Abstract
Poly(3-hydroxyalkanoate)s (PHAs) are a class of polymers receiving attention because of their potential as renewable, biodegradable and high-technology properties. Unlike most short chain length (scl) PHAs such as poly(3-hydroxybutyrate) (PHB), medium chain length (mcl) PHAs such as poly(3-hydroxyoctanoate) (PHO) exhibit low crystallinity and are elastomeric in character. PHB-b–PEG-b–PHO block copolymers can combine both properties in block copolymer matrix. In this study, we report the synthesis of the block copolymers combining the PHB and PHO blocks. Transamidation reactions of PHB with polyethylene glycol with primary amine yield equimolar amounts and PHB with amine ends. PHO reacts with the modified PHB containing the amine end to give PHB-b–PEG-b–PHO block copolymers. Structural analysis of the products was performed by using 1H–, 13C, heteronuclear single quantum coherence NMR techniques. Thermal and mechanical properties of the block polymers were also evaluated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(3-hydroxyalkanoate)s (PHA)s are a class of polymers receiving attention because of their potential as renewable, biodegradable and high-technology properties. Scl-PHAs are too rigid and brittle. In contrast, mcl-PHAs are elastomeric but have very low mechanical strength. Therefore, for packaging materials, biomedical applications, tissue engineering and other specific applications, the physical and mechanical properties of microbial polyesters need to be diversified and improved [1,2,3,4,5,6,7]. Their high hydrophobic character limits the use in drug delivery systems. The preparation of block copolymers containing hard and soft segments represents an efficient way to engineer thermoplastic materials. The improvement of their mechanical properties and hydrophilic character is still a main challenge for the polymer scientists [8,9,10,11,12,13,14,15]. For example, carboxylic end group of PHO was reacted with PEG of various molecular weights with amine end to synthesize block copolymers composed of PHO and PEG [16]. The transesterification catalyzed in the melt was used to produce diblock copolymers of poly([R]-3-hydroxybutyric acid), PHB, and monomethoxy poly(ethylene glycol), mPEG, in a one-step process. Bacterial PHB of high molecular weight is depolymerized by consecutive and partly simultaneous reactions: pyrolysis and transesterification. The formation of diblocks is accomplished by the nucleophilic attack from the hydroxyl end group of a mPEG molecule catalyzed by bis(2-ethylhexanoate) tin [17]. Poly(3-hydroxyoctanoate) (PHO) films were treated with plasma and then treated with acryl amide solutions in order to prepare films with surfaces that contained different amounts of amide groups [18]. Sparks and Scholz synthesized cationic PHA by the reaction of the pendent epoxidized double bonds with diethanol amine [19]. We have recently attached pendant poly(N-isopropyl acryl amide) onto the unsaturated medium chain length PHA via RAFT technique [20]. Thiol-ene click reactions were also used in the synthesis of amphiphilic microbial polyester [21, 22]. Dai et al. [23] reported the synthesis of block copolymers derived from PHB and PHO oligomers via enzyme catalyzed polycondensation.
In a recent work [24], novel poly(ester-urethane) block copolymers were synthesized by use of telechelic hydroxylated poly-[(R)-3-hydroxyoctanoate] with a number-average molecular weight (Mn) of 2400 as the amorphous soft segment, telechelic hydroxylated poly-[(R)-3-hydroxybutyrate] (PHB-diol) with a Mn of 2600 as the crystalline hard segment and l-lysine methyl ester diisocyanate (LDI) as the junction unit. Reaction of the PHO-diol, PHB-diol and LDI at a ratio of 1:1:2 in the presence of dibutyltin dilaurate as catalyst afforded the PHO-b-PHB block copolymers [24]. PHB–PHO block copolymers can be also synthesized by the fermentation using Escherichia coli. PHA copolymers comprising 3HB and 3HHx were produced by fermentation of wild-type Aeromonas hydrophila using lauric acid as described, and a strain of Ralstonia eutropha genetically modified to express the PHA synthase gene from Pseudomonas fluorescens GK-1317 [25,26,27]. Elastomer block copolymer synthesis using ring opening polymerization from their related lactones was also reported [26]. In addition, PHB–PHO block copolymer synthesis via hydroxyl-carboxylic acid condensation reaction or anionic polymerization was reported [28,29,30].
