Abstract
Growth and development of plants is controlled by external and internal signals. Key internal signals are those generated by hormones and the circadian clock. We highlight interactions between the circadian clock and hormonal signalling networks in regulating the physiology and growth of plants. Microarray analysis has shown that a significant proportion of transcripts involved in hormonal metabolism, catabolism, perception and signalling are also regulated by the circadian clock. In particular, there are interactions between the clock and abscisic acid, auxin, cytokinin and ethylene signalling. We discuss the role of circadian modulation (‘gating’) of hormonal signals in preventing temporally inappropriate responses. A consideration of the daily changes in physiology provides evidence that circadian gating of hormonal signalling couples the rhythmic regulation of carbon and water utilisation to rhythmic patterns of growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The circadian clock is an internal timekeeper that is an adaptation to day/night cycles providing, in Arabidopsis thaliana, competitive advantage (Dodd et al. 2005) and probably an increase in fitness (Green et al. 2002). Circadian clocks regulate many processes in plants, including leaf and stomatal movements, photosynthesis and growth and they also contribute to photoperiodism (Gardner et al. 2006; Hotta et al. 2007; Imaizumi and Kay 2006). In recent years the importance of circadian regulation of signalling pathways in the daily life of plants has been demonstrated by studies showing that the circadian clock modulates (or ‘gates’) signalling events (Covington and Harmer 2007; Dodd et al. 2006; Fowler et al. 2005; Hotta et al. 2007; Salter et al. 2003). Gating of a response by the clock results in variations in the intensity of that response depending on the time of day that the initial stimulus was applied. The molecular mechanisms by which gating occurs are unknown but one possibility is that one or more components involved in the transduction of the signal are circadian-regulated (specific examples of gating and a discussion of the possible mechanisms by which it could occur are in Figs. 2, 3 and 4 of Hotta et al. 2007).
Circadian gating demonstrates that there is an interaction between the circadian and hormonal signalling networks. System-wide analyses have suggested that the interactions between hormonal and circadian signalling might be considerable. In A. thaliana, estimates of the circadian control of gene expression from microarray analyses of oscillating transcript abundance in constant light vary from about 6% to 15% of the genome (Dodd et al. 2007; Edwards et al. 2006; Harmer et al. 2000). Within these datasets there is considerable overlap between circadian-, auxin- and abscisic acid (ABA)-regulation of transcripts (Dodd et al. 2007; Covington and Harmer 2007). The number of rhythmically regulated transcripts is probably even higher than the above estimates. Analysis of the regulation of transcripts, in various combinations of constant and rhythmic conditions and entrainment, demonstrated that 89% of detected transcripts can be rhythmic, when all conditions are considered (Michael et al. 2008). These studies suggest that rhythmic regulation of transcripts, including those transcripts involved in hormonal metabolism, catabolism, perception and signalling, is the norm, indicating a strong influence of time-of-day on hormonal responses.
The Arabidopsis thaliana circadian clock
An autonomous circadian timekeeper is probably present in every cell of A. thaliana. The structure of the A. thaliana circadian clock is complex and the distinctions between input pathways that entrain the clock, the central oscillator that generates rhythms and the output pathways controlling physiology, are blurred. The A. thaliana circadian clock consists of a series of interconnecting feedback loops that, in addition to regulating outputs, also gate both inputs to the oscillator and other signalling pathways (Fig. 1; Gardner et al. 2006; Hotta et al. 2007). The high degree of interconnectivity between components provides robustness to the molecular oscillator (Locke et al. 2006; Zeilinger et al. 2006).
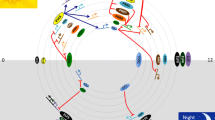
The circadian clock of Arabidopsis thaliana. The central oscillator consists of multiple interlocking transcriptional feedback loops and the small signalling molecules cADPR and Ca2+ act in the cytosol to form another feedback loop. Components of the clock associated with light perception are labelled with a sun. Phytochromes (PHY) and cryptochromes (CRY) are the principal photoreceptors that mediate light input to the clock and can themselves be regulated by the clock. ELF3 is also circadian regulated and gates light input to the clock (Covington et al. 2001; McWatters et al. 2000). Component X is predicted from mathematical modelling (Locke et al. 2005). Arrows indicate positive relationships and bars negative relationships. The mechanism by which the clock controls oscillations of cADPR and the means by which cADPR and Ca2+ input back into the clock are unknown; these relationships are therefore indicated by dotted lines. LUX is LUX ARRHYTHMO (Hazen et al. 2005), all other abbreviations are described in the text
The phase of the circadian clock is adjusted to match the day/night cycle by entrainment through the phytochromes and cryptochromes (Millar 2004; Salomé and McClung 2005). Light input to the circadian oscillator is important in the morning to match the phase of the internal oscillator with dawn and in the morning and evening to allow for day length measurement. At dawn, light signals activate a loop of morning-expressed genes consisting of LHY (LATE ELONGATED HYPOCOTYL), CCA1 (CIRCADIAN CLOCK ASSOCIATED1), PRR9 (PSEUDORESPONSE REGULATOR9) and PRR7 (Locke et al. 2006; Zeilinger et al. 2006). LHY and CCA1 are two light-activated MYB-like transcription factors that promote the expression of PRR7 and PRR9, two genes of unknown biochemical function. PRR7 and PRR9 in turn feedback to repress LHY and CCA1, thus forming a negative feedback loop active in the morning.
