other forms of social organization but instead respond in flexible ways to opportunities and actively pursue or passively accept changes in their social status. In the sample of 12 groups, 19 adult females and 22 adult males were residents at some point in time. Irrespective of the duration of these individuals’ group membership, 42% of females and 68% of males experienced pair-living and at least one other type of group structure. Some individuals lived through multiple changes from pair-living to multi-male/single-female stages and back. For females who experienced non-pair-living periods, these times amounted to roughly 50% of the 12-year census period (range 33–100%); the corresponding value for males was ca. 60% (range 9–100%). These data illustrate that a wide spectrum from exclusive pair-living to exclusive multi-male/single-female grouping and various stages in between exist at KY and that non-pair-living is not a transitional stage, but for many adults represents a substantial portion of their prime reproductive years.
In summary, our long-term data indicate that, although a majority of gibbon groups are pair-living, breeding groups with more than two adults (excluding groups with adult offspring), particularly adult males, are no exception (Reichard 2009). In the sample of 12 well-known groups censused annually over 12 years (1999–2010, N = 146 units), we found an average of 25% of groups to be multi-male/single-female (Table 11.1). We believe these data are representative for the population as a whole because the values are similar to an earlier, larger census that included non-habituated groups (Reichard 2009). Importantly, some multi-male groups were always present, and in some years made up 33% or more of groups (Table 11.1). Based on long-term demographic records (Reichard 2009), most multi-male groups consisted of two adult males living with an unrelated female, i.e., a female neither one of the males had grown up with. Two groups were each composed of three adult males and one adult female and persisted for about 2 and 4 years, respectively. Group structures besides pair-living and multi-male/single-female units such as multi-female/multi-male, and multi-female/single-male have also been observed (Reichard 2009), but they are rare and, to our knowledge, have not resulted in stable breeding units.
Nevertheless, the occurrence of three multi-female/single-male groups is interesting as it illustrates the context-dependent social flexibility in this population. We twice discovered multi-female/single-male groups in which each of two females carried a nursing infant. Unfortunately in the first case, we did not know the group’s social history and thus could not exclude the possibility that a daughter had conceived with the group’s adult male, who had very likely replaced the female’s presumed father. About 2 years and 4 months after the group had been discovered, one of the females disappeared with her offspring. In 2010, we witnessed a second group with two dependent infants. This time, we knew the social history of individuals and could confirm that a daughter gave birth a year after her mother. This was probably not the result of an incestuous mating, because the current male immigrated in 2007 and thus was unlikely to be the father of the female who had given birth recently. However, only a genetic study could confirm the kin relationships in this group. The third multi-female/single-male group formed after a young adult male and an adolescent female joined a young, unrelated adult female. The trio lived peacefully together for several years until the time of the younger female’s sexual maturity, when the older female became increasingly aggressive. The younger female left before sexual behavior with the male was witnessed. Thus, so far we can only confirm pairs and multi-male/single-female groups as reproductive units in the KY population.
Our knowledge of the complexity of social flexibility still continues to grow. Most recently, for example, we began to recognize yet another formerly unknown status of individuals. The surprising observation is that two males sometimes associate with a group and at other times spend long periods by themselves; we have termed this “semi-solitariness>”. The situation is radically different from “floating” commonly used to describe a period when an unmated individual seeks a mate following natal dispersal. In contrast, the two semi-solitary males are older and come from established multi-male groups. Whether they are searching for mates is unclear. For example, Frodo is a nearly 30 year-old male who was thought to have left his multi-male group permanently in 2007, after he was absent from the group for several months. In 2008, however, he re-appeared and occasionally traveled again with his former group. At first, we speculated that he was perhaps visiting while transitioning into another group; a phenomenon we have repeatedly witnessed with young adult males during the process of natal dispersal. Over the past 2 years, however, we realized that he sometimes foraged alone in the familiar home range. His periods alone lasted from a few hours to several days. He did not attempt to immigrate into or even contact a group other than his previous group. Interestingly, he could re-join this social group peacefully and was tolerated without signs of agonism by the resident male and female. At present, Frodo lives partly with a group and partly alone and thus is semi-solitary.
The second case of semi-solitariness concerns Cassius II, who is also at least 30 years old. In early 2010, his putative son secondarily dispersed into a neighboring group and shortly thereafter Cassius II also appeared in this group. Unlike Frodo, he either spends time with his former group or the neighboring group or is by himself. He commutes between the two groups primarily during intergroup encounters and presently shows a preference for staying in the overlap area between the two adjacent home ranges. From our observations, it seems that he travels temporarily with whichever group is in the overlap area and he rarely follows either group deeper into its home range. Like Frodo, his integration into both groups seems unproblematic, with his arrival often preceded by soft hoots and loud vocalizations, but without aggression. However, both of these semi-solitary males seem subordinate to the resident males in the groups they join because they do not call during duets and also otherwise behave like secondary males in multi-male groups (Barelli et al. 2008b).
