Abstract
Parasitic nematodes are known as important pathogens that cause problems for human and animal health. Some of them naturally inhabit the marine environment, where they are widespread and can be found in a variety of different hosts. Food-borne zoonoses via aquatic animals are most often linked to anisakid nematodes of the genera Anisakis Dujardin, 1845, Contracaecum Railliet and Henry, 1912, and Pseudoterranova Mozgovoi, 1951. These are commonly found in the digestive tract of marine mammals, and infect aquatic invertebrates and vertebrates as intermediate hosts. The most widely distributed whale worms Anisakis spp. involve cetaceans as final and planktonic copepods, euphausiids, squids and teleosts as intermediate or paratenic hosts. Painful infections of the digestive tract in humans originate through consumption of raw or semi-raw fisheries products, for example fish and squid. Recent molecular studies revealed the existence of morphologically similar but genetically different cryptic species (‘sibling species’) within the anisakids. Among these, A. simplex (s.s.) is responsible for the highest number of recorded human infections. Molecular studies of Anisakis larvae from various parts of the world Oceans demonstrate an uneven species distribution, with A. simplex (s.s.) being limited to the northern hemisphere. Another species, A. typica, has not yet been connected to this disease, and seems to be restricted to the tropical regions. This chapter presents the present state of knowledge about this widespread group of fish parasites, including the importance as human pathogens, their life cycle biology, biogeography and phylogeny. The distribution of the currently recognized Anisakis species is summarized and combined with the number of known cases of human anisakiasis. We suggest that pathogenicity for humans is different among the Anisakis siblings, providing a possible explanation for uneven disease records worldwide. The possibility of a changing risk of anisakidosis in the time of climate change is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Parasitism, a form of symbiosis, is one of the most successful modes of life (Palm and Klimpel 2007). More than half of all plant and animal species on earth are parasites, and probably no organism avoids parasitic infection during its lifetime (Palm and Klimpel 2007). Including approximately 256 families and more than 40,000 known species, the phylum Nematoda is one of the most species rich and abundant invertebrate Taxon (Anderson 2000; McClelland 2005). Beside free-living nematodes in freshwater, marine and terrestrial habitats (McClelland 2005), their parasitic forms use plants, animals and humans as host organisms at a global scale (e.g. Blaxter et al. 1998).
Gastrointestinal parasitic nematodes are known to cause a wide range of diseases and have consequences for human and animal health. They impose a significant economic burden as parasites of domestic animals, reduce productivity, and require elaborate and expensive control methods (e.g. Parkinson et al. 2004; Audicana and Kennedey 2008). Infections of humans cause substantial mortality and morbidity, resulting in about 2.9 billion infected people worldwide (Parkinson et al. 2004). Most important are the hookworms (e.g. Ancylostoma spp.), ascarids (Ascaris spp.), whipworms (e.g. Trichuris trichiura) and filarial nematodes that cause lymphatic filariasis (e.g. Brugia malayi) or elephantiasis (Wuchereria bancrofti) and African river blindness (e.g. Onchocerca volvulus) (e.g. Parkinson et al. 2004). Humans also become accidental hosts for nematodes that cannot complete their life cycles inside them, but can cause disease problems or initiate immune hypersensitivity states or allergies. The consumption of raw or undercooked fish regularly leads to food-borne zoonoses, most commonly caused by larvae of the anisakid nematode genera Anisakis, Contracaecum and Pseudoterranova (Sakanari and McKerrow 1989; Kaneko 1991; Audicana et al. 2002; Palm 2004).
Since the 1960s, the term anisakiasis had been used for a human disease caused by the third-stage larvae (L3) of members of the family Anisakidae. In 1988, a standardized nomenclature recommended three different terms: (1) anisakidosis caused by any members of the family Anisakidae, (2) anisakiasis caused by members of the genus Anisakis, and (3) pseudoterranovosis caused by members of the genus Pseudoterranova (e.g. Audicana et al. 2003; Audicana and Kennedey 2008). The first case of a human infection with Anisakis sp. was reported for the Netherlands (Van Thiel 1962) from an eosinophilic intestinal lesion in a patient. Ishikura and Kikuchi (1990) recorded 12,586 cases of anisakiasis between 1968 and 1989 in Japan. The number of cases is increasing worldwide, with ~50 cases annually in the USA and ~500 cases in Europe, over 95% of them from The Netherlands, Germany, France and Spain (e.g. Plath et al. 2001; Audicana et al. 2002; Fuentes et al. 2002). To date, over 14,000 anisakiasis cases have been reported, approximately 95% from Japan (Audicana et al. 2002). The parasite transmission is clearly related to the consumption of raw or semi-cooked fish. Especially Japanese sushi and sashimi, Dutch salted or smoked herring, Nordic gravlax (dry, cured salmon), Hawaiian lomi-lomi (raw salmon), German rollmop (rolled fillet of marinated/pickled herring), South American cebiche and Spanish boquerones en vinagre (pickled anchovies) are regular pathways of infection (e.g. Petersen et al. 1993; Audicana et al. 2002; Palm 2004).
The present communication summarizes the current state of knowledge on zoonotic anisakid nematodes, their pathogenicity, life cycle biology, biogeography and phylogeny. We suggest that in addition to different special dishes and food preferences, a characteristic distribution pattern of the currently recognized Anisakis species is responsible for an unequal regional distribution of known cases of anisakiasis so far. We assess the risk that might result from potentially changing infection levels in the time of increasing anthropogenic influence and climate change. This is especially important for an increasing number of people that use marine food products for their daily needs.
2 Genetic Identification
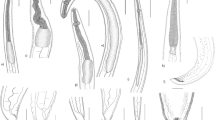
An accurate identification of nematodes at any particular life cycle stage is essential for the diagnosis of nematode infections, and consequently an important part of disease surveillance and control. Identification of nematodes from marine vertebrates has been based on morphological characters, such as the size and shape of the spiculae (sexual organs) in adult males, and head structures and papillae that regularly occur on the body surface. Larval identification used the orientation of the excretory pores, the arrangement and separation of the digestive tract into oesophagus, ventricle and attaching structures such as caeca and appendices, and the shape of the tail (Fig. 11.1). Even accompanied with morphometric information, generic and especially species identification has been difficult, leading to a high number of erroneous identifications. This promoted molecular methods for a better and more reliable species diagnosis.