LHY and CCA1 proteins accumulate through the day and repress another loop of the clock composed of evening-expressed genes. In the afternoon, LHY and CCA1 proteins are degraded relieving the repression of expression of GI (GIGANTEA) and TOC1 (TIMING OF CAB EXPRESSION1/PRR1) which form a feedback loop in which GI activates TOC1 expression and TOC1 in turn represses GI expression. GI interacts with, and stabilizes ZTL (ZEITLUPE), a component of the E3 ubiquitin ligase that targets TOC1 for degradation (Han et al. 2004; Kim et al. 2007; Más et al. 2003). ZTL is unusual in this pathway because ZTL transcripts are not under circadian control. However, the circadian alterations in GI abundance confer rhythmic changes to ZTL protein levels. The interaction between ZTL and GI is enhanced by blue light, with ZTL acting as the photoreceptor. This leads to increased ZTL stability at the end of the day when GI and TOC1 begin to accumulate (Kim et al. 2007; Más et al. 2003). At night, the GI-ZTL interaction is reduced and ZTL is freed, presumably to target TOC1 for degradation at the end of the night. This, coupled with the activation of TOC1 expression by GI, results in peak expression of TOC1 in the first half of the night. The loop of evening expressed genes is coupled back to the ‘morning’ loop by indirect activation of LHY and CCA1 expression by TOC1 (Locke et al. 2006; Zeilinger et al. 2006).
The interactions between ZTL and GI, and ZTL and TOC1 occur in the cytosol and are examples of post-translational effects that are critical to the robustness and stability of the clock. Post-translational processes contributing to plant circadian timing include phosphorylation (Daniel et al. 2004), ribosylation (Panda et al. 2002) and proteosome-dependent degradation (Más et al. 2003, Han et al. 2004). Rhythms are therefore likely to be an emergent property of the entire cellular system, incorporating both cell physiology and transcriptional feedback loops. The importance of post-translational cytosolic events in the circadian clock has been emphasized by the presence in A. thaliana of a loop that incorporates the cytosolic signalling molecule cyclic adenosine diphosphate ribose (cADPR) (Fig. 1; Dodd et al. 2007). In mammals, cADPR is an important intracellular Ca2+-releasing agent that acts via the Ca2+-induced Ca2+ release ryanodine receptor pathway rather than the inositol-(1,4,5)-trisphosphate (Ins(1,4,5)P3) pathway (Lee 2002). cADPR is metabolised from NAD by the enzyme ADP-ribosyl cyclase and a by-product of this reaction is nicotinamide which can be used to inhibit cADPR production (Sethi et al. 1996). Plants respond to cADPR by releasing Ca2+ from the vacuole (Allen et al. 1995) and the ER (Navazio et al. 2001), however the receptors involved and the enzyme producing cADPR have not been characterized at the molecular level. In addition to its role in the circadian clock, cADPR has been implicated in a number of stress related responses (Hunt et al. 2004). Circadian oscillations of [cADPR] are driven by the transcriptional feedback loops of the circadian oscillator. Inhibition of cADPR production by nicotinamide abolishes circadian oscillations of [Ca2+]cyt and lengthens the circadian period of CCA1, LHY, TOC1 and CAB2 expression and the circadian period of leaf movement. This indicates that cADPR is required to drive circadian oscillations of [Ca2+]cyt and also modulates the nuclear transcriptional feedback loops of the A. thaliana circadian oscillator (Dodd et al. 2007). Recently, it has been demonstrated that nicotinamide lengthens the period of the mammalian circadian clock in a manner similar to that in plants. The mechanism by which this occurs has not been explored but the period-lengthening is independent of the inhibitory effects of nicotinamide on SIRT1, a deacetylase that regulates mammalian circadian clock gene expression by counteracting the acetyltransferase activity of CLOCK (Asher et al. 2008). The similarity of the period lengthening effects of nicotinamide on the plant and mammalian oscillator are striking, because in both plants, and the mammalian suprachiasmatic nucleus, circadian oscillations of [Ca2+]cyt are driven by cADPR-sensitive mechanisms (Ikeda et al. 2003; Dodd et al. 2007). The existence of a loop of cytosolic signalling molecules within the circadian clock is likely to enhance stability and allow for both modulation of the circadian clock by stress signals and also circadian modulation of stress signalling, because both cADPR and [Ca2+]cyt participate in the transduction of biotic and abiotic stresses (Dodd et al. 2007).
It has also been proposed that nyctohemeral (daily) oscillations of [Ca2+]cyt are driven by CAS, a Ca2+-receptor, with Ins(1,4,5)P3 acting as the Ca2+-mobilizing second messenger (Tang et al. 2007). It was proposed that CAS, initially thought to be located in the plasma membrane, senses rhythmic changes in apoplastic [Ca2+] caused by changes in water flux as a consequence of stomatal rhythms (Tang et al. 2007). A central tenet of this hypothesis is that rhythms of [Ca2+]cyt and stomatal movements are in phase. However, in the short circadian period mutant toc1-1, [Ca2+]cyt and stomatal rhythms become uncoupled (Xu et al. 2007). In toc1-1, stomatal rhythms have a period of ~21 h in constant light (LL, Somers et al. 1998), whilst [Ca2+]cyt rhythms have a period of ~24 h (Xu et al. 2007) suggesting that these two rhythms are not functionally linked. Circadian rhythms of [Ca2+]cyt are insensitive to the phospholipase C inhibitor U73122, but are completely abolished by nicotinamide. This suggests that Ins(1,4,5)P3 does not contribute to oscillations of [Ca2+]cyt in constant conditions (Dodd et al. 2007). A recent report provides evidence that CAS is not located on the plasma membrane but rather is mainly localized to chloroplasts as an integral thylakoid membrane protein with the N-terminus Ca2+-binding region probably on the stromal side (Nomura et al. 2008). Whilst it seems unlikely that CAS acts as a plasma membrane receptor linked to phospholipase C to drive circadian oscillations of [Ca2+]cyt, it is probable that CAS has an important function in the generation of rhythmic Ca2+ signals because the chloroplast is a source of organellar Ca2+ rhythms (Johnson et al. 1995; Sai and Johnson 2002).
