Abstract
Nitrogen (N) fertilizer use in managed forest ecosystems is increasing in the United States and worldwide to enhance social, economical and environmental services. However, the effects of N-fertilization on production and consumption of greenhouse gases (GHGs), especially carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) in managed forest ecosystems are poorly understood, unlike in agriculture where effects are well documented. Therefore, a review of the available literature was conducted to comprehend the effects of N-fertilization on CO2, CH4 and N2O emissions in managed forest ecosystems to summarize sources, sinks, and controlling factors, as well as potential mitigation strategies and research gaps to reduce GHG emissions. This review clearly identifies the importance of N-fertilizer management practices on CO2, CH4 and N2O emissions. Potential N management practices to mitigate GHG emissions in managed forest ecosystems include improving N uptake efficiency, identifying and managing spatial variation in soil fertility, using the right fertilizer source at the right time, adopting appropriate methods of N-fertilizer application, and introducing nitrification/denitrification inhibitors. Nitrogen-fertilizer response is affected by soil physical (e.g., moisture, drainage, bulk density, and texture), chemical (e.g., nutrient availability, labile carbon, soil pH, and C/N ratio) and local climatic factors (e.g., temperature, relative humidity, and rainfall). Therefore, the interactions of these factors on GHG emissions need to be considered while evaluating N-fertilizer management practices. Existing studies are often limited, focusing primarily on temperate forest ecosystems, lacking estimation of net emissions considering all three predominant soil-derived GHGs, and were often conducted on a small scale, making upscaling challenging. Therefore, large-scale studies conducted in diverse climates, evaluating cumulative net emissions, are needed to better understand N-fertilization effects on GHG emissions and develop mitigation strategies. Mitigation strategies and research gaps have also been identified, which require the collaborative efforts of forest owners, managers, and scientists to increase adoption of N-fertilization best management practices and understand the importance of N-fertilizer management strategies in reducing emissions and enhancing the net GHG sink potential for managed forest ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Greenhouse gases (GHGs) affect Earth’s climate by absorbing solar infrared radiation in the lower atmosphere. Concentrations of atmospheric GHGs, specifically carbon dioxide (CO2), nitrous oxide (N2O), and methane (CH4), drive large scale and long-term climate patterns that have both environmental and economic implications. Although GHGs occur naturally in the atmosphere, anthropogenic activities have elevated their concentrations. For example, the global atmospheric concentration of CO2 increased from a pre-industrial (before 1750) value of 280–379 ppm in 2005, which spiked to above 400 ppm several times in April of 2013 (IPCC 2007a; Monastersky 2013). Similarly, pre-industrial global atmospheric concentrations of N2O increased from 270 to 319 ppb and CH4 from 715 ppb to 1,774 ppb by 2005 (IPCC 2007a). The global warming potentials (GWPs, over a 100-year time frame) for N2O and CH4 are 310 and 21 times higher than that of CO2, respectively (IPCC 2007b).
Forests have received much interest in mitigating climate change (McKinley et al. 2011). They cover about 30 % of the global land area and consequently can play a significant role in regulating global climate through their capacity to be a significant sink for CO2 and CH4 as well as a source for N2O (Pilegaard et al. 2006; Smith et al. 2003). The world’s forests absorb 8.8 Pg of CO2 each year with an average sequestration rate of 3.81 Mg CO2 ha−1 year−1. The current carbon (C) stock in the world’s forests is estimated at 861 ± 66 Pg, with approximately 44 % in soil (to 1 m depth), 42 % in live biomass (above and below ground), 8 % in deadwood, and 5 % in litter (Pan et al. 2011).
In this context, forest ecosystems in the U.S. can play a critical role in the global C cycle because forested areas are estimated to cover 323 million ha and represent a high capacity for C storage (152 Pg of CO2) in biomass and soil (USDA Forest Service 2010; FIDO 2011). This has a major impact on regional and global sources of CO2, absorbing as much as 25–50 % of CO2 emitted annually from fossil-fuel combustion in the US (US Climate Change Science Program 2008). Forests of the US (including vegetation, soils, and harvested wood) accounted for approximately 86 % of total 2010 net CO2 flux (USEPA 2012). Land use, land-use change and forestry activities between 1990 and 2010 resulted in a net C sequestration of 1.07 Pg CO2 Eq in the US (USEPA 2012). This is a 22 % increase from 1990, primarily due to an increase in the rate of net C accumulation in forest C stocks including aboveground and belowground tree biomass and harvested wood pools. This sequestration represents an offset of 19 % of CO2 emissions and 16 % of total GHGs emissions (McKinley et al. 2011; USEPA 2012). This illustrates the potential for C-sequestration in managed forests and overall reduction of GHG emissions. For example, forest management activities such as replanting, thinning, fertilization, drainage, species or genotype selection, and optimizing rotation lengths can enhance C-sequestration (Liski et al. 2001; IPCC 2006).
Increasing use of N fertilizer in agriculture and managed forest ecosystems has caused a large perturbation to the global N cycle since the industrial revolution, thereby significantly increasing net N2O emissions (Grip and Jansson 2012; Pinder et al. 2012). The IPCC has reported that 10 kg N2O–N is emitted for every 1,000 kg of N-fertilizer applied in a managed system (IPCC 2006). Although managed forests have a large C sink potential, the impacts of N-fertilizer management on GHG emissions have not been well documented. Nitrogen added through fertilization, which is a common practice in managed pine plantations (Albaugh et al. 2007), has been shown to increase N2O emissions (Papen et al. 2001; Brown et al. 2012), while simultaneously decreasing the sink strength of CH4 (Sitaula and Bakken 1993; Mochizuki et al. 2012). Judicious application of N-fertilizer and use of newer fertilizer technologies could decrease future N2O emissions without impacting productivity and other ecosystem services. Thus, optimizing N-management has a significant potential to reduce net GHG emissions from managed forest ecosystems, including the regeneration phase of forestry.
Extensive studies have been conducted on use of N-fertilizer and its impacts on growth in managed forest ecosystems (e.g., Elliot and Fox 2006; Fox et al. 2007; Haase et al. 2007; Fujinuma et al. 2011; Ring et al. 2011; Vallack et al. 2012). However, the effects of N-fertilization on production and consumption of GHGs (CO2, CH4, and N2O) in managed forest ecosystems are poorly understood despite the fact that: (1) forest fertilization is used to stimulate tree growth, reduce rotation age, and sequester C, and (2) acreage under forest fertilization has increased from 0.8 million ha in 1990 to 4.9 million ha in 2004 in southeastern United States (Albaugh et al. 2007). This calls for a detailed review and synthesis of available studies on the impacts of N-fertilizer management on GHG emissions to strengthen the knowledge in this field and identify critical knowledge gaps. Therefore, the main objective of this paper is to review and synthesize literature related to inorganic N-fertilization (typically ~200 kg N ha−1) impacts on greenhouse gas emissions in managed forest ecosystems, and present the best current knowledge available and future research needs.
Carbon dioxide emissions in forest ecosystems
Source and sink of carbon-dioxide
Forests, especially in the northern hemisphere, are a significant sink of CO2 (Ciais et al. 1995). Forest ecosystems in the US accounted for 86 % of total 2010 net CO2 flux (USEPA 2012). They contribute to the C budget by releasing and removing CO2 from the atmosphere (Schlesinger and Bernhardt 2013). Trees constantly remove CO2 from the atmosphere through photosynthesis, which converts atmospheric C to organic C. Subsequently, CO2 can leave forest ecosystems through respiration (both autotrophic and heterotrophic), the major mechanism for CO2 flux from forest ecosystems (Fig. 1).
Effects of nitrogen inputs on the processes that regulate the fluxes of N2O, CO2 and CH4 in forest ecosystems (Conrad 1996; Schimel 2000; Thirukkumaran and Parkinson 2000; Philips and Fahey 2007; Liu and Greaver 2009; Canfield et al. 2010; Watanabe and Ortega 2011; Bernal et al. 2012; Brown et al. 2012)
Nitrogen-fertilizer applications alter the emissions of CO2 by regulating plant and microbial activities that are related to soil processes (e.g., N and C cycling) (Fig. 1). Especially in N limited forest ecosystems, N-addition plays a critical role in stimulating plant growth and consequently enhancing CO2 uptake (Fleischer et al. 2013; Law 2013; Bowden et al. 2004). Usually, N fertilization increases tree growth resulting in an increase in the C sink and net C balance of forest ecosystems (LeBauer and Treseder 2008; Liu and Greaver 2009; Tyree et al. 2013). For example, Albaugh et al. (2012) reported that the application of 224 kg N ha−1 with 28 kg of P ha−1 sequestered 19.2 Mg ha−1 of CO2 equivalent as additional stem growth. Increase in plant N content due to N-fertilization also enhances autotrophic respiration (Griffin et al. 1997; Reich et al. 2008) and litter decomposition (Berg and Laskowski 2006).
At early stages, in the first year, N application has shown to increase soil respiration in hardwood stands compared to pines due to increased productivity of the former. However after 13 years of continuous N-fertilization, soil respiration was observed to be suppressed by 41 % in both stands due to reduced microbial activity (Bowden et al. 2004). Mo et al. (2008) and Janssens et al. (2010) also reported decreases in soil microbial and root biomass due to N-fertilization resulting in a decrease in decomposition rates and increase in soil C sequestration. Application of N-fertilizer at the rate of 50 kg N ha−1 year−1 to the tropical forest ecosystems of Puerto Rico with high background N availability did not change litter fall productivity. However, mineral-associated soil C concentrations increased significantly, although live fine roots and labile soil C fractions did not (Cusack et al. 2011). Nitrogen-fertilization can also lead to soil acidification, affecting soil microbial activity, and thereby litter decomposition and heterotrophic respiration (Tonon et al. 2010). Decrease in live fine roots and labile soil C fractions resulted in a decrease in CO2 emissions in fertilized plots.
Nitrogen-fertilizer application has been reported to increase (Gough and Seiler 2004), decrease (Haynes and Gower 1995, Maier and Kress 2000), or have no effect (Lee and Jose 2003) on CO2 emissions from the soil surface, owing to different controlling factors. Therefore, the effects of N fertilization on soil respiration are equivocal, showing varied responses on autotrophic (roots) and heterotrophic (soil organisms) components of soil respiration, the balance of which determines the overall change in soil respiration.
Controls of CO2 emissions
Various factors, alone or in combination, can affect CO2 emissions from forest soils. Carbon dioxide emissions from soils are controlled by (1) soil or litter-soil interface temperature (Butnor et al. 2003; Wang et al. 2003; Vose and Bolstad 2007; Peichl et al. 2010), (2) soil moisture (Bouma and Bryla 2000; Qi and Xu 2001), (3) the amount and quality of soil organic matter (Abbas and Fares 2009), (4) N-fertilization or tissue N content (Pregitzer et al. 1998; Maier and Kress 2000; Burton et al. 2002; Bowden et al. 2004; Philips and Fahey 2007; Jassal et al. 2010; Albaugh et al. 2012), and (5) C:N ratio (Klemedtsson et al. 2005).