(a) Habitus of the anisakid nematode Pseudoterranova decipiens E isolated from the liver of the icefish Chaenocephalus aceratus. (b) Anterior end of the third-stage larvae (L3) of P. decipiens s.l. (left) from smelt (Osmerus eperlanus) and Anisakis simplex s.l. from herring (Clupea harengus). (c) Anterior end of the third-stage larva of A. typica with the boring tooth. (d, e) Posterior end of A. typica with the mucron. (f) Nematode larvae (L3) isolated from Wadden Sea fish; left – P. decipiens s.l., right – A. simplex s.l. (g) Numerous anisakid nematode larvae in the viscera of the icefish C. aceratus
Molecular techniques have the advantage that they allow analyses of the parasite DNA, securing species identification and providing data for phylogenetics. Genomic DNA sequences evolve at different rates, with non-coding, non-transcribed sequences of ribosomal DNA (rDNA) and mitochondrial DNA (mtDNA) evolving faster than those that encode essential proteins or nuclear DNA (nDNA), respectively. Molecular anisakid nematode identification started with allozyme analyses including restriction fragment length polymorphism techniques (PCR-RFLPs of ITS-DNA, e.g. D’Amelio et al. 2000; Kijewska et al. 2002; Pontes et al. 2005). The next approach was direct sequencing of rDNA, including the highly variable internal transcribed spacers ITS1-2 and the conserved 5.8S rDNA region; direct sequencing of the 28S (LSU rDNA) and complete internal transcribed spacer (ITS-1, 5.8S, ITS-2) rDNA (e.g. Li et al. 2005; Nadler et al. 2005; Zhu et al. 2000a, b, 2001, 2002) and mitochondrial cytochromoxidase 1 and 2 (mtDNA cox1, cox2) sequence analyses (e.g. Valentini et al. 2006; Cross et al. 2007; Mattiucci and Nascetti 2008; Mattiucci et al. 2008a, b) followed. Also micro-satellites can be used to distinguish the species among populations. These studies identified the existence of “sibling species” within the ascaridoids, being morphologically very similar but genetically different, having distinct host preferences, life cycles and geographical distribution (e.g. Mattiucci et al. 1997, 2005; Zhu et al. 2002; Nadler et al. 2005; Marques et al. 2006; D’Amelio et al. 2007; Klimpel et al. 2007, 2008, 2010; Mattiucci and Nascetti 2008).
Within the family Anisakidae the genus Contracaecum includes two sibling species complexes, the (1) C. osculatum complex with the five species C. osculatum A, B, C (C. osculatum s.s.), D, E, and the (2) C. ogmorhini complex with the two species C. ogmorhini (s.s.) and C. margolisi, and additionally C. radiatum (e.g. Mattiucci and Nascetti 2008; Shamshi et al. 2009a, b). The Pseudoterranova decipiens complex consists of six different species (P. decipiens s.s. = P. decipiens B, P. krabbei, P. bulbosa, P. azarasi, P. decipiens E, P. cattani) (Mattiucci et al. 2007; Mattiucci and Nascetti 2008). Most recently Klimpel et al. (2007, 2008, 2010) and Palm et al. (2008) studied the species identity within the genus Anisakis by using the following protocol.
Genomic DNA isolation and purification followed amplification of the rDNA region (ITS-1, 5.8S, ITS-2), and flanking sequences (=ITS+), using the primers NC5 (5′-GTA GGT GAA CCT GCG GAA GGA TCA TT-3′) and NC2 (5′-TTA GTT TCT TTT CCT CCG CT-3′) (Zhu et al. 2000a). The PCR reaction (50 μL) includes 25 μL of Master-Mix (Peqlab Biotechnology GmbH, Erlangen, Germany) containing dNTPs, MgCl2, buffer, and Taq-Polymerase, 3 μL of each primer, 14 μL aqua dest. and 5 μL genomic DNA. Each PCR reaction is performed in a thermocycler (Biometra or Peqlab, Germany) under the following conditions: after initial denaturation at 91°C for 1 min, 40 cycles of 94°C for 45 s (denaturation), 55°C for 45 s (annealing), and 72°C for 45 s (extension), followed by a final extension at 72°C for 10°min. PCR products were examined on 1% agarose gels. A 100-bp ladder marker (peqGOLD, Erlangen, Germany) is used to estimate the size of the PCR products. For anisakid nematode identification, the PCR products must be purified with an E.Z.N.A. Cycle-Pure Kit (Peqlab Biotechnology GmbH, Erlangen, Germany), followed by sequencing of a total volume of 7 μL, including 2 μL of primer (individually) and 5 μL of the PCR product (250 ng/μL). Both spacers and the 5.8S gene from each PCR product are sequenced in both directions, using the primers NC5, NC13 (forward; 5′-ATC GAT GAA GAA CGC AGC-3′), NC13R (reverse; 5′-GCT GCG TTC TTC ATC GAT-3′), XZ1R (reverse; 5′-GGA ATG AAC CCG ATG GCG CAA T-3′), and NC2.
These studies revealed the existence of nine species, six belonging to two sibling species complexes, the (1) A. simplex complex with A. simplex (s.s.), A. pegreffii, and A. simplex C, the (2) A. physeteris complex with A. physeteris, A. brevispiculata and A. paggiae, and the three species A. typica, A. ziphidarum und A. nascettii (e.g. Klimpel et al. 2008, 2010; Mattiucci and Nascetti 2008; Fig. 11.2).
Anisakis spp. final cetacean host distribution in the A. physeteris and A. simplex complexes (Cetacea-families; I Physeteridae; II Kogiidae; III Ziphiidae, Delphinidae; IV Delphinidae; V Ziphiidae, Delphinidae; VI Physeteridae, Ziphiidae, Neobalaenidae, Delphinidae; VII Phocoenidae, Balaenopteridae, Monodontidae, Delphinidae)
3 Life Cycle Ecology of Anisakid Nematodes
According to Anderson (1984, 1996), parasitic nematodes first evolved in terrestrial hosts and were only able to invade aquatic environments after the development of heteroxeny (the use of intermediate hosts) and paratenesis (the use of transport hosts). Intermediate hosts support larval growth and development to a stage where the nematode is capable of infecting its definitive host. Both intermediate and paratenic hosts participate in the temporal and spatial dispersal of the parasite, thereby increasing the likelihood of transmission into the final host (e.g. McClelland 2005). Heteroxeny is the common life cycle pattern of marine ascaridoid nematodes such as Anisakis, Contracaecum, and Pseudoterranova. Transmission pathways are habitat-dependent and usually involve a broad spectrum of invertebrates and intermediate or paratenic fish hosts (McClelland 2005; Klimpel and Rückert 2005; Palm and Klimpel 2007).
The life cycle of anisakid nematodes follows the general nematode life cycle pattern, including four larval stages (L1–L4) and the adults in the final host. The heteroxenous life cycle involves a variety of hosts that are transferred through the marine food chain. Most important are the three genera Anisakis (whales), Contracaecum (bird, seals), and Pseudoterranova (seals), that can be distinguished morphologically and according to their final host spectrum (e.g. Klimpel et al. 2008, 2010; Mattiucci and Nascetti 2008). Life cycle studies of these nematodes have been limited by difficulties in maintaining them alive in the laboratory, culturing sufficient numbers of parasite-free experimental hosts, and creating effective exposure (e.g. Køie and Fagerholm 1995; Køie et al. 1995; Køie 2001; Klimpel et al. 2004, 2008, 2010; Mattiucci and Nascetti 2008). However, empirical studies on the distribution and abundance of anisakid larvae in the intermediate and final hosts have revealed important insights into the life cycle biology of these parasites. Being responsible for anisakiasis and of high importance for human health, the whaleworms Anisakis spp. mainly infect toothed whales and a range of pelagic schooling fish worldwide. Thus, the life cycle can be considered to take place in the pelagic environment, with some seals and baleen whales getting accidentally infected (e.g., Køie et al. 1995; Hays et al. 1998a,b; Køie 2001; Klimpel et al. 2004, 2010). Life cycle stages include four larval stages (L1–L4), within the eggs (L1–L3) and subsequently in the intermediate or paratenic hosts (L3), and as preadults (L4) and adults in the cetacean final hosts (Fig. 11.3). The nematode eggs are excreted with the faeces and embryonate in seawater (Køie 2001). Køie et al. (1995) found larvae surrounded by two cuticles prior to hatching. They were surrounded by sheaths with lateral extensions and were able to float, enabling them to use mainly pelagic crustaceans as intermediate hosts (Køie et al. 1995). During ingestion by the crustacean first intermediate host, the larvae are most probably released from the second stage cuticle by the action of the mouthparts. This allows the third stage larvae to penetrate the gut prior to establishing themselves in the haemocoel (Køie et al. 1995). Larger invertebrates (mainly copepods, euphausiids) and smaller fish are thought to be important second intermediate hosts, and various predatory fish species and cephalopods serve as paratenic hosts. If small fishes are preyed upon by larger piscivorous fishes, the larvae are capable of re-infecting the latter without further moulting. Consequently, piscivorous hosts may accumulate enormous numbers of larvae (Lile 1998). Cetaceans acquire the nematodes by preying upon the intermediate hosts. To date, a total of 34 cetacean species have been found to harbour Anisakis spp. (e.g. Klimpel et al. 2008; Mattiucci and Nascetti 2008; Fig. 11.4).