The uncoupling of [Ca2+]cyt and stomatal rhythms in the toc1-1 mutant as well as the absence of [Ca2+]cyt rhythms in the short circadian period cca1-1 mutant, indicates that there are multiple, genetically separable, circadian oscillators in A. thaliana (Xu et al. 2007). The different circadian oscillators could be located in the same or different cell types (Gardner et al. 2006; Xu et al. 2007). Cell-type specific expression of the clock component PRR3 may contribute to the differential behavior of circadian clocks in A. thaliana (Para et al. 2007).
Interactions between hormone signalling networks and the circadian clock
The physiological processes controlled by plant hormones overlap considerably with those regulated by the circadian clock, indicating possible cross-talk between circadian and hormonal signalling networks. It was noticed as early as 1937 that the sensitivity of plants to auxin varied over the day (Went and Thimann 1937), indicative of what is now known as circadian gating of auxin signalling (Covington and Harmer 2007). Hormonal-clock interactions are not restricted to auxin but might also include ABA and possibly cytokinins and ethylene.
Gating of responses to ABA
ABA regulates events from seed germination right through to seed formation, affecting both vegetative and reproductive processes (Finkelstein and Rock 2002). ABA is also involved in responses to numerous stresses, including water-deficit, salt, hypoxia and cold and affects responses to wounding and pathogen attack. The circadian clock influences many of these processes. There is highly significant overlap between the transcripts regulated by the circadian clock or daily light/dark cycles and ABA (Mizuno and Yamashino 2008). Transcripts regulated by the closely related methyl jasmonate signalling pathway also overlap with those regulated in day/night cycles (Mizuno and Yamashino 2008). In contrast, transcripts regulated by ethylene, cytokinin, giberellic acid and brassinosteroids do not, or only weakly significantly, overlap with rhythmically-regulated transcripts in constant light and day/night cycles (Dodd et al. 2007; Mizuno and Yamashino 2008).
Stomatal movements are an example of a physiological process regulated by both the circadian clock and ABA. Rhythmic stomatal movements anticipate dawn and dusk (Fig. 2), and the opening of stomata before dawn is effected by activation of inward K+ channels (Lebaudy et al. 2008). During water-deficit stress ABA reduces the size of the stomatal pore. When water-deficit stress is mild, ABA is less effective at closing stomata in the morning than in the afternoon (Correia et al. 1995; Fig. 2). This may be an example of circadian gating of ABA signalling which ensures stomata open to facilitate CO2 uptake in the cool of the morning, when transpiration will be lower, but are closed in the heat of the afternoon if water supply is limiting (Webb 1998).
Gating of responses in stomata, hypocotyl elongation and cold signalling. Stomatal aperture (a), relative growth rate of hypocotyl elongation (b, modified from Nozue et al. 2007) and RD29A transcript abundance (c, Smith et al. 2004) are rhythmic in 12:12 LD cycles. The responses of stomata (a) and the hypocotyl (b) to various stimuli are gated by the clock, with blue triangles showing the approximate times when stimuli have greater effect. a Stimuli affecting stomata are divided into those that promote opening and those that promote closure. b A Low R:FR ratio and auxin increase hypocotyl elongation maximally at the times indicated. c In cold signalling, a LT stimulus (snowflake) can induce transcription of the CBF1-3 transcription factors as well as increases in [Ca2+]cyt. These responses are also gated, and the approximate time of the greatest response to LT, in constant light conditions, is indicated by the blue arrows. The experiments summarised in this figure were performed in various photoperiods of light and dark, or constant light and therefore the gating effects might not be directly applicable to a 12:12 LD cycle. To provide a conceptual summary we have included the data from all conditions. A 12:12 LD cycle is indicated by white and black rectangles for reference
The ABA signalling pathway includes many components in common with cold signalling and plant responses to cold can include an increase in ABA. As with ABA-signalling, there is evidence that cold-signalling is gated by the circadian clock. One function of the cold-signalling pathway is the induction of freezing tolerance in response to prior exposure to non-damaging low temperatures (LT). In A. thaliana, part of the freezing-tolerance mechanism is the LT-induction of the CBF1-3 transcription factors (C-REPEAT BINDING FACTORs also known as DEHYDRATION-RESPONSIVE ELEMENT-BINDING PROTEIN 1b, DREB1c and DREB1a, respectively; Vogel et al. 2005) that in turn drive the expression of about 200 genes which form the CBF regulon. The CBFs also confer tolerance to drought and salt stress (Fowler and Thomashow 2002; Liu et al. 1998; Maruyama et al. 2004). Expression of CBF1-3 is circadian regulated and induction of CBF1-3 in response to LT has a 24 h rhythm indicative of circadian gating of LT signalling (Fowler et al. 2005). The maximum LT-induced increase in transcription of CBF1-3 occurs when plants are exposed to LT 4 h after dawn (Fig. 2) and there is no temporal difference in the LT stimulation of CBF1-3 expression in arrhythmic CCA1 overexpressors (CCA1-ox). A functional circadian clock is, therefore, required for the rhythmic sensitivity of CBF1-3 transcription to LT (Fowler et al. 2005).
