Abstract
A family of promising polyhydroxyalkanoate(PHA) polyesters called Nodax™ class PHA copolymers, consisting of (R)-3-hydroxyalkanoate comonomer units with medium-size-chain side groups and (R)-3-hydroxybutyrate, are described. The bio-based biodegradable plastics made from renewable resources will be commercially available from Meredian. Because of the unique design of the molecular structure, the Nodax™ class PHA copolymers have a set of useful attributes, including polyolefin-like thermomechanical properties, polyester-like physicochemical properties, and interesting biological properties. Therefore, broad ranges of industrial and consumer product applications are anticipated. The structure and properties of the new PHA copolymers, as well as processing and conversion to various products are reviewed with some historical background of the development and future commercialization plans.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Polyhydroxyalkanoate (PHA) is one of the promising bio-based biodegradable plastics made from renewable resources (Doi 1990). It is currently made by the bacterial fermentation of biomass, but a future possibility exists to produce PHA by using higher organisms (Poirier et al. 1995). Among a large number of known PHAs (Steinbüchel 1995), the so-called Nodax™ class PHA copolymers (Poliakoff and Noda 2004; Noda et al. 2005a, b), which will become available from Meredian (Bainbridge, GA, USA), are showing great potential to be a plastic resin of general utility across a broad array of applications.
The early development of this class of PHA copolymers was initiated by Procter & Gamble (Cincinnati, OH, USA) around the late 1980s. During the several decades of intensive research effort, Procter & Gamble accumulated a broad base of intellectual properties associated with this class of materials (Noda 1996, 1999). In 2007, Meredian took over the Nodax™ technology from Procter & Gamble for the full commercialization of this class of bioplastics. A large-scale commercial production of the PHA copolymers is scheduled to start in a few years. Given this new development, a comprehensive description of this class of material may be of interest to scientists and engineers in the field.
PHA copolymers having the Nodax™ class molecular structure exhibit a unique set of combined useful attributes, including polyolefin-like thermomechanical properties, polyester-like physicochemical properties, and interesting biological properties (Satkowski et al. 2001; Federle et al. 2002; Noda et al. 2004, 2005a, b). Therefore, broad ranges of industrial and consumer product applications are anticipated for this class of material. In this chapter, the basic structure and properties of the PHA copolymers, as well as their processing and potential conversion to various products, are reviewed with some historical background of the development and future commercialization plans.
2 Molecular Structure
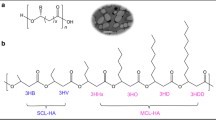
The general molecular structure of the so-called Nodax™ class PHA copolymers is a random copolymer of predominantly (R)-3-hydroxybutyrate (3HB) and other (R)-3-hydroxyalkanoate (3HA) comonomer units, as shown schematically in Fig. 1. The secondary 3HA comonomer units must have side groups consisting of at least three carbon atoms. Examples of such 3HA units with medium-chain-length (mcl) side groups include (R)-3-hydroxyhexanoate (3HHx), (R)-3-hydroxyoctanoate (3HO), (R)-3-hydroxydecanoate (3HD), and (R)-3-hydroxyoctadecanoate (3HOd). Such PHA copolymers typically consist of at least 50 mol% 3HB and at least 2 mol% secondary mcl-3HA units.
The functional architecture of this class of PHA copolymers is substantially different from that of more familiar types of PHAs, such as poly[(R)-3-hydroxybutyrate] (PHB) homopolymer or poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] (PHBV) copolymer. The size of the side groups in the conventional PHAs is limited only to short-side-chain types with no more than two carbon atoms. Although PHAs with only one or two carbon side groups may be viewed essentially as linear polymers, PHA copolymers with mcl side groups belong to a broad class of moderately branched polymers. The inclusion of a small amount of mcl-3HA units into the PHA polymer backbone leads to some profound changes in important physical properties of this class of copolymers.
The motivation behind the molecular design of NodaxTM class PHA copolymers closely follows that of the well-known industrial polyolefin linear low density polyethylene (LLDPE). LLDPE is a random copolymer of ethylene with a small amount of α-olefin units, such as 1-butene or 1-hexene, which will result in the formation of the polymer chain structure with mcl alkyl side group branches. In a similar manner, one can envision the possibility of creating a polymer structure of LLDPE with a PHA backbone having short alkyl side chains, as depicted in Fig. 2.
