Abstract
Chalcones, flavanones, dihydrochalcones, and aurones are categorized into minor flavonoids. However, these compounds take the significant roles in plant kingdom. These minor flavonoids are unique to plants and are an essential part of their success in adapting to life as sedentary organisms in diverse and inconstant surrounding. Furthermore, these compounds are subclasses of the interesting naturally occurring flavonoids in view of their structural pattern as well as biochemical and pharmacological relevance. It seems that they are important not only for plants but also for animals including human beings. This chapter deals with these minor flavonoids.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
1 Chalcone
1.1 Introduction
Chalcones, 1,3-diaryl-2-propen-1-ones, belong to the plant flavonoid family. The name “chalcone” comes from a Greek word chalcos (bronze). Chemically, they consist of open-chain flavonoids in which the two aromatic rings (A and B) are joined by a three-carbon α,β-unsaturated carbonyl system. Chalcones possess the conjugated double bond and a completely delocalized π-electron system on both aromatic rings. Although chalcone skeleton is the initial intermediate structure used in biosynthesis of all flavonoids, chalcones are one of the minor subclasses of flavonoids.
1.2 Occurrence of Chalcones
Chalcones were originally discovered in plants as the yellow flower pigments of Coreopsis and other yellow-rayed Compositae. After that, they have subsequently been found in other plant families including Solanaceae, Anacardiaceae, Caesalpiniaceae, Piperaceae, and Apiaceae. 6′-Deoxychalcones were known to be chemical constituents of leguminous plants, but it is scarcely reported that they were found in other plant species. A lot of chalcones were isolated from either Compositae (Asteraceae) or Leguminosae (Fabaceae), two families well known to accumulate these. Besides, various prenylchalcones can be found in hop plants (Cannabaceae).
1.3 Phytochemistry of Chalcones
Chalcone numbering is shown in Fig. 61.1. A vast number of naturally occurring chalcones are polyhydroxylated in the aromatic rings. Usually, chalcones have hydroxyl group at C2′-, C4′-, and/or C6′-positions in A-ring because the A-ring is biosynthesized via the acetate-malonate pathway. Prenylchalcones have prenyl group(s) between the hydroxyl groups. Cyclization of the prenyl group and the adjoining hydroxyl group produces pyrano ring on the aromatic ring. Chalcones, which do not have an oxygen function at the 2′-position, are called retrochalcones. The B-ring generally has a hydroxyl group at C4-position. They are both intermediates and end products in flavonoid biosynthesis, act as defensive compounds, participate in plant-insect interactions, and contribute to the medicinal value of herbs.
1.4 Biosynthesis of Chalcones
Chalcones are biosynthesized through the combination pathway of acetate-malonate and shikimate pathways [1–3]. Malonyl-CoA is synthesized from acetyl-CoA, whereas p-coumaroyl-CoA originates from phenylalanine, which is produced via the shikimate pathway. Chalcone synthase (CHS) catalyzes the significant step of chalcone biosynthesis. It first condenses a phenylpropanoid-CoA (e.g., p-coumaroyl-CoA) with three units of malonyl-CoA and cyclizes the resulting tetraketide intermediate to afford a chalcone (e.g., naringenin-chalcone). 6′-Deoxychalcones (e.g., isoliquiritigenin) having a resorcinol-type A-ring are biosynthesized by coactions of NADPH-dependent chalcone reductase and CHS (Scheme 61.1).
1.5 Biological Activities of Chalcones
Considerable attention has been devoted to research of chalcones [4–6], which are distributed in fruits, spices, tea, and soy-based foodstuff, because of their interesting and potential pharmacological activities. Naringenin-chalcone is found in Compositae, Lamiaceae, and Solanaceae [7]. Naringenin-chalcone is one of the predominant flavonoids found in tomatoes (Solanum lycopersicum) and accumulates almost exclusively in the tomato peel [8]. This compound inhibited histamine release with an IC50 value of 68 μg/ml and was found to be the most promising antiallergic polyphenol of this extract [9]. The effect on the production of naringenin-chalcone proinflammatory mediators in lipopolysaccharide (LPS)-stimulated macrophages has been examined. This compound inhibited the production of tumor necrosis factor (TNF)-α, monocyte chemoattractant protein (MCP)-1, and nitric oxide (NO) by LPS-stimulated RAW 264.7 macrophages in a dose-dependent manner [10]. Naringenin-chalcone exhibited anti-inflammatory properties by inhibiting the production of proinflammatory cytokines in the interaction between adipocytes and macrophages. In addition, the oral administration of naringenin-chalcone was shown to suppress Th2 cytokine production from CD4 T cells in the spleen and to attenuate allergic airway inflammation and airway hyperreactivity [11]. The eating naringenin-chalcone could contribute to the prevention and improvement of insulin resistance and related metabolic syndrome [12]. Isosalipurposide, the glucoside derivative of naringenin-chalcone isolated from Nymphaea caerulea or Helichrysum maracandicum, exhibited the inhibition of ROS (reactive oxygen species) generation in HL60 cells [13] and strong antiproliferative activity against cultured cells of SENCAR mouse in vitro [14]. Isoliquiritigenin is found in Glycyrrhiza species (Leguminosae) such as G. uralensis, G. glabra, G. inflata, Lardizabalaceae, and Amaryllidaceae [15–19]. Isoliquiritigenin is known as a natural aldose reductase inhibitor [20]. It was reported to possess antioxidative and super oxide scavenging activities [16], antiplatelet aggregation effect [21], estrogenic property [22], and inhibitory on xanthine oxidase activity in vitro [23]. And it demonstrates its anti-inflammatory effect by inhibiting LPS-induced iNOS and COX-2 expression via the attenuation of NF-κB in RAW 264.7 macrophages [24]. Interestingly, isoliquiritigenin was also found to inhibit cocaine-induced dopamine release by modulating GABAB receptor [25]. Besides, it was found that isoliquiritigenin had good effects on inhibition proliferation, including apoptosis and locking cell cycle progression in the G1 phase against human lung cancer A549 cells [26]. It induced cell cycle arrest and p21 expression in these cells [27], apoptosis and p53-expression in human liver carcinoma Hep G2 cells [28], and also induces apoptosis by depolarizing mitochondrial membranes in human prostate cancer DU145 cells [29]. Moreover, isoliquiritigenin induced monocytic differentiation of human leukemia HL60 cells [30]. It was reported that isoliquiritigenin has the ability to protect cells from AA (arachidonic acid) and iron-induced ROS production and mitochondrial dysfunction; the cytoprotective effect mediated via AMPK (AMP-activated protein kinase)-dependent GSK3β (glycogen synthase kinase-3β) inactivation. Isoliquiritigenin is useful to protect mitochondria from an iron catalyzed burst of oxidative stress [31]. 2′-O-Methyl isoliquiritigenin isolated from the root of Dalbergia odorifera T. Chen had the obvious antioxidative effect and the inhibitory effect of decrease of glutathione (GSH) level of rat lens induced by UV irradiation [32] (Fig. 61.2).
Butein (2′,3,4,4′-tetrahydroxychalcone) can be isolated from stembark of cashews and Rhus verniciflua Stokes. Past investigations suggested that butein exhibits anticarcinogenic effects. The organic extract purified from R. verniciflua Stokes inhibited the growth of transformed hepatic cells but not the untransformed parent cells [33], whereas butein alone could induce G(2)/M phase arrest in Hep G2 cells [34]. Its antiproliferative or pro-apoptotic effects can be brought about through downregulating STAT3-related gene expressions [35] and inhibiting telomerase activity [36]. This compound can also resensitize the TRAIL-resistant leukemia cells undergoing apoptosis upon TRAIL treatment [37] and reduce clonogenic growth of human breast cancer UACC-812 cells [38]. In addition, butein can suppress the proliferation of many human cancers including colon carcinoma, osteosarcoma, and hepatic stellate cells in vitro [39–43]. Sappanchalcone isolated from sappan lignum (the dried heartwood of Caesalpinia sappan) showed rapid vasorelaxant activity on the mesenteric artery [44]. This chalcone exhibited the anti-inflammatory effect in LPS-induced human periodontal ligament HPDL cells by protecting from H2O2 [45]. Sappanchalcone suppressed human oral cancer cell (HN4 and HN12) growth and induces apoptosis through the activation of p53-dependent mitochondrial, p38, ERK, JNK, and NF-κB signaling [46]. Additionally, other biological effects of this compound involve the inhibition of β-hexosaminidase release [47] and anti-influenza virus activity [48]. Flavokawin A and B were isolated from kava (Piper methysticum) with anti-inflammatory activity. They inhibited TNF-α-induced degradation and translocation of p50 and p65 NF-κB subunits from the cytoplasm to the nucleus [49]. Flavokawin B produced pronounced antinociception effect against both chemical and thermal models of pain in mice that exhibited both peripheral and central analgesic activity [50]. Cardamonin isolated from Alpinia rafflesiana, Artemisia absinthium, or Syzygium samarangense inhibited NO and prostaglandin E2 (PGE2) production from LPS- and IFN-γ-activated RAW 264.7 macrophages by the inhibition of p65NF-κB nuclear translocation due to prevention of I-κBα phosphorylation, which subsequently caused the accumulation of I-κBα [51, 52]. This chalcone also inhibited the generation of the stable thromboxan metabolite, thromboxan B2 (TxB2), via both COX-1 and COX-2 pathways; generation of intracellular ROS; and secretion of TNF-α from RAW 264.7. Cardamonin demonstrated its cytotoxic activity against human colon cancer SW-480 cells [53].