Overall, semi-solitariness seems to be rare, although we believe previous cases might have passed unnoticed because we never expected individuals of the 12-year census period to live alone almost secretively, and our data collection has always focused on individuals in identifiable groups. In the past, phases of semi-solitariness, if they occurred, were categorized as “transitional” and thus did not make it into publication, even when we were not sure about the whereabouts of “transient individuals” until they reappeared in other groups. The reasons for semi-solitariness are unclear. Perhaps it is an alternative strategy to the subordinate, secondary status in multi-male groups, because both semi-solitary males are affiliated with multi-male/single-female groups. The presence of mature sons in the neighborhood may also importantly influence flexible group membership in this population, but further speculation must await knowledge of kin relations.
The recent observations highlight the great importance of time depth in understanding social dynamics and evolutionary forces of male–male competition and female mate choice that shape reproductive strategies in primates, perhaps particularly in apes, who express an impressive range of behavioral flexibility (van Schaik et al. 2004). Interpretations of group dynamics would have been very different had our study ceased after 5 or 10 years. We illustrate this point with an example of known transitions in and out of study group “A” (Fig. 11.2), although the argument applies to the entire study population. At each 5-year interval, the group composition of several groups involved would have looked different and consequently would have been interpreted differently with regard to the social and mating system of the population (Table 11.2).
Finally, we can ask why this flexibility (particularly in forming small multi-male units) was not recognized in earlier studies of wild gibbons. Perhaps when social histories of individuals were not known well, all too often additional adult males were considered adult sons of a breeding pair. At KY, however, longitudinal records of many groups allowed us to detect the presence of multi-male/single-female groups.
3.2 Female Reproductive Strategies>
Our understanding of reproductive strategies of white-handed gibbon females has undergone dramatic changes during the past two decades. Although they were initially thought to be passive and monogamous recipients of males’ socio-sexual strategies, it is now clear that gibbon females actively pursue their own reproductive interests, just like other mammalian females who are pair-living or form small polyandrous groups (Griffith et al. 2002; Wolff and Macdonald 2004; Munshi-South 2007). Following a plethora of molecular studies of female reproductive strategies in pair-living birds, the classic concept of female sexual monogamy has been shattered in most pair-living species. Recent molecular genetics and endocrinology studies have changed the perception of female reproductive interests, to which white-handed gibbon females are no exception.
Primate females may generally gain from multiple mating. Polyandrous mating during fertile periods might increase the probability of conception (van Noordwijk and van Schaik 2000) or of having their offspring sired by males who produce the most competitive sperm (Small 1989; Dixson 1998). Copulating with many males may also function to confuse paternity, which is advantageous in species with a high risk of infanticide (Hrdy 1979; Nunn 1999; van Schaik et al. 2000). Moreover, if a female preferentially copulates with her social partner compared to other males, as we found for KY gibbons, she might additionally benefit from her mate’s raised paternity probability because her mate will be the most likely protector should her next infant be attacked by other males (van Schaik et al. 1999, 2004; Palombit et al. 2000; Buchan et al. 2003; Moscovice et al. 2009) or predators (van Schaik and Hörstermann 1994) and/or because he will defend a territory against intruders (Goldizen 2003).
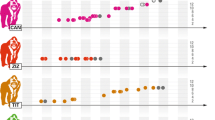
Analyses of proximate aspects of reproductive strategies depend on reliable information about endocrine mechanisms and reproductive physiology that underlie interactions between hormonal and behavioral factors. Our studies have confirmed that monitoring ovarian function in wild gibbons is feasible (Barelli and Heistermann 2009), and that females exhibit behavioral and non-behavioral reproductive status cues that are displayed during both the fertile and non-fertile phase of the ovarian cycle. During a recent study (2003–2005), we found that although females’ mating activity was skewed toward one preferred male (i.e., the primary male), half of the studied females (N = 10) lived in multi-male groups and each one also copulated with the second, subordinate male (i.e., the secondary male) in her group. Mating with a primary male increased during the fertile phase (Barelli et al. 2008b). Primary males in multi-male gibbon groups performed most copulations and had priority of access to fertile females. However, copulations by secondary males were distributed widely through female cycles, and these males had mating opportunities during periovulatory periods (Fig. 11.3). Copulating with both males even continued into non-fertile days of the menstrual cycle when conception was highly unlikely (as well as during pregnancy when conception was impossible), which contrasts strongly with the still widespread view that white-handed gibbons are socio-sexually monandrous and focused on single partners; instead, KY white-handed gibbon females are often sexually polyandrous (Barelli et al. 2008b, Reichard 2009).
