Abstract
Dormancy in vegetative buds of perennial plants plays an important role for surviving harsh environmental conditions. Identifying the genetic and physiological mechanisms regulating dormancy in these vegetative structures will allow manipulation of plant growth and development in both crops and weeds. Model plants have been used to study the physiological effects that photoperiod and temperature impart on dormancy regulation in perennial buds. At the molecular level, models derived through analysis of the transcriptome have shed new light on multiple cellular pathways and physiological processes associated with dormancy transitions and, in some cases, have revealed overlap with pathways regulating flowering and cold acclimation. In this chapter, we discuss proposed models based on advances to our understanding of physiological and molecular factors affecting dormancy regulation in vegetative buds of perennials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Short Photoperiod
- Woody Perennial
- Leafy Spurge
- Short Vegetative Phase
- Dehydration Responsive Element Binding
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 Introduction
Vegetative buds contain meristems in various stages of growth and development, which posses the capacity to serve as reservoirs for potential vegetative and/or floral development following extremes in seasonal weather conditions. In perennials, these buds are formed on vegetative propagules such as bulbs, corms, roots, rhizomes, stolons, and tubers or, in the case of trees they also exist as apical or axillary buds during the growing and nongrowing season (Anderson et al. 2001). During the perennial life cycle, axillary and apical buds of most woody tree species and shrubs or underground adventitious buds of herbaceous species transition through the various stages of dormancy (para-, endo-, and eco-dormancy) as defined by Lang et al. (1987). During the growing season, meristematic development in paradormant axillary or adventitious buds is under the control of physiological signals generated external to the buds (this process is also referred to as correlative inhibition or apical dominance) (Fig. 5.1). In autumn, shortening photoperiod and/or cool temperature induces endodormancy (also referred to as innate dormancy) through internal signals and processes within the bud that prevent growth even if external physiological signals are removed and the plants are returned to growth-promoting conditions. Establishment of endodormancy prevents initiation of new shoot growth from buds during autumn when the environment can rapidly fluctuate between growth-promoting and nonpromoting conditions. As a result, endodormancy prevents buds from establishing new shoot growth that would be susceptible to environmental stress (e.g., frost and dehydration) and would thus deplete plants of buds needed to generate vegetative growth the following growing season (Chao et al. 2007; Rohde and Bhalerao 2007; Volaire and Norton 2006). Extended periods of extreme temperatures are generally required to release buds from endodormancy. However, these environmental extremes also prevent further growth, a process known as ecodormancy. In this chapter, we will discuss the environmental factors that affect physiological and molecular signaling mechanisms associated with seasonal transitions in well-defined phases of plant bud dormancy; particularly the induction and release from endodormancy by environmental signals such as photoperiod and/or temperature, and its overlap with flowering pathways in woody and herbaceous perennials.
Overview of dormancy status during seasonal bud development in herbaceous and woody perennials. Diagram shows developmental comparison of underground adventitious buds of herbaceous (leafy spurge) and apical or axillary buds of woody (poplar) plants during seasonal transitions in well-defined phases of para-, endo-, and eco-dormancy. Abbreviations: ABA abscisic acid, GA gibberellic acid, IPT isopentenyl transferase, SL strigolactone
5.1.1 Bud Phenology in Model Perennials
Determination of vegetative or floral bud development is dependent on specific environmental signals such as exposure to extended cold temperatures or specific photoperiods (Chouard 1960; Rohde and Bhalerao 2007). However, across plant species, the developmental context of buds from annuals, woody perennials, and herbaceous perennials is different. For example, in most woody perennials, such as poplar (Populus spp.), the mature growing apices transition to overwintering buds, while in herbaceous perennials such as leafy spurge (Euphorbia esula), the mature growing apices senesce and die, which is similar to the model annual plant Arabidopsis thaliana. However, unlike Arabidopsis, where the whole plant dies, adventitious buds located on the persisting underground crown and root system of leafy spurge overwinter for renewed seasonal shoot growth. Since buds of model herbaceous and woody perennials exhibit differences in their phenotypic development (Fig. 5.1), each will be discussed separately. Although this chapter focuses on dormancy in perennials, where appropriate, information obtained from studies with Arabidopsis will be included to bridge gaps in proposed models.
5.1.1.1 Woody Perennials
Several model dicot systems have been used to study bud dormancy and flowering in woody perennials. In the spring, apical buds of poplar initiate growth (often referred to as bud break) to become the new growing shoot meristem (Yuceer et al. 2003). In poplar, preformed early leaves in the apical buds rapidly expand, while leaf primordia on the shoot continue to differentiate to become what is referred to as later leaves. Axillary buds formed at the base of early preformed leaves remain vegetative, while axillary buds that form at the base of the later leaves are programmed to become floral buds in adult trees (see Fig. 5.1). The early preformed leaves may provide the florigenic signal required for the axillary buds of the late-formed leaves to differentiate into floral organs (Yuceer et al. 2003). However, the floral buds that form at the base of later leaves will not flower until they have overwintered. After exposure to 1–2 weeks of short photoperiod, apical meristems cease growth and the leaf primordia differentiate into scales rather than leaves, a process known as bud set. If these buds are placed back under long photoperiod conditions, they still resume growth, indicating that the limited short photoperiods induce ecodormancy. However, subjecting apical buds to 4–6 weeks of short photoperiod prevents growth upon return to long photoperiod conditions, indicating that exposure of buds to extended short photoperiods induces endodormancy. These buds also become partially cold acclimated after 4–6 weeks under short photoperiod conditions; although additional cold temperatures enhance cold hardiness (Welling et al. 1997). Once buds are endodormant, they remain so until extended exposure to cold temperatures reestablish growth competence.
Other woody perennials used to study bud dormancy include chestnut (Castanea sativa) (Ramos et al. 2005), grape (Vitis spp.) (Fennell and Hoover 1991; Mathiason et al. 2008; Schnabel and Wample 1987; Wake and Fennell 2000), various other fruit crops such as apple (Malus spp.) (Foster et al. 2003; Heide and Prestrud 2005) and members of the Prunus family such as peach (Bielenberg et al. 2004; Li et al. 2009) and apricot (Yamane et al. 2008), and to lesser extents other woody perennials such as red osier dogwood (Cornus sericea) (Smithberg and Weiser 1968; Svendsen et al. 2007). Like poplar, these species follow similar bud developmental phenology, with the formation of an apical meristem (sometimes referred to as a terminal vegetative bud) and axillary buds forming either vegetative or floral buds depending on their maturity requirements, relative position on the stem, and/or light/temperature conditions during development. In grape, bud meristems that overwinter develop 6–9 nodes over the growing season; each node develops a leaf with a vegetative axillary bud along with a tendril or floral bud opposite the leaf. At nodes 4–6, the opposing bud generally forms a flower, while the other bud forms a tendril (Pratt 1971). In apple, the apical bud and those closest to the apical bud become committed to flowering, while more distal buds remain committed to vegetative production (Foster et al. 2003). In both grape and apple, floral bud development occurs in the growing season that precedes flowering, similar to poplar (Foster et al. 2003; Pratt 1971).
