Abstract
As in other highly sexually dimorphic, group-living animals, reproduction in gorillas has been largely viewed as the outcome of competition among males. However, females may exert choice via dispersal decisions or choice of partner in multimale groups, and males may also mate selectively. Here, we examine the paternity of 79 wild mountain gorilla offspring born into four groups characterized by stable dominance hierarchies and the presence of mature offspring of the dominant male. We found that on average the dominant male sires the majority (72 %) of the offspring in stable multimale groups and subordinate males also produce offspring, particularly when dominant males become older or the number of competing males increases. Although expected to disperse to avoid inbreeding, only half of the maturing daughters of dominant males left the group in which their father maintained dominance. However, in all five cases of reproduction by a resident daughter of a dominant male, a subordinate male was the sire of the offspring. As females commonly initiate and end copulations, and dominant males may prefer mating with fully mature females, both male and female mate preferences in addition to male competition apparently play a role in reproductive patterns in multimale groups, emphasizing the complexity of social dynamics in one of our closest living relatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inbreeding avoidance, the avoidance of production of offspring with increased homozygosity and reduced fitness due to breeding by relatives, has long been seen as a fundamental factor driving dispersal (Dobson 1982; Pusey 1987; Clutton-Brock 1989; Wolff 1994; Lukas and Clutton-Brock 2011). In social mammals, members of the dispersing sex face the challenges of becoming established in a new group, while members of the philopatric sex may benefit from cooperative interactions with same sex group members, including close relatives that they have known their entire lives (Silk 2009). Although male-biased dispersal is typical for social mammals, habitual female dispersal occurs in the closest relatives of humans, the chimpanzees and gorillas (Lukas and Clutton-Brock 2011). A key insight explaining the occurrence of female dispersal is the observation that female mammals habitually disperse when the breeding tenure of resident males exceeds the age at which females commence breeding (Clutton-Brock 1989), a conclusion reinforced by a recent phylogenetically explicit comparative analysis (Clutton-Brock and Lukas 2012).
Dispersal is thus the only means of inbreeding avoidance for a female reaching maturity in a group in which her father retains his position as the only breeding male. However, many social mammal groups contain multiple males, and male social dominance rank is a powerful but not absolute determinant of male reproductive success, with subordinate males usually obtaining some paternities (e.g., Paul et al. 1993; Wickings et al. 1993; Hoelzel et al. 1999; Ortega et al. 2003). The reproductive success of subordinate males is often seen as the outcome of the dominant male’s reduced ability to control group reproduction when faced with multiple sexually receptive females or, alternatively, as part of a strategy by the dominant to retain subordinate group members (Altmann 1962; Altmann et al. 1996; Setchell et al. 2005; Boesch et al. 2006; Kutsukake and Nunn 2006; Wroblewski et al. 2009). The presence of multiple males provides the potential for female mate choice, although females may find it difficult to exert choice in promiscuously mating groups containing multiple sexually coercive males (Clutton-Brock and Parker 1995; Wroblewski et al. 2009; Stumpf and Boesch 2010). Data are limited due to the necessity of genetically establishing paternities of adult group members, but in spotted hyenas and capuchin monkeys, mature daughters of long-term dominant males are reported to prefer mating with recent immigrant males and female capuchins apparently avoid producing offspring with their dominant father (Muniz et al. 2006; Honer et al. 2007).
With routine dispersal by both sexes and groups containing one or several males, mountain gorillas allow examination of the relationships between inbreeding avoidance and female dispersal, male reproductive competition, and mate choice by either sex. Female gorillas are always associated with a social group, which in western gorilla (Gorilla gorilla) and most eastern gorilla (Gorilla beringei) groups is led by a single mature “silverback” male (Harcourt and Stewart 2007). Genetic data suggest that this male is the sire of all group offspring (Bradley et al. 2004). However, approximately half of mountain gorilla (Gorilla beringei beringei) groups contain multiple adult males of breeding age whose interactions are consistent with a linear dominance hierarchy (Robbins 1999; Gray et al. 2013). About half of females in such multimale groups leave their natal group before producing their first offspring, and all mature males potentially mate with group females (Watts 1990; Robbins 1999; Robbins et al. 2009). However, dominant males obtain the majority of the matings with older females, while subordinate males primarily obtain matings with younger, nulliparous females (Robbins 1999). Males may disrupt matings by other males or be aggressive towards females, and despite being half the size of males, females are able to initiate and terminate copulations (Watts 1990; Robbins 1999). These dynamics therefore provide an opportunity for an examination of the factors affecting male reproductive success with regard to both male intrasexual competition and the possibility of intersexual selection via female or male mate choice.