In this study, the synthesis of a series of block copolymers of PHB, PEG and PHO via transamidation reaction is reported. PHB was reacted with polyethylene glycol with two primary amine terminals in order to obtain PHB with one primary amine end. PHB with primary amine end was then reacted with different amounts of PHO to prepare a series of PHB-b–PEG-b–PHO block copolymers. The products obtained were characterized in detail.
Results and discussion
Novel PHB-b–PEG-b–PHO block copolymers were obtained from brittle PHB and elastomeric PHO blocks via transamidation reactions. In the first step, reaction of PHB with PEG with two amine terminals resulted in PHB with an amine terminal derivative: PHB–PEG amide (PHB-b-PEG-NH2). Fractional precipitation was used to purify PHB–PEG diblock copolymer. White solid PHB–PEG diblock copolymer was obtained in medium yield (ca. 50 wt%). Table 1 shows the results and the synthesis conditions of the PHB-b-PEG block copolymers in different reaction times at 110 °C. (Weight ratio of PEGNH2/PHB = 1, while molar ratio is 234, as taken Mw values.) 1H NMR spectra of the PHB-b-PEG block copolymers are seen in Fig. 1. Characteristic PEG signals were observed in all 1H NMR at δ = 3.75 ppm. PHB contents of the diblock copolymers were varying in range from 6.5 to 13 mol% with changing reaction time, as listed in Table 1. Longer polymerization times cause higher PEG inclusion in block copolymer but lower molar mass of the product. Because of the increase in PEG content in longer polymerization time, water uptake of the block copolymers increases.
PEG content in the PHB-b-PEG diblock copolymers was calculated as PEG mol content % from their 1H NMR spectra. PHB contents of the diblock copolymers were varying in range from 6.5 to 13 mol%, as listed in Table 1. When compared the Mn of the precursor (187,000 g/mol), dramatic decrease in molecular weight of the diblock copolymer was observed. Mn of PHB-b-PEG oligomers was in range from 3760 to 7240 g/mol. Clearly, PHB is degraded fast during the amidation reaction in the presence of PEG-NH2.
PHB derivatives with amine terminals were then reacted with PHO to obtain PHB-b–PEG-b–PHO block copolymers. The reaction steps for the synthesis of the block copolymers were shown in Scheme 1. For the amidation reactions, a series of the mixtures of the PHB-b-PEG and PHO were prepared as 0.1–0.5, 0.2–0.5, 0.3–0.5 and 0.5–0.5 g, respectively. As taken the molar masses as 12,200 g/mol for PHB–PEG-3 and 171,000 g/mol for PHO, the molar ratios of the mixtures were 2.8, 5.60, 8.42 and 14.1, respectively. Then, the block copolymers were coded as PHB-b–PEG-b–PHO (01–05), PHB-b–PEG-b–PHO (02–05), PHB-b–PEG-b–PHO (03–05), and PHB-b–PEG-b–PHO (05–05).
The products obtained in the reaction steps were all characterized by NMR technique. Characteristic PEG signals of the triblock copolymers were observed in all 1H NMR spectra at δ = 3.7 ppm. PEG contents in the triblock copolymers were calculated as PEG mol content % from their NMR spectra in range from 0.5 to 2.1, which are listed in Table 2.
The transamidation reaction of the PHB – b-PEG with PHO resulted in PHB-b–PEG-b–PHO block copolymers. Reaction conditions and GPC results are seen in Table 2. The molar masses of the triblock copolymers were changing from 96,000 to 123,000 g/mol, while that precursor PHO was 87,000. Block copolymers had the higher molecular weight than the precursor PHO. This increase in the molar mass also confirms the amidation reaction. The GPC chromatograms can be seen in SI—Figure 1 in Supporting information.
Structural analysis of the block copolymers was performed using FT-IR and 1H and heteronuclear single quantum coherence (HSQC) NMR techniques.
The characteristic signals of the block copolymers are observed in FT-IR spectra in Fig. 2. Signals of C=O (ester carbonyl) and –C–O– (ester and ether C–O bonds) can be seen at 1730 and 1100 cm−1, respectively. C=O stretching band of amide groups was rarely seen in a tiny signal at 1650 cm−1 because there is only two –CONH groups in a very big polymer chain. For example, polymer chain with Mn 97,000 g/mol contains only two amide groups. So, FT-IR spectra of the products confirm the strong ester groups and very weak amide carbonyl. Because of this, the further structural characterization was carried out using 1H NMR and HSQC NMR techniques.