Frequency of female copulation (number of copulations/day in which copulations occurred) with primary males (black dots) and secondary males (white dots) related to the day of ovulation (day ‘0’). Frequency of female copulation is calculated by first averaging the frequency for each female separately, and secondly across these individual values to yield a representative composite frequency that equally balanced individual contributions (see Barelli et al. 2008a, b)
Mating during pregnancy has also been suggested as a mechanism to confuse paternity and reduce the risk of infanticide in case of a male change (van Noordwijk and van Schaik 2000; van Schaik et al. 2000). It is noteworthy here that the only lactating females we have so far witnessed to become sexually active were two females who had just experienced male changes. While still carrying nursing infants, the females developed sexual swellings>. In one case, the relationship with the new male was tense despite copulations. A few weeks after the male take-over, the female increasingly refused copulation attempts and stayed out of close proximity, and in the days prior to the disappearance of the female’s infant some grappling and screaming was noticed. This female’s sexual activity might have been a tactic to protect her suckling infant from harm by deceptively signaling receptivity to the new male. The anecdote is consistent with predictions of the sexual selection hypothesis for male infanticide (van Schaik et al. 2000): (1) The new male was unknown in the area and therefore can be assumed to have had a zero probability of having fathered the female’s current offspring. (2) The new male had an increased chance of fathering the female’s subsequent offspring because he remained with the female (and still is paired with her) and she mated with him during her subsequent cycle. (3) The female gave birth faster again than she would have had the infant survived. For this female, two of the three previous interbirth intervals between surviving infants were 3 years and one was 3 years and 8 months long, but the interbirth interval following the disappearance of the infant was only 2 years and 3 months.
3.2.1 Gibbon Sexual Swellings and Their Functional Significance
In strictly pair-living, monandrous females, sexual swellings are not expected to evolve (Nunn 1999) because male–male competition is low or absent and no selection pressure exists for females to advertise their fertile periods to a pair mate. However, gibbon females often mate polyandrously, and groups at KY frequently have two adult males that both maintain sexual relationships with the group female. It is thus not surprising that white-handed gibbons have sexual swellings (Barelli et al. 2007). These cyclical sexual swellings are admittedly small compared to the well-known, exaggerated sexual swellings of chimpanzees, baboons, and some macaques, but despite their modest size they follow the same physiological principles. Based on faecal progestogen profiles of 8 females over 15 menstrual cycles, we found that in 80% of cycles, ovulation overlapped tightly with the maximum swelling phase (duration: Ø 9.3 days; 42.8% of cycle length). In fact, the probability of ovulation peaked on average on day three of the maximum swelling period, although the timing between maximum swelling and probability of ovulation varied between days − 1 to day 13 of the swelling period and three times an ovulation fell outside the maximum swelling phase (Barelli et al. 2007). Thus, in analogy to sexual swelling patterns in primates living in multi-male social systems (Deschner et al. 2004; Engelhardt et al. 2005; Fürtbauer et al. 2010), KY gibbons also exhibit cyclical sexual swellings during their menstrual cycle that do not precisely indicate the day of ovulation.
To understand sexual swellings occurring outside the menstrual cycle better, we also tested five pregnant and six lactating females. Surprisingly, different swellings phases were noticeable also in pregnant females (and in similar proportions compared to cycling females), but not in lactating females, who were rarely swollen. We conclude that despite their smaller size, gibbons’ sexual swellings probably serve functions similar to those suggested for primates with exaggerated swellings. In support of such an interpretation, female sexual activity corresponds with the size of the sexual swelling. Primary males, but not secondary males, copulate more frequently with cycling and pregnant females who are maximally swollen than with those females who are not or are only partially swollen (Barelli et al. 2008b).
Over the last 30 years, several hypotheses have been proposed to explain the evolution of conspicuous sexual swellings in species in which females mate with multiple males (reviewed in Zinner et al. 2004), whereas moderate or small sexual swellings have rarely been considered. Exaggerated swellings are hypothesized to increase paternity certainty, reliably advertise changes in female reproductive status (“obvious-ovulation hypothesis”: Hamilton 1984), or provide information on female quality (“reliable-quality indicator hypothesis”: Pagel 1994). They may also function to confuse paternity if ovulation does not precisely occur at peak swelling and thereby allow females to mate with multiple males when potentially fertile (“best-male hypothesis”: Clutton-Brock and Harvey 1976; “many-male hypothesis”: Hrdy 1981; Hrdy and Whitten 1987). Lastly, the “graded signal hypothesis” (Nunn 1999) posits that exaggerated swellings indicate the probability of ovulation, without allowing a male to precisely pinpoint the day of ovulation, thus giving a female more freedom to manipulate males’ mating interests, particularly in species where males are larger than and dominant to females. Following this last hypothesis, the highest probability of ovulation should occur close to peak swelling size, but because of the prolonged duration of receptivity associated with a prolonged display of the sexual signal, females might mate with other males when ovulation is less likely but still possible (Nunn 1999). Our sexual swelling data on gibbons are in line with the graded signal hypothesis suggesting that it can also be applied to less conspicuous swellings (Barelli et al. 2008b; Reichard 2009). The occurrence of sexual swellings in gibbons (Nadler et al. 1993; Cheyne and Chivers 2006) may be related to the widespread flexibility in social organization revealed by recent research (Fuentes 2000; Lappan 2007a; Malone and White 2008; Reichard 2009).