Xanthohumol, desmethylxanthohumol, 4′-methylxanthohumol, and 2′-O-methyl-3′-prenylchalconaringenin can be found in hop plants (Humulus lupulus). Xanthohumol is the most abundant prenylchalcone present in hops (concentrations up to 1 %, w/w). It is accompanied by its homologue, desmethylxanthohumol, albeit in lower concentrations [54]. Xanthohumol, desmethylxanthohumol, and 4′-methylxanthohumol were reported to exhibit strong antioxidative effects in ORAC assay [55]. Xanthohumol has been suggested to have potential cancer chemopreventive activities by inhibiting human breast MCF-7, colon HT-29, ovarian cancer A-2780 [56], and B-chronic lymphocytic leukemia cell proliferation in vitro [57]. This also showed antiangiogenic properties in vitro and in vivo where it inhibited proliferation of endothelial and Kaposi’s sarcoma-derived tumor cells in vitro, prevented angiogenesis in the Matrigel sponge model, and reduced Kaposi’s sarcoma xenograft growth in vivo. The antiangiogenic effects of xanthohumol correlated with a block of NF-κB activation and decreased phosphorylation of Akt [58, 59]. Isobavachalcone, which is a prenylchalcone found in Angelica keiskei, Dorstenia barteri, or Psoralea corylifolia, has been demonstrated to exhibit cancer antipromotive and antiproliferative activity [60]. Previous studies have shown that isobavachalcone exerts inhibitory effect against skin tumor promotion in vivo mouse skin carcinogenesis [61], and the ability of this chalcone to induce apoptosis in neuroblastoma IMR-32 and NB-39 cells has been reported [62]. Isobavachalcone significantly reduced pro-caspase-3 and pro-caspase-9 and subsequently increased the level of cleaved caspase-3 and cleaved caspase-9 in both cell lines. In addition, isobavachalcone demonstrated strong antifungal activity against various fungi, Candida albicans, C. glabrata, Microsporum audorium, and Trichophyton rubrum [63]. Crotaorixin isolated from the aerial parts of Crotalaria orixensis and medicagenin, which is a diprenylchalcone, isolated from the roots of Crotalaria medicagenia exhibited the high antimalarial activity. They showed 100 % inhibition of maturation of Plasmodium falciparum parasites from ring stage to schizont stage at low concentrations [64] (Fig. 61.3).
Licochalcones such as licochalcone A, B, and C isolated from licorice (the root and rhizome of Glycyrrhiza spp. G. uralensis, G. glabra, and G. inflate) are classified into the retrochalcones (chalcones which do not have an oxygen function at the 2′-position). The content of licochalcone A in licorice was found to be very high [65]. Previous studies showed that licochalcone A possessed radical scavenging [65], antileishmanial [66], and antispasmodic effects [67]. This chalcone has been used to treat various abdominal spasmodic symptoms in Japan [68]. Licochalcone A is well known to be a natural antiparasitic agent. Licochalcone A is a potent membrane-active compound that transforms normal erythrocytes into echinocytes in parallel with the inhibition of growth of P. falciparum cultures. The erythrocyte membrane-modifying effect was also transiently observed in vivo in mice after intravenous administration [69]. This compound exhibited the antimicrobial activity by inhibiting the growth of Staphylococcus aureus, Bacillus subtilis, and the activity of Helicobacter pylori [70, 71]. Furthermore, licochalcone A significantly inhibited LPS-induced NF-κB activation. This chalcone specifically inhibited the phosphorylation of p65 NF-κB at serine 276, leading to the inhibition of NF-κB transactivation [72]. Licochalcone A also has anticancer effects, induced apoptosis in MCF-7, HL60, and human prostate cancer LNCaP cells, and arrested G2 and late-G1 in human prostate cancer PC-3 cells [73–75]. Licochalcone B showed the high antioxidative effect stronger than that of licochalcone A on the 5-lipoxygenase-dependent peroxidation in arachidonate metabolism [76]. Licochalcone C also exhibited the anti-inflammatory activity by inhibiting NF-κB signaling [77]. Recently, chalcone glycosides, which do not have an oxygen function at the 2′-position, were isolated from the aerial parts of Brassica rapa L. “hidabeni” [78]. These 4′-O-β-d-glucopyranosyl-4-hydroxy-3′-methoxychalcone and 4′-O-β-d-glucopyranosyl-3′,4-dimethoxychalcone markedly inhibited antigen-stimulated degranulation in rat basophilic leukemia RBL-2H3 cells. The inhibitory effects were mainly due to suppression of intracellular Ca2+ elevation by suppression of intracellular ROS production through NOX inactivation [79]. Moreover, 4′-O-β-d-glucopyranosyl-3′,4-dimethoxychalcone inhibited LPS-induced iNOS expression and NO production in rat immortalized microglia HAPI cells. The inhibitory effect is due to the prevention of phosphorylation of signal transduction and activator of translocation 1 (STAT1) [80] (Fig. 61.4).
Lonchocarpin, which is a pyranochalcone, isolated from Lonchocarpus sericeus showed significant antiplatelet effect. The effect was suggested to be mediated by phosphodiesterase activity by inhibition or elevation of intracellular levels of adenosine 3′:5′-cyclic monophosphate and guanosine 3′:5′-cyclic monophosphate [81]. Crotaramosmin isolated from Crotalaria ramosissima showed weak antimalarial activity and strong antileishmanial effect [64].
Carthamin, which is a bichalcone, occurs in the tubular flowers at a late phase of the flowering stage in safflower Carthamus tinctorius [82]. This compound is known to be called Natural Red 26. Carthamin has been extensively used as a natural food color additive and created to cosmetics for geisha and kabuki artists for a long time in Japan. Carthamin administration could improve the blood fluidity by decreasing whole blood viscosity [83]. Kurzichalcolactones A and B, which have an unprecedented carbon side chain on the chalcone A-ring, were isolated from Cryptocarya kurzii with cytotoxic activity against human epidermoid carcinoma KB cells [84] (Fig. 61.5).
2 Flavanone
2.1 Introduction
Within the plant secondary metabolites of flavonoids, flavanones define one of the minor subclasses. They may be called dihydroflavones. The basic chemical structure of flavanones involves two benzene rings (A and B), which are linked by a heterocyclic ring (C). The most characteristic point of flavanone structures is that the C-ring is saturated. Flavanones have an asymmetric carbon at C2-position. They are also interesting compounds because they are the obligate intermediates in flavonoid biosynthesis. Although flavanones are minor chemical constituents of plants, they have attracted a lot of attention in chemistry and biological sciences.
2.2 Occurrence of Flavanones
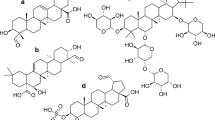
Flavanones occur in several plant families including Libiatae, Annonaceae, Acanthaceae, Compositae, Leguminosae, and also Rutaceae [85]. Flavanones having a hydroxyl group at 5-position in A-ring constitute the majority of flavonoids in Rutaceae fruits such as sweet (Citrus sinensis), sour oranges (C. aurantium), and their relatives. All orange-type citrus fruits contain the flavanone aglycones naringenin and hesperetin, but they rarely occur as free aglycones in the fruit itself. The dominant flavanone glycosides in sweet oranges (C. sinensis) are hesperidin and narirutin, whereas in sour oranges (C. aurantium), the two predominant flavanone glycosides are neohesperidin and naringin. The major difference between the flavanone glycosides of sweet and sour oranges is in their sugar moieties, which influence taste. The sugar rutinose (6-O-α-l-rhamnosyl-β-d-glucose) causes the flavanones hesperidin and narirutin to have a neutral taste and is relatively high in sweet oranges, tangerines, and tangors. The sugar neohesperidose (2-O-α-l-rhamnosyl-β-d-glucose) is high in tangelos and sour oranges and imparts a tangy or bitter taste to the glycosides neohesperidin and naringin [86]. The representative citrus flavanones and their glycosides are shown in Fig. 61.6. On the other hand, various flavanones, which do not have a hydroxyl group at 5-position, can be found in Leguminosae (Fabaceae) family.
2.3 Phytochemistry of Flavanones
Flavanone numbering is shown in Fig. 61.7. As a rule, flavanones have hydroxyl groups at 5- and 7-positions in A-ring and 4′-position in B-ring. And flavanones exist as glycosides in nature. Naturally occurring flavanones usually have the 2S-configuration, but racemization can occur during extraction. Flavanones can be easily converted to isomeric chalcones in alkaline media (or vice versa in acidic media) provided that there is a hydroxyl substituent at 2′- or 6′-position of the chalcone. In general, the physical properties of flavanones are greatly different from that of flavones. The content of flavanones may control the sweetness or bitterness of fruits. The flavanones are less soluble than the chalcones, tend to separate first in fractional crystallization, and are easily precipitated at low pH, especially if solutions are chilled or frozen.
2.4 Biosynthesis of Flavanones
The enzyme chalcone isomerase (CHI) is the second key enzyme of flavonoid biosynthesis in higher plants and catalyzes the conversion of chalcones to their corresponding flavanones. Chalcones having a hydroxyl group at C2′-position, especially those further possessing a 6′-hydroxy substitution, are spontaneously converted into a racemic mixture of the respective 2 S- and 2R-flavanones. However, they are stereospecifically isomerized into 2S-flavanones more rapidly by CHI than spontaneous conversion in plants (Scheme 61.2). Flavanones have a chiral center at C-2 position so that naturally occurring members are often optically active.