One arm of the cold-signalling pathway involves ABA-mediated increases in cADPR and [Ca2+]cyt leading to the expression of a suite of cADPR- and Ca2+-responsive genes such as RD29A (RESPONSIVE TO DESICCATION29A) (also known as LOW TEMPERATURE INDUCED78 and COLD REGULATED78; Viswanathan and Zhu 2002; Wu et al. 2003). The transcript levels of RD29A, [Ca2+]cyt and [cADPR] are all circadian-regulated (Dodd et al. 2006, 2007; Johnson et al. 1995). In addition, the acute responses of both RD29A and [Ca2+]cyt to cold are gated by the circadian clock, with maximum responsiveness during the subjective day (Dodd et al. 2006; Fig. 2). The involvement of cADPR in a loop of the circadian oscillator is suggestive of a mechanism by which gating of ABA and cold signalling might occur as a consequence of the circadian control of the abundance of the transducing second messengers. Maximal induction by LT of CBF1-3, RD29A and [Ca2+]cyt during the day might indicate that a different set of responses to cold are required at this time than at night. Because it is usual for the temperature to drop at night and increase during the day, a cold stimulation during the day could be indicative of the onset of autumn and winter. Possibly, long term responses in preparation for winter are required if cold is perceived during the day.
The high degree of interconnection between ABA signalling and the circadian clock is further exemplified by evidence that ABA can affect circadian oscillator period. The period of the rhythmic changes in promoter activity of CCA1 (a core oscillator gene), AtGRP7/CCR2 (GLYCINE-RICH RNA-BINDING PROTEIN7/COLD, CIRCADIAN RHYTHM AND RNA BINDING 2; a possible slave oscillator component) and CAB2 (CHLOROPHYLL A/B-BINDING PROTEIN2; a marker gene for clock output), are lengthened in response to exogenous application of ABA in LL (Hanano et al. 2006). Consistent with this, the period of AtGRP7/CCR2 promoter activity rhythms are shortened in the ABA-deficient mutant aba2 (abscisic acid absent2) in constant darkness (Hanano et al. 2006). Whether the effects of ABA on circadian period can be explained due to the effects of ABA on [cADPR] remains to be determined. Transient alterations of [cADPR] have little effect on the oscillator, and prolonged increases in [cADPR] might shorten period (Dodd et al. 2007) in contrast to the reported ABA-induced lengthening of period (Hanano et al. 2006).
Gating of responses to auxin
Auxin is a potent regulator of plant growth and development (Leyser 2005). The levels of both free indole acetic acid (IAA) and conjugated-IAA fluctuate in a circadian manner in A. thaliana (Jouve et al. 1999). IAA is required for the circadian rhythms of growth of the flowering stem (Jouve et al. 1999), showing that auxin responses are intimately connected to processes also controlled by the clock. This is also suggested by overlap between some auxin-regulated and circadian transcriptomes (Covington and Harmer 2007; Dodd et al. 2007). Genes involved in every aspect of auxin signalling are circadian-regulated, including biosynthesis, efflux, inactivation and perception (Covington and Harmer 2007). In addition, many auxin-induced and repressed genes are also circadian-regulated (Covington and Harmer 2007). The circadian clock can drive rhythms of expression from a synthetic auxin-responsive promoter, and the clock can gate transcriptional and hypocotyl growth responses to auxin, but auxin has only weak effects on circadian oscillator function (Covington and Harmer 2007). In general, transcriptional and growth responses to auxin tend to peak in the subjective night and be lower during subjective day and this is broadly consistent with the rhythmic growth of the hypocotyl (Fig. 2). In LL, the peak rate of hypocotyl elongation occurs at subjective dusk and growth stops entirely around subjective dawn (Dowson-Day and Millar 1999), whereas in light/dark cycles (LD), the peak of growth is shifted to around dawn (Fig. 2; Nozue et al. 2007). It is not only auxin-induced growth and transcriptional changes that are subject to gating by the circadian clock; stomatal opening can be induced by auxin and the hormone is less effective at inducing stomatal opening in Commelina communis during the night than during the day (Fig. 2; Snaith and Mansfield 1985). This is in contrast to the phase in which auxin is most effective in promoting hypocotyl elongation and transcriptional activation, suggesting that different gating mechanisms modulate different responses, possibly through cell-specific components of the circadian gating mechanisms.
Circadian rhythms of ethylene production
The levels of ethylene emission from A. thaliana plants oscillate with a circadian period partially due to the circadian control of the abundance of transcripts encoding 1-amino-cyclopropane-1-carboxylic acid (ACC) synthases (ACSs) which make ACC, the precursor of ethylene (Thain et al. 2004). Among other things, ethylene affects cell growth and shape and is involved in hypocotyl elongation (Abeles et al. 1992) but the function of rhythmic ethylene emission is unclear. Ethylene has no effect on the clock or rhythmic outputs (Thain et al. 2004) and maximal ethylene production in the middle of the day does not coincide with maximal hypocotyl elongation (Nozue et al. 2007). Similarly, ACC has no consistent effects on the oscillator (Hanano et al. 2006). Rhythmic hypocotyl elongation persists in ethylene biosynthesis and signalling mutants, including etr1-1, ein4-1 and eto2-1, suggesting ethylene is not required for rhythmic growth (Thain et al. 2004). However, ethylene has other roles, including involvement in responses to biotic and abiotic stresses, which could represent targets for circadian ethylene signals (Abeles et al. 1992) and these remain to be explored.