The effect of incorporating such short branches on the physical properties of highly crystallizable polymers such as polyethylene is well known. The branches act as a molecular defect, which disrupts the excessive regularity of the polymer chain and consequently lowers the melt temperature (T m) and crystallinity. High-density polyethylene or PHB homopolymer without sizable side groups has high T m and crystallinity. The material, therefore, is relatively hard and brittle and may be useful for making bottles and milk jugs. In contrast, copolymers with medium-size-chain side groups have much lower T m and crystallinity. They become substantially more flexible and ductile and are suitable for making films and other soft articles.
3 Preparation Methods
3.1 Chemical Synthesis
It is noteworthy to point out that most of the Nodax™ class PHA copolymers studied in the early days were initially prepared by the classic chemical synthesis based on the ring-opening polymerization of chiral derivatives of lactone monomers (Schechtman and Kemper 1997; Federle 2002). The chemical synthesis program was pushed forward on the basis of the careful analysis of the biochemical synthetic pathway of existing PHA, which clearly indicated the possible existence of microorganisms capable of producing this type of copolymers. A large-scale production of PHA copolymers by the polymerization of rather expensive chiral monomers was certainly not a commercially very attractive option. On the other hand, a small-scale chemical synthesis in the laboratory allowed the researchers to examine the properties of a variety of new PHA copolymers, which had not yet been discovered in biological systems. The availability of a large number of novel PHA samples made by the chemical synthesis indeed greatly contributed to the early buildup of the broad intellectual properties related to this class of PHA copolymers (Noda 1996). The corresponding biosynthesis of the same PHA copolymers occurred much later, after the discovery of suitable microorganisms.
Figure 3 shows the basic scheme for the polymer synthesis. Details of the early chemical synthesis of mcl-3HA-containing PHA copolymers have been described elsewhere (Schechtman and Kemper 1997). It was recognized that sufficiently high molecular weights must be obtained to test the properties of these materials to their fullest potential. Particular care was taken to ensure random comonomer distribution along the chain and to ensure a high degree of isotacticity to closely approximate biosynthesized materials. Chiral 3-alkyl-β-propiolactone monomers were polymerized with zinc alkoxide initiator in dry toluene.
Chemical synthesis of PHA copolymer by ring-opening polymerization (Noda et al. 2005b)
3.2 Biosynthesis
Many researchers in the past searched for the appropriate microorganisms capable of producing random copolymers of 3HB and mcl-3HA, now known as Nodax™ class PHA copolymers. Although some microbes were found to produce polymers containing various 3HA units, their specific origin as to where these 3HA came from was not certain (Brandl et al. 1989; Huisman et al. 1989; Timm and Steinbüchel 1990). Indeed, many of the early studies indicated that different 3HA fragments came from blends of PHB homopolymer and copolymers of mcl-3HA without 3HB units (Timm et al. 1990). Scientists from Kaneka in Japan (Shiotani and Kobayashi 1994) were the first to report the definitive discovery of microorganisms capable of producing copolymers of 3HB and 3HHx. By the mid-1990s, many other researchers started reporting the biosynthesis of various 3HA copolymers by using transgenic microorganisms (Abe et al. 1994; Caballero et al. 1995, Kato et al. 1996). Thus, the biosynthetic production of moderately branched PHA copolymers by a fermentation process has become possible (Lee et al. 2000; Chen et al. 2001).
The metabolic pathways utilized to produce poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate] (PHBHx) copolymer is shown in Fig. 4 (Noda et al. 2005a). Two units of acetyl-CoA forms the acetoacetyl-CoA with phaA thiolase, which is then converted to 3-hydroxybutyryl-CoA with phaB reductase. Parallel to these steps are the other metabolic pathways involving fatty acid biosynthesis (phaG) and fatty acid oxidation (phaJ, OAR, MFP), leading to the other 3-hydroxyacyl-CoA units. Finally, the copolymerization of 3HB-CoA and 3HA-CoA with phaC PHA synthase results in the production of this type of PHA copolymers. Figure 5 shows examples of microorganisms making different types of moderately branched PHA copolymers (Noda et al. 2005a).