Endodormancy induction in some grape varieties require short photoperiod conditions, while other varieties require both short photoperiod and cold temperatures (Fennell and Hoover 1991; Schnabel and Wample 1987). A similar phenomenon has been noted in northern vs. southern ecotypes of red osier dogwood (Smithberg and Weiser 1968; Svendsen et al. 2007). In apple and pear (Pyrus spp.), temperature alone appears to regulate endodormancy induction and release (Heide and Prestrud 2005). In all these systems, once endodormancy is released, buds develop into either vegetative meristems or flowers depending on their predetermined state.
5.1.1.2 Herbaceous Perennials
Unlike most woody perennials, which develop new vegetative growth from apical or axillary buds located on above ground stems and branches, the aerial portions of herbaceous perennials seasonally die back to ground level, and development of new stems and shoots occurs from adventitious buds that are underground or at the soil surface. Compared to woody perennials, substantially fewer herbaceous perennial models are used to study well-defined phases of dormancy. Leafy spurge has emerged as a prominent model system to study dormancy transitions in herbaceous perennials (Chao et al. 2005).
Leafy spurge is a wild flower common to road sides and pasture lands in central and eastern Europe, but has become an invasive weed in the Northern Great Plains of the US and Canada. Leafy spurge reproduces sexually by seeds, or asexually by vegetative reproduction from an abundance of underground adventitious buds. Dormancy-imposed inhibition of new shoot growth from underground adventitious buds has long been considered a key characteristic leading to the persistence and invasiveness of herbaceous perennial weeds such as leafy spurge, Canada thistle (Cirsium arvense), field bindweed (Convolvulus arvensis), etc. As a result, the nonuniform emergence of vegetative shoots, resulting from dormancy-imposed inhibition of growth, is one of the key characteristics allowing many weedy plants to escape conventional control measures. Understanding the pathways and networks that regulate dormancy in weedy perennials could identify new targets for manipulating plant growth and reduce economic costs to land managers worldwide.
In herbaceous perennials like leafy spurge, bud and flower development, as well as dormancy transition, deviate from those described for woody perennials. In field settings, leafy spurge forms new adventitious buds on the underground portion of its stem (often referred to as the crown; see Fig. 5.1) and on roots. During late spring to early summer, usually after flowering has occurred, new crown buds form (Anderson et al. 2005). However, visible crown buds will also form on stems of greenhouse-grown, nonflowering plants within 2–3 months after propagation from shoot cuttings. Like axillary buds of other models, crown and root buds will not develop into new shoots during the growing season unless the above ground portion of the plant dies or is removed. During late summer and early autumn, these paradormant buds transition to a state of endodormancy which is often marked by bud expansion. Following extended cold treatment, endodormant buds transition to an ecodormant state at which point they simultaneously become both growth and floral competent (Anderson et al. 2005). Leafy spurge plants that have transitioned through both endo- and eco-dormancy generate new shoot growth followed by flowering after several weeks when returned to growth-conducive environments (Doğramaci et al. 2010; Foley et al. 2009).
Potato (Solanum tuberosum) is another model system used to study bud dormancy. In potato, short photoperiod exposure perceived by the leaves induces underground stolons to develop tubers with axillary buds (Rodríguez-Falcón et al. 2006). Once formed, these buds are endodormant (Sonnewald 2001). In addition to the substantial body of work on potato bud endodormancy at the physiological level (Suttle 2004, 2008), there is evidence that seasonal regulation of floral and tuber-inducing signaling pathways might also overlap at the molecular level (Rodríguez-Falcón et al. 2006) and that endodormancy maintenance may involve chromatin remodeling (Law and Suttle 2004).
5.2 Environmental Regulation
Light and/or temperature are important environmental signals affecting transitions between well-defined phases of dormancy and the underlying mechanisms regulating these transitions are a major focus in this review (Figs. 5.2 and 5.3). These environmental signals influence the physiology of buds and alter their ability to initiate new vegetative growth under growth-conducive conditions (Allona et al. 2008; Anderson et al. 2001; Cao et al. 2008; Franklin 2009; Horvath et al. 2003; Rohde and Bhalerao 2007). Interestingly, in leafy spurge, cold temperatures can induce endodormancy, but an extended period of cold temperature (referred to as vernalization in relation to flowering) signals a dual response that initiates growth- and floral-competence (Foley et al. 2009). In poplar, proteins normally associated with flowering in Arabidopsis, such as FLOWERING LOCUS T (FT), TERMINAL FLOWER 1 (TFL1), PHYTOCHROME A (PHYA), and CONSTANS (CO), have also been associated with regulating growth cessation and endodormancy (Böhlenius et al. 2006; Eriksson 2000; Ruonala et al. 2008). In Arabidopsis, genes encoding these proteins are impacted by both light and temperature (Franklin 2009; Kobayashi and Weigel 2007; Michaels 2009; Nozue and Maloof 2006; Penfield 2008). However, although it has been suggested that signal transduction pathways regulating flowering and endodormancy converge (Horvath 2009), there are still many unresolved questions on how these pathways interact.
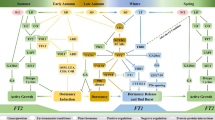
Based on information gathered from both annuals and perennials, this proposed model represents a simplified review of the multiple pathways linking environmental input signals to dormancy regulation and flowering. Both phytochromes and cryptochromes couple input signals from photoperiod and temperature to circadian clock associated proteins. Circadian control involves feedback loop interactions among a host of proteins such as CCA1, ELF3, GI, LHY, PRR9, PRR7 and PRR5, TOC1, and ZTL. Output from entrainment of the circadian clock genes modify the expression of an additional signal transduction cascade that originates from PHYA and CRY2. This portion of the leaf photoperiod measuring mechanism acts through FKF1, GI, CDF1, and the floral promoter CO, which partially controls expression of floral integrators like FT. FT is generally transported through the phloem to the apical meristem. Vernalization mediates the epigenetic repression of FLC or FLC-LIKE, which encodes a MADS-box transcription factor that also represses the expression of floral pathway integrators such as FT. Long photoperiods also inhibit expression of SVP/DAM, which encodes other MADS-box transcription factors that inhibits FT and CENL1. Although not shown in this diagram, there are multiple points of crosstalk between circadian regulation and cold responses that also modify the activation of floral and endodormancy responses
Proposed model of environmental, physiological, and genetic factors impacting dormancy transitions in perennial buds. Red arrows/bars indicate genes, hormones, metabolites, or environmental condition that have proven, in at least one perennial system, to induce/inhibit effects on targets, while black arrows/bars indicate suspected induction/inhibition on targets. Short daylight affects PHYA and GA which impact growth cessation and endodormancy induction. Short daylight also induces ethylene production which may induce ABA (through induction of NCED1) to enhance and perhaps maintain endodormancy. Short daylight and or short-term cold are suspected of inducing DAM genes, the product of which likely act to inhibit FT expression to regulate endodormancy induction. Short-term cold also induced ABA accumulation in the buds which may enhance endodormancy. Long-term cold inhibits the expression of some (but not all) DAM genes, reduces ABA content, and releases buds from endodormancy. “?” represents contradictory data where short day clearly induces PHYA expression yet overexpression of transgenic PHYA inhibits endodormancy
5.2.1 Light
Photoperiod is generally considered the primary signal regulating endodormancy induction in many perennials (Howe et al. 1996; Jeknic and Chen 1999; Li et al. 2003; Smithberg and Weiser 1968; Wake and Fennell 2000), with the notable exceptions in several Rosaceae family members (Heide and Prestrud 2005). In Arabidopsis and poplar, photoperiod is perceived by photoreceptors such as phytochrome, which perceives the ratio of red to far-red light (Eriksson 2000; Franklin 2009), and in Arabidopsis cryptochrome (CRY) is also known to perceive and transduce blue light signals (Cashmore et al. 1999; McClung 2006). These and other molecular input signals interact with and respond to components of the circadian clock (see Fig. 5.2), which controls diurnal rhythms in annuals and perennials (Harmer 2009; McClung 2006; Robertson et al. 2008; Rodríguez-Falcón et al. 2006).