Cytokinins can regulate clock function
Cytokinins promote cell division and shoot formation as well as many other growth processes (Sakakibara 2006) and there is some evidence for interactions between cytokinins and the clock (Hanano et al. 2006; Salomé et al. 2006; Zheng et al. 2006). Endogenous application of cytokinin affects the phase but not the period of the clock (Hanano et al. 2006; Salomé et al. 2006; Zheng et al. 2006) and increases transcription of LHY and CCA1 but suppresses transcription of TOC1 (Zheng et al. 2006). The effect of cytokinin on the clock is thought to be mediated by ARR4 (ARABIDOPSIS RESPONSE REGULATOR4), a gene responsive to cytokinin that also acts as an input to the core oscillator (Salomé et al. 2006). In terms of cytokinin-modulation of circadian rhythms, the arr4 mutant is insensitive to cytokinin (Zheng et al. 2006) whereas the ARR4-ox mutant is hypersensitive to cytokinin (Hanano et al. 2006). Cytokinin and light signalling co-regulate many processes (Thomas et al. 1997) and, as light signalling is gated by the clock (Hotta et al. 2006), it might be informative to further investigate the interactions between cytokinin and circadian signalling.
A day in the life of a plant: physiological significance of interactions between the clock and hormones
Plants gamble the daily consumption of water and carbon against anticipated later replenishment. During the day, C3 and C4 plants loose water when the stomata are open leading to a decrease in water potential. At night, when the stomata are closed, water uptake is greater than water loss and water potential increases (Fig. 3). Similarly, C3 and C4 plants consume the transitory starch reserve at night, with the anticipation of replenishing this reserve with carbon fixed during the following day (Fig. 3). A consideration of the daily changes in physiology provides evidence that circadian gating of hormonal signalling couples this rhythmic regulation of reserve utilisation to rhythmic growth patterns, presumably because growth requires carbon for raw materials and water for turgor to drive cell expansion. Circadian gating of hormonal signalling also alters physiological responses to prevent temporally inappropriate responses from occurring, thus increasing the overall fitness of the plant.
Daily changes in starch content and water potential. During the day excess photosynthetic carbon is stored as starch in anticipation of night time demand, and at night the starch is metabolised at a constant rate until stores are depleted at dawn (a). This basic pattern is maintained, with a period of acclimation, in long and short days (redrawn from Gibon et al. 2004). Water potential (ψw) decreases during the day, when stomata are open, and starts to increase around dusk when stomata are more closed in both long and short days (b; redrawn from Hamilton and Davies 1988). Yellow indicates the photoperiod
In the early morning, stomata are relatively insensitive to ABA, allowing carbon uptake through open stomatal pores before the heat of the afternoon, even in moderately water-deficit stressed plants (Correia et al. 1995; Fig. 2). Stomatal opening is also favoured by high sensitivity to blue light at dawn (Fig. 2), ensuring the stomata open to maximise carbon fixation during the day (Webb 1998). Similarly, transcripts encoding proteins involved in photosynthesis are particularly sensitive to light in the morning (Harmer et al. 2000). As the day proceeds, water is lost by transpiration through the open stomata and leaf water potential decreases (Hamilton and Davies 1988; Fig. 3). This rhythmic loss of water during the day might explain the accumulation of ABA-upregulated and stress-related genes during the day (Harmer et al. 2000; Kreps et al. 2002; Mizuno and Yamashino 2008). In the early afternoon, guard cells become more sensitive to ABA and, if there is water-deficit stress, stomatal aperture is reduced, thereby decreasing transpiration at a time when the air temperature is likely to be highest (Figs. 2 and 3).
At night, the stomata close, water reserves are replenished through the roots and the plant becomes a net consumer of carbon (Figs. 2 and 3). Transitory starch, produced from sucrose during the day and stored in the leaves, is metabolised at night to support sucrose synthesis and metabolism (Fig. 3; Smith and Stitt 2007). The plant metabolises carbon to a set point at the anticipated end of the night, ensuring that starch reserves last the entire night but are also completely consumed. If the night is artificially extended, the carbon becomes depleted before the end of the night and growth is severely inhibited (Fig. 3; Gibon et al. 2004). Once entrained to the long night, the rate of starch production is increased during the day and the rate of consumption at night is decreased (Gibon et al. 2004; Fig. 3). This leads to a reduction in growth rate to accommodate the decrease in metabolites available for growth and prevents complete inhibition of growth (Smith and Stitt 2007). Entrainment to the long night is thought to occur through a combination of signalling networks involving sugar signalling and sensing, light and the clock (Smith and Stitt 2007). Sugar metabolism and signalling networks also interact with the ABA and ethylene signalling networks (León and Sheen 2003) and the auxin and cytokinin signalling networks (Moore et al. 2003; Rolland et al. 2006).