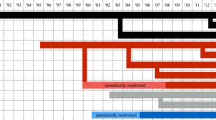
Our previous study of male reproductive skew in multimale groups of mountain gorillas monitored by the Karisoke Research Center focused on male competition and showed that the dominant male did not completely monopolize group reproduction (Bradley et al. 2005). However, as we noted at the time, the presence of only two to three silverbacks in each of the four research groups and the small number of subordinate male sirings (six of 39) limited exploration of factors relating to male competition or mate choice. In the subsequent 10 years, the four main study groups increased in size and at times contained as many as 14 males (Caillaud et al. 2013). In addition, until recently the groups remained remarkably stable both in membership and dominance relations, with no change in the identities of the respective dominant males for 10 years or longer.
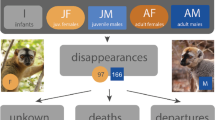
Here, we use genetic analysis to determine the paternity of 97 offspring, including 79 individuals born into four mountain gorilla groups containing multiple mature males and experiencing a lengthy tenure by the dominant male. The presence of mature offspring of the dominant male in his social group has several interesting aspects. First, although maturing females may avoid inbreeding with their still-dominant father by emigrating, the presence of multiple mature males may allow nondispersing females an alternative way to avoid inbreeding. Second, the presence of mature male offspring of the dominant male means that we can examine the association between a subordinate male’s relatedness to the dominant male and his reproductive share. Third, we can assess how well other types of relatives avoid inbreeding. Finally, we can test whether the increasing sizes of the groups may influence male reproductive skew by eroding dominant control of reproduction.
Material and methods
Noninvasive fecal samples for genetic analysis have been collected since 1999, and details of sample collection and genetic analysis are provided in the Supplemental Information. In brief, we genotyped DNA extracts at 16 autosomal microsatellite loci and used CERVUS 3.0 to analyze the completed genotypes (Kalinowski et al. 2007). As we did previously (Bradley et al. 2005), we considered as potential sires all males older than 7 years who were present in the mother’s social group at the estimated time of conception of a given offspring. There were one to 14 potential sires per offspring (average 5.8). Using CERVUS, we conducted simulations assuming either five or nine potential sires and assuming that 10 % of potential sires were related at the level of half-siblings (R = 0.25). The simulations assuming five and nine potential sires were applied to datasets consisting of offspring with six or fewer potential sires and seven or more potential sires per offspring, respectively. Results did not differ when we used simulations with different numbers of potential sires or increased proportions of relatives among the potential sires. In addition to employing CERVUS, we compared offspring, mother and potential sire genotypes for genotypic incompatibilities (“mismatches”) and found results consistent with the CERVUS assignments.
We checked for significant differences in the frequency of subordinate siring among groups by fitting a logistic regression model with group as a single factor (four levels). We tested for an effect of the group on subordinate siring frequency with an analysis of deviance, comparing the log-likelihood of this model with that of the null model.
We used generalized linear mixed models (GLMMs) to examine the effects of several factors upon paternity outcomes. As a result of long-term observation combined with genetic analysis, we typically know the natal group and often the identities of the parents of the mothers and potential sires in this study and so can assess the relationship between mothers and potential sires and categorize them as parent–offspring, maternal sibling, paternal sibling, or unrelated. For each offspring, we included the identities of the mother, offspring, and the candidate sires as random factors, because specific values of these variables could appear numerous times in the model. Standardized rank of the candidate sires was a predictor variable. Standardized ranks of males were calculated as previously described using behavioral information to identify the top-ranked silverback male (Stoinski et al. 2009b). Designation of relative rank for additional males is hampered by the infrequency of agonistic contact between males within groups, and so, we assigned the next highest rank to all other silverbacks, who were assumed to be dominant over “blackbacks” (defined here as all males between 7 and 12 years of age). Another predictor variable was the degree of paternal kinship of the candidate sire to the mother of the offspring. This was coded as two separate binary variables: one indicating whether they were paternal siblings and one indicating whether the male was the mother’s father. This allows for these quite different forms of kinship to take different effects in the model. Maternal kinship of the potential sires to the mother was included, coded by a single variable with three levels (0 = no relationship, 0.5 maternal sibling, 1.0 mother–son). We also included paternal and maternal relationship of each male to the dominant male as similar predictor variables with three levels each, coding the value for the dominant male himself as zero. Because male competitive ability may decrease after a certain age, we also included male age as a predictor variable. All predictors with more than two levels were scaled to have unit standard deviation, and male age was square-root transformed. The number of males was appropriately transformed and included in the model as an offset, thereby ensuring that in the absence of any predictor effects, the probability of siring assigned to each male would be 1/(number of males in group). The response variable was whether or not the candidate male was the sire of the offspring (0 = no, 1 = yes).