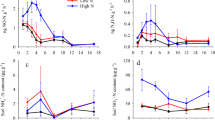
The impacts of forest N-fertilization on CO2 emissions have varied widely. Of the 12 studies of the effect of N-fertilization on soil CO2 flux in managed forest, especially under high rates of fertilizer addition (e.g., ~150 kg N ha−1), six studies reported that N-fertilization decreased soil CO2 emissions by 15–46 % (Table 1). These studies do not indicate any strong relationship of CO2 flux with climate or forest type, but in general pine forests have shown to decrease CO2 emissions and hardwood to increase CO2 emissions, especially in the first year of application. This increase in CO2 emissions is attributed to increased productivity and microbial communities (Brumme and Beese 1992; Bowden et al. 2004). Nitrogen fertilization at 167 kg N ha−1 year−1 of hardwood stands in New York and New Hampshire resulted in a decrease in both decomposition of SOM and heterotrophic (microbial) respiration in the rhizosphere (Philips and Fahey 2007). Similarly, Tyree (2005) reported lower heterotrophic respiration (produced during the decomposition of organic matter by soil organisms found within the soil profile) but increased autotrophic (root) respiration in fertilized loblolly pine ecosystems (Pinus taeda L.). Increases in the GHG sink due to N addition in forest ecosystems are mostly due to stimulated large CO2 uptake from increased primary productivity (Liu and Greaver 2009), reduction in microbial activity and biomass (Fog 1988; Thirukkumaran and Parkinson 2000; Wallenstein et al. 2006), and decrease in root and/or mycorrhizal growth or respiration (Haynes and Gower 1995). Nitrogen fertilization can have the greatest influence on the sink potential in nutrient poor soils, as reported from studies conducted at eleven Swedish coniferous forests (Arnebrant et al. 1996).
In contrast, other studies have been shown to increase CO2 emissions by 6–41 % after N-fertilizer application (Table 1). Increase in CO2 emissions was observed in varied climatic conditions from temperate forests in Germany and the U.S. to subtropical forests of China, especially at an early stage of plantation (Table 1; Brumme and Beese 1992; Deng et al. 2010). Jassal et al. (2010) observed an increase in forest floor CO2 emissions due to an increase in autotrophic (including rhizospheric) soil respiration after application of N-fertilizer until four months. Lastly, there were a few studies that reported no N-fertilizer effect on CO2 emissions in pine plantations (Table 1; Castro et al. 1994; Maljanen et al. 2006). These observations may be attributed to acidic soil with pH ranging from 3.6 to 4.3, which might have limited the decomposition process and root activity, responsible for the CO2 production from soil (Maljanen et al. 2006).
Responses to N fertilization are varied, but appear to have some trends when constrained by stand type, age class, soil properties, etc. More studies are required to better understand the variability within each of these settings, particularly where forest management can be used to mitigate GHG emissions.
Management practices to increase CO2 sink
Our ability to manage forest resources sustainably is an important priority for the environment, ecosystem services and the economy. The use of long-lived wood and wood products as an energy source has been shown to be a low-cost C capture (Birdsey et al. 2000; Lippke et al. 2011). About 2 tonnes of wood produced can sequester 1 tonne of C from the atmosphere, assuming 50 % C in wood (Karchesy and Koch 1979). Furthermore, life-cycle analyses reveal that the wood products used in construction store more C and use less fossil energy than steel, concrete, or brick, whose manufacture is energy intensive and produces substantial GHG emissions. Although wood products do not permanently sequester C from the atmosphere, they do sequester it for the life of the product, which can be >100 years (Lippke et al. 2011).
Another management factor that needs consideration is forest fertilization (Albaugh et al. 2012). Fertilizer application rates should be reduced where wood is used for paper production because of high life cycle GHG emissions and short half-life of paper products (Gan et al. 2012). Nitrogen-fertilizer application usually enhances C sequestration in forest ecosystems, ranging from 20 to 70 kg C sequestered per kg of atmospheric N deposited, with most C gains occurring in aboveground biomass (Shan et al. 2001; Hyvönen et al. 2008; Pinder et al. 2013). However, the impacts on belowground C are complex and varied, but usually small (Nave et al. 2009). Intensive management and fertilization of young forest plantations can convert forested ecosystems from a C source to a C sink by increasing C sequestration potential through enhanced primary productivity compared to natural forests (Maier and Kress 2000, Albaugh et al. 2012). For example, N-fertilization in temperate deciduous forests can reduce soil CO2 emissions by 19 % (Table 1; Bowden et al. 2000), but must be considered in the context of other soil-derived GHG emissions.
Methane emissions in forest ecosystems
Source and sink of CH4
Methane is the second most abundant GHG and is 21 times more potent than CO2 (IPCC 2007b). Production of CH4 by methanogenic archaea in wetlands is a major source while consumption by CH4 oxidizing bacteria in upland soils is a major sink. The average annual CH4 emission can be 10 times larger at undrained sites relative to drained (Arnold et al. 2005). Since 1750, atmospheric CH4 concentrations increased by 8.5 ± 1.3 Gt CO2-eq. year−1 (Montzka et al. 2011). Forest soils can act as both sources and sinks of CH4. Well-drained upland forest soils are one of the most important global biological sinks for CH4 (Smith et al. 2000; Dutaur and Verchot 2007; Megonigal and Guenther 2008), where methanotrophic bacteria assimilate CH4 into the microbial biomass as organic C and oxidize it to CO2 to gain energy (King 1997; Xu and Inubushi 2004; Dutaur and Verchot 2007; USEPA 2010). Methanotrophic bacteria play a vital role in GHG budgets, especially for forest ecosystems, because they are the only biological sink for CH4. Although methanotrophic bacteria are the only organisms using CH4, they also emit CO2, usually in proportion to about 50 % of the CH4 consumed. Methane sink strength of soils depends on oxidation by methanotrophic bacteria, which is influenced by environmental factors that control oxidation rates. Globally, forest soils are a net sink of atmospheric CH4, with estimates from 1.8 to 11.8 Tg CH4–C yr−1 (IPCC 2007b). Specifically, tropical forest soils contribute 28 % (6.2 Tg yr−1) to the global CH4 sink (Veldkamp et al. 2013). Methane consumption rates for aerobic temperate forest soils can be up to 3.17 mg CH4–C m−2 day−1 (Steudler et al. 1989).
On the other hand, it has been demonstrated that flooded forest soils are CH4 sources (McKenzie et al. 1998; Rice et al. 2010). Major sources of CH4 include wetlands, ruminant animals, rice paddies, biomass burning and fossil fuel production (IPCC 2007b). Although CH4 is emitted mostly in wetland soils, it can also be found in tropical and upland soils during high rainfall or wet seasons (Keller and Reiners 1994). For example, Frankenberg et al. (2005), using space-borne near-infrared absorption spectroscopy, mapped the global CH4 distribution and observed unexpectedly high CH4 concentrations over tropical rainforests, revealing that emission inventories considerably underestimated forest CH4 sources. They reported that model simulation indicated a tropical CH4 source of around 120 Tg year−1. Another study by Crutzen et al. (2006) reported a CH4 source of 78 Tg year−1 from tropical forests, again suggesting that CH4 sources cover a wider array of soil types, biomes, and climates than previously thought.
Controls of CH4 emissions
The major factors regulating production or consumption of CH4 from soils are: (1) water-filled pore space (Adamsen and King 1993; Castro et al. 1995; Brumme and Borken 1999; Smith et al. 2000; Borken and Brumme 2009; Jassal et al. 2011; Aronson et al. 2012; Gundersen et al. 2012; Veldkamp et al. 2013), (2) N status or N-fertilization of soil (Steudler et al. 1989; Castro et al. 1995; Sitaula et al. 1995a; Smith et al. 2000; Papen et al. 2001; Fender et al. 2012; Gundersen et al. 2012; Mochizuki et al. 2012; Wood and Silver 2012), and (3) soil temperature (Castro et al. 1995; MacDonald et al. 1997; Smith et al. 2000; Veldkamp et al. 2013). Other factors shown to affect CH4 production or consumption in forest soils include pH (Sitaula et al. 1995a; Sparks 1995; Brumme and Borken 1999; Smith et al. 2000; Xu and Inubushi 2004; Borken and Brumme 2009), bulk density (Smith et al. 2000; Teepe et al. 2004), soil texture (Boeckx et al. 1997), forest type (Hudgens and Yavitt 1997), and stand age (Gundersen et al. 2012).
One of the major factors influencing CH4 oxidation is soil N status, which is often manipulated through forest N-fertilization. N limitation of CH4 oxidation is common in both wetland as well as upland soils (Bodelier and Laanbroek 2004). The effects of forest N-fertilization on CH4 production or consumption has been studied intensively (Table 2). Out of the 12 studies we found that focused on CH4 responses to forest N-fertilization, nine were conducted in temperate forests and only three in tropical forests. Ten studies reported inhibitory effects of forest N-fertilization on CH4 uptake, ranging from 5 to 95 % reduction, and two studies did not observe any effect. Inhibitory effects of N-fertilization on CH4 oxidation have been observed in temperate coniferous forest (Castro et al. 1994; Schnell and King 1994; Sitaula et al. 1995a; Xu and Inubushi 2004), temperate deciduous forest (Chan et al. 2005), mixed deciduous woodland (Dobbie and Smith 1996), and tropical forests (Castro et al. 1994).
Zhang et al. (2008a), in a rehabilitated tropical mixed pine-broadleaf forest and a disturbed tropical pine forest, reported that N-fertilization had no inhibitory effect on CH4 uptake. However, in a fertile, mature tropical broadleaf evergreen forest, N-fertilization at rates of 50, 100, and 150 kg N ha−1 inhibited soil CH4 uptake by 6, 14, and 32 %, respectively (Zhang et al. 2008a). Thus, the response of N-fertilization to soil CH4 uptake also depends on the land-use history and on the soil N status of the forest. However, an earlier study by Castro et al. (1995) reported that high-fertility sites can have 2–3 times greater CH4 uptake rates than low-fertility sites, which may be due to CH4 oxidizers being N-limited for growth, especially in unfertilized forest soils (Papen et al. 2001). This shows the variability of N-fertilization on the site productivity and capacity of land for CH4 oxidation potential.
These impacts of forest N-fertilization on CH4 consumption and production are controlled by various factors that affect N availability as depicted in Fig. 2. The main mechanism by which N fertilizers inhibits CH4 oxidation, as well as toxic effects resulting from hydroxylamine and nitrite, is ammonia, either from ammonification or ammonium based N-fertilization, which competes with CH4 for the methane monooxygenase activity for reduction (Schnell and King 1994; Bodelier 2011; Alam and Jia 2012).
