Abstract
It is estimated that around 170 Tg N year−1 of reactive nitrogen (N) is produced on a global basis by industry, fossil fuel burning, and biological N fixation. It is applied to land both deliberately to help produce enough food and fiber, and indirectly via atmospheric deposition as pollution. These human interventions to the N cycle, which is naturally highly conservative to loss pathways, are contributing to climate change effects, for example, by enhancing nitrous oxide (N2O) emissions. Nitrogen-driven carbon (C) storage through increased plant growth in non-forested or agricultural systems may be modest, but increased N deposition has been shown to substantially increase carbon dioxide (CO2) uptake by certain forests. Climate change drivers such as elevated CO2 and temperature can further influence the terrestrial C and N cycling and alter soil N availability, which constrains the CO2 sink capacity of earth’s biosphere. In this chapter, an attempt has been made to evaluate the impacts of anthropogenic drivers on the terrestrial N cycle with implications for soil health and climate change. Consequences of changes in soil health parameters such as N availability, carbon sequestration, and acidification in relation to managing fertilizer N use in agro-ecosystems with the aim of increasing productivity but reducing greenhouse gas emissions have been discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Nitrogen (N) is the primary nutrient limiting plant growth throughout the world. While 78.08% of the earth’s atmosphere is N, this is not readily available for use by the majority of living organisms, because the N2 molecules do not easily enter into chemical reactions. The exception to this is biological N fixation, which until the preindustrial age was the only process where N2 molecules were converted to reactive N forms, such as ammonium (NH +4 ) and nitrate (NO −3 ). Biological N fixation is a process undertaken by bacteria, living in soil or symbiotically within plant root nodules. However, in the postindustrial age, reactive N is increasingly being added through manufactured fertilizers, which accounts for more than half of the annual amount of N fixed by human activities (Schlesinger 2009). Burning of fossil fuels where the air becomes so hot that the N2 molecules break apart constitutes another anthropogenic-driven N addition to terrestrial ecosystems. The N, which is lost from soils in gaseous forms to the atmosphere, can also redistribute across the landscape via wet and dry deposition.
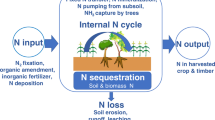
A large amount of soil N exists in organic forms, which depending upon carbon (C)-to-N ratios and the chemical composition of the soil organic matter can be mineralized by microbes, transforming it into plant available forms, NH +4 and NO −3 (mineralization) or immobilizing the mineral N forms to organic forms (immobilization). During microbially mediated processes of nitrification and denitrification, the reactive N is lost from soil as nitrous oxide (N2O), a powerful greenhouse gas. The N cycle (Fig. 6.1) is completed through the process of denitrification, in which many soil microorganisms use NO −3 as the electron acceptor and return N2 molecules to the atmosphere, especially under highly anaerobic conditions (see Chap. 8). Losses of reactive N from the soil can also occur via leaching as NO −3 , runoff, erosion, and ammonia volatilization (Fig. 6.1).
The contribution to climate change by human-induced changes in the N cycle at regional and global scales is becoming more widely acknowledged. Concurrently, controlling the impact of agriculture on N cycling and vice versa is a growing challenge for sustainable development (Gruber and Galloway 2008). Increasing temperatures and atmospheric CO2 levels, the two components of climate change, also influence a number of pathways in the terrestrial N cycle. For example, Rustad et al. (2000) reported that an increase in atmospheric temperature leads to significant overall increase in N mineralization and nitrification in terrestrial ecosystems. On the other hand, the direct influence of increasing atmospheric CO2 is mainly limited to plant leaves (photosynthesis, stomatal aperture, and perhaps respiration), but there may be indirect effects on soil N transformations, mediated through the changes in the aboveground biomass and belowground C allocation (Koch and Mooney 1996). For example, Müller et al. (2009) observed that gross N mineralization and immobilization were increased in a temperate grassland soil under elevated CO2 while nitrification was decreased. Thus, the effects of climate change on soil N transformations can be complex, and the long-term implications on N retention and N use efficiency are unclear.
The application of synthetic N fertilizers together with the development and introduction of rice and wheat cultivars has helped food production to keep pace with human population growth since the late 1960s. However, excessive use of N fertilizers can negatively influence soil properties, leading to decreased levels of exchangeable Ca, Mg, and K, reduced effective cation exchange capacity and acidification of soil, along with increased N leaching and production of N2O. Management practices, such as the introduction of legumes into crop rotations, tillage practices, and stubble retention versus incorporation, also influence N availability and supply in agro-ecosystems, thereby providing significant implications for soil fertility and the environment.
In this chapter, an attempt has been made to evaluate the impacts of anthropogenic drivers (such as climate change, N deposition, and land management) on the terrestrial N cycle along with implications for soil health and climate change. Consequences of changes in soil health parameters such as N availability, C sequestration, and acidification in relation to managing fertilizer N use in agro-ecosystems with the aim of increasing N use efficiency and crop productivity but reducing greenhouse gas emissions have been discussed.
2 Nitrogen in the Biosphere
Globally, the creation of reactive N has increased nearly 50% between 1890 and 1990 (Galloway and Cowling 2002) and continues to increase every year. Although the creation of reactive N is dominated by agricultural activities, via the production and consumption of N fertilizers and N fixation by legumes, production of energy from fossil fuels also plays an important role (Table 6.1). The residence time of reactive N in vegetation and soils varies widely (ten to several hundred years) depending on the type of vegetation and its age. The residence time of reactive N affects the permanence of the biospheric N sink. An adequate understanding of the fate of the reactive N in the biosphere is lacking; mass-balance studies of agricultural fields indicate that more than 50% of N in synthetic fertilizers escapes from the point of application (Deutsch et al. 2007). Globally, ~10% of the N applied as fertilizer is contained in food (Galloway and Cowling 2002); most of the remaining reactive N is lost to the environment during food production. Some of the N applied to agricultural land becomes unavailable to crop and pasture species through immobilization processes and can remain in the soil for long periods of time (Jagadamma et al. 2007).
Increasing emissions of nitrogen oxide (NO) from fossil fuel burning and NH3 emissions associated with intensification of agriculture has led to a three- to five-fold increase in N emissions over the last century (Denman et al. 2007). Global NO and NH3 emissions are mainly terrestrial in origin. In the year 2000, 52.1 Tg N year−1 of NO and 64.6 Tg N year−1 of NH3 were emitted with between 30 and 50% of NO and around 40% of NH3 deposited on the open ocean and coastal zones, while the rest is believed to have been distributed across terrestrial ecosystems (Dentener et al. 2006). Both NO and NH3 emissions are predicted to increase further in many regions during the twenty-first century due to the growth of the global population leading to increased demand for food, particularly animal protein (Dentener et al. 2006). By 2100, N deposition over land may increase by a factor of 2.5 (Lamarque et al. 2005). Along with emissions of NOx from fossil fuel combustion, gaseous forms of N emanating from agricultural soils produce an N enrichment of natural ecosystems, with documented effects on forests, deserts, grasslands, coastal ecosystems, and oceans (Boulart et al. 2006; Brooks 2003; Scavia and Bricker 2006).
It is estimated that 9 Tg N year−1 may be accumulating in the terrestrial biosphere in pools with residence times of ten to several hundred years (Schlesinger 2009). Atmospheric deposition of N on land has increased due to anthropogenic activities by ~46 Tg N year−1 (Galloway et al. 2004), with about one-third of the increase deposited in forests (18 Tg N year−1) (Hudson et al. 1994), where trees provide a long-term sink for N in biomass. Studies indicate that ~25–30% of the N applied as fertilizer or through N deposition is retained in tree biomass (Nadelhoffer et al. 1999), and a similar amount is retained in forest soils (Schlesinger 2009). If ~50% of N deposition is stored within the ecosystem, then ~9 Tg N year−1 might be sequestered in terrestrial woody biomass (Schlesinger 2009). Some of this N is returned to the atmosphere as NOx or N2O when woody biomass is burned. As summarized by Schlesinger (2009), anthropogenic N applied to land as fertilizer and as N deposition is ~124 Tg N year−1. The current estimate of reactive N produced by industry, biological nitrogen fixation, and from fossil fuel combustion is ~170 Tg N year−1 (Table 6.1). The budget for the terrestrial portion of the N cycle is thus not balanced and is thus linked with several serious environmental problems, such as increasing the greenhouse effect, reducing the protective ozone layer, adding to smog, contributing to acid rain, and contaminating drinking water.