In a second analysis, we focused on the factors affecting the dominant male’s probability of siring. For each offspring, we included as random factors the identity of the mother and the dominant male. Predictor variables were the following: the dominant male’s tenure length at the date of conception, the number of breeding-age males in the group, whether the mother was the dominant male’s daughter, the mother’s parity, and the number of breeding-age females in the group. Because of multicollinearity due to correlations between tenure length or number of males and mother as dominant male’s daughter or parity, we fit four separate models, each including either tenure length or number of males, and either mother as dominant male’s daughter or parity. For this analysis, we excluded the three offspring sired when BEE was dominant because the starting date of his tenure is not precisely known. The response variable was whether the sire was the dominant male or any subordinate male (1 = dominant male, 0 = any subordinate male). Tenure length was square root-transformed, numbers of males and females were log-transformed, and all predictors with more than two levels were scaled in order to have unit standard deviation.
Finally, in order to assess the factors influencing which subordinate males sires when the dominant male does not, we constructed a model similar to the first but excluded the dominant male, included only the offspring sired by a subordinate male, and included two binary variables corresponding to whether each subordinate male was once a dominant male or would become a dominant male in any group by the end of 2012.
GLMMs were fit using the function “glmer,” in the lme4 package in R (Bates et al. 2013; R Core Team 2014) as detailed in the online supplemental information. In all GLMM analyses, significance was determined using chi-square tests in the full model (before dropping any predictors), with Holm–Bonferroni correction for multiple comparisons. Backward elimination of predictors was then performed using the function “drop1,” until Akaike information criterion (AIC) was minimized. Receiver operating characteristic (ROC) curve analysis was done on the reduced models, using the ROCR package in R (Sing et al. 2005). We interpreted fit values in the reduced models. The area under the ROC curve (AUC) is a value ranging from 0 to 1 that specifies how accurately a given model can predict binary outcomes based on data (Bradley 1997). A value of 1 corresponds to perfect performance, whereas 0.5 is chance performance. In our case, the model is a GLMM, the outcomes are whether a given male sired a given offspring or not, and the data are the predictor variables which were used to fit the model. The AUC takes both sensitivity and specificity of the model-based prediction into account.
Results
Dominant males’ share of paternity in stable multimale groups
We used the genotypes of 150 gorillas at 16 autosomal microsatellite loci to determine the paternity of 97 gorillas conceived over two decades (1985–2008) in eight social groups (Supplementary Information Table S1). Seventy-nine of the offspring were conceived in multimale groups characterized by male dominance hierarchies stable for multiple years, and we focus on these cases in the further analyses. Of these 79 offspring, 57 were sired by the dominant male and 22 by a subordinate male, so that the dominant male sired an average of 72 % of offspring in stable multimale groups (CI 0.60–0.82 exact binomial confidence interval) (Table 1). Excluding the short sample of BEE’s tenure, we find that the dominant male’s share of reproduction does not vary among the four male tenures sampled (p = 0.571, logistic regression with analysis of deviance).
Sirings by subordinate males appear more prevalent during the later phase of the dominant male’s career (Fig. 1). This corresponds to the period when dominant males are older, potentially face more competition from increasing numbers of maturing males in the group, and may have mature daughters in the group. There are five cases in which the daughter of the dominant male reproduced, and in each case, the dominant male did not sire the offspring, a striking result given the dominant male’s overall large share of reproduction. There were no other cases in which the daughter of any male residing in the group reproduced.
Reproductive success of individual dominant males as a function of his age (x-axes) and the total number of competing males (y-axes). Colored dots represent offspring sired by the dominant male, gray dots are offspring sired by a subordinate male, and gray dots containing an asterisk are offspring sired by a subordinate male with the daughter of the dominant male
Factors influencing any male’s probability of siring
In order to systematically assess the importance of factors influencing an individual male’s probability of siring, we constructed a generalized linear mixed model (GLMM) using standardized male rank, male age, male paternal or maternal genetic relationship to the mother, and male paternal or maternal genetic relationship to the dominant male as predictor variables. Because we observed that dominant males did not breed with their daughters but females did occasionally have offspring with paternal brothers, we included in our model the possibility that male paternal kinship to the mother might have a dichotomous effect depending on whether the male is the mother’s father or brother.
The full model containing all predictors indicated that both being the father of the mother and rank had highly significant effects upon the probability of siring (Table 2a). The estimated effect for being the father of the mother is not reliable because this predictor quasi-separates the data (i.e., whenever we have full kinship, there is no siring, so we have too much freedom in choosing a fit value). This does not affect the estimates for the other predictors.