Schematic life cycle of Anisakis species. The pelagic life cycle of Anisakis spp. follows the general nematode life cycle pattern, including four larval stages (L1–L4) and the adults in the cetacean final host. The heteroxenous life cycle involves a variety of invertebrate and vertebrate hosts that are transferred through the marine food chain (Kuhn 2010)
Cetacean and pinniped final hosts of anisakid nematode species. (a) Dolphins (Fam. Delphinidae) are the most common final hosts of Anisakis typica from subtropical and tropical marine waters. (b) Minke whale (Balaenoptera bonaerensis, Fam. Balaenopteridae) as potential final hosts for Anisakis spp. in the Southern Ocean (Antarctica). (c) Beaked whales (Fam. Ziphiidae) in the Southern Ocean (Antarctica) are final hosts of Aniskais ziphidarum. (d) Southern elephant seal (Mirounga leonina), (e) Weddell seal (Leptonychotes weddellii) and (f) Leopard seal (Hydrurga leptonyx) are final hosts of the nematode genera Contracaecum and Pseudoterranova in the Southern Ocean (Antarctica)
In contrast to the whaleworms, the sealworms of the genus Pseudoterranova seem to be restricted to a benthic life cycle (e.g. Palm et al. 1994; Køie et al. 1995; Palm 1999; McClelland 2002). Partially embryonated eggs, passed in the faeces of a seal, settle on the sea bed where they complete development to the third stage larvae (L3) and hatch. Newly hatched larvae are still ensheathed in the cuticle of the previous second larval stage (L2) and attach to the substrate caudally (e.g. Køie et al. 1995; Anderson 2000; McClelland 2002, 2005). When ingested by benthic crustaceans (e.g. amphipods, gammarids, isopods, harpacticoid copepods), they exsheath inside the first intermediate host, penetrate into the haemocoel and begin to grow. These hosts serve to enhance transmission to a larger array of benthic macro-invertebrates as second intermediate hosts, where the larval sealworms grow in length (e.g. Anderson 2000, McClelland 2002, 2005). At this point they become infective to fish and also to seals. The invertebrate hosts are usually ingested by primary benthic teleosts, including juveniles of larger demersal fish species. The larvae penetrate the gut wall of the fish and establish themselves in the internal organs or the musculature, where they continue to grow in length. Large, piscivorous fish may serve as second/third fish or paratenic hosts that accumulate the larval worms (Palm 1999; Anderson 2000; McClelland 2002, 2005). Following ingestion by the seal definitive host, infective third stage larvae (L3) escape from the bodies of the fish or invertebrate, embed their anterior part into the gastric mucosa, mature and reproduce. Ten marine mammal species belonging to the Otariidae and Phocidae have been recorded as final hosts (e.g. Mattiucci and Nascetti 2008; Fig. 11.4).
Nematodes of the genus Contracaecum seem to have equally complex life cycles involving benthic and pelagic invertebrates (e.g. crustaceans, squid) and fish (e.g. Klöser et al. 1992; Køie and Fagerholm 1995; Køie et al. 1995). They are also transmitted to pelagic and demersal fish by for example euphausiids, shrimps and small fish that are found near bottom in daytime but feed pelagically at night. In total 12 marine mammal species including specimens from the families Ottariidae and Phocidae and different fish-eating sea birds (e.g. of the genera Larus, Pelecanus, Phalacrocorax) have been identified as final hosts in the Contracaecum life cycle (e.g. Torres et al. 1983; Farjallah et al. 2008; Mattiucci and Nascetti 2008; Shamshi et al. 2009a; Fig. 11.4).
4 Phylogeny and Host Range of Anisakid Nematodes (Anisakis spp.)
Traditional nematode taxonomy was based on a limited number of criteria, such as the shape of the oesophagus, male and female reproductive organs and life cycle patterns (e.g. Wijova et al. 2006). Recent morphology-based classifications split the nematodes into the classes Secernentea (= Phasmidea, = Rhabditea) and Adenophorea ( = Aphasmidea, = Enoplea), including most terrestrial and parasitic species and most marine species, respectively (Dorris et al. 1999). There were considerable obstacles for an accurate identification especially of the larval forms. For this reason, earlier classification systems were not compatible with each other, and there was no universally accepted nematode phylogeny during the last century (e.g. Blaxter et al. 2000; De Ley and Blaxter 2002; Meldal et al. 2007). A study of the small subunit (SSU) or 18S rRNA for a wide range of major nematode taxa concluded convergent evolution in many lineages, requiring revision of the morphology-based higher-level classification (Blaxter et al. 1989; Dorris et al. 1999). Although most recent datasets of the SSU rRNA genes comprise more than 300 taxa (Blaxter 2003), sampling remains strongly biased towards some groups and poor for other important taxa.
The nematodes divide into the three major clades Dorylaimia (clade I, free-living, invertebrate, vertebrate and plant parasites from the marine and terrestrial environment), Enoplia (clade II, marine and plant parasite species) and Chromadoria, which include the Rhabditida (marine free-living species, few terrestrial/freshwater representatives). The Rhabditida separate into the Spirurina (clade III, only animal parasites), Tylenchina (clade IV, animal-, plant- and fungus-parasitic and free-living groups) and Rhabditina (clade V, free-living and parasitic species) (e.g. De Ley and Blaxter 2002; Parkinson et al. 2004; De Ley 2006). Parasitism of both plants and animals seems to have arisen multiple times within nematode evolution, and all major clades include parasites (Blaxter et al. 1989; De Ley and Blaxter 2002; Parkinson et al. 2004). The Spirurina include five infraorders (Ascaridomorpha, Spiruromorpha, Rhigonematomorpha, Oxyuridomorpha, Gnathostomatomorpha) and one additional superfamily, the Dracunculoidea. With exception of the invertebrate parasites of the Rhigonematomorpha, all others include both invertebrate and vertebrate parasites (De Ley 2006; Wijova et al. 2006). The Ascaridomorpha are most closely related to the Spiruromorpha and Rhigonematomorpha, a clade that also comprises terrestrial and marine parasites (De Ley and Blaxter 2002; De Ley 2006), among these the family Anisakidae with Anisakis, Contracaecum and Pseudoterranova. While the former infects whales as final hosts, Pseudoterranova occurs in seals and Contracaecum in a range of aquatic hosts, including seals and birds.