Although “the graded signal hypothesis” offers the most comprehensive explanation for the patterns of sexual swellings, it does not explain the presence of sexual swellings during pregnancy and lactation. Developing a swelling during pregnancy may help maintain the male’s sexual interest and mating activity, which can create paternity confusion and reduce the risk of infanticide (van Schaik et al. 1999; Engelhardt et al. 2005) and perhaps decrease a male’s interest in EPCs and thereby allow the female to benefit from his permanent presence. Thus, flexible mating behavior and imprecise sexual swelling signals in wild gibbons are consistent with the theory of paternity confusion. Moreover, the clear association between sexual swelling size and copulation frequency supports our interpretation that the small swellings in gibbons attracts male sexual interest and are analogous to the exaggerated swellings of Old World monkeys and great apes.
4 White-Handed Gibbon Life History
Understanding primate life history strategies depends critically on data from wild, unprovisioned, and naturally reproducing populations (Brockman and van Schaik 2005; Cords and Chowdhury 2010; Fürtbauer et al. 2010). Our gibbon project has now reached a time-depth that allows us to begin to assess some life history variables. Perhaps knowledge of basic life history parameters should generally guide our perception of the time-depth of field studies because the number of generations contributing to a data set may be biologically more meaningful than the number of field seasons, a common proxy often used in relation to the labels “long-term” and “short-term” study. For example, a short-term study of a few years on a mouse lemur population represents a greater biological time-depth and perhaps sample size than a decade-long study of a few individuals of an orangutan population.
From a life-history perspective, our study is still in its infancy. Gibbons’ adult group sizes are small and their life history is extremely slow for a primate of such small size (Ross 2004; Reichard and Barelli 2008). We still lack, for example, data on the maximum or even average lifespan>. A few old individuals whom we have known for a long time have disappeared and probably died, but others who appear to be of similar age are still alive, and reliable, systematic birth records only exist for the population since the early 1990s (Reichard and Barelli 2008). To estimate a minimum age of the oldest adult females with unknown birth dates, we used long-term records of date of first appearance in the population and added to this the average years until first reproduction (see below). The data indicate that females may live to age 40 or older, although they tend to begin to “disappear” by this age, probably because they die (Table 11.3). The oldest female in our sample is alive at age 43 and some females continued to reproduce between 30 and 40 years of age, although the sample of females alive past 30 years of age is small. It is clear that wild gibbon females enjoy a long life span compared to other primates of similar mass (i.e., 5–6 kg). Unfortunately, females with known birth dates will still be nowhere near the end of their potential reproductive careers or lives at ages of 15–25 years, so our knowledge of female life histories is still incomplete (Table 11.3).
Data on age at first reproduction are available for five females, who gave birth for the first time on average at age 10.5 ± 1.2 years (range 8.4–12.8 years). We don’t know the exact onset of menarche yet, but for two sub-adult females who displayed their first elongated vulva with a conspicuous mass of pink tissue at approximately 8 years of age (Hima: 8 years and 109 days; Rung: 8 years and 49 days; Barelli et al. 2007), no distinct cyclic pattern in progestogen levels (follicular and luteal components of the menstrual cycle) was detected by that age. Two precisely known gestation periods of two females lasted 184 and 195 days respectively (Barelli et al. 2007), which is shorter than the commonly assumed 210 days gestation period for white-handed gibbons (van Tienhoven 1983). If we subtract gestation length from age at first birth we can conservatively measure sexual maturity to occur at the latest at the age of first conception, which occurred on average at the age of 10.0 ± 1.5 years (range 7.8–12.2 years, N = 5) in these females. An interesting difference existed among the five females because the female with the earliest onset of reproduction (8.4 years) was the only female still residing in her natal group. This group had two simultaneously breeding females for several months, because her mother had given birth a year earlier (see above), until the daughter’s infant disappeared for unknown reasons. At the time of writing, the young female still resides with her natal group at age 9 years and 3 months. Pre-dispersal reproduction is exceptional in our population and most females disperse at the age of 7–8 years (Brockelman et al. 1998). Overall, white-handed gibbons at KY begin reproducing much later than monkeys of similar body mass and, remarkably, at about the same age as female mountain gorillas (Okamoto et al. 2000; Nakagawa et al. 2003; Wich et al. 2004; Hsu et al. 2006; Fürtbauer et al. 2010; Di Fiore et al. 2011).
Detailed data are also available for female interbirth intervals (IBI). The average population IBI between surviving offspring of habituated KY females (N = 11, 1990–2009) was 3.4 ± 0.7 years (range 34–71 months, N = 22 IBI). Adding one exceptional IBI of 14.4 years (173 months) increases the average IBI to 3.9 ± 0.4 years (N = 23 IBI). The one exceptionally long IBI was surprising because copulations were observed across most years. Prior to her most recent birth, the female was considered post-reproductive for 9.8 years according to Caro et al. (1995). The anecdote illustrates the danger of assessing female reproductive status behaviorally, which might be particularly misleading in long-lived apes. Death of a suckling infant significantly shortens birth intervals to an average 2.2 ± 0.7 years (range 11–36 months, N = 9 IBI; t-test: t (29) = 4.64, p < 0.001), although great variation naturally exists in this measure because it depends on variables such as infant age at death or a mother’s age or body condition. The shortest IBI recorded after an infant’s death was 11 months, which meant that a female conceived only 3 months after she had lost an infant, and the longest was 3.1 years, which closely resembles the mean IBI in the population.