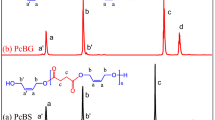
1H NMR spectra of the triblock copolymers are seen in Fig. 3. Characteristic signals were observed. PEG contents of the diblock copolymers were also calculated from their spectra. The characteristic signals were all signed on the spectra. In this manner, a is –CH3 of PHO, b and f are −CH2’s of PHO and –CH3 of PHB, d and g are CH2–COO– of PHO and PHB, and e is –CH–O– of PHB and PHO. Most signals of the PHB and PHO in 1H NMR spectra of PHB and PHO overlapped each other. Therefore, HSQC NMR spectrum of the block copolymer was taken because the exact characterization of the blocks separately is needed.
HSQC NMR spectra help us to confirm the presence of PHB and PHO copolymers in the obtained block copolymers. The characteristic signals of methyl groups of PHB and PHO were confirmed in the HSQC NMR spectrum. Figure 4 shows HSQC NMR spectrum of a PHB-b–PEG-b–PHO triblock copolymer. The red circles show CH2 groups, while the blue colors show –CH3 and –CH– groups. The two different blue color circles at 0.8 and 1.2 ppm differentiate the methyl groups of PHO (at δ = 0.8 ppm, PHO) and PHB (at δ = 1.2 ppm, PHB). Therefore, two different methyl groups also strongly confirm the PHB–PHO block copolymer structure. The presence of the PEG units is also confirmed by arising signal at 3.6 ppm in 1H NMR spectrum. The exact structural confirmation of the triblock copolymer, PHB-b–PEG-b–PHO, was carried out using both 1H and HSQC NMR techniques.
Thermal analysis of the block copolymers was performed using DSC and TGA instruments. DSC traces of PHB-b–PEG-b–PHO block copolymers can be seen in Fig. 5. They were containing two main melting points related to PHO (Tm 50–54 °C) and PHB blocks (Tm 155–158 °C). Originally, because PEG2000 blocks [33] and PHO blocks have Tm’s in the same range, melting transitions of PEG blocks were overlapped with those of PHO blocks [24]. In case of PHB-b–PEG-b–PHO (05–05, Fig. 5d) there is a small melting transition at 92 °C. This small melting transition may result from different combinations of the triblock. Generally, PHO, medium chain length PHA, has Tg at around − 30 °C like in case of Fig. 5c, d. Figure 5a, b shows Tg in this range overlapped more or less Tm of a trace of impurity coming from small organic molecule.
Decomposition temperatures were measured using thermogravimetric analysis instrument. Decomposition temperatures were about 260 °C for PHB-b–PEG-b–PHO copolymers while precursor decomposition temperature of PHO was 280 °C. Triblock copolymers show slightly lower onset decomposition as PHB-b-PEG constituent increases. Similarly, maximum decomposition temperature of the triblock copolymers was lower than that of the precursor. Among the tri block copolymers, some correlation was observed such as the increase in PHB–PEG inclusion in triblock copolymer causes slightly increase in thermal resistance.
Decomposition temperatures are listed in Table 3.
Mechanical strength
Stress–strain results of PHB-b–PEG-b–PHO block copolymers were obtained from a Zwick instrument. Brittle PHB gains flexibility with addition of PHO blocks. Mechanical strength and elongation of the triblock copolymers were lower than that of the PHO, while very brittle PHB gained flexibility. Increase in PHB content in the triblock copolymers causes slightly decrease in both mechanical strength and the elongation staying still having elastomeric property. Table 3 contains the results of the stress–strain measurement for the triblock copolymers. Triblock copolymers reached elongation levels ranging from 50 to 64%. When compared the crosslinked PHA elastomers reported in a recent article [32], the block copolymers obtained in this work exhibits the higher mechanical strength than the crosslinked PHA elastomers based on autoxidized unsaturated PHAs. Thermal resistance of the triblock copolymers increases because of PHB units when compared to that of PHO homopolymer.
Water uptake of the PHB-conjugate was measured to understand the hydrophilicity of the polymer derivatives. By this way, the high hydrophobic PHB and PHO (water uptake approx. 1%) gain hydrophilicity. As amine terminated PEG increase in feed, PHB is degraded and molar mass decreases. Because of this, water uptake increases in PHB–PEG diblock copolymers (Table 1). In case of triblock copolymers, PHB–PEG–PHO, quite high water uptake but less than that of the PHB–PEG diblock copolymers was obtained (Table 2).