Most Anisakis siblings have been identified from toothed whales, especially from the Delphinidae and Ziphiidae. The phylogenetic relationships within Anisakis together with their most common final hosts are illustrated in Fig. 11.2. The nine known species divide according to their host range into two major clades, the A. physeteris sibling species complex and the other six species. Three of them form the clade of the A. simplex sibling species complex. They are sister to the other three species, A. typica, A. ziphidarum and A. nascettii, the latter two combined on a single clade. Anisakis typica is restricted to dolphins (Delphinidae) from subtropical and tropical waters and to a single species of the family Pontoporidae (e.g. Mattiucci et al. 2002, 2005; Klimpel et al. 2008; Palm et al. 2008). Anisakis ziphidarum and A. nascettii have been reported so far only from the Ziphiidae. The A. simplex sibling species complex typically infects toothed but also baleen whales. Anisakis simplex (s.s.) parasitizes oceanic cetaceans of the families Delphinidae, Monodontidae, Phocoenidae, and Balaenopteridae mainly in the North Atlantic and Pacific Oceans. Anisakis pegreffii also utilizes the family Delphinidae as final hosts, however, additionally infecting the Ziphiidae, Physeteridae, and Neobalaenidae (Mattiucci et al. 1997) mainly in the entire Atlantic and Mediterranean but also in Australia. Anisakis simplex C infects toothed whales of the families Delphinidae and Ziphiidae in the southern hemisphere, extending its range of distribution into the North Pacific. Species within the A. physeteris sibling species complex are host specific for the Kogiidae and Physeteridae. Anisakis brevispiculata and A. paggiae have been recorded from kogiids mainly in the Mid- and Southern Atlantic Ocean (Mattiucci and Nascetti 2006; Valentini et al. 2006), and the cosmopolitan A. physeteris is known from physeterids (Fig. 11.5).
5 Biography of Anisakis spp.
Species of the genus Anisakis are distributed worldwide. The biogeography of Anisakis spp. follows a variety of factors that, in combination, lead to zoogeographical distribution patterns: (1) the final host distribution, (2) the host specificity in the final and intermediate hosts, (3) migration patterns of second and paratenic hosts, and (4) the characteristic life cycle (Kellermanns et al. 2007; Klimpel et al. 2008, 2010; Palm et al. 2008). These factors enable Anisakis siblings to explore all kinds of marine environments, from the shallow seas and open ocean into the deep-sea (e.g. Palm and Klimpel 2008). Most Anisakis siblings have been reported from the temperate, subtropical, and tropical waters between the equator and 35° North and South, while some species seem to be most common in the boreal regions of the Atlantic and Pacific (Fig. 11.5). Within Antarctic waters (Southern Ocean), these nematodes are at the most southern range of their distribution and to our knowledge extremely scarce (Klimpel et al. 2010).
Most detailed records on the zoogeography have been reported for A. simplex (s.s.), a common and highly abundant nematode in the North Atlantic and Pacific Oceans. According to the genetic identification, this species is limited to the northern hemisphere. The most closely related species, A. pegreffii, is known from the central Atlantic and the Mediterranean Sea, with some records from the most southern tips of the South American, African and Australian continents. Klimpel et al. (2010) identified specimens from migrating myctophids from the Southern Ocean off the South Shetlands that were genetically identical to specimens from China. The third species within the A. simplex complex, A. simplex C, has been also recorded from South America, Africa and Australia and around the South Shetland Islands in the Antarctic Ocean. Klimpel et al. (2010) analyzed the ITS-1, 5.8S and ITS-2 rDNA regions of A. simplex C specimens from the Antarctic Ocean, finding them identical to specimens from Pacific Canada and California, confirming the extensive range of distribution for this species. Because the parasites were found only in migrating myctophids coupled with rare findings from other teleosts in the Antarctic Ocean (also A. pegreffii), the authors concluded that these specimens originated from outside the Antarctic. Consequently, they can be considered at the most southern range of distribution in the Southern Ocean, and an earlier molecular record of A. simplex C from the elephant seal Mirounga leonina was interpreted as an accidental case of infection.
The two most closely related species within the A. physeteris complex, A. paggiae and A. brevispiculata, have been recorded so far only from the Atlantic Ocean, with most records in the northern hemisphere and a single record from the South African coast. Anisakis physeteris has been recorded only from the central and north Atlantic and the Mediterranean. However, because this species typically infects sperm whales that are known for an extensive zoogeographical distribution, the parasite might follow the distribution pattern of their final hosts. According to Klimpel et al. (2008) and Mattiucci and Nascetti (2008), A. ziphidarum and A. nascettii are typical parasites of ziphiid whales. The former has been recorded from the same localities as A. paggiae and A. brevispiculata, only from the Atlantic Ocean, and only a few records have reported A. nascettii from the waters of the central Atlantic Ocean, South Africa (SE Atlantic Ocean) and New Zealand (SW Pacific Ocean) (Mattiucci and Nascetti 2008; Mattiucci et al. 2009). A unique distribution pattern is known so far for A. typica, a species that has been described as circumtropical. According to Palm et al. (2008), several genotypes exist for this anisakid, however, there is no information on morphological differences between them. It can be concluded that following the extensive range of distribution of their mammalian final hosts and the low host specificity in migrating intermediate and paratenic hosts, anisakid nematodes have extensive ranges of distribution. This may explain why they are among the most common fish parasites recorded during common fish parasitological examinations.
6 Pathogenicity and Zoonotic Potential
The research interest in anisakid nematodes is based on the ability of the parasite larvae to survive in humans when ingested alive. Besides having zoonotic potential, anisakid larvae in the teleosts as well as the adults in marine mammals are several centimetres in length, and can cause pathological effects in their hosts. According to Dailey (2001), gastritis or ulcers have been often found in association with aggregations of L3, L4 and adult stages of anisakids (Anisakis, Contracaecum, Pseudoterranova) in the stomach and upper intestine of pinnipeds, cetaceans and sea otters. The symptoms of heavy infections include diarrhoea, dehydration and anaemia (e.g. McClelland 2005). Intestinal perforations leading to peritonitis and death have been attributed to Contracaecum and Pseudoterranova infections in sea lions and sea otters, respectively.
Nematode parasites of marine vertebrates may also be pathogenic to their intermediate hosts. Larval Pseudoterranova spp. caused erratic behaviour and death in experimentally infected marine crustaceans (McClelland 1990). Various larval anisakids (Anisakis, Contracaecum, Pseudoterranova) have been connected to mechanical compression or necrosis of the liver, lesions in the gut wall, viscera and musculature, depletion of lipids and mortality in heavily infected marine fish (Rohde 1984; Williams and Jones 1994; McClelland 2005). However, even a high intensity of infestation with Pseudoterranova decipiens and Contracaecum spp. in Antarctic fish (Klöser et al. 1992; Palm 1999; Palm et al. 1994, 1998, 2007) or the frequent infestation of the Atlantic and Baltic Sea herring (e.g. Szostakowska et al. 2002; Levsen and Lunestad 2010) with A. simplex (s.s.) has no visible effect on the host’s fitness. Some fish populations are commonly associated with very high anisakid burden, closely related to the abundance of their final hosts in the area of investigation (e.g. Des Clers 1991; Des Clers and Andersen 1995; Lile 1998).