We can also calculate infant and juvenile mortality and the length of the juvenile period in our population. Infant mortality during the first year was 11.1% (N = 54 infants born) and until weaning it was 25.6% (N = 43 infants surviving from birth to weaning), which is moderate to low, compared to many other primates (Wright 1995; Boesch and Boesch-Achermann 2000; Robbins et al. 2004; Strier et al. 2006; Carter et al. 2008; Cords and Chowdhury 2010). Juvenile mortality between weaning and 5 years of age remained low at 8.8% (N = 34 weaned infants) but rose to 13.6% (N = 22 juveniles older than 5 years) if the period between weaning and dispersal is considered. Overall, the juvenile period in gibbons is very long. Considering that weaning occurs between 24 and 30 months (Treesucon 1984) and ends the latest with first conception (see above), female gibbons spend about 7 years as non-reproductive immatures.
5 Conclusions
The most dramatic change in our understanding of gibbons, as we see it, has been the shift from a socio-sexual monogamy model toward a dynamic community based-model in which individuals, although living in small social units and on small, group-specific home ranges, are connected to a much larger social sphere that involves permanent exchanges and interactions across core social unit’s socio-spatial boundaries. Individuals call to each other in loud songs, they frequently meet in overlapping areas between group home ranges, and females visibly signal fertile periods to males in their vicinity with modest sexual swellings. Males seem to be more socially mobile than females, as predicted by sexual selection theory (Altmann 1990), given that they move more frequently between groups than females do. Females are more often the long-term occupants of home ranges and female take-overs of breeding groups usually involve younger females taking over the home range of old females whom they oust from the groups. Interestingly, so far we have not encountered an ousted female again, whereas ousted males frequently reappear in other groups and our long-term records show that some males transfer three and four times.
The dynamic community model is well suited to incorporate the recent wealth of unexpected findings that have emerged across gibbon taxa (Palombit 1994a, b; Malone and Oktavinalis 2006; Lappan 2007a, b; Lappan and Whittaker 2009). The social dynamics of gibbon communities are also in line with new findings of female reproductive strategies. Females mate polyandrously (Barelli et al. 2008b; Reichard 2009) and their moderate sexual swellings (Barelli et al. 2007) probably allow them to increase male–male competition to achieve EPCs, and more broadly to manipulate male sexual activities, all of which may benefit their own reproductive interests. However, reproductive strategies will not be fully understood until we have molecular paternity data.
References
Albers HJ, Grinspoon E (1997) A comparison of the enforcement of access restrictions between Xishuangbanna Nature Reserve (China) and Khao Yai National Park (Thailand). Environ Conserv 24:351–362
Altmann J (1990) Primate males go where the females are. Anim Behav 39:193–195
Asensio N, Brockelman WY, Malaivijitnond S, Reichard UH (2011) Gibbon travel paths are goal oriented. Anim Cogn 14:395–405
Barelli C, Heistermann M (2009) Monitoring female reproductive status in white-handed gibbons (Hylobates lar) using fecal hormone analysis and patterns of genital skin swellings. In: Lappan S, Whittaker DJ (eds) The gibbons: new perspectives on small ape socioecology and population biology. Springer, Berlin, pp 313–325
Barelli C, Heistermann M, Boesch C, Reichard UH (2007) Sexual swellings in wild white-handed gibbon females (Hylobates lar) indicate the probability of ovulation. Horm Behav 51:221–230
Barelli C, Boesch C, Heistermann M, Reichard UH (2008a) Female white-handed gibbons (Hylobates lar) lead group movements and have priority of access to food resources. Behaviour 145:965–981
Barelli C, Heistermann M, Boesch C, Reichard UH (2008b) Mating patterns and sexual swellings in pair-living and multimale groups of wild white-handed gibbons, Hylobates lar. Anim Behav 75:991–1001
Bartlett TQ (2009a) The gibbons of Khao Yai: seasonal variation in behavior and ecology. Pearson Prentice Hall, Upper Saddle River, NJ
Bartlett TQ (2009b) Seasonal home range use and defendability in white-handed gibbons (Hylobates lar) in Khao Yia National Park, Thailand. In: Lappan S, Whittaker DJ (eds) The gibbons: new perspectives on small ape socioecology and population biology. Springer, Berlin, pp 265–275
Boesch C, Boesch-Achermann H (2000) The chimpanzees of the Taï forest: behavioural ecology and evolution. Oxford University Press, Oxford
Boonpragob K, Homchantara N, Coppins BJ, McCarthy PM, Wolseley PA (1998) An introduction to the lichen flora of Khao Yai National Park, Thailand. Bot J Scotland 50:209–219