2.5 Biological Activities of Flavanones
Naringenin, which is abundant in grapefruits (Citrus × Paradisi) and other citrus fruits, has been shown to inhibit microsomal lipid peroxidation [87], nonenzymatic lipid peroxidation [88], and ascorbic acid-induced malondialdehyde (MDA) formation. Hesperetin showed a similar level of inhibition. Naringenin, however, had no effect on ferrous sulfate-induced MDA production [88]. This flavanone was found to inhibit TxB2 production in platelets stimulated with either thrombin or AA (arachidonic acid), whereas the glycoside form naringin was inactive [89]. Naringenin also inhibited the formation of oxygenated metabolites in platelets stimulated with thrombin and inhibited AA-induced platelet aggregation. Although eriodictyol offered protection against TNF-α-induced cytotoxicity in murine fibroblast L-929 cells, naringenin was not protective [90]. Naringenin and hesperetin have beneficial effects on cardiovascular diseases involving vasodilation. They displayed a concentration-dependent inhibition of the agonist-induced contractile responses [91]. It was known that naringenin was able to traverse the blood-brain barrier [92] and exert a diverse array of neuronal effects through their ability to interact with the protein kinase C (PKC) signaling pathways [93]. Naringenin can bind to both estrogen receptors, ER-α and ER- β [94]. Importantly, naringenin competed more effectively with 17-β-estradiol for binding to ER-β than for ER-α. Interaction of naringenin with ER-β may be relevant for cardiovascular effects as this receptor is present in significant amount in arterial tissue [95]. Furthermore, results of anticarcinogenesis experiments indicated that naringenin, but not naringin, inhibited aflatoxin B1-induced carcinogenesis [96] and that naringenin caused cytotoxicity and apoptosis via a transient induction of caspase-3 activity in HL60 cells [97]. Additionally, it exhibited strong antiproliferative activity in various cancer cells, and its treatment dose showed no toxic effect on normal cells [98–101]. Narirutin, which is found in immature oranges, was reported to inhibit airway inflammation in the allergic mouse model [102]. The anti-inflammatory effect is likely to be associated with the reduction in the ovalbumin (OVA)-induced increases of interleukin (IL)-4 and immunoglobulin E (IgE). Naringin, which is found in the peels of citrus fruits such as grapefruit, C. hassaku, and others, is hydrolyzed to a major metabolite, naringenin which readily crosses the blood-brain barrier. It has been reported to possess antiviral, antihypertensive, and neuroprotective effects [103–105]. Naringin has potent antioxidative activity which has been observed in various in vitro and in vivo animal models [106, 107]. This compound also has metal chelating, free radical scavenging properties and offers some protection against mutagenesis and lipid peroxidation [108]. The antioxidative effects have been shown to be similar to GSH (glutathione). Naringin plays the important role in regulating antioxidative capacity by increasing superoxide dismutase (SOD) and catalase (CAT) activities and by upregulating the gene expression of SOD, CAT, and glutathione peroxidase (GPx) [109]. In cholesterol metabolism, naringin is known to act as an inhibitor for a hydromethylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in the mevalonate pathway [110]. In addition, naringin enhanced the proliferation of cells including rat osteosarcoma UMR106, mouse osteoblastic MC3T3-E1, and mouse leukemia P388 cells [99, 111, 112]. Hesperetin is reported to be a powerful radical scavenger and a promoter of cellular antioxidant defense-related enzyme activities [113]. This compound exhibited anti-inflammatory activity by inhibiting of LPS-induced expression of the COX-2 gene in RAW 264.7 macrophages [114]. Hesperetin is a potent chemopreventive agent; its supplementation during the initiation, post-initiation, and entire period stages of colon carcinogenesis in the male rat model in vivo significantly reversed these activities [115]. Administration of hesperetin to 1,2-dimethylhydrazine (DMH)-treated rats decreased the tumor incidence and the number of aberrant crypt foci with simultaneous enhancement of tissue lipid peroxidation, glutathione S-transferase (GST), GPx, SOD, and CAT activities [116]. Hesperetin induced Notch homolog 1 (NOTCH1) expression in human gastrointestinal carcinoid (BON) cells, subsequently suppressing tumor cell proliferation and bioactive hormone production [117]. Therapeutically useful properties of hesperidin, which is found in the peels of citrus fruits (C. aurantium var. daidai, C. natsudaidai, and C. unshiu), have also been described. Hesperidin can prevent microvascular leakage by virtue of its vasoprotective action through the inhibition of the enzyme hyaluronidase which is reported to regulate the permeability of capillary walls and supporting tissues [118]. Additionally, it has been demonstrated that hesperidin can decrease blood cell and platelet aggregation, believed to be beneficial in cases of capillary permeability and fragility [119]. Besides, their effect on vascular permeability and ocular blood flow, both hesperidin and hesperetin, demonstrate strong antioxidative properties [120]. The antioxidative activity is through their ability to quench oxidative radical chain reactions and can thus help preserve neuronal health. Hesperidin also exhibited significant anti-inflammatory activity by modulating the prostaglandin synthesis and COX-2 gene expression pathways [114]. Hesperidin has been reported to possess analgesic, hypolipidemic, antihypertensive, and diuretic activity [121–123]. Another potential therapeutic application of hesperidin is its anticancer activity mediated through the suppression of cell proliferation [124, 125]. Neohesperidin also showed antiproliferative activity in Hep G2 cells [126] and the antiallergic effect on dermatitis in mice [127]. Eriodictyol, which is abundant in lemon, was found to reduce NO production from LPS-stimulated RAW 264.7 macrophages [128]. The inhibitory effect was found to be caused by blockage of NF-κB activation and phosphorylation of p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinases 1 and 2 (ERK1/2), and c-Jun N-terminal kinase (JNK). It has been reported that eriocitrin, which is a lemon (C. limon) flavanone, is effective in the prevention of oxidative damages caused by acute exercise-induced oxidative stress in the rat liver [129]. Neoeriocitrin showed free radical scavenging activity, the inhibition of superoxide formation [130], and the better effect than naringin on proliferation and osteogenic differentiation in mouse preosteoblast MC3T3-E1 cells [131]. Isosakuranetin, which is found in blood oranges (C. sinensis), grapefruits, and others, exhibited the neuroprotective effect. This compound increased cell viability and catalase activity (CA) and decreased membrane damage, ROS generation, intracellular calcium level ([Ca2+]i), and caspase-3 activity in H2O2-treated PC12 cells [132]. Interestingly, isosakuranetin is known to be an allelopathic molecule and acts by affecting K+ uptake and K+-dependent acid extrusion [133].
Liquiritigenin is extracted from Glycyrrhizae radix, a herbal that is frequently used to treat injury or swelling or for detoxification in traditional medicine. Liquiritigenin is also one of the major active compounds of menopausal formula 101 (MF101), a herbal extract used in clinical trials for the treatment of hot flushes and night sweats in post-menopausal women [134]. Liquiritigenin is shown to be a selective agonist of ER-β [135] and that targeting this receptor may be associated with anti-inflammatory effects. Liquiritigenin inhibited NO and TNF-α production induced by LPS in RAW 264.7 macrophages [136]. Studies have already proven that liquiritigenin exerts cytoprotective effects against heavy metal-induced toxicity in cultured hepatocytes [137] and has protective effects against liver injuries induced by acetaminophen and buthione sulfoximine (BSO) in rats [138]. Besides, liquiritigenin has been reported to have the choleretic effect and the ability to induce hepatic transporters and phase II enzymes [139] and inhibit amyloid β-peptide-induced neurotoxicity, not only in hippocampal neurons [140], but also in rats [141]. In addition, liquiritigenin inhibited the growth of human gastric carcinoma SGC-7901, human hepatocellular carcinoma SMMC-7721, and human colorectal cancer Lovo cells [142]. Liquiritin, an active component of Glycyrrhiza uralensis, might be a good candidate for treating various neurodegenerative diseases including Alzheimer’s disease or Parkinson’s disease [143]. This compound showed neuroprotection and neurotrophism on primary cultured hippocampal cells [144]. Liquiritin provided obviously neuroprotective effect on middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia/reperfusion (I/R), the effect attributable to its antioxidative and antiapoptosis activities [145]. It has been reported that liquiritin produced significant antidepressant effects in the forced swimming test and tail-suspension test in mice [146]. Prunin showed the inhibitory effect on caffeine N3-demethylation, a marker activity of CYP1A2, in human liver microsomes [147]. Additionally, prunin was tested against Gram-positive and Gram-negative bacteria, yeasts, and molds. Prunin showed no inhibitory effect against the microorganisms assayed but stimulated growth of Pseudomonas aeruginosa and different Bacillus sp. [148] (Fig. 61.8).
Isobavachin, which is a prenylated flavanone, isolated from Psoralea corylifolia showed cytotoxicity against rat hepatoma H4IIE and rat glioma C6 cells [149], promoting effect on neurogenesis of mouse embryonic stem cells by prenylation of protein [150]. Lavandulylated flavanones such as kurarinol and kurarinone were isolated from the root of Sophora flavescens. Kurarinol is known to be a natural tyrosinase inhibitor; this compound markedly inhibited melanin synthesis [151, 152]. And kurarinol is a potent inhibitor of sortase A, an enzyme that plays a key role in cell wall protein anchoring and virulence in Staphylococcus aureus [153]. Kurarinol and kurarinone were reported to exhibit hypolipidemin effects in cholesterol-fed rats [154]. In addition, these flavanones also showed significant inhibitory activities against intracellular ROS levels as well as NF-κB activation [155] (Fig. 61.9).
3 Dihydrochalcone
3.1 Introduction
Dihydrochalcones, 1,3-diphenylpropan-1-ones, are natural phenolics related to chalcones. They consist of the C6–C3–C6 skeleton structure, two aromatic rings connected by a C3 chain. The difference with chalcones is that dihydrochalcones lack a double bond at C2–C3 position.
3.2 Occurrence of Dihydrochalcones
In contrast to the ubiquitously present flavonoids, occurrence of dihydrochalcones is limited. In apple trees, the major subclass of flavonoids is represented by dihydrochalcones, which are found in large amounts (up to 5% of dry weight) in leaves and immature fruits. Although they were thought for a long time to be exclusive of Malus sp., dihydrochalcones have been reported in several other genera like Balanophora, Fragaria, and Symplocos. Nowadays, dihydrochalcones seem to be restricted to approximately 30 plant families, especially Rosaceae, Rutaceae, Lauraceae, and Leguminosae.
3.3 Phytochemistry of Dihydrochalcones
Dihydrochalcone numbering is the same as the chalcone ones. The substituted pattern of hydroxyl group of dihydrochalcones resembles that of chalcones, too. Their function in planta remains unresolved. They have been hypothesized to act as UV filters in leaves, and a role in resistance to pathogens has been suggested.
3.4 Biosynthesis of Dihydrochalcones
Whereas p-coumaroyl-CoA is the precursor for the naringenin-chalcone and further flavonoid formation, p-dihydrocoumaroyl-CoA is required for the biosynthesis of dihydrochalcones such as phloretin. It is assumed that p-dihydrocoumaroyl-CoA is formed from p-coumaroyl-CoA by a NADPH-dependent dehydrogenase. Dihydrochalcones are produced by the common CHS with equal chalcone biosynthesis [156, 157] (Scheme 61.3). However, in past radiolabeled biosynthetic experiments, p-dihydrocoumaric acid was not detected as the intermediate.