Biosynthetic pathway of producing PHA copolymer (Noda et al. 2005b)
4 Properties
4.1 Biological Properties
One of the very promising properties of biologically produced PHA copolymers is their rapid biodegradability (Noda et al. 2005a). Unlike any other type of biodegradable plastics, PHAs biodegrade under not only aerobic but also anaerobic conditions. Furthermore, PHA copolymers comprising mcl-3HA have a relatively low crystallinity compared with PHB homopolymer of PHBV copolymers. The lowered crystallinity in turn results in a very rapid degradation rate with microbial enzymes. Figure 6 show the degradation profiles of 14C-labeled poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyoctanoate] (PHBO) samples under aerobic and anaerobic conditions. Details of the experimental protocols are reported elsewhere (Federle et al. 2002).
Biodegradation of poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyoctanoate] (PHBO) copolymer (Noda et al. 2005b)
The result shows that this type of PHA copolymers can undergo a rapid biodegradation process, with or without oxygen. The PHA copolymer is shown to be readily mineralized by biodegradation to water and carbon dioxide, and in the case of the anaerobic condition also a small amount of methane. About 15–20% of the material is actually incorporated into the biomass.
It was found that the rates of both anaerobic and aerobic biodegradation of PHA copolymers are almost comparable to those of cellulose. This favorable biodegradability profile of PHA copolymers has a major practical implication. Articles made of this class of polymers may be safely disposed of or flushed away in a common underwater environment, especially household septic tanks, where the access of free atmospheric oxygen is somewhat limited. Thin films and nonwoven fabrics made of PHA copolymers indeed undergo rapid biodegradation in a simulated septic tank at a rate comparable to tissue paper. A similar anaerobic biodegradation activity is expected in other environments, such as below the surface of rice paddies, rivers, and lakes.
It is also important to point out that the biodegradation of PHA copolymers will not take place unless the surrounding conditions support the biotic activities of microbes. The presence of moisture is essential for the degradation of even highly biodegradable materials, such as PHA and cellulose, as clearly demonstrated by the fact that books can be kept in a dry library for centuries. Likewise, commercial products made of PHA will not spontaneously biodegrade on the shelf, as long as they are kept in the ordinary low biotic environment. The same is true for a well-kept landfill with minimal water seepage, where disposed of articles are safely entombed. Thus, the biodegradation of products made of PHA copolymers may be designed and controlled according to the specific storage and use conditions.
4.2 Thermal Properties and Crystallinity
4.2.1 Melt Temperature
PHB homopolymer has a very high T m, close to 180°C, which is close to its thermal decomposition temperature (Marchessault et al. 1990). The T m of PHA may be controlled by the inclusion of 3HA comonomers along with 3HB units in a manner analogous to LLDPE. The initial attempt to improve the properties of PHB homopolymer was to incorporate (R)-3-hydroxyvalerate (3HV) units into the polymer chain. Such a copolymer, PHBV, with very short ethyl side groups randomly distributed along the polymer chain, did indeed show some reduced T m and crystallinity when a sufficient amount of 3HV units was incorporated. However, the efficacy of T m and crystallinity lowering for a given amount of 3HV comonomer was surprisingly small.
It turned out that the molecular difference between 3HV and 3HB units with only one methylene group was too small to effectively disrupt the molecular regularity of the polymer chain. The ethyl side groups of 3HV comonomer units can be largely incorporated along with 3HB units without much structural disruption into the crystal lattice of PHB consisting of polymer chains with a relatively open helical conformation (Marchessault et al. 1990). Thus, the anticipated level of T m and crystallinity lowering could not be achieved. It is difficult to bring down the T m of PHBV much below 150°C by the moderate amount of incorporation of 3HV units. The relatively high T m of PHBV imposes a definite practical limitation on the utility of PHBV copolymer as well as PHB homopolymer as general purpose commodity thermoplastics. The extent of thermal degradation during the melt processing of PHA becomes a major issue when the process temperature approaches the thermal degradation temperature of PHA. The thermomechanically induced degradation of PHA becomes noticeable at process temperature as low as 150°C (Satkowski et al. 2001).