Conclusion
Environmental issue pushes the polymer scientists to prepare ecofriendly plastics from renewable resources. Copolymer from PHB and PHO can be a remedy for this purpose. Transamidation reactions can be applied to combine brittle PHB and elastic PHO blocks in a copolymer chain. Biodegradable flexible microbial packaging materials can be obtained by this way. PEG blocks in the block copolymer structure gain some hydrophilicity in order to use in the medical applications such as drug delivery systems. These amidation reactions can be applied commercially in extruder systems. Wholly biodegradability of these new copolymers is also important for tissue engineering. PHB-b–PEG-b–PHO block copolymers obtained by transamidation reactions can be promising biomaterials for biotechnology and industry.
Experimental
Materials
Poly(3-hydroxy butyrate) (PHB), microbial polyester (Mn 187 000 g/mol, Mw/Mn 2.5, Biomer Inc.) was supplied from BIOMER (Germany) [31]. Poly(3-hydroxy octanoate), (PHO), (Mn 87,200 g/mol, Mw/Mn 1.967), was produced by feeding Pseudomonas oleovorans from octanoic acid in the TUBITAK-MAM Food Research Institute, Gebze—Kocaeli Turkey [11]. Poly(ethylene glycol) bis(2-aminopropyl ether) with Mw 2000 g/mol (PEG–2003NH2) was a gift from Huntsman Corporation (Switzerland). Tin (2-ethyl hexanoate) and the other chemicals used in this work were supplied from Sigma-Aldrich.
Characterization
Proton and carbon NMR spectra were acquired at a temperature of 25 °C with an Agilent NMR 600 MHz NMR (Agilent, Santa Clara, CA, USA) spectrometer equipped with a 3 mm broadband probe. Acquisition parameters included a 45° hard pulse angle, a sweep width of 14 ppm, 1.7 s acquisition time, 0.1 s pulse delay and continuous WALTZ-16 broadband 1H decoupling. Up to 2000 scans were collected per sample, corresponding to ~ 1 h of collection time. FT-IR spectra of the polymer samples were recorded using PerkinElmer FT-IR Spectrometer 100.
Molecular weights were determined by gel permeation chromatography instrument, Viscotek GPC max Auto sampler system, consisting of a pump, three ViscoGEL GPC columns (G2000H HR, G3000H HR and G4000H HR), and a Viscotek differential refractive index (RI) detector with a CHCl3 flow rate of 1.0 mL/min at 30 °C. The RI detector was calibrated with PS standards having narrow molecular weight distribution. Data were analyzed using Viscotek OmniSEC Omni-01 software. Thermal analysis of the obtained polymers was carried out under nitrogen using a TA Q2000 DSC and Q600 Simultaneous DSC-TGA (SDT) series thermal analysis systems. Differential Scanning Calorimeters (DSC) measures temperatures and heat flows associated with thermal transitions in the polymer samples obtained. The dried sample was heated from − 60 to 120 °C under nitrogen atmosphere heating from 20 to 600 °C at a rate of 10 °C/min. Thermogravimetric analysis (TGA) measures weight loss under nitrogen atmosphere at a rate of 10 °C/min.
Zwick/Roell tensile testing machine using a 50 kg load cell with a stretch speed 100 mm/min was for stress–strain measurements of the rectangular shape with size 0.16 × 10 × 50 mm solvent cast film samples from CHCl3. Samples were dried at room temperature under vacuum for 1 weeks prior to measurement. Three samples were repeated in each stress–strain test.
Reaction of PHB with PEG2000-diamine to obtain PHB-b-PEG2000-NH2
PHB was undergone to transamidation with PEG-diamine under reflux condition in chloroform. As an example for the transamidation reaction, 10 g of PHB and 0.1 g of tin (2-ethyl hexanoate) were dissolved in 300 mL of chloroform, and the solution was refluxed for 1 h. Then, 10 g of PEG-diamine was added into this solution and continued refluxing for 2 more hours. The solvent was evaporated by using a rotary evaporator. The resulting crude copolymer was washed with petroleum ether and dried under vacuum overnight at 40 °C. To complete the transamidation reaction, the white polymer powder was cured at 110 °C for 1 h. The cured polymer was redissolved in 50 mL of chloroform and precipitated into 300 mL of methanol. Pure PHB–PEG–NH2 obtained was filtered and dried under vacuum overnight at 40 °C.