3.5 Biological Activities of Dihydrochalcones
Phloretin and its glycoside phloridzin are abundantly present in apples (Malus × domestica), especially in the peel, and strawberries. Phloretin has been reported to display antioxidative properties [158] and to prevent cytokine-induced expression of endothelial adhesion molecules and to reduce activation of human platelet activation [159]. In addition, phloretin may be beneficial for reducing insulin resistance through its potency to regulate adipocyte differentiation and function [160, 161]. Phloretin is a penetration enhancer in the delivery of lidocaine, which is a common local anesthetic and antiarrhythmic drug, through skin [162, 163]. Phloretin has been reported to inhibit the growth of human acute lymphoblastic leukemia MOLT4 cells in vitro [164] and Fisher bladder carcinoma and rat mammary adenocarcinoma cells in vivo [165]. And phloretin induced apoptosis B16 melanoma 4A5 cells by inhibition of glucose transmembrane transport [166]. Phloridzin, which is mainly distributed in plants of Malus, is known to be an antidiabetic agent. This compound inhibited intestinal glucose uptake via the sodium d-glucose cotransporter and similarly inhibited renal glucose reabsorption [167, 168]. Correction of hyperglycemia with phloridzin has been shown to normalize the effects of insulin on glucose metabolism in the liver and other peripheral tissues such as muscle and adipose tissue in diabetic rat models [169]. In addition, phloridzin improved hyperglycemia but not hepatic insulin resistance in a transgenic mouse model of type 2 diabetes [170]. Other biological functions of phloridzin involve estrogenic and antiaging activity and the inhibitory effect against the three human concentrative nucleoside transporters hCNT1, hCNT2, and hCNT3 [171–173]. Sieboldin and trilobatin, which can be found in apple leaves, were reported to contribute to the antioxidative activity and blocking effects of bacterial spread of apples [174]. Trilobatin inhibited against α-glucosidase and α-amylase linked to type 2 diabetes [175] (Fig. 61.10).
Neohesperidin dihydrochalcone, which is a non-nutritive sweetening agent of oranges, inhibited DPPH radical, lipid peroxidation, inflammation-related ROS, and xanthine oxidase activity [176–178]. Aspalathin, a dihydrochalcone C-glycoside, is the most abundant flavonoid in rooibos (Aspalathus linearis), which is well known as a herbal tea in many countries. Unfermented rooibos plant material contains between 4% and 12% aspalathin. Aspalathin also has beneficial effects on glucose homeostasis in type 2 diabetes through stimulating glucose uptake in muscle tissues and insulin secretion from pancreatic β-cells [179]. Aspalathin appeared to have in vitro antioxidative and antimutagenic effects [180–182]. Nothofagin, which is found in Aspalathus linearis, has been reported to exhibit the antioxidative and antimutagenic effects as same as aspalathin [182, 183] (Fig. 61.11).
Crotaramin and crotin, which are pyranodihydrochalcones isolated from Crotalaria ramosissima, exhibited weak inhibition of maturation of Plasmodium falciparum (NF-54) parasites [64]. Dimeric dihydrochalcones verbenachalcone and littorachalcone were isolated from the aerial parts of Verbena littoralis. These compounds were reported to act as enhancers of nerve growth factor (NGF)-mediated neurite outgrowth and axonal branching in rat pheochromocytoma PC12D cells [184, 185] (Fig. 61.12).
4 Aurone
4.1 Introduction
Aurones, 2-benzylidene-coumaran-3-ones, belong to the subclass of plant flavonoids that provides the bright yellow color of some important ornamental flowers. The name “aurone” comes from a Latin word aurum (= gold) because of the golden yellow color of the pigments. They consist the three-ring C6―C3―C6 system, and the heterocyclic C-ring is the five-membered ring [186]. Aurones are structurally the isomeric of flavones.
4.2 Occurrence of Aurones
Aurones are found in a number of flowers of some Scrophulariaceae (e.g., snapdragon [Antirrhinum majus]) and Compositae (e.g., Coreopsis, Cosmos, and Dahlia). The yellow snapdragon flower is probably one of the best sources of aurones in the vacuoles of the epidermal cells of the flowers. In some other plant species, however, aurones are also found in the bark, wood, leaves, seedlings, and nectar. In 2001, the occurrence of aurone (4′-chloroaurone) in marine organisms has been reported [187]. However, its structure was revised to 3-(4′-chloroisocoumarin) later [188].
4.3 Phytochemistry of Aurones
Aurone numbering is shown in Fig. 61.13. Naturally occurring aurones are polyhydroxylated in the aromatic rings. On the whole, aurones have hydroxyl groups at 4- and 6-positions in A-ring and 4′-position in B-ring. Aurones have been described as phytoalexins, used by the plant as defense agents against various infections.
4.4 Biosynthesis of Aurones
Biosynthesis of aurones from chalcones involves dual chemical transformation of chalcones such as hydroxylation of the B-ring moiety and oxidative cyclization (2′,α-dehydrogenation) to give the aurone structure [2]. Several lines of evidence suggest that a single enzyme, which is called aureusidin synthase (AmAS1), catalyzes both these transformations. Aureusidin can be produced from either naringenin-chalcone or isoliquiritigenin, whereas bracteatin arises solely from isoliquiritigenin. The mechanistic investigations showed that AmAS1 acts as an oxygenase. It was established that AmAS1 is responsible for the transformation of a variety of chalcones to aurones [189]. According to the screening of a panel of hydroxylated and glycosylated chalcones, it is found that only chalcones, which are hydroxylated at C2′ and C4′ positions, are transformed to aurones (Scheme 61.4). Furthermore, the AmAS1 is definitely specific to chalcones because flavanones are inert for the enzyme action.
4.5 Biological Activities of Aurones
Aureusidin which is found in snapdragon, maritimetin which is an anthochlor pigment of Coreopsis tinctoria and Baeria chrysostoma, and bracteatin which is isolated from Helichrysum bracteatum have been studied with regards to their radical scavenging potential using density functional theory (DFT) [190–192]. 4,4′,6-Trihydroxyaurone isolated from Pterocarpus santalinus and Smilax bracteata and hispidol which can be found in Trichilia hispida were able to induce significant tyrosinase inhibition. In particular, 4,4′,6-trihydroxyaurone was highly active when compared to kojic acid [193]. Sulfuretin isolated from the heartwood of Rhus verniciflua is an active antirheumatoid arthritis agent. This compound showed significant inhibitory effects on hind paw edema and trypsin inhibitor activity induced by Freund’s complete adjuvant reagent (FCS reagent) and on vascular permeability caused by acetic acid [194]. In addition, sulfuretin exhibited the anti-inflammatory effect by the suppression of NF-κB transcription activity via the inhibitory regulation of IκB kinase β-phosphorylation in LPS-induced RAW 264.7 macrophages [195]. Also, sulfuretin demonstrated the antidiabetogenic effect by the suppression of NF-κB activation [196] (Fig. 61.14).
5 Conclusions
The minor flavonoids are important subclasses of plant polyphenols. Especially, chalcones and flavanones are the obligate intermediates in flavonoid biosynthesis. These naturally occurring compounds also play significant roles as pigments, phytoalexins, and signaling molecules in pathogenesis and symbiosis. Additionally, considerable attention has been devoted to these compounds because of their potential pharmaceutical applications in recent years. For this reason, they are an object of continuously growing interest among the scientists. The attention is mainly drawn to the common skeleton and possibilities for its modifications guided by mechanistic and structure-activity relationship studies.