Different anthropogenic or N-cycling soil processes that increases NH4 + such as N-mineralization of organic matter, application of N-fertilizer containing NH4–N (e.g., ammonium sulphate) (Saari et al. 2004), or release from clay particles (Green et al. 1994) can inhibit CH4 oxidation and reduce CH4 uptake through methanotrophic oxidation of NH4 + instead of CH4 (Harmsen and Van Schreven 1955; Steudler et al. 1989; Green et al. 1994, Neff et al. 1994; Konda et al. 2012). Reduction in CH4 uptake is also possible through changes in the N-cycle after fertilization, which induces a shift in the microbial population of CH4-oxidizing bacteria to NH4-oxidizers, thereby reducing CH4 uptake (Castro et al. 1994). Inhibitory effects on CH4 consumption can be detected shortly after N-fertilization and can persist for long periods (Smith et al. 2000; Papen et al. 2001). Additionally, high rates of nitrification can cause toxic effects on methanotrophs by producing NO2 − and NH2OH (Schnell and King 1994; King and Schnell 1994). Therefore, nitrification rate is negatively correlated with CH4 uptake (Sitaula and Bakken 1993). As such, earlier studies (Hutsch et al. 1994; Willison et al. 1995) have also shown a slight inhibitory effect of nitrate (NO3 −) on CH4 oxidation. More recent studies further support these claims, reporting that NO3 − addition can have a greater inhibitory effect on CH4 oxidation in managed forest soils (Wang and Ineson 2003; Reay and Nedwell 2004), especially to the sites with low soil N (Mochizuki et al. 2012). Few studies (Wang and Ineson 2003; Xu and Inubushi 2004) have reported strong inhibitory effects of NO3 − additions on CH4 uptake compared to NH4 addition in coniferous forest soils. In contrast, Wood and Silver (2012) in humid tropical forest observed the positive relationship between CH4 consumption and soil NO3. Ammonium and NO3 stimulate CH4 uptake by Methylomicrobium (a gamma-proteobacterium) under high methane concentration (10,000 ppm), but on the contrary inhibit CH4 uptake by Methylocystis (an alpha-proteobacteirum) in forest soils (Mohanty et al. 2006). However, at a lower CH4 concentration (1,000 ppm), only NO3 − showed an inhibitory effect. In an incubation study, CH4 uptake was observed after 27 years of N-fertilization with a high rate of 600 kg ha−1 (Borjesson and Nohrstedt 1998). This indicates that in the long-run, increased C retention in forest soils after N-fertilization can lead to increases in CH4 uptake.
Management practices to increase CH4 uptake
One of the major factors that can counteract atmospheric increases of CH4 is the consumption of CH4 by soils. Forest soils can exhibit a highly dynamic pattern in terms of CH4 flux rates, with a net uptake shifting to net emission when soil becomes submerged (McNamara et al. 2006). Human activities have reduced the soil sink for atmospheric CH4 by converting undisturbed forests to agricultural and urban land (Dobbie et al. 1996).
Type of fertilizer applied, or form of available N, can have significant impacts on CH4 oxidation potential in forest soils. For example, applying KNO3, which has low potential to inhibit CH4 oxidation, can increase CH4 uptake (Xu and Inubushi 2004). Other means to enhance CH4 uptake are by reducing flooded condition, reducing acidity, decreasing compaction, and increasing C retention. Soil hydrology controls the direction and magnitude of CH4 flux rates (Hutsch et al. 1994; Borjesson and Nohrstedt 1998; Sitaula et al. 2000; Christiansen et al. 2012). Therefore, improving soil drainage has been proposed as a mitigation measure that can reduce CH4 emission from wet soils (Castro et al. 1995; Papen et al. 2001; Dalal and Allen 2008; Guckland et al. 2009; Christiansen et al. 2012) and even convert it to a CH4 sink (Von Arnold et al. 2005; Guckland et al. 2009; Christiansen et al. 2012); however, in the process, it has been shown to release soil C (Smith et al. 2003) and should be considered in the context of all soil-derived GHG fluxes. Practices that increase C retention in forest soils could lead to increased CH4 oxidation in the long-term (Borjesson and Nohrstedt 1998). For example, wood ash application in boreal spruce forest soil has been shown to increase CH4 consumption (Maljanen et al. 2006). Soil acidity adversely affects atmospheric CH4 consumption (Smith et al. 2000; Benstead and King 2001; Borken and Brumme 2009). Therefore, lime application in forest soil can increase CH4 consumption by 25–560 % due to improvement in the chemical, biological, and physical condition of the soils (Borken and Brumme 1997).
Nitrous oxide emissions in forest ecosystems
Source and sink of N2O
Nitrous oxide is a powerful GHG in the Earth’s atmosphere, 310 times more potent than CO2, and is involved in the destruction of the stratospheric ozone layer (Ehhalt et al. 2001; IPCC 2007b). Globally, soils are the largest source of N2O, accounting for 65 % of total global emissions (Dalal et al. 2003; Smith and Conen 2004; IPCC 2007a, b). It is important to note that N2O is increasing in the Earth’s atmosphere by ≈0.3 % year−1 in the recent past (Denman et al. 2007; Davidson 2009). Although N2O emissions have decreased by 3.2 % in the US, direct emission from forest fertilization increased by 455 % since 1990 (USEPA 2012). The application of synthetic fertilizers to US forest soils in 2010 resulted in direct N2O emissions of 0.4 Tg CO2 Eq. (USEPA 2012). This increase is likely to continue in the absence of mitigation efforts (Fisher et al. 2007; Galloway et al. 2008). Ecosystem losses of N from 24 to 53 % are attributed to denitrification (Houlton et al. 2006), which is induced by high soil moisture when sufficient substrate (i.e., NO3) is available (Dobbie et al. 1999). N2O and nitric oxide (NO) are emitted by nitrification and denitrification. Depending on the soil saturation in water, nitrification or denitrification may contribute more to the production of N2O with only denitrifying bacteria able to reduce N2O to N2 under complete anoxia. Under well-drained conditions, nitrification would be the main source of N2O (Fig. 1).
Several studies have reported that significant amounts of N2O are emitted from forest ecosystems, with estimates ranging from 2.4 to 5.7 Tg N2O–N year−1 (Brumme et al. 1999; IPCC 2007b). Forest fertilization has become a common silvicultural practice where soil available-N is deficient (Chapin et al. 2002; Fox et al. 2007) or annual atmospheric N deposition is <2 kg N ha−1 (Galloway et al. 2004). A mature forest which is rich in N and SOC and has a low C:N ratio, has been observed to be a hotspot for N2O emissions after application of N-fertilizer (Zhang et al. 2008b). Usually, a positive effect of N-fertilization has been reported on N2O production from N- and P-limited forest soils via both nitrification and denitrification, two separate pathways for N2O production (Fig. 1) (Matson et al. 1992; Castro et al. 1994; Sitaula et al. 1995b; Billore et al. 1996; Hall and Matson 1999; Ventera et al. 2003; Jassal et al. 2008, 2010; Zhang et al. 2008b). However, the peak N2O emissions induced by N-fertilization only last for a short period from 2 to 3 weeks after fertilization in the summer and also in soil thawing periods in early spring for the first year. But in the second year, the elevation of N2O emissions is significant for N-fertilizer application of >100 kg N ha−1 yr−1 (Peng et al. 2011). Elevated precipitation increases soil water content and thereby increases N2O efflux, especially in combination with added N-fertilizer (Niboyet et al. 2011; Brown et al. 2012). However, studies have reported that N-fertilizer applications in temperate forest do not necessarily result in large emission of N2O (Bowden et al. 2000).
A significant N2O sink has been observed in managed forests of Canada (Kellman and Kavanaugh 2008) and Europe (Goldberg and Gebauer 2009; Inclán et al. 2012), as well as in natural forests of South Korea (Berger et al. 2013). Nitrogen-limited dry forest ecosystems can usually serve as a sink for atmospheric N2O (Castro et al. 1993; Bowden et al. 2000; Papen et al. 2001; Rosenkranz et al. 2006; Goldberg and Gebauer 2009). The finding of soil as a sink for atmospheric N2O can be explained by the presence of denitrifier population in soil, which in the absence of soil NO3, can use atmospheric N2O as an electron acceptor for the production of N2 (Papen et al. 2001). It is important to understand these findings, and the controlling factors or processes involved, in order to manage forest ecosystems to mitigate increases in atmospheric N2O.
Controls of N2O emissions
Effects of N-fertilizer application on soil N2O emissions are primarily controlled by soil N status, and thus practices that affect N mineralization or the form and amount of N-fertilizer applied (Corre et al. 1999; Ambus et al. 2006; Liu and Greaver 2009: Konda et al. 2012). However, fertilization effects are greatly dependent on soil conditions and local climate. Water filled pore space (WFPS), or soil water content, is a secondary controller of N2O emissions (Corre et al. 1999; Garcia-Montiel et al. 2001; Kellman and Kavanaugh 2008; Koehler et al. 2009; Berger et al. 2013). However, in a tropical forest with a pronounced dry season, soil moisture might be the primary factor controlling N2O emissions (Koehler et al. 2009). The magnitude of N2O emissions has been shown to increase with increasing WFPS (Abbasi and Adams 2000). In a silt loam soil, for example, autotrophic nitrification is the predominant source of N2O at 35–60 % WFPS, and denitrification predominates above 70 % WFPS (Davidson 1991; Bateman and Baggs 2005). Heterotrophic nitrification accounted for 20 % of N2O emitted at 50 % WFPS in arable soils where the main source of available N is NH4 +. A WFPS around 60 % offers optimal conditions for nitrification accounting for 81 % of N2O emitted. Nitrous oxide emissions during heterotrophic nitrification would be expected to be greater in acidic soils such as in conifer forest, where autotrophic nitrification is often inhibited (Pedersen et al. 1999; Laverman et al. 2000). Other factors controlling N2O emissions are labile C availability (Szilas et al. 1998; Davidson et al. 2000; Galloway et al. 2008; Goldberg and Gebauer 2009; Weslien et al. 2009; Goldberg et al. 2010; Brown et al. 2012; Christiansen et al. 2012; Wood and Silver 2012), soil temperature (Kellman and Kavanaugh 2008; Ullah and Moore 2011), soil pH (Sitaula et al. 1995b; Weslien et al. 2009; Peichl et al. 2010; Christiansen et al. 2012), soil texture (Berger et al. 2013), amount of throughfall (Borken and Beese 2005; Zona et al. 2013), C:N ratio (Ambus et al. 2006; Ullah and Moore 2011), land use or forest composition (Brumme et al. 1999; Ambus et al. 2006; Eickenscheidt et al. 2011), and age of forest stand (Gundersen et al. 2012).
The reviews on the effect of forest N-fertilization on N2O emissions have been summarized in Table 3. Our review indicates that, in general, forest N-fertilization increases N2O emissions ranging from 20 to >500 % compared to unfertilized controls. A large increase in N2O emissions of >fivefold after N-fertilization in tropical forest of Panama is due to the presence of an organic layer in which nitrification increased significantly following N application (Koehler et al. 2009). Nitrogen fertilization in combination with increased precipitation and temperature can encourage soil N2O emissions (Brown et al. 2012). Where precipitation is more variable, N2O and NO emissions shift in importance, with N2O flux dominating in wet months (Verchot et al. 1999). Yet, increases in soil temperature alone may (Malchair et al. 2010) or may not increase soil N2O emissions (Niboyet et al. 2011; Brown et al. 2012).
Few studies (Bowden et al. 2000; Steudler et al. 2002; Maljanen et al. 2006) fail to observe significant increases in N2O emissions after forest N-fertilization. This may be possible because: (1) trees may be better competitors for available N, (2) smaller amounts of N (33.3 kg N ha−1 per fertilization) were applied compared to other studies, and (3) WFPS was well below the optimum of 60–70 % for maximum denitrification (Garcia-Montiel et al. 2001; Steudler et al. 2002). Additionally, lack of fertilization responses on N2O emissions in these studies were also due to rapid N cycling, reducing N2O release especially in fine textured soils (Bowden et al. 2000) and N-rich forest soil (Maljanen et al. 2006).