We then used backward elimination of predictors to find the preferred model with the minimal AIC. The selected model had an area under the ROC curve of 0.94, suggesting good predictive ability (Bradley 1997). This model included only an intercept, whether the male is the father of the mother, and rank (Table 2). The odds ratio, which is given by exp(estimate), aids in interpreting the values of the estimates (Table 2a). The odds ratio of the probability of siring is p(sire)/(1 – p(sire)). The baseline value of this is 0.24, indicating that the probability of siring is about one third of the probability of not siring. The predictors’ odds ratios act multiplicatively on this. For a dominant male, the effect of rank is to multiply his odds ratio by 2.64*6.12, where the 2.64 results from the scaling of the rank predictor, and 6.12 is the odds ratio. His odds ratio thus becomes 3.88, i.e., almost four-to-one odds of siring. If the female is his daughter, his probability of siring is effectively zero.
The model suggests that inbreeding is avoided by the dominant male and/or female recognizing when the female is his daughter. However, an alternative hypothesis is that the dominant male avoids breeding with females that are nulliparous, as they may be his daughters. To test whether this can also explain the data, we first examined the correlation between two binary predictors: whether a male is the female’s father and whether the male is dominant and, simultaneously, the female is nulliparous. Correlation was 0.47 (95 % CI = 0.40–0.54, Fisher’s exact test), suggesting that female parity can be used to predict kinship.
We then fit a model including all previous predictors, except that the full paternal kinship factor was replaced by a binary factor that was 1 if the male was dominant and the female nulliparous, and 0 otherwise. This factor behaved similarly to the full paternal kinship factor: it had high significance and was retained in the reduced model (Table 2b). The reduced model also achieved an area under the ROC curve of 0.94.
Factors influencing siring by the dominant male
Having established that the dominant male is expected to sire most group offspring except those of his daughters, we then constructed a second model to ask which additional factors influenced siring by the dominant male. Specifically, we wanted to investigate why dominant males monopolize reproduction less in the later portions of their dominance tenures (Fig. 1). We included the dominant male’s tenure length rather than his age as a predictor variable but note that the two measures are highly correlated (0.95, 95 % CI = 0.93–0.97).
In our data, the tenure length of the dominant male is also highly correlated with the number of males in the group (0.77, 95 % CI = 0.66–0.85). This correlation persisted after appropriate transforms were applied to one or both of the variables. When a model was fit that included both of these predictors, multicollinearity was evident (dropping one predictor caused the significance of the other predictor to change dramatically). Therefore, we cannot completely separate the effects of these two predictors. Similarly, the parity of a given female was significantly negatively correlated with whether she was the dominant’s daughter (−0.42, 95 % CI = −0.59to −0.21), and these two factors also showed multicollinearity.
We therefore proceeded by fitting four separate models, each containing either tenure length or number of males, and either parity or whether the mother was the dominant male’s daughter. Each model also contained, for each offspring, the number of reproductive-age females present as a predictor, and the identities of the dominant male and female as random effects.
Tenure length or number of males were each significant in all models in which they were included, except for one model in which number of males had an insignificant effect after Bonferroni correction (p = 0.07, Table 3). Similarly, parity or whether the female is the dominant male’s daughter were each significant in all models in which they were included (Table 3).
When we include whether the female is the dominant male’s daughter as a predictor, then our model suggests, for example, that a dominant male with three competitors is expected to have an 87 % chance of siring an offspring, which decreases to 64 % when the number of competitors is ten. Similarly, a dominant male 3 years into his tenure has a 94 % chance to sire a given offspring, while after 7 years as dominant male his chance is 80 %. ROC curves for the four different models were similar, with area under the curve ranging from 0.80 to 0.94.
Factors influencing which subordinate male sires
Finally, we focused only on the 22 offspring not sired by the dominant male in order to ask what factors influence which subordinate male successfully sires an offspring. Of these 22 offspring, there were 19 cases in which multiple subordinate males were competing for paternity. These 19 offspring were sired by eight males 9 to 29 years in age who sired one to five offspring each and one to four subordinate males were successful per group. Two offspring (IGZ, RWE) were sired by males less than 12 years old and thus considered incompletely mature “blackback” males, while one (ISH) was sired by a deposed dominant male. We considered the predictors rank, age, relatedness to the dominant male, relatedness to the mother, whether the subordinate male was once a dominant male, and whether the subordinate male would become the dominant male in some group by the end of 2012. We found that only rank was a significant predictor with an area under the ROC curve of 0.95 (Supplementary Information Table S2). However, we note that our power to find a significant effect was weak with numerous predictors and only 19 cases.
Avoidance and occurrence of inbreeding and female dispersal
Turning from the models, we examined in detail the occurrences of breeding by relatives. The five cases in which the dominant male and his daughter did not produce an offspring occurred in two groups and include three instances where CAN was dominant (IMH, UBZ, NGU) and two cases where SHI was dominant (ITE, UBK). In four of these cases, the offspring was sired by a subordinate male unrelated to the mother, and in one case (ITE), the father was the maternal sibling of the mother.