Most recent research activities relate to the zoonotic potential of anisakids to infest humans. Epidemiological studies in Japan have indicated that anisakiasis was most frequent in coastal human populations (Audicana et al. 2002). Most common transmission routes are raw, undercooked and lightly marinated seafood (see Petersen et al. 1993; Palm 2004), for example of the spotted chub mackerel (Scomber japonicus) and Japanese flying squid (Todarodes pacificus) in Japan (Audicana et al. 2002; Audicana and Kennedey 2008). In western Europe, herring (Clupea harengus) is the main species involved, and in Spain, most cases can be related to the consumption of pickled anchovies (Engraulis encrasicolus) and raw sardines (Sardina pilchardus) (Audicana et al. 2002; Audicana and Kennedey 2008). Archetypal cases of anisakiasis or anisakiosis (Couture et al. 2003), involving the penetration of the alimentary tract and associated organs and causing clinical symptoms (e.g. nausea, severe epigastric pain, vomiting, allergy, diarrhoea), have been reported largely from Japan and other Asian countries (e.g. McClelland 2005). Anisakis larvae have been diagnosed as the disease-causing pathogen in most cases, the remainder being attributed to an infection with Pseudoterranova larvae (McClelland 2005). According to Smith (1999) and McClelland (2005) most cases of Pseudoterranova infection in Europe and the US have been largely asymptomatic, being diagnosed after the expulsion of the nematodes by coughing, vomiting or defaecation.
Helminth infections often induce chronic rather than acute disease, even in cases of very high levels of parasites. This results from parasite’s adaptations to evade the host immune response to secure their own survival. Human anisakidosis is peculiar because these parasites are not adapted to humans, and more than 90% of cases are caused by a single larva (Kagei and Isogaki 1992; Daschner et al. 2000; Audicana and Kennedey 2008). Differences may therefore be expected between A. simplex pathogenesis and that caused by other helminths in humans. An example of this is filariasis, in which there is a high and persistent burden of parasites, possibly resulting from host–parasite coevolution in order to optimize their mutual survival (e.g. Mitchell 1991; Taylor et al. 2005; Audicana and Kennedey 2008). Overt hypersensitivity reactions are rare unless provoked by natural or drug-induced death of the parasites residing in tissues. This contrasts with Anisakis infections, where allergic reactions seem to be common in humans.
Over the last few years, studies have indicated that, as with other helminth infections, the pathological changes occurring within the gastrointestinal tract are the combined result of the direct action of the larva during tissue invasion and the complex interaction between the host immune system and the substances released by, or contained within, the parasite. Allergies caused by the anisakid larvae in fish consumers have been of major concern. In the reported allergic cases of people from northern Spain, cooked hake (Merluccius merluccius) closely followed anchovies as the main pathway of infection. In Germany especially rolled fillet of marinated herring (rollmop) and fried smelt (Osmerus eperlanus) are a common source of infection with allergic reactions to A. simplex (s.s) in the former and P. decipiens in the latter.
Human infection by anisakid nematodes, especially Anisakis species, induces stimulation of both T helper type 1 and 2 (Th1, Th2) responses, and provokes a strong specific immune response by antibody isotypes, the immunoglobulin (Ig) IgE, IgG, IgA and IgM (Kennedey 2000; Cho et al. 2005; Audicana and Kennedey 2008). According to Anadón et al. (2009) more than 10.0% of gastrointestinal anisakiasis may be accompanied by allergic symptoms. Some studies have detected the presence of anti-Anisakis IgE antibodies in more than 10.0% of healthy subjects, suggesting the existence of a high number of infected patients who do not develop clinical symptoms (Anadón et al. 2009). In contrast to marine mammals, Anisakis larvae do not usually reach the adult stage in humans and the larvae die over a specific period after infection. Therefore, it is likely that the immune response against Anisakis allergens from the third and/or fourth-stage larvae occurs in response to two consecutive antigenic stimuli, for example (1) the excretory/secretory (ES) and cuticle antigens while the larvae is alive and (2) the cuticle and protease-resistant somatic and ES antigens, after the larvae die (Anadón et al. 2009). Previous studies have shown that the Anisakis ES allergens are the most clinically important, as they are targeted by most of the anti-Anisakis IgE antibodies induced during infections by this parasite (Anadón et al. 2009). To date several ES and somatic Anisakis allergens have been characterized and cloned, including Anis s 1 and Anis s 7 as probably the most important ES allergens, and have been reported in 85–100.0% of infected humans (Anadón et al. 2009). The Anis s 2 (paramyosin) and Ani s 3 (tropomyosin) are somatic Anisakis allergens that cross-react with other common allergens. Other allergens such as Ani s 4 (cystain), Ani s 6 (serine protease inhibitor), Ani s 5, Anis s 8 and Ani s 9 (the latter three among to the SXR/RAL-2 family proteins) are minor ES allergens which were reported from fewer than 50.0% of infected humans (Anadón et al. 2009).
7 Ascaridoid Nematodes and Climate Change
We are living in a period of climate change. Temperatures have increased by at least 0.33°C since 1990 (ocean and land combined), and ice fields on Greenland and parts of the Antarctic continent, for example the Larsen and Wilkins shelf ice, are melting at alarming rates (e.g. Rahmstorf et al. 2007). Though proceeding at a moderate pace in terms of human life span, climate change is transforming the world’s oceans by increasing the temperature and acidity of seawater and altering atmospheric and oceanic circulation. This has consequences for species distribution and composition in the marine ecosystems, changing the biogeography and biodiversity in aquatic habitats.
The natural variability of abiotic factors such as water temperatures (resulting e.g. in frontal zones, e.g. Klimpel and Rückert 2005) and ocean circulations is relatively high, often following non-linear or cyclic patterns. Similarly, aquatic habitats suffer significant anthropogenic habitat change, mainly caused by overexploitation and unsustainable use. Because these fluctuations overlay the so far subtle effects of temperature changes that are caused by anthropogenic-induced global warming, direct studies of the future consequences for the major ecosystems are difficult. However, the study of the effects of natural climate variability on selected organisms and environments can provide valuable insights into the possible impact of global warming. Compared to terrestrial systems, marine ecosystems are expected to react more sensitively and quickly to changes in climatic conditions, with unpredictable consequences for the species composition, spatial population shifts, or a restructuring of the food webs involved (e.g. Steele 1998, 2004; Hsieh et al. 2005; Jiao 2009). For example, many Atlantic and Pacific fish stocks exhibit a close correlation with climate patterns over many decades (Klyashtorin 2001). Even small natural climatic changes can have significant effects on the marine ecosystems and their organisms.
Fish parasites can be used as biological indicators for environmental impact and change (Palm 2010). However, their potential to indicate global change scenarios has not been tested. Palm (2010) suggested that oceanic and remote marine ecosystems such as the central Pacific, mid Atlantic, Antarctic or the Polar Sea are the best candidate localities to link an effect of a changing climate directly to aquatic parasite communities at a larger scale. These regions are less affected by factors such as anthropogenic species introduction, pollutants and for example seasonal migrations. Most informative are metazoan helminths as parasite bioindicators because they are embedded within the marine food web and live in oceanic and remote environments. Ascaridoid nematodes combined with larval cestodes and possibly acanthocephalans are useful as biological indicators for host abundance (Palm 2010). The anisakids especially, due to their omnipresence, wide distribution and dependence on the availability of the large predatory final hosts such as seals and whales, are potential candidates to indicate environmental change at a larger scale. According to Marcogliese (2008), warming of coastal waters will result in a higher number of pelagic fish species that follow warmer currents northwards, resulting in increasing Anisakis spp. infection of fish. Other effects are a general shift in host ranges and the introduction of pathogens into formerly uninfected regions.