Towards the end of the night A. thaliana hypocotyl growth rate is maximal (Nozue et al. 2007). Growth is controlled by a complex interplay of multiple signalling networks through the coincidence of internal circadian and external light and dark signals as demonstrated by the rephasing of the maximal period of hypocotyl expansion from subjective dusk in LL, to around dawn in LD (Nozue et al. 2007). A model describing the mechanisms for the rhythmic regulation of growth has been proposed (Nozue et al. 2007). In this model, the transcription factors PIF4 (PHYTOCHROME-INTERACTING FACTOR4) and PIF5 promote growth. PIF4 and PIF5 transcripts are under circadian control with transcript abundance being minimal in the middle of the night and the PIF4 and PIF5 proteins are degraded in the light. Thus, two rhythmic behaviours conspire to result in maximal growth rates around dawn; circadian control of PIF4 and PIF5 transcript abundance, and rhythmic light signals causing degradation of PIF4 and PIF5 proteins. Restricting growth to the night may have a number of advantages. Turgor, the driving force for cell expansion, is highest prior to dawn when there is minimal loss of water from the stomata. In addition, focusing growth to the end of the dark period might allow judicious use of carbon, allowing the demands of growth to be compared with other metabolic demands on transitory starch reserves.
Conclusions
Daily rhythms of light and heat input necessitate rhythms of uptake and use of both water and carbon by plants. Thus it is almost inevitable that there are rhythms in the processes associated with carbon and water balance, such as growth and stomatal movements. Evolutionary pressures that promoted these rhythms in physiology are likely also to have favoured rhythmic sensitivity to the hormones that regulate physiology. Particularly important will have been the development of mechanisms to prevent responses from occurring at the wrong time of day. For example, stomatal opening in response to nocturnal accumulation of auxin will result in loss of water with no gain in carbon fixation but, this is prevented, in part, by circadian gating of stomatal responses to auxin (Webb 1998). Thus rhythmic sensitivity to auxin and ABA are likely to be a common feature of many physiological processes. Similarly, the daily light/dark cycle imposes potential rhythmic abiotic stresses of heat, cold, photodamage and water-deficit stress. This too may have resulted in evolutionary pressure to favour particular stress responses such as ABA-signalling at the times of day when these stresses might be anticipated to be greatest. Evidence is also accumulating that hormone signals can influence clock function, indicating a network in which abiotic and circadian signalling are tightly integrated. The mechanisms by which the circadian clock gates hormonal signalling are unknown, but it is likely that an output of the clock modulates the hormonal signal transduction chain (Hotta et al. 2007). During extreme stresses, gating mechanisms are likely to be overridden since survival outweighs all other activities, but during more mild stress, the clock might act to modulate stress signalling to incorporate temporal information in to the network responding to cellular demands. This will maximise carbon uptake, growth and subsequent seed set and survival.
References
Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in plant biology, 2nd edn. Academic Press, San Diego
Allen GJ, Muir SR, Sanders D (1995) Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science 268:735–737. doi:10.1126/science.7732384
Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F et al (2008) SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134:317–328. doi:10.1016/j.cell.2008.06.050
Correia MJ, Pereira JS, Chaves MM, Rodrigues ML, Pacheco CA (1995) ABA xylem concentrations determine maximum daily leaf conductance in field-grown Vitis vinifera L. plants. Plant Cell Environ 18:511–521. doi:10.1111/j.1365-3040.1995.tb00551.x
Covington MF, Harmer SL (2007) The circadian clock regulates auxin signaling and responses in Arabidopsis. PLoS Biol 5(8):e222. doi:10.1371/journal.pbio.0050222
Covington MF, Panda S, Liu XL, Strayer CA, Wagner DR, Kay SA (2001) ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell 13:1305–1315
Daniel X, Sugano S, Tobin EM (2004) CK2 phosphorylation of CCA1 is necessary for its circadian oscillator function in Arabidopsis. Proc Natl Acad Sci USA 101:3292–3297. doi:10.1073/pnas.0400163101
Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F et al (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309:630–633. doi:10.1126/science.1115581
Dodd AN, Jakobsen MK, Baker AJ, Telzerow A, Hou S-W, Laplaze L et al (2006) Time of day modulates low-temperature Ca2+ signals in Arabidopsis. Plant J 48:962–973. doi:10.1111/j.1365-313X.2006.02933.x
Dodd AN, Gardner MJ, Hotta CT, Hubbard KE, Dalchau N, Love J et al (2007) The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science 318:1789–1792. doi:10.1126/science.1146757
Dowson-Day MJ, Millar AJ (1999) Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. Plant J 17:63–71. doi:10.1046/j.1365-313X.1999.00353.x
Edwards KD, Anderson PE, Hall A, Salathia NS, Locke JC, Lynn JR et al (2006) FLOWERING LOCUS C mediates natural variation in the high-temperature response of the Arabidopsis circadian clock. Plant Cell 18:639–650. doi:10.1105/tpc.105.038315