In temperate ecosystems, photoperiod is the most reliable indicator of seasonal transitions. Thus, it is not surprising that light regulates dormancy and flowering via genes and signaling mechanisms involved in regulating the circadian clock (Smith 2000). For example, PHYA and PHYB, proteins involved in entrainment of the circadian clock (McClung 2006; Rodríguez-Falcón et al. 2006), have a significant impact on flowering in Arabidopsis (Fujiwara et al. 2008; Ishikawa et al. 2006; Mas and Yanovsky 2009; McClung 2006). PHYA also has an indisputable impact on endodormancy in poplar (Olsen et al. 1997; Ruonala et al. 2008) and PHYB plays a critical role in perception of short photoperiod and induction of tuberization in potato (Rodríguez-Falcón et al. 2006). Activation of Arabidopsis PHYB initiates a series of regulatory feedback loops formed by numerous circadian clock genes (McClung 2006). Additional research with Arabidopsis highlights the affect of photoperiod on other circadian regulating genes such as CIRCADIAN CLOCK ASSOCIATED1 (CCA1), EARLY FLOWERING3-4 (ELF3 and ELF4), GIGANTIA (GI), LATE ELONGATED HYPOCOTYL (LHY), PHYTOCHROME-INTERACTING FACTOR3 (PIF3), PSEUDO-RESPONSE REGULATORS (PRR9, PRR7 and PRR5), TIMING OF CAB1 (TOC1), LUX ARRHYTHMO (LUX), and ZEITLUPE (ZTL), (Harmer 2009; McClung 2006). These circadian clock genes interact with and modify the expression of an additional signal transduction cascade that originates from PHYA and CRY1/2. This portion of the photoperiod measuring mechanism acts through FLAVIN-BINDING, KELCH REPEAT, F-BOX1 (FKF1), CYCLING DOF FACTOR1 (CDF1), and CO (Fujiwara et al. 2008; Kobayashi and Weigel 2007; Michaels 2009; Sawa et al. 2007), and partially controls FT and TFL1 in Arabidopsis (Kobayashi et al. 1999).
In poplar, FT and CENTRORADIALIS-LIKE 1 (CENL1) (orthologue of TFL1) regulate seasonal growth cessation (Böhlenius et al. 2006), and dormancy (Ruonala et al. 2008). In Arabidopsis and poplar, FT and CENL1 also regulate flowering with FT promoting flowering and TFL1/CENL1 inhibiting flowering (Böhlenius et al. 2006; Kobayashi et al. 1999; Mohamed 2006). Interestingly, whereas wild-type Populus spp. ceased growth and set buds under short photoperiod (Eriksson 2000; Olsen 1997), short photoperiod-induced growth cessation and CO repression did not occur in PHYA overexpressing lines (Böhlenius et al. 2006); even when placed in 6 h of daylight (Olsen et al. 1997). Thus, overexpression of PHYA in Populus spp. may prevent growth cessation and endodormancy induction by up-regulating FT and CENL1 (Böhlenius et al. 2006; Ruonala et al. 2008).
Because photoperiod impacts circadian regulation and endodormancy induction, it is likely that circadian clock genes also affect endodormancy and vice versa. For example, during the transition from endodormancy to ecodormancy, numerous genes involved in circadian regulation were up-regulated in crown buds of leafy spurge (Horvath et al. 2008). A similar induction of genes involved in circadian regulation was also observed in grape, chestnut, and poplar during the transition from endodormancy to ecodormancy (Mathiason et al. 2008; Ramos et al. 2005; Ruttink et al. 2007). Interplay between several components of the circadian clock and floral-regulating MADS-box transcription factors FLOWERING LOCUS C (FLC) and SHORT VEGETATIVE PHASE (SVP) are known (Fujiwara et al. 2008; Michaels 2009; Salathia et al. 2006), and both of these factors have been shown to regulate FT in Arabidopsis (Helliwell et al. 2006; Lee et al. 2007; Searle et al. 2006).
5.2.2 Temperature
Temperature, like photoperiod, can also regulate endodormancy and flowering induction in some perennials (Foley et al. 2009; Heide and Prestrud 2005; Mouhu et al. 2009; Schnabel and Wample 1987; Smithberg and Weiser 1968; Svendsen et al. 2007). However, compared to photoperiod, considerably less is known about the role that cold plays in endodormancy induction. Cold temperatures are sufficient for inducing endodormancy in some dogwood ecotypes (Svendsen et al. 2007), Rosaceae spp. (Heide and Prestrud 2005), citrus (Moss 1969), and leafy spurge (Anderson et al. 2005; Foley et al. 2009). Interestingly, Foley et al. (2009) further discovered that crown buds must transition through a state of endodormancy to achieve fulfillment of flowering by vernalization in leafy spurge.
The circadian clock is also known to integrate low temperature responses in both annuals and perennials (Harmer 2009; Penfield 2008; Ramos et al. 2005; Samach and Wigge 2005). Thus, it is not surprising that temperature affects endodormancy in perennials (Heide 2008; Kwolek and Woolhouse 1982; Svendsen et al. 2007). In addition, there is evidence for cross talk between low temperature and phytochrome signaling (Allona et al. 2008; Benedict et al. 2006; Heschel et al. 2007; Kim et al. 2002; Olsen et al. 1997; Penfield 2008; Smith 2000), which may link temperature responses to FT and CENL1 expression and endodormancy induction.
5.2.2.1 Cold-Hardening/Cold-Acclimation
Cool autumn temperatures not only initiate endodormancy in buds of perennials but also induce a phenomenon important for survival referred to as cold hardening or cold acclimation. Cold hardening protects the cellular constituents from damage as a result of dehydration and freezing (Guy 1990; Thomashow 2001), and in Arabidopsis cold-regulated gene expression likely occurs through a process involving circadian evening elements (Mikkelsen and Thomashow 2009). Several transcription factors induced by cold temperatures bring about global changes in gene expression that play an important role in the hardening/acclimation process. Among the best studied of these transcription factors is a family of AP2 DNA-binding transcription factors known as C-REPEAT BINDING FACTOR (CBF) 1-4, also known as DEHYDRATION RESPONSIVE ELEMENT BINDING FACTOR (DREB). CBFs are themselves believed to be regulated by another transcription factor known as INDUCER of CBF EXPRESSION (ICE) 1. ICE1 is present at normal growing temperatures but is either activated or interacts with a protein that is activated by cold (Thomashow 2001). PHYA has been implicated as a transduction pathway mediator; either by PHYA-mediated phosphorylation, or by modulation of proteolysis of ICE1 (Benedict et al. 2006; also see Penfield 2008). Interestingly, overexpression of PHYA appears to inhibit short photoperiod-induced cold hardening in poplar (Olsen et al. 1997). In trees, PHYA may prevent short photoperiod induced cold hardening and growth cessation through maintenance of gibberellic acid (GA) levels (see Allona et al. 2008).