Climate change might have a direct affect on the parasite species but also indirect effects through changes in the distribution and abundance of their intermediate and final hosts (Marcogliese 2008). Especially parasites with complex life cycles, or those in poikilothermic hosts, may be disproportionately affected by global warming (Marcogliese 2008). Induced ice melting in most northern and southern habitats must have consequences for the polar seal and whale populations, and also their parasites. Under global change scenarios, increased sea surface temperature in the northern Arctic might shrink seal populations, reducing the number of final hosts and consequently the number of worms in the fish. On the other hand, more ice-free waters allow the large migrating whale populations to extend their range of distribution into more northern and southern habitats, into formerly ice-covered regions. It has been demonstrated that A. simplex C and A. pegreffii are at the most southern range of distribution in the Southern Ocean (Klimpel et al. 2010). Higher water temperatures in the high Antarctic might lead to a higher abundance of the whaleworm Anisakis in formerly unrepresented regions. Consequently, while the numbers of the sealworms Pseudoterranova and possibly Contracaecum decrease under shrinking and changing final host populations, other anisakids (Anisakis) might be able to extend their numbers. This would also have consequences for the zoonotic potential of these worms. A higher worm abundance of A. simplex (s.s.) in fish of the North Atlantic and Pacific waters will result in higher transmission rates to humans, and probably cause an increasing conflict potential through the consumption of marine fish.
References
Anadón AM, Romarís F, Escalante M, Rodríguez E, Gárate T, Cuéllar C, Ubeira FM (2009) The Anisakis simplex Anis s 7 major allergen as an indicator of true Anisakis infections. Clin Exp Immunol 156:471–478
Anderson RC (1984) The origins of zooparasitic nematodes. Can J Zool 62:317–328
Anderson RC (1996) Why do fish have so few roundworm (nematode) parasites? Environ Biol Fish 6:1–5
Anderson RC (2000) Nematode parasites of vertebrates – their development and transmission, 2nd edn. CAB International, Wallingford
Audicana MT, Kennedey MW (2008) Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev 21(2):360–379
Audicana MT, Ansotegui IJ, De Corres LF, Kennedey MW (2002) Anisakis simplex: dangerous – dead and alive? Trends Parasitol 18:20–25
Audicana MT, del Pozo MD, Iglesias R, Ubeira FM (2003) Anisakis simplex and Pseudoterranova decipiens. In: Miliotis MD, Bier JW (eds) International handbook of foodborne pathogens. Marcel Dekker, New York, pp 613–636
Blaxter ML (2003) Nematoda: genes, genomes and the evolution of parasitism. Adv Parasitol 54:102–197
Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK (1989) A molecular evolutionary framework for the phylum Nematoda. Nature 392:71–75
Blaxter ML, Dorris M, De Ley P (2000) Patterns and processes in the evolution of animal parasitic nematodes. Nematology 2:43–55
Cho TH, Park HY, Cho S, Sohn J, Yoon YW, Cho JE, Cho SW (2005) The time course of biological and immunochemical allergy states induced by Anisakis simplex larvae in rats. Clin Exp Immunol 143:203–208
Couture C, Measures L, Gagnon J, Desbiens C (2003) Human intestinal anisakiosis due to consumption of raw salmon. Am J Surg Pathol 27:1167–1172
Cross MA, Collins C, Campbell N, Watts PC, Chubb JC, Cunningham CO, Hatfield EMC, MacKenzie K (2007) Levels of intra-host and temporal sequence variation in a large CO1 sub-units from Anisakis simplex sensu stricto (Rudolphi 1809) (Nematoda: Anisakidae): implications for fisheries management. Mar Biol 151:695–702
D’Amelio S, Mathiopoulus KD, Santos C, Pugachev ON, Webb SC, Picanço M, Paggi L (2000) Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: Ascaridoidea) defined by polymerase chain reaction-based restriction fragment length polymorphism. Int J Parasitol 30:223–226
D’Amelio S, Barros NB, Ingrosso S, Fauquier DA, Russo R, Paggi L (2007) Genetic characterization of members of the genus Contracaecum (Nematoda: Anisakidae) from fish-eating birds from west-central Florida, USA, with evidence of new species. Parasitology 134:1041–1051
Dailey MD (2001) Parasitic diseases. In: Dierauf LA, Gulland MD (eds) Marine mammal medicine. CRC Press, Boca Raton, pp 357–379
Daschner A, Alonso-Gomez A, Cabanas R, Suarez-de-Parga JM, Lopez Serrano MC (2000) Gastroallergic anisakiasis: borderline between food allergy and parasitic disease – clinical and allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J Allergy Clin Immunol 105:176–181
De Ley P (2006) A quick tour of nematode diversity and the backbone of nematode phylogeny. In: WormBook (ed) The C. elegans Research Community, WormBook 1.41.1. The C. Elegans Research Community, The C. Elegans Research Community, http://www.WormBook.org
De Ley P, Blaxter ML (2002) Systematic position and phylogeny. In: Lee DL (ed) The biology of nematodes. Taylor & Francis, London, pp 1–30
Des Clers S (1991) Functional relationship between sealworm (Pseudoterranova decipiens, Neamtoda, Ascaridoidea) burden and host size in Atlantic cod (Gadus morhua). Proc R Soc Lond B 245:85–89
Des Clers S, Andersen K (1995) Sealworm (Pseudoterranova decipiens) transmission to fish trawled from Hvaler, Oslofjord, Norway. J Fish Biol 46:8–17
Dorris M, De Ley P, Blaxter ML (1999) Molecular analysis of nematode diversity and the evolution of parasitism. Parasitol Today 15:188–193
Farjallah S, Merella P, Ingrosso S, Rotta A, Slimane BB, Garippa G, Said K, Busi M (2008) Molecular evidence for the occurrence of Contracaecum rudolphii A (Nematoda: Anisakidae) in shag Phalacrocorax aristotelis (Linnaeus) (Aves: Phalacrocoracidae) from Sardinia (western Mediterranean Sea). Parasitol Int 57:437–440
Fuentes V, Barranco R, Sanchez I, Sierra Z, Cabrerizo S, Vicente ME, Rubio M, Baeza ML (2002) Subclinical Anisakis simplex sensitization: a five-year follow up. J Allergy Clin Immunol 109:S218
Hays R, Measures LN, Huot J (1998a) Euphausiids as intermediate hosts of Anisakis simplex in the St. Lawrence estuary. Can J Zool 76:1226–1235
Hays R, Measures LN, Huot J (1998b) Capelin (Mallotus villosus) herring (Clupea harengus) as paratenic hosts of Anisakis simplex, a parasite of beluga (Delphinapterus leucas) in the St. Lawrence estuary. Can J Zool 76:1411–1417
Hsieh CH, Glaser SM, Lucas AJ, Sugihara G (2005) Distinguishing random environmental fluctuations from ecological catastrophes for the North Pacific Ocean. Nature 435:336–340
Ishikura H, Kikuchi K (1990) Intestinal anisakiasis in Japan. Springer, Tokyo
Jiao Y (2009) Regime shift in marine ecosystems and implications for fisheries management, a review. Rev Fish Biol Fish 19:177–191