Brockelman WY (2009) Ecology and the social system of gibbons. In: Lappan S, Whittaker DJ (eds) The gibbons: new perspectives on small ape socioecology and population biology. Springer, Berlin, pp 211–239
Brockelman WY, Reichard U, Treesucon U, Raemaekers JJ (1998) Dispersal, pair formation and social structure in gibbons (Hylobates lar). Behav Ecol Sociobiol 42:329–339
Brockman DK, van Schaik CP (2005) Seasonality and reproductive function. In: Brockman DK, van Schaik CP (eds) Seasonality in primates: studies of living and extinct human and non-human primates. Cambridge University Press, Cambridge, pp 269–306
Brodie JF, Helmy OE, Brockelman WY, Maron JL (2009) Bushmeat poaching reduces the seed dispersal and population growth rate of a mammal-dispersed tree. Ecol Appl 19:854–863
Buchan JC, Alberts SC, Silk JB, Altmann J (2003) True paternal care in a multi-male primate society. Nature 425:179–181
Caro TM, Sellen DW, Parish A, Frank R, Brown DM, Voland E, Borgerhoff Mulder M (1995) Termination of reproduction in nonhuman and human female primates. Int J Primatol 16:205–220
Carter ML, Pontzer H, Wrangham RW, Peterhans JK (2008) Skeletal pathology in Pan troglodytes schweinfurthii in Kibale National Park, Uganda. Am J Phys Anthropol 135:389–403
Cheyne SM, Chivers DJ (2006) Sexual swellings of female gibbons. Folia Primatol 77:345–352
Clutton-Brock TH, Harvey PH (1976) Evolutionary rules and primates societies. In: Bateson PPG, Hinde RA (eds) Growing points in ethology. Cambridge University Press, Cambridge, pp 195–237
Cords M, Chowdhury S (2010) Life history of Cercopithecus mitis stuhlmanni in the Kakamega Forest, Kenya. Int J Primatol 31:433–455
Deschner T, Heistermann M, Hodges K, Boesch C (2004) Female sexual swelling size, timing of ovulation, and male behavior in wild West African chimpanzees. Horm Behav 46:204–215
Di Fiore A, Link A, Campbell CJ (2011) The Atelines: behavioral and socioecological diversity in a New World radiation. In: Campbell CJ, Fuentes A, MacKinnon KC, Bearder SK, Stumpf RM (eds) Primates in perspective, 2nd edn. Oxford University Press, Oxford, pp 155–188
Dixson AF (1998) Primate sexuality: comparative studies of the prosimians, monkeys, apes and human beings. Oxford University Press, Oxford
Engelhardt A, Hodges JK, Niemitz C, Heistermann M (2005) Female sexual behavior, but not sex skin swelling, reliably indicates the timing of the fertile phase in wild long-tailed macaques (Macaca fascicularis). Horm Behav 47:195–204
Fuentes A (2000) Hylobatid communities: changing views on pair bonding and social organization in hominoids. Yrbk Phys Anthropol 43:33–60
Fürtbauer I, Schülke O, Heistermann M, Ostner J (2010) Reproductive and life history parameters of wild female Macaca assamensis. Int J Primatol 31:501–517
Gale GA, Round PD, Pierce AJ, Nimnuan S, Pattanavibool A, Brockelman WY (2009) A field test of distance sampling methods for a tropical forest bird community. Auk 126:439–448
Goldizen AW (2003) Social monogamy and its variations in callitrichids: do these relate to the costs of infant care? In: Reichard UH, Boesch C (eds) Monogamy: mating strategies and partnerships in birds, humans and other mammals. Cambridge University Press, Cambridge, pp 232–247
Goodall J (1986) The chimpanzees of Gombe: patterns of behavior. Belknap, Cambridge, MA
Grassman LI Jr, Tewes ME, Silvy NJ, Kreetiyutanont K (2005) Ecology of three sympatric felids in a mixed evergreen forest in north-central Thailand. J Mammal 86:29–38
Griffith SC, Owens IPF, Thuman KA (2002) Extra-pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212
Hamilton WJ III (1984) Significance of paternal investment by primates to the evolution of adult male-female associations. In: Taub DM (ed) Primate paternalism. Van Nostrand Reinhold, New York, pp 309–335
Hrdy SB (1979) Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol Sociobiol 1:13–40
Hrdy SB (1981) The women that never evolved. Harvard University Press, Cambridge, MA
Hrdy SB, Whitten PL (1987) Patterning of sexual activity. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 370–384
Hsu MY, Lin JF, Agoramoorthy G (2006) Effects of group size on birth rate, infant mortality and social interactions in Formosan macaques at Mt Longevity, Taiwan. Ethol Ecol Evol 18:3–17
Jensen A, Meilby H (2010) Returns from harvesting a commercial non-timer forest product and particular characteristics of harvesters and their strategies: Aquilaria crassna and Agarwood in Lao PDR. Econ Bot 64:34–45
Kitamura S, Yumoto T, Poonswad P, Noma N, Chuailua P, Plongmai K, Maruhashi T, Suckasam C (2004) Pattern and impact of hornbill seed dispersal at nest trees in a moist evergreen forest in Thailand. J Trop Ecol 20:545–553