Transition from para- to endo-dormancy in leafy spurge crown buds, which coincided with decreasing night temperatures, are paralleled by up-regulation of cold-hardening transcripts with homology to Arabidopsis DREB A4 (At2g35700) and ICE1-LIKE (Doğramaci et al. 2010; Horvath et al. 2008). Since cold-induced expressions of some DREB/CBF-family members are gated by the circadian clock in Arabidopsis (Fowler et al. 2005), these particular regulators of transcription might also have some impact or overlap with pathways affecting dormancy transitions in response to low temperature, particularly in plants such as apple or leafy spurge that rely primarily on cold-induced endodormancy.
5.2.2.2 Vernalization
Although cold hardening generally occurs concomitantly with endodormancy induction, extended cold temperatures both break endodormancy and induce floral competency in numerous perennials (Anderson et al. 2005; Chouard 1960; Foley et al. 2009; Nishikawa et al. 2007). Chouard (1960) originally hypothesized that there might be a connection between vernalization and release from endodormancy. This idea was later expanded to suggest that mechanisms such as chromatin modification might play an underling role in endodormancy in perennials such as leafy spurge, because buds need to “remember” that they are dormant, particularly during brief warm spells common during late autumn and early winter (Horvath et al. 2003). This mechanism was later shown to be true in the case of vernalization in Arabidopsis (Amasino 2004; Henderson and Dean 2004; Sung and Amasino 2004). Further support of this hypothesis in perennials comes from identification of differentially expressed chromatin-modifying genes during endodormancy induction and release in leafy spurge, poplar (in both apical and cambial meristems), grape, and potato (Campbell et al. 2008; Doğramaci et al. 2010; Druart et al. 2007; Horvath et al. 2008; Or et al. 2000; Ruttink et al. 2007).
In contrast to perennials, the mechanisms by which extended cold temperature induces floral competence in winter annuals, such as Arabidopsis, is well understood and information is accumulating on vernalization processes in other species (Alexandre and Hennig 2008). During vernalization, the chromatin structure in the promoter and 5′ coding regions of the floral repressor FLC is altered to prevent expression of FLC, even after multiple rounds of cell division (He and Amasino 2005). The key players in regulating chromatin modifications are VERNALIZATION INSENSITIVE3 (VIN3), VERNALIZATION1 (VRN1), and 2 (VRN2) (Amasino 2004; Sung and Amasino 2004). In Arabidopsis, extended cold temperatures up-regulate VIN3 by unknown mechanisms and once induced, VIN3 along with VRN1, VRN2, and LIKE HETEROCHROMATIN PROTEIN1 (LHP1) specifically alter the methylation and acetylation of histones on the FLC promoter to block transcription (Henderson and Dean 2004; Sung and Amasino 2004; Sung et al. 2006).
Chromatin remodeling regulates other flowering genes besides FLC. Chromatin alterations also regulate FT to some extent and TFL2, another protein similar to LHP1 (Takada and Goto 2003). Likewise, the gene EARLY BOLTING IN SHORT DAYS (EBS), that is similar to a group of bromo/homeodomain containing zinc finger proteins associated with chromatin-modifying complexes, impacts FT expression (Pineiro et al. 2003). Mutations in other chromatin-modifying proteins such as various members of the SWITCH/SUCROSE NONFERMENTABLE (SWI/SNF) protein complexes, POLYCOMB group proteins, and other known chromatin-modifying proteins also impact floral timing and development (Farrona et al. 2004; Henderson and Dean 2004; Noh and Noh 2006), and may also impact endodormancy maintenance in perennials (Doğramaci et al. 2010; Horvath et al. 2008). However, the role that epigenetic factors play in regulating endodormancy induction and release in perennial buds requires additional support to confirm the function of chromatin-modifying genes differentially expressed during these transitions.
5.3 Genetic/Physiological Model(s) for Regulation of Dormancy Transitions
Data presented so far highlights that signal transduction pathways affected by both cold and light signaling converge on the expression of FT and CENL1 to affect endodormancy and flowering (Fig. 5.2 and Table 5.1). Both of these genes are also regulated by SVP and FLC in Arabidopsis, two specific floral-regulating MADS-box transcription factors (Fujiwara et al. 2008; Lee et al. 2007; Michaels 2009). In perennial species, DORMANCY ASSOCIATED MADS-box (DAM) genes, which are closely related to SVP, have been identified as playing a role in endodormancy induction. DAM genes were first identified as having a role in dormancy through map-based cloning of the EVERGROWING locus in peach (Bielenberg et al. 2004). The evergrowing (earlier called evergreen) peach varieties contain a mutation that prevents induction of dormancy under short daylight conditions (Li et al. 2009), in contrast to that observed for wild-type varieties (Diaz 1974). Sequencing of the EVERGROWING locus of wild type and mutant lines revealed a deletion of a series of MADS-box genes in the mutant line (Bielenberg et al. 2008).
It has been hypothesized that induction of DAM genes may be required for down-regulating FT and CENL1 during the initiation of growth cessation and/or endodormancy in some perennial species (Horvath et al. 2008; Horvath 2009), since SVP down-regulates FT in Arabidopsis (Fujiwara et al. 2008; Lee et al. 2007) and FT and CENL1 influences growth cessation and dormancy in trees (Böhlenius et al. 2006; Ruonala et al. 2008). This hypothesis is supported by potential FT- and CENL1-LIKE genes from leafy spurge being down-regulated concomitantly with DAM induction during endodormancy (Horvath et al. 2008). Additionally, Horvath et al. (2010) found that FT expression was down-regulated in transgenic Arabidopsis lines overexpressing leafy spurge DAM1, and these transgenic lines also had reduced bolt height and delayed flowering compared to wild type. Although the functionality of the putative FT-LIKE gene from leafy spurge has not been verified, the results were consistent with the hypothesis that DAM regulates FT/CENL1. Research is continuing in multiple perennials to determine if DAM gene expression has any direct impact on dormancy, flowering, or FT expression.
The induction of DAM genes may also play a role in regulating transitional phases of dormancy in other perennials. For example, DAM genes from raspberry (Rubus idaeus) (Mazzitelli et al. 2007), potato (Campbell et al. 2008), apricot (Yamane et al. 2008), peach (Bielenberg et al. 2008; Li et al. 2009), and likely poplar (see Horvath et al. 2008) have been implicated in bud dormancy regulation. Differential expression of DAM genes has been observed during dormancy-inducing short photoperiod conditions in Populus spp. and Prunus spp. (Ruttink et al. 2007; Yamane et al. 2008) and during cold-induced endodormancy induction of leafy spurge (Horvath et al. 2010). Additionally, putative cis-acting elements similar to circadian-regulating evening elements, affecting diurnal regulation, are conserved in the promoters of DAM genes of poplar and leafy spurge (Horvath et al. 2008), but CBF-binding sites (Fowler et al. 2005) are only observed in the leafy spurge DAM1 promoter (Horvath et al. 2008). Thus among perennial species, the presence of CBF sites in the promoters of DAM genes could explain why cold is the primary signal-inducing endodormancy in some perennial species, as opposed to other perennial species where photoperiod primarily regulates endodormancy induction.