Kagei A, Isogaki I (1992) A case of acute abdominal syndrome caused by the presence of a large number of Anisakis simplex larvae. Int J Parasitol 22:251–253
Kaneko JJ (1991) Parasite hazards of public health significance to U.S. consumers of raw fish. IAAAM Proc 22:130–134
Kellermanns E, Klimpel S, Palm HW (2007) Molecular identification of ascaridoid nematodes from the deep-sea onion-eye grenadier (Macrourus berglax) from the East Greenland Sea. Deep Sea Res Pt I 54:2194–2202
Kennedey MW (2000) Immune response to Anisakis simplex and other ascarid nematodes. Allergy 55(Suppl 59):7–13
Kijewska A, Rokicki J, Sitko J, Wegrzyn G (2002) Ascaridoidea: a simple DNA assay for identification of 11 species infecting marine and freshwater fish, mammals and fish-eating birds. Exp Parasitol 101:35–39
Klimpel S, Rückert S (2005) Life cycle strategy of Hysterothylacium aduncum to become the most abundant anisakid fish nematode in the North Sea. Parasitol Res 97:141–149
Klimpel S, Palm HW, Rückert S, Piatkowski U (2004) The life cycle of Anisakis simplex in the Norwegian Deep (northern North Sea). Parasitol Res 94:1–9
Klimpel S, Kellermanns E, Palm HW, Moravec F (2007) Zoogeography of fish parasites of the pearlside (Maurolicus muelleri), with genetic evidence of Anisakis simplex (s.s.) from the Mid-Atlantic Ridge. Mar Biol 152:725–732
Klimpel S, Kellermanns E, Palm HW (2008) The role of pelagic swarm fish (Myctophidae: Teleostei) in the oceanic life cycle of Anisakis sibling species at the Mid-Atlantic Ridge, Central Atlantic. Parasitol Res 104:43–53
Klimpel S, Busch MW, Kuhn T, Rohde A, Palm HW (2010) The Anisakis simplex complex off the South Shetland Islands (Antarctica): endemic populations versus introduction through migratory hosts. Mar Ecol Prog Ser 403:1–11
Klöser H, Plötz J, Palm H, Bartsch A, Hubold G (1992) Adjustment of anisakid nematode life cycles to the high Antarctic food web as shown by Contracaecum radiatum and C. osculatum in the Weddell sea. Antarct Sci 4:171–178
Klyashtorin L (2001) Climate change and long-term fluctuations of commercial catches. The possibility of forecasting. FAO, Rome
Køie M (2001) Experimental infections of copepods and sticklebacks Gasterosteus aculeatus with small ensheathed and large third-stage larvae of Anisakis simplex (Nematoda, Ascaridoidea, Anisakidae). Parasitol Res 87:32–36
Køie M, Fagerholm HP (1995) The life cycle of Contracaecum osculatum (Rudolphi, 1802) sensu stricto (Nematoda, Ascaridoidea, Anisakidae) in view of experimental infections. Parasitol Res 81:481–489
Køie M, Berland B, Burt MDB (1995) Development to third-stage larvae occurs in the eggs of Anisakis simplex and Pseudoterranova decipiens (Nematoda, Ascaridoidea, Anisakidae). Can J Fish Aquat Sci 52:134–139
Kuhn T (2010) Molecular studies on marine ascaridoid nematodes-Diploma thesis, Heinrich Heine University, Düsseldorf
Levsen A, Lunestad BT (2010) Anisakis simplex third stage larvae in Norwegian spring spawning herring (Clupea harengus L.), with emphasis on larval distribution in the flesh. Vet Parasitol 171(3-4):247–253. doi:10.1016/j.vetpar.2010.03.039
Li A, D’Amelio S, Paggi L, He F, Gasser RB, Lun Z, Abollo E, Turchetto M, Zhu X (2005) Genetic evidence for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) and the validity of Contracaecum septentrionale (Kreis, 1955) (Nematoda: Anisakidae). Parasitol Res 96:361–366
Lile NK (1998) Alimentary tract helminths of four pleuronectid flatfish in relation to host phylogeny and ecology. J Fish Biol 53:945–953
Marcogliese DJ (2008) The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev Sci Tech 27:467–484
Marques JF, Cabral HN, Busi M, D’Amelio S (2006) Molecular identification of Anisakis species from Pleuronectiformes off the Portuguese coast. J Helminthol 80:47–51
Mattiucci S, Nascetti G (2006) Molecular systematics, phylogeny and ecology of anisakid nematodes of the genus Anisakis Dujardin, 1845: an update. Parasite 13:99–113
Mattiucci S, Nascetti G (2008) Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host–parasite co-evolutionary processes. Adv Parasitol 66:47–148
Mattiucci S, Nascetti G, Cianchi R, Paggi L, Arduino P, Margolis L, Brattey J, Webb SC, D’Amelio S, Orecchia P, Bullini L (1997) Genetic and ecological data on the Anisakis simplex complex with evidence for a new species (Nematoda, Ascaridoidea, Anisakidae). J Parasitol 83:401–416
Mattiucci S, Paggi L, Nascetti G, Portes SC, Costa G, Di Beneditto AP, Ramos R, Argyrou M, Cianchi R, Bullini L (2002) Genetic markers in the study of Anisakis typica (Diesing, 1890): larval identification and genetic relationships with other species of Anisakis Dujardin, 1845 (Nematoda: Anisakidae). Syst Parasitol 51:159–170
Mattiucci S, Nascetti G, Dailey M, Webb SC, Barros N, Cianchi R, Bullini L (2005) Evidence for a new species of Anisakis Dujardin, 1845: morphological description and genetic relationships between congeners (Nematoda: Anisakidae). Syst Parasitol 61:157–171
Mattiucci S, Abaunza P, Damiano S, Garcia A, Santos MN, Nascetti G (2007) Distribution of Anisakis larvae identified by genetic markers and their use for stock characterization of demersal and pelagic fish from European waters: an update. J Helminthol 81:117–127
Mattiucci S, Paoletti M, Webb SC, Sardella N, Timi JT, Berland B, Nascetti G (2008a) Genetic relationships among species of Contracaecum Railliet and Henry, 1912 and Phocascaris Höst, 1932 (Nematoda: Anisakidae) from pinnipeds based on mitochondrial cox2 sequences, and congruence with allozyme data. Parasite 15:408–419
Mattiucci S, Paoletti M, Olivero-Verbel J, Baldiris R, Arroyo-Salgado B, Garbin L, Navone G, Nascetti G (2008b) Contracaecum bioccai n. sp. from the brown pelican Pelecanus occidentalis (L.) in Colombia (Nematoda: Anisakidae): morphology, molecular evidence and its genetic relationship with congeners from fish-eating birds. Syst Parasitol 69(2):101–121
Mattiucci S, Paoletti M, Webb SC (2009) Anisakis nascettii n.sp. (Neamtoda: Anisakidae) from beaked whales of the southern hemisphere: morphological description, genetic relationships between congeners and ecological data. Syst Parasitol 74:199–217
McClelland G (1990) Larval sealworm (Pseudoterranova decipiens) infections in benthic macrofauna. In: Bowen WD (ed) Population biology of sealworm (Pseudoterranova decipiens) in relation to its intermediate and seal hosts. Canadian Bulletin of Fisheries & Aquatic Sciences, Ottawa, pp 47–65
McClelland G (2002) The trouble with sealworms (Pseudoterranova decipiens species complex, Nematoda): a review. Parasitology 124:S183–S203
McClelland G (2005) Nematoda (roundworms). In: Rohde K (ed) Marine parasitology. CABI Publishing, Wallingford, pp 104–115