Abbreviations
- AmAS1:
-
Aureusidin synthase
- CHI:
-
Chalcone isomerase
- CHS:
-
Chalcone synthase
- CoA:
-
Coenzyme A
- COX:
-
Cyclooxygenase
- LPS:
-
Lipopolysaccharide
- NADPH:
-
Reduced nicotinamide adenine dinucleotide phosphate
- NF:
-
Nuclear factor
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
- TNF:
-
Tumor necrosis factor
References
Dewick PM (2002) Medicinal natural products (a biosynthetic approach), 2nd edn. Wiley
Mander L, Liu H-W (2010) Comprehensive natural products II (chemistry and biology), vol 1. Elsevier Ltd, Oxford
Shirley BW (1996) Flavonoid biosynthesis: ‘new’ functions for an ‘old’ pathway. Trends Plant Sci 1:377
Batovska DI, Todorova IT (2010) Trends in utilization of the pharmacological potential of chalcones. Curr Clin Pharmacol 5:1
Dimmock JR, Elias DW, Beazely MA, Kandepu NM (1999) Bioactivities of chalcones. Curr Med Chem 6:1125
Nowakowska Z (2007) A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem 42:125
Davis KM, Bloor SJ, Spiller GB, Deroles SC (1998) Production of yellow color in flowers: redirection of flavonoid biosynthesis in Petunia. Plant J 13:259
Rivero RW, Ruiz JM, Garcia PC, Lopez LLR, Sanchez E, Romeo L (2001) Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci 160:315
Yamamoto T, Yoshimura M, Yamaguchi F, Kouchi T, Tsuji R, Saito M, Obata A, Kikuchi M (2004) Anti-allergic activity of naringenin chalcone from a tomato skin extract. Biosci Biotechnol Biochem 68:1706
Hirai S, Kim Y-II, Goto T, Kang M-S, Yoshimura M, Obata A, Yu R, Kawada T (2007) Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci 81:1272
Iwamura C, Shinoda K, Yoshimura M, Watanabe Y, Obata A, Nakayama T (2010) Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells. Allergol Int 59:67
Horiba T, Nishimura I, Nakai Y, Abe K, Sato R (2010) Naringenin chalcone improves adipocyte functions by enhancing adiponectin production. Mol Cell Endocrinol 323:208
Agnihotri VK, ElSohly NH, Khan SI, Smillie TJ, Khan IA, Walker LA (2008) Antioxidant constituents of Nymphaea caerulea flowers. Phytochemistry 69:2061
Yagura T, Motomiya T, Ito M, Honda M, Iida A, Kiuchi F, Tokuda H, Nishino H (2008) Anticarcinogenic compounds in the Uzbek medicinal plant. Helichrysum maracandicum. J Nat Med 62:174
Kape Y, Parniske M, Brandt S, Werner D (1992) Isoliquiritigenin, a strong nod gene- and glyceollin resistance-inducing flavonoid from soybean root exudate. Appl Environ Microbiol 58:1705
Haraguchi H, Ishikawa H, Mizutani K, Tamura Y, Kinoshita T (1998) Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata. Bioorg Med Chem 6:339
Cao Y, Wang Y, Ji C, Ye J (2004) Determination of liquiritigenin and isoliquiritigenin in Glycyrrhiza uralensis and its medicinal preparations by capillary electrophoresis with electrochemical detection. J Chromatogr A 1042:203
Pan X, Kong L, Zhang Y, Cheng C, Tan R (2000) In vitro inhibition of rat monoamine oxidase by liquiritigenin and isoliquiritigenin isolated from Sinofranchetia chinensis. Acta Pharmacol Sin 21:949
Ramadan M, Kamel M, Ohtani K, Kasai R, Yamasaki K (2000) Minor phenolics from Crinum bulbispermum bulbs. Phytochemistry 54:891
Aida K, Tawata M, Shindo H, Onaya T, Sasaki H, Yamaguchi T, Chin M, Mitsuhashi H (1990) Isoliquiritigenin: a new aldose reductase inhibitor from Glycyrrhizae Radix. Planta Med 56:254
Tawata M, Aida K, Noguchi T, Ozaki Y, Kume S, Sasaki H, Chin M, Onaya T (1992) Anti-platelet action of isoliquiritigenin, an aldose reductase inhibitor in licorice. Eur J Pharmacol 212:87
Tamir S, Eizenberg M, Somjen D, Izrael S, Vaya J (2001) Estrogen-like activity of glabrene and other constituents isolated from licorice root. J Steroid Biochem Mol Biol 78:291
Kong LD, Zhang Y, Pan X, Tan RX, Cheng CHK (2000) Inhibition of xanthine oxidase by liquiritigenin and isoliquiritigenin isolated from Sinofranchetia chinensis. Cell Mol Life Sci 57:500
Kim J-Y, Park SJ, Yun K-J, Cho Y-W, Park H-J, Lee K-T (2008) Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-κB in RAW 264.7 macrophages. Eur J Pharmacol 584:175–84
Kim J-Y, Park SJ, Yun K-J, Cho Y-W, Park H-J, Lee K-T (2008) Isoliquiritigenin suppresses cocaine-induced extracellular dopamine release in rat brain through GABAB receptor. Eur J Pharmacol 587:124
Hsu YL, Kuo PL, Chiang LC, Lin CC (2004) Isoliquiritigenin inhibits the proliferation and induces the apoptosis of human non-small cell lung cancer A549 cells. Clin Exp Pharmacol Physiol 31:414
Li T, Satomi Y, Katoh D, Shimada J, Baba M, Okuyama T, Nishino H, Kitamura N (2004) Induction of cell cycle arrest and p21CIP1/WAF1 expression in human lung cancer cells by isoliquiritigenin. Cancer Lett 207:27
Hsu YL, Kuo PL, Lin CC (2005) Isoliquiritigenin induces apoptosis and cell cycle arrest through p53-dependent pathway in Hep G2 cells. Life Sci 77:279
Juang JI, Lim SS, Choi HJ, Shin HK, Kim EJ, Chung WY, Park KK, Park JHY (2006) Isoliquiritigenin induces apoptosis by depolarizing mitochondrial membranes in prostate cancer cells. J Nutr Biochem 17:689
Li D, Wang Z, Chen H, Wang J, Zheng Q, Shang J, Li J (2009) Isoliquiritigenin induces monocytic differentiation of HL-60 cells. Free Radic Biol Med 46:731
Choi SH, Kim YW, Kim SG (2010) AMPK-mediated GSK3β inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem Pharmacol 79:1352
Yu X, Wang W, Yang M (2007) Antioxidant activities of compounds isolated from Dalbergia odorifera T. Chen and their inhibition effects on the decrease of glutathione level of rat lens induced by UV irradiation Food Chem 104:715–720
Son YO, Lee KY, Lee JC, Jang HS, Kim JC, Jeon YM, Jang YS (2005) Selective antiproliferative and apoptotic effects of flavonoids purified from Rhus verniciflua Stokes on normal versus transformed hepatic cell lines. Toxicol Lett 155:115
Moon DO, Kim MO, Choi YH, Hyun JW, Chang WY, Kim GY (2010) Butein induces G2/M phase arrest and apoptosis in human hepatoma cancer cells through ROS generation. Cancer Lett 288:204
Pandey MK, Sung B, Ahn KS, Aggarwal BB (2009) Butein suppresses constitutive and inducible signal transducer and activator of transcription (STAT) 3 activation and STAT3-regulated gene products through the induction of a protein tyrosine phosphatase SHP-1. Mol Pharmacol 75:525
Moon DO, Kim MO, Lee JD, Choi YH, Kim GY (2009) Butein suppresses c-Myc-dependent transcription and Akt-dependent phosphorylation of hTERT in human leukemia cells. Cancer Lett 286:172
Kim N (2008) Butein sensitizes human leukemia cells to apoptosis induced by tumor necrosis factor-related apoptosis inducing ligand (TRAIL). Arch Pharm Res 31:1179
Samoszuk M, Tan J, Chorn G (2005) The chalcone butein from Rhus verniciflua Stokes inhibits clonogenic growth of human breast cancer cells co-cultured with fibroblasts. BMC Complement Altern Med 5:5
Kim NY, Pae HO, Oh GS, Kang TH, Kim YC, Rhew HY, Chung HT (2001) Butein, a plant polyphenol, induces apoptosis concomitant with increased caspase-3 activity, decreased Bcl-2 expression and increased bax expression in HL-60 cells. Pharmacol Toxicol 88:261
Wang Y, Chan FL, Chen S, Leung LK (2005) The plant polyphenol butein inhibits testosterone-induced proliferation in breast cancer cells expressing aromatase. Life Sci 77:39
Yit CC, Das NP (1994) Cytotoxic effect of butein on human colon adenocarcinoma cell proliferation. Cancer Lett 82:65
Jang HS, Kook SH, Son YO, Kim JG, Jeon YM, Jang YS, Choi KC, Kim J, Han SK, Lee KY, Park BK, Cho NP, Lee JC (2005) Flavonoids purified from Rhus verniciflua Stokes actively inhibit cell growth and induce apoptosis in human osteosarcoma cells. Biochim Biophys Acta 1726:309
Lee SH, Seo GS, Kim HS, Woo SW, Ko G, Sohn DH (2006) 2′,4′,6′-Tris(methoxymethoxy) chalcone attenuates hepatic stellate cell proliferation by a heme oxygenase-dependent pathway. Biochem Pharmacol 72:1322
Sasaki Y, Suzuki M, Matsumoto T, Hosokawa T, Kobayashi T, Kamata K, Nagumo S (2010) Vasorelaxant activity of Sappan Lignum constituents and extracts on rat aorta and mesenteric artery. Biol Pharm Bull 33:1555
Jeong GS, Lee DS, Li B, Lee HJ, Kim EC, Kim YC (2010) Effects of sappanchalcone on the cytoprotection and anti-inflammation via heme oxygenase-1 in human pulp and periodontal ligament cells. Eur J Pharmacol 644:230
Lee YM, Kim YC, Choi BJ, Lee DW, Yoon JH, Kim EC (2011) Mechanism of sappanchalcone-induced growth inhibition and apoptosis in human oral cancer cells. Toxicol In Vitro 25:1782
Yodsaoue O, Cheenpracha S, Karalai C, Ponglimanont C, Tewtrakul S (2009) Anti-allergic activity of principles from the roots and heartwood of Caesalpinia sappan on antigen-induced β-hexosaminidase release. Phytother Res 23:1028
Liu AL, Shu SH, Qin HL, Lee SM, Wang YT, Du GH (2009) In vitro anti-influenza viral activities of constituents from Caesalpinia sappan. Planta Med 75:337
Folmer F, Blasius R, Morceau F, Tabudravu J, Dicato M, Jaspars M, Diederich M (2006) Inhibition of TNFα-induced activation of nuclear factor κB by kava (Piper methysticum) derivatives. Biochem Pharmacol 71:1206
Mohamad AS, Akhtar MN, Zakaria ZA, Perimal EK, Khalid S, Mohd PA, Khalid MH, Israf DA, Lajis NH, Sulaiman MR (2010) Antinociceptive activity of a synthetic chalcone, flavokawin B on chemical and thermal models of nociception in mice. Eur J Pharmacol 647:103