Kitamura S, Suzuki S, Yumoto T, Chuailua P, Plongmai K, Poonswad P, Noma N, Maruhashi T, Suckasam C (2005) A botanical inventory of a tropical seasonal forest in Khao Yai National Park, Thailand: implications for fruit-frugivore interactions. Biodiv Conserv 14:1241–1262
Kitamura S, Yumoto T, Noma N, Chuailua P, Maruhashi T, Wohandee P, Poonswad P (2008) Aggregated seed dispersal by wreathed hornbills at a roost site in a moist evergreen forest of Thailand. Ecol Res 23:943–952
Lappan S (2007a) Social relationships among males in multimale siamang groups. Int J Primatol 28:369–387
Lappan S (2007b) Patterns of dispersal in Sumatran siamangs (Symphalangus syndactylus): preliminary mtDNA evidence suggests more frequent male than female dispersal to adjacent groups. Am J Primatol 69:692–698
Lappan S, Whittaker DJ (2009) The gibbons: new perspectives on small ape socioecology and population biology. Springer, Berlin
Lynam AJ, Round PD, Brockelman WY (2006) Status of birds and large mammals in Thailand’s Dong Phayayen - Khao Yai Forest Complex. Biodiversity Research and Training (BRT) Program and Wildlife Conservation Society, Bangkok
Malone NM, Oktavinalis H (2006) The socioecology of the silvery gibbon (Hylobates moloch) in the Cagar Alam Leuweung Sanchang (CALS), West Java, Indonesia. Am J Phys Anthropol 129(suppl 42):124
Malone NM, White FJ (2008) The socioecology of Javan gibbons (Hylobates moloch): tests of competing hypotheses. Am J Phys Anthropol 135(suppl 46):148
Moscovice LR, Heesen M, Di Fiore A, Seyfarth RM, Cheney DL (2009) Paternity alone does not predict long-term investment in juveniles by male baboons. Behav Ecol Sociobiol 63:1471–1482
Munshi-South J (2007) Extra-pair paternity and the evolution of testis size in a behaviorally monogamous tropical mammal, the large treeshrew (Tupaia tana). Behav Ecol Sociobiol 62:201–212
Nadler RD, Dahl JF, Collins DC (1993) Serum and urinary concentrations of sex hormones and genital swelling during the menstrual cycle of the gibbon. J Endocrinol 136:447–455
Nakagawa N, Ohsawa H, Muroyama Y (2003) Life-history parameters of a wild group of West African patas monkeys (Erythrocebus patas patas). Primates 44:281–290
Nunn CL (1999) The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim Behav 58:229–246
Okamoto K, Matsumura S, Watanabe K (2000) Life history and demography of wild moor macaques (Macaca maurus): summary of 10 years of observations. Am J Primatol 52:1–11
Pagel M (1994) The evolution of conspicuous oestrous advertisement in Old World monkeys. Anim Behav 27:1333–1341
Palombit RA (1994a) Dynamic pair bonds in hylobatids: implications regarding monogamous social systems. Behaviour 128:65–101
Palombit RA (1994b) Extra-pair copulations in a monogamous ape. Anim Behav 47:721–723
Palombit RA, Cheney DL, Fischer J, Johnson S, Rendall D, Seyfarth RM, Silk JB (2000) Male infanticide and defense of infants in chacma baboons. In: van Schaik CP, Janson CH (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge, pp 123–152
Perry S, Manson JH (2008) Manipulative monkeys: the capuchins of Lomas Barbudal. Harvard University Press, Cambridge, MA
Raemaekers JJ, Raemaekers PM (1985) Field playback of loud calls to gibbons (Hylobates lar): territorial, sex-specific and species-specific responses. Anim Behav 33:481–493
Raemaekers JJ, Raemaekers PM, Haimoff EH (1984) Loud calls of the gibbon (Hylobates lar): repertoire, organisation and context. Behaviour 91:146–189
Reichard UH (1991) Zum Sozialverhalten einer Gruppe freilebender Weiβhandgibbons (Hylobates lar). MSc thesis, Göttingen University, Göttingen
Reichard UH (1995) Extra-pair copulation in a monogamous gibbon (Hylobates lar). Ethology 100:99–112
Reichard UH (1998) Sleeping sites, sleeping places, and presleep behavior of gibbons (Hylobates lar). Am J Primatol 46:35–62
Reichard UH (2003) Social monogamy in gibbons: the male perspective. In: Reichard UH, Boesch C (eds) Monogamy: mating strategies and partnerships in birds, humans and other mammals. Cambridge University Press, Cambridge, pp 190–213
Reichard UH (2009) Social organization and mating system of Khao Yai white-handed gibbons, 1992–2006. In: Lappan S, Whittaker DJ (eds) The gibbons: new perspectives on small ape socioecology and population biology. Springer, Berlin, pp 347–384
Reichard UH, Barelli C (2008) Life history and reproductive strategies of Khao Yai Hylobates lar: implications for social evolution in apes. Int J Primatol 29:823–844
Reichard UH, Sommer V (1997) Group encounters in wild gibbons (Hylobates lar): agonism, affiliation, and the concept of infanticide. Behaviour 134:1135–1174
Robbins MM, Bermejo M, Cipolletta C, Magliocca F, Parnell RJ, Stokes E (2004) Social structure and life-history patterns in western gorillas (Gorilla gorilla gorilla). Am J Primatol 64:145–159