An additional case of avoidance of father–daughter reproduction occurred in Beetsme’s group (offspring IHU), but outside the period of stable dominance relationships we are focusing on here. In addition, although father–daughter reproduction has not been observed, the dominant male CAN reproduced in a multimale group with his mother PUC in 1998 and 2003 to produce MAF and NDW, respectively. BWE reproduced with his mother GIN in 2007, but this occurred in a one-male group.
One means by which a female may avoid inbreeding with a dominant father is dispersal. During the time period analyzed here, we find just five instances of dispersal by daughters of the dominant male (three from BEE group, two from PAB group) (Supplemental Information). These contrast with the five cases of resident daughters (three in PAB group, two in SHI group) producing offspring sired by subordinate males in their natal groups.
A second means by which daughters of the dominant male may avoid inbreeding is through mating preferences. It has been theorized that females should be “socially inhibited” from breeding with co-resident males who were known to them as adults during maturation (Watts 1990). In accordance with this prediction, in all five cases of reproduction by a daughter of the dominant male, we find that the sires of the offspring were less than 9 years older (mean = 5.4, range 3.6 to 8.5 years) than the mothers and so were juveniles themselves when the mothers were infants. In contrast, the two dominant males were 18 (PAB) and 19 (SHI) years older than their reproductively capable daughters.
Given that matings between relatives are inconsistently avoided and mature half-siblings are present in the social groups, we can estimate the proportion of sampled offspring produced by parents related at the half-sibling level or greater. In the majority (52 of 57) of cases in which the dominant male sired the offspring, he and the mother were not known to be related. In addition to the aforementioned two cases in which the dominant male in a multimale group reproduced with his mother, there were three occasions on which a dominant male reproduced with a maternal sister (offspring TUR, KRB, and DUS). Of the 22 offspring sired by subordinate males, the subordinate male and the mother were in one case maternal siblings (ITE), in three cases paternal siblings (offspring TEG, KRN, and IYO; TEG and IYO have the same mother), and unrelated in the remaining 18 cases. Overall, the incidence of inbreeding was the similar whether the dominant or subordinate male was the sire (dominant male sirings, 5 of 57 cases, subordinate male sirings, 4 of 22 cases). In sum, the parents of 70 of the 79 offspring conceived in multimale groups and analyzed here were not related on the level of half-sibling or higher, while two offspring were produced by mother–son matings and seven offspring were produced by half-sibling matings.
Discussion
We assigned paternity of offspring born into several groups of mountain gorillas containing numerous competing males living in stable social circumstances to ask how increasing levels of male competition, greater tenure length, and the presence of relatives affect male reproductive share. We find that intrasexual competition plays an important role, with 72 % of offspring assigned to the dominant male. However, this proportion is notably lower than the 85 % estimated in a previous study employing a subset of 39 offspring (Bradley et al. 2005), and we find evidence that within group avoidance of inbreeding plays an important role in mountain gorilla reproduction.
Because the tenure of dominant males can often exceed the time it takes for their daughters to mature, female gorillas are expected to exert a form of mate choice by dispersing from their natal group (Clutton-Brock 1989), and we indeed found that half of ten daughters of reigning dominant males dispersed upon reaching maturity. However, just as many females remained and reproduced in their natal groups and in no case was the dominant male the father of the offspring. Our model accordingly found that the probability of a dominant male siring his daughter’s offspring is effectively zero, while on average he has almost two-to-one odds of siring any other offspring. Although paternity relationships were not known, using data from the late 1970s and mid-1980s, Watts found that about half of 15 natal females emigrated before giving birth, while the others (8 females) reproduced in the natal group and in each case these females were able to copulate with males who were not their presumed fathers (Watts 1990).
Turning to other primates, a dramatic reduction of dominant reproductive monopolization resulting from apparent inbreeding avoidance by dominant fathers and daughters has also been observed in the white-faced capuchin monkey (Muniz et al. 2006). Avoidance of matings with familiar paternal relatives has also been reported in wild savanna baboons (Alberts 1999), captive Barbary macaques (Kuester et al. 1994), and wild northern muriquis (Strier et al. 2011). Although female dispersal is usually routine in chimpanzees, in a population of chimpanzees in which only ∼50 % of females disperse, it was observed that males were disinterested and females resistant to matings with maternal relatives and only one case of mother–son inbreeding was recorded (Wroblewski et al. 2009).