3 Nitrogen Deposition, Soil Health, and Climate Change
The response of terrestrial ecosystems to N depositions can be interpreted to some extent from the conceptual “nitrogen saturation” model described by Aber et al. (1989). The N-limited systems initially retain the deposited N by using it for plant and microbial growth as well as via accumulation in plant biomass and soil organic matter. However, when inputs of N begin to exceed the biotic demands within the ecosystem, the excess N can be potentially lost via leaching and gaseous emissions (Matson et al. 2002). The nitrogen saturation model of Aber et al. (1989) predicts an eventual decrease in net primary production, occurring due to the loss of cations and nutrient imbalances. Plants may be less important than soils in retaining N when inputs are relatively low, especially in the range expected from current levels of atmospheric N depositions. However, chronic N additions may increase rates of soil N mineralization because of the incorporation of additional N into organic matter that could decrease its C:N ratio (Aber et al. 1998).
As soils in most agro-ecosystems already receive substantial inputs of N as fertilizer to achieve better crop yield, additional input through N deposition would not be expected to have a large effect on the cycling and sequestration of C in plant biomass or soil (Reay et al. 2008), unless N fertilization strategies are revised to account for additional N input through atmospheric deposition. Whereas, the unintended fertilization of forest ecosystems in the form of atmospheric N deposition has not only stimulated forest growth, but also affected soil microbial activity, and the cycling of C and nutrients in soils (Janssens et al. 2010).
Forest ecosystems contain a large part of the terrestrial C store, and also control the transfer of C between the atmosphere and the soil (Dixon et al. 1994). Since net primary production in both temperate and tropical forests is strongly N limited (LeBauer and Treseder 2008; Vitousek and Howarth 1991), even a small stimulation in tree growth rate in forest ecosystems due to an increase in the reactive N supply may cause a large change in the C sink capacity of forests. Nevertheless, a large amount of CO2 that is taken up from the atmosphere by forest trees through photosynthesis are returned rapidly (back to the atmosphere) through respiration by roots and decomposer communities in soil (Bhupinderpal-Singh et al. 2003; Högberg et al. 2001; Högberg et al. 2008). Since C stocks in soil exceed those in vegetation by about 2:1 in northern temperate forests to over 5:1 in boreal forests (Dixon et al. 1994; Schlesinger 1997), changes in soil C stocks can be more important than changes in vegetation C stocks for a positive forest C budget (Medlyn et al. 2005).
The response of soil C to changing N deposition will be dictated by the balance between N-induced increases in C inputs, due to increased plant growth, and C outputs via soil organic C decomposition and microbial respiration (see Chaps. 5 and 7). However, only a few experiments have studied the effects of long-term additions of small amounts of N on soil organic matter decomposition and microbial respiration (Janssens et al. 2010). A meta-analysis by Knorr et al. (2005a) indicated that litter decomposition was stimulated with N deposition at sites containing high quality (low-lignin) litters, whereas it was decreased at sites containing low-quality (typically high-lignin) litters. Fog (1988) observed a positive relationship between C:N ratio and CO2 evolution rate in the litter and humus layers in European forests, but no clear relationship was observed in the mineral soil (Persson et al. 2000). In two Swedish forest fertilization experiments, Persson et al. (2000) added N annually for up to 27 years. A 30% reduction in the mineralization rate was observed in the mor (litter) layer where N had been applied at a rate of 60 kg ha−1 year−1 in comparison to unfertilized plots. Similarly, Franklin et al. (2003) showed that 100 years of fertilization with N applied at a rate of 30 kg ha−1 year−1 could result in a doubling (1.3 kg C m−2) of the amount of C stored in the mor layer. About 60% of this increase is estimated to be the result of decreased decomposition rate and the rest a result of increased litter production. A recent meta-analysis carried out by Janssens et al. (2010) also suggests that N deposition impedes organic matter decomposition and promotes C retention in temperate forest soils where N is not limiting microbial growth. According to Ågren et al. (2001), the retention of more C in N-fertilized forest soils may result from decreased growth rate of decomposers due to formation of recalcitrant compounds. The N-induced decrease in decomposition rate may also result from increased decomposer’s C-use efficiency (production-to-assimilation ratio) (Ågren et al. 2001; Franklin et al. 2003).
Conversely, anthropogenic N deposition can have a detrimental impact on terrestrial ecosystems through soil acidification and a consequential reduction in plant biodiversity (Galloway et al. 2003). During the acidification process, soils release base cations, such as calcium and magnesium, neutralizing the increase in acidity. However, over time and with continued addition of N, the base cations can be depleted, at which time aluminum (Al3+) is released from soil minerals, often reaching toxic levels. The acidification response may depend on the form of N added, the net balance between proton-producing and consuming processes, and the buffering capacity of the soil (Uehara and Gillman 1981). Soil acidification leads to reduced microbial N immobilization (Venterea et al. 2004). Most temperate-zone soils are buffered by base cations, which are replaced by Al3+ at pH ranges below pH 4.5, while many tropical soils are highly weathered, depleted in primary minerals and poorly buffered (Uehara and Gillman 1981), and hence these soils may be prone to acidification through N additions.
4 Interaction Between N and C Cycling in Relation to Climate Change
With human activities exerting so much impact on different systems of the planet, the interactions between the N cycle, the C cycle, and climate are expected to become an increasingly important determinant of the earth system (Gruber and Galloway 2008). Although understanding of the consequences of human alteration of the N cycle, and possible strategies to manage these, has increased over the last two decades, not enough emphasis has been placed on the study of the interactions of N cycling with that of C, and how these cycles interact with the climate system. In the recent past, the C cycle-climate change models generally assumed a strong CO2 fertilization effect and did not consider N limitation of the terrestrial biosphere. Thus, these models may have overestimated the ability of the terrestrial biosphere to act as a CO2 sink in the future (Thornton et al. 2009) and predict a slow rise in the atmospheric CO2 and a slow rate of climate change (Hungate et al. 2003). Furthermore, N limitation may become more pronounced in some ecosystems as atmospheric CO2 concentration increases (Luo et al. 2004; Reich et al. 2006a). On the other hand, future increase in temperature may enhance soil N mineralization, thereby counteracting any adverse effects of elevated CO2 on N availability (Hovenden et al. 2008). Additionally, interactions between C:N ratios in plants and soils, increased soil fertility, and microbial activity are also not very well understood and may need to be addressed in climate models. Thus the nature and importance of N–C-climate interactions are becoming increasingly pressing. The central question is: how will the availability of N affect the capacity of earth’s biosphere to continue to absorb C from the atmosphere and hence mitigate climate change (Gruber and Galloway 2008)? However, increased supply of fertilizer N for food production may also have unintended negative environmental consequences such as elevated levels of N2O production and increasing eutrophication of waterways. Thus, a tight coupling between N availability and plant uptake is desired and necessary to prevent detrimental environmental impacts.
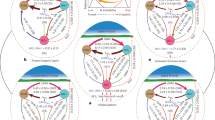
The concentration of N2O in the atmosphere is primarily determined by the magnitudes of nitrification and denitrification in soil – the two central microbial processes of the N cycle that result in gaseous emissions of N from soil. Over the past 60,000 years, close correspondence between atmospheric CO2 levels, temperature, and atmospheric N2O concentrations demonstrate that the N cycle is closely coupled to variations in the climate system and C cycle (Gruber and Galloway 2008). Nevertheless, anthropogenic alterations of the global C and N cycles appear to have led to the highest atmospheric concentrations of CO2 and N2O in 650,000 years (Forster et al. 2007). A significant correlation between the flux of CO2 and N2O from soils has been reported by Xu et al. (2008). The N2O flux from soil may increase globally with rising atmospheric CO2 and temperature, because these climate change drivers can alter N mineralization–immobilization turnover processes (Hoyle et al. 2006; Müller et al. 2009), and in turn nitrification and denitrification (Fig. 6.2).