Fortunately, it was later discovered that, unlike the ethyl side group of the 3HV unit, side chains having more than three carbons cannot be incorporated into the crystal lattice structure of PHB. Random incorporation of comonomer units, such as 3HHx, 3HO, and 3HD, which are rejected from the crystal structure, is an effective way of disrupting the excessive regularity of PHB homopolymer. Thus, it has become possible to dramatically reduce the crystallinity of PHA copolymers by adjusting the level of 3HA comonomer units having a medium chain of at least three carbons or more distributed along with the dominant 3HB comonomer units (Noda 1996; Satkowski et al. 2001).
Figure 7 shows the representative T m data of PHA copolymers measured by differential scanning calorimetry as a function of the level of various 3HA comonomers, which are different from the dominant 3HB repeat units of the copolymer (Noda et al. 2005b). It should be noted that there are two very distinct groups of PHA copolymers showing different T m-lowering trends. The T m of PHBV copolymer does not change much from that of PHB homopolymer even at the level of 7 mol% incorporation of 3HV units. In contrast, the T m of new PHA copolymers, consisting of mcl-3HA comonomer units, such as 3HHx, 3HO, and 3HD, is substantially lower than that of PHBV. Interestingly, the efficacy of T m lowering for a given mole percentage incorporation of comonomer is essentially the same for all mcl-3HA comonomers. Thus, any mcl-3HA acts as the effective disruption of the regular structure of PHB, as long as the side group consists of at least three carbons.
Melt temperature of PHA copolymers (Noda et al. 2005b)
4.2.2 Crystallinity
Crystallinity of articles made of PHA copolymers is another property which can be adjusted by the incorporation of mcl-3HA units. Crystallinity influences the stiffness of the material. Because of the strict molecular stereoregularity of PHA created by the biosynthesis, the crystallinity of PHB homopolymer is known to become very high, often well in excess of 50%. Such high crystallinity results in the excessively hard and brittle material not well suited for many practical applications. The incorporation of mcl-3HA comonomer units, which cannot be incorporated into the PHB crystal lattice structure, should be an effective disruption to the excessive level of crystallinity.
Figure 8 shows the effect of 3HA incorporation into the PHB chain on the crystallinity of PHA copolymers measured by X-ray diffraction (Noda et al. 2005b). Again, two very distinct trends for the comonomer content dependence are observed. Although the level of crystallinity is not much affected by the incorporation of the 3HV unit, other 3HA units all systematically lower the crystallinity of PHA copolymers. As in the case of T m lowering, the effect of mcl-3HA seems to be independent of the side group size, as long as it contains at least three carbons. Thus, both PHBHx and PHBO copolymers, with propyl and pentyl side groups respectively, show a similar crystallinity-lowering trend. Even poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyoctadecanoate] (PHBOd), a PHA copolymer with side groups much longer than 15 carbon atoms, shows a similar trend.
Crystallinity of PHA copolymers (Noda et al. 2005b)
Although the T m of PHA copolymers can be widely adjusted with the mcl-3HA comonomer composition, it is often set between 100 and 150°C to achieve the processing properties of typical commodity thermoplastics such as polyethylene. The crystallinity in this copolymer composition range is 20–40%, which produces very flexible low density polyethylene (LDPE) like materials.
4.2.3 Glass-Transition Temperature
The glass-transition temperature T g is another important thermal property of plastics with strong practical implications. It is well recognized that the T g is closely associated with the segmental mobility of polymer chains, which in turn governs the toughness and other physical properties of the material. Unfortunately, the T g of PHB homopolymer is somewhat high, such that the material becomes brittle upon cooling below 0°C. Even at room temperature, the high T g and the limited polymer chain segmental mobility of PHB negatively affect the toughness and impact resistance of the material.
The incorporation of 3HA comonomer units into the PHB polymer chain reduces the T g of copolymers (Marchessault et al. 1990). Figure 9 shows the T g of various PHA copolymers with different lengths of side-chain groups (Noda et al. 2005b). There is a clear trend that the T g of PHA copolymer is more effectively lowered by the incorporation of 3HA units with longer side chains. Thus, PHBHx, with a propyl side chain, has a lower T g than PHBV, with an ethyl group, and PHBO, with a pentyl group, or poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxydecanoate] (PHBD), with a heptyl group, has an even lower T g for a given level of 3HA incorporation. A higher level of 3HA incorporation also tends to lower the T g of PHA copolymers.