Several points suggest that in these gorillas, both daughters and dominant fathers may exhibit behaviors that reduce the occurrence of inbreeding. As in many mammals, because females invest more in each offspring and have more limited reproductive potential, they are expected to be the sex with greater fitness incentives for avoiding inbreeding (Trivers 1972). In practical terms, although male gorillas are approximately twice the size of females, females initiate most copulations and can effectively end or avoid copulations and thus likely exercise mate choice (Watts 1990). For a female to avoid copulating with her father, she must first recognize him. We found that the subordinate male fathers of offspring produced by the nondispersing daughters of the dominant male were substantially younger than the dominant males, suggesting that females may use relative age as a cue to avoid mating with their fathers. Recognition of paternal relatives is generally a difficult task in group living primates (Silk 2009), but may be facilitated in mountain gorillas by the high proportion of time spent by immature offspring in proximity to the dominant male (Rosenbaum et al. 2011).
With regard to behavior of the dominant male, the lack of father–daughter inbreeding is consistent with the observation that the dominant male tolerates copulations between his putative nulliparous or parous daughters and subordinate males (Watts 1990). Indeed, in our analysis of the factors influencing success by the dominant male, we found that the female was nulliparous in all instances of father–daughter inbreeding avoidance. Although definitive paternity data are lacking, there are no known offspring resulting from presumptive father–daughter matings during an earlier study period in the 1970s–1980s (Watts 1990). A more recent study considering reproductive behavior in the BEE, PAB, and SHI groups during a subset (2003–2007) of our study period showed that dominant males monopolized a higher proportion of copulations with multiparous females than did the next-highest ranked males (Stoinski et al. 2009a), suggesting that although dominant males have been observed to copulate with their daughters, they prefer females of proven reproductive capability (Watts 1990; Robbins 1999), as has also been suggested for dominant male chimpanzees (Muller et al. 2006).
An alternative explanation for the lack of offspring produced by the breeding of daughters with fathers is that although such copulations occur, these do not result in viable offspring. Our observations of offspring with related parents, including three cases of mother–son inbreeding, argue against this possibility. The lack of inbreeding avoidance in these cases is puzzling, as the female would be expected to recognize her adult offspring (Silk 2009). It may be that in the context of a population displaying routine male and female dispersal and occasional group fissions, females tend to survive and reproduce in a group led by their dominant son too infrequently for this process to drive a bias against such matings.
Our models showed that the degree of relatedness between subordinate males and the dominant male (father–son, maternal brothers, paternal brothers) has no effect on the probability of reproduction by subordinate males, although our statistical power is limited with only 22 offspring sired by subordinate males. However, the approximately doubled lifetime reproductive success (i.e., fitness) predicted for males who stay and queue in the natal community relative to those who disperse, even if such queuing males do not reproduce while subordinate, means that the dominant male should not need to “allow” unrelated subordinates to breed as a means to retain them in the group for group defense (Robbins and Robbins 2005; Kutsukake and Nunn 2006). Instead, we find that the reduced paternity share exhibited by dominant males in the latter phases of their tenure is highly correlated with the number of adult males in the group as well as tenure length. Since the effects of these factors cannot be separated, this suggests that tenure length—which is correlated with dominant age and likely also physical condition affecting male competitive ability and/or attractiveness to females—along with the number of competitors significantly affects the probability of siring by dominants. We did not see an effect of group size, or of the number of females in the group, as might have been expected if dominant males faced increasing difficulty in monopolizing reproduction due to an increasing number of simultaneously receptive females as formalized in the priority-of-access model (Altmann 1962).
Our results, by suggesting that mountain gorilla females living in multimale groups may effectively avoid breeding with their dominant male father, raise the question of why female mountain gorillas in multimale groups do routinely disperse (Robbins et al. 2009). A study using 30 years of observational data from this population found that about 60 % of nulliparous females dispersed from their natal group, regardless of whether the natal group contained one or multiple males and that these females did not exhibit a preference for joining single or multimale groups (Robbins et al. 2013). Fitness benefits to females, such as interbirth intervals, did not differ between single and multimale groups, nor did the incidence of infanticide, suggesting that female preferences for a particular group type do not drive female dispersal (Robbins et al. 2013). Traits such as marked sexual dimorphism and relatively small testes, as observed in mountain gorillas, are typically found in species where reproduction occurs in groups containing one male and multiple females (Harcourt et al. 1981). Indeed, routine female dispersal from groups containing a single male is the norm in western gorillas and presumably the ancestral gorilla pattern. It may simply be that while mountain gorilla females in multimale groups may mate with subordinate males and thereby avoid the potential fitness costs of inbreeding with the related dominant male, this strategy is too new or too infrequent to counter a female tendency towards dispersal.