Ahmad S, Israf DA, Lajis NH, Shaari K, Mohamed H, Wahab AA, Ariffin KT, Hoo WY, Aziz NA, Kadir AA, Sulaiman MR, Somchit MN (2006) Cardamonin, inhibits pro-inflammatory mediators in activated RAW 264.7 cells and whole blood. Eur J Pharmacol 538:188–194
Israf DA, Khaizurin TA, Syahida A, Lajis NH, Khozirah S (2007) Cardamonin inhibits COX and iNOS expression via inhibition of p65NF-κB nuclear translocation and Iκ-B phosphorylation in RAW 264.7 macrophage cells. Mol Immunol 44:673–679
Hatziieremia S, Gray AI, Ferro VA, Paul A, Plevin R (2006) The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFκB signalling pathways in monocytes/macrophages. Br J Pharmacol 149:188
Stevens JF, Taylor AW, Clawson JE, Deinzer ML (1999) Fate of xanthohumol and related prenylflavonoids from hops to beer. J Agric Food Chem 47:2421
Vogel S, Ohmayer S, Brunner G, Heilmann J (2008) Natural and non-natural prenylated chalcones: synthesis, cytotoxicity and anti-oxidative activity. Bioorg Med Chem 16:4286
Miranda CL, Stevens JF, Helmrich A, Henderson MC, Rodriguez RJ, Yang Y-H, Deinzer ML, Barnes DW, Buhler DR (1999) Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem Toxicol 37:271
Lust S, Vanhoecke B, Janssens A, Philippe J, Bracke M, Offner F (2005) Xanthohumol kills B-chronic lymphocytic leukemia cells by an apoptotic mechanism. Mol Nutr Food Res 49:844
Dell’Eva R, Ambrosini C, Vannini N, Piaggio G, Albini A, Ferrari N (2007) AKT/NF-κB inhibitor xanthohumol targets cell growth and angiogenesis in hematologic malignancies. Cancer 110:2007–2011
Albini A, Dell’Eva R, Vené R, Ferrari N, Buhler DR, Noonan DM, Fassina G (2006) Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF-κB and Akt as targets. FASEB J 20:527
Jing H, Zhou X, Dong X, Cao J, Zhu H, Lou J, Hu Y, He Q, Yang B (2010) Abrogation of Akt signaling by Isobavachalcone contributes to its anti-proliferative effects towards human cancer cells. Cancer Lett 294:167
Akihisa T, Tokuda H, Hasegawa D, Ukiya M, Kimura Y, Enjo F, Suzuki T, Nishino H (2006) Chalcones and other compounds from the exudates of Angelica keiskei and their cancer chemopreventive effects. J Nat Prod 69:38
Nishimura R, Tabata K, Arakawa M, Ito Y, Kimura Y, Akihisa T, Nagai H, Sakuma A, Kohno H, Suzuki T (2007) Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol Pharm Bull 30:1878
Mbaveng AT, Ngameni B, Kuete V, Simo IK, Ambassa P, Roy R, Bezabih M, Etoa FX, Ngadjui BT, Abegaz BM, Meyer JJ, Lall N, Beng VP (2008) Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae). J Ethnopharmacol 116:483
Narender T, Shweta TK, Srinivasa Rao M, Srinivasa K, Puri SK (2005) Prenylated chalcones isolated from Crotalaria genus inhibit in vitro growth of the human malaria parasite Plasmodium falciparum. Bioorg Med Chem Lett 15:2453
Shibata S (2000) A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi 120:849
Chen M, Zhai L, Christensen SB, Theander TG, Kharazmi A (2001) Inhibition of fumarate reductase in Leishmania major and L. donovani by chalcones. Antimicrob Agents Chemother 45:2023–2029
Friis-Moller A, Chen M, Fuursted K, Christensen SB, Kharazmi A (2006) In vitro antimycobacterial and antilegionella activity of licochalcone A from Chinese licorice roots. Planta Med 68:416
Nagai H, He JX, Tani T, Takao T (2007) Antispasmodic activity of licochalcone A, a species-specific ingredient of Glycyrrhiza inflata roots. J Pharm Pharmacol 59:1421
Ziegler HL, Hansen HS, Staerk D, Christensen SB, Hageratrand H (2004) The antiparasitic compound licochalcone a is a potent echinocytogenic agent that modifies the erythrocyte membrane in the concentration range where antiplasmodial activity is observed. Antimicrob Agents Chemother 48:4067
Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T (2002) Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci 71:1449
Chen M, Christensen SB, Blom J, Lemmich E, Nadelmann L, Fich K, Theander TG, Kharazmi A (1993) Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob Agents Chemother 37:2550
Furusawa J, Funakoshi-Tago M, Tago K, Mashino T, Inoue H, Sonoda Y, Kasahara T (2009) Licochalcone A significantly suppresses LPS signaling pathway through the inhibition of NF-κB p65 phosphorylation at serine 276. Cell Signal 21:778
Rafi MM, Rosen RT, Vassil A, Ho CT, Zhang H, Ghai G, Lambert G, Dipaola RS (2000) Modulation of bcl-2 and cytotoxicity by licochalcone-A, a novel estrogenic flavonoid. Anticancer Res 20:2653
Yo Y-T, Shieh G-S, Hsu K-F, Wu C-L, Shiau A-L (2009) Licorice and licochalcone-A induce autophagy in LNCaP prostate cancer cells by suppression of Bcl-2 expression and the mTOR pathway. J Agric Food Chem 57:8266
Fu Y, Hsieh T-C, Guo J, Kunicki J, Lee MYWT, Darzynkiewicz Z, Wu JM (2004) Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem Biophys Res Commun 322:263
Hatano T, Kagawa H, Yasuhara T, Okuda T (1988) Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull 36:2090
Furusawa J, Funakoshi-Tago M, Mashino T, Tago K, Inoue H, Sonoda Y, Kasahara T (2009) Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-κB p65 in LPS signaling pathway. Int Immunopharmacol 9:499
Ninomiya M, Efdi M, Inuzuka T, Koketsu M (2010) Chalcone glycosides from aerial parts of Brassica rapa L. ‘hidabeni’, turnip. Phytochem Lett 3:96–99
Itoh T, Ninomiya M, Nozawa Y, Koketsu M (2010) Chalcone glycosides isolated from aerial parts of Brassica rapa L. hidabeni’ suppress antigen-stimulated degranulation in rat basophilic leukemia RBL-2 H3 cells. Bioorg Med Chem 18:7052–7057
Hara H, Nakamura Y, Ninomiya M, Mochizuki R, Kamiya T, Aizenman E, Koketsu M, Adachi T (2011) Inhibitory effects of chalcone glycosides isolated from Brassica rapa L. hidabeni’ and their synthetic derivatives on LPS-induced NO production in microglia. Bioorg Med Chem 19:5559–68
Fontenele JB, Leal LKAM, Ferreira MAD, Silveira ER, Viana GSB (2005) Antiplatelet Effect of Lonchocarpin and Derricin Isolated from Lonchocarpus sericeus. Pharm Biol 43:726
Takahashi Y, Miyasaka N, Tasaka S, Miura I, Urano S, Hikichi K, Ikura M, Matsumoto T, Wada M (1982) Constitution of two coloring matters in the flower petals of Carthamus tinctorius L. Tetrahedron Lett 23:5163
Li H-X, Han S-Y, Wang X-W, Ma X, Zhang K, Wang L, Ma Z-Z, Tu P-F (2009) Effect of the carthamins yellow from Carthamus tinctorius L. on hemorheological disorders of blood stasis in rats. Food Chem Toxicol 47:1797–802
Fu X, Sévenet T, Remy F, Païs M, Hamid A, Hadi A, Zeng LM (1993) Flavanone and chalcone derivatives from Cryptocarya kurzii. J Nat Prod 56:1153
Brahmachari G (2008) Naturally occurring flavanones: an overview. Nat Prod Commun 3:1337
Peterson JJ, Dwyer JT, Beecher GR, Bhagwat SA, Gebhardt SE, Haytowitz DB, Holden JM (2006) Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: a compilation and review of the data from the analytical literature. J Food Compos Anal 19:S66
Laughton MJ, Evans PJ, Moroney MA, Hoult JRS, Halliwell B (1991) Inhibition of mammalian 5-lipoxygenase and cyclo-oxygenase by flavonoids and phenolic dietary additives. Relationship to antioxidant activity and to iron ion-reducing ability Biochem Pharmacol 42:1673–81
Ratty AK, Das NP (1988) Effects of flavonoids on nonenzymic lipid peroxidation: structure-activity relationship. Biochem Med Metab Biol 39:69
Corvazier E, Maclouf J (1985) Interference of some flavonoids and nonsteroidal anti-inflammatory drugs with oxidative metabolism of arachidonic acid by human platelets and neutrophils. Biochim Biophys Acta 835:315
Habtemariam S (1997) Flavonoids as inhibitors or enhancers of the cytotoxicity of tumor necrosis factor-α in L-929 tumor cells. J Nat Prod 60:775
Herrera MD, Zarzuelo A, Jimenez J, Marhuenda E, Duarte J (1996) Effects of flavonoids on rat aortic smooth muscle contractility: structure-activity relationships. Gen Pharmacol 27:273
Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Evans R-C (2003) Interaction between flavonoids and the blood-brain barrier: in vitro studies. J Neurochem 85:180
Vauzour D, Vafeiadou K, Rice-Evans C, Williams RJ, Spencer JP (2007) Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J Neurochem 103:1355
Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson A (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 139:4252
Register TC, Admas MR (1998) Coronary artery and cultured aortic smooth muscle cells express mRNA for both the classical estrogen receptor and the newly described estrogen receptor beta. J Steroid Biochem Mol Biol 64:187
Guengerich FP, Kim DH (1990) In vitro inhibition of dihydropyridine oxidation and aflatoxin B1 activation in human liver microsomes by naringenin and other flavonoids. Carcinogenesis 11:2275
Chen YC, Shen SC, Lin HY (2003) Rutinoside at C7 attenuates the apoptosis-inducing activity of flavonoids. Biochem Pharmacol 66:1139
Kanno S, Shouji A, Asou K, Ishikawa M (2003) Effects of naringin on hydrogen peroxide-induced cytotoxicity and apoptosis in P388 cells. J Pharmacol Sci 92:166
Kanno S, Shouji A, Hirata R, Asou K, Ishikawa M (2004) Effects of naringin on cytosine arabinoside (Ara-C)-induced cytotoxicity and apoptosis in P388 cells. Life Sci 75:353