Evidence presented in this review suggests that PHYA may play a role in seasonal growth cessation and endodormancy induction through regulation of FT and CENL1. However, release from endodormancy by extended cold temperatures likely does not involve these genes, at least in perennials such as leafy spurge, where FT- and CENL1-LIKE genes are not up-regulated following endodormancy release (Horvath et al. 2008). Likewise, PHYA signaling is unlikely in endodormant buds from most temperate perennials since leaves (the primary photoreceptor organs) are not present during the transition from endodormancy to ecodormancy. Observing the impact of FT- and CENL1-LIKE gene expression in buds of perennials to determine if FT and CENL1 expression is sufficient for endodormancy release across multiple species should help to clarify their functional role in endodormancy maintenance. However, since some DAM genes are preferentially expressed only during endodormancy (Horvath et al. 2008; Li et al. 2009; Yamane et al. 2008), DAM could be part of a mechanism involved in controlling both induction and release of endodormancy in perennials.
5.3.1 Hormones
Hormones such as abscisic acid (ABA), GA, cytokinin, auxin, and ethylene are implicated in various aspects of growth cessation, bud set, and endodormancy in both woody and herbaceous perennials (Allona et al. 2008; Chao et al. 2006, 2007; Horvath et al. 2003, 2008; Horvath 2009; Olsen 2006; Rodríguez-Falcón et al. 2006; Rohde and Bhalerao 2007). Since photoperiod impacts endodormancy induction and affects circadian responses, it is likely that circadian responses could impact hormone levels affecting dormancy and vice versa (Alabadi and Blázquez 2009; Covington and Harmer 2007; Nozue and Maloof 2006; Robertson et al. 2008). In addition, cold night temperatures combined with inhibition of GA accumulation are sufficient for inducing ecodormancy and bud set in PHYA overexpressing poplar lines (Mølmann et al. 2005), and cold temperature also blocks growth through the action of hormones, such as ABA and GA (see Figs. 5.1 and 5.3). There is evidence that ABA levels as well as oxidative stress may play some role in endodormancy release in perennials (Arora et al. 2003; Destefano-Beltrán et al. 2006; Or 2009). Additionally, auxin responses are impacted by conditions that induce endodormancy (Anderson et al. 2005; Horvath et al. 2008; Schrader et al. 2004), and a node that links the clock and auxin networks has recently been identified (Rawat et al. 2009). For many years, auxin was considered the primary hormone regulating the transition from paradormancy to growth (Beveridge 2006). However, the lack of apically derived auxin transport into buds, and the contradictory role of auxin requirements for bud growth pose unanswered questions as to the mechanisms by which auxin controlled bud outgrowth. Cytokinins have been noted for promotion of cell division and organ formation that are invoked as key regulatory signals promoting axillary bud outgrowth when the apical meristem is removed, and auxin is known to negatively regulate cytokinin biosynthesis in Arabidopsis (Nordstrom et al. 2004). A strigolactone, whose production appears to be dependent on polar auxin transport in annuals, has also been implicated in inhibiting shoot branching (Gomez-Roldan et al. 2008) and appears to directly impact bud growth (see Fig. 5.1). It will be interesting to determine if this new hormone plays any role in transitions between paradormancy and endodormancy in perennials.
Data obtained from poplar, leafy spurge, and potato provides evidence that a transient spike in ethylene (or ethylene perception) precedes, and is necessary for, the initiation of endodormancy (Horvath et al. 2008; Ruttink et al. 2007; Suttle 1998). Research done on ABA accumulation in Citrus suggests that ethylene may directly induce the key ABA biosynthetic gene 9-CIS-EPOXYCAROTENOID DIOXYGENASE (NCED1) (Rodrigo and Alquezar 2006). In turn, catabolism of ABA during dormancy release in potato may, in part, be correlated to decreased levels of NCED (Destefano-Beltrán et al. 2006). In leafy spurge, ABA levels were elevated during endodormancy but dropped following the transition to ecodormancy (Horvath et al. 2008). At least ten other genes associated with ethylene production or ethylene responses were highly expressed during paradormancy, but were repressed later during endo- and eco-dormancy (Horvath et al. 2008).
5.3.2 Sugar
Sugar levels have been correlated with the transition of vegetative buds from paradormancy to endodormancy (Anderson et al. 2005; Arora et al. 2003; Chao et al. 2006). The effect of sugars on regulating well-defined phases of vegetative bud dormancy has been investigated using the model perennial weed, leafy spurge. Sugars from leaves of leafy spurge have been linked to suppression of underground adventitious bud growth during paradormancy (Horvath 1999), and further research has shown that glucose or sucrose can inhibit root bud growth in a mechanism reversed by GA (Chao et al. 2006; Horvath et al. 2002). In addition, sugar signaling is suspected to play an important role in maintaining paradormancy by affecting cell cycle progression at the G1/S phase (Horvath et al. 2002). Shoot removal causes a rapid degradation of starch and a decline in sucrose, concurrent with the transition from paradormancy to active shoot growth (Chao et al. 2006; Horvath et al. 2002). In contrast, during autumn senescence, conversion of starch to sucrose occurs in leafy spurge underground adventitious buds, which is also paralleled by a transition from paradormancy to endodormancy (Anderson et al. 2005). In parallel with these transitions, transcript levels of a leafy spurge starch degrading enzyme, β-amylase, were 1,000-fold higher in endodormant buds and 16,000-fold higher in ecodormant buds than that of paradormant buds (Chao and Serpe 2009). Genes involved in starch degradation were also up-regulated in the cambial meristems of poplar during endodormancy (Schrader et al. 2004). Based on these and other findings, it is proposed that both sugars and their metabolism may play a role in regulating vegetative bud dormancy through cross talk with hormones (Fig. 5.1) (Anderson et al. 2001, 2005; Horvath et al. 2003). Specifically, it has been suggested that sugars are antagonistic to GA perception and likely play a role in signaling pathways required for inducing endo- and eco-dormancy (Chao et al. 2007).
5.4 Conclusions
Vegetative buds have an essential role in the perennial life cycle, and also serve as a mechanism for plant survival after periods of harsh environmental stress. Evolution of this process allowed plants to adapt to seasonal changes in environment and to expand across vast areas of land in temperate regions of the world; an adaptation that could be adversely challenged by global warming. The ability of meristematic cells within these buds to enter and exit well-defined phases of dormancy in response to environmental signals ensures appropriate timing of both vegetative growth and flowering. Consequently, meristematic cells of perennial buds are governed by specific internal signal transduction pathways that respond in concert to external environmental cues. Advances in our understanding of these processes now suggests that photoperiod and temperature signal transduction pathways affecting dormancy likely converge and/or share components with signaling pathways regulating flowering, including similar transcription factors, chromatin remodeling genes, and biochemical signals and receptors.