Although our data show that nondispersing females avoid breeding with their fathers in multimale groups, we cannot with these limited data examine if nondispersing or dispersing females more effectively avoid other forms of inbreeding. Further, with only 22 cases of sirings by subordinate males, our data show only that relative rank influences which subordinate male sires, and additional data would be needed to examine the effect of relatedness between the subordinate males and females. However, the finding that multiple subordinate males sire offspring in a given group may suggest that different females may have different preferences. Nonetheless, 9 of 79 offspring analyzed here had parents related at least as half-siblings and the incidence does not differ for offspring sired by the dominant or subordinate males, suggesting that other types of inbreeding (primarily between half-siblings) are not effectively avoided in these multimale groups. Our findings are consistent with recent genome analyses of mountain gorillas which describe overall low levels of genetic variation consistent with small long-term effective populations sizes as well as patterns of homozygosity indicative of recent inbreeding (Xue et al. 2015).
Future studies focused upon further elucidation of inter- and intrasexual selective processes in mountain gorillas can take advantage of recent large-scale changes in group composition and demography (Caillaud et al. 2013) to examine the reproductive consequences of male and female dispersal decisions. It is clear that even after more than 40 years of study, continued observations of the Karisoke mountain gorillas are needed to appreciate their population dynamics and the insights they provide into sexual selection.
References
Alberts SC (1999) Paternal kin discrimination in wild baboons. Proc R Soc Lond B 266:1501–1506
Altmann SA (1962) A field study of the sociobiology of the rhesus monkey, Macaca mulatta. Ann N Y Acad Sci 102:338–435
Altmann J, Alberts SC, Haines SA et al (1996) Behavior predicts genetic structure in a wild primate group. Proc Natl Acad Sci U S A 93:5797–5801
Bates D, Maechler M, Bolker B, Walker S (2013) lme4: linear mixed-effects models using Eigen and S4. R package version1.0-5, http://CRAN.Rproject.org/package=lme4
Boesch C, Kohou G, Nene H, Vigilant L (2006) Male competition and paternity in wild chimpanzees of the Tai forest. Am J Phys Anthropol 130:103–115
Bradley AP (1997) The use of the area under the roc curve in the evaluation of machine learning algorithms. Pattern Recogn 30:1145–1159
Bradley BJ, Doran-Sheehy DM, Lukas D, Boesch C, Vigilant L (2004) Dispersed male networks in western gorillas. Curr Biol 14:510–513
Bradley BJ, Robbins MM, Williamson EA, Steklis HD, Steklis NG, Eckhardt N, Boesch C, Vigilant L (2005) Mountain gorilla tug-of-war: silverbacks have limited control over reproduction in multimale groups. Proc Natl Acad Sci U S A 102:9418–9423
Caillaud D, Ndagijimana F, Vecellio V, Stoinski TS (2013) Individual and group level factors shape the social sphere of individual mountain gorillas (Gorilla b. beringei). Am J Phys Anthropol 150:92
Clutton-Brock TH (1989) Female transfer and inbreeding avoidance in social mammals. Nature 337:70–72
Clutton-Brock TH, Lukas D (2012) The evolution of social philopatry and dispersal in female mammals. Mol Ecol 21:472–492
Clutton-Brock TH, Parker GA (1995) Sexual coercion in animal societies. Anim Behav 49:1345–1365
Dobson SF (1982) Competition for mates and predominant juvenile male dispersal in mammals. Anim Behav 30:1183–1192
Gray M, Roy J, Vigilant L, Fawcett K et al (2013) Genetic census reveals increased but uneven growth of a critically endangered mountain gorilla population. Biol Conserv 158:230–238
Harcourt AH, Stewart KJ (2007) Gorilla society: what we know and don’t know. Evol Anthropol 16:147–158
Harcourt AH, Harvey PH, Larson SG, Short RV (1981) Testis weight, body weight and breeding system in primates. Nature 293:55–57
Hoelzel AR, Le Boeuf BJ, Reiter J, Campagna C (1999) Alpha-male paternity in elephant seals. Behav Ecol Sociobiol 46:298–306
Honer OP, Wachter B, East ML, Streich WJ, Wilhelm K, Burke T, Hofer H (2007) Female mate-choice drives the evolution of male-biased dispersal in a social mammal. Nature 448:798–801
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
Kuester J, Paul A, Arnemann J (1994) Kinship, familiarity and mating avoidance in barbary macaques, Macaca sylvanus. Anim Behav 48:1183–1194
Kutsukake N, Nunn C (2006) Comparative tests of reproductive skew in male primates: the roles of demographic factors and incomplete control. Behav Ecol Sociobiol 60:695–706
Lukas D, Clutton-Brock TH (2011) Group structure, kinship, inbreeding risk and habitual female dispersal in plural-breeding mammals. J Evol Biol 24:2624–2630
Muller MN, Thompson ME, Wrangham RW (2006) Male chimpanzees prefer mating with old females. Curr Biol 16:2234–2238
Muniz L, Perry S, Manson JH, Gilkenson H, Gros-Louis J, Vigilant L (2006) Father-daughter inbreeding avoidance in a wild primate population. Curr Biol 16:R156–R157
Ortega J, Maldonado JE, Wilkinson GS, Arita HT, Fleischer RC (2003) Male dominance, paternity, and relatedness in the Jamaican fruit-eating bat (Artibeus jamaicensis). Mol Ecol 12:2409–2415
Paul A, Kuester J, Timme A, Arnemann J (1993) The association between rank, mating effort, and reproductive success in male Barbary macaques (Macaca sylvanus). Primates 34:491–502
Pusey AE (1987) Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol Evol 2:295–299
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/
Robbins MM (1999) Male mating patterns in wild multimale mountain gorilla groups. Anim Behav 57:1013–1020
Robbins AM, Robbins MM (2005) Fitness consequences of dispersal decisions for male mountain gorillas (Gorilla beringei beringei). Behav Ecol Sociobiol 58:295–309
Robbins AM, Stoinski T, Fawcett K, Robbins MM (2009) Leave or conceive: natal dispersal and philopatry of female mountain gorillas in the Virunga volcano region. Anim Behav 77:831–838
Robbins AM, Gray M, Basabose A, Uwingeli P, Mburanumwe I, Kagoda E, Robbins MM (2013) Impact of male infanticide on the social structure of mountain gorillas. PLoS ONE 8, e78256
Rosenbaum S, Silk JB, Stoinski TS (2011) Male-immature relationships in multi-male groups of mountain gorillas (Gorilla beringei beringei). Am J Primatol 73:356–365
Setchell JM, Charpentier M, Wickings EJ (2005) Mate guarding and paternity in mandrills: factors influencing alpha male monopoly. Anim Behav 70:1105–1120
Silk JB (2009) Nepotistic cooperation in non-human primate groups. Philos T Roy Soc B 364:3243–3254
Sing T, Sander O, Beerenwinkel N, Lengauer T (2005) ROCR: visualizing classifier performance in R. Bioinformatics 21:3940–3941
Stoinski TS, Perdue BM, Legg AM (2009a) Sexual behavior in female western lowland gorillas (Gorilla gorilla gorilla): evidence for sexual competition. Am J Primatol 71:587–593
Stoinski TS, Vecellio V, Ngaboyamahina T, Ndagijimana F, Rosenbaum S, Fawcett KA (2009b) Proximate factors influencing dispersal decisions in male mountain gorillas, Gorilla beringei beringei. Anim Behav 77:1155–1164
Strier KB, Chaves PB, Mendes SL, Fagundes V, Di Fiore A (2011) Low paternity skew and the influence of maternal kin in an egalitarian, patrilocal primate. Proc Natl Acad Sci U S A 108:18915–18919
Stumpf RM, Boesch C (2010) Male aggression and sexual coercion in wild West African chimpanzees, Pan troglodytes verus. Anim Behav 79:333–342
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man, 1871-1971. Aldine-Atherton, Chicago, pp 136–179
Watts DP (1990) Mountain gorilla life histories, reproductive competition, and sociosexual behavior and some implications for captive husbandry. Zoo Biol 9:185–200
Wickings EJ, Bossi T, Dixson AF (1993) Reproductive success in the mandrill, Mandrillus sphinx: correlations of male dominance and mating success with paternity, as determined by DNA fingerprinting. J Zool 231:563–574
Wolff JO (1994) More on juvenile dispersal in mammals. Oikos 71:349–352
Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE (2009) Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim Behav 77:873–885
Xue Y, Prado-Martinez J, Sudmant PH et al (2015) Mountain gorilla genomes reveal the impact of long-term population decline and inbreeding. Science 348:242–245
Acknowledgments
We gratefully acknowledge the Rwandan Government and National Park authorities for their long-term commitment to gorilla conservation and support of the Karisoke Research Center. The authors express their gratitude to the many Karisoke field assistants and researchers who painstakingly gathered the decades of demographic and genetic data used in this study, and in particular V. Vecellio and F. Ndagijimana for their work in coordinating these efforts. We thank C. Lang and A. Abraham for contributing to the laboratory analyses, M. Schreiber for drawing the figure, and D. Lukas and A. Robbins for discussion. This research was financially supported by the Max Planck Society. The Dian Fossey Gorilla Fund International (DFGFI) also gratefully acknowledges the public and private agencies, foundations, and individuals that have provided support for the Karisoke Research Center (see www.gorillafund.org for a list of supporters).
Ethical standards
All research complied with the laws of the countries in which it was performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. I. M. Dunbar
Rights and permissions
About this article
Cite this article
Vigilant, L., Roy, J., Bradley, B.J. et al. Reproductive competition and inbreeding avoidance in a primate species with habitual female dispersal. Behav Ecol Sociobiol 69, 1163–1172 (2015). https://doi.org/10.1007/s00265-015-1930-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1930-0