Meldal BHM, Debenham NJ, De Ley P, De Ley IT, Vanfleteren JR, Vierstraete AR, Bert W, Borgonie G, Moens T, Tyler PA, Austen AR, Blaxter ML, Rogers AD, Lambshead PJD (2007) An improved molecular phylogeny of the Nematoda with special emphases on marine taxa. Mol Phylogenet Evol 42:622–636
Mitchell GF (1991) Co-evolution of parasites and adaptive immune response. Immunol Today 12:A2–A5
Nadler SA, D’Amelio S, Dailey MD, Paggi L, Siu S, Sakanari JA (2005) Molecular phylogenetics and diagnosis of Anisakis, Pseudoterranova, and Contracaecum from Northern Pacific marine mammals. J Parasitol 91:1413–1429
Palm HW (1999) Ecology of Pseudoterranova decipiens (Krabbe, 1878) (Nematoda: Anisakidae) from Antarctic waters. Parasitol Res 85:638–646
Palm HW (2004) The Trypanorhyncha diesing, 1863. PKSPL-IPB Press, Bogor
Palm HW (2010) Fish parasites as biological indicators in a changing world: Can we monitor environmental impact and global change? Springer, Berlin
Palm HW, Klimpel S (2007) Evolution of parasitic life in the ocean. Trends Parasitol 23:10–12
Palm HW, Klimpel S (2008) Metazoan fish parasites of Macrourus berglax Lacepéde, 1801 and other macrourids of the North Atlantic: invasion of the deep sea from the continental shelf. Deep Sea Res Pt II 55:236–242
Palm HW, Andersen K, Klöser H, Plötz J (1994) Occurrence of Pseudoterranova decipiens (Nematoda) in fish from the southeastern Weddell Sea (Antarctic). Polar Biol 14:539–544
Palm HW, Reimann N, Spindler M, Plötz J (1998) The role of the rock cod Notothenia coriiceps in the life cycle of Antarctic parasites. Polar Biol 19:399–406
Palm HW, Klimpel S, Walter T (2007) Demersal fish parasite fauna around the South Shetland Islands: high species richness and low host specificity in deep Antarctic waters. Polar Biol 30:1513–1522
Palm HW, Damriyasa IM, Linda OIBM (2008) Molecular genotyping of Anisakis Dujardin, 1845 (Nematoda: Ascaridoidea: Anisakidae) larvae from marine fish of Balinese and Javanese waters, Indonesia. Helminthologia 45:3–12
Parkinson J, Mitreva M, Whitton C, Thomson M, Daub J, Martin J, Schmid R, Hall N, Barrell B, Waterston RH, McCarter JP, Blaxter ML (2004) A transcriptomic analysis of the phylum Nematoda. Nat Genet 36:1259–1267
Petersen F, Palm HW, Möller H, Cuzi MA (1993) Flesh parasites of fish from central Philippine waters. Dis Aquat Org 15:81–86
Plath F, Holle A, Zendeh D, Möller FW, Barten M, Reisinger EC, Liebe S (2001) Anisakiasis des Magens – ein Fallbericht aus Deutschland. Z Gastroenterol 39:177–180
Pontes T, D’Amelio S, Costa G, Paggi L (2005) Molecular characterization of larval anisakid nematodes from marine fishes of Madeira by a PCR-based approach, with evidence for a new species. J Parasitol 91:1430–1434
Rahmstorf S, Cazenave A, Church JA, Hansen JE, Keeling RF, Parker DE, Somerville RCJ (2007) Recent climate observations compared to projections. Science 316:709
Rohde K (1984) Diseases caused by Metazoans: helminths. In: Kinn O (ed) Diseases of marine animals, IV, Part 1st edn. Biologische Anstalt Helgoland, Hamburg, pp 193–319
Sakanari JA, McKerrow JH (1989) Anisakiasis. Clin Microbiol Rev 2:278–284
Shamshi S, Norman R, Gasser R, Beveridge I (2009a) Redescription and genetic characterization of selected Contracaecum spp. (Nematoda: Anisakidae) from various hosts in Australia. Parasitol Res 104:1507–1525
Shamshi S, Norman R, Gasser R, Beveridge I (2009b) Genetic and morphological evidences for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) Nematoda: Anisakidae) in Australia. Parasitol Res 105:829–838
Smith JW (1999) Ascaridoid Nematodes and pathology of the alimentary tract and its associated organs in vertebrates, including man: a literature review. Helminthol Abstr 68:49–96
Steele JH (1998) Regime shifts in marine ecosystems. Ecol Appl 8:33–36
Steele JH (2004) Regime shifts in the ocean: reconciling observations and theory. Prog Ocean 60:135–141
Szostakowska B, Myjak P, Kur J (2002) Identification of aniskaid nematodes from the Southern Baltic Sea using PCR-based methods. Mol Cell Probes 16:111–118
Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM (2005) Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol 174:4924–4933
Torres P, Sierpe V, Schlatter R (1983) Occurrence of Contracaecum rudolphii in new hosts in Chile. Z Parasitenkd 69:397–399
Valentini A, Mattiucci S, Bondanelli P, Webb SC, Mignucci-Giannone A, Colom-Llavina MM, Nascetti G (2006) Genetic relationships among Anisakis species (Nematoda: Anisakidae) inferred from mitochondrial cox2 sequences, and comparison with allozyme data. J Parasitol 92:156–166
Van Thiel PH (1962) Anisakiasis. Parasitology 52:16–17
Wijova M, Moravec F, Horak A, Lukes J (2006) Evolutionary relationships of Spirurina (Nematoda: Chromadorea: Rhabditida) with special emphasis on dracunculoid nematodes inferred from SSU rRNA gene sequences. Int J Parasitol 36:1067–1075
Williams H, Jones A (1994) Parasitic worms of fish. Taylor & Francis, London
Zhu XQ, Gasser RB, Jacobs DE, Hung GC, Chilton NB (2000a) Relationships among some ascaridoid nematodes based on ribosomal DNA sequence data. Parasitol Res 86:738–744
Zhu XQ, D’Amelio S, Paggi L, Gasser RB (2000b) Assessing sequence variation in the internal transcribed spacers of ribosomal DNA within and among members of the Contracaecum osculatum complex (Nematoda: Ascaridoidea: Anisakidae). Parasitol Res 86:677–683
Zhu X, D’Amelio S, Hu M, Paggi L, Gasser RB (2001) Electrophoretic detection of population variation within Contracaecum ogmorhini (Nematoda: Ascaridoidea: Anisakidae). Electrophoresis 22:1930–1934
Zhu XQ, D’Amelio S, Palm HW, Paggi L, George-Nascimento M, Gasser RB (2002) SSCP-based identification of members within the Pseudoterranova decipiens complex (Nematoda: Ascaridoidea: Anisakidae) using genetic markers in the internal transcribed spacers of ribosomal DNA. Parasitology 124:615–623
Acknowledgements
The present study was financially supported by the German Research Council (DFG KL 2087/1-1, 1–2), by the Research and Innovation funds of the Heinrich-Heine-University Düsseldorf, and the Gesellschaft für Ichthyologie e.V. (GiF). Dr. R. Bray (The Natural History Museum London) provided helpful comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Klimpel, S., Palm, H.W. (2011). Anisakid Nematode (Ascaridoidea) Life Cycles and Distribution: Increasing Zoonotic Potential in the Time of Climate Change?. In: Mehlhorn, H. (eds) Progress in Parasitology. Parasitology Research Monographs, vol 2. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-21396-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-21396-0_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-21395-3
Online ISBN: 978-3-642-21396-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)