Transamidation reactions of PHO with primary amine ended PHB
PHB with a primary amine end was used in the transamidation reaction with PHO to obtain PHB -b – PEG-b- PHO block copolymers. For example, 0.10 g of PHB–PEG–NH2 (Mw = 12,200 g/mol) and 0.50 g of PHO (Mw = 171,000 g/mol) were dissolved in 10 mL of chloroform under Argon. After the transamidation reaction was completed, the solution was poured into 300 mL of methanol to precipitate the block copolymer. Polymer obtained was redissolved in chloroform and reprecipitated from methanol for further purification. It was dried under vacuum at room temperature for a week.
Water uptake measurements
To do this experiment, polymer film obtained from solvent casting using chloroform solution was dried under vacuum at 40 °C for 24 h. Then the dried film soaked into distilled water at room temperature for 24 h. The water uptake of the polymer was calculated using the following equation:
where ms is weight of swollen polymer film and md is weight of dried polymer film.
References
Lenz RW, Marchessault RH (2005) Bacterial polyesters: biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules 6:1–8
Hazer B, Steinbüchel A (2007) Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl Microbiol Biotechnol 74:1–12
Hazer DB, Kilicay E, Hazer B (2012) Poly (3-hydroxyalkanoate)s: diversification and biomedical applications. A state of the art review. Mater Sci Eng C Mater Biol Appl 32:637–647
Marchessault RH, Dou H (2011) Ramsay, microbial medium chainlength poly[(R)-3-hydroxyalkanoate] shows liquid crystal behaviour. J Int J Biol Macromol 48:271–275
Nguyen S, Marchessault RH (2006) Graft copolymers of methyl methacrylate and poly([R]-3-hydroxybutyrate) macromonomers as candidates for inclusion in acrylic bone cement formulations: compression testing. J Biomed Mater Res B Appl Biomater 1:5–12
Hazer B (1996) Poly (β-hydroxy nonanoate) and Polystyrene or poly (methyl methacrylate) graft copolymers: microstructure characteristics and mechanical and thermal behavior. Macromol Chem Phys 197:431–441
Hazer B (2015) Simple synthesis of amphiphilic poly(3-hydroxy alkanoate)s with pendant hydroxyl and carboxylic groups via thiol-ene photo click reactions. Polym Degrad Stab 119:159–166
Zhang DM, Cui FZ, Luo ZS, Lin YB, Zhao K, Chen GQ (2000) Wettability improvement bacterial polyhydroxy alkanoates via ion implantation. Surf Coat Technol 131:350–354
Nguyen S, Marchessault RH (2004) Synthesis and properties of graft copolymers based on poly(3-hydroxybutyrate) macromonomers. Macromol Biosci 4:262–268
Nguyen S, Marchessault RH (2005) Atom transfer radical copolymerization of bacterial poly(3-hydroxybutyrate) macromonomers and methyl methacrylate. Macromolecules 38:290–296
Koçer H, Borcaklı M, Demirel S, Hazer B (2003) Production of bacterial polyesters from some various new substrates by Alcaligenes eutrophus and Pseudomonas oleovorans. Turk J Chem 27:365–373
Scandola M, Focarete ML, Adamus G, Sikorska W, Baranowska I, Swierczek S, Gnatowski M, Kowalczuk M, Jedlinski Z (1997) Polymer blends of natural poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and a synthetic atactic poly(3-hydroxybutyrate). Characterization and biodegradation studies. Macromolecules 30:2568–2574
Zinn M, Witholt B, Egli T (2001) Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv Drug Deliv Rev 53:5–21
Arkin AH, Hazer B (2002) Chemical modification of chlorinated microbial polyesters. Biomacromolecules 3:1327–1335
Li J, Li X, Ni X, Leong KW (2003) Synthesis and characterization of new biodegradable amphiphilic poly(ethylene oxide)-b-poly[(R)-3-hydroxy butyrate]-b-poly(ethylene oxide) triblock copolymers. Macromolecules 36:2661–2667
Kim HY, Ryu JH, Chu CW, Son GM, Jeong YI, Kwak TW, Kim DH, Chung CW, Rhee YH, Kang DH et al (2014) Paclitaxel-incorporated nanoparticles using block copolymers composed of poly (ethylene glycol)/poly (3-hydroxyoctanoate). Nanoscale Res Lett 9:525