Glass-transition temperature of PHA copolymers (Noda et al. 2005b)
4.3 Mechanical Properties
PHB homopolymer, and to some extent even PHBV copolymers, have traditionally suffered from the excessively stiff and brittle nature arising from the relatively high crystallinity of the material. The mechanical properties of PHB and PHBV are said to be similar to those of isotactic polypropylene (PP), but the lower ductility and impact strength prevent PHB and PHBV from effectively replacing commercial PP. In contrast, the properties of mcl-3HA containing PHA copolymers are closer to those of high-grade polyethylene (Satkowski et al. 2001). Because of the effective control of the excess crystallinity afforded by the incorporation of mcl-3HA units, the ductility and strength of the material are substantially increased. This improvement has made Nodax™ class copolymer an excellent candidate for the replacement for the existing general purpose commodity plastic resins.
Figure 10 shows typical tensile stress–strain curves of solution-cast PHA copolymer films after being aged for 7 days. The incorporation of 3HA units into the PHB polymer chain tends to decrease the stiffness and simultaneously increase the ultimate elongation of the material. These effects are more pronounced with mcl-3HA having longer side chains. The ability to produce remarkably tough and highly elastic material upon careful elongation under proper conditions is one of the interesting features of this class of PHA copolymers (Melik and Noda 2004). Such “hard elastic” films and fibers can be deformed over several times the size and are still able to snap back to the original dimension, as demonstrated in Fig. 11.
The stiffness of PHA copolymers can be controlled by the incorporation of mcl-3HA comonomer units (Noda et al. 2005b). Figure 12 shows Young’s modulus of various PHA copolymers systematically decreases with the addition of 3HA comonomers. The effect of reduced crystallinity by incorporating more mcl-3HA is apparent. The incorporation of a higher level of mcl-3HA results in lower crystallinity, which in turn makes the material softer. The value of Young’s modulus of PHA varied between that of very stiff polymers, such as poly(lactic acid) (PLA) and PP, and much softer material, such as LDPE.
Young’s modulus of PHA copolymers (Satkowski et al. 2001)
The stiffness of semicrystalline PHA is controlled not only by the overall crystallinity but also by the mechanical properties of the amorphous region of the material. This is shown by the effect of the length of the comonomer side chains, which influences the stiffness. Figure 13 shows Young’s modulus of three different PHA copolymer samples with an identical level of mcl-3HA comonomers measured at room temperature (Noda et al. 2005b). Figure 8 indicates the expected crystallinity of these samples containing 10 mol% 3HA should be about 40%. However, the stiffness of the three samples is remarkably different, and PHA copolymer with longer side chain groups tends to be much softer.
Young’s modulus of PHA copolymers (Noda et al. 2005b)
The difference in the overall stiffness of semicrystalline PHA copolymers arises from the difference in the ductility of the amorphous region of the samples. The molecular segmental mobility of the amorphous portion of PHA is strongly influenced by the difference between the T g and the end-use temperature. Thus, a PHA copolymer with a longer side chain group, which has lower T g, should exhibit less overall stiffness, even if the crystallinity might be similar. A similar trend for the influence of the length of the side chain on the flexural modulus is shown in Fig. 14. Again, the increase in the side chain length resulted in much more flexible material.
Flexural modulus of PHA copolymers (Noda et al. 2005b)
4.4 Other Useful Properties
Many important physical properties, such as thermal and mechanical properties, of mcl-3HA containing semicrystalline PHA copolymers are relatively similar to those of conventional polyolefins. In contrast, its chemical properties are strongly affected by the presence of polar functional groups found in the molecular structure of polyester. For example, the surface energy of a PHA film is much higher than that of polyethylene, resulting in the superior wetting and ink printability useful for applications in flexible coatings and laminated papers. In addition to surface properties, PHA generally shows good compatibility and dispersibility for additives, such as pigments and fillers, as well as other biodegradable plastics, especially PLA (Noda et al. 2005a).
The effect of the presence of medium-sized branches on the physical properties also shows up in the solubility. Unlike the highly crystalline PHB and PHBV, mcl-3HA-containing PHA copolymers are readily soluble in so-called green solvents, such as ethyl acetate and acetone. It is possible to extract the polymer from biomass without using halogen-containing solvents such as chloroform (Noda and Schechtman 1999). This favorable solubility profile provides extra flexibility and economy in the extraction and purification of PHA copolymers.