It is not surprising that many of the genes involved in circadian response pathways have been linked to dormancy transitions, since buds of several woody and herbaceous perennial species use seasonal changes in photoperiod as a reliable signal for regulating endodormancy induction. Additional studies are still needed to identify what, if any, role circadian regulated genes play in dormancy transitions, and to understand why some circadian-regulating genes are up-regulated or constitutively expressed during cold periods associated with endo- and eco-dormancy (Horvath et al. 2008; Ramos et al. 2005). Clock regulators must undergo temporal synchronization to impact responses that allow plant buds to adjust to seasonal changes. As a consequence, understanding how photoperiod and temperature modify expression of specific clock genes could provide insight into the mechanisms through which these environmental factors regulate dormancy and survival.
Duplication of DAM genes may have led to specialization of these genes for unique roles in the regulation of dormancy and flowering. Nearly all of the genes mentioned in this review are members of gene families. Understanding the evolution of these gene families and the specific factors controlling both their regulation and function is needed to determine what role, if any, individual members have in dormancy regulation. Comparative transcriptomics indicates that numerous genes involved in multiple physiological, developmental, and biochemical responses show conserved patterns of expression during endodormancy transitions and during floral transitions. However, additional research is needed to determine the specific functions and interactions that these multiple signaling and molecular pathways have on transitions in well-defined phases of bud dormancy in woody and herbaceous perennial plants.
References
Alabadi D, Blázquez MA (2009) Molecular interactions between light and hormone signaling to control plant growth. Plant Mol Biol 69:409–417
Alexandre CM, Hennig L (2008) FLC or not FLC: the other side of vernalization. J Exp Bot 59(6):1127–1135
Allona I, Ramos A, Ibáñez C, Contreras A, Casado R, Aragoncillo C (2008) Review. Molecular control of winter dormancy establishment in trees. Span J Agric Res 6:201–210 (Special issue)
Amasino R (2004) Vernalization, competence, and the epigenetic memory of winter. Plant Cell 16(10):2553–2559
Anderson JV, Chao WS, Horvath DP (2001) A current review on the regulation of dormancy in vegetative buds. Weed Sci 49:581–589
Anderson JV, Gesch RW, Jia Y, Chao WS, Horvath DP (2005) Seasonal shifts in dormancy status, carbohydrate metabolism, and related gene expression in crown buds of leafy spurge. Plant Cell Environ 28(12):1567–1578
Arora R, Rowland LJ, Tanino K (2003) Induction and release of bud dormancy in woody perennials: a science comes of age. HortScience 38(5):911–921
Benedict C, Geisler M, Trygg J, Hunter N, Hurry V (2006) Consensus by democracy. Using meta-analyses of microarray and genomic data to model the cold acclimation signaling pathway in arabidopsis. Plant Physiol 141:1219–1232
Beveridge CA (2006) Axillary bud outgrowth: sending a message. Curr Opin Plant Biol 9:35–40
Bielenberg DG, Wang Y, Fan S, Reighard GL, Scorza R, Abbott AG (2004) A deletion affecting several gene candidates is present in the evergrowing peach mutant. J Hered 95(5):436–444
Bielenberg DG, Wang Y, Li Z, Zhebentyayeva T, Fan S, Reighard GL, Scorza R, Abbott AG (2008) Sequencing and annotation of the evergrowing locus in peach [Prunus persica (L.) Batsch] reveals a cluster of six MADS-box transcription factors as candidate genes for regulation of terminal bud formation. Tree Genet Genomes 4:495–507
Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312:1040–1043
Campbell MA, Segear E, Beers L, Knauber D, Suttle J (2008) Dormancy in potato tuber meristems: chemically induced cessation in dormancy matches the natural process based on transcript profiles. Funct Integr Genomics 8:317–328
Cao Y, Dai Y, Cui S, Ma L (2008) Histone H2B monoubiquitination of the chromatin of FLOWERING LOCUS C regulates flowering time in Arabidopsis. Plant Cell 20:2586–2602
Cashmore AR, Jarillo JA, Wu J-Y, Liu D (1999) Cryptochromes: blue light receptors for plants and animals. Science 284:760–765
Chao WS, Serpe MD (2009) Changes in the expression of carbohydrate metabolism genes during three phases of bud dormancy in leafy spurge. Plant Mol Biol 73:227–239
Chao WS, Horvath DP, Anderson JV, Foley ME (2005) Potential model weeds to study genomics, ecology, and physiology in the 21st century. Weed Sci 53(6):929–937
Chao WS, Serpe MD, Anderson JV, Gesch RW, Horvath DP (2006) Sugars, hormones, and environment affect the dormancy status in underground adventitious buds of leafy spurge (Euphorbia esula). Weed Sci 54:59–68
Chao WS, Foley ME, Horvath DP, Anderson JV, Chao WS, Foley ME, Horvath DP, Anderson JV (2007) Signals regulating dormancy in vegetative buds. Int J Plant Dev Biol 1(1):49–56, ISSN 1749-4753
Chouard P (1960) Vernalization and its relations to dormancy. Annu Rev Plant Physiol 11:191–238
Covington MF, Harmer SL (2007) The circadian clock regulates auxin signaling and responses in Arabidopsis. PLoS Biol 5(8):e222. doi:10.1371/journal.pbio.0050222
Destefano-Beltrán L, Knauber D, Huckle L, Suttle JC (2006) Effects of postharvest storage and dormancy status on ABA content, metabolism, and expression of genes involved in ABA biosynthesis and metabolism in potato tuber tissues. Plant Mol Biol 61:687–697
Diaz MD (1974) Vegetative and reproductive growth habits of evergreen peach trees in Mexico. Proceedings from the 19th International Horticulture Congress, vol 18, Warsaw, p 525
Doğramaci M, Horvath DP, Chao WS, Foley ME, Christoffers MJ, Anderson JV (2010) Extended low temperature impacts dormancy status, flowering competence, and transcript profiles in crown 571 buds of leafy spurge. Plant Mol Bio 73:207–226
Druart N, Johansson A, Baba K, Schrader J, Sjodin A, Bhalerao RR, Resman L, Trygg J, Moritz T, Bhalerao RP (2007) Environmental and hormonal regulation of the activity-dormancy cycle in the cambial meristem involves stage-specific modulation of transcriptional and metabolic networks. Plant J 50:557–573
Eriksson ME (2000) The role of phytochrome A and gibberellins in growth under long and short day conditions. Studies in hybrid aspen. Swedish University of Agricultural Sciences, Umea, ISSN 1401-6230
Farrona S, Hurtado L, Bowman JL, Reyes JC (2004) The Arabidopsis thaliana SNF2 homolog AtBRM controls shoot development and flowering. Development 131(20):4965–4975
Fennell A, Hoover E (1991) Photoperiod influences growth, bud dormancy, and cold-acclimation in Vitis-Labruscana and V-Riparia. J Am Soc Hortic Sci 116(2):270–273
Foley ME, Anderson JV, Horvath DP (2009) The effects of temperature, photoperiod, and vernalization on regrowth and flowering competence in Euphorbia esula (Euphorbiaceae) crown buds. Botany 87:986–992
Foster T, Johnston R, Seleznyova A (2003) A morphological and quantitative characterization of early floral development in apple (Malus x domestica Borkh.). Ann Bot 92:199–206