Finkelstein RR, Rock CD (2002) Abscisic acid biosynthesis and response. In: Somerville CR, Meyerowitz EM (eds) The arabidopsis book. American Society of Plant Biologists, Rockville, pp 1–48
Fowler SG, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14:1675–1690. doi:10.1105/tpc.003483
Fowler SG, Cook D, Thomashow MF (2005) Low temperature induction of Arabidopsis CBF1, 2 and 3 is gated by the circadian clock. Plant Physiol 137:961–968. doi:10.1104/pp.104.058354
Gardner MJ, Hubbard KE, Hotta CT, Dodd AN, Webb AAR (2006) How plants tell the time. Biochem J 397:15–24. doi:10.1042/BJ20060484
Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J et al (2004) Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39:847–862. doi:10.1111/j.1365-313X.2004.02173.x
Green RM, Tingay S, Wang ZY, Tobin EM (2002) Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol 129:576–584. doi:10.1104/pp.004374
Hamilton DA, Davies PJ (1988) Mechanism of export of organic material from the developing fruits of pea. Plant Physiol 86:956–959
Han L, Mason M, Risseeuw EP, Crosby WL, Somers DE (2004) Formation of an SCFZTL complex is required for proper regulation of circadian timing. Plant J 40:291–301. doi:10.1111/j.1365-313X.2004.02207.x
Hanano S, Domagalska MA, Nagy F, Davis SJ (2006) Multiple phytohormones influence distinct parameters of the plant circadian clock. Genes Cells 11:1381–1392. doi:10.1111/j.1365-2443.2006.01026.x
Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T et al (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290:2110–2113. doi:10.1126/science.290.5499.2110
Hazen SP, Schultz TF, Pruneda-Paz JL, Borevitz JO, Ecker JR, Kay SA (2005) LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proc Natl Acad Sci USA 102:10387–10392. doi:10.1073/pnas.0503029102
Hotta CT, Gardner MJ, Hubbard KE, Baek SJ, Dalchau N, Suhita D et al (2007) Modulation of environmental responses of plants by circadian clocks. Plant Cell Environ 30:333–349. doi:10.1111/j.1365-3040.2006.01627.x
Hunt L, Lerner F, Ziegler M (2004) NAD—new roles in signalling and gene regulation in plants. New Phytol 163:31–44. doi:10.1111/j.1469-8137.2004.01087.x
Ikeda M, Sugiyama T, Wallace CS, Gompf HS, Yoshioka T, Miyawaki A et al (2003) Circadian dynamics of circadian and nuclear Ca2+ in single suprachiasmatic nucleus neurones. Neuron 38:253–263. doi:10.1016/S0896-6273(03)00164-8
Imaizumi T, Kay SA (2006) Photoperiodic control of flowering: not only by coincidence. Trends Plant Sci 11:550–558. doi:10.1016/j.tplants.2006.09.004
Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A et al (1995) Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science 269:1863–1865. doi:10.1126/science.7569925
Jouve L, Gaspar T, Kevers C, Greppin H, Degli Agosti R (1999) Involvement of indole-3-acetic acid in the circadian growth of the first internode of Arabidopsis. Planta 209:136–142. doi:10.1007/s004250050615
Kim WY, Fujiwara S, Suh SS, Kim J, Kim Y, Han L et al (2007) ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 449:356–360. doi:10.1038/nature06132
Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, Harper JF (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol 130:2129–2141. doi:10.1104/pp.008532
Lebaudy A, Vavasseur A, Hosy E, Dreyer I, Leonhardt N, Thibaud JB et al (2008) Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc Natl Acad Sci USA 105:5271–5276. doi:10.1073/pnas.0709732105
Lee HC (2002) Cyclic ADP-ribose and NAAPD: structures, metabolism and functions. Kluwer Academic Publishers, Boston
León P, Sheen J (2003) Sugar and hormone connections. Trends Plant Sci 8:110–116. doi:10.1016/S1360-1385(03)00011-6
Leyser O (2005) Auxin distribution and plant pattern formation: how many angels can dance on the point of PIN? Cell 121:819–822. doi:10.1016/j.cell.2005.06.005
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K et al (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Locke JCW, Southern MM, Kozma-Bognár L, Hibberd V, Brown PE, Turner MS et al (2005) Extension of a genetic network model by iterative experimentation and mathematical analysis. Mol Syst Biol 1:1744–4292. doi:10.1038/msb4100018
Locke JCW, Kozma-Bognár L, Gould PD, Fehér B, Kevei E, Nagy F et al (2006) Experimental validation of a predicted feedback loop in the multi-oscillator clock of Arabidopsis thaliana. Mol Syst Biol 2:59. doi:10.1038/msb4100102
Maruyama K, Sakuma Y, Kasuga M, Ito Y, Seki M, Goda H et al (2004) Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J 38:982–993. doi:10.1111/j.1365-313X.2004.02100.x
Más P, Kim WY, Somers DE, Kay SA (2003) Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature 426:567–570. doi:10.1038/nature02163
McWatters HG, Bastow RM, Hall A, Millar AJ (2000) The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature 408:716–720. doi:10.1038/35047079
Michael TP, Mockler TC, Breton G, McEntee C, Byer A, Trout JD et al (2008) Network discovery pipeline elucidates conserved time-of-day specific cis-regulatory modules. PLoS Genet 4(2):e14. doi:10.1371/journal.pgen.0040014
Millar AJ (2004) Input signals to the plant circadian clock. J Exp Bot 55:277–283. doi:10.1093/jxb/erh034
Mizuno T, Yamashino T (2008) Comparative transcriptome of diurnally oscillating genes and hormone-responsive genes in Arabidopsis thaliana: insight into circadian clock-controlled daily responses to common ambient stresses in plants. Plant Cell Physiol 49:481–487. doi:10.1093/pcp/pcn008
Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX et al (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light and hormonal signaling. Science 300:332–336. doi:10.1126/science.1080585
Navazio L, Mariani P, Sanders D (2001) Mobilization of Ca2+ by cyclic ADP-ribose from the endoplasmic reticulum of cauliflower florets. Plant Physiol 125:2129–2138. doi:10.1104/pp.125.4.2129
Nomura H, Komori T, Kobori M, Nakahira Y, Shiina T (2008) Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J 53:988–998. doi:10.1111/j.1365-313X.2007.03390.x
Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL et al (2007) Rhythmic growth explained by coincidence between internal and external cues. Nature 448:358–361. doi:10.1038/nature05946
Panda S, Poirier GG, Kay SA (2002) tej Defines a role for poly(ADP-ribosyl)ation in establishing period length of the arabidopsis circadian oscillator. Dev Cell 3:51–61. doi:10.1016/S1534-5807(02)00200-9
Para A, Farré EM, Imaizumi T, Pruneda-Paz JL, Harmon FG, Kay SA (2007) PRR3 is a vascular regulator of TOC1 stability in the Arabidopsis circadian clock. Plant Cell 19:3462–3473. doi:10.1105/tpc.107.054775
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709. doi:10.1146/annurev.arplant.57.032905.105441
Sai J, Johnson CH (2002) Dark-stimulated calcium ion fluxes in the chloroplast stroma and cytosol. Plant Cell 14:1279–1291. doi:10.1105/tpc.000653
Sakakibara H (2006) CYTOKININS: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57:431–449. doi:10.1146/annurev.arplant.57.032905.105231
Salomé PA, McClung CR (2005) What makes the Arabidopsis clock tick on time? A review on entrainment. Plant Cell Environ 28:21–38. doi:10.1111/j.1365-3040.2004.01261.x
Salomé PA, To JPC, Kieber JJ, McClung CR (2006) Arabidopsis response regulators ARR3 and ARR4 play cytokinin-independent roles in the control of circadian period. Plant Cell 18:55–69. doi:10.1105/tpc.105.037994
Salter MG, Franklin KA, Whitelam GC (2003) Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature 426:680–683. doi:10.1038/nature02174
Sethi JK, Empson RM, Galione A (1996) Nicotinamide inhibits cyclic ADP-ribose-mediated calcium signalling in sea urchin eggs. Biochem J 319:613–617
Smith AM, Stitt M (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30:1126–1149. doi:10.1111/j.1365-3040.2007.01708.x
Smith SM, Fulton DC, Chia T, Thorneycroft D, Chapple A, Dunstan H et al (2004) Diurnal changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and posttranscriptional regulation of starch metabolism in Arabidopsis leaves. Plant Physiol 136:2687–2699. doi:10.1104/pp.104.044347
Snaith PJ, Mansfield TA (1985) Responses of the stomata to IAA and fusicoccin at the opposite phases of an entrained rhythm. J Exp Bot 36:937–944. doi:10.1093/jxb/36.6.937
Somers DE, Webb AAR, Pearson M, Kay SA (1998) The short-period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development 125:485–494
Tang RH, Han S, Zheng H, Cook CW, Choi CS, Woerner TE et al (2007) Coupling diurnal cytosolic Ca2+ oscillations to the CAS-IP3 pathway in Arabidopsis. Science 315:1423–1426. doi:10.1126/science.1134457
Thain SC, Vandenbussche F, Laarhoven LJ, Dowson-Day MJ, Wang ZY, Tobin EM et al (2004) Circadian rhythms of ethylene emission in Arabidopsis. Plant Physiol 136:3751–3761. doi:10.1104/pp.104.042523
Thomas TH, Hare PD, Van Staden J (1997) Phytochrome and cytokinin responses. Plant Growth Regul 23:105–122. doi:10.1023/A:1005906609158
Viswanathan C, Zhu JK (2002) Molecular genetic analysis of cold-regulated gene transcription. Philos Trans R Soc Lond B Biol Sci 357:877–886. doi:10.1098/rstb.2002.1076
Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF (2005) Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J 41:195–211. doi:10.1111/j.1365-313X.2004.02288.x
Webb AAR (1998) Stomatal rhythms. In: Lumsden PJ, Millar AJ (eds) Biological rhythms and photoperiodism in plants. Bios Scientific Publications, Oxford, pp 66–79
Went FW, Thimann KV (1937) Phytohormones. The Macmillan Company, New York, p 294
Wu Y, Sanchez JP, Lopez-Molina L, Himmelbach A, Grill E, Chua NH (2003) The abi1-1 mutation blocks ABA signalling downstream of cADPR action. Plant J 34:307–315. doi:10.1046/j.1365-313X.2003.01721.x
Xu X, Hotta CT, Dodd AN, Love J, Sharrock R, Lee YW et al (2007) Distinct light and clock modulation of cytosolic free Ca2+ oscillations and rhythmic CHLOROPHYLL A/B BINDING PROTEIN2 promoter activity in Arabidopsis. Plant Cell 19:3474–3490. doi:10.1105/tpc.106.046011
Zeilinger MN, Farré EM, Taylor SR, Kay SA, Doyle FJ 3rd (2006) A novel computational model of the circadian clock in Arabidopsis that incorporates PRR7 and PRR9. Mol Syst Biol 2:58. doi:10.1038/msb4100101
Zheng B, Deng Y, Mu J, Ji Z, Xiang T, Niu Q-W et al (2006) Cytokinin affects circadian-clock oscillation in a phytochrome B- and Arabidopsis response regulator 4-dependent manner. Physiol Plant 127:277–292. doi:10.1111/j.1399-3054.2006.00660.x
Acknowledgements
The authors thank AN Dodd for critical reading. Research is funded by the Biotechnology and Biological Sciences Research Council (UK), Engineering and Physical Sciences Research Council (UK), the Royal Society of London, the Isaac Newton Trust and Corpus Christi College Cambridge.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robertson, F.C., Skeffington, A.W., Gardner, M.J. et al. Interactions between circadian and hormonal signalling in plants. Plant Mol Biol 69, 419–427 (2009). https://doi.org/10.1007/s11103-008-9407-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-008-9407-4