A superior barrier property is another one of the interesting features of PHA copolymers. Table 1 shows the relative values of the water vapor and oxygen gas transmission rate for films made of various plastics. Even though PHA films can be very flexible, if the crystallinity is kept low, they exhibit excellent an barrier property for oxygen, carbon dioxide, and odors. Although PHA films are not as good as polyolefins, they also act as a reasonable barrier for moisture vapor. The optical transparency of thin films is also excellent for a flexible material.
PHA has much higher stability against hydrolysis compared with PLA. PHA does not decompose under normal temperature and humidity, and the level of hydrolytic degradation during the processing is low for a polyester resin. Interestingly, however, it degrades rapidly in alkaline solution at a high temperature (Noda 2005). Figure 15 shows the chemical digestion rate of PHA and other polyesters. That means PHA can be readily digested chemically during a process such as a de-inking step in paper recycling.
5 Processing and Conversion to Products
PHA copolymers can be converted into various forms and products, such as films, sheets, fibers, nonwovens, molded articles, pulps, powders, coating materials, laminates, and composites, using conventional processing steps such as extrusion and thermoforming. This broad processability of PHA copolymers arises from the melting point lowering resulting from the incorporation of mcl-3HA and superior compatibility of the material. Figure 16 shows a rough guideline for the design space of the type of copolymers which can be used for different applications (Noda et al. 2005a). Factors important to the conversion processes include the 3HA composition of copolymers, average molecular weights and molecular weight distributions, and the presence of processing aids such as nucleating agents and plasticizers. Figure 17 shows some examples of prototypes made from PHA copolymers.
Product design space of PHA copolymers (Noda et al. 2005a)
6 Production and Commercialization
Meredian is committed to moving forward with large-scale production of PHA and will begin its operations in 2009 utilizing a pilot facility to validate production and process design prior to construction of its first full-scale PHA production facility in 2010. Meredian will focus its planned annual output of over 600 million pounds on specific market applications where the greatest value is provided to the marketplace. Meredian has an objective to build production facilities that are strategically located to support key customers while minimizing transportation costs.
7 Concluding Remarks
A family of promising PHA polyesters called Nodax™ class PHA copolymers, consisting of 3HA comonomer units with medium-size-chain side groups and 3HB, which will become commercially available from Meredian were described. Because of the unique design of the molecular structure, the Nodax™ class PHA copolymers have a set of useful attributes, including polyolefin-like thermomechanical properties, polyester-like physicochemical properties, and interesting biological properties, suggesting broad ranges of industrial and consumer product applications.
Advances in PHA technology will enable Meredian to be well positioned to serve the growing demand for materials produced from renewable resources. Leading companies around the world are focused on sustainable initiatives and the utilization of products produced exclusively from renewable starting materials that do not compete with the food supply and that also support multiple end-of-life options. Commercial success will be dependent upon the effectiveness of the current supply chain to efficiently transition to these new materials which will be required to meet ever-increasing demands for increased functionality. The properties of PHA within the portfolio of those described in this chapter have the unique capability to meet this challenge and exceed expectations.