Fowler SG, Cook D, Thomashow MF (2005) Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiol 137:961–968
Franklin KA (2009) Light and temperature signal crosstalk in plant development. Curr Opin Plant Biol 12:63–68
Fujiwara S, Oda A, Yoshida R, Niinuma K, Miyata K, Tomozoe Y, Tajima T, Nakagawa M, Hayashi K, Coupland G, Mizoguchi T (2008) Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering in Arabidopsis. Plant Cell 20:2960–2971
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Becard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–195
Guy CL (1990) Cold acclimation and freezing tolerance: role of protein metabolism. Annu Rev Plant Phys Plant Mol Biol 41:187–223
Harmer SL (2009) The circadian system in higher plants. Ann Rev Plant Biol 60:357–377
He Y, Amasino RM (2005) Role of chromatin modification in flowering-time control. Trends Plant Sci 10(1):30–35
Heide OM (2008) Interaction of photoperiod and temperature in the control of growth and dormancy of Prunus species. Sci Hort 115:309–314
Heide OM, Prestrud AK (2005) Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol 25(1):109–114
Helliwell CA, Wood CC, Robertson M, Peacock WJ, Dennis ES (2006) The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J 46:183–192
Henderson IR, Dean C (2004) Control of Arabidopsis flowering: the chill before the bloom. Development 131:3829–3838
Heschel MS, Selby J, Butler C, Whitelam GC, Sharrock RA, Donohue K (2007) A new role for phytochromes in temperature-dependent germination. New Phytol 174:735–741
Horvath DP, Sung S, Kim D, Anderson JV (2010) Characterization, expression and function of DORMANCY ASSOCIATED MADS-BOX genes from leafy spurge. Plant Mol Biol 73:169–179
Horvath DP (1999) Role of mature leaves in inhibition of root bud growth in Euphorbia esula L. Weed Sci 47:544–550
Horvath DP (2009) Common mechanisms regulate flowering and dormancy. Plant Sci 177:523–531
Horvath DP, Chao WS, Anderson JV (2002) Molecular analysis of signals controlling dormancy and growth in underground adventitious buds of leafy spurge. Plant Physiol 128(4):1439–1446
Horvath DP, Anderson JV, Chao WS, Foley ME (2003) Knowing when the grow signals regulating bud dormancy. Trends Plant Sci 8(11):534–539
Horvath DP, Chao WS, Suttle JC, Thimmapuram J, Anderson JV (2008) Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.). BMC Genomics 9:536–552
Howe GT, Gardner G, Hackett WP, Furnier GR (1996) Phytochrome control of short-day-induced bud set in black cottonwood. Physiol Plant 97:95–103
Ishikawa M, Kiba T, Chua NH (2006) The Arabidopsis SPA1 gene is required for circadian clock function and photoperiodic flowering. Plant J 46:736–746
Jeknic Z, Chen THH (1999) Changes in protein profiles of poplar tissues during the induction of bud dormancy by short-day photoperiods. Plant Cell Physiol 40(1):25–35
Kim H-J, Kim Y-K, Park J-Y, Kim J (2002) Light signaling mediated by phytochrome plays an important role in cold-induced gene expression through the C-repeat/dehydration responsive element (C/DRE) in Arabidopsis thaliana. Plant J 29(6):693–704
Kobayashi Y, Weigel D (2007) Move on up, it’s time for change-mobile signals controlling photoperiod-dependent flowering. Genes Dev 21:2371–2384
Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286:1960–1962
Kwolek AVA, Woolhouse HW (1982) Studies on the dormancy of Calluna-Vulgaris (L) Hull, during winter – the effect of photoperiod and temperature on the induction of dormancy and the annual cycle of development. Ann Bot 49(3):367–376
Lang GA, Early JD, Martin GC, Darnell RL (1987) Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. HortScience 22:371–377
Law RD, Suttle JC (2004) Changes in histone H3 and H4 multi-acetylation during natural and forced dormancy break in potato tubers. Physiol Plant 120(4):642–649
Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH (2007) Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev 21:397–402
Li C, Junttila O, Ernstsen A, Heino P, Palva ET (2003) Photoperiodic control of growth, cold acclimation and dormancy development in silver birch (Betula pendula) ecotypes. Physiol Plant 117:206–212
Li Z, Reighard GL, Abbott AG, Bielenberg DG (2009) Dormancy-associated MADS genes from the EVG locus of [Purnus persica (L.) Batsch] have distinct seasonal and photoperiodic expression patterns. J Exp Bot 60:3521–3530
Mas P, Yanovsky MJ (2009) Time for circadian rhythms: plants get synchronized. Curr Opin Plant Biol 12:574–579
Mathiason K, He D, Grimplet J, Venkateswari J, Galbraith DW, Or E, Fennell A (2008) Transcript profiling in Vitis riparia during chilling requirement fulfillment reveals coordination of gene expression patterns with optimized bud break. Funct Integr Genomics. doi:10.1007/s10142-008-0090-y
Mazzitelli L, Hancock RD, Haupt S, Walker PG, Pont SDA, McNicol J, Cardle L, Morris J, Viola R, Brennan R, Hedley PE, Taylor MA (2007) Co-ordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. J Exp Bot 58:1035–1045
McClung CR (2006) Plant circadian rhythms. Plant Cell 18:792–803
Michaels SD (2009) Flowering time regulation produces much fruit. Curr Opin Plant Biol 12:75–80
Mikkelsen MD, Thomashow MF (2009) A role for circadian evening elements in cold-regulated gene expression in Arabidopsis. Plant J 60:328–339
Mohamed R (2006). Expression and function of Populus homologs to TERMINAL FLOWER1 genes: roles in onset of flowering and shoot phenology. PhD dissertation, Oregon State University, Corvallis, OR
Mølmann JA, Asante DKA, Jensen JB, Krane MN, Ernstsen A, Junttila O, Olsen JE (2005) Low night temperature and inhibition of gibberellin biosynthesis override phytochrome action and induce bud set and cold acclimation, but not dormancy in PHYA overexpressors and wild-type of hydrid aspen. Plant Cell Environ 28:1579–1588
Moss GI (1969) Influence of temperature and photoperiod on flower induction and inflorescence development in sweet orange – (Citrus Sinensis L Osbeck). J Hort Sci Biotech 44(4):311–320
Mouhu K, Hytönen T, Folta K, Rantanen M, Paulin L, Auvinen P, Elomaa P (2009) Identification of flowering genes in strawberry, a perennial SD plant. BMC Plant Biol 9:122. doi:10.1186/1471-2229-9-122
Nishikawa F, Endo T, Shimada T, Fujii H, Shimizu T, Omura M, Ikoma Y (2007) Increased CiFT abundance in the stem correlates with floral induction by low temperature in Satsuma mandarin (Citrus unshiu Marc.). J Exp Bot 58(14):3915–3927
Noh B, Noh YS (2006) Chromatin-mediated regulation of flowering time in Arabidopsis. Physiol Plant 126(4):484–493
Nordstrom A, Tarkowski P, Tarkowska D, Norbaek R, Astot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci 101:8039–8044
Nozue K, Maloof J (2006) Diurnal regulation of plant growth. Plant Cell Environ 29:396–408
Olsen JE (2006) Mechanisms of dormancy regulation. Acta Hortic 727:157–165
Olsen JE, Junttila O, Nilsen J, Eriksson ME, Martinussen I, Olsson O, Sandberg G, Moritz T (1997) Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant J 12(6):1339–1350
Or E (2009) Grape bud dormancy release-the molecular aspect. In: Roubelakis-Angelakis KA (ed) Grapevine molecular physiology and biotechnology, 2nd edn. Springer Science and Business Media B.V. doi 10.1007/978-90-481-2305-6_1.