Ross C (2004) Life histories and the evolution of large brain size in great apes. In: Russon A, Begun DR (eds) The evolution of thought: evolutionary origins of great ape intelligence. Cambridge University Press, Cambridge, pp 122–139
Round PD, Gale GA (2008) Changes in the status of Lophura pheasants in Khao Yai National Park, Thailand: a response to warming climate? Biotropica 40:225–230
Singhrattna N, Rajagopalan B, Kumar KK, Clark M (2005) Internannual and interdecadal variability of Thailand summer monsoon season. J Climate 18:1697–1708
Small MF (1989) Female choice in nonhuman primates. Yrbk Phys Anthropol 32:103–127
Smitinand T (1977) Plants of Khao Yai National Park. New Thammada Press Ltd., Bangkok
Smitinand T (1989) Thailand. In: Campbell DG, Hammond HD (eds) Floristic inventory of tropical countries: the status of plant systematic, collections, and vegetation, plus recommendations for the future. The New York Botanical Gardens, New York, pp 63–82
Sommer V, Reichard U (2000) Rethinking monogamy: the gibbon case. In: Kappeler PM (ed) Primate males: causes and consequences of variation in group composition. Cambridge University Press, Cambridge, pp 159–168
Strier KB, Dib LT, Figueira JEC (2002) Social dynamics of male muriquis (Brachyteles arachnoides hypoxanthus). Behaviour 139:315–342
Strier KB, Boubli JP, Possamai CB, Mendes SL (2006) Population demography of northern muriquis (Brachyteles hypoxanthus) at the Estação Biológica de Caratinga/Reserva particular do Patrimônio Natural-Felìciano Miguel Abdala, Minas Gerais, Brazil. Am J Phys Anthropol 130:227–237
Tangtam N (1992) Khao Yai ecosystem: the hydrological role of Khao Yai National Park. In: Proceedings of the International Workshop on Conservation and Sustainable Development, AIT/Bangkok and Khao Yai National Park, Bangkok, Thailand, 22–26 April 1991. Asian Institute of Technology, Bangkok, pp 345–363
Treesucon U (1984) Social development of young gibbons (Hylobates lar) in Khao Yai National Park, Thailand. MSc thesis, Mahidol University, Bangkok
van Noordwijk MA, van Schaik CP (2000) Reproductive patterns in eutherian mammals: adaptations against infanticide? In: van Schaik CP, Janson CH (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge, pp 322–360
van Schaik CP, Hörstermann M (1994) Predation risk and the number of adult males in a primate group: a comparative test. Behav Ecol Sociobiol 35:261–272
van Schaik CP, van Noordwijk MA, Nunn CL (1999) Sex and social evolution in primates. In: Lee PC (ed) Comparative primate socioecology. Cambridge University Press, Cambridge, pp 204–240
van Schaik CP, Hodges JK, Nunn CL (2000) Paternity confusion and the ovarian cycles of female primates. In: van Schaik CP, Janson CH (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge, pp 361–387
van Schaik CP, Preuschoft S, Watts DP (2004) Great ape social systems. In: Russon AE, Begun DR (eds) The evolution of thought: evolutionary origins of great ape intelligence. Cambridge University Press, Cambridge, pp 190–209
van Tienhoven A (1983) Reproductive physiology of vertebrates. Cornell University Press, Ithaca, NY
Watts DP (2002) Reciprocity and interchange in the social relationships of wild male chimpanzees. Behaviour 139:343–370
Wells RS (1991) The role of long-term study in understanding the social structure of a bottle nose dolphin community. In: Pryor K, Norris KS (eds) Dolphin societies: discoveries and puzzles. University of Berkley Press, Berkley, pp 199–226
Wich SA, Utami-Atmoko SS, Mitra Setia T, Rijksen HD, Schürmann C, van Hooff JARAM, van Schaik CP (2004) Life history of wild Sumatran orangutans (Pongo abelii). J Hum Evol 47:385–398
Wolff JO, MacDonald DW (2004) Promiscuous females protect their offspring. Trends Ecol Evol 19:127–134
Wright PC (1995) Demography and life history of free-ranging Propithecus diadema edwardsi in Ranomafana National Park, Madagascar. Int J Primatol 16:835–854
Zhang L, Brockelman WY, Allen MA (2008) Matrix analysis to evaluate sustainability: the tropical tree Aquilaria crassna, a heavily poached source of agarwood. Biol Conserv 141:1676–1686
Zinner DP, Nunn CL, van Schaik CP, Kappeler PM (2004) Sexual selection and exaggerated sexual swellings of female primates. In: Kappeler PM, van Schaik CP (eds) Sexual selection in primates: new and comparative perspectives. Cambridge University Press, New York, pp 71–89
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Reichard, U.H., Ganpanakngan, M., Barelli, C. (2012). White-Handed Gibbons of Khao Yai: Social Flexibility, Complex Reproductive Strategies, and a Slow Life History. In: Kappeler, P., Watts, D. (eds) Long-Term Field Studies of Primates. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-22514-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-22514-7_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-22513-0
Online ISBN: 978-3-642-22514-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)