References
Abe H, Doi Y, Fukushima T, Eya H (1994) Biosynthesis from gluconate of a random copolyester consisting of 3-hydroxybutyrate and medium-chain-length 3-hydroxyalkanoates by Psedomonas sp. 61–3. Int J Biol Macromol 16:115–119
Brandl H, Knee EJ, Fuller RC, Gross RA, Lenz RW (1989) Ability of the phototrophic bacterium Rhodosprillum rubrum to produce various poly(β-hydroxyalkanoates): potential sources for biodegradable polyesters. Int J Biol Macromol 11:49–55
Caballero KP, Karel SF, Register RA (1995) Biosynthesis and characterization of hydroxybutyrate-hydroxycaproate copolymers. Int J Biol Macromol 17:86–92
Chen GQ, Zhang G, Park SJ, Lee SY (2001) Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl Microbiol Biotechnol 57:50–55
Doi Y (1990) Microbial polyesters. VCH, New York
Federle TW, Barlaz MA, Pettigrew CA, Kerr KM, Kemper JJ, Nuck BA, Schechtman LA (2002) Anaerobic biodegradation of aliphatic polyesters: poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) and poly(ε-caprolactone). Biomacromolecules 3:813–822
Huisman G, Leeuw O, Eggink G, Witholt B (1989) Synthesis of poly-3-hydroxyalkanoate is a common feature of fluorescent Pseudomonads. Appl Environ Microbiol 55:1949–1954
Kato M, Bao HJ, Kang CK, Fukui T, Doi Y (1996) Production of a novel copolyester of 3-hydroxybutyric acid and medium-chain-length 3-alkanoic acids by Psedomonas sp. 61–3 from sugars. Appl Microbiol Biotechnol 45:363–370
Lee SH, Oh DH, Ahn WS, Lee Y, Choi J, Lee SY (2000) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanaote) by high-cell-density cultivation of Aeromonas hydrophila. Biotechnol Bioeng 67:240–244
Marchessault RH, Monasterios CJ, Morin FG, Sundarajan PR (1990) Chiral poly(b-hydroxyalkanoates): an adaptable helix influenced by the alkane side-chain. Int J Biol Macromol 12:158–165
Melik DH, Noda I (2004) polymer products comprising soft and elastic biodegradable polyhydroxyalkanoate copolymer compositions and methods of preparing such polymer products. US Patent 6,794,023 B1
Noda (1996) Biodegradable copolymers and plastic articles comprising biodegradable copolymers. US Patent 5,498,692
Noda (1999) Films and absorbent articles comprising a biodegradable polyhydroxyalkanoate comprising 3-hydroxybutyrate and 3-hydroxyhexanoate comonomer units. US Patent 5,990,271
Noda I (2005) Plastic articles digestible by hot alkaline treatment. US Patent 6,872,802 B2
Noda I, Schechtman LA (1999) Solvent extraction of polyhydroxyalkanoates from biomass. US Patent 5,942,597
Noda I, Satkowski MM, Dowrey AE, Marcott C (2004) Polymer alloy of Nodax copolymers and poly(lactic acid). Macromol Biosci 4:269–275
Noda I, Bond EB, Green PR, Melik DH, Narasimhan K, Schechtman LA, Satkowski MM (2005a) Preparation, properties, and utilization of biobased biodegradable Nodax™ copolymers. In: Cheng HN, Gross RA (eds) Polymer biocatalysis and biomaterials. American Chemical Society, Washington, pp 280–291
Noda I, Green PR, Satkowski MM, Schechtman LA (2005b) Preparation and properties of a novel class of polyhydroxyalkanoate copolymers. Biomacromolecules 6:580–586
Poirier Y, Schechtman SC, LA SMM, Noda I (1995) Synthesis of high-molecular-weight poly([R]-(–)-3-hydroxybutyrate) in transgenic Arabidopsis thaliana plant cells. Int J Biol Macromol 17:7–12
Poliakoff M, Noda I (2004) Plastic bags, sugar cane and advanced vibrational spectroscopy: taking green chemistry to the third world. Green Chem 6:G37–G38
Satkowski MM, Melik DH, Autran J-P, Green PR, Noda I, Schechtman LA (2001) Physical and processing properties of polyhydroxyalkanoate copolymers. In: Doi Y, Steinbüchel A (eds) Polyesters II – properties and chemical synthesis, vol 3b, Biopolymers. Wiley, Weinheim, pp 231–263
Schechtman LA, Kemper JJ (1997) Polymerization of beta-substituted beta-propiolactones initiated by alkylzinc alkoxide. US Patent 5,648,452
Shiotani T, Kobayashi G (1994) Copolymer and method for producing thereof. US Patent 5,292,860
Steinbüchel A (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett 128:219–228
Timm A, Steinbüchel A (1990) Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudonomas aeruginosa and other fluorescent pseudomonads. Appl Environ Microbiol 56:3360–3367
Timm A, Byrom D, Steinbüchel A (1990) Formation of blends of various poly(3-hydroxyalkanoic acids) by a recombinant strain of Pseudomonas oleovorans. Appl Microbiol Biotechnol 33:296–301
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Noda, I., Lindsey, S.B., Caraway, D. (2010). Nodax™ Class PHA Copolymers: Their Properties and Applications. In: Chen, GQ. (eds) Plastics from Bacteria. Microbiology Monographs, vol 14. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-03287-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-642-03287-5_10
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-03286-8
Online ISBN: 978-3-642-03287-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)