Ravenelle F, Marchessault RH (2002) One-step synthesis of amphiphilic diblock copolymers from bacterial poly([R]-3-hydroxybutyric acid). Biomacromolecules 3:1057–1064
Kim HW, Chung CW, Kim SS, Kim YB, Rhee YH (2002) Preparation and cell compatibility of acrylamide-grafted poly(3-hydroxyoctanoate). Int J Biol Macromol 30:129–135
Sparks J, Scholz C (2008) Synthesis and characterization of a cationic poly(β-hydroxyalkanoate). Biomacromolecules 9:2091–2096
Toraman T, Hazer B (2014) Synthesis and characterization of the novel thermoresponsive conjugates based on poly(3-hydroxy alkanoates). J Polym Environ 22:159–166
Hazer B (2010) Amphiphilic poly (3-hydroxy alkanoate)s: potential candidates for medical applications. Int J Polym Sci. https://doi.org/10.1155/2010/423460
Babinot J, Guigner JM, Renard E, Langlois V (2012) Poly(3-hydroxyalkanoate)-derived amphiphilic graft copolymers for the design of polymersomes. Chem Commun 48:5364–5366
Dai S, Xue L, Zinn M, Li Z (2009) Enzyme-catalyzed polycondensation of polyester macrodiols with divinyl adipate: a green method for the preparation of thermoplastic block copolyesters. Biomacromolecules 10:3176–3181
Andrade AP, Neuenschwander P, Hany R, Egli T, Witholt B, Li Z (2002) Synthesis and characterization of novel copoly(ester–urethane) containing blocks of poly-[(R)-3-hydroxyoctanoate] and poly-[(R)-3-hydroxybutyrate]. Macromolecules 35:4946–4950
Tappel RC, Kucharski JM, Mastroianni JM, Stipanovic AJ, Nomura CT (2012) Biosynthesis of poly[(R)-3-hydroxyalkanoate] copolymers with controlled repeating unit compositions and physical properties. Biomacromolecules 13:2964–2972
Noda I, Green PR, Satkowski MM, Schechtman LA (2005) Preparation and properties of a novel class of polyhydroxyalkanoate copolymers. Biomacromolecules 6:580–586
Chen GQ, Zhang G, Park SJ, Lee SY (2001) Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl Microbiol Biotechnol 57:50–55
Ke Y, Zhang XY, Ramakrishna S, He LM, Wu G (2016) Synthetic routes to degradable copolymers deriving from the biosynthesized polyhydroxyalkanoates: a mini review. Express Polym Lett 10:36–53
Adamus G, Sikorska W, Janeczek H, Kwiecie M, Sobota M, Kowalczuk M (2012) Novel block copolymers of atactic PHB with natural PHA for cardiovascular engineering: synthesis and characterization. Eur Polym J 48:621–631
Iwata T, Doi Y, Kasuya KI, Inoue Y (1997) Visualization of enzymatic degradation of poly[(R)-3-hydroxybutyrate] single crystals by an extracellular PHB depolymerase. Macromolecules 30:833–839
Neugebauer D, Rydz J, Goebel I, Dacko P, Kowalczuk M (2007) Synthesis of graft copolymers containing biodegradable poly(3-hydroxybutyrate) chains. Macromolecules 40:1767–1773
Hazer B, Hazer DB, Çoban B (2010) Synthesis of microbial elastomers based on soybean oil. Autoxidation kinetics, thermal and mechanical properties. J Polym Res 17:567–577
Li SM, Rashkov I, Espartero JL, Manolova N, Vert M (1996) Synthesis, characterization, and hydrolytic degradation of PLA/PEO/PLA triblock copolymers with long poly(l-lactic acid) blocks. Macromolecules 29:57–62
Acknowledgements
This work was supported by the Bülent Ecevit University Research Funds (#BEU – 2013 – 72118496 - 02 and BEU - 2013 – 72118496 - 03). The authors thank to Prof. Dr. Mahmut Köse and Prof. Dr. İbrahim Demirtaş for their valuable discussion about the NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hazer, B., Akyol, E., Şanal, T. et al. Synthesis of novel biodegradable elastomers based on poly[3-hydroxy butyrate] and poly[3-hydroxy octanoate] via transamidation reaction. Polym. Bull. 76, 919–932 (2019). https://doi.org/10.1007/s00289-018-2410-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2410-2