Or E, Vilozny I, Eyal Y, Ogrodovitch A (2000) The transduction of the signal for grape bud dormancy breaking induced by hydrogen cyanamide may involve the SNF-like protein kinase GDBRPK. Plant Mol Biol 43(4):483–494
Penfield S (2008) Temperature perception and signal transduction in plants. New Phytol 179:615–628
Pineiro M, Gomez-Mena C, Schaffer R, Martinez-Zapater JM, Coupland G (2003) EARLY BOLTING IN SHORT DAYS is related to chromatin remodeling factors and regulates flowering in Arabidopsis by repressing FT. Plant Cell 15(7):1552–1562
Pratt C (1971) Reproductive anatomy in cultivated grapes – A Review. Am J Enol Vitic 22(2):92–109
Ramos A, Perez-Solis E, Ibanez C, Casado R, Collada C, Gomez L, Aragoncillo C, Allona I (2005) Winter disruption of the circadian clock in chestnut. Proc Natl Acad Sci USA 102(19):7037–7042
Rawat R, Schwartz J, Jones MA, Sairanen I, Cheng Y, Andersson CR, Zhao Y, Ljung K, Harmer SL (2009) REVEILLE1, a Myb-like transcription factor, integrates the circadian clock and auxin pathways. Proc Natl Acad Sci USA 106:16883–16888
Robertson FC, Skeffington AW, Gardner MJ, Webb AAR (2008) Interactions between circadian and hormonal signaling in plants. Plant Mol Biol 69:419–427
Rodrigo MJ, Alquezar B (2006) Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J Exp Bot 57(3):633–643
Rodríguez-Falcón M, Bou J, Prat S (2006) Seasonal control of tuberization in potato: conserved elements with the flowering response. Annu Rev Plant Biol 57:151–180
Rohde A, Bhalerao RP (2007) Plant dormancy in the perennial context. Trends Plant Sci 12:217–223
Ruonala R, Rinne PLH, Kangasjarvi J, van der Schoot C (2008) CENL1 expression in the rib meristem affects stem elongation and the transition to dormancy in Populus. Plant Cell 20:59–74
Ruttink T, Arend M, Morreel K, Storme V, Rombauts S, Fromm J, Bhalerao RP, Boerjan W, Rohde A (2007) A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 19(8):2370–2390
Salathia N, Davis SJ, Lynn JR, Michaels SD, Amasino RM, Millar AJ (2006) FLOWERING LOCUS C-dependent and -independent regulation of the circadian clock by the autonomous and vernalization pathways. BMC Plant Biol 6:10. doi:10.1186/1471-2229-6-10
Samach A, Wigge PA (2005) Ambient temperature perception in plants. J Plant Biol 8:483–486
Sawa M, Nusinow DA, Kay SA, Imaizumi T (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318:261–265
Schnabel BJ, Wample RL (1987) Dormancy and cold hardiness in Vitis-Vinifera L Cv White Riesling as influenced by photoperiod and temperature. Am J Enol Vitic 38(4):265–272
Schrader J, Moyle R, Bhalerao R, Hertzberg M, Lundeberg J, Nilsson P, Bhalerao RP (2004) Cambial meristem dormancy in trees involves extensive remodeling of the transcriptome. Plant J 40:173–187
Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, Amasino RA, Coupland G (2006) The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Gene Dev 20:898–912
Smith H (2000) Phytochromes and light signal perception by plants – an emerging synthesis. Nature 407:585–591
Smithberg MH, Weiser CJ (1968) Patterns of variation among climatic races of red-osier dogwood. Ecology 49(3):495–505
Sonnewald U (2001) Control of potato tuber sprouting. Trends Plant Sci 6(8):333–335
Sung S, Amasino RM (2004) Vernalization and epigenetics: how plants remember winter. Curr Opin Plant Biol 7:102–108
Sung S, He Y, Eshoo TW, Tamada Y, Johnson L, Nakahigashi K, Goto K, Jacobsen SE, Amasino RM (2006) Epigenetic maintenance of the vernalized state in Arabidopsis thaliana requires LIKE HETEROCHROMATIN PROTEIN 1. Nat Genet 38(6):706–710
Suttle JC (1998) Involvement of ethylene in potato microtuber dormancy. Plant Physiol 118(3):843–848
Suttle JC (2004) Physiological regulation of potato tuber dormancy. Am J Potato Res 81:253–262
Suttle JC (2008) Effects of synthetic phenylurea and nitroguanidine cytokinins on dormancy break and sprout growth in russet burbank minitubers. Am J Potato Res 85:121–128
Svendsen E, Wilen R, Stevenson R, Liu R, Tanino KK (2007) A molecular marker associated with low-temperature induction of dormancy in red osier dogwood (Cornus sericea). Tree Physiol 27(3):385–397
Takada S, Goto K (2003) TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell 15(12):2856–2865
Thomashow MF (2001) So what’s new in the field of plant cold acclimation? Lots! Plant Physiol 125:89–93
Volaire F, Norton M (2006) Summer dormancy in perennial temperate grasses. Ann Bot 98:927–933
Wake CMF, Fennell A (2000) Morphological, physiological and dormancy responses of three Vitis genotypes to short photoperiod. Physiol Plant 109:203–210
Welling A, Kaikuranta P, Rinne P (1997) Photoperiodic induction of dormancy and freezing tolerance in Betula pubescens. Involvement of ABA and dehydrins. Physiol Plant 100:119–125
Yamane H, Kashiwa Y, Ooka T, Tao R, Yonemori K (2008) Suppression subtractive hybridization and differential screening reveals endodormancy-associated expression of an SVP/AGL24-type MADS-box gene in lateral vegetative buds of Japanese apricot. J Am Soc Hortic Sci 133(5):708–716
Yuceer C, Land SB Jr, Kubiske ME, Harkess RL (2003) Shoot morphogenesis associated with flowering in Populus deltoides (Salicaceae). Am J Bot 90:196–206
Acknowledgments
Special thanks to Cetin Yuceer for providing the poplar pictures used in Fig. 5.1. The first and second authors contributed equally.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Anderson, J.V., Horvath, D.P., Chao, W.S., Foley, M.E. (2010). Bud Dormancy in Perennial Plants: A Mechanism for Survival. In: Lubzens, E., Cerda, J., Clark, M. (eds) Dormancy and Resistance in Harsh Environments. Topics in Current Genetics, vol 21. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-12422-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-642-12422-8_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-12421-1
Online ISBN: 978-3-642-12422-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)