Kanno S, Tomizawa A, Ohtake T, Koiwai K, Ujibe M, Ishikawa M (2006) Naringenin-induced apoptosis via activation of NF-κB and necrosis involving the loss of ATP in human promyeloleukemia HL-60 cells. Toxicol Lett 166:131
Sabarinathan D, Mahalakshmi P, Vanisree AJ (2011) Naringenin, a flavanone inhibits the proliferation of cerebrally implanted C6 glioma cells in rats. Chem-Biol Interact 189:26
Funaguchi N, Ohno Y, La BL, Asai T, Yuhgetsu H, Sawada M, Takemura G, Minatoguchi S, Fujiwara T, Fujiwara H (2007) Narirutin inhibits airway inflammation in an allergic mouse model. Clin Exp Pharmacol Physiol 34:766
Kaul TN, Middleton E, Ogra PL (1985) Antiviral effect of flavonoids on human viruses. J Med Virol 15:71
Reshef N, Hayari Y, Goren C, Boaz M, Madar Z, Knobler H (2005) Antihypertensive Effect of Sweetie Fruit in Patients With Stage I Hypertension. Am J Hypertens 18:1360
Lu YH, Su MY, Huang HY, Li L, Yuan CG (2010) Protective effects of the citrus flavanones to PC12 cells against cytotoxicity induced by hydrogen peroxide. Neurosci Lett 484:6
Rajadurai M, Prince PS (2009) Naringin ameliorates mitochondrial lipid peroxides, antioxidants and lipids in isoproterenol-induced myocardial infarction in wistar rats. Phytother Res 23:358
Cavia-Saiz M, Busto MD, Pilar-Izquierdo MC, Ortega N, Perez-Mateos M, Muñiz P (2010) Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J Sci Food Agric 90:1238
Jagetia GC, Venkatesha VA, Reddy TK (2003) Naringin, a citrus flavonone, protects against radiation-induced chromosome damage in mouse bone marrow. Mutagenesis 18:337
Jeon SM, Bok SH, Jang MK, Lee MK, Nam KT, Park YB, Rhee SJ, Choi MS (2001) Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci 69:2855
Kim HJ, Oh GT, Park YB, Lee MK, Seo HJ, Choi MS (2004) Naringin alters the cholesterol biosynthesis and antioxidant enzyme activities in LDL receptor-knockout mice under cholesterol fed condition. Life Sci 74:1621
Wu JB, Fong YC, Tsai HY, Chen YF, Tsuzuki M, Tang CH (2008) Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur J Pharmacol 588:333
Wong RW, Rabie AB (2006) Effect of naringin on bone cells. J Orthop Res 24:2045
Kim JY, Jung KJ, Choi JS, Chong HY (2004) Hesperetin: a potent antioxidant against peroxynitrite. Free Radic Res 38:761
Hirata A, Murakami Y, Shoji M, Kadoma Y, Fujisawa S (2005) Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res 25:3367
Aranganathan S, Nalini N (2009) Efficacy of the potential chemopreventive agent, hesperetin (citrus flavanone), on 1,2-dimethylhydrazine induced colon carcinogenesis. Food Chem Toxicol 47:2594
Aranganathan S, Selvam JP, Nalini N (2009) Hesperetin exerts dose dependent chemopreventive effect against 1,2-dimethyl hydrazine induced rat colon carcinogenesis. Invest New Drugs 27:203
Zarebczan B, Pinchot SN, Kunnimalaiyaan M, Chen H (2011) Hesperetin, a potential therapy for carcinoid cancer. Am J Surgery 201:329
Beiler JM, Martin GJ (1948) Inhibition of hyaluronidase action by derivatives of hesperidin. J Biol Chem 174:31
Garg A, Garg S, Zaneveld LJ, Singla AK (2001) Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother Res 15:655
Zhang J, Stanley RA, Melton LD, Skinner MA (2007) Inhibition of lipid oxidation by phenolic antioxidants in relation to their physicochemical properties. Pharmacology 1:180. Online
Galati EM, Monforte MT, Kirjavainen S, Forestieri AM, Trovato A, Tripodo MM (1994) Biological effects of hesperidin, a citrus flavonoid. (Note I): antiinflammatory and analgesic activity. Farmaco 40:709–12
Monforte MT, Trovato A, Kirjavainen S, Forestieri AM, Galati EM, Lo Curto RB (1995) Biological effects of hesperidin a Citrus flavonoid. (note II): hypolipidemic activity on experimental hypercholesterolemia in rat. Farmaco 50:595–9
Galati EM, Trovato A, Kirjavainen S, Forestieri AM, Rossitto A, Monforte MT (1996) Biological effects of hesperidin, a Citrus flavonoid. (Note III): antihypertensive and diuretic activity in rat. Farmaco 51:219–21
Tanaka T, Makita H, Ohnishi H, Mori H, Satoh K, Hara A, Sumida T, Fukutani K, Tanaka T, Ogawa H (1997) Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis in rats by flavonoids diosmin and hesperidin, each alone and in combination. Cancer Res 57:246
Tanaka T, Makita H, Kawabara K, Mori H, Kakumoto M, Satoh K, Hara A, Sumida T, Tanaka T, Ogawa H (1997) Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis 18:957
Bellocco E, Barreca D, Lagana G, Leuzi U, Tellone E, Ficarra S, Kotyk A, Galtieri A (2009) Influence of l-rhamnosyl-d-glucosyl derivatives on properties and biological interaction of flavonoids. Mol Cell Biochem 321:165
Itoh K, Masuda M, Naruto S, Murata K, Matsuda H (2009) Antiallergic activity of unripe Citrus hassaku fruits extract and its flavanone glycosides on chemical substance-induced dermatitis in mice. J Nat Med 63:443
Lee JK (2011) Anti-inflammatory effects of eriodictyol in lipopolysaccharide-stimulated Raw 264.7 murine macrophages. Arch Pharm Res 34:671–9
Minato K, Miyake Y, Fukumoto S, Yamamoto K, Kato Y, Shimomura Y, Osawa T (2003) Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sci 72:1609
Yu J, Wang L, Walzem RL, Miller EG, Pike LM, Patil BS (2005) Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J Agric Food Chem 53:2009
Li L, Zeng Z, Cai G (2011) Comparison of neoeriocitrin and naringin on proliferation and osteogenic differentiation in MC3T3-E1. Phytomedicine 18:985
Hwang S-L, Yen G-C (2009) Modulation of Akt, JNK, and p38 activation is involved in citrus flavonoid-mediated cytoprotection of PC12 cells challenged by hydrogen peroxide. J Agric Food Chem 57:2576
Sacco S, Maffei M (1997) The effect of isosakuranetin (5,7-dihydroxy 4′-methoxy flavanone) on potassium uptake in wheat root segments. Phytochemistry 46:245
Kupfer R, Swanson L, Chow S, Staub RE, Zhang YL, Cohen I, Christians U (2008) Oxidative in vitro metabolism of liquiritigenin, a bioactive compound isolated from the Chinese herbal selective estrogen β-receptor agonist MF101. Drug Metab Dispos 36:2261
Mersereau JE, Levy N, Staub RE, Baggett S, Zogric T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, Leiman DC (2008) Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist. Mol Cell Endocrinol 283:49
Kim YW, Zhao RJ, Park SJ, Lee JR, Cho IJ, Yang CH, Kim SG, Kim SC (2008) Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-κB-dependent iNOS and proinflammatory cytokines production. Br J Pharmacol 154:165
Kim SC, Byun SH, Yang CH, Kim CY, Kim JW, Kim SG (2004) Cytoprotective effects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and cytochrome c-mediated PARP cleavage). Toxicology 197:239
Kim YW, Ki SH, Lee SJ, Lee SJ, Kim CW, Kim SC, Kim SG (2006) Liquiritigenin, an aglycone of liquiritin in Glycyrrhizae radix, prevents acute liver injuries in rats induced by acetaminophen with or without buthionine sulfoximine. Chem-Biol Interact 161:125
Kim YW, Kang HE, Lee MG, Hwang SJ, Kim SC, Lee CH, Kim SG (2009) Liquiritigenin, a flavonoid aglycone from licorice, has a choleretic effect and the ability to induce hepatic transporters and phase-II enzymes. Am J Physiol Gastrointest Liver Physiol 296:G372
Liu RT, Zou LB, Lu QJ (2009) Liquiritigenin inhibits Aβ25-35-induced neurotoxicity and secretion of Aβ1-40 in rat hippocampal neurons. Acta Pharmacol Sin 30:899
Liu RT, Zou LB, Fu JY, Lu QJ (2010) Effects of liquiritigenin treatment on the learning and memory deficits induced by amyloid β-peptide (25-35) in rats. Behav Brain Res 210:24
Higo H, Zhang S-P, He L, Li F, Liu Y, Cai Y-Q (2008) Anti-proliferative effects and induction of apoptosis by liquiritigenin in human hepatocellular carcinoma SMMC-7721 cells. J Trad Med 25:160
Chen ZA, Wang JL, Liu RT, Ren JP, Wen LQ, Chen XJ, Bian GX (2009) Liquiritin potentiate neurite outgrowth induced by nerve growth factor in PC12 cells. Cytotechnology 60:125
Yang Y, Bian GX, Lu QJ (2008) Neuroprotection and neurotrophism effects of liquiritin on primary cultured hippocampal cells. China J Chin Mater Med 33:931
Sun Y-X, Tang Y, Wu A-L, Liu T, Dai X-L, Zheng Q-S, Wang Z-B (2010) Neuroprotective effect of liquiritin against focal cerebral ischemia/reperfusion in mice via its antioxidant and antiapoptosis properties. J Asian Nat Prod Res 12:1051
Wang WX, Hu XY, Zhao ZY, Liu P, Hu YC, Zhou JP, Zhou DF, Wang ZB, Guo D, Guo HZ (2008) Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog Neuropsychopharmacol Biol Psychiatry 32:1179
Lee H, Yeom H, Kim YG, Yoon CN, Jin C, Choi JS, Kim B-R, Kim D-H (1998) Structure-related inhibition of human hepatic caffeine N3-demethylation by naturally occurring flavonoids. Biochem Pharmacol 55:1369
Celiz G, Audisio MC, Daz M (2010) Antimicrobial properties of prunin, a citric flavanone glucoside, and its prunin 6″-O-lauroyl ester. J Appl Microbiol 109:1450
Wätjen W, Weber N, Lou Y-J, Wang ZQ, Chovolou Y, Kampkötter A, Kahl R, Proksch P (2007) Prenylation enhances cytotoxicity of apigenin and liquiritigenin in rat H4IIE hepatoma and C6 glioma cells. Food Chem Toxicol 45:119