Potential nitrogen–carbon-climate interactions. The main anthropogenic drivers of these interactions during the twenty-first century are shown. Plus signs indicate that the interaction increases the amount of the factor shown; minus signs indicate a decrease; question marks indicate an unknown impact (or, when next to a plus or minus sign, they indicate a high degree of uncertainty). Arrow thickness denotes strength of interaction. Only selected interactions are shown (adapted by permission from Macmillan Publishers Ltd: Nature, Gruber and Galloway (2008))
Terrestrial C stocks have been altered by increasing atmospheric CO2 concentration and N deposition, as well as by changing land use (Matson et al. 2002; see Chap. 1). In 2005, agriculture accounted for an estimated emission of 5.1–6.1 Gt CO2-eq year−1, which is 10–12% of total global anthropogenic emissions of greenhouse gases (Smith et al. 2007a). There are four main global sinks for these emissions: the atmosphere, the oceans, tropical vegetation, and temperate and boreal vegetation, mainly forests. It has long been recognized that N limitations often constrain C accumulations in mid- and high-latitude ecosystems, such as temperate and boreal forests (Tamm et al. 1982). Deposition of N to forests ranges between 1 and 100 kg ha−1 year−1; the smaller amounts generally occur in remote forests in rural areas at high latitudes and the large amounts in industrialized central Europe (Jarvis and Fowler 2001). However, there exists uncertainty as to the extent and for how long high annual rates of N additions will be able to stimulate the production of mature forests. Recent findings on plant responses to elevated CO2 concentrations also confirm that low N availability can constrain C sequestration in terrestrial ecosystems (Luo et al. 2004; Reich et al. 2006b).
Thornton et al. (2007) and Sokolov et al. (2008) conducted modeling studies by introducing prognostic C and N cycle interactions in the stand-alone land surface component of an atmosphere-ocean general circulation model (AOGCM) and in a reduced-complexity climate model. They observed that the land-atmosphere components of the global climate-C cycle feedback are fundamentally influenced by C–N cycle interactions. Thornton et al. (2007) demonstrated that N limitation significantly reduced the stimulation of net C uptake on land associated with increased CO2 concentration in the atmosphere. This should lead to predictions of higher CO2 concentration in the atmosphere for a given level of fossil fuel consumption in a coupled climate-C cycle simulation. Studies carried out by Thornton et al. (2007) and Sokolov et al. (2008) predict a 53–78% reduction in the effect of elevated CO2 on land C sink strength due to C–N coupling. Thornton et al. (2007) showed that C–N interaction fundamentally alters the terrestrial C cycle response to interannual variability in temperature and precipitation. Sokolov et al. (2008) found that the introduction of C–N coupling in a reduced-complexity climate model produced a change in the sign of the terrestrial C cycle response to warming, switching from a strong positive feedback in which warming leads to a net release of C from the terrestrial biosphere, to a weak negative feedback in which warming leads to a modest uptake of C aided by increased N mineralization. More recently, Thornton et al. (2009) demonstrated a weaker dependence of net terrestrial C flux on soil moisture changes and a stronger positive growth response to warming in tropical regions, than that predicted by a similar AOGCM model implemented without terrestrial C–N interactions.
5 Nitrogen in Agro-Ecosystems, Soil Health, and Climate Change
5.1 Nitrogen Cycling in Natural Versus Managed Ecosystems
An understanding of soil N turnover in natural or unmanaged ecosystems can provide clues about how to efficiently manage N fertilization while maintaining soil health and crop production. In natural ecosystems, atmospheric deposition and biological N2 fixation constitute the external sources of N which then is returned to the soil either as plant litter or as residues from herbivore-based food chain. The accumulation of organic forms of N in soil is a characteristic feature of unmanaged terrestrial ecosystems. Mineral N released through mineralization–immobilization turnover can be taken up by plant roots or is lost from the system. Natural ecosystems often exhibit a high degree of temporal and spatial synchrony and synlocation between the release and uptake of N by mixed plant communities. This results in a minimal transfer across system boundaries relative to the extent of N cycling within the ecosystem, consequently leading to minimal losses via nitrate leaching or gaseous emissions (Christensen 2004). On the other hand, agro-ecosystems are often relatively open with respect to N cycling. In agro-ecosystems, besides inputs of N through deposition and biological N2 fixation, N is also applied externally as chemical fertilizers or organic manures to compensate for the N removed in exported in harvested yield. In modern agro-ecosystems, the removal of as much as 300 kg N ha−1 in harvested aboveground portions of the crops each year necessitates substantial inputs of N to maintain productivity (Cassman et al. 2002). The intensive nature of agricultural cropping systems exhibits large N uptake during active but often relatively short growth phase. If the supply of N in synthetic fertilizers is not sufficient to meet crop demands in agro-ecosystems, soils can become N depleted. Reduced synlocation and synchrony in the N turnover and reduced return of organic matter to the soil adversely affect N use efficiency and soil health in managed agro-ecosystems. Furthermore, changes in hydrology through irrigation and drainage, and changes in soil structure through tillage, can further change the N dynamics within agro-ecosystems in comparison to those of natural systems.
5.2 Fertilizer N Use Effects on Soil Health in Agro-Ecosystems
One of the most important soil health parameters in relation to both climate change and N management is organic matter content of the soil. It is in fact a key indicator of soil health because it plays a role in a number of vital functions affecting soil fertility, productivity, and the environment. Managing soil organic matter for a maximum contribution to soil health and resilience presents a conundrum. Decomposition and mineralization of organic matter are required for functions such as providing energy and nutrients, whereas maintaining or increasing the quantity of organic matter can have positive effects on chemical, physical, and biological properties of soil (see Chaps. 2 and 5).
Maintenance of soil organic matter is an important goal in agriculture, both in terms of sustaining soil fertility and sequestering atmospheric CO2. As C is sequestered in a soil, the availability of N can decrease as N binds with the organic matter and precluding it from participation in the N cycle. Thus, the role of C:N ratio in soil organic matter should also be considered in assessing fertilizer N use efficiency and designing long-term N management strategies. With growing dependence on chemical N fertilizer, the assertion has often been made that these inputs are a positive factor in maintaining or increasing soil organic matter as higher yields enhance the input of crop residues. Such a view is, however, at odds with the long-term changes in soil C reported for the Morrow Plots, the world’s oldest experimental site under continuous corn (Khan et al. 2007). For example, after 40–50 years of applying chemical fertilizers that exceeded corn N removal by 60–190%, a net decline in soil C occurred despite the incorporation of residues, which is consistent with the data from numerous cropping experiments involving synthetic N fertilization (Khan et al. 2007). These data implicate fertilizer N in promoting the decomposition of crop residues and soil organic matter through the stimulation of microbial activity. Several contrary reports are also available in the literature, which show that N fertilization increases soil organic C sequestration in certain ecosystems (Khan et al. 2007). According to Khan et al. (2007), such contradictory observations may be due to the following reasons: (1) absence of baseline data in assessing soil organic C changes; (2) soil organic C data represent only a very limited depth of surface soil; or (3) the study period is inadequate for detecting the changes. Furthermore, fertilizer N applications may reduce microbial activity if acidity generated during NH +4 oxidation is not controlled (Vanotti et al. 1997), thereby leading to a decline in the rate of organic matter mineralization. Nitrogen additions in certain ecosystems have also been shown to adversely affect the activity of lignin-degrading microorganisms or enhance lignin-N complexes resistant to decomposition, thereby favoring organic matter accumulation in soil (Fog 1988).
To fully realize the potential benefits of N fertilizers, application rates must be adequate but not excessive, so as to maximize the economic profitability of crop production while minimizing microbial oxidation of residue C and native soil organic matter. This strategy merits serious consideration because soils hold more than twice as much C as the atmosphere, and even a minor change in terrestrial CO2 balance could have a significant global impact (Powlson 2005). After reviewing data from long-term experiments worldwide to quantify the long-term impacts of N fertilizers on soil N cycling, Glendining and Powlson (1995) concluded that N applications increased N recycling from crop residues, root turnover, and exudates. Initially, mainly labile fractions of soil N were influenced from these inputs, but over years and decades the fractions of soil organic N that cycle more slowly were also affected. Soil productivity can be enhanced both through N fertilization and by growing legumes in rotation, although the two management strategies may have different influences on nutrient cycling and soil health (Liebig et al. 2002).
Logically, soil should gain N if fertilizer inputs exceed grain removal. However, Mulvaney et al. (2009) recorded a decline in total soil N similar to that recorded in soil organic C in the Morrow plots (Khan et al. 2007). This decline is in agreement with numerous long-term baseline data sets from chemical-based cropping systems involving a wide variety of soils, geographic regions, and tillage practices, as listed by Mulvaney et al. (2009). Furthermore, these trends from long-term experiments reveal that chemical fertilization is often ineffective in preventing soil N depletion, even in cases involving an ample input of N and greater production and incorporation of crop residues. As mentioned above, these effects are more likely due to the fact that mineral N, particularly in the form of ammoniacal fertilizers, can stimulate microbial carbon decomposition, thereby promoting the decay of added crop residues and also the indigenous organic matter that serves as the major reservoir of soil N.
When N used in the synthesis of plant biomass is returned to soil, it exists in equilibrium with a larger and more stable pool associated with humus. This equilibrium is shifted toward immobilization when the input of C is higher than N and toward mineralization when the input of N is higher than C. The ultimate effect in the latter case may be a net loss of applied organic N through crop uptake, leaching, or gaseous emissions of mineralized N. Dissolved organic N may also be lost through profile transport. As reviewed by van Kessel et al. (2009), dissolved organic N losses can range from a minimum of 0.3 kg N ha−1 year−1 in a clover-based pasture to a maximum of 127 kg N ha−1 year−1 in a grassland to which urine was applied. Mulvaney et al. (2009) have compiled a global data set to compare net mineralization with and without N fertilization and at different N application rates, which demonstrates the inherent potential of synthetic N to enhance microbial decomposition. This data set is remarkably consistent in documenting more rapid mineralization for fertilized than unfertilized soils, and in many cases identifying a positive effect from increasing the rate of N applied. Although Powlson et al. (2010) has criticized the way Mulvaney et al. (2009) interpreted the data from long-term experiments, they agree that the observation of significant soil C and N declines in subsoil layers deserves further consideration.
5.2.1 Fertilizer N Management in Agro-Ecosystems
In agro-ecosystems, crops do not always use N efficiently when it is applied through fertilizers, manures, and other sources (Galloway et al. 2003, Cassman et al. 2003). The unutilized N is susceptible to various loss pathways – leaching as nitrate, ammonia volatilization, denitrification, and runoff (Fig. 6.1). By reducing these losses, improved N use efficiency can also lead to reduced off-site N2O emissions. Fertilizer N use efficiency can be improved by: (a) adjusting application rates based on precise estimation of crop needs; (b) using slow- or controlled-release fertilizer forms or nitrification inhibitors; (c) improved timing of N application; (d) placing the N more precisely into the soil to make it more accessible to crops roots; or (e) by avoiding N applications in excess of immediate plant requirements (Robertson 2004; Paustian et al. 2004; Bijay-Singh 2008; Monteny et al. 2006). Landholders apply N fertilizer on the assumption that in managed agro-ecosystems this is the sole source of plant available N. But considerable evidence obtained from 15N-tracer investigations suggests that plant uptake is generally greater from native soil N than from N applied via synthetic fertilizers, even with excessive fertilization (Olson 1980; Kitur et al. 1984; Reddy and Reddy 1993; Stevens et al. 2005). Saito and Ishii (1987) showed that native soil N is an important source of N that is used by crops in managed agro-ecosystems. They investigated the uptake of N by maize from soil N and fertilizer N sources, from 12 soils, and showed that native soil N dictates the efficiency of applied fertilizer N as well as the quantity of N lost from the soil-plant system. Additionally, improvements in N use efficiency can indirectly lead to reduced greenhouse gas emissions from N fertilizer manufacture.
Given the fundamental coupling of microbial C and N cycling, the dominant occurrence of both elements in soil organic forms, and the close correlation between soil C and N mineralization (Dou et al. 2008), the practices that lead to loss of soil organic C will also have serious implications for the storage of N in soil. The loss of organic N decreases soil productivity and the agronomic efficiency of fertilizer N and has been implicated in reports of yield stagnation and the decline of grain production (Mulvaney et al. 2009). As already discussed, even with intensive fertilization, soil reserves usually supply the bulk of N uptake by nonleguminous crops. Thus, a decrease in soil N supply is inherently detrimental to productivity, although crop yields may be sustained or even increase because of introduction of improved varieties or due to higher fertilizer application rate despite the lower incremental return per unit of N applied. Eventually, however, soil degradation is likely to lead to a decline or stagnation in yield, an emerging concern for input-intensive agriculture.
5.3 Nitrogen Availability in Relation to Non-CO2 Emissions and Soil C Sequestration
The basic principles of sound N management are well known and need to be promoted within the context of decreasing N losses from the soil-plant system including greenhouse gas emissions in the form of N2O as well as for increasing economic profitability of crop production. It follows from the discussion in Sect. 6.5.2 that high N use efficiency can be achieved by avoiding excessive N applications and by synchronizing N supply with crop demand. The latter is more easily achieved when N is supplied in split doses using fertilizers compared to where organic N sources are used (both imported organic materials such as animal manures, and legumes or residues grown on site), where the release of nutrients is controlled by biological mineralization processes. In general, the release of N from organic sources continues beyond the period of crop production and when applied in amounts large enough to meet the nutrient requirement of agro-ecosystems can contribute to leaching losses and off-site pollution problems, including additional generation of N2O (Duxbury 2006). Research has also shown that emissions of N2O from cropland are higher when organic sources of N rather than inorganic fertilizers are used (Duxbury et al. 1982). As shown in Table 6.2, there exists a large global potential of N availability through N fixation by leguminous plants via intercropping and off-season cropping (Badgley et al. 2007) and from livestock feces (Niggli et al. 2009). Nitrogen additions through these routes can also contribute to large N2O emissions from soil (Rochette and Janzen 2005).
According to Smith et al. (2007a), agriculture accounted for 47 and 58% of total anthropogenic emissions of methane (CH4) and N2O during 2005. Methane and N2O contributed 3.3 and 2.8 Gt CO2-eq year−1, respectively, to the atmosphere. Despite large annual exchanges of CO2 between the atmosphere and agricultural lands, the fluxes are estimated to be approximately balanced with net CO2 emissions ~0.04 Gt CO2-C year−1 only. However, capturing CO2 as soil organic matter can contribute to improved soil health and productivity (Drinkwater et al. 1998) and potentially mitigate climate change. In fact, soil C sequestration (enhanced sinks) is the mechanism responsible for most of the mitigation potential in the agriculture sector, with an estimated 89% contribution to the technical potential (per area estimate of mitigation potential for different greenhouse gases multiplied by the area for that practice in each region) (Smith et al. 2007a). In general, agronomic practices that increase crop yield and generate higher inputs of organic residue can lead to increased soil C storage, although reports do exist that N fertilizer use on soils to achieve higher crop yield may lead to the loss of organic C from soils (Khan et al. 2007; see Sect. 6.5.2). No-till agriculture is usually considered a useful agronomic technique to reduce the rate of loss of organic C and N from soils as opposed to conventional tillage practices (see Chap. 9). However, the overall rate of soil C sequestration under no-till agriculture may depend on local soil and climatic conditions, and hence the reported effects are inconsistent and not well-quantified globally (Smith and Conen 2004; Li et al. 2005; see Chap. 9). Emissions of N2O per hectare can be reduced by adopting cropping systems with reduced reliance on fertilizers; for example, the use of rotations with legume crops (West and Post 2002). But, as already mentioned, legume-derived N can also serve as a source of N2O. Furthermore, cultivated wetland rice soils emit significant quantities of CH4 (Yan et al. 2003). This source of CH4 emissions can be reduced by draining wetland rice during the growing season (Smith and Conen 2004; Yan et al. 2003). However, any reduction in CH4 emissions achieved due to this strategy may be partly offset by increased N2O emissions under aerobic conditions.
The oxidation of CH4 by CH4-oxidizing microorganisms (methanotrophs) in soil is important in defining the global CH4 budget (see Chaps. 9 and 10). Oxic soils are a net sink while wetland soils are a net source of atmospheric CH4. The consumption of CH4 in upland as well as lowland systems may be inhibited by NH +4 produced in soil or added through ammoniacal fertilizers because NH +4 can competitively inhibit CH4 oxidation by methanotrophs (Bedard and Knowles 1989). However, NH +4 -based fertilization has also been demonstrated to stimulate CH4 consumption in rice paddies, especially when methanotrophs are N limited (Bodelier and Laanbroek 2004). Available literature reveals that N limitation of CH4 consumption occurs in a variety of lowland soils, upland soils, and sediments. Obviously, depriving CH4-oxidizing bacteria of a suitable source of N hampers their growth and activity.
5.4 Global Significance of N2O Emissions from Terrestrial and Oceanic Sources
Based on data pertaining to N2O flux from soils, wetlands, and the sea, globally ~2.6–3.9% of the denitrification flux consists of N2O (Denman et al. 2007; Seitzinger et al. 2006), which is increasing in earth’s atmosphere by ~0.3% per year (Denman et al. 2007). Schlesinger (2009) has calculated recent changes in global denitrification by dividing the observed increase in N2O in earth’s atmosphere (~4 Tg N year−1) by an estimate of the ratio of N2O to the total N2 + N2O produced by denitrifiers. For wetland, stream, and lake sediments, the values for this ratio are always low because the denitrification flux consists of greater proportion of N2 than N2O (Kralova et al. 1992; Riley and Matson 2000). For upland ecosystems, a significant percentage of the efflux due to denitrification occurs as N2O. The mean ratios N2O/(N2 + N2O) for soils under natural or recovering vegetation, agricultural soils, and freshwater wetlands and flooded soils are 0.492 ± 0.066, 0.375 ± 0.035, and 0.082 ± 0.024, respectively (Schlesinger 2009). According to Schlesinger (2009), assuming that the rise in N2O in the Earth’s atmosphere is solely from denitrification in terrestrial ecosystems and if 53% of denitrification occurs in upland agricultural soils with a N2O/(N2 + N2O) ratio of 0.37, and 47% occurs in wetlands with a ratio of 0.08 (Bouwman et al. 2005), then the weighted mean ratio becomes 0.23 and the calculated total rate of denitrification is now 17 Tg N year−1 greater than in preindustrial times (Schlesinger 2009).
6 Nitrogen Management Under Future Climate Change Scenarios
As already discussed, sequestration of C in the soil is a mechanism that could potentially be responsible for significant mitigation potential for climate change. According to Smith et al. (2007a), reduction in CO2, CH4 and N2O emissions from soils can contribute 89, 9, and 2%, respectively, to the total mitigation potential. Of course, the level of adoption of appropriate measures, effectiveness of these in enhancing C sinks, and persistence of mitigation, as influenced by future climatic trends, economic conditions, and social behavior will determine the exact contribution. The projected changes in climate in coming decades may influence emissions of greenhouse gases from agriculture and the effectiveness of practices adopted to minimize these emissions. For example, increasing atmospheric CO2 concentrations may affect the functioning of terrestrial ecosystems through changes in plant growth rates, plant litter composition, drought tolerance, and N demands (van Groenigen et al. 2005; Jensen and Christensen 2004). Similarly, atmospheric N deposition and increasing temperature may affect the net C sequestration potential of forests and crop production systems. Increasing temperatures are likely to have a positive effect on forest and crop production in colder regions due to a longer growing season (Smith et al. 2007b), but increasing temperatures may also accelerate decomposition of soil organic matter, releasing stored soil C into the atmosphere (Knorr et al. 2005b; Smith et al. 2007b). Increasing mineralization rates will also elevate the rate with which N is released from soil organic matter and therefore its availability for uptake by plants. Similarly, higher rates of N deposition are expected to increase plant growth, but may alter C sequestration and soil N2O emissions in unexpected directions. Furthermore, complex interactions between the climate change drivers and C and N cycles may produce high levels of uncertainties on the potential effects of climate change on soil N availability and CO2 emissions (see Sects. 6.3 and 6.4).
With the prediction that the increase in atmospheric CO2 levels by 2100 will result in an additional warming of up to several degrees Celsius (Forster et al. 2007), one can expect the intensification of N cycle in terrestrial ecosystems, in addition to direct anthropogenic inputs (Gruber and Galloway 2008). Thus, future management of the N cycle in terrestrial ecosystems is expected to be highly challenging. A meta-analysis of data generated in different soils and ecosystems carried out by Rustad et al. (2001) revealed that warming significantly increases N mineralization and nitrification. Furthermore, Groffman et al. (2009) found that N cycling processes are more sensitive to variations in soil moisture induced by global warming. It was observed that net N mineralization and nitrification were slower in warmer climate, but these processes were more driven by lower soil moisture content than higher temperature. In northeast China, Hu et al. (2009) observed that N mineralization rate in the soil under meadow steppes increased significantly due to a 2°C rise in soil surface temperature, although warming did not have a significant influence on total N content. In a semi-arid soil, Hoyle et al. (2006) observed that nitrification increased linearly with temperature and dominated over immobilization for available ammonium in soil incubated above 20°C, indicating that nitrification is often the principal process influencing NH +4 consumption. These findings suggest that the N mineralization–immobilization turnover at soil temperatures above 20°C is not tightly coupled, and there is a high potential for loss of N as nitrate.
Information about soil solution inorganic nitrogen (NH +4 + NO −3 ) is important from the point of fertilizer N management. Shaw and Harte (2001) recorded no increase in soil solution N despite increases in mineralization rate. This result was ascribed to increased plant uptake. While Ineson et al. (1998) showed a decrease, Peterjohn et al. (1994) observed no change in soil solution inorganic N concentration upon soil heating. According to Rustad et al. (2000), soil solution inorganic concentrations will increase only in N-saturated ecosystems, whereas most N will be taken up by vegetation in N-limited systems. Verburg (2005) found that by increasing air temperatures by 3°C in summer and 5°C in winter, soil inorganic pools increased indicating an enhanced mineralization which exceeded plant demand and led to leaching losses.
There is good reason for concern about sustaining world food production by supplying additional N because crop N uptake originates largely from the native soil source rather than fertilizer N (see Sect. 6.5.2.1). In view of the changing scenario with regard to N cycle process under a warmer climate, the immediate need is to use scientific and technological advances that can increase input efficiencies. The most important aspect of this strategy would be to more accurately match the input of ammoniacal N to crop N requirement by accounting for site-specific variations in soil N supplying capacity and by synchronizing application with plant N uptake. Across a wide range of crop-growing conditions, information to reduce uncertainty in synchronizing crop N demand and soil N supply can contribute substantially to reducing fertilizer application rates and the associated economic and environmental costs. In the long term, a transition toward agricultural diversification using legume-based crop rotations, which provide a valuable means to reduce the intensity of ammoniacal fertilization with the input of less reactive organic N, may be required. A number of management options which have limited potential now are also likely to have increased potential in the long term. Examples include better use of fertilizer through precision farming, wider use of slow and controlled-release fertilizers and adoption of nitrification inhibitors.
An analysis based on measurements and model studies as reported by Reay et al. (2008) indicates that the likelihood of greatly enhanced global CO2 sequestration resulting from future changes in N deposition is low. A doubling of the N emission levels in the year 2000 may achieve approximately three billion tonnes of additional CO2 sequestration in northern and tropical forests each year. However, using emission factor given by Crutzen et al. (2007), this would induce global annual emissions of 2.7 billion tonnes of CO2 equivalent, in the form of N2O, via increased nitrification and denitrification on land and in the oceans. Such pollution-swapping would greatly offset any climate change mitigation benefits of N deposition. Under such situations, protection of the existing terrestrial C sinks from deforestation and land use changes, rather than possible enhanced C sequestration through N deposition, will provide greater climate change mitigation benefits in coming decades (Reay et al. 2008).
7 Summary and Conclusions
The large generation of reactive N by industry, fossil fuel burning, and biological N2 fixation has been able to maintain adequate availability of N for crop plants, thereby ensuring ample production of food and fiber to meet the increasing demand of a growing population. However, ~10% of the applied N remains in food and the remainder generally ends up in the environment, polluting the atmosphere and water bodies, and influencing the climate. The N cycle in natural ecosystems is highly conservative to loss pathways, but it is heavily disrupted in managed agro-ecosystems, leading to enhanced N2O emissions and N leaching. Production of fertilizer N for application in managed agro-ecosystems is still accelerating, and this trend is not likely to change in the near future. Furthermore, many terrestrial and marine ecosystems will continue to receive an increasing supply of reactive N produced by industry and fossil fuel burning, and also indirectly via atmospheric deposition. Questions remain as to the likely consequences for terrestrial C sink capacity, specifically whether increasingly heavy inputs of reactive N will serve to significantly reduce rising atmospheric CO2 concentrations.
The evidence for changes in soil C stocks under N deposition scenarios is contradictory, with some studies suggesting that soil C may decrease while others suggest no change or significant increases in soil C. It remains a challenge to obtain a clear understanding of the impacts of N additions through fertilizer application or atmospheric N deposition on soil health. Furthermore, changes in soil health due to excessive external N inputs such as acidification, cation exchange capacity, greenhouse gas emissions, and nutrient leaching are likely to have serious negative implications for plant productivity and the environment.
Climate change drivers such as elevated CO2 and temperature can influence the terrestrial C and N cycling and alter soil N availability, which constrain the CO2 sink capacity of earth’s biosphere. As C–N interaction fundamentally alters the terrestrial C cycle response to interannual variability in temperature and precipitation (see Sect. 6.4), future atmospheric CO2 concentration and associated anthropogenic climate change may be accurately predicted as climate change models introduce C–N interactions in their land components.
Effects of global warming on different processes and pathways of the N cycle are going to be very complex as several interactions will be involved and the overall effects are likely to be site specific. Management of N fertilizers in agro-ecosystems that improve fertilizer N use efficiency by avoiding excessive N applications and synchronizing N supply with crop demand should help produce enough food for growing populations with minimal contributions to climate change effects.
References
Aber JD, Nadelhoffer KJ, Steudler P, Melillo JM (1989) Nitrogen saturation in northern forest ecosystems – hypothesis revisited. Bioscience 39:378–386
Aber JD, McDowell W, Nadelhoffer K, Magill A, Berntson G, Kamakea M, McNulty S, Currie W, Rustad L, Fernandez I (1998) Nitrogen saturation in temperate forest ecosystems. Bioscience 48:921–934
Ågren GI, Bosatta E, Magill AH (2001) Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 128:94–98
Badgley C, Moghtader J, Quintero E, Zakem E, Chappell MJ, Avilés-Vàzquez K, Samulon A, Perfecto I (2007) Organic agriculture and the global food supply. Renewable Agric Food Syst 22:86–108
Bedard C, Knowles R (1989) Physiology, biochemistry, and specific inhibitors of CH4, NH +4 , and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev 53:68–84
Bhupinderpal-Singh, Nordgren A, Lofvenius MO, Högberg MN, Mellander PE, Högberg P (2003) Tree root and soil heterotrophic respiration as revealed by girdling of boreal Scots pine forest: extending observations beyond the first year. Plant Cell Environ 26:1287–1296
Bijay-Singh (2008) Crop demand-driven site specific nitrogen applications in rice and wheat – some recent advances. Indian J Agron 53:157–166
Bodelier PLE, Laanbroek HJ (2004) Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol Ecol 47:265–277
Boulart C, Flament P, Gentilhomme V, Deboudt K, Migon C, Lizon F, Schapira M, Lefebvre A (2006) Atmospherically-promoted photosynthetic activity in a well-mixed ecosystem: significance of wet deposition events of nitrogen compounds. Estuar Coast Shelf Sci 69:449–458
Bouwman AF, Van Drecht G, Knoop JM, Beusen AHW, Meinardi CR (2005) Exploring changes in river nitrogen export to the world’s oceans. Global Biogeochem Cyc 19:GB1002. doi:10.1929/2004GB0002314
Brooks M (2003) Effects of increased soil nitrogen on the dominance of alien annual plants in the Mojave desert. J Appl Ecol 40:344–353
Cassman KG, Dobermann A, Walters D (2002) Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31:132–140
Cassman KG, Dobermann A, Walters DT, Yang H (2003) Meeting cereal demand while protecting natural resources and improving environmental quality. Annu Rev Environ Res 28:315–358
Christensen BT (2004) Tightening the nitrogen cycle. In: Schjønning P, Elmholt S, Christensen BT (eds) Managing soil quality: challenges in modern agriculture. CAB International, Wallingford, pp 47–67
Crutzen PJ, Mosier AR, Smith KA, Winiwarter W (2007) N2O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. Atmos Chem Phys Discuss 7:11191–11205
Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, Hauglustaine D, Heinze C, Holland E, Jacob D, Lohmann U, Ramachandran S, da Silva Dias PL, Wofsy SC, Zhang X (2007) Couplings between changes in the climate system and biogeochemistry. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 499–587
Dentener F et al. (2006) Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Global Biogeochem Cyc 20:GB4003. doi:10.1029/2005GB002672
Deutsch C, Sarmiento JL, Sigman DM, Gruber N, Dunne JP (2007) Spatial coupling of nitrogen inputs and losses in the ocean. Nature 445:163–167
Dixon RK, Brown S, Houghton RA, Solomon AM, Trexler MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263:185–190
Dou F, Wright AL, Hons FM (2008) Sensitivity of labile soil organic carbon to tillage in wheat-based cropping systems. Soil Sci Soc Am J 72:1445–1453
Drinkwater LE, Wagoner P, Sarrantonio M (1998) Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 396:262–265
Duce RA, LaRoche J, Altieri K, Arrigo KR, Baker AR, Capone DG, Cornell S, Dentener F, Galloway J, Ganeshram RS, Geider RJ, Jickells T, Kuypers MM, Langlois R, Liss PS, Liu SM, Middelburg JJ, Moore CM, Nickovic S, Oschlies A, Pedersen T, Prospero J, Schlitzer R, Seitzinger S, Sorensen LL, Uematsu M, Ulloa O, Voss M, Ward B, Zamora L (2008) Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science 320(5878):893–897
Duxbury JM (2006) Soil carbon sequestration and nitrogen management for greenhouse gas mitigation. In: Climate change and agriculture: promoting practical and profitable responses, pp IV-5–IV-7. www.climateandfarming.org/pdfs/FactSheets/IV.2Soil.pdf. Accessed 17 Aug 2009
Duxbury JM, Bouldin DR, Terry R, Tate RL (1982) Emissions of nitrous oxide from soils. Nature 298:462–464
Erisman JW, Sutton MA, Galloway J, Klimont Z, Winiwarter W (2008) How a century of ammonia synthesis changed the world. Nat Geosci 1:636–639
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW, Haywood J, Lean J, Lowe DC, Myhre G, Nganga J, Prinn R, Raga G, Schulz M, Van Dorland R (2007) Changes in atmospheric constituents and in radiative forcing. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Franklin O, Högberg P, Ekblad A, Ågren GI (2003) Pine forest floor carbon accumulation in response to N and PK additions – bomb 14C modelling and respiration studies. Ecosystems 6:644–658
Galloway JN, Cowling EB (2002) Reactive nitrogen and the world: two hundred years of change. Ambio 31:64–71
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53:341–356
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland C, Green P, Holland E, Karl DM, Michaels AF, Porter JH, Townsend A, Vorosmarty C (2004) Nitrogen cycles: past, present and future. Biogeochemistry 70:153–226
Glendining MJ, Powlson DS (1995) The effects of long-continued applications of inorganic nitrogen fertilizer on soil organic nitrogen – a review. In: Lal R, Stewart BA (eds) Soil management: experimental basis for sustainability and environmental quality. Advances in Soil Science Series. Lewis, Boca Raton, pp 385–446
Groffman PJ, Hardy JP, Fisk MC, Fahey TJ, Driscoll CT (2009) Climate variation and soil carbon and nitrogen cycling processes in a northern hardwood forest. Ecosystems 12:927–943
Gruber N, Galloway JN (2008) An Earth-system perspective of the global nitrogen cycle. Nature 451:293–296
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Hogberg MN, Nyberg G, Ottosson-Lofvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Högberg P, Hogberg MN, Gottlicher SG, Betson NR, Keel SG, Metcalfe DB, Campbell C, Schindlbacher A, Hurry V, Lundmark T, Linder S, Nasholm T (2008) High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytol 177:220–228
Hovenden MJ, Newton PCD, Carran RA, Theobald P, Wills KE, Vander Schoor JK, Williams AL, Osanai Y (2008) Warming prevents the elevated CO2-induced reduction in available soil nitrogen in a temperate, perennial grassland. Global Change Biol 14:1018–1024
Hoyle FC, Murphy DV, Fillery IRP (2006) Temperature and stubble management influence microbial CO2–C evolution and gross N transformation rates. Soil Biol Biochem 38:71–80
Hu L, Yang H, Wang W, Guo J (2009) Soil nutrient responses to one year of simulated global warming and nitrogen deposition on the Songnen meadow steppes, northeast China. http://www.paper.edu.cn/index.php/default/selfs/downpaper/huliangjun326400-self-200906-20. Accessed 27 July 2010
Hudson RJM, Gherini SA, Goldstein RA (1994) Modeling the global carbon cycle nitrogen-fertilization of the terrestrial biosphere and the missing CO2 sink. Global Biogeochem Cyc 8:307–333
Hungate B, Dukes JS, Shaw MR, Luo Y, Field CB (2003) Nitrogen and climate. Science 302:1512–1513
IFA (International Fertilizer Industry Association) (2009) Statistics. http://www.fertilizer.org/
Ineson P, Benham DG, Poskitt J, Harrison AF, Taylor K, Woods C (1998) Effects of climate change on nitrogen dynamics in upland soils. 1. A soil warming study. Global Change Biol 4:153–161
Jagadamma S, Lal R, Hoeft RG, Nafziger ED, Adee EA (2007) Nitrogen fertilization and cropping systems effects on soil organic carbon and total nitrogen pools under chisel-plow tillage in Illinois. Soil Tillage Res 95:348–356
Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze ED, Tang J, Law BE (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Jarvis PG, Fowler DG (2001) Forests and the atmosphere. In: Evans J (ed) The forests handbook, vol 1. Blackwell Science, Oxford, pp 229–281
Jensen B, Christensen BT (2004) Interactions between elevated CO2 and added N: effects on water use, biomass, and soil 15N uptake in wheat. Acta Agric Scand 54B:175–184
Khan SA, Mulvaney RL, Ellsworth TR, Boast CW (2007) The myth of nitrogen fertilization for soil carbon sequestration. J Environ Qual 36:1821–1832
Kitur BK, Smith MS, Blevins RL, Frye WW (1984) Fate of 15N-depleted ammonium nitrate applied to no-tillage and conventional tillage corn. Agron J 76:240–242
Knorr M, Frey SD, Curtis PS (2005a) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Knorr W, Prentice IC, House JI, Holland EA (2005b) Long-term sensitivity of soil carbon turnover to warming. Nature 433:298–301
Koch GW, Mooney HA (eds) (1996) Carbon dioxide and terrestrial ecosystems. Academic, San Diego
Kralova M, Masscheleyn PH, Lindau CW, Patrick WH (1992) Production of dinitrogen and nitrous oxide in soil suspensions as affected by redox potential. Water Air Soil Pollut 61:37–45
Lamarque J-F, Kiehl J, Brasseur G, Butler T, Cameron-Smith P, Collins WJ, Granier C, Hauglustaine D, Hess P, Holland E, Horowitz L, Lawrence M, Mckenna D, Merilees P, Prather M, Rasch P, Rotman D, Shindell D, Thornton P (2005) Assessing future nitrogen deposition and carbon cycle feedback using a multimodel approach: Analysis of nitrogen deposition. J Geophys Res 110:D19303. doi: 10.1029/2005JD005825
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Li C, Frolking S, Butterbach-Bahl K (2005) Carbon sequestration in arable soils is likely to increase nitrous oxide emissions, offsetting reductions in climate radiative forcing. Clim Change 72:321–338
Liebig MA, Varvel GE, Doran JW, Wienhold BJ (2002) Crop sequence and nitrogen fertilization effects on soil properties in the Western Corn Belt. Soil Sci Soc Am J 66:596–601
Luo Y, Su B, Currie WS, Dukes JS, Finzi AC, Hartwig U, Hungate BA, McMurtrie RE, Oren R, Parton WJ, Pataki DE, Shaw MR, Zak DR, Field CB (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731–739
Matson P, Lohse KA, Hall SJ (2002) The globalization of nitrogen deposition: consequences for terrestrial ecosystems. Ambio 31:113–119
Medlyn BE, Berbigier P, Clement R, Grelle A, Loustau D, Linder S, Wingate L, Jarvis PG, Sigurdsson BD, McMurtrie RE (2005) The carbon balance of coniferous forests growing in contrasting climatic conditions: a model-based analysis. Agric Forest Meteorol 131:97–124
Monteny G-J, Bannink A, Chadwick D (2006) Greenhouse gas abatement strategies for animal husbandry. Agric Ecosyst Environ 112:163–170
Müller C, Rütting T, Abbasi MK, Laughlin RJ, Kammann C, Clough TJ, Sherlock RR, Kattge J, Jäger H-J, Watson CJ, Stevens RJ (2009) Effect of elevated CO2 on soil N dynamics in a temperate grassland soil. Soil Biol Biochem 41:1996–2001
Mulvaney RL, Khan SA, Ellsworth TR (2009) Synthetic nitrogen fertilizers deplete soil nitrogen: a global dilemma for sustainable cereal production. J Environ Qual 38:2295–2314
Nadelhoffer KJ, Emmett BA, Gundersen P, Kjønaas OJ, Koopmans CJ, Schleppi P, Tietema A, Wright RF (1999) Nitrogen deposition makes a minor contribution to carbon sequestration in temperate forests. Nature 398:145–148
Niggli U, Fließbach A, Hepperly P, Scialabba N (2009) Low greenhouse gas agriculture: mitigation and adaptation potential of sustainable farming systems. Rev. 2 – 2009. Food and Agriculture Organization of the United Nations, Rome, 21 pp
Olson RV (1980) Fate of tagged nitrogen fertilizer applied to irrigated corn. Soil Sci Soc Am J 44:514–517
Paustian K, Babcock BA, Hatfield J, Lal R, McCarl BA, McLaughlin S, Mosier A, Rice C, Robertson GP, Rosenberg NJ, Rosenzweig C, Schlesinger WH, Zilberman D (2004) Agricultural mitigation of greenhouse gases: science and policy options, CAST (Council on Agricultural Science and Technology) Report, R141 2004. ISBN 1-887383-26-3, 120 pp
Persson T, Karlsson PS, Seyferth U, Sjöberg RM, Rudebeck A (2000) Carbon mineralisation in European forest soils. In: Schulze ED (ed) Carbon and nitrogen cycling in European forest ecosystems, Ecological Studies 142. Springer, Berlin, pp 257–275
Peterjohn WT, Melillo JM, Steudler PA, Newkirk KM, Bowles FP, Aber JD (1994) Responses of trace gas fluxes and N availability to experimentally elevated soil temperatures. Ecol Appl 4:617–625
Powlson D (2005) Will soil amplify climate change? Nature 433:204–205
Powlson DS, Jenkinson DS, Johnston AE, Poulton PR, Glendining MJ, Goulding KWT (2010) Comments on “Synthetic nitrogen fertilizers deplete soil nitrogen: a global dilemma for sustainable cereal production,” by R.L. Mulvaney, S.A. Khan, and T.R. Ellsworth in the Journal of Environmental Quality 2009 38:2295–2314. J Environ Qual 39:749–752
Reay DS, Dentener F, Smith P, Grace J, Feely RA (2008) Global nitrogen deposition and carbon sinks. Nat Geosci 1:430–437
Reddy GB, Reddy KR (1993) Fate of nitrogen-15 enriched ammonium nitrate applied to corn. Soil Sci Soc Am J 57:111–115
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J (2006a) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Reich PB, Hungate BA, Luo Y (2006b) Carbon-nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide. Ann Rev Ecol Evol Syst 37:611–636
Riley WJ, Matson PA (2000) NLOSS: a mechanistic model of denitrified N2O and N2 evolution from soil. Soil Sci 165:237–249
Robertson GP (2004) Abatement of nitrous oxide, methane and other non-CO2 greenhouse gases: the need for a systems approach. In: Field CB, Raupach MR (eds) The global carbon cycle. Integrating humans, climate, and the natural world, SCOPE 62. Island Press, Washington, pp 493–506
Rochette P, Janzen HH (2005) Towards a revised coefficient for estimating N2O emissions from legumes. Nutr Cycl Agroecosyst 73:171–179
Rustad LE, Melillo JM, Mitchell MJ, Fernandez IJ, Steudler PA, McHale PJ (2000) Effects of soil warming on C and N cycling in northern U.S. forest soils. In: Mickler R, Birdsey R, Hom J (eds) Responses of northern U.S. forests to environmental change. Springer, Berlin, pp 357–381
Rustad LE, Campbell J, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net N mineralization, and aboveground plant growth in experimental ecosystem warming. Oecologia 126:543–562
Saito M, Ishii K (1987) Estimation of soil nitrogen mineralization in corn-grown fields based on mineralization parameters. Soil Sci Plant Nutr 33:555–566
Scavia D, Bricker SB (2006) Coastal eutrophication assessment in the United States. Biogeochemistry 79:187–208
Schlesinger WH (1997) Biogeochemistry, an analysis of global climate change. Academic, San Diego
Schlesinger WH (2009) On the fate of anthropogenic nitrogen. Proc Natl Acad Sci USA 106:203–208
Seitzinger S, Harrison JA, Böhlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, van Drecht G (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16:2064–2090
Shaw MR, Harte J (2001) Response of nitrogen cycling to simulated climate change: differential responses along a subalpine ecotone. Global Change Biol 7:193–210
Smith KA, Conen F (2004) Impacts of land management on fluxes of trace greenhouse gases. Soil Use Manage 20:255–263
Smith P, Martino D, Cai Z, Gwary D, Janzen H, Kumar P, McCarl B, Ogle S, O’Mara F, Rice C, Scholes B, Sirotenko O (2007a) Agriculture. In: Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA (eds) Climate change 2007: mitigation: contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 498–540. http://www.ipcc.ch/ipccreports/ar4-wg3.htm
Smith P, Martino D, Cai Z, Gwary D, Janzen HH, Kumar P, McCarl BA, Ogle SM, O’Mara F, Rice C, Scholes RJ, Sirotenko O, Howden M, McAllister T, Pan G, Romanenkov V, Schneider UA, Towprayoon S (2007b) Policy and technological constraints to implementation of greenhouse gas mitigation options in agriculture. Agric Ecosyst Environ 118:6–28
Sokolov AP, Kicklighter DW, Melillo JM, Felzer BS, Schlosser CA, Cronin TW (2008) Consequences of considering carbon-nitrogen interactions on the feedbacks between climate and the terrestrial carbon cycle. J Clim 21:3776–3796
Stevens WB, Hoeft RG, Mulvaney RL (2005) Fate of nitrogen-15 in a long-term nitrogen rate study: II. Nitrogen uptake efficiency. Agron J 97:1046–1053
Tamm CO, Lake JV, Miller HG (1982) Nitrogen cycling in undisturbed and manipulated boreal forest. Phil Trans R Soc Lond 296(1082):419–425
Thornton PE, Lamarque J-F, Rosenbloom NA, Mahowald NM (2007) Influence of carbon-nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Global Biogeochem Cyc 21:GB4018. doi:10.1029/2006GB002868
Thornton PE, Doney SC, Lindsay K, Moore JK, Mahowald N, Randerson JT, Fung I, Lamarque J-F, Feddema JJ, Lee Y-H (2009) Carbon-nitrogen interactions regulate climate-carbon cycle feedbacks: results from an atmosphere-ocean general circulation model. Biogeosci Discuss 6:3303–3354
Uehara G, Gillman G (1981) The mineralogy, chemistry, and physics of tropical soils with variable charge clays. Westview, Boulder, 160pp
US Geological Survey (2008) Nitrogen (fixed) – ammonia. In: Mineral commodity summaries 2008. U.S. Department of the Interior, U.S. Geological Survey, pp 118–119
van Groenigen KJ, Gorissen A, Six J, Harris D, Kuikman PJ, van Groenigen JW, van Kessel C (2005) Decomposition of 14C-labeled roots in a pasture soil exposed to 10 years of elevated CO2. Soil Biol Biochem 37:497–506
van Kessel C, Clough T, van Groenigen JW (2009) Dissolved organic nitrogen: an overlooked pathway of nitrogen loss from agricultural systems? J Environ Qual 38:393–401
Vanotti MB, Bundy LG, Peterson AE (1997) Nitrogen fertilizer and legume-cereal rotation effects on soil productivity and organic matter dynamics in Wisconsin. In: Paul EA et al (eds) Soil organic matter in temperate agroecosystems: long-term experiments in North America. CRC, Boca Raton, pp 105–119
Venterea RT, Groffman PM, Verchot LV, Magill AH, Aber JD (2004) Gross nitrogen process rates in temperate forest soils exhibiting symptoms of nitrogen saturation. For Ecol Manage 196:129–142
Verburg PSJ (2005) Soil solution and extractable soil nitrogen response to climate change in two boreal forest ecosystems. Biol Fertil Soils 41:257–261
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea – how can it occur? Biogeochemistry 13:87–115
West TO, Post WM (2002) Soil organic carbon sequestration rates by tillage and crop rotation: a global data analysis. Soil Sci Soc Am J 66:1930–1946
Xu X, Tian H, Hui D (2008) Convergence in the relationship of CO2 and N2O exchanges between soil and atmosphere within terrestrial ecosystems. Global Change Biol 14:1651–1660
Yan X, Ohara T, Akimoto H (2003) Development of region-specific emission factors and estimation of methane emission from rice field in East, Southeast and South Asian countries. Global Change Biol 9:237–254
Acknowledgments
I appreciate constructive comments from two anonymous reviewers as well as the editors, especially the senior editor, on earlier drafts.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Bijay-Singh (2011). The Nitrogen Cycle: Implications for Management, Soil Health, and Climate Change. In: Singh, B., Cowie, A., Chan, K. (eds) Soil Health and Climate Change. Soil Biology, vol 29. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-20256-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-642-20256-8_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-20255-1
Online ISBN: 978-3-642-20256-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)