Nitrogen-limited forest ecosystems can function as a sinks for atmospheric N2O (Papen et al. 2001). In these ecosystems, N2O uptake from the atmosphere into the soil can be catalyzed by soil denitrifiers which use N2O from the atmosphere instead of NO3 − as an electron acceptor for denitrification. On the other hand, forest N-fertilization can alter these dynamics and change soils from a net sink to a net source of atmospheric N2O (Papen et al. 2001). Usually, the effects of N-fertilization on N2O emission is triggered by rainfall events (Borken and Beese 2005; Zona et al. 2013) as soil moisture is one of the major drivers of N2O emissions. Drought has been observed to decrease soil N2O emissions and resulting soil N2O consumption. Berger et al. (2013) observed N2O consumption in sandy-loam soils of temperate deciduous forests in South Korea during early summer drought which then switched to a N2O source during the monsoon season especially when WFPS was >36 %. Soils consume N2O with varying magnitude, most likely in anoxic microsites throughout the soil profile, but the potential is larger in organic than in mineral forest soils, possibly due to higher organic C levels and C:N ratios (Ambus et al. 2006; Frasier et al. 2010; Ullah and Moore 2011).
Management practices to reduce N2O emissions
Nitrous oxide emissions are affected by forest management, and particularly N management. But, scaling up prediction of these GHG exchanges in time and space from plot level studies remains a great challenge (Schulze et al. 2009). This is because of the fact that hot spots (small but reactive areas) and hot events (brief periods of high fluxes) frequently account for much of the N2O gas exchange (Groffman et al. 2009). Improving N uptake efficiency (NUE) is important for increasing forest productivity while maintaining environmental quality and the economic efficiency of fertilization. Increasing NUE is possible by applying the right amount at the right time and adopting the best method of application through the right type or source of N-fertilizers. Selecting and applying an appropriate N source, which leads to low N2O emission potential, such as NO3 − compared to NH4 +, can reduce nitrification driven N2O emissions (Sitaula and Bakken 1993). However, Ambus et al. (2006) reported that NO3 − (in addition to NO2 −) is the dominant substrate for denitrification driven N2O emissions. Thus, the type of fertilizer must be considered in the context of soil drainage/moisture and other obvious environmental quality concerns. Enhanced efficiency fertilizers such as slow-release fertilizers, coated urea fertilizers, controlled-release fertilizers, and stabilized fertilizers (nitrification or urease inhibitors) can minimize the potential for nutrients loss to the environment (McCarty and Bremner 1989; Hall 2005; Cahill et al. 2010). These fertilizers are commonly used for lawn care, golf courses, and agriculture (Shaviv 2001; Obreza and Rouse 2006; Morgan et al. 2009) but need to be explored for use in forest ecosystems. Nitrapyrin (2-chloro-6-trichloromethyl-pyridine) has been the inhibitor mostly used (Taylor 1983; Menéndez et al. 2012; Burzaco et al. 2013) although allylthiourea (Hall 1984; Jäntti et al. 2012) has also been used. These inhibitors block the NH4 + to NH2OH step of ammonia oxidation (Campbell and Aleem 1965). Effectiveness of these inhibitors depends on concentration of inhibitors, strains of nitrifiers, substrate added, and loss of nitrapyrin through volatilization or degradation (Bedard and Knowles 1989).
Additional factors that can interact with fertilizer-N causing N2O emissions are soil acidity, moisture level and forest management. Soil acidification can have a strong effect on N2O emission in forest soils (Sitaula et al. 1995b). Liming of acidic forest soils has been shown to reduce N2O emissions by 9–62 % (Borken and Brumme 1997; Klemedtsson et al. 1997) in addition to reducing soil acidity and increasing growth and vigor of forest trees especially sugar maple (Long et al. 1997). Management of the water level can also be effective in reducing N2O emissions. However, there may be a trade-off with an increase in CH4 emissions with soil water management (Gundersen et al. 2012). Applying small amounts of N enough for plant N uptake when WFPS is < 60 % can reduce N2O emissions (Garcia-Montiel et al. 2001). Nitrous oxide emission measurement solely from inter-row soil can underestimate N2O emissions by 44–67 % (Cai et al. 2012), because annual N2O emission can decrease with distance from the tree (Pang et al. 2009). Therefore, a better understanding of the N-fertilizer effects on soil and forest management is vital for the mitigation of GHGs.
Forest regeneration strategies
Increased global demand for timber and other forest products will increase the need of forests to produce enough raw materials to satisfy societal needs. This could result in increased deforestation and, consequently, higher soil GHG emissions, primarily as CO2. Generally, little to no change in soil C is observed when land is quickly regenerated following harvesting, regardless of intensity (Johnson and Curtis 2001). This illustrates the importance of developing the next cohort of trees as quickly as possible given that CO2 emissions can be high the longer the soils are subjected to the increased temperatures and microbial activity associated with diminished canopy cover. Winjum et al. (1992) estimated that land available for reforestation and regeneration (natural and managed) could sequester 2.2–5.6 Gt C in the high-latitude zone over a 50-year period.
The magnitude of GHG emissions following harvest varies from site to site, geographical location (e.g., tropical vs. temperate forests), and management. For example, tropical forests may emit more GHGs immediately following harvest due to high decomposition rates, but these losses are quickly offset by rapid stand development. Additionally, the amount of C sequestered in wood products during a 50–100 year period is also much higher. On the other hand, the magnitude of loss in temperate regions is largely dependent on relative latitude. If we examine and compare the two highest producing temperate forests in the world, Douglas-fir (Pseudotsuga menziesii) and western hemlock (Tsuga heterophylla) in the Pacific Northwest (PNW) and loblolly pine (Pinus taeda) in the southeast US, Douglas-fir and western hemlock forests have slower decomposition rates than loblolly pine forests due to relative latitude. However, saw-timber rotation lengths are 40–50 years for Douglas-fir versus 25–30 years for loblolly pine so a few extra rotations are possible with loblolly pine over a 100 year period. This can lead to significantly more C accretion vs. a single rotation of 100 years (Johnson et al. 2003).
Sound sustainable forest management practices, specifically through proper and rapid forest regeneration (natural or artificial) immediately following harvest and debris management (i.e., forest residues and forest floor), is key to minimizing soil GHG emissions. For example, PNW stands are primarily cable logged, leaving the majority of debris on site. In combination with exceptional soil/site productivity and minimal site preparation (i.e., tillage), large GHG losses are likely low. If compared to loblolly pine in the south-eastern US, decomposition rates are accelerated due to the hot humid conditions but stands fully occupy sites more quickly and have shorter rotation lengths. These forests often have a lot of soil disturbance from site preparation (i.e., bedding, ripping, and tillage); however, unlike agriculture where 100 % of land is cultivated/disturbed, only about 20–25 % of land is site prepared, leaving large amounts of debris and the majority of forest floor intact. Silvicultural systems that promote natural regeneration such as variable retention of seed tree regimes can also enhance C stocks through quick regeneration (Archambault et al. 2008). Whether natural or artificial regeneration strategies are deployed, rapid forest regeneration can increase C absorption capacity thereby reducing the amount of GHG emissions to the atmosphere (IPCC 2006; Johnson and Curtis 2001).
Mitigation strategies
Without substantial mitigation efforts, emissions of GHGs will continue to increase as need for food, fiber, and energy increases. Land use and its management practices impact a variety of ecosystem processes (e.g., photosynthesis, respiration, nitrification, methane oxidation, ammonification, denitrification, decomposition, and combustion), which can result in significant GHGs emissions. Reversing forest losses through restoration, improvement, and conservation of forest land is a vital step for GHG mitigation (Jackson and Baker 2010). Improved N-fertilizer management practices, restoration of previously cleared forest land, and sustainable harvesting can result in an increase in net GHG sink potential. These mitigation strategies require the collaborative effort of forest owners, managers and scientists.
Based on the findings of this review, GHG mitigation strategies in managed forest require an integrated management plan taking into account several of the following:
-
1.
Nitrogen uptake efficiency Future GHG mitigation strategies need to involve the identification of fertilizer management practices that increase N uptake efficiency. Nitrogen uptake efficiency can be increased by managing N-fertilizer with appropriate form, rate, timing, placement, and by using controlled or slow release N-fertilizers.
-
2.
Nitrogen fertilization, especially in nutrient poor soils, increases cumulative GHG sink potential in forest ecosystems, mostly due to stimulated productivity.
-
3.
Identification of temporal and spatial variability: Special attention should be given to variation of soil fertility. Soil and foliar analysis provides an estimate of N-availability, which helps guide N-fertilizer rate decisions. Tools such leaf area index (LAI) using remote sensing are best to optimize nitrogen uptake efficiency and reduce gaseous loss.
-
4.
Use of inhibitors Nitrification and denitrification processes can be reduced using inhibitors.
-
5.
Carbon sequestration Best management practices that include avoiding deforestation or conversion of forest to other land uses, encouraging afforestation, increasing harvest interval, promoting urban forestry, and storing C in long lived forest products, need to be adapted to increase C retention in forest ecosystems.
-
6.
Managing soil acidity for optimal nutrient uptake Managing soil acidity by avoiding the use of acidic fertilizers and by liming of acidic forest soils has shown to reduce N2O emissions and increase CH4 consumption.
-
7.
Consideration of forest type Coniferous forest soils can have N2O fluxes three times lower than deciduous forest soils. Methane uptake is also higher from sites dominated by coniferous species.
-
8.
Soil moisture/drainage Improving soil drainage has been proposed as a mitigation measure to reduce CH4 emissions from wet soils, but more work is needed to determine concomitant contributions to N2O emissions.
-
9.
Extension education Forest N-fertilizer management practices need to be transferred to those that own and manage forest lands in order to encourage adoption of fertilizer best management practices for the benefit of reducing emissions of GHGs.
Conclusions
Nitrogen-fertilizer use in managed forest ecosystems is increasing in order to provide social, economic and environmental services. This review has indicated several direct and indirect contributions of N-fertilization on the emissions of CO2, CH4 and N2O GHGs. Most significantly is the need to improve N uptake efficiency through better N-fertilizer management practices to minimize GHG emissions. Nitrogen-fertilizer responses on soil production or consumption of GHGs are mainly affected by biological processes. They are also affected by different physical (i.e., temperature, moisture, rainfall, drainage, bulk density, and texture) and chemical (i.e., nutrient availability, labile C, soil pH, and C/N ratio) factors within managed forest ecosystems. Therefore, the multiple factors affecting N-fertilizer response on GHG emissions need to be simultaneously considered while evaluating N-fertilizer management practices for a particular site.
Additionally, managing one GHG (e.g., CH4) might increase emissions of other GHGs (e.g., N2O). Therefore, managing GHG emission effects of N-fertilization in managed forest ecosystems should evaluate cumulative net emissions considering all three major GHGs (CO2, CH4 and N2O) in order to estimate the net benefit (Law 2013). The effects of fertilization on emissions of individual gasses are often evaluated, but studies evaluating impacts on all three GHGs are needed to understand the net impact on global warming potential (GWP). If all three major GHGs are considered in the budget calculations, proper N-fertilization may decrease GWP by stimulating a biological CO2 sink (Albaugh et al. 2012), despite contrary effects on other GHGs. Consideration of the trade-offs are critical.
Limited studies are available on N-fertilizer response to GHGs emissions from managed forest ecosystems and the available studies are of small scale. The prediction and upscaling of GHG exchange is challenging as the findings from small scale studies have shown a wide variation in terms of N fertilizer use and GHG emissions. Generalizing these findings at higher scales can be erroneous. Therefore, large-scale studies or surveys are needed to better estimate GHG budgets and GWP implications of forest fertilization. Furthermore, GHG fluxes are often governed by high magnitude, short-term fluxes that are highly variable spatially. Special attention should be given to this spatial and temporal dynamic in order to increase the accuracy of GHG estimates.
Furthermore, methane uptake inhibition effects of N-fertilizer application have been well documented for the temperate managed forest ecosystems but only one study was available for a tropical climate. Therefore, there is a need for better understanding of N-fertilization effects on CH4 uptake outside of temperate latitudes.
In conclusion, these identified gaps in our scientific understanding require the collaborative attention of forest owners, managers, and scientists. Recent worldwide increases in the use of N fertilizers to enhance ecosystem services from managed forest have also increased concerns about potential increase of GHG fluxes from managed forests. This review identifies strategies, cumulatively considered as best management practices for N fertilization, that have the potential to enhance the CO2 and CH4 sink capacity and simultaneously mitigate N2O sources from managed forest ecosystems as an important GHG mitigation option for climate change strategies.
References
Abbas F, Fares A (2009) Soil organic carbon and carbon dioxide emission from an organically amended Hawaiian tropical soil. Soil Sci Soc Am J 73:995–1003
Abbasi MK, Adams WA (2000) Gaseous N emission during simultaneous nitrification–denitrification associated with mineral N fertilisation to a grassland soil under field conditions. Soil Biol Biochem 32:1251–1259
Adamsen APS, King GM (1993) Methane consumption in temperate and sub-arctic forest soils. Glob Biogeochem Cycles 5:319–334
Alam MS, Jia Z (2012) Inhibition of methane oxidation by nitrogenous fertilizers in a paddy soil. Front Microbiol 3(246):1–13
Albaugh TJ, Allen HL, Fox TR (2007) Historical patterns of forest fertilization in the Southeastern United States from 1969 to 2004. South J Appl For 31:129–137
Albaugh TJ, Vance ED, Gaudreault C, Fox TR, Allen HL, Stape JL, Rubilar RA (2012) Carbon emissions and sequestration from fertilization of pine in the southeastern United States. For Sci 58:419–429
Ambus P, Zechmeister-Boltenstern S, Butterbach-Bahl K (2006) Sources of nitrous oxide emitted from European forest soils. Biogeosciences 3:135–145
Archambault L, Delisle C, Larocque GR (2008) Forest regeneration 50 years following partial cutting in mixedwood ecosystems of southern Quebec, Canada. For Ecol Manag 257(2):703–711
Arnebrant K, Baath E, Söderström B, Nohrstedt H (1996) Soil microbial activity in eleven Swedish coniferous forests in relation to site fertility and nitrogen fertilization. Scand J Forest Res 11:1–6
Arnold KV, Nilsson M, Hanell B, Weslied P, Klemedtsson L (2005) Fluxes of CO2, CH4 and N2O from drained organic soils in deciduous forests. Soil Biol Biochem 37:1059–1071
Aronson EL, Vann DR, Helliker BR (2012) Methane flux response to nitrogen amendment in an upland pine forest soil and riparian zone. J Geophys Res Biogeol 117:G3. doi:10.1029/2012JG001962
Bateman EJ, Baggs EM (2005) Contribution of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fertil Soils 41:379–388
Bedard C, Knowles R (1989) Physiology, biochemistry, and specific inhibitors of CH4, NH4 +, and CO oxidation by methanotrophs and nitrifiers. Microb Rev 53:68–84
Benstead J, King GM (2001) The effect of soil acidification on atmospheric methane uptake by a Maine forest soil. FEMS Microbiol Ecol 34:207–212
Berg B, Laskowski R (2006) Litter decomposition: a guide to carbon and nutrient turnover. Adv Ecol Res 38. Elsevier Academic Press, San Diego, p 421
Berger S, Jung E, Köpp J, Kang H, Gebauer G (2013) Monsoon rains, drought periods and soil texture as drivers of soil N2O fluxes -Soil drought turns East Asian temperate deciduous forest soils into temporary and unexpectedly persistent N2O sinks. Soil Biol Biochem 57:273–281
Bernal S, Hedin LO, Likens GE, Stefan G, Buso DC (2012) Complex response of the forest nitrogen cycle to climate change. Proc Natl Acad Sci USA 109:3406–3411
Billore SK, Numata M, Minami K (1996) Nitrous oxide emission from grassland and forest soils through nitrification. Curr Sci 70:1010–1012
Birdsey R, Alig R, Adams D (2000) Mitigation activities in the forest sector to reduce emissions and enhance sinks of greenhouse gases. In: Joyce LA, Birdsey R (eds) The impact of climate change on America’s forests: A technical document supporting the 2000 USDA Forest Service RPA Assessment. Gen. Tech. Rep. RMRS-GTR-59. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, pp 112–131
Bodelier PLE (2011) Interactions between nitrogenous fertilizers and methane cycling in wetland and upland soils. Curr Opin Environ Sustain 3:379–388
Bodelier PLE, Laanbroek HJ (2004) Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol Ecol 47:265–277
Boeckx P, Cleemput OV, Villaralvo I (1997) Methane oxidation in soils with different textures and land use. Nutr Cycl Agroecosyst 49:91–95
Borjesson G, Nohrstedt HO (1998) Short- and long-term effects of nitrogen fertilization on methane oxidation in three Swedish forest soils. Biol Fertil soils 27:113–118
Borken W, Beese F (2005) Control of nitrous oxide emissions in European beech, Norway spruce and Scots pine forests. Biogeochemistry 76:141–159
Borken W, Brumme R (1997) Liming practices in temperate forest ecosystems and the effects on CO2, N2O and CH4 fluxes. Soil Use Manage 13:251–257
Borken W, Brumme R (2009) Methane uptake by forest soils. Ecol Stu 208 Part B 369–385
Bouma TJ, Bryla DR (2000) On the assessment of root and soil respiration for soils of different textures: interactions with soil moisture contents and soil CO2 concentrations. Plant Soil 227:215–221
Bowden RD, Melillo JM, Steudler PA (1991) Effects of nitrogen additions on annual nitrous-oxide fluxes from temperate forest soils in the northeastern United States. J Geophys Res 96:9321–9328
Bowden RD, Rullo R, Stevens GR, Steudler PA (2000) Soil fluxes of carbon dioxide, nitrous oxide, and methane at a productive temperate deciduous forest. J Environ Qual 29:268–276
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P (2004) Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard forest. For Ecol Manag 196:43–56
Brown JR, Blankinship JC, Niboyet A, van Groenigen KJ, Dijkstra P, Roux XL, Leadley PW, Hungate BA (2012) Effects of multiple global change treatments on soil N2O fluxes. Biogeochemistry 109:85–100
Brumme R, Beese F (1992) Effects of liming and nitrogen fertilization on emissions of CO2 and N2O from a temperate forest. J Geophys Res Atmos 97:12851–12858
Brumme R, Borken W (1999) Site variation in methane oxidation as affected by atmospheric deposition and type of temperate forest ecosystem. Glob Biogeochem Cycles 13:493–501
Brumme R, Borken W, Finke S (1999) Hierarchical control on nitrous oxide emission in forest ecosystems. Glob Biogeochem Cycles 13:1137–1148
Burton AJ, Pregitzer KS, Ruess RW, Hendrick RL, Allen MF (2002) Root respiration in North American forests: effects of nitrogen concentration and temperature across biomes. Oecologia 131:559–568
Burton AJ, Pregitzer KS, Crawford JN, Zogg GP, Zak DR (2004) Simulated chronic NO3 deposition reduces soil respiration in northern hardwood forests. Glob Change Biol 10:1080–1091
Burzaco JP, Smith DR, Vyn TJ (2013) Nitrous oxide emissions in Midwest US maize production vary widely with band-injected N fertilizer rates, timing and nitrapyrin presence. Environmental Research Letters, 8(3), 035031. IP Address: 128.173.38.238
Butnor JR, Johnsen KH, Oren R, Katul GG (2003) Reduction of forest floor respiration by fertilization on both carbon dioxide-enriched and reference 17-year-old loblolly pine stands. Glob Change Biol 9:849–861
Cahill S, Osmond D, Israel D (2010) Nitrogen release from coated urea fertilizers in different soils. Commun Soil Sci Plant Anal 41:1245–1256
Cai Y, Ding W, Luo J (2012) Spatial variation of nitrous oxide emission between interrow soil and interrow plus row soil in a long-term maize cultivated sandy loam soil. Geoderma 181–182:2–10
Campbell NER, Aleem MIH (1965) The effect of 2-chloro, 6-(trichloromethyl) pyridine on the chemoautotrophic metabolish of nitrifying bacteria. 1. Ammonia and hydroxylamine oxidation by Nitrosomonas. Antonie Van Leeuwenhoek 31:124–136
Canfield DE, Glazer AN, Falkowski PG (2010) The evolution and future of Earth’s nitrogen cycle. Science 330:192–196
Castro MS, Steudler PA, Melillo JM, Aber JD, Millham S (1993) Exchange of N2O and CH4 between the atmosphere and soils in spruce-fir forests in the northeastern United States. Biogeochemistry 18:119–135
Castro MS, Peterjohn WT, Melillo JM, Steudler PA, Gholz HL, Lewis D (1994) Effect of nitrogen fertilization on the fluxes of N2O, CH4, and CO2 from soils in a Florida slash pine plantation. Can J For Res 24:9–13
Castro MS, Steudler PA, Melillo JM, Aber JD, Bowden RD (1995) Factors controlling atmospheric methane consumption by temperate forest soils. Glob Biogeochem Cycles 9:1–10
Chan ASK, Steudler PA, Bowden RD, Gulledge J, Cavanaugh CM (2005) Consequences of nitrogen fertilization on soil methane consumption in a productive temperate deciduous forest. Biol Fertil Soils 41:182–189
Chapin FS, Matson PA, Mooney HA (2002) Principles of terrestrial ecosystem ecology. Springer, New York
Christiansen JR, Vesterdal L, Gundersen P (2012) Nitrous oxide and methane exchange in two small temperate forest catchments—effects of hydrological gradients and implication for global warming potential of forest soils. Biogeochemistry 107:437–454
Ciais P, Tans PP, Trolier M, White JWC, Francey RJ (1995) A large northern hemisphere terrestrial CO2 sink indicated by 13C/12C of atmospheric CO2. Science 269:1098–1102
Conrad R (1996) Soil microorganism as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microb Rev 60:609
Corre MD, Pennock DJ, Kessel C, Kirkelliott D (1999) Estimation of annual nitrous oxide emissions from a transitional grassland-forest region in Saskatchewan, Canada. Biogeochemistry 44:29–49
Crutzen PJ, Sanhueza E, Brenninkmeijer CAM (2006) Methane production from mixed tropical savanna and forest vegetation in Venezuela. Atmos Chem Phys Discuss 6:3093–3097
Cusack DF, Whendee L, Silver MS, Torn SDB, Firestone MK (2011) Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 92:621–632
Dalal RC, Allen DE (2008) Greenhouse gas fluxes from natural ecosystems. Aust J Bot 56:369–407
Dalal RC, Wang WJ, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Aust J Soil Res 41:165–195
Davidson EA (1991) Fluxes of nitrous oxide and nitric oxide from terrestrial ecosystems. A global inventory of nitric oxide emissions from soils. In: Rogers JE, Whitman WB (eds) Microbial production and consumption of greenhouse gases: methane, nitrogen oxides, and halomethanes. American Society for Microbiology, Washington, pp 219–235
Davidson EA (2009) The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat Geosci 2:659–662
Davidson EA, Keller M, Ericson HE, Verchot L, Louis V, Veldkamp E (2000) Testing a conceptual model of soil emissions of nitrous and nitric oxides. Bioscience 50:667–680
Deng Q, Zhou G, Liu J, Liu S, Duan H, Zhang D (2010) Responses of soil respiration to elevated carbon dioxide and nitrogen addition in young subtropical forest ecosystems in China. Biogeosciences 7:315–328
Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, Hauglustaine D, Heinze C, Holland E, Jacob D, Lohmann U, Ramachandran S, da Silva Dias PL, Wofsy SC, Zhang X (2007) Couplings between changes in the climate system and biogeochemistry. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate Change 2007: the physical science basis. Contribution of working group i to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp 499–587
Dobbie KE, Smith KA (1996) Comparision of CH4 oxidation rates in woodland, arable and set aside soils. Soil Biol Biochem 28:1357–1365
Dobbie KE, Smith KA, Prieme A, Christensen S, Degorska A, Orlanski P (1996) Effect of land use on the rate of methane uptake by surface soils in northern Europe. Atmos Environ 30(7):1005–1011
Dobbie KE, McTaggart IP, Smith KA (1999) Nitrous oxide emissions from intensive agriculture systems: variations between crops and seasons, key driving variables, and mean emission factors. J Geophys Res 104:26891–26899
Dutaur L, Verchot LV (2007) A global inventory of the soil CH4 sink. Global Biogeochem Cycles 21:GB4013
Ehhalt D, Prather M, Dentener F, Derwent R, Dlugokencky EJ, Holland E, Isaksen I, Katima J, Kirchhoff V, Matson P, Midgley P, Wang M, Berntsen T, Bey I, Brasseur G, Buja L, Collins WJ, Daniel JS, DeMore WB, Derek N, Dickerson R, Etheridge D, Feichter J, Fraser P, Friedl R, Fuglestvedt J, Gauss M, Grenfell L, Grubler A, Harris N, Hauglustaine D, Horowitz L, Jackman C, Jacob D, Jaegle L, Jain AK, Kanakidou M, Karlsdottir S, Ko M, Kurylo M, Lawrence M, Logan JA, Manning M, Mauzerall D, McConnell J, Mickley LJ, Montzka S, Muller JF, Olivier J, Pickering K, Pitari G, Roelofs GJ, Rogers H, Rognerud B, Smith SJ, Solomon S, Staehelin J, Steele P, Stevenson DS, Sundet J, Thompson A, van Weele M, von Kuhlmann R, Wang Y, Weisenstein D, Wigley TM, Wild O, Wuebbles DJ, Yantosca R, Joos F, McFarland M (2001). Atmospheric chemistry and greenhouse gases. In: Houghton J et al (eds) IPCC climate change 2001: the scientific basis. Cambridge Univ. Press, pp 240–287
Eickenscheidt N, Brumme R, Veldkamp E (2011) Direct contribution of nitrogen deposition to nitrous oxide emissions in a temperate beech and spruce forest—a 15 N tracer study. Biogeoscience 8:621–635
Elliot JR, Fox TR (2006) Effects of a controlled release fertilizer on the nitrogen dynamics of mid-rotation loblolly pine plantation in the Piedmont, Virginia. Gen. Tech. Rep. SRS-92. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southern Research Station, pp 124–128
Fender A, Pfeiffer B, Gansert D, Leuschner C, Daniel R, Jungkunst HF (2012) The inhibiting effect of nitrate fertilization on methane uptake of a temperate forest soil is influenced by labile carbon. Biol Fertil Soils 48:621–631
FIDO (Forest Inventory Data Online) (2011) Carbon in U.S. forests. USDA Forest Service (Accessed on 8/9/2011) http://www.fia.fs.fed.us/
Fisher BS, Nakicenovic N, Alfsen K, Corfee Morlot J, de la Chesnaye F, Hourcade J Ch, Jiang K, Kainuma M, Rovere E La, Matysek A, Rana A, Riahi K, Richels R, Rose S, van Vuuren D, Warren R (2007) Issues related to mitigation in the long term context. In: Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA (eds) Climate Change 2007: Mitigation. Contribution of working group III to the fourth assessment report of the Inter-governmental Panel on Climate Change, Ch. 3 Cambridge University Press, Cambridge
Fleischer K, Janssens IA, Gianelle D, Dolman AJ, Rebel KT, van der Molen MK, Erisman JW, Wassen MJ, van Loon EE, Montagnani L, Gough CM, Herbst M (2013) The contribution of nitrogen deposition to the photosynthetic capacity of forests. Glob Biogeochem Cycles 27:187–199
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Fox TR, Allen HL, Albaugh TJ, Rubilar R, Carlson CA (2007) Tree nutrition and forest fertilization of pine plantations in the southern United States. South J Appl For 31:5–11
Frankenberg C, Meirink JF, van Weele M, Platt U, Wagner T (2005) Assessing methane emissions from global space-borne observations. Science 308:1010–1014
Frasier R, Ullah S, Moore TR (2010) Nitrous Oxide Consumption Potentials of Well-drained Forest Soils in Southern Quebec, Canada. Geomicrobiol J 27:53–60
Fujinuma R, Balster NJ, Hyung-Kyung L (2011) Reduced rates of controlled-release fertilizer lower potential nitrogen leaching from a Wisconsin bare-root tree nursery. Proceedings of the 17th Central Hardwood Forest Conference GTR-NRS-P-78. pp 347–357. http://www.nrs.fs.fed.us/pubs/gtr/gtr-p-78papers/37fujinumap78.pdf
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger RP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vorosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Gan J, Smith CT, Langeveld JWA (2012) Effects of considering greenhouse gas consequences on fertilizer use in loblolly pine plantations. J Environ Manage 113:383–389
Garcia-Montiel DC, Steudler PA, Neill C, Melillo JM, Piccolo MC, Cerri CC (2001) Controls on soil nitrogen oxide emissions from forest and pastures in the Brazilian Amazon. Glob Biogeochem Cycles 15:1021–1030
Goldberg SD, Gebauer G (2009) Drought turns a central European Norway spruce forest soil from an N2O source to a transient N2O sink. Glob Change Biol 15:850–860
Goldberg SD, Knorr KH, Blodau C, Lischeid G, Gebauer G (2010) Impact of altering the water table height of an acidic fen on N2O and NO fluxes and soils concentrations. Glob Change Biol 16:220–223
Gough CM, Seiler JR (2004) Belowground carbon dynamics in loblolly pine (Pinus taeda) immediately following diammonium phosphate fertilization. Tree Physiol 24:845–851
Green CJ, Blackmer AM, Yang NC (1994) Release of fixed ammonium during nitrification in soils. Soil Sci Soc Am J 58:1411–1415
Griffin KL, Bashkin MA, Thomas RB, Strain BR (1997) Interactive effects of soil nitrogen and atmospheric carbon dioxide on root/rhizosphere carbon dioxide efflux from loblolly and ponderosa pine seedlings. Plant Soil 190:11–18
Grip H, Jansson P (2012) Modelling 100 years of C and N fluxes at fertilized Swedish mountainous spruce forests. In: Haigh MJ, Hofer T, Krecek J, Kubin E (eds) Management of mountain watersheds. Springer, The Netherlands, pp 200–206
Groffman PM, Butterbach-Bahl K, Fulweiler RW, Gold AJ, Morse JL, Stander EK, Tague C, Tonitto C, Vidon P (2009) Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 93:49–77
Guckland A, Flessa H, Prenzel J (2009) Controls of temporal and spatial variability of methane uptake in soils of a temperate deciduous forest with different abundance of European beech (Fagus sylvatica L.). Soil Biol Biochem 41:1659–1667
Gundersen PP, Christiansen JR, Alberti G, Brüggemann N, Castaldi S, Gasche R, Kitzler B, Klemedtsson L, Lobo-do-Vale R, Moldan F, Rutting T, Schleppi P, Weslien P, Zechmeister-Boltenstern S (2012) The response of methane and nitrous oxide fluxes to forest change in Europe. Biogeosciences 9:3999–4012
Haase DL, Alzugaray P, Rose R, Jacobs DF (2007) Nutrient-release rates of controlled-release fertilizers in forest soil. Commun Soil Sci Plant Anal 38:739–750
Hall GH (1984) Measurement of nitrification rates in lake sediments: comparision of the nitrification inhibitors nitrapyrin and allylthiourea. Microb Ecol 10:25–36
Hall A (2005) Benefits of enhanced-efficiency fertilizer for the environment. In: International Fertilizer Association International workshop on enhanced-efficiency fertilizers. Frankfurt, Germany, 28–30 June, 2005. The Mosaic Company, USA
Hall SJ, Matson PA (1999) Nitrogen oxide emissions after nitrogen addition in tropical forests. Nature 400:152–155
Harmsen GW, Van Schreven DA (1955) Mineralization of organic nitrogen in soil. Adv Agron 7:299–398
Haynes BE, Gower ST (1995) Belowground carbon allocation in unfertilized and fertilized red pine plantations in northern Wisconsin. Tree Physiol 15:317–325
Houlton BZ, Sigman DM, Hedin LO (2006) Isotopic evidence for large gaseous losses from tropical rainforests. Proc Natl Acad Sci 103(23):8745–8750
Hudgens DE, Yavitt JB (1997) Land-use effects on soil methane and carbon dioxide fluxes in forests near Ithaca, New York. Ecoscience 4:214–222
Hutsch BW, Webster CP, Powlson DS (1994) Methane oxidation in soil as affected by land use, soil pH and N fertilization. Soil Biol Biochem 26:1613–1622
Hyvönen R, Persson T, Andersson S, Olsson B, Ågren GI, Linder S (2008) Impact of long-term nitrogen addition on carbon stocks in trees and soils in northern Europe. Biogeochemistry 89:121–137
Inclán R, Uribe C, Sánchez L, Sánchez DM, Clavero Á, Fernández AM, Morante R, Blanco A, Jandl R (2012) N2O and CH4 fluxes in undisturbed and burned holm oak, scots pine and pyrenean oak forests in central Spain. Biogeochemistry 107:19–41
Intergovernmental Panel on Climate Change (IPCC) (2006) 2006 IPCC guidelines for national greenhouse gas inventories. Eggleston HS, Buendia L, Miwa K, Nagra T, Tanabe K (eds) Prepared by the national greenhouse gas inventories programme. Institute for Global Environmental Strategies, Japan
Intergovernmental Panel on Climate Change (IPCC) (2007a) Climate Change 2007: Synthesis report. Contribution of Working Groups I, II, and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In: Pachauri RK, Reisinger A (eds) IPCC Geneva, Switzerland 2, p 104. http://www.ipcc.ch/publications_and_data/publications_ipcc_fourth_assessment_report_synthesis_report.htm
Intergovernmental Panel on Climate Change (IPCC) (2007b) Climate Change 2007: The physical science basis. Contribution of Working Groups I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon SD, Manning QM, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, Chapter 2, pp 212–213. http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter2.pdf
Jackson RB, Baker JS (2010) Opportunities and constraints for forest climate mitigation. Bioscience 60:698–707
Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze ED, Tang J, Law BE (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Jäntti H, Leskinen E, Stange CF, Hietanen S (2012) Measuring nitrification in sediments–comparison of two techniques and three 15NO measurement methods. Isot Environ Health Stud 48:313–326
Jassal RS, Black TA, Chen B, Roy R, Nesic Z, Spittlehouse DL, Trofymow JA (2008) N2O emissions and carbon sequestration in a nitrogen-fertilized Douglas fir stand. J Geophys Res 113(G04013):10. doi:10.1029/2008JG000764
Jassal RS, Black TA, Trofymow JA, Roy R, Nesic Z (2010) Soil CO2 and N2O flux dynamics in a nitrogen-fertilized pacific northwest douglas-fir stand. Geoderma 157:118–125
Jassal RS, Black TA, Roy R, Ethier G (2011) Effect of nitrogen fertilization on soil CH4 and N2O fluxes, and soil and bole respiration. Geoderma 162:182–186
Johnson DW, Curtis PS (2001) Effects of forest management on soil C and N storage: meta-analysis. For Ecol Manag 140(2):227–238
Johnson DW, Todd DE, Tolbert VR (2003) Changes in ecosystem carbon and nitrogen in a Loblolly pine plantation over the first 18 years. Soil Sci Soc Am J 67:1594–1601
Karchesy J, Koch P (1979) Energy production from hardwoods growing on southern pine sites. US Department of agriculture. Forest service. General Technical Report SO-24
Keller M, Reiners WA (1994) Soil-atmosphere exchange of nitrous oxide, nitric oxide, and methane under secondary succession of pasture to forest in the Atlantic lowlands of Costa Rica. Glob Biogeochem Cycles 8:399–409
Kellman L, Kavanaugh K (2008) Nitrous oxide dynamics in managed northern forest soil profiles: is production offset by consumption? Biogeochemistry 90. Isotopic evidence for large gaseous losses from tropical rainforests 115–128
King GM (1997) Responses of atmospheric methane consumption by soils to global climate change. Glob Change Biol 3:351–362
King GM, Schnell S (1994) Ammonium and nitrite inhibition of methane oxidation by Methylobacter albus BG8 and Methylosinus trichosporium OB3b at low methane concentrations. Appl Environ Microb 60:3508–3513
Klemedtsson L, Klemedtsson A, Moldan F, Weslien P (1997) Nitrous oxide emission from Swedish forest soils in relation to liming and simulated increased N-deposition. Biol Fertil Soils 25:290–295
Klemedtsson L, Arnold KV, Weslien P, Gundersen P (2005) Soil CN ratio as a scaler parameter to predict nitrous oxide emissions. Glob Change Biol 11:1142–1147
Koehler B, Corre MD, Veldkamp E, Wullaert H, Wright SJ (2009) Immediate and long-term nitrogen oxide emissions from tropical forest soils exposed to elevated nitrogen input. Glob Change Biol 15:2049–2066
Konda R, Ohta S, Ishizuk S, Heriyano J, Wicaksono A (2012) N2O and CH4 fluxes from Acacia mangium plantation soils in response to nitrogen application and FH layer removal. Anadolu J Agric Sci 25:53–57
Laverman AM, Zoomer HR, Engelbrecht D, Berg MP, van Straalen NM, van Versveld HW, Verhoef HA (2000) Soil layer-specific variability in net nitrification and denitrification in an acid coniferous forest. Biol Fertil Soils 32:427–434
Law B (2013) Nitrogen deposition and forest carbon. Nature 496:307–308
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Lee K, Jose S (2003) Soil respiration, fine root production, and microbial biomass in cottonwood and loblolly pine plantations along a nitrogen fertilization gradient. For Ecol Manage 185:263–273
Lippke B, Elaine O, Harrison R, Skog K, Gustavsson L, Sathre R (2011) Life cycle impacts of forest management and wood utilization on carbon mitigation: knowns and unknowns. Carbon Manage 2:303–333. www.future-science.com
Liski J, Pussinen A, Pingoud K, Makipaa R, Karjalainen T (2001) Which rotation length is favourable to carbon sequestration? Can J For Res 31:2004–2013
Liu L, Greaver TL (2009) A review of nitrogen enrichment effects on three biogenic GHGs: the CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecol Lett 12:1103–1117
Long RP, Horsley SB, Lilja PR (1997) Impact of forest liming on growth and crown vigor of sugar maple and associated hardwoods. Can J For Res 27:1560–1573
MacDonald JA, Skiba U, Sheppard LJ, Ball B, Roberts JD, Smith KA, Fowler D (1997) The effect of nitrogen deposition and seasonal variability on methane oxidation and nitrous oxide emission rates in an upland spruce plantation and moorland. Atmos Environ 31:3693–3706
Magill AH, Aber JD, Hendricks JJ, Bowden RD, Melillo JM, Steudler PA (1997) Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol Appl 7:402–415
Maier CA, Kress LW (2000) Soil CO2 evolution and root respiration in 11 year-old loblolly pine (Pinus taeda) plantations as affected by moisture and nutrient availability. Can J For Res 30:347–359
Malchair S, De Boeck HJ, Lemmens CMHM, Ceulemans R, Merckx R, Nijs I, Carnol M (2010) Diversity–function relationship of ammonia-oxidizing bacteria in soils among functional groups of grassland species under climate warming. Appl Soil Ecol 44:15–23
Maljanen M, Jokinen H, Saari A, Strommer R, Martikainen PJ (2006) Methane and nitrous oxide fluxes, and carbon dioxide production in boreal forest soil fertilized with wood ash and nitrogen. Soil Use Manage 22:151–157
Matson PA, Gower ST, Volkmann C, Billow C, Grier CC (1992) Soil nitrogen cycling and nitrous oxide flux in a Rocky Mountain Douglas-fir forest: effects of fertilization, irrigation and carbon addition. Biogeochemistry 18:101–117
McCarty GW, Bremner JM (1989) Inhibition of nitrification in soil by heterocyclic nitrogen compounds. Biol Fertil Soils 8(3):204–211
McKenzie C, Schiff S, Aravena R, Kelly C, Louis VS (1998) Effect of temperature on production of CH4 and CO2 from peat in a natural and flooded boreal forest wetland. Clim Change 40:247–266
McKinley DC, Ryan MG, Birdsey RA, Giardina CP, Harmon ME, Heath LS, Houghton RA, Jackson RB, Morrison JF, Murray BC, Pataki DE, Skog KE (2011) A synthesis of current knowledge on forests and carbon storage in the United States. Ecol Appl 21:1902–1924
McNamara NP, Chamberlain PM, Piearce TG, Sleep D, Black HI, Reay DS, Ineson P (2006) Impact of water table depth on forest soil methane turnover in laboratory soil cores deduced from natural abundance and tracer 13C stable isotope experiments. Isot Environ Healt Stud 42:379–390
Megonigal JP, Guenther AB (2008) Methane emissions from upland forest soils and vegetation. Tree Physiol 28:491–498
Menéndez S, Barrena I, Setien I, González-Murua C, Estavillo JM (2012) Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions. Soil Biol Biochem 53:82–89
Mo JM, Zhang W, Zhu WX, Gundersen P, Fang YT, Li DJ, Wang H (2008) Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob Change Biol 14:403–412
Mochizuki Y, Koba K, Yoh M (2012) Strong inhibitory effect of nitrate on atmospheric methane oxidation in forest soils. Soil Biol Biochem 50:64–166
Mohanty SR, Bodelier PL, Floris V, Conrad R (2006) Differential effects of nitrogenous fertilizers on methane-consuming microbes in rice field and forest soils. Appl Environ Microbiol 72:1346–1354
Mojeremane W, Rees RM, Mencuccini M (2012) The effects of site preparation practices on carbon dioxide, methane and nitrous oxide fluxes from a peaty gley soil. Forestry 85:1–15
Monastersky R (2013) Global carbon dioxide levels near worrisome milestone. Nature 497:13–14
Montzka SA, Dlugokencky EJ, Butler JH (2011) Non-CO2 greenhouse gases and climate change. Nature 476:43–50
Morgan KT, Cushman KE, Sato S (2009) Release mechanisms for slow- and controlled release fertilizers and strategies for their use in vegetable production. Hortic Technol 19:10–12
Nave LE, Vance ED, Swanston CW, Curtis PS (2009) Impacts of elevated N inputs on north temperate forest soil C storage, C/N, and net N-mineralization. Geoderma 153:231–240
Neff JC, Bowman WD, Holland EA, Fisk MC, Schmidt SK (1994) Fluxes of nitrous oxide and methane from nitrogen-amended soils in a Colorado alpine ecosystem. Biogeochemistry 27:23–33
Niboyet A, Le Roux X, Dijkstra P, Hungate BA, Barthes L, Blankinship JC, Brown JR, Field CB, Leadley PW (2011) Testing interactive effects of global environmental changes on soil nitrogen cycling. Ecosphere 2 Article 56
Obreza TA, Rouse RE (2006) Long-term response of’Hamlin’ orange trees to controlledrelease nitrogen fertilizers. Hortic Sci 41:423–426
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala S, McGuire DA, Piao S, Rautiainen A, Sitch S, Hayes D (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–992
Pang JZ, Wang XK, Ouyang YJ, Liu XJ (2009) Nitrous oxide emissions from an apple orchard soil in the semiarid Loess Plateau of China. Biol Fertil Soil 46:37–44
Papen H, Daum M, Steinkamp R, Butterbach-Bahl K (2001) N2O and CH4-Fluxes from soils of a N-limited and N-fertilized spruce forest ecosystem of the temperate zone. J Appl Biol 75:159–163
Pedersen H, Dunkin KA, Firestone MK (1999) The relative importance of autotrophic and heterotrophic nitrification in aconifer forest soil as measured by 15 N tracer and pool dilution techniques. Biogeochemistry 44:135–150
Peichl M, Arain MA, Ullah S, Moore TR (2010) Carbon dioxide, methane, and nitrous oxide exchanges in an age-sequence of temperate pine forests. Glob Change Biol 16:2198–2212
Peng Q, Qi Y, Dong Y, Xiao S, He Y (2011) Soil nitrous oxide emissions from a typical semiarid temperate steppe in inner Mongolia: effects of mineral nitrogen fertilizer levels and forms. Plant Soil 342:345–357
Philips RP, Fahey TJ (2007) Fertilization effects on fine root biomass, rhizosphere microbes and respiratory fluxes in hardwood forest soils. New Phytol 176:655–664
Pilegaard K, Skiba U, Ambus P, Beier C, Brüggemann N, Butterbach-Bahl K, Dick J, Dorsey J, Duyzer J, Gallagher M, Gasche R, Horvath L, Kitzler B, Leip A, Pihlatie MK, Rosenkranz P, Seufert G, Vesala T, Westrate H, Zechmeister-Boltenstern S (2006) Factors controlling regional differences in forest soil emission of nitrogen oxides (NO and N2O). Biogeosciences 3:651–661
Pinder RW, Davidson EA, Goodale CL, Greaver TL, Herrick JD, Liu L (2012) Climate change impacts of US reactive nitrogen. Proc Natl Acad Sci 109:7671–7675
Pinder RW, Bettez ND, Bonan GB, Greaver TL, Wieder WR, Schlesinger WH, Davidson EA (2013) Impacts of human alteration of the nitrogen cycle in the US on radiative forcing. Biogeochemistry 114:25–40
Pregitzer KS, Laskowski MJ, Burton AJ, Lessard VC, Zak DR (1998) Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiol 18:665–670
Qi Y, Xu M (2001) Separating the effects of moisture and temperature on soil CO2 efflux in a coniferous forest in the Sierra Nevada mountains. Plant Soil 237:15–23
Reay DS, Nedwell DB (2004) Methane oxidation in temperate soils: effects of inorganic N. Soil Biol Biochem 36:2059–2065
Reich PB, Tjoelker MG, Pregitzer KS, Wright IJ, Oleksyn J, Machado JL (2008) Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol Lett 11:793–801
Rice AL, Butenhoff CL, Shearer MJ, Teama D, Rosenstiel TN, Khalil MAK (2010) Emissions of anaerobically produced methane by trees. Geophys Res Lett 37:L03807. doi:10.1029/2009GL041565
Ring ERE, Jacobson SJS, Högbom LHL (2011) Long-term effects of nitrogen fertilization on soil chemistry in three Scots pine stands in Sweden. Can J Forest Res 41:279–288
Rosenkranz P, Brüggemann N, Papen H, Xu Z, Seufert G, Butterbach-Bahl K (2006) N20 no and CH4 exchange and microbial n turnover over a Mediterranean pine forest soil. Biogeosciences 3:121–133
Saari A, Rinnan R, Martikainen PJ (2004) Methane oxidation in boreal forest soils: kinetics and sensitivity to pH and ammonium. Soil Biol Biochem 36:1037–1046
Schimel J (2000) Rice, microbes and methane. Nature 403:375–376
Schlesinger WH, Bernhardt ES (2013) Biogeochemistry: an analysis of global change. Academy Press, MA
Schnell S, King GM (1994) Mechanistic analysis of ammonium inhibition of methane consumption in forest soils. Appl Environ Microbiol 60:3514–3521
Schulze ED, Luyssaert S, Ciais P, Freibauer A, Janssens IA, Soussana JF, Smith P, Grace J, Levin I, Thiruchittampalam B, Heimann M, Dolman AJ, Valentini R, Bousquet P, Peylin P, Peters W, Roedenbeck C, Etiope G, Vuichard N, Wattenbach M, Nabuurs GJ, Poussi Z, Nieschulze J, Gash JH (2009) Importance of methane and nitrous oxide for Europe’s terrestrial greenhouse-gas balance. Nat Geosci 2:842–850
Shan J, Morris LA, Hendrick RL (2001) The effects of management on soil and plant carbon sequestration in slash pine plantations. J Appl Ecol 38:932–941
Shaviv A (2001) Advances in controlled-release fertilizers. Adv Agron 71:1–49
Sitaula BK, Bakken LR (1993) Nitrous oxide release from spruce forest soil: relationships with nitrification, methane uptake, temperature, moisture and fertilization. Soil Biol Biochem 25:1415–1421
Sitaula BK, Bakken LR, Abrahamsen B (1995a) N-fertilization and soil acidification effects on N2O and CO2 emission from temperate pine forest soil. Soil Biol Biochem 27:1401–1408
Sitaula BK, Bakken LR, Abrahamsen G (1995b) CH4 uptake by temperate forest soil: effect of N input and soil acidification. Soil Biol Biochem 27:871–880
Sitaula BK, Hansen S, Sitaula JIB, Bakken LR (2000) Methane oxidation potentials and fluxes in agricultural soil: effects of fertilization and soil compaction. Biogeochemistry 48:323–339
Smith KA, Conen F (2004) Impacts of land management on fluxes of trace greenhouse gases. Soil Use Manage 20:255–263
Smith KA, Dobbie KE, Ball BC, Bakken LR, Sitaula BK, Hansen S, Brumme R, Borken W, Christensen S, Prieme A, Fowler D, Macdonald JA, Skiba U, Klemedtsson L, Kasimir-Klemedtsson A, Degorska A, Orlanski P (2000) Oxidation of atmospheric methane in Northern European soils, comparison with other ecosystems, and uncertainties in the global terrestrial sink. Glob Change Biol 6:791–803
Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A (2003) Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur J Soil Sci 54:779–791
Sparks DL (1995) Environmental soil chemistry. Academic Press, New York, p 267
Steudler PA, Bowden RD, Mellilo JM, Aber JD (1989) Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature 341:314–316
Steudler PA, Garcia-Montiel DC, Piccolo MC, Neill C, Melillo JM, Feigl BJ, Cerri CC (2002) Trace gas responses of tropical forest and pasture soils to N and P fertilization. Glob Biogeochem Cy 16:1023
Szilas CP, Borggaard OK, Hansen HCB, Rauer J (1998) Potential iron and phosphate mobilization during flooding of soil material. Water Air Soil Pollut 106:97–109
Taylor BE (1983) Assays of microbial nitrogen transformation, pp 809–837. In: Carpenter EJ, Capone DG (eds) Nitrogen in the marine environment. Academic Press, Inc., New York
Teepe R, Brumme R, Beese F, Ludwig B (2004) Nitrous oxide emission and methane consumption following compaction of forest soils. Soil Sci Soc Am J 68:605–611
Thirukkumaran CM, Parkinson D (2000) Microbial respiration, biomass, metabiolic quotient and litter decomposition in a lodgepole pine forest floor amended with nitrogen and phosphorus fertilizers. Soil Biol Biochem 32:59–66
Tonon G, Sohi S, Francioso O, Ferrari E, Montecchio D, Gioacchini P, Ciavatta C, Panzacchi P, Powlson D (2010) Effect of soil pH on the chemical composition of organic matter in physically separated soil fractions in two broadleaf woodland sites at Rothamsted, UK. Eur J Soil Sci 61:970–979
Tyree MC (2005) The short-term effects of fertilization on total soil CO2 efflux, heterotrophic, and autotrophic respiration of loblolly pine (Pinus taeda L.). M.Sc. thesis Virginia Polytechnic Institute and State University. Blacksburg, Virginia. http://scholar.lib.vt.edu/theses/available/etd-09062005181026/unrestricted/Thesis_Tyree.pdf
Tyree M, Seiler J, Maier C (2013) Site-specific forest management: matching genotypes and silviculture to optimize carbon sequestration. In: Guldin JM (ed) Proceedings of the 15th biennial southern silvicultural research conference. e-Gen. Tech. Rep. SRS-GTR-175. U.S. Department of Agriculture, Forest Service, Southern Research Station, Asheville, pp 343–348. http://www.srs.fs.usda.gov/pubs/43656#sthash.qJignKCp.dpuf
Ullah S, Moore TR (2011) Biogeochemical controls on methane, nitrous oxide, and carbon dioxide fluxes from deciduous forest soils in eastern Canada. J Geophys Res-Biogeol (2005–2012) 116:G03. doi:10.1029/2010JG001525
US Climate Change Science Program (2008) Our changing planet. The US Climate Change Science Program for fiscal year 2008. A report by the Climate Change Science Program and the subcommittee on global change research, A supplement to the President’s budget for fiscal year 2008. Executive Office of the President of the United States, Washington, DC, USA
U.S. Department of Agriculture, Forest Service (2010) U.S. forest and Carbon: Some important facts. Forest Service’s Forest Inventory and Analysis. Forest Carbon Briefing Paper-12 Oct 2010. http://www.fs.fed.us/rmrs/docs/forest-carbon/fact-sheet.pdf
USEPA (2010) Methane and nitrous oxide emissions from natural sources. United States Environmental Protection Agency, Office of Atmospheric Programs,1200 Pennsylvania Ave., NW Washington, DC 20460. EPA 430-R-10-001. http://www.epa.gov/methane/pdfs/Methane-and-Nitrous-Oxide-Emissions-From-Natural-Sources.pdf
USEPA (2012) Inventory of U.S. greenhouse gas emissions and sinks: 1990–2010. U.S.Environmental Protection Agency, 1200 Pennsylvania Ave. Washington, DC. http://www.epa.gov/climatechange/Downloads/ghgemissions/US-GHG-Inventory-2012-Main-Text.pdf
Vallack HW, Leronni V, Metcalfe DB, Högberg P, Ineson P, Subke JA (2012) Application of nitrogen fertilizer to a boreal pine forest has a negative impact on the respiration of ectomycorrhizal hyphae. Plant Soil 352:405–417
Veldkamp E, Koehler B, Corre MD (2013) Indications of nitrogen-limited methane uptake in tropical forest soils. Biogeosciences Discussions 10(3):6007–6037
Ventera RT, Groffman PM, Verchot LV (2003) Nitrogen oxide gas emissions from temperate forest soils receiving long-term nitrogen inputs. Glob Change Biol 9:346–357
Verchot LV, Davidson EA, Cattanio JH, Ackerman IL, Erickson HE, Keller M (1999) Land use change and biogeochemical controls of nitrogen oxide emissions from soilsin eastern Amazonia. Glob Biogeochem Cycles 13:31–46
Von Arnold K, Nilsson M, Hanell B, Weslien P, Klemedtsson L (2005) Fluxes of CO2, CH4 and N2O from drained organic soils in deciduous forests. Soil Biol Biochem 37:1059–1071
Vose JM, Bolstad PV (2007) Biotic and abiotic factors regulating forest floor CO2 flux across a range of forest age classes in the southern Appalachians. Pedobiologia 50:577–587
Wallenstein MD, McNulty S, Fernandez IJ, Boggs J, Schlesinger WH (2006) Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. For Ecol Manag 222:459–468
Wang ZP, Ineson P (2003) Methane oxidation in a temperate coniferous forest soil: effects of inorganic N. Soil Biol Biochem 35:427–433
Wang C, Bond-Lamberty B, Gower ST (2003) Soil surface CO2 flux in a boreal black spruce fire chronosequence. J Geophys Res 108(D3):8224. doi:10.1029/2001JD000861
Watanabe MDB, Ortega E (2011) Ecosystem services and biogeochemical cycles on a global scale: valuation of water, carbon and nitrogen processes. Environ Sci Policy 14:594–604
Weslien P, Klemedtsson AK, Borjesson G, Klemedtsson L (2009) Strong pH influence on N2O and CH4 fluxes from forested organic soils. Eur J Soil Sci 60:311–320
Willison TW, Webster CP, Goulding KWT, Powlson DS (1995) Methane oxidation in temperate soils: effects of land-use and the chemical form of nitrogen fertilizer. Chemosphere 30:539–546
Winjum JK, Dixon RK, Schroeder PE (1992) Estimating the global potential of forest and agroforest management practices to sequester carbon. Water Air Soil Poll 64:213–227
Wood TE, Silver WL (2012) Strong spatial variability in trace gas dynamics following experimental drought in a humid tropical forest. Glob Biogeochem Cycles 26:GB3005. doi:10.1029/2010GB004014
Xu X, Inubushi K (2004) Effects of N sources and methane concentrations on methane uptake potential of a typical coniferous forest and its adjacent orchard soil. Biol Fertil Soils 40:215–221
Xu X, Han L, Luo X, Han S (2011) Synergistic effects of nitrogen amendments and ethylene on atmospheric methane uptake under a temperate old-growth forest. Adv Atmos Sci 28:843–854
Zhang W, Mo J, Zhou G, Gundersen P, Fang Y, Lu X, Zhang T, Dong S (2008a) Methane uptake responses to nitrogen deposition in three tropical forests in southern China. J Geophys Res 113:D11116. doi:10.1029/2007jd009195
Zhang W, Mo J, Yu G, Fang Y, Li D, Lu X, Wang H (2008b) Emissions of nitrous oxide from three tropical forests in Southern China in response to simulated nitrogen deposition. Plant Soil 306:221–236
Zona D, Janssens IA, Aubinet M, Gioli B, Vicca S, Fichot R, Ceulemans R (2013) Fluxes of the greenhouse gases (CO2, CH4 and N2O) above a short-rotation poplar plantation after conversion from agricultural land. Agric For Meteorol 169:100–110
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shrestha, R.K., Strahm, B.D. & Sucre, E.B. Greenhouse gas emissions in response to nitrogen fertilization in managed forest ecosystems. New Forests 46, 167–193 (2015). https://doi.org/10.1007/s11056-014-9454-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-014-9454-4