Wang D-Y, Hu YZ, Kong S-S, Yu Y-P, Zhu D-Y, Lou Y-J (2011) Promoting effects of isobavachin on neurogenesis of mouse embryonic stem cells were associated with protein prenylation. Acta Pharmacol Sin 32:425
Hyun SK, Lee W-H, Jeong DM, Kim Y, Choi JS (2008) Inhibitory effects of kurarinol, kuraridinol, and trifolirhizin from Sophora flavescens on tyrosinase and melanin synthesis. Biol Pharm Bull 31:154
Ryu YB, Westwood IM, Kang NS, Kim HY, Kim JH, Moon YH, Park KH (2008) Kurarinol, tyrosinase inhibitor isolated from the root of Sophora flavescens. Phytomedicine 15:612
Oh I, Yang W-Y, Chung S-C, Kim T-Y, Oh K-B, Shin J (2011) In vitro sortase A inhibitory and antimicrobial activity of flavonoids isolated from the roots of Sophora flavescens. Arch Pharm Res 34:217
Kim HY, Jeong DM, Lung HJ, Jung YJ, Yokozawa T, Choi JS (2008) Hypolipidemic effects of Sophora flavescens and its constituents in poloxamer 407-induced hyperlipidemic and cholesterol-fed rats. Biol Pharm Bull 31:73
Jung HA, Jeong D-M, Chung HY, Lim HA, Kim JY, Yoon NY, Choi JS (2008) Re-evaluation of the antioxidant prenylated flavonoids from the roots of Sophora flavescens. Biol Pharm Bull 31:908
Gosch C, Halbwirth H, Kuhn J, Miosic S, Stich K (2009) Biosynthesis of phloridzin in apple (Malus domestica Borkh.). Plant Sci 176:223–231
Gosch C, Halbwirth H, Stich K (2010) Phloridzin: biosynthesis, distribution and physiological relevance in plants. Phytochemistry 71:838
Lee KW, Kim YJ, Kim DO, Lee HJ, Lee CY (2003) Major phenolics in apple and their contribution to the total antioxidant capacity. J Agric Food Chem 51:6516
Stangl V, Lorenz M, Ludwig A, Grimbo N, Guether C, Sanad W, Ziemer S, Martus P, Baumann G, Stangl K (2005) The flavonoid phloretin suppresses stimulated expression of endothelial adhesion molecules and reduces activation of human platelets. J Nutr 135:172
Hassan M, Yazidi CE, Landrier J-F, Lairon D, Margotat A, Amiot M-J (2007) Phloretin enhances adipocyte differentiation and adiponectin expression in 3 T3-L1 cells. Biochem Biophys Res Commun 361:208
Hassan M, Yazidi CE, Malezet-Desmoulins C, Lairon D, Amiot M-J, Margotat A (2010) Gene expression profiling of 3T3-L1 adipocytes exposed to phloretin. J Nutr Biochem 21:645
Valenta C, Cladera J, O’Shea P, Hadqraft J (2001) Effect of phloretin on the percutaneous absorption of lignocaine across human skin. J Pharm Sci 90:485
Auner BG, Valenta C (2004) Influence of phloretin on the skin permeation of lidocaine from semisolid preparations. Eur J Pharm Biopharm 57:307
Devi MA, Das NP (1993) In vitro effects of natural plant polyphenols on the proliferation of normal and abnormal human lymphocytes and their secretions of interleukin-2. Cancer Lett 69:191
Nelson JAS, Falk RE (1993) The efficacy of phloridzin and phloretin on tumor cell growth. Anticancer Res 13:2287
Kobori M, Shinmoto H, Tsushida T, Shinohara K (1997) Phloretin-induced apoptosis in B16 melanoma 4A5 cells by inhibition of glucose transmembrane transport. Cancer Lett 119:207
Boccia MM, Kopf SR, Baratti CM (1999) Phlorizin, a competitive inhibitor of glucose transport, facilitates memory storage in mice. Neurobiol Learn Mem 71:104
Raja MM, Tyagi NK, Kinne RKH (2003) Phlorizin recognition in a C-terminal fragment of SGLT1 studied by tryptophan scanning and affinity labeling. J Biol Chem 278:49154
Rossetti L, Smith D, Shulman GI, Zawalich W, DeFronzo RA (1987) Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79:1510
Zhao H, Yakar S, Gavrilova O, Sun H, Zhang Y, Kim H, Setser J, Jou W, LeRoith D (2004) Phloridzin improves hyperglycemia but not hepatic insulin resistance in a transgenic mouse model of type 2 diabetes. Diabetes 53:2901
Wang J, Chung MH, Xue B, Ma H, Ma C, Hattori M (2010) Estrogenic and antiestrogenic activities of phloridzin. Biol Pharm Bull 33:592
Xiang L, Sun K, Lu J, Weng Y, Taoka A, Sakagami Y, Qi J (2011) Anti-aging effects of phloridzin, an apple polyphenol, on yeast via the SOD and Sir2 genes. Biosci Biotechnol Biochem 75:854
Toan SV, To KK, Leung GP, de Souza MO, Ward JL, Tse CM (2003) Genomic organization and functional characterization of the human concentrative nucleoside transporter-3 isoform (hCNT3) expressed in mammalian cells. Pfügers Arch 447:195
de Bernonville TD, Gaucher M, Guyot S, Durel C-E, Dat JF, Brisset M-N (2011) The constitutive phenolic composition of two Malus × domestica genotypes is not responsible for their contrasted susceptibilities to fire blight. Environ Exp Bot 74:65
Dong H-Q, Li M, Zhu F, Liu F-L, Huang J-B (2012) Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against α-glucosidase and α-amylase linked to type 2 diabetes. Food Chem 130:261
Nakamura Y, Watanabe S, Miyake N, Kohno H, Osawa T (2003) J Agric Food Chem 51:3309
Suarez J, Herrera MD, Marhuenda E (1998) In vitro scavenger and antioxidant properties of hesperidin and neohesperidin dihydrochalcone. Phytomedicine 5:469
Choi J-M, Yoon B-S, Lee S-K, Hwang J-K, Ryang R (2007) Antioxidant properties of neohesperidin dihydrochalcone: inhibition of hypochlorous acid-induced DNA strand breakage, protein degradation, and cell death. Biol Pharm Bull 30:324
Kawano A, Nakamura H, Hata S, Minakawa M, Miura Y, Yagasaki K (2009) Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine 16:437
von Gadow A, Joubert E, Hansmann CF (1997) Comparison of the antioxidant activity of Aspalathin with that of other plant phenols of Rooibos tea (Aspalathus linearis), α-Tocopherol, BHT, and BHA. J Agric Food Chem 45:632
Joubert E, Winterton P, Britz TJ, Ferreria D (2004) Superoxide anion and α, α-diphenyl-β-picrylhydrazyl radical scavenging capacity of rooibos (Aspalathus linearis) aqueous extracts, crude phenolic fractions, tannin and flavonoids. Food Res Int 37:133
Snijman PW, Swanevelder SJ, Joubert E, Green IR, Gelderblom WCA (2007) The antimutagenic activity of the major flavonoids of rooibos (Aspalathus linearis): some dose-response effects on mutagen activation-flavonoid interactions. Mutat Res 631:111
Snijman PW, Joubert E, Ferreira D, Li X-C, Ding Y, Green IR, Gelderblom WCA (2009) Antioxidant activity of the dihydrochalcones Aspalathin and Nothofagin and their corresponding flavones in relation to other Rooibos (Aspalathus linearis) flavonoids, epigallocatechin gallate, and trolox. J Agric Food Chem 57:6678
Li Y-S, Matsunaga K, Kato R, Ohizumi Y (2001) Verbenachalcone, a novel dimeric dihydrochalcone with potentiating activity on nerve growth factor-action from Verbena littoralis. J Nat Prod 64:806
Li Y, Ishibashi M, Chen X, Ohizumi Y (2003) Littorachalcone, a new enhancer of NGF-mediated neurite outgrowth, from Verbena littoralis. Chem Pharm Bull 51:872
Boumendjel A (2003) Aurones: a subclass of flavones with promising biological potential. Curr Med Chem 10:2621
Atta-Ur-Rahman CMI, Hayat S, Khan AM, Ahmed A (2001) Two new aurones from marine brown alga Spatoglossum variabile. Chem Pharm Bull 49:105
Venkateswarlu S, Panchagnula GK, Gottumukkala AL, Subbaraju GV (2007) Synthesis, structural revision, and biological activities of 4′-chloroaurone, a metabolite of marine brown alga Spatoglossum variabile. Tetrahedron 63:6909
Nakayama T (2002) Enzymology of aurone biosynthesis. J Biosci Bioeng 94:487
Nenadis N, Sigalas MP (2008) A DFT study on the radical scavenging activity of maritimetin and related aurones. J Phys Chem A 112:12196
Nenadis N, Sigalas MP (2011) A DFT study on the radical scavenging potential of selected natural 3′,4′-dihydroxy aurones. Food Res Int 44:114
Kumar KS, Kumaresan R (2011) A quantum chemical study on the antioxidant properties of aureusidin and bracteatin. Int J Quant Chem 111:4483
Okombi S, Rival D, Bonnet S, Mariotte A-M, Perrier E, Boumendjel A (2006) Discovery of benzylidenebenzofuran-3(2 H)-one (Aurones) as inhibitors of tyrosinase derived from human melanocytes. J Med Chem 49:329
Choi J, Yoon B-J, Huh K, Park K-Y, Lee K-T, Park H-J (2002) Anti-rheumatoidal effect of sulfuretin isolated from the heartwood of Rhus veniciflua in rats and mice. Nutraceuticals and Food 7:347
Shin J-S, Park YM, Choi J-H, Park H-J, Shin MC, Lee YS, Lee K-T (2010) Sulfuretin isolated from heartwood of Rhus verniciflua inhibits LPS-induced inducible nitric oxide synthase, cyclooxygenase-2, and pro-inflammatory cytokines expression via the down-regulation of NF-κB in RAW 264.7 murine macrophage cells. Int Immunopharmacol 10:943–50
Song M-Y, Jeong G-S, Kwon K-B, Ka S-O, Jang H-Y, Park J-W, Kim Y-C, Park B-H (2010) Sulfuretin protects against cytokine-induced β-cell damage and prevents streptozotocin-induced diabetes. Exp Mol Med 42:628
Go ML, Wu X, Liu XL (2005) Chalcones: an update on cytotoxic and chemoprotective properties. Curr Med Chem 12:483
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Ninomiya, M., Koketsu, M. (2013). Minor Flavonoids (Chalcones, Flavanones, Dihydrochalcones, and Aurones). In: Ramawat, K., Mérillon, JM. (eds) Natural Products. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-22144-6_62
Download citation
DOI: https://doi.org/10.1007/978-3-642-22144-6_62
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-22143-9
Online ISBN: 978-3-642-22144-6
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics